Abstract
Background
MetS are common throughout the world, including Ethiopia. These have traditionally been treated using medicinal plants, particularly in rural areas where they are freely accessible. This systematic review tried to investigate the treatment of MetS with Ethiopian medicinal herbs and made recommendations for more validation research. A careful analysis of the literature was also conducted on the therapeutic effects of these and other Ethiopian medicinal plants with hepatoprotective and antihypertensive activities.
Methods
The relevant keywords “Ethnomedicinal + hypertension,” “Ethnopharmacological + hypertension,” “Ethnomedicinal + hepatitis, jaundices, and liver disease,” “Ethnopharmacological + hepatic disorder,” and “Ethnomedicinal + weight loss” were used to search for relevant articles in the major electronic scientific databases, including PubMed, Science Direct, Web of Science, and Google Scholar. The search strategy included all articles with descriptions that were accessible until April 30, 2022. The study's subjects, methods, or year of publication were no restrictions in the search. The outcomes were compiled using descriptive statistics.
Results
Fifty-four (54) studies were examined in the review that satisfied the inclusion and exclusion criteria for the treatment of MetS in Ethiopia. The most often used ethnobotanical plant species for the treatment of hypertension and hepatic disorders were Moringa stenopetala and Croton macrostachyus. Both hepatic and hypertensive disorders were treated more frequently with leaves (52% and 39%, respectively) than with roots (20% and 13%, respectively). Some intriguing studies came from an ethnobotanical investigation into medicinal herbs' hepatoprotective and antihypertensive properties. The most often investigated medicinal plant for its antihypertensive effects is Moringa stenopetala.
Conclusion
The study revealed that Ethiopians often use anti-MetS herbal remedies. We advocate the experimental validation of the commonly used medicinal plants with the identification of active compounds and the development of effective alternative drugs for the treatment of MetS.
1. Introduction
Metabolic syndrome (MetS), a cluster of interrelated metabolic disorders, is becoming more common around the world. According to the International Diabetes Federation, MetS affects around 25% of the world's adult population, and its prevalence is expected to rise in the next few decades [1]. MetS are on the rise and pose a serious threat to public health, especially in countries in sub-Saharan Africa with limited resources [2]. Governments in underdeveloped countries have already spent billions of dollars to tackle the widespread effects of MetS and related risk factors [3]. The emergence of risk factors for MetS and an increase in its incidence worldwide have all been related to genetic, epigenetic, and environmental factors [4]. The adoption of sedentary lifestyles, which are defined by low physical activity or exercise and the intake of high-energy foods, is also to blame for this epidemic [5]. The risk factors for MetS are being addressed through dietary modifications and the use of pharmaceutical drugs that primarily target specific biochemical pathways involved in food metabolism [6]. Pharmaceutical medications usually cost a lot of money, have poor patient compliance, and have been associated with the emergence of a variety of undesirable side effects with prolonged usage. In addition, they are monotherapeutic, concentrating on just a few health outcomes associated with metabolic dysregulation. Alternative and complementary approaches to the management of metabolic diseases must be studied and developed urgently. Herbal remedies should be used in these alternate MetS risk factor management strategies. Medicinal plants are defined as any plant or plant preparation that has beneficial therapeutic and/or preventive properties or that provides health-promoting properties and temporary relief [7]. Medicinal plants are now accepted by healthcare providers as having a role to play in the management and prevention of metabolic disorders [8]. The use of herbal medicine is no longer limited to developing countries; it has grown into a multibillion-dollar industry that spans all demographic and socioeconomic groups [9]. Medicinal plants include pharmacodynamic bioactive compounds that have a therapeutic impact that is additive and synergistic in the treatment of metabolic disorders [10]. Most pharmaceutical drugs are derived from medicinal plants using local knowledge and then isolating the main active compounds [11]. Plant material utilized in the preparation of medicinal remedies could be used as a template for the development of pharmaceutical drugs. The identification of beneficial phytochemical compounds in medicinal plants and their application in the treatment of MetS have reduced the financial burden of relying on costly synthetic pharmaceutical drugs. According to the WHO, even in the presence of pharmaceutical drugs, most rural and urban-based communities in Africa still rely on traditional remedies for their primary healthcare [12]. When compared to some of the pharmaceutical drugs now being used in the management of metabolic disorders, another driving factor in the usage of medicinal plants is the impression that they are free of adverse side effects and acute toxicity [13]. Despite the fact that some people prefer to use medicinal plants due to their perceived safety, scientific validation is required to ensure the safety and consistency of medicinal preparations. In fact, the WHO recommends demonstrating safety before determining the therapeutic benefit of medicinal plants used in primary care [14]. In this review, we looked at how medicinal plants are currently being used or studied in Ethiopia to treat and prevent MetS risk factors such as obesity, cardiovascular disease, and liver disease.
2. Methods
2.1. Search Strategy
Scientific search engines such as Google Scholar, PubMed, Scopus, Science Direct, and Research Gate were used to look up Ethiopia, “Ethnomedicinal + hypertension,” “Ethnopharmacological + hypertension,” “Ethnomedicinal + hepatitis, jaundices, and liver disease,” “Ethnopharmacological + hepatic disorder,” and “Ethnomedicinal + weight loss.” The search was conducted without regard to the subjects, methods, or year of publication.
2.2. Inclusion and Exclusion Criteria
Our inclusion criteria were as follows: (i) articles must be written in English; (ii) articles must be field studies (surveys); (iii) studies must provide complete ethnobotanical information; and (iv) studies should include medicinal plants with antihypertensive and hepatoprotective activities. Exclusion criteria included (i) articles with no study areas or scientific plant names, (ii) articles with only an abstract, (iii) articles written in a non-English language, (iv) newspapers, (v) reviews, and (vi) for species reported as “sp.” without a species name, such as Euphorbia sp., which was not counted because other Euphorbia species were present.
2.3. Assessment of Methodological Quality
Before being included in the review, all 54 papers were critically appraised using established procedures to ensure methodological validity [15]. Preferred Reporting of Systematic Reviews and Meta-Analysis (PRISMA) criteria were employed to ensure scientific rigor (see selection process in Figure 1).
Figure 1.
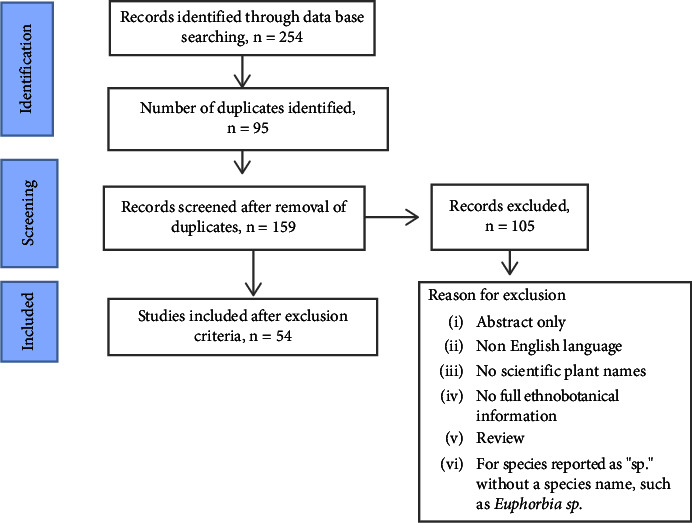
Flow chart used for the design of the current review.
2.4. Data Abstraction and Review Process
Using the inclusion/exclusion criteria, the articles underwent screening. The following information was extracted from each study using abstraction forms: scientific, family, plant parts used, methods of preparation and mode of action, extraction solvent utilized, models used, and effects of pharmacological medicinal plants. The International Plant Name Index (https://www.ipni.org) and the Kew Botanical Garden plant name database (https://www.kew.org) were used to verify species names and synonyms. Data extraction was carried out twice independently, after which the datasheet was checked for methodological compliance and any errors were fixed. The results were summarized by descriptive statistics.
3. Result and Discussion
3.1. Literature Search Results
The scanning of databases yielded two hundred fifty-four (254) relevant articles, 95 of which were duplicates. After analyzing our inclusion and exclusion criteria, one hundred five (105) articles were excluded, and the remaining fifty-four (54) articles were included (Figure 1).
