Abstract
Antigen-detecting rapid diagnostic testing (Ag-RDT) has contributed to containing the spread of SARS-CoV-2 variants of concern (VOCs). In this study, we proposed a biomimetic clamp assay for impedimetric SARS-CoV-2 nucleocapsid protein (Np) detection. The DNA biomimetic clamp (DNA-BC) is formed by a pair of Np aptamers connected via a T20 spacer. The 5'- terminal of the DNA-BC is phosphate-modified and then anchored on the surface of the screen-printed gold electrode, which has been pre-coated with Au@UiO-66-NH2. The integrated DNA-material sensing biochip is fabricated through the strong Zr−O−P bonds to form a clamp-type impedimetric aptasensor. It is demonstrated that the aptasensor could achieve Np detection in one step within 11 min and shows pronounced sensitivity with a detection limit of 0.31 pg mL–1. Above all, the aptasensor displays great specificity and stability under physiological conditions as well as various water environments. It is a potentially promising strategy to exploit reliable Ag-RDT products to confront the ongoing epidemic.
Keywords: Antigen test, SARS-CoV-2 Nucleocapsid protein, Aptamer, Impedimetric biosensor, Screen-printed electrode
1. Introduction
Since 2020, the SARS-CoV-2 virus has been continuously mutating. The new Omicron variants, which are among the most transmissible ones, have resulted in a surge of asymptomatic infections and aggravated the COVID-19 epidemic [1]. Importantly, it should be mentioned that a considerable proportion of convalescent individuals have experienced prolonged and iterative sequela of multiple organs [2]. Therefore, accurate, rapid, and universal testing strategies are imperative for the early diagnosis of viral infections and environmental monitoring. The antigen-detecting rapid diagnostic testing (Ag-RDT) is conducive to improving the capability and accuracy of early diagnosis such that subsequent quarantine measures can be taken as early as possible [3]. Currently, the dominant Ag-RDT products are antibody-based lateral flow assay (LFA) immunochromatographic strips [4], [5]. Despite the clear advantages of being convenient to operate and affordable to purchase, the detection limit of Ag-RDT is not ideal and often produces false positives. Alternatively, the electrochemical techniques have provided solutions in developing biosensors for diverse analytes benefiting from the high sensitivity and rapid signal readout [6], [7]. For instance, Salinas et al. developed an amperometric rotation biosensor for the enzymatic determination of cholesterol[8]. Electrochemical impedance spectroscopy (EIS), another powerful technique, can reflect delicate changes of the electroactive species and electrolytes in proximity to the electrode surface and eliminate the multiple procedures [9]. It is label-free, sensitive, non-destructive, and can straightforwardly quantify the analyte, thus emerging as a viable method for SARS-CoV-2 viral antigen detection.
Aptamers are a class of single-stranded nucleic acid sequences with high-affinity recognition of targets comparable to antibodies and widely serve as recognition elements in fabricating aptamer-based biosensors (aptasensor) [10], [11]. Bivalent or multivalent aptamers have their inherent characteristics of superior sensitivity and specificity, greatly reducing false-positive results. Thereby, Zhang et al. selected dual aptamers to fabricate an electrochemical biosensor for the detection of SARS-CoV-2 spike (S) protein [12]. Idili et al. applied aptamers to propose an electrochemical sensor enabling rapid, reagentless, and quantitative measurement of the SARS-CoV-2 spike protein in its clinical range [13]. Adeel et al. reported a label-free electrochemical aptasensor for the detection of SARS-CoV-2 spike protein based on carbon cloth sputtered gold nanoparticles[14]. However, the mutation of SARS-CoV-2 occurs during the S-gene coding, especially, Omicron variants showcase high number of mutations on the spike protein (23 amino acid substitutions) and its RBD (15 amino acid substitutions) so that the above methods, to some extent, are not advantaged towards meeting the detection of other SARS-CoV-2 variants [15]. Comparatively, nucleocapsid protein (Np), as the most abundant SARS-CoV-2 antigen, is high conserved and crucial for virus transcription and replication [16], [17]. It can be released in individuals' blood, saliva, and serum after SARS-CoV-2 infection [18], [19], [20]. Therefore, Np is an important target for diagnosing early infection and challenging the variants of concern whereas there was scarcely any dual aptamers-based eletrochemical biosensors for Np detection.
Herein, we proposed a biomimetic clamp assay by dimerizing the dual aptamers of Np-A48 and Np-A61 [21] to function as the handles linked via a T20 spacer to grasp the Np. The gold nanoparticles (AuNPs) decorated amino-functionalized UiO-66 (Au@UiO-66-NH2) were modified on the surface of screen-printed gold electrodes (SPGEs) to form the adsorption sites and enhance the electrical conductivity. The DNA biomimetic clamp (DNA-BC) was anchored on the surface of the composite via Zr-O-P bonds to construct an electrochemical aptasensor. Our study provides a disposable sensing biochip with the features of high sensitivity, portability, and low cost, one step in determining Np, leading to promising industrialization potential and commercialization prospects to achieve early diagnosis of SARS-CoV-2 infection.
