Abstract
Background
Both restricted inverse kinematic alignment (iKA) and gap balancing aim for a balanced total knee arthroplasty by adjusting femoral component position based on ligamentous gaps. However, iKA targets a native tibial joint line vs resecting perpendicular to the mechanical axis. This study compares how these 2 techniques impact the balance and laxity throughout flexion and joint line obliquity (JLO), arithmetic hip-knee-ankle angle (aHKA), and the coronal plane alignment of the knee (CPAK).
Methods
Two surgeons performed 75 robot-assisted iKA total knee arthroplasties. A digital joint tensioner collected laxity data throughout flexion before femoral resection. The femoral component position was determined using predictive gap-planning to optimize the balance throughout flexion. Planned gap balancing (pGB) simulations were performed for each case using neutral tibial resections. Mediolateral balance, laxity, and CPAK were compared among pGB, planned iKA (piKA), and final iKA.
Results
Both piKA and pGB had similar mediolateral balance and laxity, with mean differences <0.4 mm. piKA had a lower mean absolute difference from native JLO than pGB (3 ± 2° vs 7 ± 4°, P < .001). aHKA was similar (P > .05) between pGB and piKA. piKA recreated a more native CPAK distribution, with types I-V being the most common ones, while most pGB knees were of type V, VII, and III. Final iKA and piKA had similar mediolateral balance and laxity, with a root-mean-square error <1.4 mm.
Conclusions
Although balance, laxity, and aHKA were similar between piKA and pGB, piKA better restored native JLO and CPAK phenotypes. The neutral tibial resection moved most pGB knees into types V, VII, and III. Surgeons should appreciate how the alignment strategy affects knee phenotypes.
Keywords: Restricted inverse kinematic alignment, Gap balancing, Total knee arthroplasty, Computer-assisted, Robotic-assisted
Introduction
The goal of improving outcomes in total knee arthroplasty (TKA) has led to the development of various alternate implant alignment strategies [[1], [2], [3], [4], [5]]. Gap balancing (GB) prioritizes balancing the soft tissue envelope by first resecting the tibia neutral to the mechanical axis and then adjusting the femoral component position to achieve balanced gaps in extension and flexion [2,6]. Tibia-first restricted inverse kinematic alignment (iKA) is an evolution of GB which aims to restore the native tibial joint line while targeting a specific soft tissue balance profile throughout flexion by adjusting the femoral component position from the patient’s native femoral anatomy and allowing some lateral flexion laxity [7]. While a neutral tibial resection has long been thought to be a requirement for better long-term outcomes and implant survivorship [8], studies have shown that restoring the native joint line may improve clinical outcomes while not hindering survivorship [[3], [4], [5]]. The impact of a neutral vs an inclined tibial joint line on gap balance throughout the range of flexion has not been investigated.
Simulation is a convenient method to directly compare the surgical outcomes of 2 TKA techniques in individual patients. Until recently, few studies have simulated TKA using patient-specific soft tissue laxity data due to the historically invasive nature of this process [9]. Intraoperative robotic technology can now accurately quantify joint laxity throughout the arc of flexion in TKA [10]. Recent studies have used these data to simulate different implant alignment techniques in individual patients to understand the effects of alignment technique on joint laxity and balance [11]. This is important clinically as small changes in balance and laxity have been correlated with improvements in TKA clinical outcomes [6].
The coronal plane alignment of the knee (CPAK) classification is a tool for analyzing knee phenotypes in native, arthritic, and replaced knees. CPAK uses a simple 9-category classification for knee phenotypes by plotting tibial and femoral joint line obliquity (JLO) against arithmetic hip-knee-ankle (aHKA) alignment, providing a useful way to compare phenotypes between TKA techniques [[12], [13], [14]].
This study therefore aimed to compare how iKA and GB alignment strategies impact mediolateral (ML) balance, laxity, and resection patterns, as well as JLO, aHKA, and CPAK type using patient-specific gap data collected under controlled ligament loading during robot-assisted TKA [12]. Lastly, this study investigates the ability of a predictive algorithm to predict the final balance and laxity in iKA. The study hypothesizes that (1) planned iKA (piKA) will have similar ML imbalance and laxity compared to planned GB (pGB) but will better restore native JLO with greater tibial varus, greater femoral valgus, and less external femoral rotation; (2) piKA will better restore the native CPAK than pGB; and (3) there will be a good agreement between piKA and final iKA (fiKA) ML balance and laxity.
Methods
Eighty-two consecutive robot-assisted iKA TKAs performed by 2 surgeons at 2 centers were retrospectively reviewed after obtaining ethics approval from an independent institutional review board (Bellberry Ltd. approval no. 2020-08-764-A-1). Patients with prior knee trauma, surgery, or gross ligamentous deficiency were excluded. Patients were included if final laxity data were captured by the digital robotic ligament tensioner. Seven cases were excluded for missing data, and 75 were included: 30 from 1 surgeon and 45 from the other. The indication for TKA for all patients was end-stage osteoarthritis. Cases were performed between March 2020 and December 2021. Both surgeons have over 20 years of experience in knee arthroplasty and have performed over 300 kinematically aligned knees with modified standard instruments and over 100 kinematically aligned knees with robotic assistance prior to this study.
The mean patient age was 72 ± 8 years, with a body mass index of 30 ± 7 kg/m2. Sixty-five percent of patients were female. The mean preoperative flexion contracture was 6 ± 5°, and the mean preoperative coronal deformity was 4 ± 5° varus.
