Abstract
Rationale & Objective
We sought to elicit patient preferences regarding the use of plasma exchange in antineutrophil cytoplasmic antibody-associated vasculitis (AAV) and its tradeoffs of risk of kidney failure and risk of serious infection.
Study Design
Patient survey.
Setting & Participants
The online survey was circulated to adults with AAV via kidney and vasculitis networks in Canada, the United Kingdom, and the United States.
Outcomes
Respondents reviewed the estimated 1-year risks of kidney failure and serious infection in AAV with and without plasma exchange across 5 serum creatinine categories (150, 250, 350, 450, and 600 μmol/L). For each scenario, participants indicated whether or not they would choose plasma exchange.
Analytical Approach
Responses were assessed with multilevel multivariable logistic regression models to identify predictors of respondent choice regarding treatment with plasma exchange.
Results
The 470 respondents from the 13 countries (United States 61.7%, United Kingdom 20.0%, Canada 13.8%, and other countries 4.5%) had a mean age of 58.6 (SD 14.3) years, 70.2% women. Respondents were more likely to choose plasma exchange in scenarios at high risk of kidney failure and serious infection (creatinine level of 350 or 450 μmol/L) compared with lower risk scenarios or the highest risk scenario. However, 145 (30.9%) chose plasma exchange across all scenarios, whereas 80 (17.0%) declined plasma exchange across all scenarios. Respondents from the United Kingdom (OR, 2.61; 95% CI, 1.09-6.22) who received previous dialysis (OR, 2.70; 95% CI, 1.12-6.52) or received previous plasma exchange (OR, 5.62; 95% CI, 2.72-11.61) were more likely to choose plasma exchange, whereas older respondents (OR, 0.98; 95% CI, 0.96-0.99 per 1 year increase) were less likely.
Limitations
Unclear generalizability to non–English-speaking, older, and less health literate adults, possible responder bias, survivor bias, lack of individualized risk assessments for kidney failure, and serious infection.
Conclusions
Patients with AAV do not express a consistent choice for plasma exchange, which highlights the need for shared decision making.
Index Words: ANCA-associated vasculitis, plasma exchange, survey
Plain Language Summary.
Recent evidence shows that plasma exchange decreases the risk of kidney failure and increases the risk of serious infection but does not affect overall mortality in antineutrophil cytoplasmic antibody-associated vasculitis (AAV). In this online patient survey that was circulated to adults with AAV via kidney and vasculitis networks, 470 respondents reviewed 5 scenarios with varying estimated 1-year risks of kidney failure and serious infection with and without plasma exchange based on baseline serum creatinine levels. In each scenario, respondents were asked whether they would choose treatment with plasma exchange. We identified independent predictors of choosing treatment with plasma exchange, including age, country, kidney function, and previous receipt of dialysis/plasma exchange, but overall, there was heterogeneity in treatment decisions, which highlights the need for individualized shared decision making.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare1 multisystem disease2 with a mortality of up to 25% at 4.5 years3 due to active vasculitis or adverse events related to immunosuppression.4 Kidney involvement is common in AAV5 and can result in kidney failure requiring dialysis or kidney transplantation.6 Both kidney and lung involvement are associated with increased morbidity and mortality in patients with AAV.7
AAV is initially treated with immunosuppression to control the disease and prevent progressive organ damage.8, 9, 10, 11, 12, 13, 14, 15, 16 Plasma exchange is a method of mechanically removing antibodies, such as ANCA, and is an adjunctive treatment for AAV that involves the replacement of a patient’s blood volume to rapidly decrease ANCA, which is are thought to drive disease manifestations.17,18 After the recent publication of plasma exchange and glucocorticoids in severe ANCA-associated vasculitis (PEXIVAS),19 we completed an updated systematic review/meta-analysis of plasma exchange in AAV20 that informed a clinical practice guideline.21 In this meta-analysis, plasma exchange proved to have no impact on mortality but decreased the risk of kidney failure while increasing the risk of serious infection.
As a part of the guideline process, engaging 4 patient partners with AAV and one of their caregivers helped inform patient values and preferences regarding plasma exchange.22 In the Grading of Recommendations, Assessment, Development and Evaluations process, uncertainty and variability in patient values and preferences affect the strength of recommendations.23 We explored patient and caregiver treatment decisions for plasma exchange with regards to its benefits and harms at different baseline risks of kidney failure and serious infection. This informed the strength of our recommendations for plasma exchange in AAV across the spectrum of risk with a strong recommendation encompassing >90% of fully informed patients and a weak (conditional) recommendation encompassing 50%-90% of fully informed patients. To better understand treatment decisions for plasma exchange in AAV and thus to inform clinical practice and future guideline recommendations, we conducted an international survey of a larger number of adult patients with AAV.
