PURPOSE
The US Food and Drug Administration–expanded access program (EAP) uses a single patient use (SPU) mechanism to provide patient access to investigational agents in situations where no satisfactory or comparable therapy is available. Genomic profiling of de novo and relapsed or refractory childhood cancer has led to increased identification of new drug targets in the last decade. The aim of this study is to examine the SPU experience for genomically targeted therapies in patients with pediatric cancer.
PATIENTS AND METHODS
All genomically targeted therapeutic SPUs obtained over a 5-year period were evaluated at four large pediatric cancer programs. Data were collected on the type of neoplasm, agents requested, corresponding molecularly informed targets, and clinical outcomes.
RESULTS
A total of 45 SPUs in 44 patients were identified. Requests were predominantly made for CNS and solid tumors (84.4%) compared with hematologic malignancies (15.6%). Lack of an available clinical trial was the main reason for SPU initiation (64.4%). The median time from US Food and Drug Administration submission to approval was 3 days (range, 0-12 days) and from Institutional Review Board submission to approval was 5 days (range, 0-50 days). Objective tumor response was seen in 39.5% (15 of 38) of all evaluable SPUs. Disease progression was the primary reason for discontinuation of drug (66.7%) followed by toxicity (13.3%).
CONCLUSION
SPU requests remain an important mechanism for pediatric access to genomically targeted agents given the limited availability of targeted clinical trials for children with high-risk neoplasms. Furthermore, this subset of SPUs resulted in a substantial number of objective tumor responses. The development of a multi-institutional data registry of SPUs may enable systematic review of toxicity and clinical outcomes and provide evidence-based access to new drugs in rare pediatric cancers.
INTRODUCTION
Development of targeted therapeutics accompanied by advances in next-generation sequencing (NGS) has ushered in an era of precision medicine in pediatric oncology within the last decade.1-3 Although the availability and utility of broad sequencing platforms have markedly increased, access to targeted therapies through clinical trials for pediatric patients as compared with adults remains a multifactorial challenge.4-7 Recognition of the need for trials evaluating targeted agents for children with cancer has led to the development of important initiatives such as the NCI-COG Pediatric MATCH trial (NCT03155620).8 Despite these opportunities for access, many patients remain ineligible or are logistically unable to enroll on such clinical research studies. Furthermore, the time from first-in-human to first-in-child clinical trials is long, averaging 6.5 years.9
CONTEXT
Key Objective
Genomic profiling of pediatric cancers frequently reveals potential drug targets; however, access to genomically targeted agents for children remains limited. The single patient use (SPU) mechanism established by the US Food and Drug Administration under its expanded access program provides a mechanism for patients to receive investigational agents when no alternative therapies or clinical trials exist. This study examines the SPU experience for genomically targeted therapies across four large pediatric cancer centers.
Knowledge Generated
Genomically targeted agents were recurrently acquired via SPUs across all institutions, most commonly for CNS and solid tumors. Objective tumor responses were seen in approximately 40% of patients across a broad range of mutation types in hematologic, solid tumor, and CNS neoplasms.
Relevance
The SPU mechanism provides meaningful access to genomically targeted agents for children with cancer. Establishing SPU registries for data collection and sharing would help accelerate drug development for pediatric cancer.
The US Food and Drug Administration (FDA)–expanded access program (EAP) is a mechanism for providing patients with access to investigational agents before FDA approval and outside clinical trials, by using a single patient use (SPU) request, in situations where no satisfactory or comparable therapy is available.10,11 Since 2009, the use of SPUs has rapidly expanded with the FDA authorizing 99% of the SPU applications in FY2010-2015.12 Feit et al13 reviewed SPU in adult and pediatric patients at a single institution and showed that a markedly higher percentage of pediatric patients (34%) received access through SPUs compared with adult patients, while making up only 2% of the overall population at the treating center. They established that the utility of SPUs is broad and can be of meaningful benefit in a small, heavily pretreated patient population. More recently, Shulman et al14 examined the use of SPUs at a single pediatric cancer center over a 12-year period. Most of the indications for filing SPU requests in this study were dictated by the histologic diagnosis of cancers rather than a genomically defined drug target.
Given the paucity of data with genomically targeted SPUs in pediatric oncology, we performed a multi-institutional retrospective review of all such approved requests at four large pediatric oncology centers in the United States. We evaluated the reasons for SPU, timelines for approval, reasons for discontinuation of the drug, and overall responses. We further characterized the specific genomic alterations and drug targets for each SPU.
