Abstract
It is extremely rare for granulomatosis with polyangiitis to form masses in the kidneys. Magnetic resonance imaging findings of renal masses caused by this disease have been infrequently reported. In this study, we report a case of renal masses caused by granulomatosis with polyangiitis with different findings. While on steroid treatment for a recently diagnosed granulomatosis with polyangiitis, a man in his 60s underwent computed tomography for a hepatic dysfunction. Computed tomography showed incidental findings of a 40 mm × 35 mm mass in the left kidney and two 8 mm × 8 mm masses in the right kidney; all masses were hypovascular. On magnetic resonance imaging, the left renal mass showed a hyperintense signal with slightly hypointense signal rim on T2-weighted imaging. The left renal mass showed a strong hypointense signal where the mass abutted the renal capsule. On diffusion-weighted imaging, the left renal mass showed an isointense signal with a hyperintense signal rim. Both right renal masses showed an isointense signal with slightly hypointense signal rim on T2-weighted imaging and hyperintense signal on diffusion-weighted imaging. Suspecting renal masses caused by the disease, the patient was then treated with steroids and methotrexate. After 6 months of treatment, both right renal masses resolved; however, the left renal mass shrank but abnormal signal remained. Based on the treatment course, it is conceivable that the renal masses were caused by granulomatosis with polyangiitis.
Keywords: Granulomatosis with polyangiitis, Renal mass, MRI
Introduction
Granulomatosis with polyangiitis (GPA) is an autoimmune multisystemic small vasculitis that belongs to the group of anti-neutrophil cytoplasmic antibody (ANCA)-associated small-vessel vasculitis [1]. Masses due to GPA are frequently seen in the nasal cavity, paranasal sinuses, and lungs, but rarely in the kidney.
Case report
Eighteen months prior, this male patient in his 60s was diagnosed with GPA; myeloperoxidase (MPO)-ANCA was positive (130 U/mL; upper limit, 3.5 U/mL) and he began treatment with steroids (20 mg/d) at our hospital. Two months prior, his steroids were reduced and azathioprine was started. Shortly thereafter, hepatic dysfunction attributed to azathioprine developed. Dynamic contrast-enhanced computed tomography (CT) performed to evaluate the liver revealed masses in both kidneys. Blood tests showed elevated liver enzymes but renal function was not decreased. Urinalysis was normal.
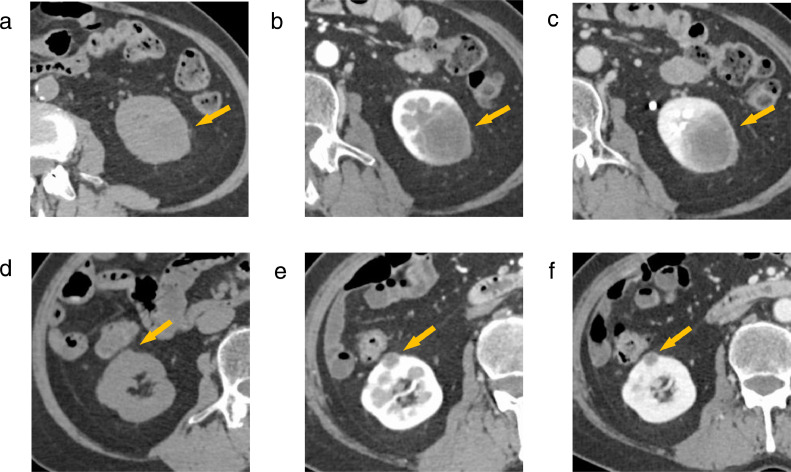
Dynamic contrast-enhanced CT revealed a 40 mm × 35 mm × 30 mm hypovascular mass with unclear margins in the lower left kidney (Fig. 1a-c). Two 8 mm × 8 mm × 8 mm masses were seen in the upper and middle cortices of the right kidney (Figs. 1d-f). Both were hypovascular with relatively clear margins. Chest CT showed multiple nodules in the lower lobes of both lungs; these nodules had resolved on follow-up chest CT after 3 months.
Fig. 1.
