Abstract
Introduction
In the context of COVID-19 pandemic, a national pharmacovigilance survey was set up in March 2020. The purpose of this survey was to ensure continuous monitoring of adverse drug reactions (ADRs) in patients with COVID-19, not only related to the drugs used in this indication but also related to all drugs administered to these patients or suspected of having promoted the infection.
Material and methods
This descriptive study was based on data extracted from the French Pharmacovigilance Database from 1 January 2020 to 30 September 2021. Misuse was also analysed through the MESANGE project. The ADRs were classified according to three groups: “drugs used to treat COVID-19”, “other drugs administered to COVID-19 positive patients” and “drugs suspected of having promoted COVID-19”. The data were also presented according to 2 periods (period one was from January to June 2020 and period two from July 2020 onwards).
Results
Among 2189 included cases, 67.1% were serious. Cases were mainly related to “other drugs administrated to COVID-19 positive patients” (58.5%) followed by “drugs used to treat COVID-19” (33.7%) and “drugs suspected of having promoted COVID-19” (7.8%). Drugs used to treat COVID-19 and their main safety profile were different depending on the period: mostly hydroxychloroquine (51%) with heart injury and lopinavir/ritonavir (42%) with liver injury for the first period, and dexamethasone (46%) with hyperglycemia and tocilizumab (28%) with liver injury for the second period. The drugs suspected of worsening COVID-19 differed in both periods especially for non-steroidal anti-inflammatory drugs mainly reported in period 1 (41.5% versus 8.2% in period 2). Other immunosuppressive drugs were in the majority in the second period (85.7%), with mainly methotrexate (15.3%), anti-CD20 (15.3%) and anti-TNF alpha (10.5%). No confirmed safety signal was identified among other drugs administered to patients with COVID-19. The profile of ADRs and suspected drugs was similar between the 2 periods. The study of misuse in outpatient settings identified in both periods mainly hydroxychloroquine, azithromycin, ivermectin and zinc ± vitamin C.
Discussion
This survey, based on real-time pharmacological and medical assessment of ADRs and weekly meetings in a specific national committee, made it possible to identify relevant safety signals which contribute to patient care with no delay. The main safety signal highlighted was serious cardiac damage under hydroxychloroquine, alone or combined with azithromycin and also with lopinavir/ritonavir. This signal has contributed to the evolution of the recommendations for these 2 drugs. The methodology of this survey has been taken over and is still going on for the pharmacovigilance monitoring of vaccines against COVID-19, for monoclonal antibodies used against COVID-19 and also for Paxlovid® (nirmatrelvir/ritonavir) which benefit from dedicated surveys.
Keywords: Pharmacovigilance survey, Drug safety, COVID-19
Abbreviations
- ADRs
adverse drug reactions
- ANSM
French Health Agency
- COVID-19
coronavirus disease 2019
- HIV
human immunodeficiency virus
- MedDRA
Medical Dictionary for Regulatory Activities
- MERS-CoV
Middle East respiratory syndrome coronavirus
- NSAID
non-steroidal anti-inflammatory drug
- RPVC
French Regional Pharmacovigilance Centres
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviations
- TNF
tumor necrosis factor
Introduction
COVID-19 pandemic was an exceptional health situation on many different levels: first, a new virus (SARS-CoV2) has been identified; second, different clinical pictures have emerged; and third, no effective drug assessed by clinical trials was available.