3.2. Medicinal Plants in the Management of Obesity
According to the World Health Organization, risk factors related to being overweight or obese account for 2.8 million deaths annually, making obesity the seventh greatest cause of mortality [16]. In Africa, the overweight population of under-fives has risen by around 24% since 2000 [16]. According to a recent systematic review and meta-analysis obesity and overweight were found to be prevalent in Ethiopian cities at 22.4% and 6.2%, respectively [17]. Obesity occurs when eating a meal with a high calorific value (carbohydrates) is combined with a decrease in physical activity to burn the calories absorbed [18]. Being overweight has been linked to a variety of comorbidities, including cardiovascular disorders (stroke and heart), type 2 diabetes mellitus, and the malignancies of breast, prostate, kidney, and colon cancer [19]. Leading a healthy lifestyle, engaging in regular physical activity, consuming less free sugars and salts, decreasing saturated fat consumption while increasing consumption of dietary vegetables and whole grains, as well as pharmacological therapies and surgical interventions, are all recommended for weight loss [20]. However, treating obesity is difficult because only 5–10% of people maintain their weight loss over time [21]. There is a reversal of weight loss when pharmacotherapy is stopped or a healthy lifestyle is abandoned [22]. Also, some of the synthetic drugs used have unfavorable side effects [23]. Herbal supplements are an alternative to pharmacological drugs for weight loss. They are effective, safe, and less expensive than pharmacological drugs. However, there is no serious attention given to obesity disease research in Ethiopia presently. In this review, we included some plants that are frequently consumed for weight loss in Ethiopia, along with their parts and preparation techniques (Table 1). The mentioned herbal remedies have not been evaluated for their safety and efficacy in the management of obesity. Consequently, both in vitro and in vivo studies were necessary.
Table 1.
List of medicinal plants and their preparation methods for the treatment of hypertension.
| Species name | Family name | Local name | Plant part used | Methods of herbal material preparation and mode of action | Ref |
|---|---|---|---|---|---|
| Verbascum sinaiticum | Scrophulariaceae | Daba KededAm | Root | Crushing the root orally | [24] |
| Trigonella foenumgraecum | Leguminosae | AbishAm | Seed | Grind, powdered, add water, and drunk | [25] |
| Syzygium guineense | Myrtaceae | DuuwanchoOr | Bark & fruit | The ripe fruits of the plant are eaten in small amounts for some time | [26] |
| Dorstenia barnimiana | Moraceae | Work BemedaAm | Root | Root powder mixed with honey and fermented for seven days is taken orally in the morning | [27, 28] |
| Brucea antidysenterica | Simaroubaceae | AballoAm | Root | Root powder mixed with honey is taken orally | [27] |
Am, Amharigna; Or, Afaan Oromoo.
3.3. Medicinal Plants in the Management of Cardiovascular Diseases
According to the World Health Organization (WHO), high blood pressure is responsible for an estimated 62% of cardiovascular diseases (CVDs) and 49 percent of ischemic heart disorders worldwide [27]. Hypertension (HTN) is a chronic medical disorder in which the blood pressure (BP) in the arteries is too high. It makes it more difficult for the heart to pump blood via the blood vessels. Hypertension affects an estimated 1.28 billion adults worldwide aged 30 to 79, with the majority (two-thirds) living in low- and middle-income nations [29]. HTN accounts for at least 45 percent of all heart disease deaths and 51 percent of all stroke deaths [30]. According to a meta-analysis of the prevalence of HTN in Ethiopia, it is on the increase, with an estimated prevalence of 19.6% [31]. In this section of the review, we looked at how medicinal plants are used in Ethiopian traditional and complementary medicine to treat liver disease. Twenty-two (22) medicinal plants from fourteen (14) families were found in this ethnobotanical review, and the traditional healer used them to treat hypertension. The plant families with the most species are Lamiaceae (n = 4), Fabaceae (n = 2), and Polygonaceae (n = 2) (Table 1). Analysis of the eligible ethnobotanical findings revealed that different parts of the medicinal plants were utilized in the preparation of MetS remedies. The antihypertensive medicinal 'plants' leaves (39%) and roots (13%) are the parts that are most frequently harvested (Figure 2). The most often cited ethnobotanical plant species for the treatment of hypertension was Moringa stenopetala (Table 2 and Figure 3). Moringa stenopetala, often known as the African Moringa or cabbage tree, is a deciduous tree native to Kenya and Ethiopia in the Moringa genus of flowering plants [54]. M. stenopetala contains alkaloids, amino acids, essential oils, fatty acids, flavonoids, phenolic compounds, and sterols [55]. Some pharmacological activities of M. stenopetala have been reported in the literature including antimicrobial [56–58], antidiabetic [59–61], antitrypanosomal [62], antimalarial [63], anti-Leishmania [64], anti-inflammatory and analgesic [65, 66], antihypertensive [67], antioxidant [61, 68, 69], anticancer [70], and thyroid function [71]. It could be more effective than other antihypertensive medicinal plants in terms of treatment.
Figure 2.
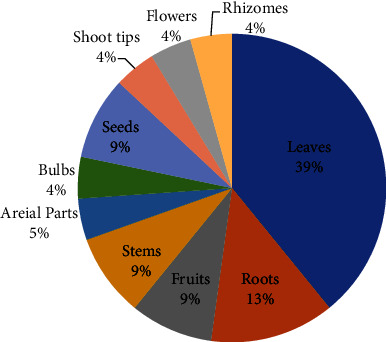
Frequency distribution of plant parts used to prepare remedies.
Table 2.
List of medicinal plants and their preparation methods for the treatment of hypertension.
| Species name | Family name | Local name | Plant part used | Methods of herbal material preparation and mode of action | Ref |
|---|---|---|---|---|---|
| Allium cepa | Liliaceae | Key shinkurt | Bulbs | The bulb is chopped, macerated in water, filtered, and drunk | [32] |
| Hordeum vulgare | Poaceae | Gebs | Seeds | Mashilla (Sorghum spp.) and Gebs (germinated barley) are baked together in the same way that bread is prepared. This is broken up and fermented with beqil (malt starter) before being brewed, distilled, and served in a shot glass | [33] |
| Thymus schimperi | Lamiaceae | Tosigne | Leaves | Tea made from boiled leaves | [33, 34] |
| Lupinus albus | Fabaceae | Gibtto | Seeds | Seeds infused in water and filtrate are taken orally | [35] |
| Rumex abyssinicus | Polygonaceae | Mekmoko | Roots | The decoction is taken on an empty stomach | [35] |
| In a blender, crush the root and combine it with the Allium sativum bulbs. Boil the combination, and then drink the hot decoction or powdered root with milk | [36, 37] | ||||
| Crinum abyssinicum | Amaryllidaceae | Yejib shinkurt | Shoot tips | Fresh shoot tips squeezed the liquid, mixed with water, drunk it | [25] |
| Citrus aurantifolia | Rutaceae | Lemon | Fruits | Lemon juice is drunk from the fruit | [25] |
| Foeniculum vulgare | Apiaceae | Ensilal | Leaves | Fresh leave of Foeniculum vulgare add to boiled tea and drink it | [25, 36] |
| Moringa stenopetala | Moringaceae | Shiferaw | Leaves | Dry/fresh leave make as tea and drink it or fresh leave boil with Allium cepa and Capsicum annuuam, add oil and taken | [25, 38–41] |
| Dovyalis abyssinica | Flacortiaceae | Yabesha Qoshm | Roots & stem tubers | Root and stem tuber is smashed with “Tela” and drunk it | [36] |
| Bersama abyssinica | Melianthaceae | Azamr | Roots & leaves | Fresh root and leave crushed and mixed with honey and taken once daily for 3 consecutive days | [42] |
| Cadaba farinosa | Capparidaceae | Qalaanqaal (som) | Roots | Chopped, boiled with meat soup, and drunk | [39] |
| Leucaena leucocephala | Fabaceae | Stems | Chopped, macerated, filtered, mixed with honey and milk, and drunk | [39] | |
| Citrus aurantium | Rutaceae | Komtatie | Flowers | Drink the fresh juice flower | [37] |
| Otostegia integrifolia | Lamiaceae | Tinjute | Leaves | Leaves are boiled in water and a cup of the solution is taken every morning until recovery | [43] |
| Acanthospermum hispidum | Asteraceae | Leaves | Leaves are crushed and boiled and one teacup is drunk at 12 h intervals for a week | [44] | |
| Salvia tiliifolia | Lamiaceae | Aqorarach | Leaves | Fresh leaf juice is mixed with little water and given Orally |
[45] |
| Rumex nepalensis | Polygonaceae | Tullet | Leaves | Fresh leaves are boiled and drunk | [46] |
| Zingiber officinale | Zingiberaceae | Gengible | Rhizomes | The rhizome is chewed | [43] |
| Rosa abyssinica | Rosaceae | Kega | Fruits | Powdered fruits are, mixed with water and drunk | [47] |
| Satureja punctata | Lamiaceae | Lomishet | Aerial parts | The decoction of the dried aerial parts of the plant is taken orally as a tea | [48] |
| Artemisia absinthium | Asteraceae | Ariti | Leaves | Pounded; chewed orally | [49] |
Figure 3.

