PURPOSE
Burkitt lymphoma (BL) has unique biology and clinical course but lacks a standardized prognostic model. We developed and validated a novel prognostic index specific for BL to aid risk stratification, interpretation of clinical trials, and targeted development of novel treatment approaches.
METHODS
We derived the BL International Prognostic Index (BL-IPI) from a real-world data set of adult patients with BL treated with immunochemotherapy in the United States between 2009 and 2018, identifying candidate variables that showed the strongest prognostic association with progression-free survival (PFS). The index was validated in an external data set of patients treated in Europe, Canada, and Australia between 2004 and 2019.
RESULTS
In the derivation cohort of 633 patients with BL, age ≥ 40 years, performance status ≥ 2, serum lactate dehydrogenase > 3× upper limit of normal, and CNS involvement were selected as equally weighted factors with an independent prognostic value. The resulting BL-IPI identified groups with low (zero risk factors, 18% of patients), intermediate (one factor, 36% of patients), and high risk (≥ 2 factors, 46% of patients) with 3-year PFS estimates of 92%, 72%, and 53%, respectively, and 3-year overall survival estimates of 96%, 76%, and 59%, respectively. The index discriminated outcomes regardless of HIV status, stage, or first-line chemotherapy regimen. Patient characteristics, relative size of the BL-IPI groupings, and outcome discrimination were consistent in the validation cohort of 457 patients, with 3-year PFS estimates of 96%, 82%, and 63% for low-, intermediate-, and high-risk BL-IPI, respectively.
CONCLUSION
The BL-IPI provides robust discrimination of survival in adult BL, suitable for use as prognostication and stratification in trials. The high-risk group has suboptimal outcomes with standard therapy and should be considered for innovative treatment approaches.
INTRODUCTION
Burkitt lymphoma (BL) is a rare mature high-grade B-cell lymphoma that globally constitutes 2% of all adult non-Hodgkin lymphomas, with an estimated incidence of 11,285 cases per year.1,2 BL is curable for many patients with intensive multiagent immunochemotherapy, despite often presenting with disseminated disease and not uncommonly with blood or CNS involvement.3,4 BL has been classified into variably defined low- and high-risk groups.5-12 However, the historically defined low-risk group constitutes only a minority (approximately 10%) of patients with localized nodal disease. By contrast, the high-risk group contains most patients (> 85%-90%) with disseminated BL and is typically not stratified further. Previous studies have analyzed age, performance status (PS), and involvement of the bone marrow or CNS as high-risk indicators.10,13,14 Collectively, no uniform agreement on clinical prognostication or reproducible risk stratification exists, posing challenges to individualized therapy, interpretation of clinical trials, and design of future studies.
CONTEXT
Key Objective
Can a Burkitt lymphoma (BL)–specific prognostic index stratify progression-free survival (PFS)?
Knowledge Generated
We derived the BL International Prognostic Index (BL-IPI) using a real-world cohort of 633 patients treated in the United States and externally validated its performance in an independent cohort of 457 patients treated in Scandinavia, the United Kingdom, Canada, and Australia. The BL-IPI includes age ≥ 40 years, lactate dehydrogenase > 3× upper limit of normal, performance status ≥ 2, and CNS involvement as risk factors, and delineated groups with low (no-risk factors, 3-year PFS = 92%), intermediate (one factor, PFS = 72%), and high risk (≥ 2 factors, PFS = 53%).
Relevance
The BL-IPI can be used to describe distribution of risk in clinical trial participants and help design future trials focused on patients who have excellent prognosis using currently available chemoimmunotherapy and the poor-risk group who are in need of novel treatment approaches.
Our objective was to develop and validate a simple prognostic index specific to adult (sporadic and immunodeficiency-related) BL that would account for its unique clinical features and that would be applicable across various geographic settings where standard immunochemotherapy regimens are applied. Because BL typically presents at a younger age than diffuse large B-cell lymphoma (DLBCL) and often with highly elevated serum lactate dehydrogenase (LDH), the index should determine the best prognostic cutoffs for these variables.
Identification of well-defined risk groups could assist clinicians in more accurately identifying prognosis for adult patients with BL. It may also help design future treatment approaches, which could involve de-escalation of intensive chemotherapy where possible or introduction of novel strategies suitable for groups that show unsatisfactory outcomes. Toward this end, we leveraged data from a large multi-institutional study of BL from the United States15 and conducted external validation (including assessment of calibration and discrimination) in an independent international data set.16
METHODS
Data Sources
Development of the BL International Prognostic Index (BL-IPI) was based on a retrospectively collected BL Real-World Evidence data set of 641 adults (age > 18 years) treated between 2009 and 2018 across 30 academic and community institutions in the United States (Data Supplement, online only).15 This study was approved by Institutional Review Boards at each center. Cases had to meet the 2016 WHO definition of BL based on the characteristic small or medium cell morphology with tingible body macrophages, the presence of MYC rearrangement (not mandatory if all other criteria were met, as observed in 10% of patients), expression of CD10 and BCL6, lack of BCL2, and proliferative fraction of approximately 100%.15,17 Twenty-one submitted cases consistent with other high-grade lymphomas (including double-hit lymphomas or DLBCL) were excluded after review of pathology reports. All staging evaluations, including bone marrow and CNS, were performed using local institutional standards, and treatments followed local practice. This analysis included 633 patients who received multiagent chemotherapy, including rituximab in > 90%.
The external derivation cohort was constructed by pooling data from a retrospective study from Australia, Canada, Denmark, Norway, and Sweden (2004-2017) with a specifically collected data set from eight hospitals in the United Kingdom (2008-2019).18 These multi-institutional registries were harmonized with regard to all essential variables (Data Supplement). All patients in the validation data set received standard immunochemotherapy, including rituximab in > 95%.
Variables and End Points
The following variables were considered for inclusion in the BL-IPI: age, sex, HIV status, PS according to the Eastern Cooperative Oncology Group (ECOG) scale, stage (I and/or II or III and/or IV), B symptoms, involvement of > 1 extranodal site, bone marrow, or CNS, the absence of MYC rearrangement, hemoglobin level, serum albumin, and serum LDH normalized to local upper limit of normal (ULN). We did not consider tumor size because of low ascertainment and unavailability in the validation data set.
We used progression-free survival (PFS) as the primary end point, defined as time from diagnosis until disease progression, recurrence, death from any cause, or last follow-up, according to the International Working Group guideline.19 Overall survival (OS) was a secondary end point.
Statistical Analysis
We estimated survival curves using the Kaplan-Meier method and examined prognostic association between explanatory variables and PFS using univariable and multivariable proportional hazard models fitted in the derivation data set. The analysis (previously reported for the entire data set)15 was focused on the 633 patients treated with immunochemotherapy. First, we inspected the continuous variables for potential nonlinear associations and determined the optimal prognostic cutoffs (Data Supplement). Because many variables were highly overlapping, we selected the most informative prognostic factors using two methods: stepwise selection and lasso for model selection, repeated in 1,000 bootstrapped samples for internal validation (see the Data Supplement for detailed criteria and methods).20 Cox models were augmented by multiple imputation using chained equations, generating 15 imputed data sets to account for missing data (assumed to be missing at random) on stage (2%), HIV (2%), extranodal involvement (2%), LDH (6%), PS (7%), hemoglobin (5%), and albumin (9%), and pooling estimates using Rubin's rules.21 Lasso was conducted using adaptive λ* selection.22,23 Both methods converged on the same four variables that were equally weighted based on observed model coefficients. We then defined the BL-IPI categories by inspection of survival curves and assessed the robustness of risk discrimination by evaluating survival estimates in informative subsets.
