Abstract
Introduction
Shoulder arthroplasty is a common treatment for shoulder arthritis. Prosthetic joint infection of the shoulder (PJIS) is a debilitating complication to the patient and the healthcare system. Incidence of infection is 0.98–5% for primary arthroplasty. The mean hospital cost for two-stage revision was approximately $35,824. The aim of this paper is to review the recent literature and collate the latest evidence to aid diagnosis and treatment of this serious complication.
Methods
A literature review was performed using PubMed and Google Scholar databases. A search strategy was adopted using the keywords: ‘infection’ AND ‘shoulder arthroplasty’ OR ‘total shoulder arthroplasty’OR ‘TSA’ OR ‘reverse shoulder arthroplasty’ OR ‘RSA’ OR ‘rTSA’. This initial search resulted in 349 articles. A PRISMA flowchart process was followed. Duplicates were removed, screening was performed and the resulting full texts were analysed and further excluded, leaving 46 articles suitable for inclusion. A PICO search strategy was also used.
Results and interpretation
Risk factors for PJIS include procedure type, trauma indications and patient factors.
The organism commonly isolated is Cutebacterium acnes, which makes diagnosis challenging due to its indolent nature. Investigations include biochemical tests, synovial aspirate, tissue cultures and radiological examinations.
Treatment depends on the depth of the infection and the patient requirements. Medical treatment with antibiotics to local debridement, cement spacer and revision arthroplasty have all been described in the literature. A multidisciplinary decision is made on the microbiological evidence and patient factors.
Conclusion
PJIS is a rare but potentially devastating complication of shoulder arthroplasty and diagnosis is often challenging. There has been much research performed recently, providing more evidence on how to optimise management.
Keywords: Shoulder, Arthroplasty, Infection, Revision
1. Introduction
Shoulder arthroplasty (total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty (RSA)) is a common and effective treatment for shoulder arthritis.1 TSA is performed on patients with an intact rotator cuff and RSA is indicated in advanced cuff arthropathy, trauma sequelae and post-fracture pathology in elderly patients and inflammatory arthropathy.1,2 Prosthetic joint infection of the shoulder (PJIS) is a debilitating complication to the patient and the healthcare system. Incidence of infection has been reported between 0.98 and 5% for primary arthroplasty.1,3,4 The economic impact is significant, with mean hospital cost for two-stage revision being approximately $35,824 5.
Historically, the majority of guidelines surrounding PJIS have been developed from lower limb arthroplasty infection studies, however there has been a recent increase in PJIS-specific literature.1
The organisms commonly isolated include Cutebacterium (C.) acnes (formerly Propionibacterium acnes), Staphylococcus aureus and Staphylococcus epidermidis.6 Diagnosis is often challenging due to the indolent nature of C. acnes. It often does not manifest classic infective features such as oedema, erythema, fever or purulent discharge. In addition, biochemical markers such as C-reactive protein (CRP), white blood cell count (WBC), Erythrocyte Sedimentation Rate (ESR) and interleukin (IL)-6 may be normal.3
Treatment depends on severity of infection and patient requirements. Medical treatment with antibiotics, local debridement, cement spacer and revision arthroplasty have all been described in the literature, with a multidisciplinary decision made based on microbiological evidence and patient factors.3