2. Experimental section
2.1. Reagents and apparatus
Synthetic oligonucleotides were ordered and HPLC-purified by General biol. Co., Ltd. (Chuzhou, China), and the sequences were listed in Table S1. The aptamers used in this study were developed by our group [20]. Zirconium (IV) chloride (ZrCl4) and amino-terephthalic acid (NH2-BDC) were purchased from Heowns Chemical Reagent (Tianjin, China). Gold chloride tetrahydrate (HAuCl4·4H2O) was obtained from Sigma-Aldrich (St. Louis, MO). Phosphate buffer saline (PBS) and bovine serum albumin (BSA) were purchased from Sangon Biotech (Shanghai, China). National center for protein science Shanghai (NCPSS) offered the plasmid of SARS-CoV-2 N protein (NFPS–P10034 Pet28a-N). A Millipore filtration system produced deionized water used throughout this experiment. All chemical reagents were of analytical grade and used without further purification.
SARS-CoV-2 Np was prepared and purified based on our previous procedures [22], and the purity was checked by SDS-PAGE (Fig. S1). A portable potentiostat (Ivium, Netherlands) was operated to record electrochemical signals while screen-printed gold electrodes (SPGEs, ref. DRP-250BT, ϕ = 4 mm) were purchased from Metrohm DropSens S.L. (Spain). A BIAcore T200 biosensor system was used to conduct surface plasmon resonance (SPR) experiments to assess the binding affinity of aptamers.
2.2. Preparation of UiO-66-NH2 and Au@UiO-66-NH2
The preparation of UiO-66-NH2 followed a reported synthetic process with some minor modifications [23]. In brief, 82.5 mg NH2-BDC, 104.9 mg ZrCl4, and 12 mL acetic acid were ultrasonically dissolved in 100 mL of N, N-Dimethylformamide (DMF) for 15 min. The resulting solution was transferred to three autoclaves which were sealed off and then heated at 120 ℃ for 24 h. Afterward, the products were collected via centrifugation at 5000 rpm for 10 min at room temperature and exchanged with DMF and ethanol. The white powder was acquired using a vacuum desiccator at 90 ℃ for 12 h.
20.0 mg UiO-66-NH2 was dispersed in 4 mL deionized water-ethanol solution and sonicated with a 1:1 vol ratio. 0.5 mL of 30 mM HAuCl4·4 H2O was added and sufficiently stirred for 4 h. Then, 2 mL of 0.05 M NaBH4 was added dropwise while kept stirring for 30 min under ice bath conditions. After centrifugation, the collected products were washed with deionized water by three times and dried to get Au@UiO-66-NH2.
2.3. Fabrication of the clamp-type impedimetric biochip
Initially, the SPGE was coated with 15 μL Au@UiO-66-NH2 aqueous suspension (1 mg mL−1) and modified through the strong amino-Au interaction after being incubated at 4 ℃ overnight. The modified SPGE was immersed in deionized water to remove the weakly bound Au@UiO-66-NH2 and dried under ambient conditions. Subsequently, 15 μL phosphate-modified DNA-BC solution (5 μM) was dropped onto the electrode to anchor the DNA-BC at 37 ℃ for 30 min. A 10 mM Tris-HCl buffer was used to remove unattached DNA-BC, then BSA (0.25 wt%) was incubated for 30 min to block the unoccupied sites and eliminate nonspecific adsorption. After being washed with deionized water, the biochip was stored at 4 ℃ before further use. The Np (0–2 ng mL−1) was diluted in PBS buffer, dropped on the biochip surface, and incubated for 5 min before electrochemical measurements.
2.4. Electrochemical measurements
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed in 5.0 mM [Fe(CN)6]3−/4− solution containing 0.25 M KCl to characterize each of the steps while fabricating the biochip. Cyclic voltammetric curves were recorded from -0.3 to 0.6 V vs. Ag /AgCl with a step of 1 mV at a scan rate of 100 mV s−1. The EIS experiments were conducted under a stable open circuit voltage (OCV) with frequency scan ranging from 100 kHz to 0.1 Hz and 5 mV as the amplitude. After the procedure finished within 360 s, the EIS Nyquist plots were processed using the built-in Ivium software.
2.5. Actual samples preparation
Saliva and serum from healthy individuals were provided from members of our group. Prior to the recovery experiments, standard samples were prepared with various spiked Np concentrations. The river water was collected from the Yueya River (Jinnan District, Tianjin, China). The wastewater samples were obtained from the sewage disposal plant of Nankai University (Tianjin, China). Syringe filters were utilized throughout to filter the above water samples. Various concentrations of Np (50, 100, 200 pg mL−1) were spiked in water samples for calculating recovery ratios to verify the universality.
3. Results and discussion
3.1. Design of DNA-BC
The performance of the aptasensor can be influenced by the length and structure of the aptamer which determines the accessibility of the target to the aptamer [24]. Multivalent aptamers not only enhance the binding affinity toward their target but also enable the increase of nucleic acid stabilities [25]. We selected the aptamers Np-A48 and Np-A61 which could bind to two distinct sites of Np exhibiting sandwich-type interaction and did not interfere with each other [21]. In a preliminary experiment, we studied the relationship between sequence truncation and affinity. The original Np-A48 and Np-A61 have a sequence of 58-nt and an affinity of 0.45 and 2.74 nM towards Np. As exhibited in Table S2 and Fig. S2, the evolutive affinity changed from 0.45 nM to 4.10 nM when the Np-A48 was truncated to 22-nt. In terms of Np-A61, the truncated sequence was determined to be 28-nt. According to the predicted secondary structure from the NUPACK server (http://www.nupack.org/), the dual mini-aptamers Np-A48t and Np-A61t reserve a core recognition domain as depicted in Scheme 1 which fold into a rigid conformation to ensure the aptamer-target interaction. Compared with the original sequences, the adoption of truncated aptamers not only preserves the recognition performance of full-length aptamers but also significantly reduces the cost of synthesis and use.