In France, several drugs were used to manage this emergent infectious disease in the early period, following recommendations proposed by the French Public Health Council on 5 and 23 March 2020 [1], including:
-
•
antiviral remdesivir, a nucleotide analogue prodrug developed for treating Ebola-related diseases and for which data were available for other coronaviruses (SARS-CoV and Middle East respiratory syndrome coronavirus [MERS-CoV]). As early as February 2020, specific pharmacodynamic data on SARS-CoV2 were available;
-
•
antimalarial drugs chloroquine and its derivative hydroxychloroquine, mainly used for its anti-inflammatory activity, for their potential antiviral action with in vitro data available for SARS-CoV and MERS-CoV. In vitro data for SARS-CoV2 were available in February 2020 for chloroquine and in March 2020 for hydroxychloroquine;
-
•
protease inhibitors lopinavir and ritonavir, used in combination since the early 2000s in the treatment of human immunodeficiency virus (HIV) and for which data have been available for other coronaviruses (SARS-CoV and MERS-CoV);
-
•
immunomodulators such as anti-Il6 (tocilizumab in particular) in connection with their potentially beneficial action in treating cytokine release syndromes.
On the 26 March 2020, after assessment of safety data reported by health professionals, the network of 31 French Regional Pharmacovigilance Centres (RPVCs) was able to alert the National Agency for the Safety of Medicines and Health Products (ANSM) on the occurrence of two serious cases of cardiovascular adverse drug reactions (ADRs) reported with hydroxychloroquine, associated with azithromycin in one case. A national pharmacovigilance survey was officially set up on the 27 March. The purpose of this survey was to ensure continuous monitoring of ADRs in patients with COVID-19 (non-clinical trials), not only related to the drugs used in this indication but also, more broadly, related to all drugs administered to these patients or suspected of having promoted the infection. The data were initially analysed daily, then weekly, and discussed in a specific ANSM committee bringing together representatives of the RPVCs and ANSM. The aim of this committee was to identify potential safety signals, consider the measures to be taken, and propose to alert health professionals and patients if necessary. The data discussed were from the pharmacovigilance survey (i.e., spontaneous notifications), clinical trials conducted in France, the French Centres for Evaluation and Information on Drug Dependence and Addictovigilance, the French Poison Control and Toxicovigilance Centres, and any other relevant source (literature, European Medicines Agency, etc.) [2]. The survey reports and a summary document were publicly available on the ANSM website [3].
We present here the main results of this ongoing survey.
Material and method
Data were extracted from the French Pharmacovigilance Database, including all ADRs reported and pharmacologically validated by the 31 RPVCs from 1 January 2020 to 30 September 2021. Extractions were done daily for the first three months and then every week.
The relationship between and ADRs and a drug was assessed using the French pharmacovigilance causality assessment method [4], [5]. The seriousness of each case was recorded according to the regulatory definition [6]. ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA).
The cases were identified in the database under the following conditions: narrative including the words « COVID » or « SARS », or ADRs coded in the high level term « Coronavirus infections », or at least one drug having an indication for coronavirus or COVID-19 disease or medical history including coronavirus or COVID disease.
Exclusion criteria were cases where COVID-19 was ruled out, and cases related to a COVID-19 vaccine since a dedicated survey had been initiated in December 2020.
Misuse analysis was also based on data collected from the MESANGE study [7]. This study aimed to implement a simple system for collecting data on drug misuse from pharmacies in Burgundy and Franche-Comté. The collection was subsequently extended to the whole of France in the context of the COVID-19 pandemic.
A pharmacovigilance specialist manually screened all cases. Cardiac ADRs were specifically assessed by an expert RPVC, and all other ADRs by another specific expert RPVC.
The cases were classified according to three groups: “drugs used to treat COVID-19”, “other drugs administered to COVID 19 positive patients”, and “drugs suspected of having promoted COVID-19”.
The data were also presented according to 2 periods (period 1 is from January to June 2020 and period 2 from July 2020 onwards) in view of the changes in the recommendations for the therapeutic management of patients with COVID-19 published by the French Public Council on 23 July 2020 [8].
Results
As of 30 September 2021, 2189 cases were included, of which 67.1% were serious. Most reporters were health professionals (98.1%) of public hospitals (96.0%). Only 4.5% of the cases were transmitted through the Ministry of Health portal.
Following the three groups of drugs, cases were mainly related to “other drugs administrated to COVID-19 positive patients” (58.5%) followed by “drugs used to treat COVID-19” (33.7%) and “drugs suspected of having promoted COVID-19” (7.8%).