Restricted iKA surgical technique
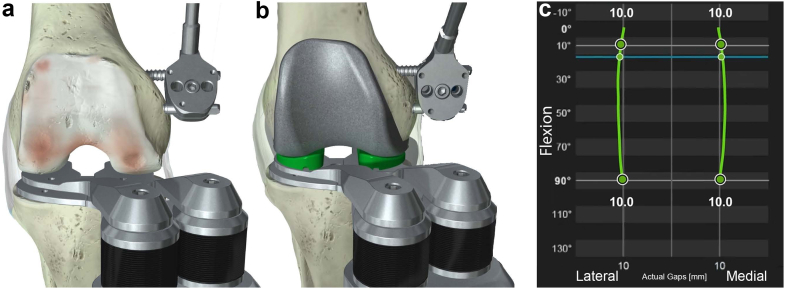
Both surgeons used the same cruciate-retaining implant (Apex; Corin Ltd., Raynham, MA) and robot-assisted system in combination with the Balance Bot, a digital robotic ligament-tensioning device (Corin Ltd., Raynham, MA) [6,10]. The digital joint tensioner was used to measure joint laxity by applying equal forces to the medial and lateral compartments independently (Fig. 1a and b) [10]. The device operates in conjunction with a surgical navigation system along with a miniature bone-mounted robotic cutting guide [15,16]. The digital joint tensioner has been shown to measure joint gaps with high repeatability, with variations in measurements within the optical tracking system accuracy [10].
Figure 1.
The digital joint tensioning device utilizes independent medial and lateral active spacing units that are controlled via the navigation system to measure joint gaps throughout the flexion range. Joint tension is selected by the surgeon. (a) Gap data collected after tibial resection are used for predictive balance. (b) Final gap data are collected with the femoral trial in place. (c) The system provides a visual representation of the joint gaps throughout the flexion range.
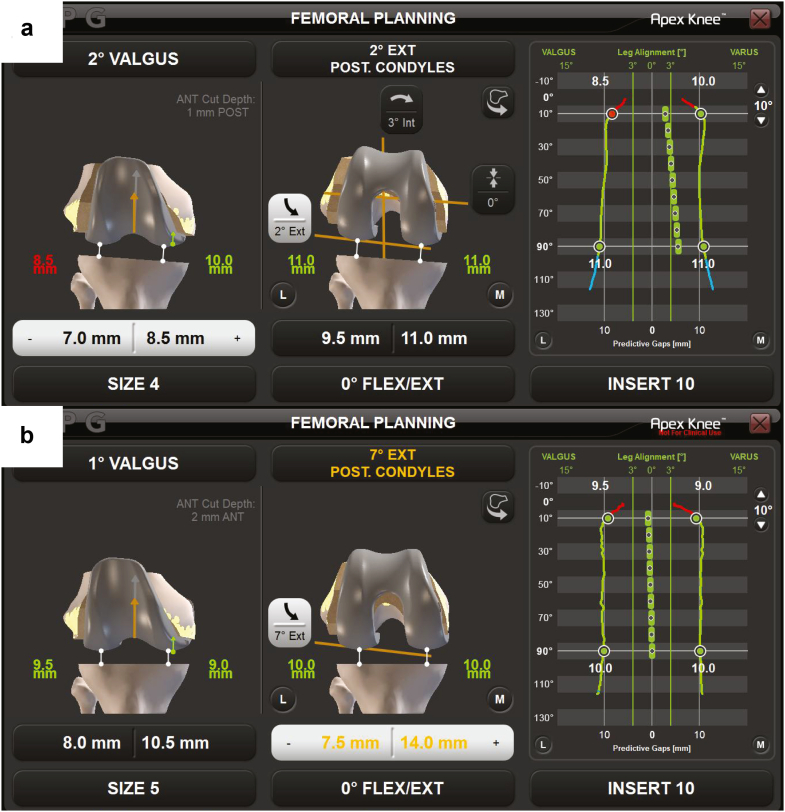
In all cases, iKA was performed using imageless robot-assisted navigation with a tibia-first workflow, similar to the method described by Murgier and Clatworthy and Winnock de Grave et al. [7,17]. A medial parapatellar approach with minimal medial release upon exposure was used. Optical trackers were fixed to the tibia and femur. Tibial registration included digitization of the medial and lateral resection depths as per the study by Murgier and Clatworthy, using the mid-coronal line of the lateral tibial plateau and a point landmarked on the tidemark of the medial plateau where cartilage wear is approximately 2 mm [17]. The femoral anatomy was registered using a 3D morphometric model [16]. An initial kinematic assessment was performed providing range of motion under manual manipulation. Using the navigation system, the tibial resection was then planned to restore the native joint line in the coronal plane, accounting for cartilage wear as described by Murgier and Clatworthy [17] and limiting resection to 6° varus and 3° valgus to the mechanical axis. The tibial resection was then performed, matching the patient’s native slope within the range of 2° – 9°, and validated using the navigation system validation plate. The mean planned posterior tibial slope was 4.7 ± 1.4° (range 2° – 9°). The digital joint tensioner was then inserted into the joint space to collect laxity data as the knee was taken through a range of motion during an initial balance assessment with forces ranging from 70 N to 90 N (Fig. 1a). The laxity data from the initial balance assessment were used as an input for the intraoperative predictive gap-planning software that virtually placed the femoral component, rendering a postoperative gap prediction throughout flexion. Femoral resections were planned to achieve stability and rectangular ML gaps in extension, while allowing for some lateral laxity as the knee moves into flexion, limiting distal femoral valgus to 6° valgus and 3° varus from the mechanical axis using the predictive gap-planning software [10]. An example femoral plan for piKA is shown in Figure 2a. Femoral resections were then executed using the robotic cutting guide. After femoral resection, the digital tensioner was inserted again to perform a final laxity assessment throughout flexion (Fig. 1b and c). Laxity was defined as the tibial insert thickness subtracted from the gap between the resected tibia and the femoral component. Planned laxity was calculated using the tibial insert thickness selected during the femoral planning stage. Final laxity was calculated using the implanted tibial insert thickness. ML balance was defined as the difference between lateral and medial laxity.
Figure 2.
Femoral planning screen for piKA (a) and pGB (b). Femoral component position can be adjusted with the computer navigation system intraoperatively. The predictive soft tissue laxity plan is then visualized throughout the flexion range.