In the validation cohort, we assessed the relative size of each BL-IPI category and computed calibration by calculating PFS and/or OS observed in each predicted risk category. We used Harrell's C concordance statistic as a measure of model discrimination. Statistical analyses were conducted using Stata/MP16.1 (StataCorp LLC, College Station, TX). All estimates are provided with 95% CIs.
RESULTS
Patient Characteristics
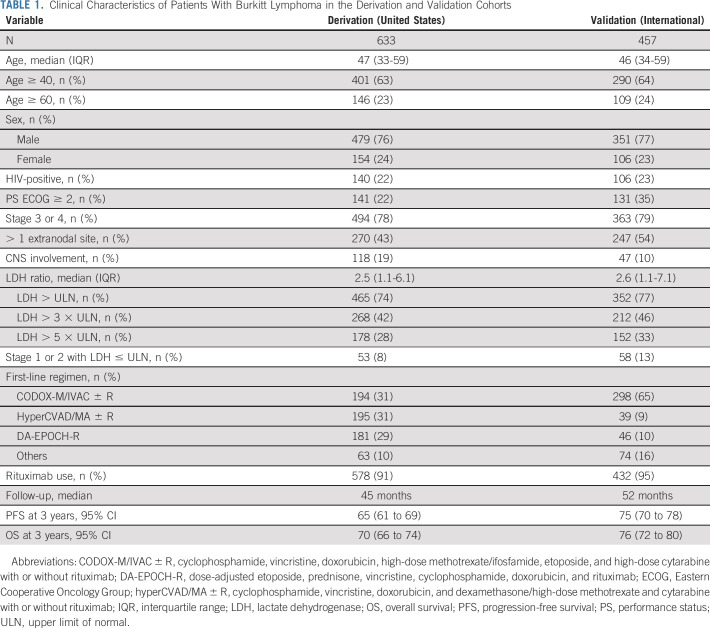
Characteristics of patients in the derivation (N = 633) and validation (N = 457) cohorts were similar (Table 1), with a median age 46-47 years, predominance of men, and 22%-23% of HIV-positive individuals. CNS involvement was more frequent in the US cohort (19%) versus the international (10%) cohort (P < .001). More than 75% of patients in both data sets had advanced-stage BL and abnormal LDH. Furthermore, only 10% had disease that might historically be qualified as low-risk (stage I or II with normal LDH).
TABLE 1.
Clinical Characteristics of Patients With Burkitt Lymphoma in the Derivation and Validation Cohorts
In the US cohort, patients received various chemotherapy regimens suggested by the National Comprehensive Cancer Network guidelines.24 By contrast, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine with or without rituximab (CODOX-M/IVAC ± R regimen) was preferentially used in the international cohort.5,7-9,25,26 More than 90% of patients received rituximab. The median follow-up was 45 and 52 months for the US and international cohorts, respectively. Median PFS or OS was not reached, but PFS estimates at 3 years were higher in the international cohort (75%; 95% CI, 70 to 78) compared with the US data (65%; 95% CI, 61 to 69; Data Supplement).
Development of the BL-IPI
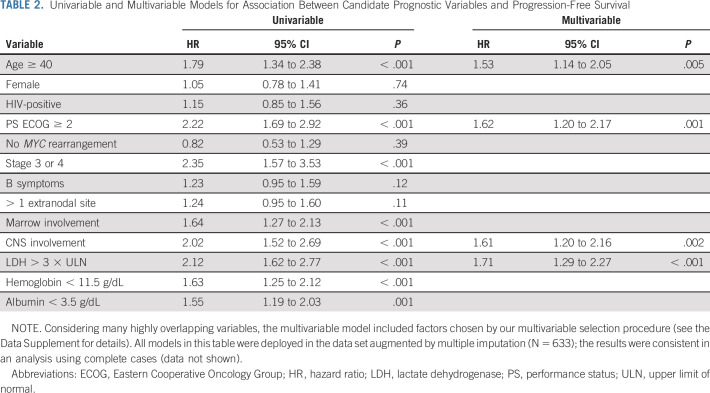
Based on the observed concordance statistics, we determined that age ≥ 40 years, LDH > 3 × ULN, hemoglobin < 11.5 g/dL, and albumin < 3.5 g/dL were optimal prognostic cutoffs in BL (Data Supplement). In univariable analyses, age ≥ 40 years, PS ECOG ≥ 2, advanced stage, involvement of the bone marrow, CNS, LDH > 3 × ULN, low hemoglobin, and low albumin were associated with inferior PFS, but only four factors were forward selected for the multivariable model: age ≥ 40 years, PS ECOG ≥ 2, LDH > 3 × ULN, and CNS involvement (Table 2). The same four variables were consistently selected by the lasso method (Data Supplement).
TABLE 2.
Univariable and Multivariable Models for Association Between Candidate Prognostic Variables and Progression-Free Survival
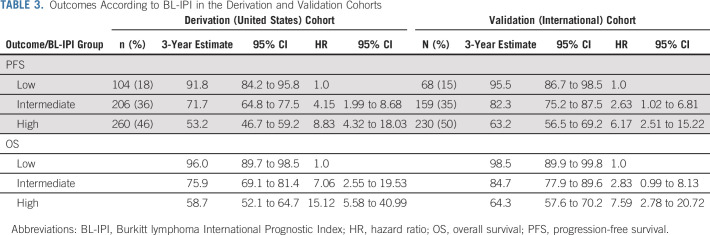
Since model coefficients for all variables were similar, we constructed the BL-IPI by assigning equal weight (one point) to each factor (Fig 1A). Because PFS (Fig 1B) and OS (Data Supplement) did not statistically significantly differ between groups with two versus three versus four factors, we combined these groups into one high-risk category. The final BL-IPI comprised three categories: low-risk (zero risk factors; 18% of patients; 3-year PFS = 92%), intermediate-risk (one risk factor; 36% of patients; 3-year PFS = 72%), and high-risk (≥ 2 factors; 46% of patients; 3-year PFS = 53%; Table 3). Median PFS was estimable only in the high-risk group (46 months; 95% CI, 19 to 53). The three-group model was prognostic for PFS (Fig 1C; log-rank P < .0001; C-statistic = 0.655) and OS (Fig 1D; P < .0001; C-statistic = 0.667), with only marginally higher concordance for the five-group version (C-statistic = 0.666 for PFS, 0.685 for OS).
FIG 1.