Frequently cited antihypertensive medicinal plants. (a) Moringa stenopetala [50]. (b) Thymus Schimperi [51]. (c) Rumex abyssinicus [52]. (d) Foeniculum vulgare [53].
3.3.1. Antihypertensive Activity of Potential Ethiopian Medicinal Plants
The antihypertensive properties of six (6) Ethiopian medicinal plants from five (10) families were investigated in Ethiopia. Male Wistar rats, guinea pigs, and Sprague-Dawley rats have all been utilized as a variety of animal models to test these herbs' potential antihypertensive effects. Blood pressure (SBP, MABP, and DBP), diuretic, natriuretic, kaliuretic, and aortic relaxation were among the parameters used to assess these plants. In all models, it was discovered that the medicinal plants had a significant antihypertensive effect. Four of the plant species included in (Table 2) have antihypertensive activity (Table 3), which supports their traditional uses. Thymus schimperi, Moringa stenopetala, Otostegia integrifolia, and Satureja punctata are a few examples. The most studied plant parts were leaves, and the most extractive solvents were aqueous.
Table 3.
Antihypertensive activities of Ethiopian medicinal plants.
| Species | Family | Plant parts used | Extracts | Models used | Effects | Ref |
|---|---|---|---|---|---|---|
| Thymus schimperi | Leaves | Aqueous (250, 500, 750 and 1000 mg/kg) | Male Wistar rats | At 500 mg/kg, the extract had the highest diuretic index. Greater doses of T. schimperi (500 mg/kg) and the standard drug captopril (20 mg/kg/day) significantly (p < 0.01) reduced SBP when compared to the salt-sucrose group | [72] | |
| Moringa stenopetala | Moringaceae | Leaves | Aqueous and 70% ethanol (250, 500, and 1000 mg/kg) | Male Wistar rats | When compared to the positive and normal control groups, which received captopril (20 mg/kg/day) and distilled water (adlibitum), the highest daily oral dose of AQ crude extract (1000 mg/kg) significantly reduced SBP, MAP, and DBP rises. At the highest dose of 70% EtOH crude extract, SBP, MAP, and DBP all significantly lowered | [73] |
| Leaves | Aqueous (10, 20, 30, and 40 mg/kg) | Guinea pigs | SBP, DBP, and MABP in normotensive anesthetized Guinea pigs declined significantly | [67] | ||
| Leaves | Aqueous (62.5, 125, 250, and 500 mg/kg) and hot tea infusion | Male Wistar rats | The diuretic, natriuretic, and kaliuretic effects of both the aqueous crude extract and the hot tea infusion of the leaves are significant (p < 0.01). The strongest diuretic efficacy was found in the aqueous crude extract (125 mg/kg) and hot tea infusion (2 tsp), which were comparable to the reference drug furosemide (10 mg/kg) | [74] | ||
| Leaves | Aqueous crude, 70% ethanol crude (1.25, 2.5, 5, and 10 mg/mL) | In vitro (thoracic aortic ring of a Guinea pig) | In pre-contracted isolated entire, spirally cut thoracic aortic strips of Guinea pigs, both extracts had a relaxing (vasodilatory) effect in a dose-dependent manner | [75] | ||
| Calpurnia aurea | Fabaceae | Seed | Methanol (15, 30, and 45 mg/kg) | Sprague-Dawley rats | In renal hypertensive and normotensive rats, blood pressure (SBP, DBP, and MABP) reduced dose-dependently and significantly after treatment | [76] |
| Syzygium guineense | Myrtaceae | Leaves | Methanol (50, 100, and 150 mg/kg) | Sprague-Dawley rats | SBP, MAP, and DBP all decreased significantly at the maximum dose of crude extract. At a concentration of 5–70 mg/mL, the extract elicited a dose-dependent relaxation of the aorta pre-contracted with KCl, with a maximal relaxation of 56.22% at the 70 mg/mL concentration | [77] |
| Otostegia integrifolia | Lamiaceae | Leaves | Methanol (250, 500 and 1000 mg/kg) | Sprague-Dawley rats | In a dose-dependent manner, blood pressure was significantly reduced. At a concentration of 6.25–125 μg/L, the extract elicited a dose-dependent relaxation of the aortic strip pre-contracted with KCl, with a maximal relaxation (100 percent) achieved at a cumulative concentration of 318.75 μg/ml | [78] |
| Satureja punctata | Lamiaceae | Aerial parts | Aqueous (10, 20 and 30 mg/kg) | Guinea pig | SBP, DBP, and MABP all decreased in a dose-dependent manner when compared to baseline hypertensive BP. At concentrations ranging from 2.5 to 40 mg/ml, the extract caused a dose-dependent relaxation of the aorta pre-contracted with KCl, with a maximal relaxation of 98.19% achieved at 40 mg/ml | [79] |
3.4. Medicinal Plants in the Management of Hepatic Diseases
The liver is one of the body's largest and most influential organs. It plays an important role in a variety of physiological processes, including macronutrient metabolism, blood volume regulation, immune system support, endocrine control of growth signaling pathways, lipid homeostasis, and xenobiotic detoxification, including drug detoxification [80]. Different illness conditions, on the other hand, affect its structure and function. Changes in lifestyle and dietary habits, contamination of food or drink, chemical and drug addiction, and hepatic infections have all contributed to an increase in the incidence of hepatic illnesses around the world. Hepatitis, cirrhosis, fatty liver, bile duct obstruction, and jaundice are the most common hepatic diseases. Globally, they constitute the leading cause of morbidity and mortality [81]. An earlier clinical investigation in Ethiopia found that liver disease was responsible for 12% of hospital admissions and 31% of hospital mortality [82]. Since a large portion of Ethiopia's population lives in poverty and has limited access to modern healthcare, traditional medicine is used to treat liver disease. Traditional medicines used to treat liver disease are thus an important topic to address in future discussions about how to treat this problem. A variety of plant species that are utilized by traditional healers and herbalists in the treatment of liver diseases have been identified through ethnobotanical studies. In this section of the review, we'll look at how medicinal plants are used in Ethiopian traditional and complementary medicine to treat liver disease. In this ethnobotanical review, twenty-six (26) medicinal plants from twenty-one (21) families were identified, and the traditional healer used them to treat liver disease. Fabaceae (n = 3) and Cucurbitaceae (n = 3) are the plant families with the most species (Table 4). This could be since these are among Ethiopia's Flora Regions' most widely spread families [90]. The eligible ethnobotanical data analysis revealed that different parts of the medicinal plants were employed to make MetS remedies. The leaves (52%) and roots (22%) of plants used as hepatic remedies are the parts that are harvested most frequently (Figure 4). Croton macrostachyus was the most commonly employed ethnobotanical plant species for the treatment of hepatic disorders (Table 4, Figure 5). Croton macrostachyus is a medium-sized monoecious or deciduous tree that grows up to 30 meters tall in tropical Africa [96]. C. macrostachyus fruits, leaves, stem bark, and twigs contain alkaloids, amino acids, anthraquinones, carbohydrates, cardiac glycosides, coumarins, essential oil, fatty acids, flavonoids, phenolic compounds, phlorotannins, polyphenols, phytosterols, saponins, sterols, tannins, terpenoids, and unsaturated sterols [97, 98]. Some pharmacological activities of C. macrostachyus have been reported in the literature including anthelmintic [99], antibacterial [100], anticonvulsant and sedative [101], antidiabetic [102], antidiarrheal [97], anti-inflammatory [103], anti-Leishmania [104], antioxidant [105], and antimalarial [106]. It could be more effective than other antihepatic medicinal plants in terms of treatment.
Table 4.