PATIENTS AND METHODS
We identified a cohort of patients age younger than 30 years who were diagnosed with pediatric neoplasms between January 1, 2014, and June 30, 2019. Patients had SPU applications completed at one of the four large pediatric cancer centers, including the Aflac Cancer and Blood Disorders Center at Children's Healthcare of Atlanta (CHOA), Cincinnati Children's Hospital Medical Center (CCHMC), Dana-Farber/Boston Children's Cancer and Blood Disorders Center (DFCI/BCH), and Memorial Sloan Kettering Cancer Center (MSKCC). Only genomically targeted SPUs informed by DNA and/or RNA sequencing results were included for this analysis. Clinical Laboratory Improvement Amendments–certified institutional and/or commercial testing platforms, namely, MSK-IMPACT, MSK-Fusion, Foundation One Heme, OncoScan, OncoPanel, and OncoKids Cancer Panel, were used to obtain these sequencing results.15-20 Patient demographics, diagnosis, treatment details, responses to treatment, and vital status were abstracted from the electronic medical records at each institution. The follow-up data cutoff date was May 20, 2020. Patients or guardians signed consent for their respective SPU protocol before initiation of therapy. Given the retrospective nature of this study, a waiver of informed consent and Health Insurance Portability and Accountability Act authorization was requested and accepted by the Institutional Review Boards (IRBs) at all four institutions.
All cancers were categorized into one of the three groups: solid tumor, hematologic malignancy, or CNS tumor. Genomic data included the gene target for each SPU including the specific mutation type for each targeted alteration. Indications for initiation of the SPU request and reason for discontinuing drug were assessed. Objective response to therapy was determined at a minimum of 6 weeks after initiation of therapy for CNS and solid tumors and at least 4 weeks after initiation of therapy for hematologic malignancies. Responses were categorized as complete response (CR) or partial response (PR). Only CR was considered a response for hematologic malignancies. In patients with stable disease or disease progression, perceived benefit, if any, was determined by the primary oncologist. All primary treating physicians were asked to assess benefit by selecting one or more of the following options for their patients: (1) symptomatic relief, (2) improved quality of life, or (3) delay in progression that allowed for additional therapy over time. Time needed for FDA approval for emergent or urgent use of targeted agents along with time from IRB submission and approval to initiation of drug therapy was calculated. Finally, duration of therapy from initiation of therapy to date of last dose and last follow-up was determined. Descriptive statistics were used to summarize the demographic information and SPU details for all patients.
RESULTS
We identified 44 patients who met criteria for inclusion in our study (Table 1). Of these, 18 patients were treated at MSKCC, nine patients were treated at CHOA and DFCI each, and eight patients were treated at CCHMC. The median age of the cohort at the time of the SPU initiation was 8 years (range, 0.42-28 years), and 54.5% were female. Overall, 72.7% were White and 77.3% were non-Hispanic or non-Latino. As of the follow-up cutoff date, 24 of 44 (54.5%) patients were alive. Within our cohort, 35 of 44 patients had relapsed or refractory disease at the time of SPU initiation and 9 of 44 had received no prior therapy other than biopsy or resection at the time of diagnosis. In terms of treatment before SPU initiation, 37 of 44 patients had received prior chemotherapy (median of two regimens), 26 of 44 patients had surgery, and 21 of 44 had received radiation therapy.
TABLE 1.
Demographic Information of all Patients (N = 44) Associated With Single Patient Use Requests

We further characterized the SPU requests for each patient. One patient had two separate SPU requests for different agents resulting in a total of 45 SPUs for 44 patients (Table 2). The most common indication for initiation of SPU was the lack of clinical trial availability (64.4%), followed by ineligibility for a clinical trial (28.9%). Among the 29 patients for whom there were no clinical trials to access the desired agent class, three had issues with the commercially available formulation for off-label use (ie, no liquid formulation for children unable to swallow pills), necessitating SPU initiation. For all SPUs, the median time from FDA submission to approval was 3 days (range, 0-12 days) and from IRB submission to approval was 5 days (range, 0-50 days), with comparable times among institutions. The median duration of therapy with the SPU requested medication for all patients was 216 days (range, 2-1,832 days). At the follow-up cutoff date, 14 of 44 (31.8%) patients remained on therapy and 30 patients had discontinued therapy, with one patient being subsequently treated with another agent via second SPU. The main reason for discontinuation of therapy was disease progression in 20 of 30 (66.7%) of all patients followed by toxicity in 4 of 30 (13.3%).