GB simulations
GB was simulated for each iKA case post hoc by importing intraoperative data into the robotic system planning software. The tibial and femoral resections were then virtually performed using the robotic system planning software. The tibial coronal resection was set to neutral (0°) to the mechanical axis. The default resection from the high side of the proximal tibia was set to the tibial implant construct thickness of 10 mm, which, if necessary, was increased to ensure a minimum of 2 mm was resected off the low side of the proximal tibia. Femoral planning was then performed to achieve equal ML balance at 10° (extension) and 90° degrees of flexion. A limit of ±6° was applied to the distal femoral coronal resection and to the femoral rotation resection. The distal femoral resection was adjusted to plan for a 1 mm tighter extension gap than flexion gap both medially and laterally, and the posterior femoral resection was adjusted to plan for a 0 mm gap in flexion both medially and laterally. Studies have reported similar trends of looser flexion space for GB and other alignment techniques [10,18,19]. If the joint laxity in all 4 quadrants (medial and lateral in flexion and extension) was looser by more than 1 mm than the target, the insert thickness was increased until at least 1 quadrant was within the threshold. An example femoral plan for pGB is shown in Figure 2b.
Data analysis
ML balance and lateral and medial laxity were all compared between piKA and pGB. The percentage of knees with >2 mm of ML imbalance was compared. Alignment was captured using the robotic system resection validation probe. ML balance and lateral and medial laxity were compared between piKA and fiKA. Laxity root-mean-square error was also compared between fiKA and piKA medially and laterally at 10°, 45°, and 90°.
The native medial proximal tibial angle (MPTA) and lateral distal femoral angle (LDFA) were calculated relative to the mechanical axis by adjusting for 2 mm of cartilage wear on the medial proximal tibia and on the medial distal femur in knees with a >3° varus deformity in the coronal plane, and similarly a 2 mm correction was applied to the lateral side in knees with a >3° valgus deformity [3,20,21]. Preoperative and postoperative coronal alignment were also recorded using the navigation system.
A CPAK analysis was performed in accordance with the methods presented by MacDessi et al. [12]. aHKA was calculated as MPTA − LDFA. JLO was calculated as MPTA + LDFA. CPAK analyses were performed for the native preoperative alignment, pGB, piKA, and fiKA. Native preoperative CPAK was calculated using the landmarks from the navigation system with the osteoarthritic wear correction factor applied. pGB and piKA used the validated tibial resection angles along with the planned femoral resection angles, while fiKA used the validated resection angles for both the tibia and femur.
Statistical analysis
Kolmogorov-Smirnov test confirmed the data were normally distributed (ML balance and resection thicknesses rejected alternative hypothesis, P > .05 in all cases) [22]. Welch's unequal variances t-tests, variance tests (F-tests), and chi-squared tests were used where appropriate in comparing achieved balance and laxity between groups. Statistical analyses were conducted using the R environment for statistical computing (version 4.1.0) [23]. Statistically significant differences are indicated in figures by “∗∗∗”/“†††” = P ≤ .001; “∗∗”/“††” = P ≤ .01; and “∗”/“†” = P ≤ .05, with “∗” and “†” denoting t- and F-tests, respectively. Significant results are highlighted in bold in all tables. A prospective matched pair means a power analysis was performed. Using an alpha of 0.05, beta of 0.8, a joint gap balance standard deviation (SD) of 1.5 mm with an equal sampling ratio, and a threshold joint balance difference of 0.5 mm, a minimum of 73 participants were required.
Results
piKA vs pGB
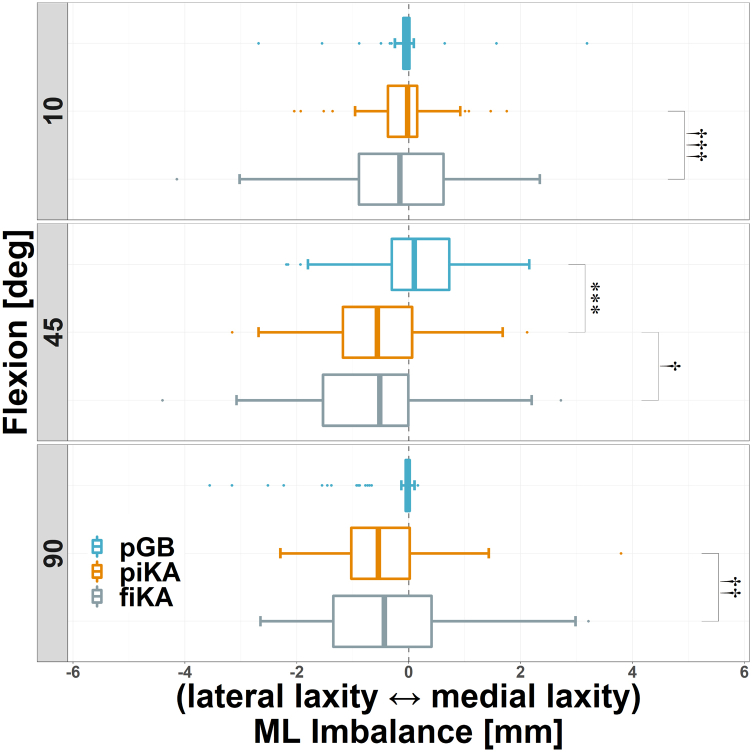
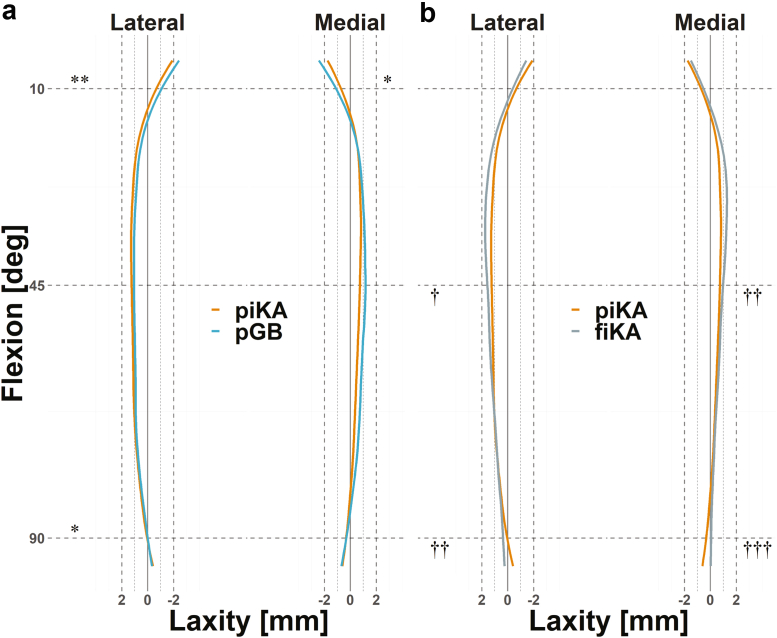
ML imbalance (mean and SD) was similar between piKA and pGB, with the only statistically significant difference being a 0.6 mm increase in relative lateral imbalance for piKA at 45° (P < .001), Figure 3 and Table 1. Compared to pGB, piKA had similar percentages of knees with ML imbalances >2 mm at 10° (1% vs 3%), 45° (4% vs 4%), and 90° (4% vs 5%) and >3 mm at 10° (1% vs 0%), 45° (0% vs 1%), and 90° (3% vs 1%), P > .95. Medial and lateral laxity were also similar for piKA and pGB, with mean differences of ≤0.4 mm throughout flexion (Figure 4a and Table 2).