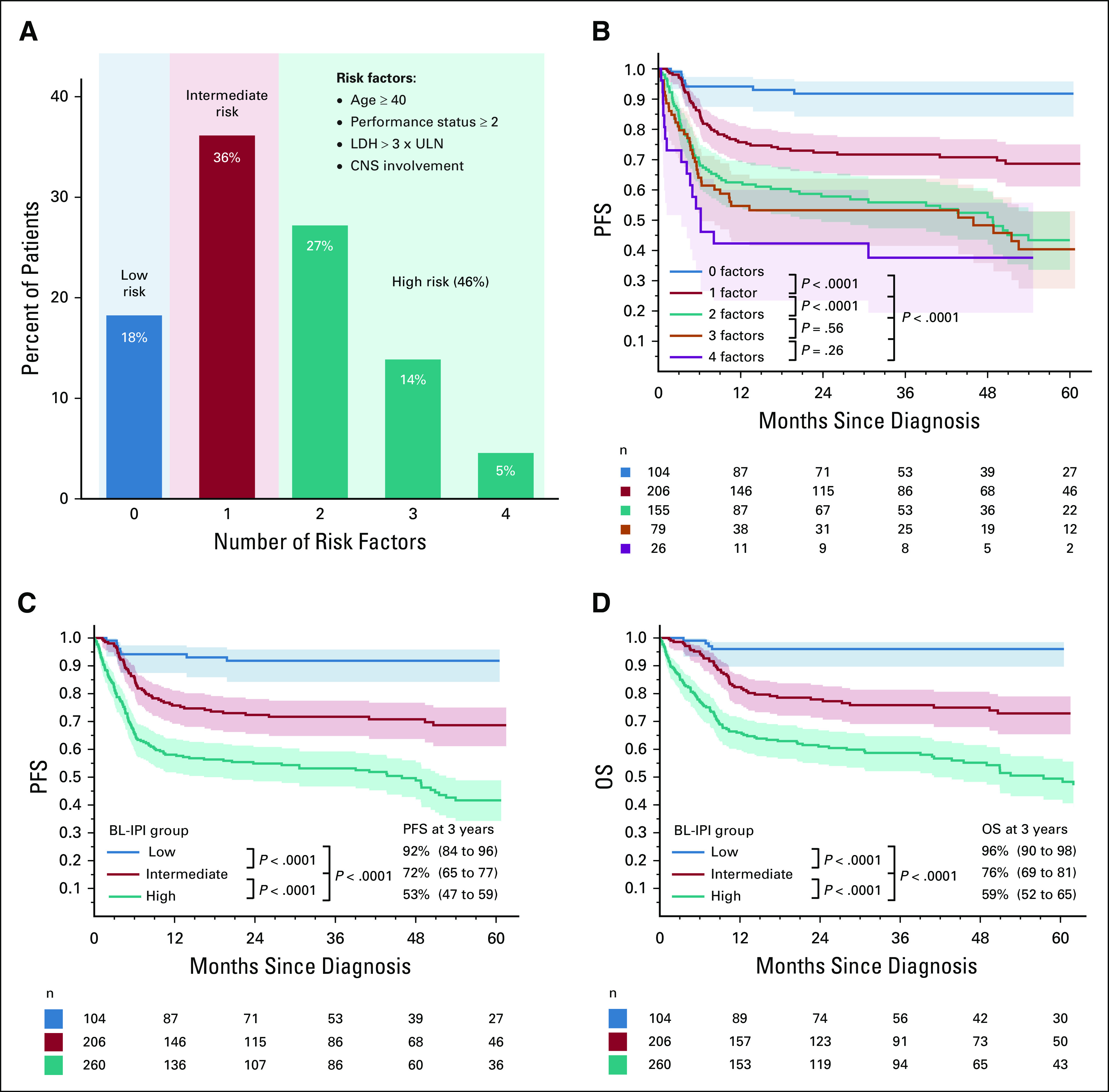
Characteristics of the BL-IPI in the derivation (US) cohort: (A) proportion of patients according to the number of risk factors; (B) PFS according to the number of risk factors; PFS of patients with two versus three, and three versus four risk factors was not statistically significantly different, and hence, these groups were combined in the high-risk category; (C) PFS according to BL-IPI risk group; (D) OS according to BL-IPI risk group; 3-year PFS and OS estimates are listed with 95% CIs (in parentheses); P values are from log-rank tests comparing groups sequentially and from an overall log-rank test. BL-IPI, Burkitt lymphoma International Prognostic Index; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; ULN, upper limit of normal.
TABLE 3.
Outcomes According to BL-IPI in the Derivation and Validation Cohorts
The BL-IPI largely recapitulated the historical designation of low-risk BL from clinical trials; however, it identified a larger group with excellent survival. Furthermore, overlap with traditional IPI categories27 was low (Data Supplement). Within the low-risk BL-IPI category, 54% had small elevations of LDH (1-3 × ULN) and 58% had advanced stage (although only 12% had marrow involvement), but prognosis of patients with these factors did not significantly differ from others within the same BL-IPI risk group (Data Supplement). Discrimination provided by the BL-IPI was slightly better than that for the traditional IPI27 (C-statistic = 0.638 for PFS, 0.657 for OS) and only marginally lower than that for a comprehensive model that included all variables significant in univariate analysis (C-statistic = 0.679 for PFS, 0.706 for OS; Data Supplement).
Subset Analyses
BL-IPI provided prognostic separation in stage III or IV BL, historically considered uniformly high-risk and constituting 78% of the US cohort. In advanced-stage BL, 3-year PFS estimates were 87%, 71%, and 52% for the low-, intermediate-, and high-risk BL-IPI categories, respectively, and 3-year OS estimates were 95%, 75%, and 57%, respectively (Data Supplement). The presence of any BL-IPI risk factor was also associated with worse prognosis in early-stage BL, with the 3-year PFS of 98% for low-risk and 73%-76% for high-risk and intermediate-risk categories (Data Supplement). BL-IPI remained prognostic in the subset of cases with confirmed MYC rearrangement (Data Supplement).
In addition, BL-IPI was prognostic in the derivation cohort among patients receiving rituximab and those without HIV infection. In the HIV-positive group, patients with high-risk BL-IPI had worse 3-year PFS (48%) than those with low risk or intermediate risk (75%-76%, Data Supplement). As delineated in the Data Supplement, BL-IPI retained prognostic value regardless of the specific chemotherapy regimen: CODOX-M/IVAC ± R (3-year PFSs of 88%, 67%, and 61%, respectively), dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R: 3-year PFSs of 87%, 73%, and 51%, respectively), or cyclophosphamide, vincristine, doxorubicin, and dexamethasone/high-dose methotrexate and cytarabine with or without rituximab (hyperCVAD/MA ± R: 3-year PFS of 100%, 80%, and 54%, respectively).
External Validation of the BL-IPI
In the validation cohort, BL-IPI categories were of similar relative size to the derivation cohort (low-risk 15%, intermediate-risk 35%, and high-risk 50% of patients, Table 3) and provided similar discrimination of PFS (C-statistic = 0.648; log-rank P < .0001; 3-year estimates of 96%, 82%, and 63% for low-, intermediate- and high-risk groups, respectively; Fig 2A) and OS (C-statistic = 0.670; P < .0001; 3-year estimates of 99%, 85%, and 64%, respectively; Fig 2B). In this international data set, BL-IPI remained prognostic in advanced- and early-stage BL (Data Supplement), among patients receiving rituximab, and those receiving CODOX-M/IVAC ± R (Data Supplement).
FIG 2.