TABLE 2.
Characteristics of Single Patient Use Requests (N = 45) in the Cohort

An objective response was not evaluable in seven SPUs. Reasons for inability to evaluate response included shorter duration of follow-up (< 6 weeks in solid or brain tumors and < 4 weeks in hematologic malignancies; 3 of 7), combination of targeted agent with chemotherapy (1 of 7), and insufficient disease burden at the time of initiation of SPU (3 of 7). Of the remaining 38 SPUs, an objective response was seen in 15 scenarios, with an overall response rate of 39.5%. Of the 15 responders, there were 3 CRs and 12 PRs. Of the three patients who achieved CR, one patient had hematologic malignancy and two had solid tumors. The duration of therapy for these three patients was 216, 531, and 1,085 days, respectively. In partial responders, nine patients had solid tumors and three had brain tumors. The median duration of therapy for partial responders was 620 days (range, 90-1,832 days). Responses were seen across eight different gene targets in all three groups of tumor types. Among nonresponders, seven (18.4%) patients had stable disease and 16 (42.1%) patients experienced disease progression as their best response.
Since SPU drug requests are frequently made when all other lines of therapy are exhausted, we sought to characterize the provider-perceived benefit of genomically targeted SPUs in patients with stable disease or disease progression. This perceived benefit of SPUs was categorized into symptomatic relief, improvement in quality of life, and slowing disease progression with the understanding that a single SPU could result in multiple perceived benefits. Of 23 patients with stable or progressive disease, 13 of 23 (56.5%) did not have any perceived benefit. Among the remaining 10 patients, 7 of 10 (70%) experienced symptomatic relief, 6 of 10 (60%) patients experienced improved quality of life, and 3 of 10 (30%) had delayed progression of disease, which allowed for further therapy.
The specific genomic alterations including their type and the drug targets were further classified for each SPU (Fig 1). Among 18 patients with CNS tumors, BRAF mutations (n = 6) were noted to be most frequent followed by FGFR1 mutations (n = 5; Fig 1A). In the 20 patients with solid tumors, the distribution was more heterogenous, with NF1 being the most common gene mutated (n = 3), followed by an equal number of patients with SMARCB1, RET, NUTM1, NTRK3, MDM2, and ALK alterations (n = 2 each). Each patient with a hematologic malignancy (n = 7) was associated with a unique mutation as depicted in Figure 1A. Mutation type varied by disease site with CNS tumor SPUs primarily targeting single-nucleotide variations, whereas gene fusions were the predominant alteration type targeted in solid tumors, followed by indels and gene amplifications (Fig 1B). Targeted alterations in hematologic malignancies primarily consisted of single-nucleotide variations and gene fusions. When stratified by genomic targets known for all agents included in this analysis, TRKA/B/C followed by FGFR1/2/3 and BRAF were the most common targets identified in the cohort (Fig 1C).
FIG 1.

Genomic alterations and drug targets across 45 SPUs: (A) genes identified with targetable mutations across the study cohort, (B) alteration mechanisms among targeted genes, and (C) SPU drug targets on the basis of corresponding genomic alterations. SNV, single-nucleotide variation; SPU, single patient use.
DISCUSSION
To our knowledge, we present the first analysis of genomically targeted SPUs for pediatric malignancies requested through the FDA EAP from four large pediatric oncology programs in the United States. In this multicenter cohort, SPUs were mainly used for treatment of patients with CNS and solid tumors with specific genomic alterations and approximately 40% of patients demonstrated an objective response. Compared with a previous pediatric cohort described in the study by Feit et al,13 the objective response rate in our population was markedly higher, 15 of 38 (39.5%) versus 6 of 48 (12.5%). In that study, the majority of pediatric patients were not treated with genomically targeted therapies. This difference highlights the effectiveness of targeting mutational vulnerabilities as a therapeutic strategy in patients with relapsed or refractory disease across cancer types.