Figure 3.
pGB (blue), piKA (orange), and fiKA (gray) ML imbalance shown with negative values representing relative lateral laxity. ∗∗∗/†††P ≤ .001, ††P ≤ .01, and †P ≤ .05 (with “∗” and “†” denoting t- and F-tests, respectively).
Table 1.
ML imbalance values, shown as mean ± SD (range).
| Flexion | pGB | piKA | fiKA | pGB vs piKA |
piKA vs fiKA |
||
|---|---|---|---|---|---|---|---|
| t-Test | F-test | t-Test | F-test | ||||
| 10° | 0 ± 0.6 (−2.7 to 3.2) | −0.1 ± 0.7 (−2 to 1.8) | −0.1 ± 1.2 (−4.1 to 2.3) | 0.694 | 0.296 | 0.800 | <0.001 |
| 45° | 0.1 ± 0.9 (−2.2 to 2.2) | −0.5 ± 0.9 (−3.2 to 2.1) | −0.7 ± 1.2 (−4.4 to 2.7) | <0.001 | 0.936 | 0.459 | 0.026 |
| 90° | −0.3 ± 0.7 (−3.6 to 0.2) | −0.5 ± 0.9 (−2.3 to 3.8) | −0.4 ± 1.3 (−2.7 to 3.2) | 0.124 | 0.098 | 0.567 | 0.002 |
Negative values represent relative lateral laxity. Significant results are shown in bold.
Figure 4.
Mean laxity profile comparisons (a) between piKA (orange) and pGB (blue) and (b) between piKA and fiKA (gray). †††P ≤ .001, ∗∗/††P ≤ .01, and ∗/†P ≤ .05.
Table 2.
Lateral and medial laxity values, shown as mean ± SD (range), and statistical test results for pGB, piKA and fiKA.
| Flexion | pGB | piKA | fiKA | pGB vs piKA |
piKA vs fiKA |
||
|---|---|---|---|---|---|---|---|
| t-Test | F-test | t-Test | F-test | ||||
| Lateral laxity | |||||||
| 10° | −1 ± 0.5 (−4.4 to 0) | −0.6 ± 1.1 (−4.3 to 1.4) | −0.4 ± 1.3 (−3.9 to 2.6) | 0.008 | - | 0.269 | 0.254 |
| 45° | 0.9 ± 1.3 (−2.6 to 4) | 1.2 ± 1.1 (−1.6 to 4.3) | 1.6 ± 1.5 (−1.9 to 5.9) | 0.101 | - | 0.086 | 0.011 |
| 90° | 0 ± 0.2 (−0.4 to 0.4) | 0.3 ± 1.2 (−4 to 3.3) | 0.5 ± 1.7 (−3.8 to 4.7) | 0.044 | - | 0.384 | 0.003 |
| Medial laxity | |||||||
| 10° | −1.1 ± 0.4 (−3.4 to 0) | −0.7 ± 1.1 (−4.1 to 1.7) | −0.6 ± 1.3 (−3.9 to 3.6) | 0.013 | - | 0.373 | 0.144 |
| 45° | 1 ± 1.2 (−1.3 to 4.4) | 0.7 ± 1 (−1.4 to 5.1) | 0.9 ± 1.4 (−2.6 to 6.8) | 0.084 | - | 0.228 | 0.002 |
| 90° | −0.3 ± 0.7 (−3.4 to 0.3) | −0.2 ± 0.8 (−2.5 to 1.9) | 0.1 ± 1.5 (−3.1 to 7.9) | 0.578 | - | 0.134 | <0.001 |
Significant results are shown in bold.
piKA more closely restored native MPTA (native = 87 ± 3°, piKA = 88 ± 2°, pGB = 90 ± 0°) and was more varus than pGB (P < .001; Table 3). piKA also more closely restored native LDFA (native = 88 ± 3°, piKA = 89 ± 3°, pGB = 91 ± 4°) and was more valgus than pGB (P < .001; Table 3). Stratifying by preoperative alignment showed piKA better replicated native MPTA for all groups, and native LDFA for varus and neutral knees (Table 4).
Table 3.
Tibial and femoral resection values, shown as mean ± SD (range), for piKA and pGB with t- and F-test P values.
| Measure | piKA | pGB | t-Test | F-test |
|---|---|---|---|---|
| MPTA (°) | 88 ± 2 (84 to 92) | 90 ± 0 (90 to 90) | <0.001 | <0.001 |
| Lateral tibia (mm) | 9 ± 1.4 (4.8 to 11.1) | 9.8 ± 0.8 (5.1 to 10) | <0.001 | <0.001 |
| Medial tibia (mm) | 7.6 ± 1.4 (3.5 to 10.8) | 6.6 ± 2.2 (2.7 to 10) | 0.002 | <0.001 |
| LDFA (°) | 89 ± 3 (82 to 96) | 91 ± 4 (84 to 97) | 0.001 | 0.156 |
| External femoral rotation (°) | 2 ± 2 (−3 to 6) | 4 ± 3 (−6 to 8) | <0.001 | 0.006 |
Significant results are shown in bold.
Table 4.