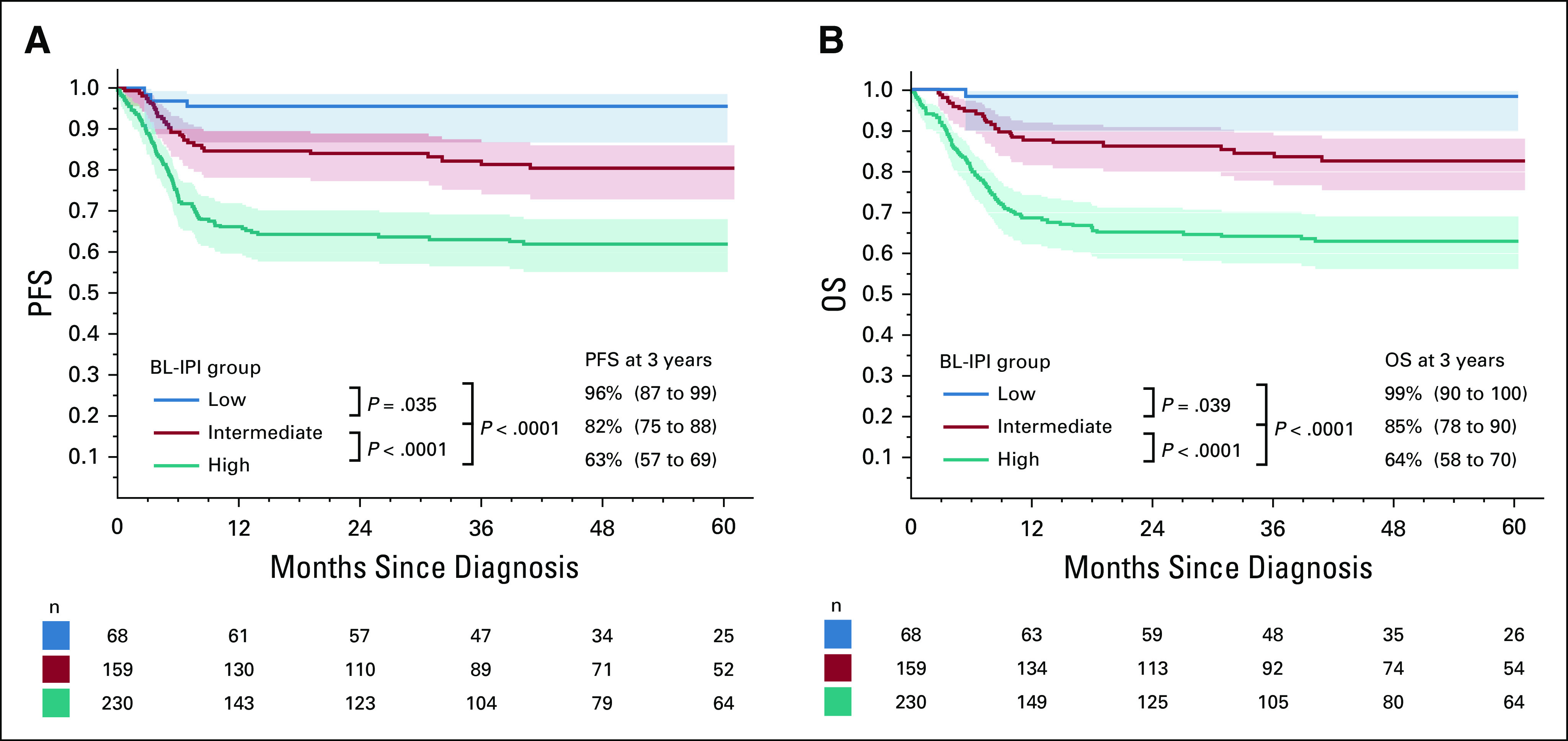
Performance of the BL-IPI in the validation (international) cohort: (A) PFS according to BL-IPI risk group, (B) OS according to BL-IPI risk group; 3-year PFS and OS estimates are listed with 95% CIs (in parentheses); P values are from log-rank tests comparing groups sequentially and from an overall log-rank test. BL-IPI, Burkitt lymphoma International Prognostic Index; OS, overall survival; PFS, progression-free survival.
DISCUSSION
The BL-IPI, as developed and validated through this large international collaboration comprising over 1,000 individual patients, provides a novel, validated prognostic index specific to sporadic and immunodeficiency-related adult BL. It permits a simple yet robust risk stratification for diverse patient cohorts treated with rituximab-based immunochemotherapy. The low-risk BL-IPI group comprised nearly 20% of patients who attained the PFS of > 90% and the OS > 95% across both data sets. Conversely, patients with high-risk BL-IPI consistently achieved 3-year PFS/OS of < 65% with modern immunochemotherapy, indicating adequate calibration of the index. Notably, the BL-IPI relies on real-world data from multiple countries and continents, supporting its utility both for clinical prognosis and for the design of prospective clinical trials. In interpreting these observations, several factors should be considered.
BL is an aggressive malignancy that can be eradicated using dose-intensive immunochemotherapy. Consequently, some studies have not differentiated treatment according to disease stage. For example, treatment was uniform (except for CNS-directed therapy) in studies using hyperCVAD/MA ± R or Cancer and Leukemia Group B protocols.28,29 Recognizing that BL may occasionally present with a localized tumor, others distinguished a low-risk category that has been variably defined using criteria of early stage, completely resected and/or nonbulky tumor, normal LDH, and good PS (Data Supplement).7,8,11,12,26,30 In clinical practice, adults meeting these criteria are uncommon. By contrast, the low-risk BL-IPI category identified 18% of patients with excellent survival, including younger individuals with slightly elevated LDH and/or advanced stage. Such patients would have been historically classified as high risk, but their outcomes do not significantly differ from others within the low-risk BL-IPI category. Therefore, this importantly expands the lower-risk group for whom less or lower intensity therapy is a viable option, although any treatment modifications need to be examined in prospective studies.
In addition, the BL-IPI provides substantial, conceptual, and practical advantage over the traditional IPI.27 Prior studies have reported many potential prognostic variables in BL, but multivariable analyses have been limited by small sample sizes and collinearity.7,10,12,13,18,31-33 The traditional IPI provided survival discrimination in some studies, mainly in a dichotomized version corresponding to early-stage disease with normal LDH.28,30,33 In a population-based study, the IPI was prognostic but was outperformed by a simpler index that used only 3 factors: age > 40 years, LDH > ULN, and PS ECOG > 1.14 Notably, the traditional IPI was not prognostic in trials using CODOX-M/IVAC (P = .89) or DA-EPOCH-R (P = .29).6,12 We found that age > 60 years and LDH > ULN, cutoffs useful for DLBCL and follicular lymphoma, were not optimal in BL, which presents at age > 60 in < 25% of cases.13,27,34 The presence of > 1 extranodal site had no significant association with PFS. LDH was abnormal in approximately 75% of patients, and minor elevations were less prognostic than the very high levels characteristic of disseminated BL. Similarly, most patients with BL present at an advanced stage, thus limiting the utility of the early or advanced dichotomization.
By contrast, CNS invasion retains major prognostic impact.10,12,35 In the phase III trial by Ribrag et al,10 abnormal LDH, albumin, and anemia predicted worse OS. Despite tailoring of treatment according to bone marrow and CNS involvement (including mandatory CNS irradiation), CNS disease was associated with worse 3-year EFS in every age stratum, including 67% with CNS involvement versus 92% without for age < 40 years. CNS and bone marrow involvement were also the principal adverse factors after treatment with DA-EPOCH-R.12 In the GMALL trial, CNS involvement (treated with 24 Gy irradiation) was associated with worse outcomes in univariable analysis, but it was no longer significant after adjustment for age, marrow involvement, LDH > ULN, and female gender.33 Similarly, CNS involvement was not prognostic in a British Columbia series, but only 8 patients had CNS involvement.32 It is possible that the adverse prognostic impact of CNS involvement may be mitigated by more intensive CNS-directed therapy than that is applied in the current practice, especially considering suboptimal adherence to intrathecal protocols observed in the US data.35 An ongoing randomized study comparing R-CODOX-M/R-IVAC versus DA-EPOCH-R for patients with newly diagnosed BL (EudraCT: 2013-004394-27) will help address the role of different chemotherapy strategies.
The BL-IPI integrates information about patient's medical status and disease burden in a parsimonious, easy-to-apply score and showed consistent calibration and discrimination when externally validated. Yet, its concordance statistic was < 0.70, which may be partly related to overall high rate of censoring (62%) known to bias the C-statistic. Patients with all four high-risk factors may have particularly poor PFS (< 40% at 3 years), but this group is so small (< 5% in both data sets) that it was more practical to include it with other higher-risk groups. Notably, concordance did not meaningfully improve with a comprehensive multivariable model using all factors statistically significant in univariate analysis. By contrast, it improved when treatment factors were included (Data Supplement), strongly suggesting that prognosis in BL is influenced by postdiagnosis events (eg, treatment tolerance and local management expertise) and/or by uncaptured molecular characteristics. Mechanisms driving the hyperproliferative biology of BL include MYC rearrangement; mutations in the centroblast transcription factor TCF3 or its negative regulator ID3; alterations of TP53, CCND3, and CDKN2A; and tonic B-cell receptor signaling.4,36 Prognostic impact of specific molecular events unfortunately has not been adequately examined, although it might be central to further improvement in prognostication for BL.