Dynamic contrast-enhanced CT. (a,d) Unenhanced, (b,e) corticomedullary phase, and (c,f) nephrogenic phase. (a-c) There is a 40 mm × 35 mm × 30 mm poorly enhancing mass in the lower left kidney with unclear margins. (d-f) There is an 8 mm × 8 mm × 8 mm poorly enhancing mass in the middle cortex of the right kidney with relatively clear margins. The CT findings of the mass in the upper cortex of the right kidney are similar (not shown).
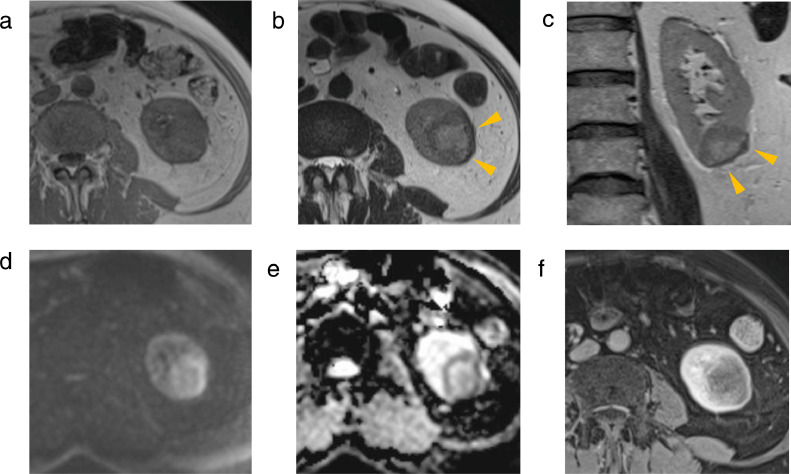
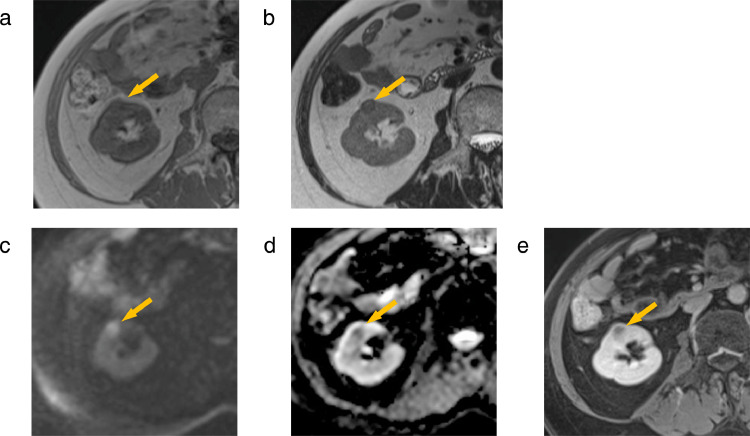
Contrast-enhanced MRI was performed (Figs. 2 and 3). On T2-weighted imaging (T2WI), the left renal mass showed a hyperintense signal with a slightly hypointense signal rim on and a strong hypointense signal where the mass abutted the renal capsule (Figs. 2b and c). On diffusion-weighted imaging (DWI) (b = 1000 s/mm2), the left renal mass showed an isointense signal with a hyperintense signal rim. On apparent diffusion coefficient map (ADCmap), the left renal mass showed a hyperintense signal with a hypointense signal rim. The ADC values in the rim was 0.87 × 10−3 mm3/s and the ADC values in the center of the mass was 1.45 × 10−3 mm3/s. The contrast effect of the left renal mass was poor. The 2 right renal masses both showed an isointense signal with a slightly hypointense signal rim onT2WI, a hyperintense signal on DWI, and a hypointense signal on ADCmap (Fig. 3). The contrast effect of the 2 right renal masses was poor.
Fig. 2.
MRI of the left renal mass. (a) T1WI, (b) T2WI, (c) coronal T2WI, (d) DWI, (e) ADCmap, and (f) contrast-enhanced image. The mass shows an isointense signal on T1WI (a), and a hyperintense signal with slightly hypointense signal rim on T2WI (b,c). The mass exhibits a strong hypointense signal where the mass borders the renal capsule on T2WI (arrowhead). DWI presents an isointense signal with a hyperintense signal rim (d). ADCmap presents a hyperintense signal with a hypointense signal rim (e). There is poor contrast enhancement (f).
Fig. 3.