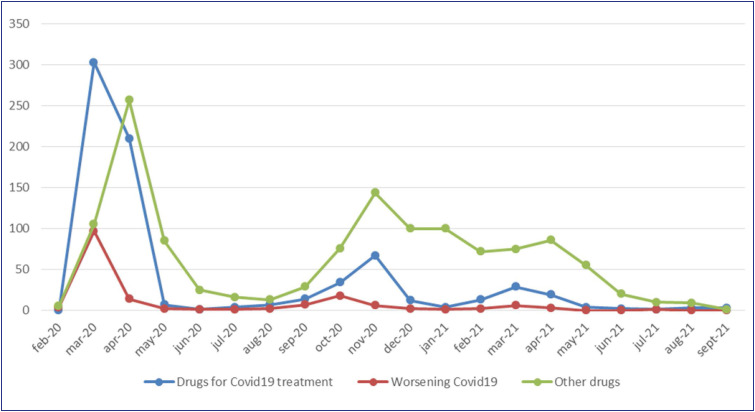
The number of cases received per week was higher in the first two months of the survey. The evolution of the number of cases received is presented in Fig. 1 .
Figure 1.
Evolution of the number of cases.
The patients were predominantly male, with a male/female ratio of 1.6. The global mean age was 64.2 years ± 17.1. The main characteristics of cases are detailed in Table 1 .
Table 1.
Main characteristics of the cases.
| Drugs used to treat COVID-19 | Other drugs administered to COVID-19 positive patients | Drugs suspected of having promoted COVID-19 | |
|---|---|---|---|
| Number of cases (% serious) | 737 (65.3%) | 1284 (65.4%) | 170 (88.2%) |
| Sex: ratio men/women | 1.8 | 1.5 | 1.5 |
| Age: mean (years) ± SD | 63.3 ± 15.6 | 66.3 ± 17.4 | 53.6 ± 16.9 |
| Period 1: n (% serious) | 521 (70.2%) | 477 (61.2%) | 118 (93.2%) |
| Period 2: n (% serious) | 216 (53.2%) | 807 (67.9%) | 49 (81.6%) |
| Period unknown: n (% serious) | 0 | 0 | 3 (0%) |
COVID: coronavirus disease; SD: standard deviation.
Drugs used to treat COVID-19
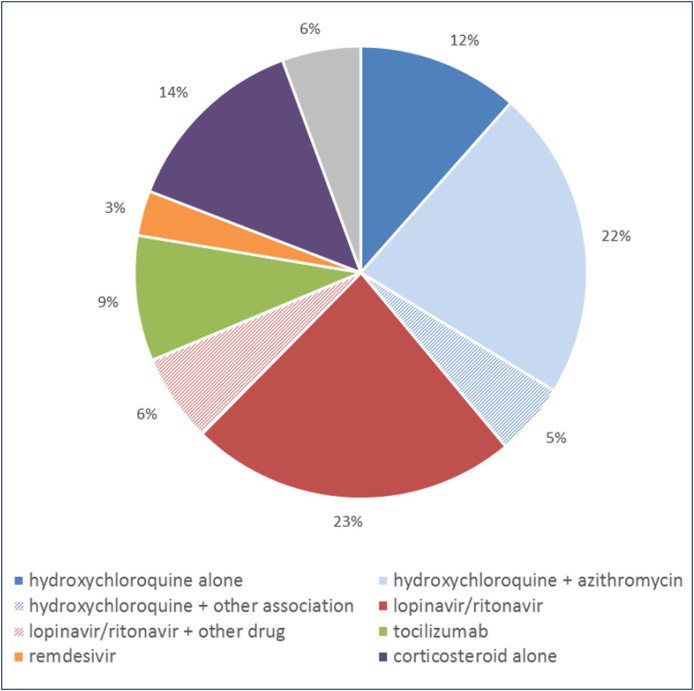
Detail of drugs administered to treat COVID-19 is shown in Fig. 2 .