MPTA and LDFA (°) broken down by preoperative HKA alignment, shown as mean ± SD (range).
| Measure | Preoperative HKA |
||
|---|---|---|---|
| Varus (>3°) |
Neutral |
Valgus (<−3°) |
|
| [N = 40, 55.6%] | [N = 25, 34.7%] | [N = 7, 9.7%] | |
| MPTA | |||
| Native | 86 ± 3 (82-92) | 88 ± 3 (83-96) | 88 ± 3 (85-92) |
| piKA | 87 ± 2 (84-91) | 89 ± 2 (86-92) | 90 ± 1 (88-90) |
| pGB | 90 ± 0 (90-90) | 90 ± 0 (90-90) | 90 ± 0 (90-90) |
| LDFA | |||
| Native | 88 ± 2 (83-93) | 87 ± 3 (81-91) | 85 ± 2 (81-88) |
| piKA | 90 ± 2 (86-96) | 88 ± 3 (84-95) | 84 ± 2 (82-88) |
| pGB | 93 ± 2 (90-97) | 89 ± 3 (84-96) | 85 ± 1 (84-88) |
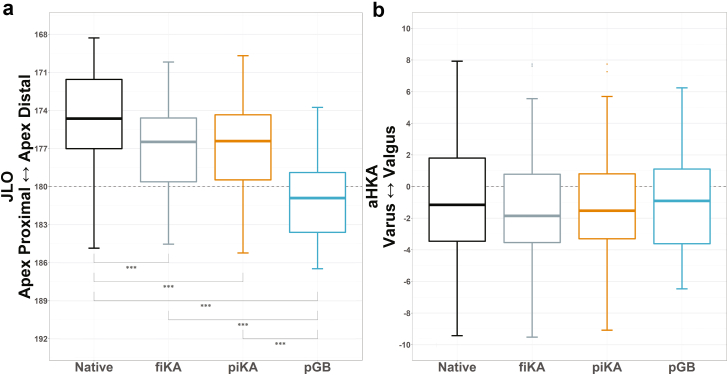
JLO for piKA and pGB were both more apex proximal than native (P < .001; Figure 5a). However, piKA more closely restored native JLO than pGB, with a mean absolute difference from native of 3 ± 2° for piKA compared to 7 ± 4° for pGB, P < .001. No differences were observed in aHKA between all groups (Figure 5b). Furthermore, there was no difference in the restoration of native aHKA between piKA and pGB, with mean absolute differences from native of 3 ± 2° and 3 ± 2°, respectively.
Figure 5.
(a) JLO and (b) aHKA comparisons between Native, fiKA, piKA, and pGB. Note the differences in JLO, while no differences are seen in aHKA. ∗∗∗P ≤ .001.
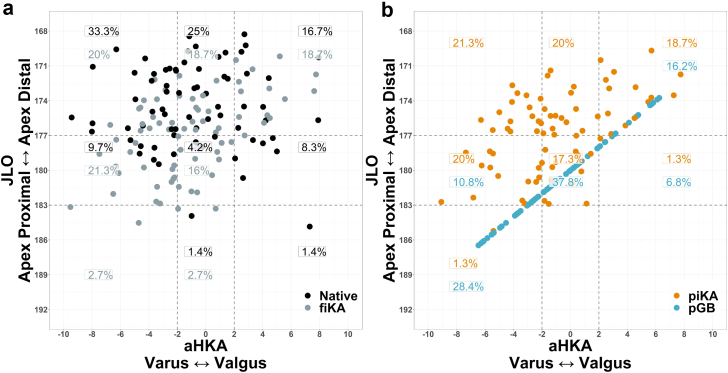
The most common native preoperative CPAK groups were groups I, II, and III (Figure 6a). piKA planned for a similar distribution of CPAK groups, with the most common being I, II, III, IV, and V (Figure 6b). pGB was planned in a way for most knees to be in groups V, VII, and III (Table 5 and Figure 6b). Less piKA knees changed CPAK groups from native compared to pGB (68% vs 92%, P < .001; Table 5). Furthermore, piKA had a lower percentage of knees experiencing a CPAK group change of >1 than pGB (Table 6).
Figure 6.
CPAK plot of aHKA against JLO showing the percentages of patients within each of the 9 CPAK types. (a) Native vs fiKA highlights the ability of iKA to restore a similar CPAK distribution to native. (b) piKA vs pGB shows the differences in the techniques, note how pGB forces the CPAK into a narrow distribution. This is due to the fact that every pGB case has a neutral tibial resection, which causes a linear relationship between JLO and aHKA.
Table 5.
Totals along with most common changes in CPAK groups from native to piKA and from native to pGB.
| Change | n | % | |
|---|---|---|---|
| Native to piKA | Total | 48 | 67.6 |
| I to II | 7 | 9.9 | |
| I to IV | 6 | 8.5 | |
| II to I | 5 | 7.0 | |
| I to V | 3 | 4.2 | |
| II to III | 3 | 4.2 | |
| Native to pGB | Total | 65 | 91.5 |
| I to VII | 12 | 16.9 | |
| I to V | 10 | 14.1 | |
| II to V | 9 | 12.7 | |
| II to VII | 4 | 5.6 | |
| III to V | 4 | 5.6 |
Table 6.
Percentages of knees who shifted CPAK categories.
| Native to piKA | Native to pGB | Chi-squared | |
|---|---|---|---|
| No change | 23/71 (32.4%) | 6/71 (8.5%) | P < .001 |
| 1 | 34/71 (47.9%) | 28/71 (39.4%) | P = .398 |
| >1 | 14/71 (19.7%) | 37/71 (52.1%) | P < .001 |
Diagonal shifts were included in >1.
Significant values highlighted in bold.
piKA vs fiKA
The mean ML imbalance, medial laxity, and lateral laxity were similar between piKA and fiKA throughout flexion (P > .05, Figure 3, Figure 4b, Table 1, and Table 2). However, variability in ML imbalance and laxity was slightly higher for fiKA than for piKA, with SD ranging from 0.2 to 0.7 mm (P < .03, Figure 3, Figure 4b, Table 1, Table 2). The root-mean-square error between fiKA and piKA laxity ranged from 1 to 1.4 mm throughout flexion (Table 7). piKA was planned to have 0.5 mm greater laxity laterally than medially at 45° and 90° (P < .01), but not at 10° (P > .05). fiKA laxity was 0.7 mm greater laterally at 45° and 0.4 mm at 90°. fiKA implanted insert thickness matched the plan in 63% of cases, was within 1 size in 93% of cases, and was within 2 sizes in 100% of cases.