Over the last decade, the genomic profiling of pediatric cancers has steadily increased with more patients with de novo and relapsed or refractory cancer having access to molecular characterization of their tumors.1,21 Although NGS platforms have enhanced our knowledge of genetic alterations in tumors, interpretation of the results and application to appropriate decision making have increased in complexity as well. For instance, data to support prioritization of agents for patients with multiple targetable lesions, or for combination therapy of targeted agents, are largely lacking for pediatric patients.2 Nonetheless, the clinical benefits of molecular tumor profiling for this population will be substantially limited without improved access to drugs that target NGS-identified vulnerabilities. Legislation aimed at increasing the repertoire of targeted agents to pediatrics has been instituted in the past three decades.22 Since 1997 when the US Congress enacted a pediatric exclusivity provision under the Food and Drug Administration Modernization Act, the incentive for drug companies to evaluate drugs in the pediatric population has slowly but steadily increased. The Pediatric Research Equity Act enacted in 2003 had minimal impact on pediatric anticancer drug development since the requirement for pediatric evaluation for most drugs used in adult cancers was waived. However, the Research to Accelerate Cures and Equity (RACE) Act of 2017 now requires evaluation of drugs in children when a molecular target is shared with adult cancers. The primary goal of these recent legislative acts is to improve access for children with malignancies to clinical trials of targeted agents.22 A subsequent study by Hwang et al23 in 2020 showed that on review of the FDA's Pediatric Molecular Target List, the RACE Act could have increased the proportion of cancer drugs potentially subject to pediatric study requirements from 0% to 78.2%. If the RACE Act is adequately enforced by regulators, it will likely result in the opening and enrollment of pediatric patients in clinical trials for newer agents, which is the preferred way to adequately assess drug dosing, adverse events, and efficacy. However, as demonstrated in the cohort of patients presented here, the FDA EAP continues to be an important mechanism for pediatric patients to receive genomically targeted agents. As mutational profiling becomes more routine across all cancer types, SPU requests may increase in frequency in the coming years.
To begin an SPU, a pharmaceutical partner must be identified who is willing to provide the agent in an appropriate formulation. The process involved in SPU creation, submission, and approval then requires a highly coordinated effort between clinicians, institutional clinical research and regulatory staff, institutional IRBs, and the FDA to ensure a timely approval of the proposed medication (Fig 2). In short, an SPU request is resource intense and a completely unfunded mandate. Within our cohort, the median time for FDA approval and IRB approval was 3 and 5 days, respectively, at highly specialized and resourced pediatric centers. However, there were instances where IRB approval required a much longer period for approval (up to 50 days), which could result in substantial delay in treating patients. It is necessary for institutional IRBs to recognize the importance of the SPU mechanism in procuring targeted agents for pediatric cancer and to convene emergent meetings for selective cases requiring urgent commencement of therapy. As the pool of genomically targeted cancer drugs expands, there will be agents administered for the first time in pediatric populations with little to no preceding clinical data regarding appropriate dose for efficacy. SPU requests in such cases should be accompanied by the ability to obtain pharmacokinetic and pharmacodynamic data from patients to enable dosage titration for efficacy.
FIG 2.