Figure 2.
Distribution of cases of ADRs related to drugs used to treat COVID-19 since the beginning of the survey (n = 737 cases). ADRs: adverse drug reactions
When compared, suspected drugs used to treat COVID-19 were different between periods 1 and 2, before and after July 2020.
In period 1, among the 521 included cases, hydroxychloroquine was the main suspected drug (found in 272 cases, 51% of suspected drugs), alone (84 cases) or in most cases associated with azithromycin (151 cases). The second most common drug reported was lopinavir/ritonavir (found in 222 cases, 42% of suspected drugs), mainly alone (175 cases). The remaining 7% was concerned tocilizumab (15 cases), azithromycin alone (13 cases) and remdesivir (9 cases).
In period 2, among the 216 included cases, the main suspected drugs were corticosteroids, especially dexamethasone (found in 124 cases, 46% of suspected drugs), followed by tocilizumab (63 cases, 28% of suspected drugs). Hydroxychloroquine was reported in 19 cases (9%), and remdesivir in 16 cases (7%). The remaining 10% were mainly macrolide antibiotics (15 cases), zinc (in 13 cases, usually with hydroxychloroquine), and ivermectin (2 cases).
ADRs reported were different depending on the implicated drug. Cardiac disorders were found in 58% of cases related to hydroxychloroquine, including 8 cases with fatal outcome and in 13% of cases related to lopinavir/ritonavir.
Hepatic disorders represented 43% of ADRs related to lopinavir/ritonavir but were also reported in 29% of tocilizumab cases, 22% for remdesivir, 12% for dexamethasone and 11% for hydroxychloroquine.
Some ADRs were more specific. Thus, ADRs were mainly cutaneous for remdesivir (30%), hyperglycaemia for dexamethasone (61%), thromboembolic events and coagulation disorders for tocilizumab (12% and 6% respectively). Renal adverse reactions were identified for lopinavir/ritonavir in 6.8% of cases and were closely monitored.
Of note, lopinavir plasma overdose was reported in 32.3% of the cases with ADRs mainly hepatic (45%) and digestive (20%) but also with no symptom (9%).
Drugs suspected of having promoted COVID-19
One hundred and seventy cases of drugs suspected of having promoted COVID-19 were identified, including 30 with a fatal outcome. The cases were mainly notified during period 1 (118 cases, 69.4%).
Some differences were found concerning suspected drugs, especially for non-steroidal anti-inflammatory drugs (NSAID), mainly reported in period 1: 49 cases (41.5%) versus 4 cases in period 2 (8.2%).
We found 124 different molecules reported in the 94 cases of “other immunosuppressive drugs”, mainly methotrexate (15.3%), anti-CD20 (15.3%) and anti-TNF alpha (10.5%).
The “other drugs” involved sitagliptin (4 cases), drugs used to treat COVID-19 (lopinavir/ritonavir, hydroxychloroquine or remdesivir) (4 cases) and papillomavirus vaccine (1 case). Details are presented in Table 2 .
Table 2.
Drugs suspected of worsening COVID-19.
| Period 1 (n = 118 cases) | Period 2 (n = 49 cases) | Period unknown (n = 3 cases) | |
|---|---|---|---|
| Non-steroidal anti-inflammatory drugs | 49 (41.5%) | 4 (8.2%) | 0 |
| Corticosteroids | 34 (28.8%) | 14 (28.6%) | 0 |
| Other immunosuppressive drugs | 49 (41.5%) | 42 (85.7%) | 3 (100%) |
| Other drugs | 8 (6.8%) | 1 (2%) | 0 |
COVID: coronavirus disease.
Other drugs administered to COVID-19 positive patients
Among the 1284 cases of ADRs of other drugs administered to COVID-19 positive patients, 840 (65.4%) were serious, including 60 with a fatal outcome.