Many new details have been identified including a recent scoring system, novel investigations (such as interleukin and alpha-defensin markers) to aid in diagnosis and a range of treatment options each with literature controversy and new evidence on varying outcomes (such as the comparison of one and two-stage revision). The aim of this paper is to review and collate the recent literature to aid diagnosis and treatment of this uncommon but serious complication of a common procedure.
2. Methods
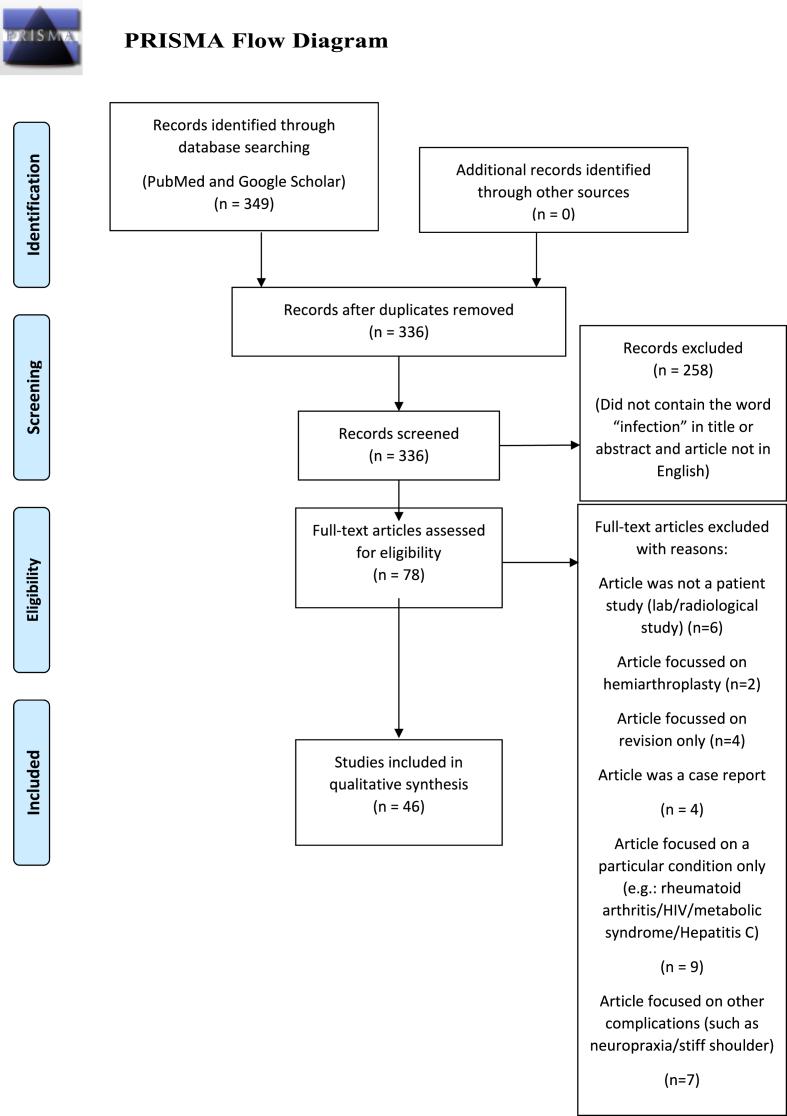
A literature review was performed using PubMed and Google Scholar databases. A search strategy was adopted using the keywords: infection AND ‘shoulder arthroplasty’ OR ‘total shoulder arthroplasty’ OR ‘TSA’ OR ‘reverse shoulder arthroplasty’ OR ‘RSA’ OR ‘rTSA’. This initial search resulted in 349 articles. A PRISMA flowchart process was followed, as outlined in Fig. 1. Duplicates were removed, leaving 336 articles. Screening was performed and 258 articles were excluded, leaving 76. The resulting full texts were analysed and further filtered using exclusion criteria, leaving 46 articles suitable for inclusion. Most of these articles were published after 2017, but there were no definite exclusions based upon date. These articles were reviewed by two separate authors. A PICO search strategy is outlined in Table 1.
Fig. 1.
PRISMA Flow diagram.
Table 1.
PICO search strategy.
| PICO element | Keywords | Search Terms | Search Strategy |
|---|---|---|---|
| Patient/Population | Patients undergoing total shoulder replacement or reverse total shoulder replacement | infection AND ‘shoulder arthroplasty’ OR ′total shoulder arthroplasty OR ‘TSA’ OR ‘reverse shoulder arthroplasty’ OR ‘RSA’ OR ‘rTSA’. | |
| Intervention |
|
||
|
|||
| Comparison |
|
Infection shoulder arthroplasty | |
| |||
| Outcome | Up to date evidence surrounding epidemiology, diagnosis, management options, success rates (including infection clearance and reinfection rates) and patient-reported outcome measures |
3. Results and Interpretation
Interpretation of these studies was structured as follows: Risk Factors, Epidemiology, Diagnosis and Management.
3.1. Risk Factors
Risk factors for PJIS can be divided into: type of procedure, indication and patient factors.
Revision arthroplasty exhibits a higher infection risk than primary, in some cases up to 32%.1 In primary arthroplasty, RSA has been shown to exhibit up to 6.11 times higher infection risk than TSA.7 One study quoted the overall PJIS rate as 2.4%.2 The higher RSA infection rate may be due to increased implant surface area, significant subacromial dead-space, decreased patient fitness and complex indications.2 The PJIS rate was 2.4% for elective arthroplasty for osteoarthritis or irreparable cuff disease, 0.9% for acute fractures, and 3.7% for fracture sequelae.2
Patient risk factors for PJIS can be classified as modifiable and non-modifiable. Modifiable risk factors include smoking and medical comorbidities; such as coagulopathy, renal disease, diabetes mellitus and rheumatological conditions (lupus erythematosus and rheumatoid arthritis).4 Intra-articular steroid injections and corticosteroid therapy also increase risk; the odds ratio of PJIS after corticosteroid intra-articular injection was 2.0 at three and six months compared to controls.3,8
Non-modifiable risk factors include gender and age. Patients younger than 65 showed a 4.0 greater odds ratio for infection than those over 654. A one-year increase in age resulted in a 5% lower risk of infection.4 Infection risk is 2.59 times greater in male than female patients.7 The combination of these factors contributed to a marked increase in risk; the Nordic Arthroplasty Register Association identified that a male undergoing RSA had an infection risk of 8%.9 Race, body mass index (BMI) and American Society of Anaesthesiologists (ASA) score were not associated with infection risk 7.
3.2. Epidemiology
The evidence on epidemiology of PJIS has been widely reported, from observational studies to meta-analyses, as summarised in Table 2.
Table 2.