MRI of the mass in middle cortex of the right kidney (arrow). (a) T1WI, (b) T2WI, (c) DWI (d) ADCmap, and (e) contrast-enhanced image. (a,b) The mass exhibits an isointense signal on T1WI and an isointense signal with a slightly hypointense signal rim on T2WI. (c) DWI presents a hyperintense signal. (d) ADCmap presents a hypointense signal. (e) There is poor contrast enhancement. The MRI findings of the mass in the upper cortex of the right kidney are similar (not shown).
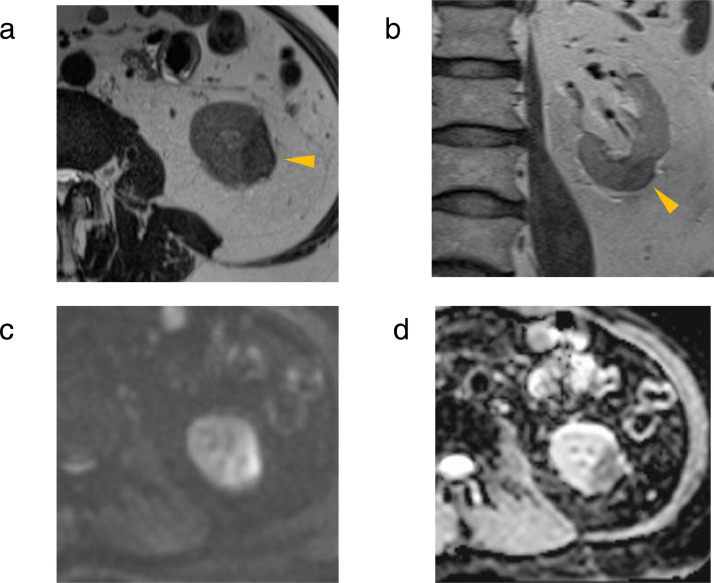
The MRI findings suggested that the renal masses were related to GPA. Azathioprine, to which the liver dysfunction was attributed, was discontinued; methotrexate was subsequently started. On follow-up MRI after 6 months, both right renal masses had resolved. The left renal mass had shrunk to 30 mm × 15 mm × 15 mm but the abnormal signals remained (Fig. 4). On T2WI, the signal of the left renal mass decreased from hyperintense to slightly hypointense. The strong hypointense signal where the mass abutted the renal capsule had shrunk but persisted (Figs. 4a and b). A hyperintense signal rim on DWI and hypointense signal rim on ADCmap persisted. On follow-up MRI after 12 months showed essentially similar findings. Based on the temporal relationship and the effect of treatment, the renal masses were attributed to GPA.
Fig. 4.
Follow-up MRI study of the left renal mass after 6 months. (a) T2WI, (b) coronal T2WI, (c) DWI, and (d) ADCmap. The left renal mass had shrunk but the abnormal signal remained. (a,b) The signal of the left renal mass decreased from hyperintense to slightly hypointense. A strong hypointense signal where the mass bordered the renal capsule had shrunk but persisted (arrowhead). (c,d) A hyperintense signal rim on DWI and a hypointense signal rim on ADCmap remained.
Discussion
The International Chapel Hill Consensus Conference (CHCG2012) defines the pathological features of GPA as necrotizing granulomatous inflammation usually involving the upper and lower respiratory tracts, necrotizing vasculitis affecting predominantly small-to-medium vessels, and necrotizing glomerulonephritis [2]. Necrotizing glomerulonephritis is the most common renal lesion caused by GPA; and necrotizing granulomatous inflammatory mass formation in the kidney is extremely rare. Approximately 60% of renal masses are solitary and 40% are multiple [3].
Contrast-enhanced CT findings of renal mass caused by GPA are often hypovascular with unclear margins [3]. Both renal masses in our patient were hypovascular, but the margins of 2 right renal masses were relatively clear.
MRI findings of renal masses caused by GPA have been reported infrequently. We were able to review 3: (1) the first reported T1WI, T2WI, DWI and ADCmap [3], (2) another reported T1WI, T2WI, and contrast-enhancement [4], and (3) the last reported only T2WI [5]. Ours is the first report of multiple renal masses with different MRI findings on initial and post-treatment studies.