Methods
Survey Design and Target Audience
Two authors (DC and MW) designed the survey and piloted it with 8 other authors (AM, ML, RAM, LF, AM, GG, DJ, PM) for sensibility and comprehension. The survey was targeted to English-speaking adults with a self-reported clinical diagnosis of AAV (granulomatosis with polyangiitis, or microscopic polyangiitis but not eosinophilic granulomatosis with polyangiitis). To reach these patients, we circulated the survey via kidney and vasculitis network email lists, an online research network, websites, and social media, including the Kidney Foundation of Canada (October 2020), Canadians Seeking Innovations and Solutions to Solve Chronic Kidney Disease network (October 2020), Vasculitis Foundation Canada (January 2021), Vasculitis UK (October 2020), and the Vasculitis Patient-Powered Research Network (V-PPRN) (December 2020). To increase survey responses, patient partners created patient-facing communications that we circulated to the above sources. The Hamilton Integrated Research Ethics Board approved the study (project number 11567). Respondents provided informed consent before beginning the anonymous survey that required answers to each question.
Patient Involvement
Patients were involved in the design and conduct of this research. Four patient partners (MF, PB, MB, TF) and a caregiver (LF) reviewed the penultimate version on the survey platform, surveymonkey.com, and suggested revisions for the final survey (see Item S1). They also circulated the survey to their respective patient networks via social media. Once the study has been published, participants and patient networks will be sent details of the results in a study newsletter developed by patient partners.
Survey Content
Respondents provided their age, sex, country, type of AAV, and degree of kidney involvement (previous kidney disease, previous dialysis, current dialysis, or kidney transplant). Respondents also provided whether they had ever received treatment plasma exchange or treatment with intravenous antibiotics and/or had been admitted to a hospital for a serious infection.
The survey provided general information about plasma exchange, including its purpose, setting, duration, vascular access, and some of its potential harms (ie, infection, bleeding, and pneumothorax). After informing respondents that plasma exchange had no overall impact on mortality, participants chose or declined plasma exchange treatment for each of 5 scenarios in which 1-year risks of kidney failure and serious infection requiring intravenous antibiotics or hospitalization with and without plasma exchange were given. The risk estimates were derived from European Vasculitis Study Group trials and a meta-analysis of plasma exchange in AAV and approximately corresponded to the risks of kidney failure and infection seen at varying serum creatinine levels (Cr; 150, 250, 350, 450, and 600 μmol/L) at the time treatment was started (Table 1).20 Respondents were randomly selected to receive the 5 scenarios in either order of increasing or decreasing risk. Importantly, as the risk of kidney failure increased, so did the risk of serious infection and so too did the absolute risk differences with plasma exchange (ie, the benefits and risks of plasma exchange increased in parallel). Risk was communicated using pictographs for each scenario (see Item S1). For each of these 5 scenarios, participants were asked, if they were a patient with a new diagnosis or relapse of AAV (with no mention of pulmonary hemorrhage), given its absolute risk decrease in kidney failure but absolute risk increase in serious infection, would they choose treatment with plasma exchange (yes or no).
Table 1.
Risks of Kidney Failure and Serious Infection at 1 Year With or Without the Plasma Exchange Across the 5 Scenarios
|
Serum Creatinine (μmol/L) |
Kidney Failure at 1 Year |
Serious Infection at 1 Year |
||||
|---|---|---|---|---|---|---|
| Without Plasma Exchange | With Plasma Exchange | Risk Difference | Without Plasma Exchange | With Plasma Exchange | Risk Difference | |
| 150 | 2/100 | 1/100 | −1/100 | 9/100 | 11/100 | +2/100 |
| 250 | 8/100 | 5/100 | −3/100 | 18/100 | 23/100 | +5/100 |
| 350 | 15/100 | 9/100 | −6/100 | 27/100 | 34/100 | +7/100 |
| 450 | 28/100 | 17/100 | −9/100 | 36/100 | 46/100 | +10/100 |
| 600 | 40/100 | 25/100 | −15/100 | 50/100 | 64/100 | +14/100 |
Note: The risk estimates were derived from European Vasculitis Study Group trials and a meta-analysis of plasma exchange in antineutrophil cytoplasmic antibody-associated vasculitis.20
Statistical Analysis
We summarized respondent characteristics and responses with means (standard deviations), medians (25th-75th percentiles), and frequencies/percentages as appropriate. Respondents were categorized into the following 5 groups: (1) those who chose plasma exchange in all scenarios; (2) those who declined plasma exchange in all scenarios; (3) those who chose plasma exchange only at higher baseline absolute risks (ie, they declined plasma exchange at lower serum Cr levels then accepted at higher serum Cr levels); (4) those who declined plasma exchange only at higher baseline absolute risks (ie, they accepted plasma exchange at lower serum Cr levels then declined at higher serum Cr levels); (5) those with other treatment decision patterns. Groups were compared using analysis of variance for continuous variables and the χ2 test for categorical variables.