Table 7.
Laxity root mean square error (mm) between piKA and fiKA.
| Flexion | Lateral | Medial |
|---|---|---|
| 10° | 1.3 | 1.3 |
| 45° | 1.3 | 1 |
| 90° | 1.4 | 1.2 |
Coronal HKA alignment remained consistent for neutral (HKA 0 ± 3°) knees from preoperative (0 ± 1°) to postoperative period (1 ± 2°) after iKA. iKA corrected knees with preoperative varus (HKA ≥ 3°) ranging from 7 ± 3° to 3 ± 2° and corrected knees with preoperative valgus (HKA ≤ −3°) ranging from −5 ± 3° to −4 ± 2°.
piKA and fiKA had similar distributions across the 9 CPAK groups, with a maximum percentage difference of 3% seen in group VIII (Figure 6a and b).
Discussion
The most important findings of this study were that (1) piKA was planned for cases with similar balance and laxity profiles and aHKA as pGB while planning for more native JLO and CPAK phenotype distribution and (2) fiKA and piKA had similar mean ML balance, laxity profiles, and CPAK phenotype distributions. The significance of these findings is that (1) iKA can achieve knee balance throughout the range of motion with better restoration of the native bone anatomy than a traditional GB technique with a neutral tibial resection, and (2) the use of a robot-assisted predictive balancing workflow allows for accurate and reproducible execution of an iKA plan, thereby reducing the risk of outlier alignment and imbalanced knees.
iKA is a relatively new technique which has similar ML balance targets to GB but differs from GB in that it aims to restore native JLO [7,24,25]. Winnock de Grave et al. describe the technique as restoring the native tibial joint line by resecting equal amounts from the medial and lateral tibia accounting for bony wear and positioning the femoral component to restore medial joint line heights in flexion and extension while allowing for lateral flexion laxity [24]. iKA differs from traditional kinematic alignment because while the tibial resection restores the prearthritic tibial joint line, the femoral component alignment does not aim to unequivocally restore the 3 kinematic axes of the knee. Winnock de Grave et al. found that compared to robot-assisted adjusted mechanical alignment (MA) a greater percentage of restricted iKA knees achieved the Patient Acceptable Symptom State thresholds for Oxford Knee Score (OKS) and satisfaction, and in varus knees, iKA led to greater OKS and satisfaction scores [25]. Furthermore, robotic-assisted iKA improved OKS scores compared to conventional adjusted MA [25].
The key differences observed between iKA and GB in the present study were around JLO and CPAK. piKA restored a more oblique and more native joint line in extension and flexion compared to pGB. This is important because restoring native JLO has been shown to optimize knee kinematics [26]. Similarly, piKA better restored the native CPAK phenotype distribution and restored more knees to their native CPAK group than pGB. pGB moved the bulk of knees into groups V, VII, and III (Figure 6b). The linear relationship observed in the CPAK distribution for pGB (Fig. 6b) is due to all pGB knees undergoing neutral (90°) tibial resections. Because MPTA is always 90° for pGB, the CPAK plot effectively becomes a linear plot of LDFA vs LDFA. piKA restored 32% of knees to their native CPAK phenotype, while pGB restored only 8.5% of knees. Sappey-Marinier et al. reported that traditional MA restored 18% of knees to their native CPAK phenotype and that apex distal knees with restored CPAK phenotypes had less postoperative pain than those that were not restored [14]. This suggests that iKA may lead to improved pain outcomes as it restores a greater percentage of CPAK phenotypes than both GB and MA. Furthermore, piKA and fiKA were within 4% of each other across each CPAK group, indicating that predictive tibia-first iKA can sufficiently predict final CPAK alignment.
Both iKA and GB contrast with traditional MA that uses soft tissue releases after the bony cuts to achieve ML balance. Due to the presence of mechanoreceptors within the soft tissue surrounding the knee joint, it is theorized that avoiding soft tissue releases during TKA could preserve the proprioceptive sensory systems integral for maintaining knee joint stability [27]. Furthermore, Vigdorchik et al. have shown that increased soft tissue releases have been negatively associated with Knee Injury and Osteoarthritis Outcome Score outcomes out to 2 years [28]. In the present study, only 1 release (posterior cructiate ligament) was recorded out of 75 iKA cases, which corresponds well with studies showing low soft tissue release rates with GB [11,29]. The iKA and GB release rates are both lower than the MA release rate that has been reported at 60% for varus knees alone [11,29,30]. These results indicate that MA may have a greater tendency to cause damage to the soft tissue mechanoreceptor system, potentially affecting proprioception and ultimately patient satisfaction [27].
Comparing fiKA to piKA shows the ability of the robotic surgical system to achieve its ML balance and laxity plan. The mean ML balance and laxity were similar between fiKA and piKA, with similar accuracy to that which has been previously reported for GB workflows with this technology [10]. fiKA was slightly more variable in midflexion and flexion; however, the differences in SDs were consistently below 0.5 mm, which is not likely to be clinically significant. These results indicate robot-assisted predictive iKA is well suited for achieving its target of a balanced soft tissue envelope throughout flexion.