The profile of ADRs and suspected drugs was similar between the 2 periods. The 10 most frequently suspected drugs were: antibiotics (476 cases, 37.1%), anticoagulants (309 cases, 24.1%), immunosuppressants (97 cases, 7.6%), proton pump inhibitors (64 cases, 5.0%), anaesthetics and neuroleptics (51 cases each, 4.0%), analgesics (48 cases, 3.7%), iodinated contrast media (47 cases, 3.7%), antifungals (39 cases, 3.0%) and antidepressants (37 cases, 2.9%).
ADRs were mainly cutaneous (27.4% of cases) and haematological (21.0% of cases). Other ADRs reported in more than 5% of cases were haemorrhages (9.6%), hepatic disorders (8.9%) and neurological disorders (7.3%). With regard to the suspected drugs, ADRs were expected in most cases.
Misuse
A focus on the misuse of medicines in outpatient setting in the pandemic context was carried out. Most identified drugs are detailed in Table 3 . Drugs in bold appear in both periods.
Table 3.
Identified misused drugs.
| Period 1 | Period 2 | |
|---|---|---|
| Drugs | Azithromycin alone Chloroquine Colloidal silver Hydroxychloroquine ± azithromycin Ivermectin Nicotine replacement products Zinc ± vitamin C |
Azithromycin alone Colchicine Hydroxychloroquine ± azithromycin Ivermectin Vitamine D Zinc ± vitamin C |
Discussion
This work presents a status report on the pharmacovigilance survey set up at the beginning of the COVID-19 pandemic. The objective of this survey, which is still ongoing, is to monitor the safety profile of medicines used in the context of the pandemic, outside clinical trials.
A pharmacovigilance survey, under good pharmacovigilance practice, is a retrospective or prospective evaluation of available safety data to identify potential safety signals, validate them and/or characterise them [9]. This survey was subject to a specific methodology adapted to the pandemic context [2].
The evolution of the number of notified cases has followed the growth of the pandemic in France [10] but with more cases reported during the first weeks.
The profile of the cases analysed was different between the two defined periods.
Regarding the cases related to drugs used to treat COVID-19: the significant difference observed between the two periods was expected since directly linked to the evolution of the recommendations. Thus, hydroxychloroquine and lopinavir/ritonavir were the drugs most widely found in the first few weeks of the survey. A safety signal was quickly highlighted in this period related to serious cardiac damage under hydroxychloroquine, alone or combined with azithromycin and also with lopinavir/ritonavir [11]. This signal was the subject of a first communication by the ANSM on 30 March 2020 [12]. In light of the data progressively collected on the putative benefit, the High Council of Public Health, in its report published on 24 May, advised against its use in outpatients or hospitalised patients, whatever the severity [13]. The decree authorising hydroxychloroquine and lopinavir/ritonavir use in COVID-19, outside of clinical trials, was repealed on 26 May 2020.
Although most adverse events were expected, this survey found a difference in the safety profile for hydroxychloroquine and also for lopinavir/ritonavir in patients managed for COVID-19 compared to their historical indications. For hydroxychloroquine, COVID-19 patients presented more cardiac disorders, especially QT interval prolongation, resulting from an interaction with azithromycin in more than 20% of cases. Hepatobiliary disorders were also significantly more frequent [14]. For lopinavir/ritonavir, patients with COVID-19 presented significantly more damage to the liver, heart and kidneys [15]. The ADRs reported before the pandemic (historic indications) were mainly gastrointestinal and cutaneous.
In the second period, dexamethasone was mainly reported. The increase in its use being linked to data on its effectiveness [8], as well as tocilizumab. No safety signal was confirmed in this second period. However, thromboembolic events and coagulation disorders have been identified as ADRs of concern for tocilizumab and are still closely monitored.
More cases of drugs suspected of having promoted COVID-19 were reported at the beginning of the survey. This is probably related to the novelty of the disease and the lack of identified risk factor at the beginning. The profile of the suspected drugs also varied, with more NSAID-related cases in the first few weeks. This signal has been the subject of a dedicated survey, several communications and publications [16], [17]. The number of reported cases declined rapidly in line with the decrease use of NSAIDs during the pandemic, probably as a result of the recommendations [18].