We constructed multilevel multivariable logistic regression models to evaluate the association between the decision to receive treatment with plasma exchange (outcome).24 Models included age, sex, country, previous dialysis, previous plasma exchange, previous serious infection, randomization, and scenario sequence as fixed effects (predictors)25 and participants as random intercepts without any interactions.26 Odds ratios (OR) and 95% confidence intervals (CIs) are presented. A sensitivity analysis included history of kidney disease or current treatment with dialysis or a kidney transplant as an alternative to use of history of dialysis. Only participants without any missing data were included without any imputation.
All statistical tests were performed using Stata release 14 (StataCorp) with a P value of <0.05 level of significance without adjustment for multiplicity.
Results
Due to the nature of the sampling strategy, a response rate could only be calculated for the V-PPRN. The V-PPRN sent a recruitment email or newsletter to 1,730 and 10,449 unique email addresses, respectively, of which 616/1,730 (35.6%) and 2,526/10,449 (24.2%) opened the emails and 247/1,730 (14.3%) and 104/10,449 (1.0%) opened the survey. Of these, a total of 311/351 (88.6% of those that opened the survey) were eligible and completed the survey.
Of 703 total respondents, 99 were excluded (8 were not interested in participating, 1 did not provide informed consent, 11 had completed the survey previously, 45 had non-AAV vasculitides, 15 had eosinophilic granulomatosis with polyangiitis, 10 had kidney disease but no vasculitis, and 9 were uncertain as to what type of vasculitis they had). Of the 604 eligible participants, 134 had incomplete data, leaving 470 respondents.
Table 2 summarizes respondent characteristics. Typical respondents were women, in their late 50s, from the United States, had a diagnosis of granulomatosis with polyangiitis, and had not previously or presently treated with dialysis or plasma exchange. Approximately half had previously experienced a serious infection.
Table 2.
Survey Respondent Characteristics (N = 470)
| Characteristic | Value |
|---|---|
| Age, year (SD) | 58.6 (14.3) |
| Sex Female, n (%) | 330 (70.2%) |
| Country, n (%) | |
| Canada | 65 (13.8%) |
| United Kingdom | 94 (20.0%) |
| United States | 290 (61.7%) |
| Othera (%) | 21 (4.5%) |
| Diagnosis, n (%) | |
| AAV | 55 (11.7%) |
| GPA | 334 (71.1%) |
| MPA | 81 (17.2%) |
| Kidney disease, n (%) | 302 (64.3%) |
| Previous dialysis, n (%) | 62 (13.2%) |
| Current dialysis/transplant, n (%) | 28 (6.0%) |
| Previous plasma exchange, n (%) | 100 (21.3%) |
| Previous serious infection, n (%) | 234 (49.8%) |
Abbreviations: AAV, antineutrophil cytoplasmic antibody-associated vasculitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis, SD, standard deviation.
Australia (n = 7), Germany (n = 3), Ireland (n = 3), South Africa (n = 2), New Zealand (n = 1), Romania (n = 1), France (n = 1), India (n = 1), Italy (n = 1), and Slovenia (n = 1).