The left renal mass showed a hyperintense signal with a slightly hypointense signal rim on T2WI, an isointense signal with a hyperintense signal rim on DWI and a hyperintense signal with a hypointense signal rim on ADCmap. These findings were also observed in previous reports [3,4]. The difference in MRI signal between the rim and the center of the lesion might be characteristic of renal mass associated with GPA. However, in a case report in which nephrectomy was performed, there was no pseudocapsule at the margins of the mass and no clear histopathologic difference between the marginal and central area [3]. The cause of the difference in MRI signal between the rim and the center of the lesion could not be determined as far as we could search. The renal mass caused by GPA was hypovascular mass on dynamic contrast-enhanced CT. Therefore, papillary renal cell carcinoma should be considered as a differential diagnosis. Typical papillary renal cell carcinoma exhibited hypointense signal on T2WI [6,7], which was different from our case and previous cases [3], [4], [5].
The right renal masses showed an isointense signal on T2WI, a hyperintense signal on DWI and a hypointense signal on ADCmap. These findings alone were difficult to differentiate from multiple lesions type of renal malignant lymphoma [8,9]. But, in our case, in addition to those findings, the masses showed a slightly hypointense signal rim on T2WI, which might be useful in the diagnosis of small renal mass due to GPA.
The left renal mass showed very similar MRI findings to those described by Yamamoto et al. [3], especially the strong hypointense signal, where the mass borders the renal capsule on T2WI. Yamamoto et al. [3] reported that the strong hypointense signal area on T2WI was localized to a markedly thickened renal capsule on pathology. This may be a characteristic MRI finding for renal masses caused by GPA.
The diameter of the right renal masses was 8 mm, and the diameter of the left renal mass was 40 mm. Yamamoto et al. reported an even larger renal mass, 70 mm in diameter, with similar characteristics to those of the left renal mass in our case [3]. The right renal masses disappeared after treatment; the left renal mass shrank but the abnormal signal persisted on MRI. Based on size and changes during the treatment interval, the left renal mass could be at a more advanced stage of necrotizing granulomatous inflammation than right renal masses; however, its impact on the MRI findings is unclear. MRI findings in renal mass due to GPA are infrequently reported. More cases need to be reviewed to characterize MRI findings.
Patient consent
The author(s) confirm that informed consent has been obtained from the involved patient(s) or if appropriate from the parent, guardian, power of attorney of the involved patient(s); and, they have given approval for this information to be published in this case report (series).
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Bacon PA. The spectrum of Wegener's granulomatosis and disease replace. N Engl J Med. 2005;325:330–332. doi: 10.1056/NEJMp048338. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Capel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Takahata K, Kamei S, Ishikawa M, Matsumoto D, Suzuki K. Granulomatosis with polyangiitis presenting as a solitary renal mass: a case report with imaging and literature review. Rad Case Rep. 2021;16:736–741. doi: 10.1016/j.radcr.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verswijvel G, Eerens I, Messiaen T, Oyen R. Granulomatous renal pseudotumor in Wegener's granulomatosis: Imaging findings in one case. Eur Radiol. 2000;10:1265–1267. doi: 10.1007/s003300000344. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor A, Balfour-Dorsey RA, George DL. Wegener's granulomatosis presenting as multiple kidney masses. Am J Med. 2002;112:82–83. doi: 10.1016/s0002-9343(01)00946-9. [DOI] [PubMed] [Google Scholar]
- 6.Doshi AM, Ream JM, Kierans AS, Bilbily M, Rusinek H, Huang W, et al. Use of MRI in differentiation of papillary renal cell carcinoma subtypes: qualitative and quantitative analysis. AJR. 2016;206:566–572. doi: 10.2214/AJR.15.15004. [DOI] [PubMed] [Google Scholar]
- 7.Allen BC, Tirman P, Jennings Clingan M, Manny J, Del Gaizo AJ, Leyendecker JR. Characterizing solid renal neoplasms with MRI in adults. Abdom Imaging. 2014;39:358–387. doi: 10.1007/s00261-014-0074-4. [DOI] [PubMed] [Google Scholar]
- 8.Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics. 2006;26:1151–1168. doi: 10.1148/rg.264055125. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, Zhu W, Wu J, Chen W. Imaging features of primary renal lymphoma. Acta Radiol. 2018;59:114–120. doi: 10.1177/0284185117706202. [DOI] [PubMed] [Google Scholar]