The BL-IPI, like other prognostic scores for non-Hodgkin lymphomas, was derived retrospectively, and thus, it should be interpreted with caution in clinical practice.27,34,37 It may help clinicians incorporate risk stratification into management decisions and can facilitate research on improved therapeutic approaches. The low-risk BL-IPI group is large enough to consider de-escalated treatment strategies. As a proof of concept, Roschewski et al12 reported 100% event-free survival in strictly defined low-burden BL (stage I or II, tumor < 7 cm, PS ECOG ≤ 1, normal LDH) using just three courses of DA-EPOCH-R (with two rituximab doses per cycle) without CNS prophylaxis. This less toxic, yet highly efficacious approach may allow introduction of novel, rationally selected targeted agents. Conversely, patients with high-risk BL-IPI need better treatment, and trials focused on this group could show significant survival increments with fewer subjects. Moreover, the consistent performance of the BL-IPI in our external validation cohort suggests that trials will benefit from harmonized stratification and comparison with real-world benchmarks.
Limitations of our study include retrospective design and lack of formal central pathology review. However, each case was diagnosed by expert academic hematopathologists, and we applied strict WHO diagnostic criteria.17 We did not have consistent data on Epstein-Barr virus detection or systematic evaluation of blood involvement by flow cytometry. Also, a prognostic index is not necessarily predictive of benefit from any therapy, and BL-IPI–derived risk estimates assume treatment with standard approaches. The index is not validated for endemic BL, and it may be less informative for patients age > 65 years, whose outcomes principally depend on comorbidity-related potential to withstand intensive treatment. The BL-IPI performance characteristics also suggest that a dynamic component incorporating postdiagnosis factors (eg, early toxicity) may provide a more individualized survival prediction. The causes of survival differences between the US and international cohorts are unclear, potentially reflecting variable staging (as suggested by varying rates of CNS involvement) or treatment paradigms, including decentralized oncology care in the United States, varying expertise, and socioeconomic disparities. Additional validation using clinical trial data could further confirm calibration of BL-IPI–based predictions with rigorously applied, uniform therapy.
In conclusion, the BL-IPI is a simple and robust prognostic model that accounts for the unique characteristics of BL. This novel, validated index can help clinicians more accurately identify prognosis for their patients with adult BL and advance targeted clinical research with the dual goals of de-escalating treatment intensity for low-risk disease and improving survival for the high-risk group through innovative approaches. Finally, the availability of BL-IPI may foster inquiry into molecular differences that lead to divergent clinical outcomes, which are not well-explained by the gross clinical indicators of disease burden.
APPENDIX 1. The Burkitt Lymphoma International Prognostic Index Consortium
Author list
Investigators are listed by country. Principal investigator names are in bold.
Australia
1. Chan Y Cheah, MBBS: Linear Clinical Research and Sir Charles Gairdner Hospital, Perth, Australia
Canada
2. Alina S. Gerrie, MD, MPH, FRCPC: BC Cancer Centre for Lymphoid Cancer and The University of British Columbia, Vancouver, BC, Canada
3. Kevin Song, MD, FRCPC: BC Cancer Centre for Lymphoid Cancer and The University of British Columbia, Vancouver, BC, Canada
Denmark
4. Tarec C. El-Galaly, MD: Aalborg University Hospital, Aalborg, Denmark
5. Lasse H. Jakobsen, PhD: Aalborg University Hospital, Aalborg, Denmark
Norway
6. Knut B. Smeland, MD: Oslo University Hospital, Oslo, Norway
Sweden
7. Fredrik Ellin, MD, PhD: Lund University, Lund, Sweden
United Kingdom
8. Anna Santarsieri, MBBS: Cambridge University Hospitals NHSFT, Cambridge, United Kingdom
9. Shireen Kassam, MBBS, FRCPath, PhD: King's College Hospital, London, United Kingdom
10. Mark Bower, MD: National Centre for HIV Malignancy, Chelsea and Westminster Hospital, London, United Kingdom
11. Alessia Dalla Pria, MD: National Centre for HIV Malignancy, Chelsea and Westminster Hospital, London, United Kingdom
12. Nicolas Martinez-Calle, MD, PhD: Nottingham University Hospitals, NHS Trust, Nottingham, United Kingdom
13. Graham P. Collins, MD, DPhil: Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
14. Xiao-Yin Zhang, PhD: Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
15. Silvia Montoto, MD: St Bartholomew's Hospital, Barts Health NHS Trust, London, United Kingdom
16. Kate Cwynarski, MBBS, PhD: University College London Hospitals NHS Foundation Trust, London, United Kingdom
17. Catherine Zhu, MD: University College London Hospitals NHS Foundation Trust, London, United Kingdom
18. Elizabeth H. Phillips, MBBS, BSc: University of Manchester, Manchester, United Kingdom
United States
19. Vaishalee P. Kenkre, MD: Carbone Cancer Center, University of Wisconsin, Madison, WI
20. Christopher D'Angelo, MD: Carbone Cancer Center, University of Wisconsin, Madison, WI
21. Alexey Danilov, MD, PhD: City of Hope Comprehensive Cancer Center, Duarte, CA
22. Nadia Khan, MD: Fox Chase Cancer Center, Philadelphia, PA
23. Allandria Straker-Edwards, MD: Fox Chase Cancer Center, Philadelphia, PA
24. Maryam Sarraf Yazdy, MD: Georgetown University Hospital, Washington, DC
25. Ayushi Chauhan, MBBS: Georgetown University Hospital, Washington, DC
26. Tatyana A. Feldman, MD: John Theurer Cancer Center, Hackensack Meridian Health School of Medicine, Hackensack, NJ
27. Lori A. Leslie, MD: John Theurer Cancer Center, Hackensack Meridian Health School of Medicine, Hackensack, NJ
28. Gabriella Magarelli, APN: John Theurer Cancer Center, Hackensack Meridian Health School of Medicine, Hackensack, NJ
29. Daniel Rector: John Theurer Cancer Center, Hackensack Meridian Health School of Medicine, Hackensack, NJ
30. Max J. Gordon, MD: Knight Cancer Institute, Oregon Health & Science University, Portland, OR