Table 3 summarizes participant responses for each scenario. In scenarios 1, 2, 3, 4, and 5, 54.9%, 56.0%, 63.4%, 61.1%, and 57.9% of participants, respectively, chose treatment with plasma exchange. Of the 470 respondents, 145 (30.9%) consistently chose plasma exchange across all scenarios, and 80 (17.0%) declined plasma exchange across all scenarios. One hundred three (21.9%) chose plasma exchange at higher baseline absolute risks (30 at Cr ≥250 μmol/L, 36 at Cr ≥350 μmol/L, 19 at Cr ≥450 μmol/L, and 18 at Cr ≥600 μmol/L), 92 (19.6%) declined plasma exchange at higher baseline absolute risks (19 at ≤Cr 250 μmol/L, 25 at Cr ≤350 μmol/L, 19 at Cr ≤450 μmol/L, and 29 at Cr 600 μmol/L≤), and 50 (10.6%) had other treatment decision patterns. Age, country, previous treatment with dialysis, current treatment with dialysis or kidney transplant, and previous plasma exchange varied between participants with different patterns of response (P < 0.05) but not sex, history of kidney disease, or history of serious infection.
Table 3.
Survey Respondent Characteristics by Response Categories
| Always Chooses Plasma Exchange | Always Declines Plasma Exchange | Chooses Plasma Exchange Only at Higher Risk | Declines Plasma Exchange Only at Higher Risk | Other Patterns | P | |
|---|---|---|---|---|---|---|
| Total | 145 (30.9%) | 80 (17.0%) | 103 (21.9%) | 92 (19.6%) | 50 (10.6%) | |
| Age, years (SD) | 57.3 (14.4) | 63.2 (13.6) | 59.0 (12.8) | 54.8 (15.8) | 61.2 (13.3) | <0.01 |
| Female sex, n (%) | 98 (67.6%) | 57 (71.3%) | 73 (70.9%) | 69 (75.0%) | 33(66.0%) | 0.57 |
| Country, n (%) | ||||||
| Canada | 17 (11.7%) | 12 (15.0%) | 18 (17.5%) | 10 (10.9%) | 8 (16.0%) | <0.01 |
| United Kingdom | 45 (31.0%) | 5 (6.3%) | 14 (13.6%) | 23 (25.0%) | 7 (14.0%) | |
| United States | 77 (53.1%) | 60 (75.0%) | 66 (64.1%) | 54 (58.7%) | 33 (66.0%) | |
| Other | 6 (4.1%) | 3 (3.8%) | 5 (4.9%) | 5 (5.4%) | 2 (4.0%) | |
| Kidney disease, n (%) (N = 302) |
103 (71.0%) | 42 (52.5%) | 69 (67.0%) | 60 (65.2%) | 28 (56.0%) | 0.05 |
| Previous dialysis, n (%) (N = 62) |
34 (23.4%) | 4 (5.0%) | 13 (12.6%) | 9 (9.8%) | 2 (4.0%) | <0.01 |
| Current dialysis/transplant, n (%) (N = 28) |
15 (10.3%) | 0 (0%) | 6 (5.8%) | 6 (6.5%) | 1 (2.0%) | 0.02 |
| Previous plasma exchange, n (%) (N = 100) |
54 (37.2%) | 3 (3.8%) | 16 (15.5%) | 18 (19.6%) | 9 (18.0%) | <0.01 |
| Previous infection, n (%) (N = 234) |
73 (50.3%) | 36 (45.0%) | 45 (43.7%) | 52 (56.5%) | 28 (56.0%) | 0.32 |
Abbreviation: SD, standard deviation.
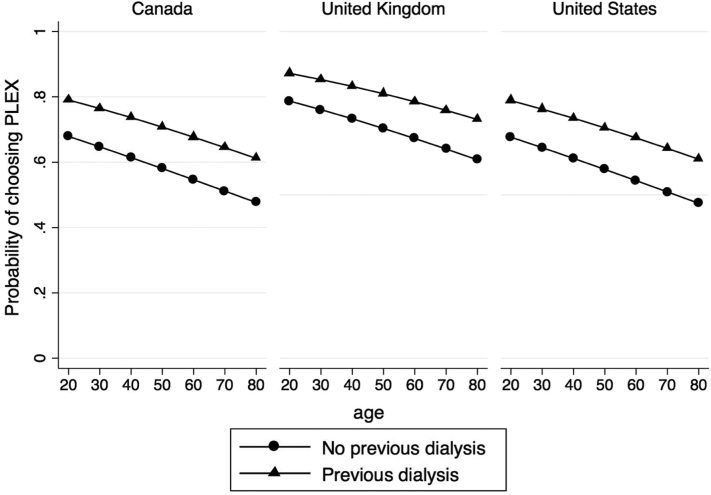
Table 4 presents the results of the multilevel multivariable logistic regression model used to identify characteristics associated with choosing plasma exchange. Younger age (OR, 0.98; 95% CI, 0.96-0.99 per 1 year increase), country (United Kingdom, OR, 2.61; 95% CI, 1.09-6.22), previous dialysis (OR, 2.70; 95% CI, 1.12-6.52), previous plasma exchange (OR, 5.62; 95% CI, 2.72-11.61), and scenarios 3 (serum Cr level, 350 μmol/L; OR, 1.93; 95% CI, 1.35-2.77) and 4 (serum Cr level, 450 μmol/L; OR, 1.61; 95% CI, 1.13-2.29) associated with choosing plasma exchange. Figure 1 shows the estimated probabilities of choosing plasma exchange by age, country, and previous dialysis and adjusted for other characteristics.