Scheme 1.
The predicted secondary structure of the truncated aptamers Np-A48t, Np-A61t, and aptamers dimerization (DApt)-inspired DNA-BC.
After homo- or hetero-dimerization, the biomimetic clamp was constructed via a polythymidine (polyT) spacer. The selection of polyT spacer depends not only on the formation of secondary structures in which these thymines may participate but also sequence stability. In a separate control experiment, we optimized the number of T in the spacer. As shown in Table S3 and Fig. S3, the DNA-BC with T20 spacer exhibited the strongest binding capacity with an affinity of 1.04 nM. Interestingly, the heterodimeric DNA-BC with the T20 spacer had a higher affinity than the homodimeric DNA-BC. Conclusively, we chose the heterodimeric A48t-T20-A61t DNA-BC for the subsequent experiments.
3.2. Principle of clamp-type impedimetric aptasensor for Np determination
The design of the DNA-BC-mediated electrochemical aptasensor is illustrated in Scheme 2. Accordingly, the as-prepared Au@UiO-66-NH2 solution was drop-cast onto the SPGE via strong amino-Au interaction. Since the porous Au@UiO-66-NH2 has a large surface area and abundant active sites of Zr4+, specific coordination between Zr4+ and phosphate group (-PO4 3-) was employed to anchor the 5’-terminal phosphated DNA-BC via Zr-O-P bonds. Then, excessive active sites on the gold electrode or Au@UiO-66-NH2 were blocked by bovine serum albumin (BSA). Finally, the integrated sensing biochip was examined to observe the change of electrochemical signals upon the addition of Np.
Scheme 2.
Schematic illustration of the design and fabrication of clamp-type impedimetric aptasensor for Np detection.
3.3. Basic characterizations of UiO-66-NH2 and Au@UiO-66-NH2
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were employed to investigate the surface morphologies and nanostructures of the UiO-66-NH2 and Au@UiO-66-NH2 composite. The TEM images of UiO-66-NH2 are illustrated in Fig. 1A, which show a regular octahedron frame and smooth surface and are in accordance with the corresponding SEM images shown in Fig. S4A. Following reducing Au nanoparticles (AuNPs) on the surface of metal-organic frameworks (MOFs), a rough morphology emerged. At the same time, the octahedral frame was maintained, confirming the successful synthesis of the Au@UiO-66-NH2 composite (Fig. 1B and Fig. S4B). The element distribution was mapped by an energy dispersive spectrometer (EDS), as presented in Fig. 1C-1 H and Fig. S5. The C, N, O, Zr, and Au are homogeneously distributed throughout an individual nanostructure of Au@UiO-66-NH2, thereby signifying the successful preparation of the composite.
Fig. 1.
Characterization of the synthetic materials. (A) TEM of UiO-66-NH2. (B) TEM of Au@UiO-66-NH2. (C) High-angle annular dark field (HAADF) image of the elemental distributions. (D-H) are individual element distributions for the C, N, O, Zr, and Au.
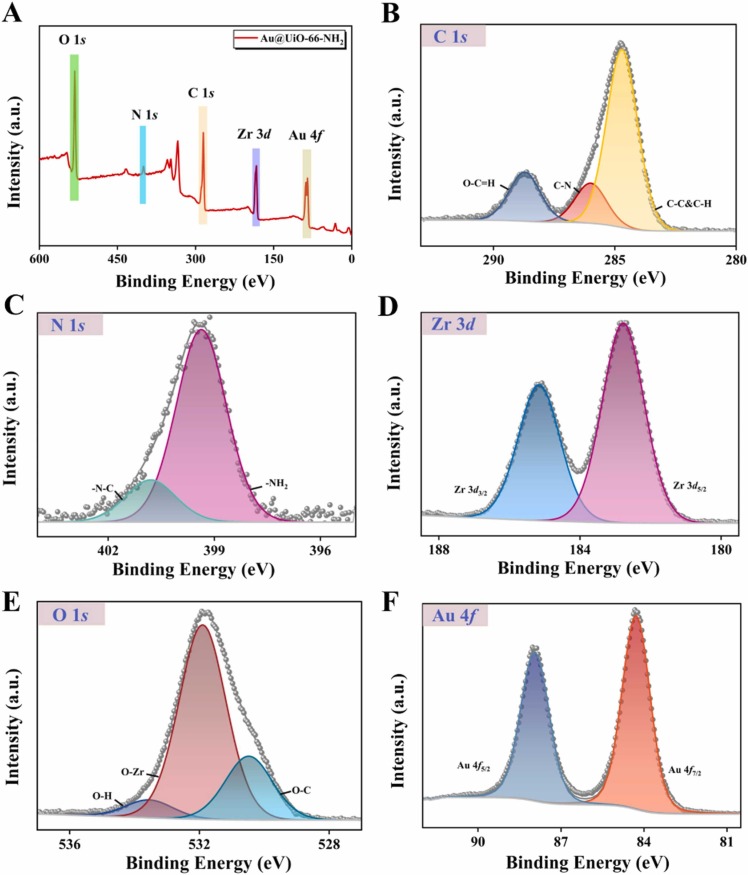
Simultaneously, X-ray photoelectron spectra (XPS) were fitted and revealed the constituents and elemental valence states of materials. Fig. S6 expressed the survey spectrum of UiO-66-NH2, mainly containing C, N, Zr, and O elements, and the corresponding spectra were exhibited in Fig. S7. The survey spectrum of Au@UiO-66-NH2 was entirely consistent with the EDC elemental mapping images ( Fig. 2A). Here, the C 1 s peaks (Fig. 2B) at binding energies (BEs) of 284.7, 286.0, and 288.7 eV indicated the existence of C−C & C−H, C−N, and O−C O, respectively on Au@UiO-66-NH2 surfaces [26]. In addition, Fig. 2C presented that the N 1 s were separated into -NH2 and -N − C peaks at BEs of 399.3 eV and 400.3 eV [27]. The Zr 3d peaks were decomposed into two peaks at the BEs of 182.9 and 185.2 eV (Fig. 2D), corresponding to the Zr 3d3/2 and 3d5/2 spectrum, respectively [28]. As depicted in Fig. 2E, the peaks of O 1 s, which were positioned at 530.5, 531.9, and 533.5 eV, were assigned to O−C, O−Zr, and O−H groups, respectively [29], [30]. In comparison with UiO-66-NH2, a pair of peaks of Au 4 f5/2 (87.9 eV) and Au 4 f7/2 (84.3 eV) were attributed to the Au 4 f spectrum (Fig. 2F) [31], further verifying the successful introduction of AuNPs in the composite.