Epidemiology of PJIS from various studies in the recent literature and key risk factors identified.
| Paper | Infection Rate | Sample size | Risk factors identified |
|---|---|---|---|
| Moeini et al.9 | 1.4% | 17,730 | Infection rate 3.1% in all RSA patients 8% in male patients |
| Kunutsor et al.10 | 0.61% | 631,854 | Male, <75 years of age, previous shoulder surgery, RSA, rotator cuff arthropathy, inpatient TSA |
| Florschutz et al.11 | 1.97% | 814 | Joints previously operated upon showed significantly higher infection rate |
| Morris et al.4 | 5% | 301 | Patients younger than 65 years old had 4.0 greater odds ratio |
| Padegimas et al.5 | 0.98% | Nutritional deficiency, drug abuse, anaemia and iron deficiency showed 2.43–2.62 greater odds ratio | |
| Shah et al.2 | 2.4% | 4396 | Reverse shoulder arthroplasty only - 2.4% for OA or irreparable rotator cuff tear, 0.9% for acute fractures and 3.7% for fracture sequelae |
Shoulder arthroplasty incidence varies between countries with around 66,000 performed yearly in the United States.3 Within the United Kingdom (UK), shoulder arthroplasty has been included in the UK National Joint Registry (NJR) since 2012 with 4859 procedures performed in 2021.12 The NJR estimates the risk of revision for infection as 0.11 per 100 years of follow up.13
3.3. Diagnosis/workup
3.3.1. Definitions of PJIS
A systematic review in 2017 identified the lack of uniform definition may have impacted diagnosis and treatment.14 The criteria were based upon hip and knee infection diagnosis protocols and due to the diagnostic challenges in PJIS, their validity and reliability was questioned15,16
In 2018, specific criteria for PJIS were developed at the International Consensus Meeting (ICM) of Orthopaedic Infections and a uniform definition was created,16,17 as outlined in Table 3.
Table 3.
“Minor Criteria” for PJIS as set out in the 2018 International Consensus Meeting (ICM) of Orthopaedic Infections.
| Unexpected wound drainage | 4 |
| Single positive tissue culture with a virulent organism | 3 |
| Single positive tissue culture with a low-virulent organism | 1 |
| Second positive tissue culture (identical low-virulence organism) | 3 |
| Humeral loosening | 3 |
| Positive frozen section (5 neutrophils in ≥5 high-power fields) | 3 |
| Positive pre-operative aspirate culture | 3 |
| Synovial neutrophil percentage >80% | 2 |
| Synovial white blood cell count >3000 cells/μL beyond 6 weeks from surgery | 2 |
| ESR >30 mm/h | 2 |
| CRP >10 mg/L | 2 |
| Elevated synovial alpha-defensin | 2 |
| Cloudy synovial fluid | 2 |
These “minor criteria”17 are weighted by importance in suspected PJIS and can be interpreted as outlined in Table 4. The likelihood of infection has been stratified into “definite infection, probable infection, possible infection and unlikely infection (Table 4).16,17 Patel et al. performed a validation study finding that unexpected wound discharge and pre-revision surgery positive cultures correlated highly with infection found at operation.18 In addition, 26% of patients with a loose humeral stem at revision were classed as “definite” or “probable” on this scale, advocating further research in this area.18
Table 4.
Definitions for likelihood of PJIS and their relevant criteria as set out in the 2018 International Consensus Meeting (ICM) of Orthopaedic Infections.
| Definite infection | Presence of a sinus tract from the skin surface to the prosthesis OR Gross intra-articular pus OR Two positive tissue cultures with identical virulent organisms |
| Probable infection | Presence of ≥6 minor criteria with an identified organism |
| Possible infection | Presence of ≥6 minor criteria without an identified organism OR < 6 minor criteria with one culture with a virulent organism OR < 6 minor criteria with 2 positive cultures with a low-virulence organism |
| Unlikely infection | <6 minor criteria with negative cultures OR < 6 minor criteria with 1 positive culture with a low-virulence organism |
3.3.2. Clinical presentation
PJIS most commonly presents with non-specific symptoms such as pain and decreased range of motion.19 A draining sinus is diagnostic, however patients rarely present with this sign.17,20 Pain is the most common presenting complaint (86%), followed by draining sinus (44%), stiffness (35%), erythema (35%), effusion (32%), fever (21%), night sweats (9%), and rigors (9%). Given the diagnostic challenges, clinicians must maintain a high index of suspicion for PJIS.19
3.3.3. Pathogens
Three main causative pathogens are recognised; C. acnes as the most common (38.9%), followed by Staphylococcus epidermidis (14.8%), and Staphylococcus aureus (14.5%).21 C. acnes is a lipophilic anaerobic bacterium commonly found in high concentrations in areas dense with sebaceous glands and moisture, such as the axilla.19 Coupled with the bacterium's lipase enzymes, this creates a perfect environment for proliferation. This may account for the higher infection rate in males, who exhibit greater concentrations of sebaceous glands.19 The incidence of C. acne growth in PJIS varied from 31 to 70%.7,19 One study found that 16.7% of revision shoulder arthroplasty cases were performed due to proven C. acnes infection.22 C. acnes triggers a minimal inflammatory response: mild and non-specific symptoms and a negligible alteration in biochemical markers and has a strong ability to form a biofilm in low-plasma regions such as prosthetic implants and bone cement.4,23 The surgical approach has been shown to affect risk of infection; the deltopectoral approach encroaches closer to the axilla, yielding higher infection rates than transdeltoid.19
S. Aureus and S. Epidermidis are gram-positive bacteria found as commensal skin organisms. PJIS caused by these pathogens tend to present with a more traditional clinical picture for acute infection with symptoms ranging from local erythema, swelling or drainage, to systemic symptoms of fevers, rigors and sepsis.16
3.3.4. Biochemical investigation
Due to C. acnes indolent nature, traditional biochemical markers: WBC, CRP and ESR are of limited diagnostic use. A systematic review reported the sensitivity of CRP as 0%–46% with specificity as 84%–95%.1 ESR had a sensitivity of 16%–42% and specificity of 65%–98%.1 WCC can show a sensitivity as low as 7%.24 Despite this, the 2018 ICM recommends obtaining these markers in the workup.17
3.3.5. Synovial aspirate
Synovial aspiration and analysis are established as the gold standard investigation for orthopaedic periprosthetic infection.17 Shoulder arthrocentesis has been reported to be of low reliability, with up to 44% “dry taps”, even under image guidance, likely due to C. acnes infection often not producing enough synovial fluid for accurate laboratory analysis.16,19 In addition, the sensitivity and specificity of biochemical markers is not currently felt to be sufficient to reliably exclude infection.16 Consequently, there is no consensus whether synovial arthrocentesis should be performed in suspected PJIS, but many sources advocate it.1 Five to six samples is suggested as the gold standard but may be difficult to obtain.3
Synovial alpha-defensin is an antimicrobial peptide that neutralises pathogens and has been used to aid diagnosis of infection in hip and knee arthroplasty to good avail with a reported sensitivity of 97%.16,19 There are currently two forms: the lab-based enzyme-linked immunosorbent assay (ELISA) and the stand-alone alpha-defensin lateral flow test. The ELISA has a reported sensitivity of 75% and specificity of 96%.16 The lateral flow has a reported sensitivity of 60% and specificity of 83%16 and there are still concerns regarding the ability to reliably confirm or exclude infection.16 Many sources describe these markers, but no protocol for their routine use was identified.