Table 4.
Multilevel Multivariable Logistic Regression Model for Choosing Plasma Exchange
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Age (per 1 y increase) | 0.98 | 0.96 | 0.99 | <0.01 |
| Male sex | 1.25 | 0.73 | 2.15 | 0.41 |
| Country (reference = Canada) | ||||
| Other | 0.74 | 0.20 | 2.74 | 0.66 |
| United Kingdom | 2.61 | 1.09 | 6.22 | 0.03 |
| United States | 0.98 | 0.48 | 1.99 | 0.96 |
| Previous dialysis | 2.70 | 1.12 | 6.52 | 0.03 |
| Previous plasma exchange | 5.62 | 2.72 | 11.61 | <0.01 |
| Previous infection | 1.14 | 0.70 | 1.85 | 0.60 |
| Scenario reference = scenario 1 (serum creatinine level, 150 μmol/L) | ||||
| scenario 2 (serum creatinine level, 250 μmol/L) | 1.08 | 0.76 | 1.54 | 0.65 |
| scenario 3 (serum creatinine level, 350 μmol/L) | 1.93 | 1.35 | 2.77 | <0.01 |
| scenario 4 (serum creatinine level, 450 μmol/L) | 1.61 | 1.13 | 2.29 | <0.01 |
| scenario 5 (serum creatinine level, 600 μmol/L) | 1.26 | 0.88 | 1.79 | 0.21 |
Note: Scenarios 1, 2, 3, 4, 5 are the 5 different cases with increasing baseline risks of dialysis and serious infection.
Abbreviations: CI, confidence interval; OR, odds ratio.
Figure 1.
Point estimates of the probability of choosing plasma exchange (PLEX) by age, country, and previous dialysis. Estimates derived by multivariable model, including age, sex, country, previous dialysis, previous plasma exchange, previous serious infection, randomization, and scenario.
Sensitivity analyses using history of kidney disease or current treatment with dialysis/kidney transplantation instead of previous did not materially change the results (Tables S1 and S2).
Discussion
In this online survey of 470 patients recruited from patient networks, almost one-third of respondents chose plasma exchange regardless of the risk of kidney failure and serious infection, one-fifth chose plasma exchange only at higher risks of kidney failure and serious infections, and one-sixth declined plasma exchange across all scenarios. The most important predictors of choosing treatment with plasma exchange were previous treatment with dialysis, previous treatment with plasma exchange, baseline risk of kidney failure/serious infection, and respondent’s country. Our findings that previous treatment with dialysis or plasma exchange were predictors of choosing treatment with plasma exchange are not surprising given likely response/survivor bias and the harms associated with dialysis, but the rationale for why being from the United Kingdom is also a significant predictor is uncertain. Unfortunately, we did not collect detailed participant information from this group, including geography or health care center information or participating in previous trials, but this is presumably related to local practice, implementation, and knowledge translation of previous trials, including plasma exchange for renal vasculitis and PEXIVAS.
The results of the study have important clinical implications. Notably, there was considerable variability in patients’ choices. A high proportion of patients declined plasma exchange under all presented scenarios or at higher absolute risks of kidney failure and serious infection (∼40% of respondents). This may be because of risks of plasma exchange, most notably serious infection, but also potentially vascular access, other complications, or perceived low risks of kidney failure (and therefore relatively small perceived benefit of plasma exchange). This supports our clinical practice guideline weak recommendations (ie, 50%-90% of fully informed patients would choose instead of >90%, which is a strong recommendation) against plasma exchange in patients with low or low-moderate risk of developing kidney failure and in favor of plasma exchange in patients moderate-high or high risk of kidney failure, which were developed with input from clinicians and only 4 patient partners and 1 caregiver.21 This demonstrates the need to engage in shared decision making when considering plasma exchange in AAV. This includes discussing plasma exchange with patients who are potentially at lower risk of kidney failure where some clinicians may not believe the potential benefits justify the potential harms or in patients where clinicians believe that the use of plasma exchange is mandatory.