List of medicinal plants and their preparation methods for the treatment of hepatic disorders.
| Species name | Family name | Local name | Plant part used | Methods of herbal material preparation and mode of action | Ref |
|---|---|---|---|---|---|
| Mentha spicata L. | Lamiaceae | Leaves | Boiling the leaves in water makes tea, or pounding the leaves and mixing them with honey makes a drink | [83] | |
| Rhus retinorrhoea | Anacardiaceae | Tilem | Roots | Rhus retinorrhoea roots, Catha edulis flowers, and Rumex nervosus roots are crushed and mixed with water and a teaspoon of salt before being drunk | [36] |
| Rumex abyssinicus | Polygonaceae | Mekmeko | Roots | The roots are crushed, powdered, and mixed with the dried and powdered meat of a bat and eaten once or twice | [32] |
| Acacia tortilis | Fabaceae | Grar | Roots | Crushed and mixed with water and consumed like tea (decoction) | [83] |
| Calpurnea aurea (Alt.) Benth | Papilionaceae | Digitta | Leaves | Fresh leaves squeezed and drunk | [25] |
| Dioscorea alata L. | Dioscoriaceae | Boye | Stems | Fresh stem cooked mixed with Allium sativum and eat | [25] |
| Acacia abyssinica | Fabaceae | Simithia | Leaves | Leave juice is given orally in the early morning for 15 days | [84] |
| Acokanthera schimperi | Apocynaceae | Merenz | Leaves | Crush, dry then fumigate | [37, 85] |
| Adhatoda schimperiana | Acanthaceae | Leaves | Three fresh leaves crushed and juice taken with cow milk in empty stomach for 3 consecutive days | [42] | |
| Treminalia brownii | Combretaceae | Aballo | Barks | Inner bark peeled, chopped, macerated in water, filtered, and drunk | [39] |
| Concocted with the bark of Croton macrostachyus and drink a cup of infusion | [86] | ||||
| Lagenaria siceraria | Cucurbitaceae | Fruits | The fruit was dissected and the patient's face was covered with the inside part of the dissected fruit | [39] | |
| Euphorbia abyssinica | Euphorbiaceae | Kulkual | Roots | Fresh root crush, immerse in water then drink or bake with bread then eat | [37] |
| Phytolacca dodecandra | Phytolaccaceae | Endod | Leaves | Fresh leave crush and drink with water | [37] |
| Leaves are crushed, squeezed and one cup of juice is taken daily for 21 days | [43] | ||||
| Rumex nervosus | Polygonaceae | Embocho | Roots | Crushed, homogenized in water, and drunk | [9] |
| Justicia shimperans | Acanthaceae | Sensel | Leaves | Leaves are pounded and juice is prepared and taken orally | [87] |
| Schinus mole | Ancardiaceae | Qundo-berbere | Leaves | The fresh leaf is crushed, mixed with water, filtered, and drink at the time of pain | [88] |
| Carica papaya | Caricaceae | Papaya | Leaves | Leaves are pounded and juice is prepared and taken | [87] |
| Cucumis ficifolius | Cucurbitaceae | Yemidir Embuy | Roots/leaves | Roots are chewed, or fresh leaf is crushed, mixed with tella/milk, and drunk it | [43, 88] |
| Croton Macrostachyus | Euphorbiaceae | Bisana | Leaves | The fresh leaf of being squeezed and one glass of juice with milk or tella is drunk for three days | [88] |
| Roots | The root bark is dried and pounded into powder and two to three spoons of powder are added to a cup containing water. Treatment is taken for 21 days | [43] | |||
| Barks | Dry bark is powdered and mixed with latex from its young twinges and applied to the wound | [89] | |||
| Leaves | Leaf powder mixed with water is taken orally for seven days | [27] | |||
| Calpurnia aurea | Fabaceae | Digita | Seeds | Dry seeds crushed and swallowed | [25] |
| Hypericum quartinianum | Hypericaceae | Ameja | Leaves | Leaf with roots of Asparagus sp. pounded and homogenized in water and given to the patient orally for three consecutive days. Half a glass is the limit for a day | [90] |
| Coffee Arabica | Rubiaceae | Buna | Barks | The bark of C. africana is powdered together with the stem bark of Croton macrostachyus, the paste is then boiled with milk and given orally | [91] |
| Dodonaea angustifolia | Sapindaceae | Kitkita | Leaves | A fist of the leaf is grounded to get half a cup of juice, which is given orally in the morning and evening until the cure | [91] |
| Verbascum sinaiticum | Scrophulariaceae | Kutitina | Roots | The fresh root is crushed, mixed with water, filtered, and drunk | [88] |
| Vitis vinifera | Vitaceae | Weyne | Leaves | Grinding the leave with Ficus carica leave separately; mix them with honey then drink 3 times a day by tea glass | [92] |
| Zehneria scabra | Cucurbitaceae | Hareg Resa | Leaves | The fresh leaf is pounded and squeezed and then drunk in half a cup of tea | [34] |
Figure 4.
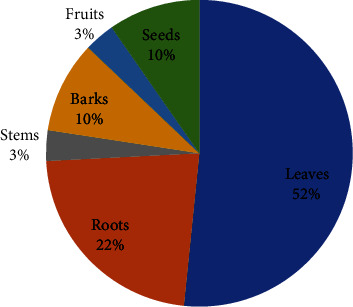
Frequency distribution of plant parts used to prepare remedies.
Figure 5.

Frequently cited antihepatic medicinal plants. (a) Croton macrostachyus [93]. (b) Cucumis ficifolius [94]. (c) Acokanthera schimperi [95].
3.4.1. Hepatoprotective Activity of Potential Ethiopian Medicinal Plants
The hepatoprotective activity of sixteen (16) Ethiopian medicinal plants from ten (10) families was investigated in Ethiopia. These plants have been scientifically tested for hepatotoxicity using a variety of experimental models, including CCl4 and paracetamol. Several parameters, including liver markers (AST, ALT, ALP, total protein, albumin, and bilirubin) and histopathological examination, were used to evaluate these plants. In animal models, all of the medicinal herbs were revealed to have a significant hepatoprotective effect. Some of the plant species listed in Table 5 have hepatoprotective activity, which supports the traditional uses listed in Table 4. These include Verbascum sinaiticum, Croton macrostachyus, Cucumis ficifolius, Justicia shimperans, Phytolacca dodecandra, Treminalia brownie, and Rumex abyssinicus. Although more polar solvents such as water, methanol, and ethanol are frequently recommended for use only in traditional preparations [119]. Significantly, the majority of the plant species studied had hepatoprotective efficacy that matched high-polarity (methanol) plant extracts in most studies. This is advantageous because it permits therapeutic components to absorb through the gut lumen into the circulatory system, where they are needed, according to Lipinski's rules of 5 [120]. As a result, active compounds interact with cell surface receptors, and polar components offer in vivo potency that is therapeutically meaningful. In oral acute toxicity tests, the majority of the test extracts exhibited LD50 values greater than or equivalent to 2000 mg/kg, which would account for the plant's safe folkloric use.
Table 5.
Hepatoprotective activity of Ethiopian medicinal plants.