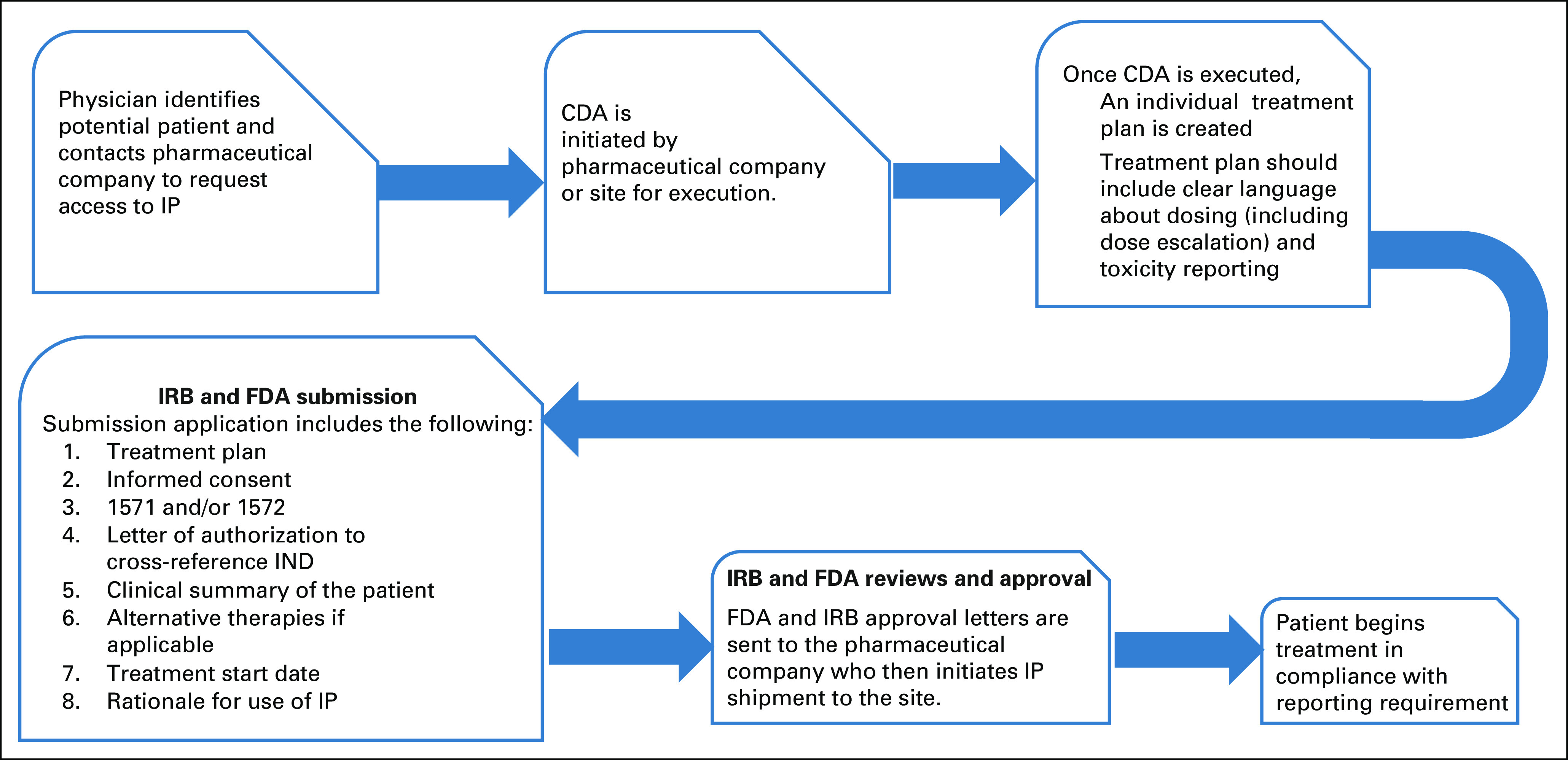
Schematic outlining the SPU approval process (single patient use IND regulatory process). CDA, Confidentiality Disclosure Agreement; FDA, US Food and Drug Administration; IND, investigational new drug; IP, investigational product; IRB, Institutional Review Board; SPU, single patient use.
Despite the mechanisms in place at many institutions to facilitate SPUs for pediatric patients, enrollment in prospective clinical trials and capture of real-world experience using these targeted agents are paramount. Early phase clinical trials enable systematic study and uniform assessment of tolerability and toxicity in the pediatric population as compared with assessments made from treating patients using the FDA EAP mechanism. However, the individual experiences from treating patients with SPUs can be used to inform the development of early phase clinical trials in children.24 Currently, no mechanisms exist for institutions to gather and share data obtained from SPU requests beyond the requirement to inform the pharmaceutical sponsor and the FDA of severe and unexpected adverse events. Data are often reported as isolated case reports or series, which might be skewed toward extraordinary responses and are not necessarily reflective of the potential broader evidence available for drug safety and efficacy. Collective data sharing may provide critical context for the future development of the SPU-provided agents in the pediatric population, offering preliminary evidence of appropriate dosing, toxicity, and target validation. Establishment of prospective registries is needed to uniformly examine the real-world data to accelerate safe and timely evidence-based access to new drugs in rare and refractory pediatric cancers.
Our study has several limitations given its retrospective nature, use of different sequencing platforms, lack of uniformity in drug initiation and treatment across institutions, and inherent differences in administrative structure and resources involved in FDA EAP requests between institutions. Although SPUs outlined here only pertain to genomically targeted drugs, SPUs were also initiated for immunotherapies and cellular therapies, which are not enumerated here. However, our study does offer a real-world perspective into the implementation, access, and responses of pediatric patients to SPU requests across the country.