Few studies previously assessed patients’ values and preferences regarding plasma exchange in AAV. As such, our study is novel and its strengths include a large sample size, international recruitment, and the involvement of patient partners to ensure the relevance and clarity of the survey. However, this study also has several limitations. The study included only respondents able to read English from mostly North America and the United Kingdom with an established diagnosis of vasculitis that was mostly granulomatosis with polyangiitis and not microscopic polyangiitis. This is potentially important because we detected some differences by country despite the relative similarity of participants included in our study, and it is possible values and preferences will vary more across more dissimilar countries. Our survey was conducted online, which may select for younger respondents with a higher degree of health literacy, the ability to comprehend complex scenarios, and the benefit/risk tradeoffs of plasma exchange. It also did not account for responder bias beyond the previous receipt of plasma exchange or dialysis (who are arguably the most informed population) or participant preconceived notions regarding the efficacy and safety of plasma exchange based on previous discussions with their clinicians or their interpretation of PEXIVAS19 outside of the survey. Furthermore, our results may be limited by survivor bias, because those respondents who previously received plasma exchange (as well as those who did not) and experienced a favorable outcome would conceivably likely make the same decision again in this survey. Again, the relative narrowness of the sample may limit generalizability but, because we found little consistency in the choice of plasma exchange even among our respondents, this reinforces the need to discuss values and preferences with patients when deciding whether to use plasma exchange in AAV. The survey was not anchored to an actual treatment decision and participants did not have the opportunity to discuss treatment decisions in detail with their physician, so preferences in the survey may not reflect decisions made in actual clinical scenarios, and we cannot predict whether or not such decisions would be more or less uniform compared with our survey scenarios. Furthermore, we did not include detailed information regarding all the possible risks of plasma exchange and the potential impact and consequences of either kidney failure or serious infection, and it is acknowledged that even for these 2 important patient outcomes, there is heterogeneity across patient factors, including age, sex/gender, comorbid conditions, and cointerventions that are not accounted for in the scenarios presented, which are derived from trial population-based data. The importance of this in decision making was commented on by some participants. However, this is not dissimilar to discussing treatment options in clinical scenarios because of the heterogeneity in frequency and severity of these events. Lastly, although the baseline risks of kidney failure and serious infection used in the survey were based on contemporary cohorts, this may change in the future given the development of novel immunosuppression and steroid sparing therapies, such as avacopan or multitarget approaches, which may modify the absolute risks (by modifying baseline risks or through interactions with plasma exchange, although unlikely) and influence decision making.
In summary, the decision to use plasma exchange in AAV based on its potential to reduce the risk of kidney failure while increasing the risk of serious infection varies substantially between patients. Clinicians treating patients with AAV at risk of kidney failure who could receive plasma exchange should engage in shared decision making to understand their patient’s values and preferences to ensure a patient-centered choice is made.
Article Information
Authors’ Full Names and Academic Degrees
David Collister, MD, PhD, Mark Farrar, Lesha Farrar, Paul Brown, Michelle Booth, Tracy Firth, Alfred Mahr, MD, MPH, PhD, Linan Zeng, MD, Mark A. Little, MD, Reem A. Mustafa, MD, MPH, PhD, Lynn A. Fussner, MD, Alexa Meara, MD, Gordon Guyatt, MD, MSc, David Jayne, MD, Peter A. Merkel, MD, MPH, and Michael Walsh, MD, PhD.
Authors’ Contributions
Research area and study design (DC, MF, LF, PB, MB, TF, AMahr, LZ, MAL, RAM, LAF, AMeara, GG, DJ, PAM, MW), data acquisition (MF, LF, PB, MB, TF, PM), data analysis and interpretation (DC), statistical analysis (DC), supervision and mentorship (MW). Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The Vasculitis Patient-Powered Research Network was supported through a Patient-Centered Outcomes Research Institute (PCORI) award (PPRN-1306-04758) and the Vasculitis Clinical Research Consortium (VCRC). The VCRC is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Science (NCATS). The VCRC is funded through a collaboration between NCATS and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54 AR057319). The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We would like to thank all participants and all patient networks involved in the study.