| Species name | Family name | Plant part used | Extracts used/dosage | Models used | Histopathology | Parameters estimated | Toxicity (LD 50) | Ref |
|---|---|---|---|---|---|---|---|---|
| Lippia adoensis | Verbenaceae | Leaves | Aqueous (200 and 400 mg/kg | CCl4-induced | Hepatocyte regeneration and peripheral mononuclear infiltration are reduced in comparison to CCl4 | Albumin and total protein levels increased, while AST, ALT, ALP, and TBIL levels reduced | — | [107] |
| Ethanol (200 and 400 mg/kg) | CCl4-induced | Hepatocyte regeneration was observed when compared to CCl4 | Total protein and albumin increased while AST, ALT, ALP, and TBIL reduced | — | [107] | |||
| Ensete ventricosum | Musaceae | Cheesman | Methanol (200 and 400 mg/kg) | Isoniazid and rifampicin- induced | Hepatocyte regeneration was observed when compared to isoniazid and rifampicin-induced hepatocyte induced | A dose of 400 mg/kg and 100 mg/kg of silymarin significantly decreased ALT, AST, ALP, and TBIL when compared to isoniazid and rifampicin | — | [108] |
| Thymus serrulatus | Lamiaceae | Aerial parts | Essential oil (200 μL/kg) | Paracetamol—induced | Except for a few inflammatory cell infiltrations, normal hepatocytes were seen in 200 μL/kg EO | When compared to paracetamol, AST, ALT, and ALP levels were reduced | — | [109] |
| Thymus schimperi | Lamiaceae | Aerial parts | Essential oil (200 μL/kg) | Paracetamol—induced | Except for certain inflammatory cell infiltrations, 200 μL/kg EO revealed normal hepatocytes | When compared to paracetamol, AST, ALT, and ALP levels were reduced | — | [109] |
| Justicia schimperiana | Acanthaceae | Leaves | Methanol (200 mg/kg) | CCl4-induced | The mice's livers were significantly protected from CCl4-induced damage | AST and ALT were significantly suppressed compared to CCl4 | 1000 | [110] |
| Verbascum sinaiticum | Scrophulariaceae | Leaves | Methanol (200 mg/kg) | CCl4-induced | The mice's livers were significantly protected from CCl4-induced damage | In comparison to CCl4- induced rats, AST and ALT were significantly reduced | — | [110] |
| Phytolacca dodecandra | Phytolaccaceae | Root | Methanol (200 and 400 mg/kg) | CCl4-induced | 200 and 400 mg/kg doses, normalized the defects in the histology of the liver of mice treated with CCl4 nearly to the level of the negative control group | ALP, ALT, AST, GGT, LDH, and bilirubin levels were all significantly lower, whereas albumin and total protein levels were significantly higher. At 400 mg/kg, the extract had a hepatoprotective effect comparable to silymarin | 2000 | [111] |
| Satureja punctata | Lamiaceae | Aerial part | Aqueous (250 and 500 mg/kg) | Nitrillotriacetate-induced | Showed a normal lobular pattern with minor necrosis and lymphocyte infiltration that was comparable to the control and silymarin-treated groups | When compared to Fe-NTA administered controls, ALP, ALT, and AST levels were considerably lower | 2000 | [112] |
| Solanecio angulatus | Asteraceae | Leaves | Methanol (200 and 400 mg/kg) | Nitrillotriacetate-induced | Not reported | ALP, ALT, and AST levels were significantly lower than Fe-NTA administered controls | 2000 | [112] |
| Cucumis ficifolius | Cucurbitaceae | Root | Methanol (125, 250, and 500 mg/kg) | CCl4-induced | Improved the histology of the liver in mice treated with CCl4 to nearly the same level as the positive control group silymarin in 500 mg/kg doses | ALP, ALT, and AST levels were lower in these animals than in CCl4-induced mice. The 500 mg/kg dose showed the greatest hepatoprotective effect | 2000 | [94] |
| Clutia abyssinica | Euphorbiaceae | Leaves | Methanol (200 and 400 mg/kg) | CCl4-induced | Inflammatory cells, vascular congestion, cellular degradation, necrosis, and vacuoles were reduced or absent | AST, ALT, and ALP levels were significantly lower than CCl4-induced controls. The higher dose (400 mg/kg) had a better hepatoprotective effect | 2000 | [113] |
| Rumex abyssinicus | Polygonaceae | Rhizome | Methanol (125, 250, and 500 mg/kg) | CCl4-induced | At 500 mg/kg, the architecture was maintained, there was modest necrosis, and there were minor lymphocytic infiltrates | AST, ALT, and ALP levels were markedly decreased and were comparable to silymarin (100 mg/kg) at 500 mg/kg | 2000 | [114] |
| Croton macrostachyus | Euphorbiaceae | Root bark | Ethanol (200 and 400 mg/kg) | Paracetamol-induced | Hepatocytes were normal and liver cells were regenerated at 400 mg/kg | In comparison to paracetamol induced the level of AST, ALT, ALP, and total bilirubin was lowered at a higher dose (400 mg/kg) | 2000 | [115] |
| Cineraria abyssinica | Asteraceae | Leaves | Methanol (200 mg/kg) | CCl4-induced | Minor necrosis and focal inflammation | AST, ALT, and ALP levels were markedly decreased and were comparable to silymarin (100 mg/kg) at 500 mg/kg | 3000 | [116] |
| Cordia africana | Boraginaceae | Stem bark | Methanol (100, 200, and 400 mg/kg) | Acetaminophen-induced | It showed moderate necrosis and vacuolar degeneration at 400 mg/kg | The level of AST, ALT, and ALP was decreased at a higher dose (400 mg/kg) compared to acetaminophen-induced | 3000 | [117] |
| Terminalia brownii | Combretaceae | Leaves | Methanol (250 and 500 mg/kg) | CCl4-induced | At 250 mg/kg, the hepatocyte cell membrane's structural integrity was only marginally protected; however, at 500 mg/kg, there was no ballooning and a significant level of protection | The levels of ALP, ALT, and AST were lower than those in mice that had been CCl4-induced. Especially in terms of preserving ALT and AST levels, the percentage of hepatoprotective activity at 500 mg/kg was comparable to the standard drug silymarin at 100 mg/kg | 5000 | [118] |
4. Conclusion
Noncommunicable diseases, as well as MetS risk factors, add significantly to Ethiopia's healthcare burden. Ethiopia has a diverse plant biodiversity with ethnobotanically and scientifically confirmed therapeutic characteristics that can and should be used to reduce the cost of providing health care. The gut microbiota's function in metabolic disorders has gotten a lot of attention recently. A large variety of plants used by indigenous people to treat various disorders, including MetS (obesity, hypertension, and hepatic problems), have been described as a result of numerous ethnobotanical investigations conducted in Ethiopia. Moringa stenopetala and Croton macrostachyus were the most commonly employed ethnobotanical plant species for the treatment of hypertension and liver diseases. Leaves were utilized as a therapeutic preparation more frequently than other parts. The antihypertensive and hepatoprotective properties of the species studied are discussed. Some ethnobotanical studies of medicinal plants investigated their antihypertensive and hepatoprotective properties, and they found some good results. Moringa stenopetala is the most commonly studied medicinal plant for its antihypertensive properties. This indicates that plants have traditionally been used to treat hypertension and liver disorders. However, there was no evidence of further study into the efficacy of some plant species that have been identified as having antihypertensive and hepatoprotective properties. More studies are needed to identify active compounds and develop successful novel drugs for the treatment of MetS.
Acknowledgments
The authors would like to acknowledge the Armauer Hansen Research Institute for providing access to various journal databases.
Data Availability
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.O’Neill S., O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obesity Reviews . 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 2.Nolan P. B., Carrick-Ranson G., Stinear J. W., Reading S. A., Dalleck L. C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Preventive medicine reports . 2017;7:211–215. doi: 10.1016/j.pmedr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudreau D., Malone D., Raebel M., et al. Health care utilization and costs by metabolic syndrome risk factors. Metabolic Syndrome and Related Disorders . 2009;7(4):305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman P. D., Hanson M. A., Buklijas T., Low F. M., Beedle A. S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nature Reviews Endocrinology . 2009;5(7):401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 5.Chen A. K., Roberts C. K., Barnard R. J. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism . 2006;55(7):871–878. doi: 10.1016/j.metabol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Moller D. E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature . 2001;414(6865):821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 7.Nigussie G. A review on traditionally used medicinal plants for scabies therapy in Ethiopia. Advances in Traditional Medicine . 2021;21(2):199–208. doi: 10.1007/s13596-020-00453-7. [DOI] [Google Scholar]
- 8.Tahir M., Gebremichael L., Beyene T., Van Damme P. Ethnobotanical study of medicinal plants in adwa district, central zone of tigray regional state, northern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2021;17(1):71–13. doi: 10.1186/s13002-021-00498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigussie G. Isolation and characterization of the roots of Rumex nervosus. Journal of Tropical Pharmacy and Chemistry . 2020;5(1):39–50. doi: 10.25026/jtpc.v5i1.241. [DOI] [Google Scholar]
- 10.Dillard C. J., German J. B. Phytochemicals: nutraceuticals and human health. Journal of the Science of Food and Agriculture . 2000;80(12):1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::aid-jsfa725>3.0.co;2-w. [DOI] [Google Scholar]
- 11.Altemimi A., Lakhssassi N., Baharlouei A., Watson D., Lightfoot D. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants . 2017;6(4):p. 42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mlilo S., Sibanda S. An ethnobotanical survey of the medicinal plants used in the treatment of cancer in some parts of Matebeleland, Zimbabwe. South African Journal of Botany . 2022;146:401–408. doi: 10.1016/j.sajb.2021.11.022. [DOI] [Google Scholar]
- 13.Naghdi N. Folklore medicinal plants used in liver disease: a review. International Journal of Green Pharmacy . 2018;12(3) [Google Scholar]
- 14.Who. Traditional Medicine Strategy: 2014-2023 . Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 15.McGinn T. G., Guyatt G. H., Wyer P. C., et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. JAMA . 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Obesity And Overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 17.Kassie A. M., Abate B. B., Kassaw M. W. Prevalence of overweight/obesity among the adult population in Ethiopia: a systematic review and meta-analysis. BMJ Open . 2020;10(8) doi: 10.1136/bmjopen-2020-039200.e039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopelman P. G. Obesity as a medical problem. Nature . 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 19.Bray G. A. Medical consequences of obesity. Journal of Clinical Endocrinology & Metabolism . 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 20.McTigue K. M., Harris R., Hemphill B., et al. Screening and interventions for obesity in adults: summary of the evidence for the US Preventive Services Task Force. Annals of Internal Medicine . 2003;139(11):933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 21.Howard A. N. The historical development, efficacy and safety of very-low-calorie diets. International Journal of Obesity . 1981;5(3):195–208. [PubMed] [Google Scholar]
- 22.Curioni C. C., Lourenço P. M. Long-term weight loss after diet and exercise: a systematic review. International Journal of Obesity . 2005;29(10):1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 23.Yanovski S. Z., Yanovski J. A. Long-term drug treatment for obesity: a systematic and clinical review. JAMA . 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alemneh D. Ethnobotanical study of plants used for human ailments in Yilmana densa and Quarit districts of west Gojjam Zone, Amhara region, Ethiopia. BioMed Research International . 2021;2021:2021–18. doi: 10.1155/2021/6615666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regassa R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. Journal of Medicinal Plants Research . 2013;7(9):517–535. [Google Scholar]
- 26.Tuasha N., Petros B., Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2018;14(1):15–21. doi: 10.1186/s13002-018-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teklehaymanot T., Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2007;3(1):12–11. doi: 10.1186/1746-4269-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. Journal of Ethnopharmacology . 2009;124(1):69–78. doi: 10.1016/j.jep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Who. Hypertension. 2021. https://www.who.int/news-room/fact-sheets/detail/hypertension .