Other individual novel markers including IL-6 may show promising reliability with sensitivity and specificity of serum IL-6 being 12.5–14% and 93–95% respectively.1 Synovial IL-6 was found to be more accurate with sensitivity and specificity of 87% and 90%, respectively.1 A combination of markers including IL-6, tumour necrosis factor-a, and IL-2 were found to have better diagnostic ability than any cytokine alone with a sensitivity of 80% a specificity of 93%, a positive predictive value of 0.87, and a negative predictive value of 0.9.25 However, large-volume data on these markers is scarce.
3.3.6. Pre-revision tissue culture
Dilisio et al. reported that tissue culture gained arthroscopically is a valuable tool in diagnosis and planning in PJIS.1 Whilst this involved a separate operation with the associated peri-operative risk and economic impact, this study found that all arthroscopic biopsy culture results were consistent with the revision surgery culture results (100% sensitivity and specificity) and positive predictive value, and negative predictive value of 1.0 26. Fluoroscopically-guided glenohumeral aspiration is an alternative with less perioperative risk. Results have been varied, with reported sensitivity ranging from 16.7% to 80%.26,27 Specificity has been reported in multiple sources as 100%26,27 as well as a positive predictive value of 100% and negative predictive value of 58.3%26
3.3.7. Radiology
It is recommended that all patients who present with postoperative pain undergo a plain radiograph where PJIS is suspected.1,3,16,19 The main signs include implant loosening and humeral osteolysis, the latter conferring a tenfold increased risk of C. acnes as the infective agent.16,19 Ultrasound scanning, and technetium bone scans are not recommended because they do not provide any additional diagnostic findings.19 Computed tomography (CT) scanning may be beneficial in assessing bone stock and current implant configuration and Magnetic Resonance Imaging (MRI) may be helpful in assessing any surrounding osteomyelitis or abscesses19
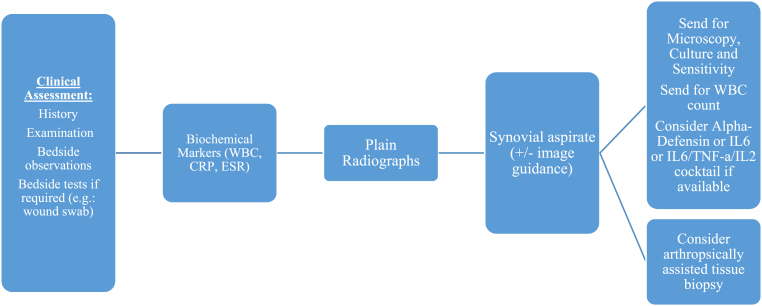
A suggested diagnostic algorithm, based upon the latest evidence as outlined above is provided in Fig. 2.
Fig. 2.
Suggested diagnostic algorithm.
3.4. Management
3.4.1. Prevention
Preventative measures can be useful in risk reduction. These can be considered as at-home measures, perioperative measures and postoperative measures.