For other drugs administered to COVID-19 positive patients, no significant difference was found between the two periods, and no safety signal has been confirmed to date. Potential signals identified in the literature were closely monitored, including heparin-induced thrombocytopenia [19] and potential adverse events related to possible overuse of paracetamol, with no confirmed signal up to date.
The focus on misuse in outpatients highlighted some drugs (hydroxychloroquine, ivermectin, vitamin D, zinc) from the first weeks until now, despite the extensive scientific evidence of their lack of efficacy and deleterious safety profile. Some drugs have been reported only on very short period (nicotine replacement products, colloidal silver), reflecting media rumours on supposed efficacy, rapidly discredited.
The strength of this survey is the rapidity with which it was set up and the adaptability of the methodology to the pandemic context. Monitoring was initially very broad, covering all drugs administered to COVID-19 positive patients, then it was subsequently adapted to practice and then limited to drugs administered to treat COVID-19 in the absence of signal needed to be followed up for other situations. Signal detection continues through the usual pharmacovigilance procedures. Data transparency should also be noticed as it contributes to the information and confidence of patients and health professionals.
The main limitation of this study is under-reporting, like all pharmacovigilance studies. A notoriety bias could not be excluded, but probably limited at the beginning of the pandemic.
Conclusion
This exceptional pandemic has been a fantastic situation to demonstrate the adaptability of the French pharmacovigilance system, which is based on the direct reporting of ADRs by healthcare professionals and patients to clinical pharmacologists from the RPVCs. Identification of relevant safety signals has been possible by real-time pharmacological and medical analysis by RPVC pharmacologists, which contributes to a rapid and qualitative patient care. The methodology of this survey has been taken over and is still going on for the pharmacovigilance monitoring of vaccines against COVID-19 [20], for monoclonal antibodies used against COVID-19 and also for Paxlovid® (nirmatrelvir/ritonavir) which benefit from dedicated surveys.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
No funding was received for the preparation of this article.
Acknowledgment
Network of the 31 French Regional Pharmacovigilance Centres, ANSM for data extraction.
References
- 1.HCSP. Avis relatif à la prise en charge des cas confirmés d’infection au virus SARS-CoV2. 5 mars 2020. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=771.[Accessed 9 January 2023].
- 2.Grandvuillemin A., Drici M.D., Jonville-Béra A.P., Micallef J., Montastruc J.L. French pharmacovigilance Network. French pharmacovigilance Network. French pharmacovigilance public system and COVID-19 pandemic. Drug Saf. 2021;44:405–408. doi: 10.1007/s40264-020-01034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ANSM. Suivi des effets indésirables des médicaments utilisés dans la prise en charge du COVID-19. 2022. http://ansm.sante.fr/dossiers-thematiques/covid-19-dispositif-renforce-de-pharmacovigilance-et-daddictovigilance. [Accessed 9 January 2023].
- 4.Miremont-Salamé G., Théophile H., Haramburu F., Bégaud B. Causality assessment in pharmacovigilance: the French method and its successive updates. Therapie. 2016;71:179–186. doi: 10.1016/j.therap.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Montastruc J.L. Pharmacovigilance and drug safety: fair prescribing and clinical research. Therapie. 2022;77:261–263. doi: 10.1016/j.therap.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 6.European medicines agency. ICH Topic E 2 A: Clinical Safety Data Management: definitions and standards for expedited reporting, June 1995, Guideline no. CPMP/ICH/377/95. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf.[Accessed 9 January 2023 (10 pp.)].