Peer Review
Received June 24, 2022 as a submission to the expedited consideration track with 4 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form October 23, 2022.
Footnotes
Complete author and article information provided before references.
Item S1: Patient Survey
Table S1: Multilevel Multivariable Logistic Regression Model for Choosing Plasma Exchange Using Previous Kidney Disease Instead of Dialysis as a Predictor
Table S2: Multilevel Multivariable Logistic Regression Model for Choosing Plasma Exchange Using Current Dialysis or Transplant Instead of Previous Dialysis as a Predictor
Supplementary Material
Item S1; Table S1-S2.
References
- 1.Watts R.A., Mooney J., Skinner J., Scott D.G., Macgregor A.J. The contrasting epidemiology of granulomatosis with polyangiitis (Wegener's) and microscopic polyangiitis. Rheumatology (Oxford) 2012;51(5):926–931. doi: 10.1093/rheumatology/ker454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette J.C., Falk R.J., Gasim A.H. Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens. 2011;20(3):263–270. doi: 10.1097/MNH.0b013e3283456731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flossmann O., Berden A., de Groot K., et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70(3):488–494. doi: 10.1136/ard.2010.137778. [DOI] [PubMed] [Google Scholar]
- 4.Little M.A., Nightingale P., Verburgh C.A., et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis. 2010;69(6):1036–1043. doi: 10.1136/ard.2009.109389. [DOI] [PubMed] [Google Scholar]
- 5.Fauci A.S., Haynes B.F., Katz P., Wolff S.M. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98(1):76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 6.Berti A., Cornec-Le Gall E., Cornec D., et al. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. 2019;34(9):1508–1517. doi: 10.1093/ndt/gfy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J.A., Dehghan N., Chen W., Xie H., Esdaile J.M., Avina-Zubieta J.A. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis. 2017;76(9):1566–1574. doi: 10.1136/annrheumdis-2016-210942. [DOI] [PubMed] [Google Scholar]
- 8.de Groot K., Adu D., Savage C.O. The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant. 2001;16(10):2018–2027. doi: 10.1093/ndt/16.10.2018. [DOI] [PubMed] [Google Scholar]
- 9.de Groot K., Harper L., Jayne D.R., et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150(10):670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 10.Jones R.B., Hiemstra T.F., Ballarin J., et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. 2019;78(3):399–405. doi: 10.1136/annrheumdis-2018-214245. [DOI] [PubMed] [Google Scholar]
- 11.Jones R.B., Tervaert J.W., Hauser T., et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 12.Stone J.H., Merkel P.A., Spiera R., et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillevin L., Pagnoux C., Karras A., et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 14.Vandergheynst F. Rituximab or azathioprine maintenance in ANCA-associated vasculitis. N Engl J Med. 2015;372(4):385–386. doi: 10.1056/NEJMc1414728. [DOI] [PubMed] [Google Scholar]
- 15.Hiemstra T.F., Walsh M., Mahr A., et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304(21):2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 16.Pagnoux C., Mahr A., Hamidou M.A., et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359(26):2790–2803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 17.Walsh M., Catapano F., Szpirt W., et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis. 2011;57(4):566–574. doi: 10.1053/j.ajkd.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennette J.C., Falk R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463–473. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- 19.Walsh M., Merkel P.A., Peh C.A., et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–631. doi: 10.1056/NEJMoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh M., Collister D., Zeng L., et al. The effects of plasma exchange in patients with ANCA-associated vasculitis: an updated systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-064604. [DOI] [PubMed] [Google Scholar]
- 21.Zeng L., Walsh M., Guyatt G.H., et al. Plasma exchange and glucocorticoid dosing for patients with ANCA-associated vasculitis: a clinical practice guideline. BMJ. 2022;376 doi: 10.1136/bmj-2021-064597. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Kunz R., et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Andrews J.C., Schünemann H.J., Oxman A.D., et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15(5):625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]
- 25.Steyerberg E.W., Eijkemans M.J., Habbema J.D. Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol. 1999;52(10):935–942. doi: 10.1016/s0895-4356(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 26.Austin P.C., Steyerberg E.W. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol. 2015;68(6):627–636. doi: 10.1016/j.jclinepi.2014.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1; Table S1-S2.