Clark et al. advocated multiple home preoperative measures including a soap and water shower on the preceding, or day of, surgery.28 This was shown to reduce the colony count of coagulase-negative staphylococcus by threefold.29 Gluconate skin preparation lowered overall positive culture rate by 28%, lowered coagulase-negative Staphylococcus culture rate by 40% and lowered C. acnes rates by 8% compared to controls.29
Perioperative measures include chlorhexidine and iodine-based preparation solutions. C. acnes was found to be present on the skin in 15% of shoulders prepared with iodine versus 7% in shoulders prepared with Chlorhexidine, therefore the latter is recommended.28 A 2% Chlorhexidine solution is often used.28
Benzoyl peroxide, often used as a topical dermatological treatment for acne, can reduce the C. acnes load by penetrating the sebaceous glands in the dermis.30 It has been shown to reduce C. acnes colonisation rates by 16% in the anterior deltoid region and 20% in the axilla28,30 without significant side-effects.3
Axillary hair clipping/shaving has been shown to increase the colonies of C. acnes bacteria compared to those unclipped, therefore this is currently not a recommended intervention unless the surgical field is better visualised by doing so.28
Operating theatre setup measures including laminar flow and body-exhaust suits are often used to provide a clean-air environment.28 Laminar flow uses a positive air pressure current to direct flow away from the operative site to create a clean zone.31 It is now considered an integral part of arthroplasty theatres.31 A recent study showed that laminar flow significantly reduced the number of bacteria colony-forming units in shoulder arthroplasty but there may be no significant reduction in contaminated air with the use of body-suits.32,33
Classical measures such as changing of surgical blades after the skin incision are effective with a 6% reduction in C. acnes bacteria colonies on fresh blades.28 Changing of surgical gloves after skin draping and before handling implants has been shown to reduce infection risk.28 Surgical site lavage with normal saline is routine however use of iodine lavage is debated, with hip and knee arthroplasty generally showing good reduction in risk of infection but no such significance reported in the spine literature.28
Preoperative screening for methicillin-resistant Staphylococcus aureus (MRSA) is generally institution-dependent.28 In the UK, all patients who undergo an elective procedure are screened for MRSA.34 The common method for this is a nasal swab and treatment with a course of topical mupirocin after positive result and intravenous vancomycin intraoperatively.28 There is currently no consensus recommendation for MRSA screening in the literature.
3.4.2. Prophylactic antimicrobials
There is currently no agreed protocol for antimicrobial prophylaxis in the literature.35 A recent systematic review found that the majority of papers recommend a cephalosporin antibiotic.35 These agents are actively bactericidal and are capable of inhibiting cell wall biosynthesis, causing bacterial lysis. They are low cost, have a good safety profile and long half-life, therefore most US sources recommend Cefazolin.28,35 In the UK, most institutions recommend their own antimicrobial agents based upon local data and microbiological consensus however many institutions recommend a cephalosporin.28,36 Vancomycin is recommended as the agent for those at high risk of MRSA, such as institution residence or long inpatient stays.28 Clindamycin is recommended for those at risk of severe penicillin allergy.35 The aforementioned systematic review suggests routine single-dose, single-agent antibiotic within 30 min (European Guidelines) to 1 h (US Guidelines) of the incision.35
Antibiotics are now a routine component of bone cement.37 Agents include tobramycin, gentamicin or vancomycin/tobramycin combination. There was a near-negligible rate of deep infection in patients with antibiotic-loaded cement compared to the controls.37 There is a paucity of data in the literature surrounding rates of cemented versus uncemented component use and the UK NJR collects data surrounding cementation but has not published figures to date.12 Intra-articular and intra-incisional antibiotics have also been recommended with intra-articular gentamicin reported to reduce deep infection by Lovallo et al.38 Intra-incisional vancomycin has been found in a recent in vitro study to be extremely effective in reducing survival of C. acnes colonies in the wound region, with no discernible effect on the cell healing morphology.39 There is no current recommendation available regarding use of topical or intra-articular antibiotics.
3.4.3. Management of established infection
There are a number of surgical treatments available for established PJIS and these should be rationalised in accordance with infection severity and patient surgical fitness.16
These can range from incision and drainage of wound collection, open washout and debridement with implant retention, single-stage revision, two-stage revision and cement spacer insertion.
Incision and drainage of wound collection has a paucity of evidence and its exclusive use is not recommended due to a recurrence of infection in 30% of cases.16,17 Formal debridement, antibiotics, implant retention (DAIR) is a procedure often used in lower limb arthroplasty.40 In shoulder arthroplasty, DAIR was historically recommended if an infection was proven within 30 days; however it has a reported failure rate of 50–63% and poor functional outcome.19 DAIR in reverse shoulder arthroplasty infection showed an 11% recurrence rate of infection therefore if considered, caution is advised.16,17,19,40