- 30.Nyakudya T. T., Tshabalala T., Dangarembizi R., Erlwanger K. H., Ndhlala A. R. The potential therapeutic value of medicinal plants in the management of metabolic disorders. Molecules . 2020;25(11):p. 2669. doi: 10.3390/molecules25112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kibret K. T., Mesfin Y. M. Prevalence of hypertension in Ethiopia: a systematic meta-analysis. Public Health Reviews . 2015;36(1):14–12. doi: 10.1186/s40985-015-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limenih Y., Umer S., Wolde-Mariam M. Ethnobotanical study on traditional medicinal plants in dega damot woreda, amhara region, north Ethiopia. International Journal of Research in Pharmacy and Chemistry . 2015;5(2):258–273. [Google Scholar]
- 33.d’Avigdor E., Wohlmuth H., Asfaw Z., Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2014;10(1):38–33. doi: 10.1186/1746-4269-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meragiaw M., Asfaw Z., Argaw M. The status of ethnobotanical knowledge of medicinal plants and the impacts of resettlement in Delanta, northwestern Wello, northern Ethiopia. Evidence-based Complementary and Alternative Medicine . 2016;2016:1–24. doi: 10.1155/2016/5060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birhanu Z. Traditional use of medicinal plants by the ethnic groups of Gondar Zuria District, North-Western Ethiopia. Journal of Natural Remedies . 2013;13(1):46–53. [Google Scholar]
- 36.Seyoum G., Zerihun G. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. African Journal of Plant Science . 2014;8(7):366–379. doi: 10.5897/ajps2013.1041. [DOI] [Google Scholar]
- 37.Chekole G., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2015;11(1):4–38. doi: 10.1186/1746-4269-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habte B. M., Kebede T., Fenta T. G., Boon H. Use of medicinal plants among Ethiopian patients with diabetes: a qualitative exploration. The Ethiopian Journal of Health Development . 2017;31(1):18–26. [Google Scholar]
- 39.Paulos B., Fenta T. G., Bisrat D., Asres K. Health seeking behavior and use of medicinal plants among the Hamer ethnic group, South Omo zone, southwestern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2016;12(1):44–13. doi: 10.1186/s13002-016-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tefera B. N., Kim Y.-D. Ethnobotanical study of medicinal plants in the hawassa zuria district, sidama zone, southern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2019;15(1):25–21. doi: 10.1186/s13002-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendimu A., Tekalign W., Asfaw B. A survey of traditional medicinal plants used to treat common human and livestock ailments from Diguna Fango district, Wolaita, southern Ethiopia. Nordic Journal of Botany . 2021;39(5) doi: 10.1111/njb.03174.njb.03174 [DOI] [Google Scholar]
- 42.Birhanu Z., Endale A., Shewamene Z. An ethnomedicinal investigation of plants used by traditional healers of Gondar town, North-Western Ethiopia. Journal of medicinal plants studies . 2015;3(2):36–43. [Google Scholar]
- 43.Araya S., Abera B., Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2015;11(1):22–25. doi: 10.1186/s13002-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eshete M. A., Kelbessa E., Dalle G. Ethnobotanical study of medicinal plants in guji agro-pastoralists, blue hora district of borana zone, oromia region, Ethiopia. Journal of medicinal plants studies . 2016;4(2):170–184. [Google Scholar]
- 45.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Current Research Journal of Biological Sciences . 2014;6(4):154–167. doi: 10.19026/crjbs.6.5515. [DOI] [Google Scholar]
- 46.Mesfin F., Seta T., Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda, Ethiopia. Ethnobotany Research and Applications . 2014;12:341–354. doi: 10.17348/era.12.0.341-354. [DOI] [Google Scholar]
- 47.Alemayehu G., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of minjar-shenkora district, north shewa zone of amhara region, Ethiopia. Journal of Medicinal Plants Studies . 2015;3(6):1–11. [Google Scholar]
- 48.Yineger H., Kelbessa E., Bekele T., Lulekal E. Plants used in traditional management of human ailments at bale mountains national park, southeastern Ethiopia. Journal of Medicinal Plants Research . 2008;2(6):132–153. [Google Scholar]
- 49.Agize M., Demissew S., Asfaw Z. Ethnobotany of medicinal plants in Loma and Gena bosa districts (woredas) of dawro zone, southern Ethiopia. Topclass Journal of Herbal Medicine . 2013;2(9):194–212. [Google Scholar]
- 50.Haseena S., Shanavas S., Ahamad T., et al. Investigation on photocatalytic activity of bio-treated α-Fe2O3 nanoparticles using Phyllanthus niruri and Moringa stenopetala leaf extract against methylene blue and phenol molecules: kinetics, mechanism and stability. Journal of Environmental Chemical Engineering . 2021;9(1) doi: 10.1016/j.jece.2020.104996.104996 [DOI] [Google Scholar]
- 51.Geneti S. T., Mekonnen G. A., Murthy H. C. A., et al. Biogenic synthesis of magnetite nanoparticles using leaf extract of Thymus schimperi and their application for monocomponent removal of chromium and mercury ions from aqueous solution. Journal of Nanomaterials . 2022;2022:1–15. doi: 10.1155/2022/5798824. [DOI] [Google Scholar]
- 52.Augustin N., Nuthakki V. K., Abdullaha M., Hassan Q. P., Gandhi S. G., Bharate S. B. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus Jacq as a dual cholinesterase inhibitor. ACS Omega . 2020;5(3):1616–1624. doi: 10.1021/acsomega.9b03693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rather M. A., Dar B. A., Sofi S. N., Bhat B. A., Qurishi M. A. Foeniculum vulgare: a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arabian Journal of Chemistry . 2016;9:S1574–S1583. doi: 10.1016/j.arabjc.2012.04.011. [DOI] [Google Scholar]
- 54.Leone A., Spada A., Battezzati A., Schiraldi A., Aristil J., Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. International Journal of Molecular Sciences . 2015;16(12):12791–12835. doi: 10.3390/ijms160612791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abd Rani N. Z., Husain K., Kumolosasi E. Moringa genus: a review of phytochemistry and pharmacology. Frontiers in Pharmacology . 2018;9:p. 108. doi: 10.3389/fphar.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eilert U., Wolters B., Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Medica . 1981;42(05):55–61. doi: 10.1055/s-2007-971546. [DOI] [PubMed] [Google Scholar]
- 57.Manilal A., Sabu K. R., Shewangizaw M., et al. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon . 2020;6(1) doi: 10.1016/j.heliyon.2020.e03303.e03303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seleshe S., Kang S. N. In vitro antimicrobial activity of different solvent extracts from Moringa stenopetala leaves. Preventive nutrition and food science . 2019;24(1):70–74. doi: 10.3746/pnf.2019.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toma A., Makonnen E., Mekonnen Y., Debella A., Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine . 2015;15(1):242–248. doi: 10.1186/s12906-015-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toma A., Makonnen E., Mekonnen Y., Debella A., Addisakwattana S. Intestinal α-glucosidase and some pancreatic enzymes inhibitory effect of hydroalcholic extract of Moringa stenopetala leaves. BMC Complementary and Alternative Medicine . 2014;14(1):180–185. doi: 10.1186/1472-6882-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Habtemariam S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: identification of the active principles. Natural Product Communications . 2015;10(3) doi: 10.1177/1934578x1501000324.1934578X1501000 [DOI] [PubMed] [Google Scholar]