The retrospective nature of this study is a limitation which can introduce various biases. To limit these, a consecutive group of TKAs were selected from each surgeon. Another limitation was the potential of surgeon-specific preferences affecting the results. However, both surgeons used a standardized technique with the same robotic system and the same cruciate-retaining implant. Another limitation is that because pGB was simulated, the results from this group did not consider any soft tissue releases that would directly impact the final laxity and balance. Because of this, piKA was used as the comparator instead of fiKA, as piKA also excludes the effects of soft tissue releases. Minimal soft tissue releases were recorded in the iKA technique, with only 1 posterior cruciate ligament release occurring across all cases. A limitation of the CPAK analysis in this study was not using X-ray imaging to calculate native CPAK and using an osteoarthritic wear correction factor based on the preopertive coronal HKA. However, the native estimate group corresponded well with the arthritic group in the study by MacDessi et al. in terms of mean MPTA (87 ± 3° vs 87 ± 2°) and mean LDFA (88 ± 3° vs 87 ± 2°) [12]. Furthermore, similar percentages of knees were observed in groups II, III, IV, VI, VII, VIII, and IX when comparing the native estimate with that of MacDessi et al. [12]. Greater discrepancies were observed in groups I and V; however, their combined totals were similar (Table 8). Lastly, the absence of clinical outcomes is a limitation of this study. However, this was not in the scope of the study, which aimed to investigate how restricted iKA impacts balance, laxity, JLO, and CPAK in comparison with a traditional GB approach. Future studies should compare how these techniques affect patient outcomes.
Table 8.
CPAK group percentages.
| Native vs MacDessi et al. | |||
|---|---|---|---|
| JLO (MPTA + LDFA) | aHKA (MPTA − LDFA) |
||
| Varus | Neutral ±2 | Valgus | |
| Apex distal JLO <177 | 33.3% vs 19.4% | 25% vs 32.2% | 16.7% vs 15.4% |
| Apex neutral JLO 180 ± 3 | 9.7% vs 9.8% | 4.2% vs 14.6% | 8.3% vs 7.4% |
| Apex proximal JLO >183 | 0% vs 0.6% | 1.4% vs 1.6% | 1.4% vs 0.4% |
Present study native (estimate) vs osteoarthritis group of the study by MacDessi et al. [12].
This is the first study to characterize the soft tissue balance and laxity profiles under constant ligament tension for iKA and compare them to simulated GB data. The results highlight that iKA achieves a well-balanced soft tissue envelope throughout flexion, while also restoring native JLO. This is an important result as it validates the iKA technique as a suitable option for the robot-assisted predictive balancing workflow.
Conclusions
Although piKA and pGB achieved similar ML balance, laxity profiles, and aHKA, piKA better restored the native joint line and CPAK phenotype with fewer knees changing CPAK type. piKA and fiKA also achieved similar ML balance and laxity profiles. Robot-assisted predictive iKA restores native JLO and achieves its ML soft tissue balance targets throughout flexion.
Conflicts of interest
A.D.O. is a paid employee of Corin. C.P. is a paid employee of Corin USA/Omni, has stock or stock options with Corin/Omni, and is in the International Society of Computer Assisted Orthopaedic Surgery Annual Meeting Scientific Review Committee. E.W. is a paid employee of Corin. S.M. receives royalties from Corin, is a paid consultant for Corin and Stryker, is an unpaid consultant for S&N, has stock or stock options with Corin, and receives research support as a principal investigator from Corin and Stryker. S.C. receives royalties from Corin Ltd., is a paid speaker for Corin and Zimmer Biomet, is a paid consultant for Corin and Zimmer Biomet, has shares in Austofix Pvt. Ltd., receives research support as a principal investigator from Corin, and is the National Chair of the Australian Society of Oerthopaedic Surgeons.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2022.101090.
Appendix A. Supplementary data
References
- 1.Oussedik S., Abdel M.P., Victor J., Pagnano M.W., Haddad F.S. Alignment in total knee arthroplasty. Bone Joint J. 2020;102-B:276–279. doi: 10.1302/0301-620X.102B3.BJJ-2019-1729. [DOI] [PubMed] [Google Scholar]
- 2.Dennis D.A., Komistek R.D., Kim R.H., Sharma A. Gap balancing versus measured resection technique for total knee arthroplasty. Clin Orthop Relat Res. 2010;468:102–107. doi: 10.1007/s11999-009-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y.S., Howell S.M., Won Y.Y., Lee O.S., Lee S.H., Vahedi H., et al. Kinematic alignment is a possible alternative to mechanical alignment in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25:3467–3479. doi: 10.1007/s00167-017-4558-y. [DOI] [PubMed] [Google Scholar]
- 4.Howell S.M. Calipered kinematically aligned total knee arthroplasty: an accurate technique that improves patient outcomes and implant survival. Orthopedics. 2019;42:126. doi: 10.3928/01477447-20190424-02. [DOI] [PubMed] [Google Scholar]
- 5.Howell S.M., Shelton T.J., Hull M.L. Implant survival and function ten years after kinematically aligned total knee arthroplasty. J Arthroplasty. 2018;33:3678–3684. doi: 10.1016/j.arth.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Wakelin E.A., Shalhoub S., Lawrence J.M., Keggi J.M., DeClaire J.H., Randall A.L., et al. Improved total knee arthroplasty pain outcome when joint gap targets are achieved throughout flexion. Knee Surg Sports Traumatol Arthrosc. 2021;30:939–947. doi: 10.1007/s00167-021-06482-2. [DOI] [PubMed] [Google Scholar]
- 7.Winnock de Grave P., Luyckx T., Claeys K., Tampere T., Kellens J., Muller J., et al. Higher satisfaction after total knee arthroplasty using restricted inverse kinematic alignment compared to adjusted mechanical alignment. Knee Surg Sports Traumatol Arthrosc. 2020;30:488–499. doi: 10.1007/s00167-020-06165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritter M.A., Davis K.E., Meding J.B., Pierson J.L., Berend M.E., Malinzak R.A. The effect of alignment and BMI on failure of total knee replacement. J Bone Joint Surg Am. 2011;93:1588–1596. doi: 10.2106/JBJS.J.00772. [DOI] [PubMed] [Google Scholar]