Revision arthroplasty is recommended for patients with established infection, who are surgically fit and have a reasonable functional demand.3 Single-stage exchange arthroplasty is a popular treatment modality traditionally in hip and knee arthroplasty and more recently in shoulder arthroplasty.3,19,41 It involves thorough synovectomy and removal of all implants and cement, with reimplantation in the same operation. The advantages include a single hospital admission and anaesthetic, resulting in lower cost and better functional result.41 Traditional criteria for single-stage revision include identification of the causative organism and lower functional demand.19 A recent systematic review identified a lower reinfection rate of 7% in single-stage compared to 21.3% in two-stage exchange.41 In addition, the combined complication rate was 17% in 1-stage compared to 32.8% in 2-stage exchange.41 Other studies, however, report no statistical difference with both achieving a success rate of over 90%.21 Single-stage revision produced the highest patient-reported outcomes, with statistically-significant superior postoperative Constant scores (mean 51) than patients undergoing a two-stage exchange (mean 44).42 As a result, it is currently recommended by the 2018 ICM consensus, with the caveat that the available reviews may be subjected to selection bias.17
Two-stage revision with removal of implants and thorough joint debridement and lavage, with introduction of a cement and antibiotic spacer at the first stage, and subsequent re-implantation at a later stage has traditionally been the “gold standard”.19 This requires multiple operations, a greater overall hospital inpatient stay, and the associated costs as well as poorer patient-reported functional outcomes.41 It has traditionally been reserved for the younger, fitter and higher demand patients or where the causative organism is unknown.3,19 Success rates have varied with 60–100% quoted across hip, knee and shoulder arthroplasty.3
For the low demand or surgically unfit patient, an antibiotic spacer containing vancomycin and/or gentamycin may be used as definitive management.19 The intention is to eradicate infection whilst providing a functional articulating shoulder with minimal pain.16 Outcomes have generally been favourable, with a small cohort study reporting no recurrent infection and good subjective and objective patient-reported outcomes: average ASES score of 54, QuickDASH of 45 and VAS score of 2.8.43,44 The average active range of motion was 68° of forward flexion and 35° of external rotation.44 A recent review highlighted 32% of cases suffered complications, including glenoid and humeral erosion as well as fracture and rotation of the spacer.45
Finally, resection arthroplasty is an available salvage procedure.3 This procedure can be fraught with complications, including poor function and pain postoperatively as well as antero-superior subluxation of the humerus.3 Clinically, 70–100% of patients are reported to be free from infection after the procedure.46 A recent systematic review provided some technical tips which may help to maximise function including retention of the tuberosities and rotator cuff insertion as well as using the same tissue planes as the initial implantation procedure to reduce tissue damage.46 Some sources report reasonable outcomes in terms of pain and function such as forward flexion of 70° and external rotation of 31° and an average Constant-Murley score of 28.8 46. Resection arthroplasty is therefore recommended as a final-resort option in PJIS. Glenohumeral arthrodesis has been reported in some sources with reasonable results, but there is a paucity of evidence in the literature surrounding its use in PJIS.46
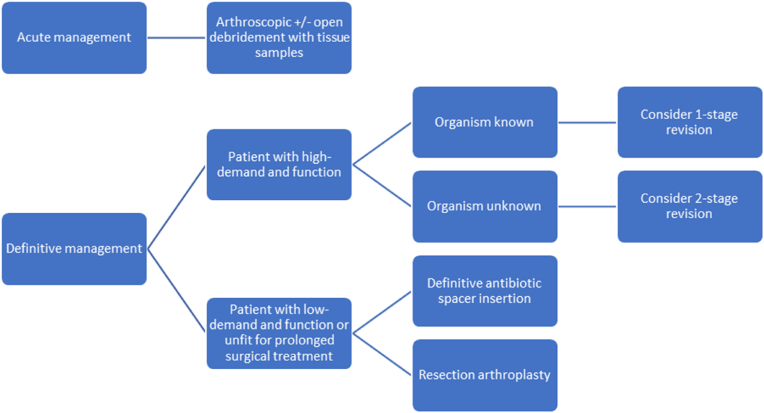
A summary of the recommended treatment modalities discussed is shown in Fig. 3.
Fig. 3.
Suggested management algorithm.
Our review has brought together much of the latest literature on PJIS (see Fig. 2, Fig. 3). The limitations include a wide range of study types including low levels of evidence such as case series and observational studies, often with small sample sizes. In addition, many of the studies were unblinded, conferring a risk of bias. However, the aim of our article is to provide a clear source of recent evidence on the diagnosis and management of PJIS.
4. Conclusion
PJIS is a rare but potentially devastating complication of shoulder arthroplasty. Diagnosis is often challenging due to the indolent and insidious nature of C. acnes; the organism frequently responsible. A new scoring system was developed in 2018 by the ICM of Orthopaedic Infections which may aid in diagnosis. Other advances include novel biochemical tests such as alpha-defensin and interleukin markers to improve investigation. The management of PJIS largely depends on the organism, and the functional demand of the patient. Management options are similar to those used in other major joint arthroplasty infections and one-stage revision appears to be the procedure of choice in the patient with moderate functional demands but two-stage revision and other salvage procedures remain options in the armoury of the treating clinician.
Informed consent (patient/guardian)
Not applicable.
Institutional ethical committee approval (for all human studies)
Not applicable.
Authors contribution
Faria: Conceptualization (data curation), Investigation, Methodology, Writing- Original draft preparation; Flood.: Writing-reviewing and editing, Data curation; Rasheed: Manuscript review; Narang: Manuscript review; Masood: Manuscript review; Bakti: Manuscript review, Supervision, Project administration Singh: Manuscript review, Supervision, Validation.
Funding statement
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
None to declare.
Acknowledgement
None to declare.
Contributor Information
Giles Faria, Email: Gilespaul.faria@nhs.net.
Catherine Flood, Email: catherineflood@nhs.net.
Abdul Rasheed Muhammed, Email: abdulrasheed.muhammed@nhs.net.
Ashish Narang, Email: ashish.narang@nhs.net.
Qazi Masood, Email: qazi.masood@nhs.net.
Nik Bakti, Email: nik.bakti@nhs.net.
Bijayendra Singh, Email: bijayendra.singh@nhs.net.