- 7.Valnet Rabier M.B., Kouassi A.S., Grandvuillemin A., MESANGE: a feasibility study of an electronic system for the reporting of drug misuse in primary care. PM1-034. Fund Clin Pharmacol. 2019;33(Suppl. S1):23–47. [Google Scholar]
- 8.HCSP. Rapport relatif à l’actualisation de la prise en charge des patients atteints de COVID-19. 20 July 2020. https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20200723_corsarcovactdelapriencha.pdf.[Accessed 9 January 2023 (291 pp.)].
- 9.Abou Taam M., Jacquot B., Ferard C., Thery A.C., Mounier C., Grandvuillemin A., et al. Réseau français des centres régionaux de pharmacovigilance. The French pharmacovigilance surveys: a French distinctiveness, a real input. Therapie. 2021;76:441–447. doi: 10.1016/j.therap.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Santé publique France. 2022. https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde.[Accessed 9 9 January 2023].
- 11.Gérard A., Romani S., Fresse A., Viard D., Parassol N., Granvuillemin A., et al. Réseau français des centres de pharmacovigilance. Off-label use of hydroxychloroquine, azithromycin, lopinavir/ritonavir and chloroquine in COVID-19: survey on cardiac adverse drug reactions by the French Network of pharmacovigilance centres. Therapie. 2020;75:371–379. doi: 10.1016/j.therap.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ANSM. Plaquenil et Kaletra : les traitements testés pour soigner les patients COVID-19 ne doivent être utilisés qu’à l’hôpital. Point d’information. 30 March 2020. https://ansm.sante.fr/actualites/plaquenil-et-kaletra-les-traitements-testes-pour-soigner-les-patients-covid-19-ne-doivent-etre-utilises-qua-lhopital.[Accessed 9 January 2023].
- 13.HCSP. COVID-19 : utilisation de l’hydroxychloroquine. May 2020. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=837.[Accessed 9 January 2023].
- 14.Lory P., Lombardi J., Lacroix C., Sanchez-Pena P., Romani S., Grandvuillemin A. Réseau français des Centres régionaux de pharmacovigilance. Comparative study of the adverse event profile of hydroxychloroquine before and during the Sars-CoV2 pandemic. Therapie. 2022;77:301–307. doi: 10.1016/j.therap.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lory P., Combret S., Michot J., Veyrac G., Chouchana L., Grandvuillemin A. Safety profile of the lopinavir/ritonavir combination before and during the SARS-CoV2 pandemic. Therapie 2022 Oct 31:S0040-5957(22)00235-9. doi: 10.1016/j.therap.2022.10.066. Epub ahead of print.https://www.em-consulte.com/article/1554514/safety-profile-of-the-lopinavirritonavir-combinati. [Accessed 9 January 2023]. [DOI] [PMC free article] [PubMed]
- 16.Micallef J., Soeiro T., Jonville-Béra A.P. French Society of pharmacology, therapeutics (SFPT). Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie. 2020;75:355–362. doi: 10.1016/j.therap.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micallef J., Soeiro T., Jonvillle-Béra A.P. COVID-19 and NSAIDs: primum non nocere. Therapie. 2020;75:514–515. doi: 10.1016/j.therap.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EPIPHARE. Usage des médicaments de ville en France durant l’épidémie de la Covid-19 – point de situation après les 8 semaines de confinement et une semaine de post-confinement. Rapport 3 du 9 juin 2020. 9 June 2020. https://ansm.sante.fr/uploads/2020/10/13/20201013-epi-phare-rapport-covid-3-1usage-medic.pdf.[Accessed 9 January 2023 (312 pp.)].
- 19.Patell R., Khan A.M., Bogue T., Merrill M., Koshy A., Bindal P., et al. Heparin induced thrombocytopenia antibodies in Covid-19. Am J Hematol. 2020;95:E295–E296. doi: 10.1002/ajh.25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacroix C., Salvo F., Gras-Champel V., Gautier S., Massy N., Valnet-Rabier M.B., et al. French Network of pharmacovigilance centres. French organization for the pharmacovigilance of COVID-19 vaccines: a major challenge. Therapie. 2021;76:297–303. doi: 10.1016/j.therap.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]