Fig. 2.
XPS spectra of the Au@UiO-66-NH2. (A) Survey of Au@UiO-66-NH2 XPS spectra. (B-F) XPS spectra of C 1 s, N 1 s, Zr 3d, O 1 s, and Au 4 f, respectively.
The Fourier transform infrared (FTIR) spectra were recorded to characterize the formation process of Au@UiO-66-NH2 further. Fig. S8 displayed the FTIR spectra of the UiO-66-NH2 and Au@UiO-66-NH2, and no substantial variation was observed. The typical peak at 3418 cm−1 belongs to the stretching vibration mode of amino groups [32]. The peaks corresponding to the N − H wagging band and strong C−N stretching vibration appeared at 771 and 1258 cm−1, respectively [33], while the peak at 1570 cm−1 can be assigned to the C−O−Zr bonding [34]. Based on the above results, it could be concluded that the Au@UiO-66-NH2 composite was successfully fabricated. Powder X-ray diffraction (PXRD) was performed to investigate the crystal and chemical structures of UiO-66-NH2 and Au@UiO-66-NH2. Fig. S9A displayed the main characteristic diffraction peaks marked at 7.28°, 8.44°, and 25.58° in the PXRD patterns of UiO-66-NH2 that were matched with the simulated patterns, thereby indicating the successful formation [35]. Besides that, it was further declared that the surface of UiO-66-NH2 was decorated with reduced AuNPs (Fig. S9B). The extra characteristic peaks marked at 38.22º, 44.48°, and 64.66º in the PXRD pattern of Au@UiO-66-NH2 represent the crystal plane of Au (111), Au (200), and Au (220), respectively, which can well correspond to JCPDS card No. 99–0056.
3.4. Electrochemical characterization of the biochip fabrication
The CV and EIS were conducted to monitor the stepwise modification process of the sensing interface using the [Fe(CN)6]3−/4− as a redox probe. The EIS data were adequately fitted through a Randles equivalent circuit (Fig. S10) in which Rct, Rs, CPE, and Zw stand for charge-transfer resistance, the resistance of the electrolyte solution, the constant phase element, and Warburg-diffusion impedance, correspondingly. In Nyquist plots, the Rct value equals the semicircle diameter and is collected to estimate the stepwise modification process. The Rct changes [ΔRct = Rct, Np - Rct, 0.25 wt% BSA] were calculated to assess the efficiencies of Np detection using this fabricated biochip.
As shown in Fig. 3A, the semicircle diameter dropped from 576.5 to 273.4 Ω (curves a and b) when Au@UiO-66-NH2 was immobilized on SPGE, suggesting that the addition of AuNPs increased the electrical conductivity. The anchoring of the DNA-BC on the surface of Au@UiO-66-NH2 triggered a restriction of the electron transport due to the electrostatic repulsion between the DNA-BC and [Fe(CN)6]3−/4− (curve c). This result indicated the successful assembling process of 5'-PO4 3- modified DNA-BC on Au@UiO-66-NH2, which was further affirmed by XPS spectra of P 2p. (Fig. S11). After the treatment with BSA, an Rct value of 2.968 kΩ accounted for the blocking effect on the electron transfer between the redox probe and the modified electrode interface (curve d). After adding 2 pg mL−1 of Np on the as-fabricated impedimetric biochip, an Rct value of 3.254 kΩ was recorded to validate the feasibility and availability of DNA-BC to grasp Np (curve e). The current variation in CV was consistent with the electron transfer resistance in EIS ( Fig. 4B). All the results demonstrated the successful fabrication of an electrochemical aptasensor for Np detection. Additionally, the electrochemical behaviors of aptasensor based on UiO-66-NH2 were also studied to compare with Au@UiO-66-NH2-based aptasensor. As shown in Fig. S12A, Curve b had a bigger semicircle than the bare SPGE (curve a) since the lack of AuNPs resulted in poor conductivity. Curves c to e were the surface of grafting DNA-BC, blocking by BSA, and incubation with 2 ng mL-1 Np in succession. The changes of Rct during the fabrication process was determined to compare the sensing performance (Fig. S12B). All the results emphasized that the Au@UiO-66-NH2-based aptasensor had a better electrochemical detection performance towards Np.