- 62.Mekonnen Y., Yardley V., Rock P., Croft S. In vitro antitrypanosomal activity of Moringa stenopetala leaves and roots. Phytotherapy Research . 1999;13(6):538–539. doi: 10.1002/(sici)1099-1573(199909)13:6<538::aid-ptr486>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 63.Kifleyohannes T., Terefe G., Tolossa Y. H., Giday M., Kebede N. Effect of crude extracts of Moringa stenopetala and Artemisia absinthium on parasitaemia of mice infected with Trypanosoma congolense. BMC Research Notes . 2014;7(1):390–397. doi: 10.1186/1756-0500-7-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinuthia G. K., Anjili C. O., Gikonyo N. K., Kigondu E. M., Ingonga J. M., Kabiru E. W. In vitro and in vivo activities of blends of crude aqueous extracts from Allium sativum L, Callistemon citrinus (Curtis) Skeels and Moringa stenopetala (Baker F) Cufodontis against Leishmania major. International Journal of Medicinal and Aromatic Plants . 2013;3(2):234–246. [Google Scholar]
- 65.Tamrat Y., Nedi T., Assefa S., Teklehaymanot T., Shibeshi W. Anti-inflammatory and analgesic activities of solvent fractions of the leaves of Moringa stenopetala Bak.(Moringaceae) in mice models. BMC Complementary and Alternative Medicine . 2017;17(1):473–510. doi: 10.1186/s12906-017-1982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mekonnen Y. Effects of ethanol extract of Moringa stenopetala leaves on Guinea‐pig and mouse smooth muscle. Phytotherapy Research . 1999;13(5):442–444. doi: 10.1002/(sici)1099-1573(199908/09)13:5<442::aid-ptr476>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 67.Mengistu M., Abebe Y., Mekonnen Y., Tolessa T. In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. African Health Sciences . 2012;12(4):545–551. [PMC free article] [PubMed] [Google Scholar]
- 68.Yalew T. A., Mekonnen Y., Retta N. Comparison of total phenolic content, free radical scavenging potential and antihyperglycemic condition from leaves extract of Moringa stenopetala and Moringa oleifera. Ethiopian Journal of public health and Nutrition . 2019;1:20–27. [Google Scholar]
- 69.Tebeka T., Libsu S. Assessment of antioxidant potential of <i>Moringa stenopetala</i> leaf extract. Ethiopian Journal of Science and Technology . 2015;7(2):93–104. doi: 10.4314/ejst.v7i2.3. [DOI] [Google Scholar]
- 70.Habtemariam S. Methodology for rapid Isolation of moringin: potential anticancer compound from the seeds of Moringa stenopetala. Pharmaceutica Analytica Acta . 2017;08(08) doi: 10.4172/2153-2435.1000558. [DOI] [Google Scholar]
- 71.Golla A. Z. Thyroid function profile, and its association to consumption of cassava and <i&gt;Moringa stenopetala&lt;/i&gt; in pregnant women. Advances in Biological Chemistry . 2013;03(05):448–454. doi: 10.4236/abc.2013.35048. [DOI] [Google Scholar]
- 72.Haji H., Makonnen E., Debella A., Geleta B. Evaluation of diuretic and antihypertensive activity of leaf extracts of <em>Thymus schimperi</em> in rats. British Journal of Pharmacology and Toxicology . 2016;7(1):1–8. doi: 10.19026/bjpt.7.2779. [DOI] [Google Scholar]
- 73.Geleta B., Makonnen E., Debella A., Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Frontiers in Pharmacology . 2016;7:p. 97. doi: 10.3389/fphar.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fekadu N., Basha H., Meresa A., Degu S., Girma B., Geleta B. Diuretic activity of the aqueous crude extract and hot tea infusion of <em>Moringa stenopetala </em>(Baker f.) Cufod. leaves in rats. Journal of Experimental Pharmacology . 2017;9:73–80. doi: 10.2147/jep.s133778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geleta B., Makonnen E., Debella A., Abebe A., Fekadu N. In vitro vasodilatory activity and possible mechanisms of the crude extracts and fractions of <em>Moringa stenopetala</em> (Baker f.) Cufod. leaves in isolated thoracic aorta of Guinea pigs. Journal of Experimental Pharmacology . 2016;8:35–42. doi: 10.2147/jep.s117545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Getiye Y., Tolessa T., Engidawork E. Antihypertensive activity of 80% methanol seed extract of Calpurnia aurea (Ait.) Benth. subsp. aurea (Fabaceae) is mediated through calcium antagonism induced vasodilation. Journal of Ethnopharmacology . 2016;189:99–106. doi: 10.1016/j.jep.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 77.Ayele Y., Urga K., Engidawork E. Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of syzygium guineense (willd) DC. Phytotherapy Research . 2010;24(10):1457–1462. doi: 10.1002/ptr.3141. [DOI] [PubMed] [Google Scholar]
- 78.Degu A., Abebe A., Engidawork E. Methanol (80%) leaf extract of Otostegia integrifolia Benth (Lamiaceae) lowers blood pressure in rats through interference with calcium conductance. BMC complementary medicine and therapies . 2021;21(1):49–11. doi: 10.1186/s12906-021-03222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hika D. Antihypertensive Activity of Aerial Parts of Satureja Punctata (Benth.) Briq.(Lamiaceae) Addis Ababa, Ethiopia: Addis Ababa University; 2015. [Google Scholar]
- 80.Williams R. Global challenges in liver disease. Hepatology . 2006;44(3):521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 81.Asadi-Samani M., Kafash-Farkhad N., Azimi N., Fasihi A., Alinia-Ahandani E., Rafieian-Kopaei M. Medicinal plants with hepatoprotective activity in Iranian folk medicine. Asian Pacific Journal of Tropical Biomedicine . 2015;5(2):146–157. doi: 10.1016/s2221-1691(15)30159-3. [DOI] [Google Scholar]
- 82.Tsega E. Current views on liver diseases in Ethiopia. Ethiopian Medical Journal . 1977;15(2):75–82. [PubMed] [Google Scholar]
- 83.Shimels A., Atinafu K., Akalu M., Getachew M. Ethnobotanical study of medicinal plants used by agro pastoralist Somali people for the management of human ailments in Jeldesa Cluster, Dire Dawa Administration, Eastern Ethiopia. Journal of Medicinal Plants Research . 2017;11(9):171–187. doi: 10.5897/jmpr2016.6292. [DOI] [Google Scholar]
- 84.Ragunathan M., Abay S. M. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, Northwestern Ethiopia. Indian Journal of Traditional Knowledge . 2009;8(2):281–284. [Google Scholar]
- 85.Giday M., Teklehaymanot T., Animut A., Mekonnen Y. Medicinal plants of the shinasha, agew-awi and amhara peoples in northwest Ethiopia. Journal of Ethnopharmacology . 2007;110(3):516–525. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Belayneh A., Bussa N. F. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2014;10(1):18–17. doi: 10.1186/1746-4269-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gabriel T., Guji T. Ethnopharmacological survey of medicinal plants in Agaro district, Jimma zone, South West Ethiopia. International Journal of Pharmaceutical Sciences and Research . 2014;5(8):p. 3551. [Google Scholar]
- 88.Kidane L., Gebremedhin G., Beyene T. Ethnobotanical study of medicinal plants in ganta afeshum district, eastern zone of tigray, northern Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2018;14(1):64–19. doi: 10.1186/s13002-018-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kassa Z., Asfaw Z., Demissew S. Ethnobotanical study of medicinal plants used by the local people in tulu korma and its surrounding areas of ejere district, western shewa zone of oromia regional state, Ethiopia. Journal of Medicinal Plants Studies . 2016;4(2):24–47. [Google Scholar]
- 90.Kefalew A., Asfaw Z., Kelbessa E. Ethnobotany of medicinal plants in ada’a district, east shewa zone of oromia regional state, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2015;11(1):25–28. doi: 10.1186/s13002-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ragunathan M., Solomon M. The study of spiritual remedies in orthodox rural churches and traditional medicinal practice in Gondar Zuria district, Northwestern Ethiopia. Pharmacognosy Journal . 2009;1(3) [Google Scholar]
- 92.Mekuanent T., Zebene A., Solomon Z. Ethnobotanical study of medicinal plants in Chilga district, Northwestern Ethiopia. Journal of Natural Remedies . 2015;15(2):88–112. doi: 10.18311/jnr/2015/476. [DOI] [Google Scholar]