- 9.Fleming B.C., Beynnon B.D. In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng. 2004;32:318–328. doi: 10.1023/b:abme.0000017542.75080.86. [DOI] [PubMed] [Google Scholar]
- 10.Shalhoub S., Lawrence J.M., Keggi J.M., Randall A.L., DeClaire J.H., Plaskos C. Imageless, robotic-assisted total knee arthroplasty combined with a robotic tensioning system can help predict and achieve accurate postoperative ligament balance. Arthroplast Today. 2019;5:334–340. doi: 10.1016/j.artd.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orsi A.D., Wakelin E.A., Plaskos C., Gupta S., Sullivan J.A. Predictive gap-balancing reduces the extent of soft-tissue adjustment required after bony resection in robot-assisted total knee arthroplasty-A comparison with simulated measured resection. Arthroplast Today. 2022;16:1–8. doi: 10.1016/j.artd.2022.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDessi S.J., Griffiths-Jones W., Harris I.A., Bellemans J., Chen D.B. Coronal plane alignment of the knee (CPAK) classification. Bone Joint J. 2021;103-B:329–337. doi: 10.1302/0301-620X.103B2.BJJ-2020-1050.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDessi S.J., Allom R.J., Griffiths-Jones W., Chen D.B., Wood J.A., Bellemans J. The importance of joint line obliquity: a radiological analysis of restricted boundaries in normal knee phenotypes to inform surgical decision making in kinematically aligned total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2022;30:2931–2940. doi: 10.1007/s00167-022-06872-0. [DOI] [PubMed] [Google Scholar]
- 14.Sappey-Marinier E., Batailler C., Swan J., Schmidt A., Cheze L., MacDessi S.J., et al. Mechanical alignment for primary TKA may change both knee phenotype and joint line obliquity without influencing clinical outcomes: a study comparing restored and unrestored joint line obliquity. Knee Surg Sports Traumatol Arthrosc. 2021;30:2806–2814. doi: 10.1007/s00167-021-06674-w. [DOI] [PubMed] [Google Scholar]
- 15.An V.V.G., Twiggs J., Leie M., Fritsch B.A. Kinematic alignment is bone and soft tissue preserving compared to mechanical alignment in total knee arthroplasty. Knee. 2019;26:466–476. doi: 10.1016/j.knee.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Shatrov J., Murphy G.T., Duong J., Fritsch B. Robotic-assisted total knee arthroplasty with the OMNIBot platform: a review of the principles of use and outcomes. Arch Orthop Trauma Surg. 2021;141:2087–2096. doi: 10.1007/s00402-021-04173-8. [DOI] [PubMed] [Google Scholar]
- 17.Murgier J., Clatworthy M. Variable rotation of the femur does not affect outcome with patient specific alignment navigated balanced TKA. Knee Surg Sports Traumatol Arthrosc. 2020;30:517–526. doi: 10.1007/s00167-020-06226-8. [DOI] [PubMed] [Google Scholar]
- 18.Joseph J., Simpson P.M., Whitehouse S.L., English H.W., Donnelly W.J. The use of navigation to achieve soft tissue balance in total knee arthroplasty - a randomised clinical study. Knee. 2013;20:401–406. doi: 10.1016/j.knee.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 19.McEwen P., Balendra G., Doma K. Medial and lateral gap laxity differential in computer-assisted kinematic total knee arthroplasty. Bone Joint J. 2019;101-B:331–339. doi: 10.1302/0301-620X.101B3.BJJ-2018-0544.R1. [DOI] [PubMed] [Google Scholar]
- 20.Lee G.-C., Wakelin E., Plaskos C. What is the alignment and balance of a total knee arthroplasty performed using a calipered kinematic alignment technique? J Arthroplasty. 2022;37:S176–S181. doi: 10.1016/j.arth.2022.01.065. [DOI] [PubMed] [Google Scholar]
- 21.Nam D., Lin K.M., Howell S.M., Hull M.L. Femoral bone and cartilage wear is predictable at 0 degrees and 90 degrees in the osteoarthritic knee treated with total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:2975–2981. doi: 10.1007/s00167-014-3080-8. [DOI] [PubMed] [Google Scholar]
- 22.Mishra P., Pandey C.M., Singh U., Gupta A., Sahu C., Keshri A. Descriptive statistics and normality tests for statistical data. Ann Card Anaesth. 2019;22:67–72. doi: 10.4103/aca.ACA_157_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Development Core Team. R . R foundation for statistical computing. 2021. A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- 24.Winnock de Grave P., Kellens J., Luyckx T., Tampere T., Lacaze F., Claeys K. Inverse kinematic alignment for total knee arthroplasty. Orthop Traumatol Surg Res. 2022;108:103305. doi: 10.1016/j.otsr.2022.103305. [DOI] [PubMed] [Google Scholar]
- 25.Winnock de Grave P., Kellens J., Tampere T., Vermue H., Luyckx T., Claeys K. Clinical outcomes in TKA are enhanced by both robotic assistance and patient specific alignment: a comparative trial in 120 patients. Arch Orthop Trauma Surg. 2022 doi: 10.1007/s00402-022-04636-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Blakeney W., Clement J., Desmeules F., Hagemeister N., Riviere C., Vendittoli P.A. Kinematic alignment in total knee arthroplasty better reproduces normal gait than mechanical alignment. Knee Surg Sports Traumatol Arthrosc. 2019;27:1410–1417. doi: 10.1007/s00167-018-5174-1. [DOI] [PubMed] [Google Scholar]
- 27.Delport H.P., Vander Sloten J., Bellemans J. New possible pathways in improving outcome and patient satisfaction after TKA. Acta Orthop Belg. 2013;79:250–254. [PubMed] [Google Scholar]
- 28.Vigdorchik J.M., Wakelin E.A., Koenig J.A., Ponder C.E., Plaskos C., DeClaire J.H., et al. Impact of component alignment and soft tissue release on 2-year outcomes in total knee arthroplasty. J Arthroplasty. 2022;37:2035–2040.e5. doi: 10.1016/j.arth.2022.04.042. [DOI] [PubMed] [Google Scholar]
- 29.Orsi A.D., Wakelin E.A., Plaskos C., Petterwood J., Coffey S. Restricted kinematic alignment achieves similar relative lateral laxity and greater joint line obliquity compared to gap balancing TKA. Knee Surg Sports Traumatol Arthrosc. 2022;30:2922–2930. doi: 10.1007/s00167-022-06863-1. [DOI] [PubMed] [Google Scholar]
- 30.Peters C.L., Jimenez C., Erickson J., Anderson M.B., Pelt C.E. Lessons learned from selective soft-tissue release for gap balancing in primary total knee arthroplasty: an analysis of 1216 consecutive total knee arthroplasties: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95:e152. doi: 10.2106/JBJS.L.01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.