References
- 1.Egglestone A., Ingoe H., Rees J., Thomas M., Jeavons R., Rangan A. Scoping review: diagnosis and management of periprosthetic joint infection in shoulder arthroplasty. Shoulder Elbow. 2019;11(3):167–181. doi: 10.1177/1758573218779076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah S.S., Gaal B.T., Roche A.M., et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: part I. JSES Int. 2020;4(4):929–943. doi: 10.1016/j.jseint.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnevialle N., Dauzères F., Toulemonde J., Elia F., Laffosse J.M., Mansat P. Periprosthetic shoulder infection: an overview. EFORT Open Rev. 2017;2(4):104–109. doi: 10.1302/2058-5241.2.160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris B.J., O'Connor D.P., Torres D., Elkousy H.A., Gartsman G.M., Edwards T.B. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(2):161–166. doi: 10.1016/j.jse.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Baghdadi Y.M.K., Maradit-Kremers H., Dennison T., et al. The hospital cost of two-stage reimplantation for deep infection after shoulder arthroplasty. JSES Open Access. 2017 Apr 19;1(1):15–18. doi: 10.1016/j.jses.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltzman M.D., Marecek G.S., Edwards S.L., Kalainov D.M. Infection after shoulder surgery. J Am Acad Orthop Surg. 2011;19(4):208–218. doi: 10.5435/00124635-201104000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Richards J., Inacio M.C.S., Beckett M., et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop. 2014;472(9):2809–2815. doi: 10.1007/s11999-014-3696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner B.C., Cancienne J.M., Burrus M.T., Griffin J.W., Gwathmey F.W., Brockmeier S.F. The timing of elective shoulder surgery after shoulder injection affects postoperative infection risk in Medicare patients. J Shoulder Elbow Surg. 2016;25(3):390–397. doi: 10.1016/j.jse.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Moeini S., Rasmussen J.V., Salomonsson B., et al. Reverse shoulder arthroplasty has a higher risk of revision due to infection than anatomical shoulder arthroplasty: 17 730 primary shoulder arthroplasties from the Nordic Arthroplasty Register Association. Bone Jt J. 2019;101-B(6):702–707. doi: 10.1302/0301-620X.101B6.BJJ-2018-1348.R1. [DOI] [PubMed] [Google Scholar]
- 10.Kunutsor S.K., Barrett M.C., Whitehouse M.R., et al. Incidence, temporal trends and potential risk factors for prosthetic joint infection after primary total shoulder and elbow replacement: systematic review and meta-analysis. J Infect. 2020;80(4):426–436. doi: 10.1016/j.jinf.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Florschütz A.V., Lane P.D., Crosby L.A. Infection after primary anatomic versus primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1296–1301. doi: 10.1016/j.jse.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Home - NJR surgeon and hospital profile. 2022. https://surgeonprofile.njrcentre.org.uk/Home/StatsIndex
- 13.Ben-Shlomo Y., Blom A., Boulton C., et al. Outcomes after joint replacement 2003 to 2020. National Joint Registry. 2021 https://www.ncbi.nlm.nih.gov/books/NBK576849/ [Google Scholar]
- 14.Hsu J.E., Somerson J.S., Vo K.V., Matsen F.A. What is a “periprosthetic shoulder infection”? A systematic review of two decades of publications. Int Orthop. 2017;41(4):813–822. doi: 10.1007/s00264-017-3421-6. [DOI] [PubMed] [Google Scholar]
- 15.Tande A.J., Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras E.S., Frantz T.L., Bishop J.Y., Cvetanovich G.L. Periprosthetic infection after reverse shoulder arthroplasty: a review. Curr Rev Musculoskelet Med. 2020;13(6):757–768. doi: 10.1007/s12178-020-09670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrigues G.E., Zmistowski B., Cooper A.M., Green A., ICM Shoulder Group Proceedings from the 2018 international consensus meeting on orthopedic infections: evaluation of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28(6S):S32–S66. doi: 10.1016/j.jse.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Patel V.V., Ernst S.M.C., Rangarajan R., Blout C.K., Lee B.K., Itamura J.M. Validation of new shoulder periprosthetic joint infection criteria. J Shoulder Elbow Surg. 2021;30(7S):S71–S76. doi: 10.1016/j.jse.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Cooper M.E., Trivedi N.N., Sivasundaram L., Karns M.R., Voos J.E., Gillespie R.J. Diagnosis and management of periprosthetic joint infection after shoulder arthroplasty. JBJS Rev. 2019;7(7):e3. doi: 10.2106/JBJS.RVW.18.00152. [DOI] [PubMed] [Google Scholar]
- 20.Sperling J.W., Kozak T.K., Hanssen A.D., Cofield R.H. Infection after shoulder arthroplasty. Clin Orthop. 2001;382:206–216. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Nelson G.N., Davis D.E., Namdari S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2016;25(8):1337–1345. doi: 10.1016/j.jse.2015.11.064. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.J., Kim J.H. Unexpected positive cultures including isolation of Propionibacterium acnes in revision shoulder arthroplasty. Chin Med J. 2014;127(22):3975–3979. [PubMed] [Google Scholar]
- 23.Hudek R., Gohlke F. [Endoprosthesis infections of the shoulder: diagnosis and therapy algorithm] Orthopä. 2013;42(7):552–559. doi: 10.1007/s00132-012-2026-4. [DOI] [PubMed] [Google Scholar]