31. Andrzej Stadnik, MPH, BS: Knight Cancer Institute, Oregon Health & Science University, Portland, OR
32. Adam J. Olszewski, MD: Lifespan Cancer Institute, The Warren Alpert Medical School of Brown University, Providence, RI
33. Adam S. Zayac, MD: Lifespan Cancer Institute, The Warren Alpert Medical School of Brown University, Providence, RI
34. Scott E. Smith, MD, PhD, FACP: Loyola University Medical Center, Maywood
35. Stephanie Berg, DO: Loyola University Medical Center, Maywood
36. Daulath Singh, MD: Loyola University Medical Center, Maywood
37. Reem Karmali, MD, MSc: Northwestern University, Chicago, IL
38. Madelyn Burkart: Northwestern University, Chicago, IL
39. Seema Naik, MD: Penn State Cancer Institute, Penn State Hershey Medical Center, Hershey, PA
40. Ryan Vaca, MD: Penn State Cancer Institute, Penn State Hershey Medical Center, Hershey, PA
41. Catherine Diefenbach, MD: Perlmutter Cancer Institute, NYU Langone Health, New York, NY
42. Yun Kyong Choi, MD: Perlmutter Cancer Institute, NYU Langone Health, New York, NY
43. Suchitra Sundaram, MD: Roswell Park Comprehensive Cancer Center, Buffalo, NY
44. Parameswaran Venugopal, MD: Rush University Medical Center, Chicago, IL
45. Seo-Hyun Kim, MD: Rush University Medical Center, Chicago, IL
46. Andrew M. Evens, DO, MSc, FACP: Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
47. Kevin A. David, MD: Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
48. Catherine Wei, MD: Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
49. Yong Lin, PhD: Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
50. Izidore S. Lossos, MD: Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, Miami, FL
51. Juan P. Alderuccio, MD: Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, Miami, FL
52. Jeremy Ramdial, MD: Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, Miami, FL
53. Asaad Trabolsi, MD: Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, Miami, FL
54. Deepa Jagadeesh, MD: Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH
55. Yusra Shao, MD: Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH
56. Agrima Mian, MBBS, MD: Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH
57. Narendranath Epperla, MD, MS: The Ohio State University James Comprehensive Cancer Center, Columbus, OH
58. David A. Bond, MD: The Ohio State University James Comprehensive Cancer Center, Columbus, OH
59. Neil Palmisiano, MD, MS: Thomas Jefferson University, Philadelphia, PA
60. Andreas K. Klein, MD: Tufts Medical Center, Boston, MA
61. Amandeep Godara, MD: Tufts Medical Center, Boston, MA
62. Kirsten M. Boughan, DO: University Hospitals Seidman Cancer Center, Cleveland, OH
63. Paolo F. Caimi, MD: University Hospitals Seidman Cancer Center, Cleveland, OH
64. Craig A. Portell, MD: University of Virginia
65. Victor M. Orellana-Noia, MD: University of Virginia
66. Manali Kamdar, MD, MBBS: University of Colorado Cancer Center, Aurora, CO
67. Bradley M. Haverkos, MD: University of Colorado Cancer Center, Aurora, CO
68. David Peace, MD: University of Illinois College of Medicine, Chicago, IL
69. Albert Ren: University of Illinois College of Medicine, Chicago, IL
70. Emma Rabinovich, MD: University of Illinois College of Medicine, Chicago, IL
71. Sarah Stettner: University of Illinois College of Medicine, Chicago, IL
72. Umar Farooq, MD: University of Iowa, Iowa City, IA
73. Seth M. Maliske, MD: University of Iowa, Iowa City, IA
74. Tycel J. Phillips, MD: University of Michigan Comprehensive Cancer Center, Dexter, MI
75. Veronika Bachanova, MD: University of Minnesota, Minneapolis, MN
76. Malvi Savani, MD: University of Minnesota, Minneapolis, MN
77. Matthew A. Lunning, DO, FACP: University of Nebraska Medical Center, Omaha, NE
78. Heather Nutsch: University of Nebraska Medical Center, Omaha, NE
79. Stephen D. Smith, MD: University of Washington/Fred Hutchinson Cancer Research Center, Seattle, WA
80. Amy Sperling, BS: University of Washington/Fred Hutchinson Cancer Research Center, Seattle, WA
81. Nishitha Reddy, MD, MBBS: Vanderbilt University Medical Center, Nashville, TN
82. Peter Martin, FRCPC, MD, MS: Weill Cornell Medicine-New York Presbyterian Hospital, New York, NY
83. Guarav Varma, MD, MPH: Weill Cornell Medicine-New York Presbyterian Hospital, New York, NY
84. Kristie A. Blum, MD: Winship Cancer Institute, Emory University, Atlanta, GA
85. Michael C. Churnetski: Winship Cancer Institute, Emory University, Atlanta, GA
PRIOR PRESENTATION
Presented in part at the 62nd ASH Annual Meeting and Exposition, Oral Presentation, December 5-8, 2020.
SUPPORT
The work of Tarec C. El-Galaly was supported by Danish Cancer Society.
AUTHOR CONTRIBUTIONS
Conception and design: Adam J. Olszewski, Mark Bower, Izidore S. Lossos, Scott E. Smith, Chan Y. Cheah, Tarec C. El-Galaly, Andrew M. Evens
Administrative support: Andrew M. Evens
Provision of study materials or patients: Adam J. Olszewski, Graham P. Collins, Veronika Bachanova, Kristie A. Blum, Kirsten M. Boughan, Mark Bower, Catherine Diefenbach, Alina S. Gerrie, Nadia Khan, Izidore S. Lossos, Seema Naik, Nishitha Reddy, Maryam Sarraf Yazdy, Knut B. Smeland, Suchitra Sundaram, Chan Y. Cheah, Andrew M. Evens
Collection and assembly of data: Adam J. Olszewski, Lasse H. Jakobsen, Graham P. Collins, Kate Cwynarski, Kristie A. Blum, Kirsten M. Boughan, Alessia Dalla Pria, Alexey Danilov, Kevin A. David, Catherine Diefenbach, Fredrik Ellin, Narendranath Epperla, Umar Farooq, Tatyana A. Feldman, Alina S. Gerrie, Deepa Jagadeesh, Manali Kamdar, Reem Karmali, Shireen Kassam, Vaishalee P. Kenkre, Seo-Hyun Kim, Andreas K. Klein, Izidore S. Lossos, Matthew A. Lunning, Peter Martin, Nicolas Martinez-Calle, Silvia Montoto, Seema Naik, Neil Palmisiano, David Peace, Elizabeth H. Phillips, Tycel J. Phillips, Craig A. Portell, Nishitha Reddy, Anna Santarsieri, Maryam Sarraf Yazdy, Knut B. Smeland, Scott E. Smith, Stephen D. Smith, Adam S. Zayac, Xiao-Yin Zhang, Catherine Zhu, Chan Y. Cheah, Tarec C. El-Galaly, Andrew M. Evens