In conclusion, FDA EAP requests for SPU of drugs remain an important mechanism for access for pediatric patients suffering from rare and refractory cancers. Clinical tumor sequencing of pediatric tumors has resulted in subsequent pursuit of genomically targeted SPUs. As a whole, genomically targeted SPUs have led to substantial objective responses across tumor types and improvement in subjective measures. Larger collaborations between treating institutions and the FDA to systematically compile and analyze data from SPUs will facilitate clinical trial development and enhance understanding of drug mechanisms and toxicities.
ACKNOWLEDGMENT
We thank our many colleagues including M. Karajannis, I. Dunkel, S. Gilheeney, S. Sait, B. Kushner, S. Modak, S. Roberts, P. Meyers, L. Wexler, C. Mazewski, D. Aguilera, T. MacDonald, M. Briones, and M. O'Brien.
Himalee S. Sabnis
Research Funding: Bristol Myers Squibb
Ahmet Zehir
Stock and Other Ownership Interests: Arcus Biosciences, Mirati Therapeutics
Honoraria: Illumina
Jason Fangusaro
Consulting or Advisory Role: Celgene
Steven G. DuBois
Consulting or Advisory Role: Bayer
Research Funding: Merck, Roche/Genentech, Lilly, Curis, Loxo, BMS, Eisai, Pfizer, Turning Point Therapeutics, Bayer, Salarius Pharmaceuticals
Travel, Accommodations, Expenses: Salarius Pharmaceuticals
Uncompensated Relationships: Y-mAbs Therapeutics Inc
Julia Glade-Bender
Research Funding: Eisai, Lilly, Loxo, Roche/Genentech, Bayer
Patents, Royalties, Other Intellectual Property: Patent on a T lymphoblastic lymphoma cell line, CUTLL1
Travel, Accommodations, Expenses: Amgen
Uncompensated Relationships: SpringWorks Therapeutics, Bristol Myers Squibb, Merck, Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/708514
Sharon M. Castellino
Research Funding: Bristol Myers Squibb
Neerav Shukla
Consulting or Advisory Role: Syndax
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Himalee S. Sabnis, David S. Shulman, Benjamin Mizukawa, Jason Fangusaro, Julia Glade-Bender, Sharon M. Castellino, Neerav Shukla
Administrative support: Chanta Whitlow, Marilyn Winchester
Provision of study materials or patients: Himalee S. Sabnis, David S. Shulman, Benjamin Mizukawa, Neerav Shukla
Collection and assembly of data: Himalee S. Sabnis, David S. Shulman, Benjamin Mizukawa, Nancy Bouvier, Vanessa A. Fabrizio, Chanta Whitlow, Marilyn Winchester, Laura Agresta, Brian Turpin, Neerav Shukla
Data analysis and interpretation: Himalee S. Sabnis, David S. Shulman, Benjamin Mizukawa, Ahmet Zehir, Daniel S. Wechsler, Steven G. DuBois, Julia Glade-Bender, Sharon M. Castellino, Neerav Shukla
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Analysis of Genomically Targeted Single Patient Use Requests for Pediatric Neoplasms
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Himalee S. Sabnis
Research Funding: Bristol Myers Squibb
Ahmet Zehir
Stock and Other Ownership Interests: Arcus Biosciences, Mirati Therapeutics
Honoraria: Illumina
Jason Fangusaro
Consulting or Advisory Role: Celgene
Steven G. DuBois
Consulting or Advisory Role: Bayer
Research Funding: Merck, Roche/Genentech, Lilly, Curis, Loxo, BMS, Eisai, Pfizer, Turning Point Therapeutics, Bayer, Salarius Pharmaceuticals
Travel, Accommodations, Expenses: Salarius Pharmaceuticals
Uncompensated Relationships: Y-mAbs Therapeutics Inc
Julia Glade-Bender
Research Funding: Eisai, Lilly, Loxo, Roche/Genentech, Bayer
Patents, Royalties, Other Intellectual Property: Patent on a T lymphoblastic lymphoma cell line, CUTLL1
Travel, Accommodations, Expenses: Amgen
Uncompensated Relationships: SpringWorks Therapeutics, Bristol Myers Squibb, Merck, Eisai
Open Payments Link: https://openpaymentsdata.cms.gov/physician/708514
Sharon M. Castellino
Research Funding: Bristol Myers Squibb
Neerav Shukla
Consulting or Advisory Role: Syndax
No other potential conflicts of interest were reported.