Fig. 3.
Electrochemical characterization of the biochip fabrication. (A) EIS and (B) CVs collected for the bare SPGE (a), Au@UiO-66-NH2/SPGE (b), DNA-BC/Au@UiO-66-NH2/SPGE (c), BSA/DNA-BC/Au@UiO-66-NH2/SPGE (d), and the integrated sensing biochip incubated with Np (2 pg mL−1) (e). (C) Influence of the DNA-BC concentration. (D) Optimization of the Np incubation time with the DNA-BC. All measurements were performed in 5.0 mM [Fe(CN)6]3−/4− solution containing 0.25 M KCl.
Fig. 4.
The performance of the DNA-BC for Np detection. (A) the EIS of the aptasensor at the different concentrations of Np (0–2 ng mL−1). (B) The relationship between the ΔRct and the concentration of Np (0–2 ng mL−1). Inset: the calibration curve for Np detection using this aptasensor, n = 3. (C) Specificity of the biochip for Np (20 pg mL−1) compared to other interfering proteins (2 ng mL−1). (D) The ΔRct of five different biochips for analyzing 20 pg mL−1 Np.
3.5. Analytical performance of the DNA-BC for Np detection
The DNA-BC concentration and the incubation time with Np were successively studied, which should play crucial roles in regulating the optimal performance of this impedimetric aptasensor. Fig. 3C showed the Rct values intensified with increasing DNA-BC concentration. The optimal concentration was determined to be 5 μM since the Rct value reached a plateau at this point. Simultaneously, it was discovered that the ΔRct increased steadily with the increase of incubation time until 5 min, so 5 min was selected as the ideal incubation time (Fig. 3D). The analytical performance of the clamp-type impedimetric biochip toward Np was inspected under optimized conditions. The Np solutions at various concentrations were dropped on the biochip and interacted with the DNA-BC. As shown in Fig. 4A, the Rct values remarkably increased as the Np increased from 0 to 2 ng mL−1. The curve responses to different Np concentrations were plotted in Fig. 4B, and the inset depicted the favorable linear relationship between theΔRct and the logarithm of Np concentration with a regression equation of ΔRct = 0.134 + 0.686 lg CNp and a correlation coefficient (R2) of 0.997. According to the 3σ rule, the detection limit could be determined to be 0.31 pg mL−1. Notably, one testing process can be completed within 11 min. This clamp-type impedimetric aptasensor is comparable or even outperform to the majority of the published methods, as summarized in Table S4. The remarkable performance is ascribed to the sensitive electrochemical system and the superior grasping efficiency of the aptamers dimerization-inspired DNA-BC.
3.6. Stability, specificity, and reproducibility of the biochip
The fabricated biochip was stored at 4 ℃ and immersed in PBS buffer after investigating the stability of the system by comparing the ΔRct for 20 pg mL−1 Np collected every day. As illustrated in Fig. S13, the ΔRct values can be well maintained for 10 days, ensuring the excellent stability of the analytical system for long-term storage.
In addition, we investigated the specificity of the fabricated impedimetric biochip towards Np by comparing it with interference analytes, including receptor binding domain (RBD), thrombin (TB), horseradish peroxidase (HRP), human serum albumin (HSA), cardiac troponin I (cTnI), and the coexisting solution. As shown in Fig. 4C, the fabricated biochip displayed apparent electrochemical impedance after being incubated with Np solution, while the influence of interference analytes on ΔRct was almost negligible even at a relatively higher concentration. Accordingly, the results suggested a potential application of the biochip in a complicated system.
Finally, high reproducibility, as another essential property, plays a vital role in the application of the biochip. To verify the performance, five parallelly prepared biochips were applied to detect Np (20 pg mL−1) by the identical procedure with triplicate measurements, and an RSD value of 2.46% was obtained (Fig. 4D), reflecting good reproducibility and accuracy.
3.7. Exploration of the potential application
It has been well-accepted that the SARS-CoV-2 virus can be transmitted through saliva, respiratory droplets, or direct contact. Moreover, the water environment can be directly contaminated with SARS-CoV-2 through infected feces and urine, which is regarded as another concerned transmission vector [36]. Seriously, the connection between water and wastewater piping systems and humans' daily activities can exacerbate community-to-community transmission of SARS-CoV-2 on the cards [37]. Therefore, monitoring SARS-CoV-2 viral components in environmental water media can provide feedback on the current level of COVID-19 infection in a community. In subsequent experiments, we quantitatively detected Np spiked in saliva, serum, and environmental water samples, including river water, and wastewater using this proposed strategy with the commercial ELISA kit as control which was deemed as the most common method for detection owing high sensitivity and good reproducibility. As the data in Table S5, the acceptable recovery from 96.7% to 104.3% with RSD below 4.5% approximated the spiked Np, which were comparable to the ELISA method, indicating that the portable clamp-type impedimetric biochip is promising for early diagnosis and water environment monitoring with high reliability and accuracy.
Finally, our method was used to detect the surrounding students who infected with COVID-19. The commercial colloidal gold immunochromatographic strips (GIS) assay was used to confirm the positive case. As shown in Fig. S14, the same nasal swab samples were firstly tested with GIS and then using our DNA-BC biochip electrode. The Rct value of patient samples was significantly larger than that of the control buffer, demonstrating the sensitivity and reliability of our method.