Data analysis and interpretation: Adam J. Olszewski, Lasse H. Jakobsen, Graham P. Collins, Kate Cwynarski, Veronika Bachanova, Kristie A. Blum, Alessia Dalla Pria, Narendranath Epperla, Umar Farooq, Alina S. Gerrie, Deepa Jagadeesh, Nadia Khan, Andreas K. Klein, Izidore S. Lossos, Matthew A. Lunning, Seema Naik, Neil Palmisiano, Tycel J. Phillips, Nishitha Reddy, Scott E. Smith, Stephen D. Smith, Suchitra Sundaram, Chan Y. Cheah, Tarec C. El-Galaly, Andrew M. Evens
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Burkitt Lymphoma International Prognostic Index
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Adam J. Olszewski
Research Funding: Genentech/Roche, TG Therapeutics, Spectrum Pharmaceuticals, Celldex
Lasse H. Jakobsen
Honoraria: Takeda
Graham P. Collins
Honoraria: Roche, Takeda, Gilead Sciences, Pfizer, Novartis, Daiichi Sankyo, Incyte, Celleron Therapeutics, MSD Oncology, BeiGene, ADC Therapeutics
Consulting or Advisory Role: Roche, Takeda, Incyte, Pfizer, MSD, Celgene, Beigene, Daiichi Sankyo, Celleron Therapeutics, ADC Therapeutics
Speakers' Bureau: Roche, Takeda, Novartis, Gilead Sciences
Research Funding: MSD Oncology, Celgene, Celleron Therapeutics, Bristol-Myers Squibb, Amgen
Travel, Accommodations, Expenses: Roche, Takeda
Kate Cwynarski
Consulting or Advisory Role: Roche, Autolus, Kite Pharma, Gilead Sciences, Takeda, Atara Biotherapeutics
Speakers' Bureau: Roche, Gilead Sciences, Janssen, Takeda
Travel, Accommodations, Expenses: Janssen, Gilead Sciences, Roche, Takeda
Veronika Bachanova
Consulting or Advisory Role: Seattle Genetics, Kite Pharma
Research Funding: Gamida Cell, Unum Therapeutics, Novartis, Incyte, Celgene
Mark Bower
Honoraria: ViiV Healthcare, Gilead Sciences, Bristol-Myers Squibb, MSD, Janssen, EUSA Pharma
Alexey Danilov
Stock and Other Ownership Interests: Abbvie
Honoraria: DAVAOncology
Consulting or Advisory Role: Gilead Sciences, Genentech/Roche, Abbvie, Verastem, TG Therapeutics, AstraZeneca, Juno Therapeutics, Teva, Bayer, Seattle Genetics, Curis, Celgene, Bristol-Myers Squibb, Karyopharm Therapeutics
Research Funding: Takeda, Gilead Sciences, Genentech/Roche, Bayer, Verastem, Bristol-Myers Squibb, AstraZeneca, Aptose Biosciences
Travel, Accommodations, Expenses: Genentech/Roche, AstraZeneca
Catherine Diefenbach
Stock and Other Ownership Interests: Gilead Sciences
Consulting or Advisory Role: Seattle Genetics, Bayer, Bristol-Myers Squibb, Genentech/Roche, Merck, Janssen, Celgene, MorphoSys
Research Funding: Seattle Genetics, Genentech, Incyte, LAM Therapeutics, Merck, Bristol-Myers Squibb, Millennium, Roche/Genentech, Janssen, MEI Pharma, Trillium Therapeutics
Narendranath Epperla
Consulting or Advisory Role: Pharmacyclics
Speakers' Bureau: Verastem, BeiGene
Umar Farooq
Honoraria: Kite Pharma
Travel, Accommodations, Expenses: Kite Pharma
Tatyana A. Feldman
Honoraria: Seattle Genetics, Pharmacyclics/Janssen, Abbvie, Bristol-Myers Squibb, Kite Pharma, Bayer, Takeda
Consulting or Advisory Role: Seattle Genetics, Bayer, Bristol-Myers Squibb
Speakers' Bureau: Seattle Genetics, Kite Pharma, Pharmacyclics, Abbvie, Janssen, Celgene
Research Funding: Bristol-Myers Squibb, Seattle Genetics, Portola Pharmaceuticals, Eisai, Kyowa Hakko Kirin, Amgen, Viracta Therapeutics, Cell Medica, Roche, Trillium Therapeutics, Pfizer, Viracta Therapeutics
Travel, Accommodations, Expenses: Seattle Genetics, Kite Pharma, Pharmacyclics, Abbvie, Takeda
Alina S. Gerrie
Honoraria: Janssen, Abbvie
Consulting or Advisory Role: Janssen, Abbvie, AstraZeneca, Sandoz
Research Funding: Abbvie, Roche Canada, Janssen, AstraZeneca
Travel, Accommodations, Expenses: Janssen
Deepa Jagadeesh
Consulting or Advisory Role: Seattle Genetics, Atara Biotherapeutics, Verastem, Kyowa Hakko Kirin
Speakers' Bureau: Verastem
Research Funding: Seattle Genetics, Regeneron, MEI Pharma, Debiopharm Group, Seattle Genetics, Trillium Therapeutics, AstraZeneca
Manali Kamdar
Consulting or Advisory Role: AstraZeneca, Celgene, Pharmacyclics/Janssen, Adaptive Biotechnologies, Karyopharm Therapeutics, Abbvie, BeiGene
Speakers' Bureau: Seattle Genetics
Reem Karmali
Honoraria: Prime Oncology, Bio Ascend
Consulting or Advisory Role: Kite/Gilead, Juno Therapeutics, Karyopharm Therapeutics
Speakers' Bureau: AstraZeneca, Kite/Gilead, BeiGene
Research Funding: Bristol-Myers Squibb, Takeda, Gilead Sciences, MedImmune, Juno Therapeutics
Travel, Accommodations, Expenses: Prime Oncology
Nadia Khan
Honoraria: Janssen
Consulting or Advisory Role: Incyte
Research Funding: Bristol-Myers Squibb
Seo-Hyun Kim
Honoraria: Seagen, Cellectar
Travel, Accommodations, Expenses: Globus Medical
Andreas K. Klein
Consulting or Advisory Role: Shire
Izidore S. Lossos
Honoraria: Janssen Biotech
Consulting or Advisory Role: Seattle Genetics, Janssen Scientific Affairs, Verastem
Research Funding: NCI
Patents, Royalties, Other Intellectual Property: Stanford-Royalties
Matthew A. Lunning
Consulting or Advisory Role: TG Therapeutics, Gilead Sciences, Janssen Oncology, Verastem, Kite Pharma, Novartis, Spectrum Pharmaceuticals, Bristol-Myers Squibb, Karyopharm Therapeutics, AstraZeneca, Acrotech Biopharma, BeiGene, Legend Biotech, ADC Therapeutics, MorphoSys, Myeloid Therapeutics, Daiichi Sankyo/Lilly
Research Funding: Celgene, Juno Therapeutics, Janssen Oncology, TG Therapeutics, Celgene, miRagen, Curis
Peter Martin
Consulting or Advisory Role: Celgene, Janssen, Bayer, Kite Pharma, BeiGene, I-Mab, MorphoSys, Sandoz, TeneoBio, Karyopharm Therapeutics, Kite/Gilead, Verastem, Cellectar, Regeneron
Research Funding: Karyopharm Therapeutics
Travel, Accommodations, Expenses: Janssen
Nicolas Martinez-Calle
Travel, Accommodations, Expenses: Abbvie
Silvia Montoto
Consulting or Advisory Role: Bayer
Speakers' Bureau: Janssen
Travel, Accommodations, Expenses: Gilead Sciences
Seema Naik
Honoraria: Sanofi, Millennium
Consulting or Advisory Role: Sanofi, Millennium
Research Funding: Genentech/Roche
Neil Palmisiano
Consulting or Advisory Role: Takeda
Research Funding: Abbvie/Genentech
David Peace
Stock and Other Ownership Interests: Bristol-Myers Squibb, Merck
Patents, Royalties, Other Intellectual Property: US Patent # 8,557,777 B2: Methods of Treating Prostate Cancer Using Prostate Specific Antigen and Tumor Endothelial Marker Peptides. October 15, 2013.