REFERENCES
- 1.Oberg JA, Glade Bender JL, Sulis ML, et al. : Implementation of next generation sequencing into pediatric hematology-oncology practice: Moving beyond actionable alterations. Genome Med 8:133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WE, Pui CH, Yang JJ: The promise and the reality of genomics to guide precision medicine in pediatric oncology: The decade ahead. Clin Pharmacol Ther 107:176-180, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuBois SG, Corson LB, Stegmaier K, et al. : Ushering in the next generation of precision trials for pediatric cancer. Science 363:1175-1181, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Seibel NL, Janeway K, Allen CE, et al. : Pediatric oncology enters an era of precision medicine. Curr Probl Cancer 41:194-200, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Forrest SJ, Geoerger B, Janeway KA: Precision medicine in pediatric oncology. Curr Opin Pediatr 30:17-24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris RE, Adamson PC: Challenges and opportunities in childhood cancer drug development. Nat Rev Cancer 12:776-782, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Laetsch TW, DuBois SG, Bender JG, et al. : Opportunities and challenges in drug development for pediatric cancers. Cancer Discov 11:545-559, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adashek JJ, LoRusso PM, Hong DS, et al. : Phase I trials as valid therapeutic options for patients with cancer. Nat Rev Clin Oncol 16:773-778, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neel DV, Shulman DS, DuBois SG: Timing of first-in-child trials of FDA-approved oncology drugs. Eur J Cancer 112:49-56, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speers MA: Providing patients with critical or life-threatening illnesses access to experimental drug therapy: A guide to clinical trials and the US FDA expanded access program. Pharmaceut Med 33:89-98, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Puthumana J, Miller JE, Kim J, et al. : Availability of investigational medicines through the US Food and Drug Administration's expanded access and compassionate use programs. JAMA Netw Open 1:e180283, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarow JP, Lurie P, Ikenberry SC, et al. : Overview of FDA's expanded access program for investigational drugs. Ther Innov Regul Sci 51:177-179, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feit NZ, Goldman DA, Smith E, et al. : Use, safety, and efficacy of single-patient use of the US Food and Drug Administration expanded access program. JAMA Oncol 5:570-572, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman DS, Kiwinda LV, Edwards S, et al. : Retrospective evaluation of single patient investigational new drug (IND) requests in pediatric oncology. Cancer Med 10:2310-2318, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JC, Offin M, Falcon C, et al. : Comprehensive molecular and clinicopathologic analysis of 200 pulmonary invasive mucinous adenocarcinomas identifies distinct characteristics of molecular subtypes. Clin Cancer Res 27:4066-4076, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Abdel-Wahab O, Nahas MK, et al. : Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood 127:3004-3014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciriello G, Miller ML, Aksoy BA, et al. : Emerging landscape of oncogenic signatures across human cancers. Nat Genet 45:1127-1133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramkissoon SH, Bandopadhayay P, Hwang J, et al. : Clinical targeted exome-based sequencing in combination with genome-wide copy number profiling: Precision medicine analysis of 203 pediatric brain tumors. Neuro Oncol 19:986-996, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiemenz MC, Ostrow DG, Busse TM, et al. : OncoKids: A comprehensive next-generation sequencing panel for pediatric malignancies. J Mol Diagn 20:765-776, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Mansfield AS, Park BH, Mullane MP: Identification, prioritization, and treatment of mutations identified by next-generation sequencing. Am Soc Clin Oncol Ed Book 38:873-880, 2018 [DOI] [PubMed] [Google Scholar]
- 22.RACE act poised to advance pediatric cancer research. Cancer Discov 10:1434, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Hwang TJ, Orenstein L, DuBois SG, et al. : Pediatric trials for cancer therapies with targets potentially relevant to pediatric cancers. J Natl Cancer Inst 112:224-228, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration : FDA briefing document–Pediatric Oncology Subcommittee of the Oncologic Drugs Advisory Committee (ODAC), June 17 and 18, 2020. https://www.fda.gov/media/138940/download [Google Scholar]