The stability of DNA aptamers has always constrained their potential applications. In the current work, we assembled divalent aptamers with MOFs. This boosted the stability and dependability of the aptamers while also enhanced the detection performance of the system. The use of dual aptamers improves the detection performance of the biosensor with a detection limit of 0.31 pg mL−1, which is 53-fold lower than that of a single aptamer in use [22]. The electrochemical impedance method eliminates the labeling of the signal probe and reduces testing costs. The point-of-care testing and portable detection strategy can well meet the needs of different scenarios. Thus, the design of our biosensor has universality in various biological detection systems and is of great significance for insight into other targets.
4. Conclusion
First, a pair of sandwich-type aptamers are dimerized to be a facile biomimetic clamp to achieve high-performance target recognition. The conductive, high-surface-area Au@UiO-66-NH2 materials provide the active interface and stabilize the aptamers. The integrated DNA-BC/Au@UiO-66-NH2/SPGE impedimetric aptasensor achieves ultrasensitive with a detection limit as low as 0.31 pg mL-1, which is comparable to or better than those of the most reported detection methods for nucleocapsid protein. Second, the collocation of biochip and portable electrochemical devices can eliminate the use of traditional large-scale instruments, offering as a rapid, on-site, cost-efficient, simple, and convenient analytical method that can be applied for point-of-care testing conducing to early diagnosis and water environment monitoring. Finally, our findings highlight the superior functionality of the nanomaterial for the stability and performance of DNA aptamer. It is feasible and reasonable to acquire the new recognition components using the truncated aptamers based on the parental aptamers that were specifically customized. We envision that the biomimetic clamp-type impedimetric aptasensor has considerable promise to combat the SARS-CoV-2 variants and even provides a different perspective on biosensor exploitation.
CRediT authorship contribution statement
Cong Han: Methodology, Validation, Formal analysis, Visualization, Software, Writing – original draft. Wenping Xing: Software, Resources, Investigation. Wenjin Li: Conceptualization, Experiment, Writing – review & editing. Xiaona Fang: Conceptualization, Resources, Software, Investigation. Jian Zhao: Resources, Software, Investigation. Feng Ge: Experiment and article revision. Wei Ding: Writing – review & editing. Pengpeng Qu: Formal analysis, Supervision, Data curation, Writing – review & editing. Zhaofeng Luo: Data curation, Project administration, Supervision. Liyun Zhang: Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge the funding support from the National Key Research and Development Program of China (No. 2020YFA0907300) and the National Natural Science Foundation of China (No. 22077069), the Natural Science Foundation of Tianjin (19JCZDJC33400, 21JCYBJC00310), and the Fundamental Research Funds for the Central Universities, Nankai University (63201111).
Biographies
Cong Han is currently a Ph.D. student at the college of life sciences, Nankai University. He received his Master's degree in 2017 from the college of biological sciences and technology, University of Jinan. His current research interests include aptasensor development and application in the early diagnosis of diseases.
Wenping Xing is currently a graduate student in grade 2020 at the college of pharmacy,Nankai University. She majors in biology and medicine, and her current research interests are the application of aptamer.
Wenjin Li received her Master's degree in 2022 from Nankai University. Her major was pharmaceutical engineering, and her research direction was biological analysis based on aptamer technology.
Xiaona Fang is now working in the Aptamer Selection Center of the Cancer Hospital of the University of Chinese Academy of Sciences, her current research interests are the SELEX of the aptamer.
Jian Zhao is currently an undergraduate student at the college of life sciences, Nankai University. His major is biotechnology.
Feng Ge is now working in the Aptamer Selection Center of the Cancer Hospital of the University of Chinese Academy of Sciences, and her research area was aptamer SELEX.
Wei Ding is a doctor of department of gynecological oncology, Tianjin central hospital of obstetrics and gynecology, Nankai university, his research area was aptamer application in clinic.
Pengpeng Qu is currently a professor at department of gynecological oncology, Tianjin central hospital of obstetrics and gynecology, Nankai university. Her research direction is oncology and the application of aptamer in gynecological oncology.
Zhaofeng Luo is currently the director of the Aptamer Selection Center of the Cancer Hospital of the University of the Chinese Academy of Sciences. And his research field is aptamer selection and application.
Liyun Zhang is currently a professor at the state key laboratory of medicinal chemical biology, college of life sciences, Nankai University. She received her Ph. D from the University of science and technology of China (2009). Dr. Zhang was a winner of the Newton International Fellowship for the researcher at the University of Oxford, United Kingdom. She joined Nankai University in 2019. Her current scientific interests are biocatalysis, synthetic biology, and aptamer-based biosensor development and application.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.snb.2023.133387.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Data will be made available on request.