Elizabeth H. Phillips
Consulting or Advisory Role: BeiGene
Tycel J. Phillips
Honoraria: Seattle Genetics, Incyte, Pharmacyclics, Bayer, Gilead Sciences, Genentech
Consulting or Advisory Role: Seattle Genetics, Pharmacyclics, Incyte, Genentech, Bayer, Gilead Sciences, Curis, Kite/Gilead, Celgene
Speakers' Bureau: Genmab
Research Funding: Abbvie, Pharmacyclics/Janssen, Bayer
Craig A. Portell
Consulting or Advisory Role: Pharmaceutical Research Associates, Genentech/Roche, Kite/Gilead, BeiGene, Aptitude Health, Janssen, Pharmacyclics, MorphoSys, Targeted Oncology
Research Funding: BeiGene, Acerta Pharma, TG Therapeutics, Genentech/Roche, Infinity Pharmaceuticals, Abbvie, Kite Pharma, Xencor, Seagen, VelosBio
Travel, Accommodations, Expenses: Kite Pharma
Nishitha Reddy
Honoraria: Seattle Genetics, Celgene, Morphosys, Cellectar
Consulting or Advisory Role: Cellectar, Morphosys, Bayer
Speakers' Bureau: Celgene, Seattle Genetics
Research Funding: Bristol-Myers Squibb
Maryam Sarraf Yazdy
Honoraria: Abbvie, Octapharm
Research Funding: Genentech
Stephen D. Smith
Consulting or Advisory Role: AstraZeneca, BeiGene, Takeda, Karyopharm Therapeutics, Kite/Gilead
Research Funding: Acerta Pharma/AstraZeneca, Ayala Pharmaceuticals, Bristol-Myers Squibb, Genentech/Roche, Ignyta, Incyte, Merck Sharp & Dohme, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, De Novo Pharmaceuticals, BeiGene, Bayer
Suchitra Sundaram
Research Funding: Loxo Oncology, Rhizen Pharmaceuticals, TG Therapeutics
Chan Y. Cheah
Honoraria: Roche/Genentech, Janssen-Cilag, TG Therapeutics, Loxo/Lilly, AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, Ascentage Pharma
Consulting or Advisory Role: Janssen-Cilag, Roche/Genentech, TG Therapeutics, Loxo/Lilly, Gilead Sciences, AstraZeneca, Bristol-Myers Squibb, Ascentage Pharma, Merck
Research Funding: Roche/Genentech, Bristol-Myers Squibb, Abbvie
Travel, Accommodations, Expenses: Roche
Tarec C. El-Galaly
Employment: Roche/Genentech
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Roche, Takeda
Other Relationship: Roche
Andrew M. Evens
Honoraria: Seattle Genetics, Pharmacyclics, Research to Practice, Miltenyi Biotec, Epizyme, Novartis, MorphoSys, Cota Healthcare, Curio Science, Targeted Oncology, WebMD, Abbvie/Pharmacyclics, HMP, Takeda, Patient Power, TG Therapeutics, PER, OncLive Clinical Congress Consultants
Consulting or Advisory Role: Seattle Genetics, Novartis, Pharmacyclics, Miltenyi Biotec, Epizyme, MorphoSys, Cota Healthcare, Abbvie, TG Therapeutics
Speakers' Bureau: Research to Practice, Curio Science
Travel, Accommodations, Expenses: Seattle Genetics, Pharmacyclics, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Molyneux EM, Rochford R, Griffin B, et al. : Burkitt's lymphoma. Lancet 379:1234-1244, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Khan G, Fitzmaurice C, Naghavi M, et al. : Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990-2017. BMJ Open 10:e037505, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson C, LaCasce A: How I treat Burkitt lymphoma in adults. Blood 124:2913-2920, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Zayac AS, Olszewski AJ: Burkitt lymphoma: Bridging the gap between advances in molecular biology and therapy. Leuk Lymphoma 61:1784-1796, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magrath I, Adde M, Shad A, et al. : Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol 14:925-934, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Mead GM, Sydes MR, Walewski J, et al. : An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: Results of United Kingdom lymphoma group LY06 study. Ann Oncol 13:1264-1274, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Mead GM, Barrans SL, Qian W, et al. : A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood 112:2248-2260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacasce A, Howard O, Lib S, et al. : Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: Preserved efficacy with decreased toxicity. Leuk Lymphoma 45:761-767, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Evens AM, Carson KR, Kolesar J, et al. : A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt's lymphoma. Ann Oncol 24:3076-3081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribrag V, Koscielny S, Bosq J, et al. : Rituximab and dose-dense chemotherapy for adults with Burkitt's lymphoma: A randomised, controlled, open-label, phase 3 trial. Lancet 387:2402-2411, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Dunleavy K, Pittaluga S, Shovlin M, et al. : Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 369:1915-1925, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roschewski M, Dunleavy K, Abramson JS, et al. : Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. J Clin Oncol 38:2519-2529, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo JJ, Winer ES, Olszewski AJ: Population-based prognostic factors for survival in patients with Burkitt lymphoma: An analysis from the Surveillance, Epidemiology, and End Results database. Cancer 119:3672-3679, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Wasterlid T, Jonsson B, Hagberg H, et al. : Population based study of prognostic factors and treatment in adult Burkitt lymphoma: A Swedish lymphoma registry study. Leuk Lymphoma 52:2090-2096, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Evens AM, Danilov AV, Jagadeesh D, et al. : Burkitt lymphoma in the modern era: Real world outcomes and prognostication across 30 US Cancer Centers. Blood 10.1182/blood.2020006926 [epub ahead of print on July 14, 2020] [DOI] [PMC free article] [PubMed]
- 16.Royston P, Altman DG: External validation of a Cox prognostic model: Principles and methods. BMC Med Res Methodol 13:33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swerdlow SH, Campo E, Pileri SA, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375-2390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakobsen LH, Ellin F, Smeland KB, et al. : Minimal relapse risk and early normalization of survival for patients with Burkitt lymphoma treated with intensive immunochemotherapy: An international study of 264 real-world patients. Br J Haematol 189:661-671, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Harrell FE Jr: Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol 69:245-247, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30:377-399, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Tibshirani R: Regression shrinkage and selection via the lasso. J R Stat Soc Ser B 58:267-288, 1996 [Google Scholar]
- 23.Zou H: The adaptive lasso and its Oracle properties. J Am Stat Assoc 101:1418-1429, 2006 [Google Scholar]
- 24.Brastianos PK, Batchelor TT: Primary central nervous system lymphoma: Overview of current treatment strategies. Hematol Oncol Clin North Am 26:897-916, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Alwan F, He A, Montoto S, et al. : Adding rituximab to CODOX-M/IVAC chemotherapy in the treatment of HIV-associated Burkitt lymphoma is safe when used with concurrent combination antiretroviral therapy. AIDS 29:903-910, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Noy A, Lee JY, Cesarman E, et al. : AMC 048: Modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood 126:160-166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The International Non-Hodgkin's Lymphoma Prognostic Factors Project : A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987-994, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Rizzieri DA, Johnson JL, Byrd JC, et al. : Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: Cancer and leukemia group B study 10 002. Br J Haematol 165:102-111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas DA, Faderl S, O'Brien S, et al. : Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 106:1569-1580, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ribera JM, Garcia O, Grande C, et al. : Dose-intensive chemotherapy including rituximab in Burkitt's leukemia or lymphoma regardless of human immunodeficiency virus infection status: Final results of a phase 2 study (Burkimab). Cancer 119:1660-1668, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Decker DP, Egan PC, Zayac AS, et al. : Treatment strategies and risk of central nervous system recurrence in high-grade B-cell and Burkitt lymphoma. Leuk Lymphoma 61:198-201, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu KY, Song KW, Connors JM, et al. : Excellent real-world outcomes of adults with Burkitt lymphoma treated with CODOX-M/IVAC plus or minus rituximab. Br J Haematol 181:782-790, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Hoelzer D, Walewski J, Dohner H, et al. : Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: Report of a large prospective multicenter trial. Blood 124:3870-3879, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solal-Celigny P, Roy P, Colombat P, et al. : Follicular lymphoma international prognostic index. Blood 104:1258-1265, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Zayac A, Evens AM, Stadnik A, et al. : Outcomes of patients with newly-diagnosed Burkitt lymphoma (BL) and central nervous system (CNS) involvement treated in the modern era: A multi-institutional real-world analysis. Blood 134:402, 2019 [Google Scholar]
- 36.Schmitz R, Ceribelli M, Pittaluga S, et al. : Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med 4:a014282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoster E, Dreyling M, Klapper W, et al. : A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111:558-565, 2008 [DOI] [PubMed] [Google Scholar]