- 24.Shields M.V., Abdullah L., Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elbow Surg. 2016;25(6):1034–1040. doi: 10.1016/j.jse.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Frangiamore S.J., Saleh A., Grosso M.J., et al. Neer Award 2015: analysis of cytokine profiles in the diagnosis of periprosthetic joint infections of the shoulder. J Shoulder Elbow Surg. 2017;26(2):186–196. doi: 10.1016/j.jse.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Dilisio M.F., Miller L.R., Warner J.J.P., Higgins L.D. Arthroscopic tissue culture for the evaluation of periprosthetic shoulder infection. J Bone Joint Surg Am. 2014;96(23):1952–1958. doi: 10.2106/JBJS.M.01512. [DOI] [PubMed] [Google Scholar]
- 27.Lapner P.L.C., Hynes K., Sheikh A. Capsular needle biopsy as a pre-operative diagnostic test for peri-prosthetic shoulder infection. Shoulder Elbow. 2019;11(3):191–198. doi: 10.1177/1758573217743943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark J.J.C., Abildgaard J.T., Backes J., Hawkins R.J. Preventing infection in shoulder surgery. J Shoulder Elbow Surg. 2018;27(7):1333–1341. doi: 10.1016/j.jse.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Murray M.R., Saltzman M.D., Gryzlo S.M., Terry M.A., Woodward C.C., Nuber G.W. Efficacy of preoperative home use of 2% chlorhexidine gluconate cloth before shoulder surgery. J Shoulder Elbow Surg. 2011;20(6):928–933. doi: 10.1016/j.jse.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Sabetta J.R., Rana V.P., Vadasdi K.B., et al. Efficacy of topical benzoyl peroxide on the reduction of Propionibacterium acnes during shoulder surgery. J Shoulder Elbow Surg. 2015;24(7):995–1004. doi: 10.1016/j.jse.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Jain S., Reed M. Laminar air flow handling systems in the operating room. Surg Infect. 2019;20(2):151–158. doi: 10.1089/sur.2018.258. [DOI] [PubMed] [Google Scholar]
- 32.Morris B.J., Kiser C.J., Laughlin M.S., et al. A localized laminar flow device decreases airborne particulates during shoulder arthroplasty: a randomized controlled trial. J Shoulder Elbow Surg. 2021;30(3):580–586. doi: 10.1016/j.jse.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Young S.W., Zhu M., Shirley O.C., Wu Q., Spangehl M.J. Do “surgical helmet systems” or “body exhaust suits” affect contamination and deep infection rates in arthroplasty? A systematic review. J Arthroplasty. 2016;31(1):225–233. doi: 10.1016/j.arth.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Who to screen for MRSA. 2014. https://www.gov.uk/government/publications/how-to-approach-mrsa-screening Gov.UK.
- 35.Longo U.G., Candela V., Facchinetti G., et al. Antibiotic prophylaxis in primary and revision shoulder replacement: a systematic review. BMC Muscoskel Disord. 2020;21(1):292. doi: 10.1186/s12891-020-03332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quality statement 2: antibiotic prophylaxis | Surgical site infection | Quality standards | Nice. 2013. https://www.nice.org.uk/guidance/qs49/chapter/quality-statement-2-antibiotic-prophylaxis
- 37.Nowinski R.J., Gillespie R.J., Shishani Y., Cohen B., Walch G., Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21(3):324–328. doi: 10.1016/j.jse.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 38.Lovallo J., Helming J., Jafari S.M., et al. Intraoperative intra-articular injection of gentamicin: will it decrease the risk of infection in total shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(9):1272–1276. doi: 10.1016/j.jse.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Miquel J., Huang T.B., Athwal G.S., Faber K.J., O'Gorman D.B. Vancomycin is effective in preventing Cutibacterium acnes growth in a mimetic shoulder arthroplasty. J Shoulder Elbow Surg. 2022;31(1):159–164. doi: 10.1016/j.jse.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Lemmens L., Geelen H., Depypere M., et al. Management of periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2021;30(11):2514–2522. doi: 10.1016/j.jse.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Aïm F., Marion B., Kerroumi Y., Meyssonnier V., Marmor S. One- or two-stage exchange for periprosthetic shoulder infection: systematic review and meta-analysis. Orthop Traumatol Surg Res OTSR. 2020;106(1):5–15. doi: 10.1016/j.otsr.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 42.George D.A., Volpin A., Scarponi S., Haddad F.S., Romanò C.L. Does exchange arthroplasty of an infected shoulder prosthesis provide better eradication rate and better functional outcome, compared to a permanent spacer or resection arthroplasty? a systematic review. BMC Muscoskel Disord. 2016;17:52. doi: 10.1186/s12891-016-0901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellegrini A., Legnani C., Macchi V., Meani E. Management of periprosthetic shoulder infections with the use of a permanent articulating antibiotic spacer. Arch Orthop Trauma Surg. 2018;138(5):605–609. doi: 10.1007/s00402-018-2870-8. [DOI] [PubMed] [Google Scholar]
- 44.Cronin K.J., Hayes C.B., Sajadi K.R. Antibiotic cement spacer retention for chronic shoulder infection after minimum 2-year follow-up. J Shoulder Elbow Surg. 2020;29(9):e325–e329. doi: 10.1016/j.jse.2020.01.065. [DOI] [PubMed] [Google Scholar]
- 45.McFarland E.G., Rojas J., Smalley J., Borade A.U., Joseph J. Complications of antibiotic cement spacers used for shoulder infections. J Shoulder Elbow Surg. 2018;27(11):1996–2005. doi: 10.1016/j.jse.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 46.Arenas-Miquelez A., Arbeloa-Gutierrez L., Familiari F., de Pablos J. Salvage procedures of the shoulder: glenohumeral arthrodesis and resection arthroplasty. Indian J Orthop. 2020;55(Suppl 1):27–37. doi: 10.1007/s43465-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]