References
- 1.Servellita V., Syed A.M., Morris M.K., Brazer N., Saldhi P., Garcia-Knight M., Sreekumar B., Khalid M.M., Ciling A., Chen P.Y., Kumar G.R., Gliwa A.S., Nguyen J., Sotomayor-Gonzalez A., Zhang Y., Frias E., Prostko J., Hackett J., Jr., Andino R., Wadford D.A., Hanson C., Doudna J., Ott M., Chiu C.Y. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 omicron and delta variants. Cell. 2022;185:1539–1548. doi: 10.1016/j.cell.2022.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamal S.M., Landers D.B., Hollenberg S.M., Turi Z.G., Glotzer T.V., Tancredi J., Parrillo J.E. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J. Am. Coll. Cardiol. 2022;79:2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I., Zavitan M., Zuckerman N., Bar-Chaim A., Kreiss Y., Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant B.D., Anderson C.E., Williford J.R., Alonzo L.F., Glukhova V.A., Boyle D.S., Weigl B.H., Nichols K.P. SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 2020;92:11305–11309. doi: 10.1021/acs.analchem.0c01975. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Wu Y., Ding L., Huang X., Xiong Y. Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Anal. Chem. 2021;145 doi: 10.1016/j.trac.2021.116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostafa I.M., Tian Y., Anjum S., Hanif S., Hosseini M., Lou B., Xu G. Comprehensive review on the electrochemical biosensors of different breast cancer biomarkers. Sens. Actuators B Chem. 2022;365 [Google Scholar]
- 7.Du X., Li Y., Zhang Z., Zhang C., Hu J., Wang X., Zhang R., Yang J., Zhou L., Zhang H., Liu M., Zhou J. An electrochemical biosensor for the assessment of tumor immunotherapy based on the detection of immune checkpoint protein programmed death ligand-1. Biosens. Bioelectron. 2022;207 doi: 10.1016/j.bios.2022.114166. [DOI] [PubMed] [Google Scholar]
- 8.Salinas E., Rivero V., Torriero A.A., Benuzzi D., Sanz M.I., Raba J. Multienzymatic-rotating biosensor for total cholesterol determination in a FIA system. Talanta. 2006;70:244–250. doi: 10.1016/j.talanta.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q.-Q., Li H.-K., Sun X.-L., Han Z.-Y., Sun J., He H. Rational incorporation of covalent organic framework/carbon nanotube (COF/CNT) composites for electrochemical aptasensing of ultra-trace atrazine. J. Mater. Chem. C. 2021;9:8043–8050. [Google Scholar]
- 10.Han C., Li Q., Ji H., Xing W., Zhang L., Zhang L. Aptamers: the powerful molecular tools for virus detection. Chem. Asian J. 2021;16:1298–1306. doi: 10.1002/asia.202100242. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Yang Z., Chai B.Z., Gao L., Zhao J., Xu X.J. An ultrasensitive electrochemical aptasensor for microcystin-LR detection using core-satellite gold nanoparticle/silver nanocluster nanoassemblies as labels for signal amplification. Sens. Actuators B Chem. 2022;371 [Google Scholar]
- 12.Zhang Z., Pandey R., Li J., Gu J., White D., Stacey H.D., Ang J.C., Steinberg C.J., Capretta A., Filipe C.D.M., Mossman K., Balion C., Miller M.S., Salena B.J., Yamamura D., Soleymani L., Brennan J.D., Li Y. High-affinity dimeric aptamers enable the rapid electrochemical detection of wild-type and B.1.1.7 SARS-CoV-2 in unprocessed saliva. Angew. Chem. Int. Ed. 2021;60:24266–24274. doi: 10.1002/anie.202110819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idili A., Parolo C., Alvarez-Diduk R., Merkoci A. Rapid and efficient detection of the SARS-CoV-2 spike protein using an electrochemical aptamer-based sensor. ACS Sens. 2021;6:3093–3101. doi: 10.1021/acssensors.1c01222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adeel M., Asif K., Alshabouna F., Canzonieri V., Rahman M.M., Ansari S.A., Guder F., Rizzolio F., Daniele S. Label-free electrochemical aptasensor for the detection of SARS-CoV-2 spike protein based on carbon cloth sputtered gold nanoparticles. Biosens. Bioelectron. X. 2022;12 doi: 10.1016/j.biosx.2022.100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., Huang B., Li D., Zhang R., Tian M., Fu L., Gao Y., Zhao X., Liu K., Qi J., Gao G.F., Wang P. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185:630–640. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H., Cui Y., Han X., Hu W., Sun M., Zhang Y., Wang P.H., Song G., Chen W., Lou J. Liquid-liquid phase separation by SARS-CoV-2 nucleocapsid protein and RNA. Cell Res. 2020;30:1143–1145. doi: 10.1038/s41422-020-00408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y., Du N., Lei Y., Dorje S., Qi J., Luo T., Gao G.F., Song H. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020;39 doi: 10.15252/embj.2020105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabiani L., Saroglia M., Galata G., De Santis R., Fillo S., Luca V., Faggioni G., D'Amore N., Regalbuto E., Salvatori P., Terova G., Moscone D., Lista F., Arduini F. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thudium R.F., Stoico M.P., Hogdall E., Hogh J., Krarup H.B., Larsen M.A.H., Madsen P.H., Nielsen S.D., Ostrowski S.R., Palombini A., Rasmussen D.B., Foged N.T. Early laboratory diagnosis of COVID-19 by antigen detection in blood samples of the SARS-CoV-2 nucleocapsid protein. J. Clin. Microbiol. 2021;59 doi: 10.1128/JCM.01001-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Yurkovetskiy L., Shen K., Luban J., Natalya D., Pascal K., Tomkins-Tinch C., Nyalile T., Wang Y., Baum A., Diehl W., Dauphin A., Carbone C., Egri S., Veinotte K., Schaffner S., Lemieux J., Munro J., Rafique A., Barve A., Sabeti P., Kyratsous C. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Microsc. Microanal. 2021;27:3260–3262. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Fang X., Liu X., Ou H., Zhang H., Wang J., Li Q., Cheng H., Zhang W., Luo Z. Discovery of sandwich type COVID-19 nucleocapsid protein DNA aptamers. Chem. Commun. 2020;56:10235–10238. doi: 10.1039/d0cc03993d. [DOI] [PubMed] [Google Scholar]