- 93.Aga W. S., Fantaye S. K., Jabasingh S. A. Biodiesel production from Ethiopian ‘Besana’-Croton macrostachyus seed: characterization and optimization. Renewable Energy . 2020;157:574–584. doi: 10.1016/j.renene.2020.05.068. [DOI] [Google Scholar]
- 94.Araya E. M., Adamu B. A., Periasamy G., Sintayehu B., Gebrelibanos Hiben M. In vivo hepatoprotective and in vitro radical scavenging activities of Cucumis ficifolius A. rich root extract. Journal of Ethnopharmacology . 2019;242 doi: 10.1016/j.jep.2019.112031.112031 [DOI] [PubMed] [Google Scholar]
- 95.Kenubih A., Belay E., Lemma K. Evaluation of the antimicrobial activity of leaf extracts of Acokanthera schimperi against various disease-causing bacteria. Journal of Experimental Pharmacology . 2021;13:889–899. doi: 10.2147/jep.s322396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edwards S., Tadese M., Hedberg I. In: Flora of Ethiopia and Eritrea Vol. 2 Part 2: Canellaceae to Euphorbiaceae . Edwards Sue, Mesfin Tadese., editors. Ethiopia: Addis Abeba University; 1995. [Google Scholar]
- 97.Degu A., Engidawork E., Shibeshi W. Evaluation of the anti-diarrheal activity of the leaf extract of Croton macrostachyus Hocsht. ex Del.(Euphorbiaceae) in mice model. BMC Complementary and Alternative Medicine . 2016;16(1):379–411. doi: 10.1186/s12906-016-1357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maroyi A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: a comprehensive review. Evidence-based Complementary and Alternative Medicine . 2017;2017:1–17. doi: 10.1155/2017/1694671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eguale T., Getachew T., Giday M., Mekonnen Y. In vitro anthelmintic activities of four Ethiopian medicinal plants against Haemonchus contortus. Pharmacologyonline . 2006;3:153–165. [Google Scholar]
- 100.Obey J. K., von Wright A., Orjala J., Kauhanen J., Tikkanen-Kaukanen C. Antimicrobial activity of Croton macrostachyus stem bark extracts against several human pathogenic bacteria. Journal of Pathogens . 2016;2016:1–5. doi: 10.1155/2016/1453428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ngo Bum E., Ngah E., Ngo Mune R., et al. Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy and Behavior . 2012;24(3):319–323. doi: 10.1016/j.yebeh.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 102.Arika W., Abdirahman Y. A., Mawia M. A., et al. In vivo antidiabetic activity of the aqueous leaf extract of Croton macrostachyus in alloxan induced diabetic mice. Pharmaceutica Analytica Acta . 2015;6(11):1–5. [Google Scholar]
- 103.Nguelefack T. B., Dutra R. C., Paszcuk A. F., de Andrade E. L., Calixto J. B. TRPV1 channel inhibition contributes to the antinociceptive effects of Croton macrostachyus extract in mice. BMC Complementary and Alternative Medicine . 2015;15(1):293–299. doi: 10.1186/s12906-015-0816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gelaw H., Adane L., Tariku Y., Hailu A. Isolation of crotepoxide from berries of Croton macrostachyus and evaluation of its anti-leishmanial activity. Journal of Pharmacognosy and Phytochemistry . 2012;1(4):15–24. [Google Scholar]
- 105.Mofor C. T., Sonfack D. C. R., Fokom R., Beng V. P., Amvam Z. P. H. Antifungal and antioxidant activity of crude extracts of three medicinal plants from Cameroon pharmacopea. Journal of Medicinal Plants Research . 2013;7(21):1537–1542. [Google Scholar]
- 106.Obey J. K., Ngeiywa M. M., Kiprono P., et al. Antimalarial activity of Croton macrostachyus stem bark extracts against Plasmodium berghei in vivo. Journal of pathogens . 2018;2018:6. doi: 10.1155/2018/2393854.2393854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasihun Y., Makonnen E., Afewerk M., Ergete W. Hepatoprotective activity of aqueous and ethanol extract of Lippia adoensis leaf against carbon tetrachloride-induced hepatotoxicity in mice. Pathology and Laboratory Medicine . 2017;1(1):5–13. [Google Scholar]
- 108.Dubiwak A. D., Damtew T. W., Senbetu M. W., et al. Hepatoprotective effect of corm of ensete ventricosum (welw.) cheesman extract against isoniazid and rifampicin induced hepatotoxicity in Swiss albino mice. Journal of Toxicology . 2021;2021:2021–8. doi: 10.1155/2021/4760455.4760455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Damtie D., Braunberger C., Conrad J., Mekonnen Y., Beifuss U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi) Journal of Essential Oil Research . 2019;31(2):120–128. doi: 10.1080/10412905.2018.1512907. [DOI] [Google Scholar]
- 110.Umer S., Asres K., Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharmaceutical Biology . 2010;48(4):461–468. doi: 10.3109/13880200903173593. [DOI] [PubMed] [Google Scholar]
- 111.Meharie B. G., Tunta T. A. Phytolacca dodecandra (phytolaccaceae) root extract exhibits antioxidant and hepatoprotective activities in mice with CCl4-induced acute liver damage. Clinical and Experimental Gastroenterology . 2021;14:59–70. doi: 10.2147/ceg.s290859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolde T., Engidawork E., Asres K., Eregete W. Evaluation of hepatoprotective activities of satureja punctata benth briq and solanecioangulatus vahl jeffrey in ferric nitrillotriacetate induced hepatotoxicity in rats. Ethiopian Pharmaceutical Journal . 2010;28(2):63–74. doi: 10.4314/epj.v28i2.1. [DOI] [Google Scholar]
- 113.Meharie B. G., Amare G. G., Belayneh Y. M. <p>Evaluation of Hepatoprotective Activity of the Crude Extract and Solvent Fractions of <em>Clutia abyssinica</em> (<em>Euphorbiaceae</em>) Leaf against CCl<sub>4</sub>-Induced Hepatotoxicity in Mice</p>. Journal of Experimental Pharmacology . 2020;12:137–150. doi: 10.2147/jep.s248677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Adamu B. A., Emiru Y. K., Sintayehu B., Araya E. M., Periasamy G., Gebrelibanos Hiben M. <p>In vivo Hepatoprotective and in vitro Radical Scavenging Activities of Extracts of <em>Rumex abyssinicus</em> Jacq. Rhizome</p>. Journal of Experimental Pharmacology . 2020;12:221–231. doi: 10.2147/jep.s258566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gebremedhin G., Tuem K. B., Kahsu A., Balasubramanian R. <p>In vitro Antioxidant and in vivo Hepatoprotective Activities of Root Bark Extract and Solvent Fractions of <em>Croton macrostachyus</em> Hochst. Ex Del. (<em>Euphorbiaceae</em>) on Paracetamol-Induced Liver Damage in Mice</p>. Journal of Experimental Pharmacology . 2020;12:301–311. doi: 10.2147/jep.s259081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sintayehu B., Bucar F., Veeresham C., Asres K. Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of cineraria abyssinica sch. Bip. exA. Rich. Pharmacognosy Journal . 2012;4(29):40–46. doi: 10.5530/pj.2012.29.6. [DOI] [Google Scholar]
- 117.Geresu G. D., Umer S., Arayaselassie M., Ashebir G., Makonnen E. Hepatoprotective effects of crude stem bark extracts and solvent fractions of Cordia africana against acetaminophen-induced liver injury in rats. Canadian Journal of Gastroenterology and Hepatology . 2022;2022:11. doi: 10.1155/2022/1449286.1449286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sintayehu B., Kakoti B. B., Kataki M. S., et al. Hepatoprotective, antixidant and anticancer activities of <i> Terminalia brownii</i> Fresen leaf abstract. Ethiopian Pharmaceutical Journal . 2018;33(1):29–38. doi: 10.4314/epj.v33i1.3. [DOI] [Google Scholar]
- 119.Willcox M. Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Medica . 2011;77(06):662–671. doi: 10.1055/s-0030-1250548. [DOI] [PubMed] [Google Scholar]
- 120.Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral natural products and herbal medicines. Journal of traditional and complementary medicine . 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