- 22.Han C., Li W., Li Q., Xing W., Luo H., Ji H., Fang X., Luo Z., Zhang L. CRISPR/Cas12a-Derived electrochemical aptasensor for ultrasensitive detection of COVID-19 nucleocapsid protein. Biosens. Bioelectron. 2022;200 doi: 10.1016/j.bios.2021.113922. [DOI] [PubMed] [Google Scholar]
- 23.Zhao P., Liu N., Jin C., Chen H., Zhang Z., Zhao L., Cheng P., Chen Y. UiO-66: an advanced platform for investigating the influence of functionalization in the adsorption removal of pharmaceutical waste. Inorg. Chem. 2019;58:8787–8792. doi: 10.1021/acs.inorgchem.9b01172. [DOI] [PubMed] [Google Scholar]
- 24.Ye H., Duan N., Gu H., Wang H., Wang Z. Fluorometric determination of lipopolysaccharides via changes of the graphene oxide-enhanced fluorescence polarization caused by truncated aptamers. Mikrochim. Acta. 2019;186:173. doi: 10.1007/s00604-019-3261-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin M., Zhang J., Wan H., Yan C., Xia F. Rationally designed multivalent aptamers targeting cell surface for biomedical applications. ACS Appl. Mater. Interfaces. 2021;13:9369–9389. doi: 10.1021/acsami.0c15644. [DOI] [PubMed] [Google Scholar]
- 26.Zeng H., Yu Z., Shao L., Li X., Zhu M., Liu Y., Feng X., Zhu X. A novel strategy for enhancing the performance of membranes for dyes separation: embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021;403:11305–11309. [Google Scholar]
- 27.Liu J., Wang X., Zhao Y., Xu Y., Pan Y., Feng S., Liu J., Huang X., Wang H., Plasma N.H.3. Functionalization of UiO-66-NH2 for highly enhanced selective fluorescence detection of U(VI) in water. Anal. Chem. 2022;94:10091–10100. doi: 10.1021/acs.analchem.2c01138. [DOI] [PubMed] [Google Scholar]
- 28.Ma J., Lu Z., Li C., Luo Y., Shi Y.E., Alam P., Lam J.W.Y., Wang Z., Tang B.Z. Fluorescence ratiometric assay for discriminating GSH and Cys based on the composites of UiO-66-NH2 and Cu nanoclusters. Biosens. Bioelectron. 2022;215 doi: 10.1016/j.bios.2022.114582. [DOI] [PubMed] [Google Scholar]
- 29.Yang J., Dai Y., Zhu X., Wang Z., Li Y., Zhuang Q., Shi J., Gu J. Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A. 2015;3:7445–7452. [Google Scholar]
- 30.Sun Z., Fan Y.Z., Du S.Z., Yang Y.Z., Ling Y., Li N.B., Luo H.Q. Conversion of fluorescence signals into optical fingerprints realizing high-throughput discrimination of anionic sulfonate surfactants with similar structure based on a statistical strategy and luminescent metal-organic frameworks. Anal. Chem. 2020;92:7273–7281. doi: 10.1021/acs.analchem.0c00907. [DOI] [PubMed] [Google Scholar]
- 31.Kumar G., Tibbitts L., Newell J., Panthi B., Mukhopadhyay A., Rioux R.M., Pursell C.J., Janik M., Chandler B.D. Evaluating differences in the active-site electronics of supported Au nanoparticle catalysts using Hammett and DFT studies. Nat. Chem. 2018;10:268–274. doi: 10.1038/nchem.2911. [DOI] [PubMed] [Google Scholar]
- 32.Yu F., Du T., Wang Y., Li C., Qin Z., Jiang H., Wang X. Ratiometric fluorescence sensing of UiO-66-NH2 toward hypochlorite with novel dual emission in vitro and in vivo. Sens. Actuators B Chem. 2022;353 [Google Scholar]
- 33.Lv S.-W., Liu J.-M., Ma H., Wang Z.-H., Li C.-Y., Zhao N., Wang S. Simultaneous adsorption of methyl orange and methylene blue from aqueous solution using amino functionalized Zr-based MOFs. Microporous Mesoporous Mater. 2019;282:179–187. [Google Scholar]
- 34.Liu M., Wang J., Yang Q., Hu N., Zhang W., Zhu W., Wang R., Suo Y., Wang J. Patulin removal from apple juice using a novel cysteine-functionalized metal-organic framework adsorbent. Food Chem. 2019;270:1–9. doi: 10.1016/j.foodchem.2018.07.072. [DOI] [PubMed] [Google Scholar]
- 35.Wang X.Y., Yin H.Q., Yin X.B. MOF@COFs with strong multiemission for differentiation and ratiometric fluorescence detection. ACS Appl. Mater. Interfaces. 2020;12:20973–20981. doi: 10.1021/acsami.0c04147. [DOI] [PubMed] [Google Scholar]
- 36.Giron-Navarro R., Linares-Hernandez I., Castillo-Suarez L.A. The impact of coronavirus SARS-CoV-2 (COVID-19) in water: potential risks. Environ. Sci. Pollut. Res. 2021;28:52651–52674. doi: 10.1007/s11356-021-16024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilevar M., Kim K.T., Lee W.H. Recent advances in biosensors for detecting viruses in water and wastewater. J. Hazard. Mater. 2021;410 doi: 10.1016/j.jhazmat.2020.124656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.