Abstract
The purpose of this study was to compare the efficacy of oral triclofos (TRI), intranasal midazolam (INM), and intranasal dexmedetomidine (IND) in achieving successful sedation in children undergoing MRI. This open-label, three-arm, randomized trial was conducted in a tertiary care teaching hospital over 18-month period. Children scheduled for MRI were enrolled. Rate of successful/adequate sedation was assessed using the Paediatric Sedation State Scale (PSSS). The primary outcome was the efficacy (successful sedation or sedation rate) of the three drugs. One-hundred and ninety-five children were included for the MRI procedure. IND was found to be superior in terms of achieving successful sedation. INM had a shorter onset and duration of sedation compared to IND and TRI, but with an increased failure rate (88.3%). Keeping INM as the reference group, it was found that the odds of sedation increased 4.1 times on changing from INM to IND (p < 0.01), and 2.26 times on changing from INM to TRI (p < 0.01). Adverse events included nasal discomfort (18.3%) in INM group; and self-limited tachycardia (4.6%) and hypotension (10.8%) in the IND group.
Conclusion: IND was more efficacious than INM or TRI for procedural sedation in children undergoing MRI without any significant adverse events.
Clinical trial registration: CTRI/2019/01/017257; date registered: 25/01/2019.
|
What is Known: • Oral triclofos (TRI) and intranasal midazolam (INM) have been used for procedural sedation in children undergoing MRI with variable success; but the experience with intranasal dexmedetomidine (IND) is limited. | |
|
What is New: • IND provides more effective sedation compared to INM or TRI for procedural sedation in children undergoing MRI, without any significant adverse events. |
Keywords: Sedation, Pediatric, Magnetic resonance imaging, Benzodiazepine, α-2 agonist, Chloral hydrate
Introduction
Procedural sedation in children for non-invasive purposes like imaging requires a moderate to deep sedation wherein the child remains in a less responsive state with the ability to independently maintain ventilation with minimal requirement of resuscitative measures [1]. Anxiety and fear of injection, or painful invasive procedures in children lead to a physiological surge of catecholamine, thereby making the experience stressful for the child and the clinician [2]. Hence, obtaining adequate magnetic resonance imaging (MRI) for different diagnostic purposes warrants the patient’s co-operation in terms of long motion-free periods of around 30 to 45 min, depending on the anatomical area selected. Motion artifacts and high decibel sound created by the magnetic field affects the quality of the imaging, and hence the diagnostic yield [3]. Since the 1980s, the standard sedation practices for MRI are the use of injectable medications that require the presence of an experienced pediatric anesthetist [4]. The oral administration of sedative drugs is an economic and oldest method of sedation, sometimes used an alternative to injectable medications [5].
The use of triclofos in infants and children undergoing painless diagnostic procedures including MRI has been studied, with evidence of efficacy and safety [6]. However, there is a dearth of data comparing it with newer non-injectable medications [7]. Intranasal route is a non-invasive novel drug administration and absorption method, bypassing the first-pass metabolism. Moreover, absorption rates and blood levels are better than oral administration [6]. Compared to intravenous administration, the intranasal route shows decreased serum concentrations with lower plasma peaks and a reduced incidence of adverse effects [6]. Midazolam acting on the GABA-A receptor is a versatile drug available for different routes of administration. Apart from sedation, it has anxiolytic, anticonvulsive, ante-grade amnestic, and muscle relaxant properties [8]. It has been tried via intranasal route apart from the routine intravenous administration with varying degrees of success as a sedative in children undergoing MRI [9]. Dexmedetomidine, a selective alpha-2 adreno-receptor agonist, has sedative, anxiolytic, and analgesic properties. It does not depress the respiratory center, making it a good replacement for the above medications. It also induces sedation which parallels natural sleep. However, there is limited data and experience from low- and middle-income countries (LMICs) on the intranasal use of dexmedetomidine as procedural sedation in children undergoing MRI [9, 10].
With this background, we conducted the present study comparing the efficacy of oral triclofos (TRI), intranasal midazolam (INM), and intranasal dexmedetomidine (IND) in achieving successful sedation during MRI, while monitoring the safety and their application in the hands of pediatricians.
Methods
This open-label, randomized, parallel group, three-arm trial was approved by the Institute Ethics Committee and was registered in the Clinical Trial Registry India (CTRI/2019/01/017257). The study was conducted from January 2019 to June 2020 and included children of 6 months to 10 years having ASA physical status I/II, and undergoing MRI. Children with upper respiratory tract infection (URTI), epistaxis or acute rhinitis, severe cardio-respiratory failure and difficult airway, and h/o allergy to the study drugs were excluded. Informed written consent from parents and assent from children of > 8 years of age were obtained prior to enrollment.
Children were evaluated with a history and physical examination before the procedure as a part of pre-procedural preparation. They were kept nil per oral (NPO) for 8 h for solid food, 4 h for breast milk, and 2 h for clear fluids as per the American College of Physicians (ACP) guidelines for Procedural Sedation Analgesia [11].
Computer-generated randomization was done with the list being prepared from the website “www.randomisation.com.” Allocation concealment was done by serially numbered opaque sealed envelope (SNOSE) technique. Children were randomized to one of the following three groups. INM group received midazolam (Neon Laboratories Ltd, Mumbai, India) as nasal spray at a dose of 0.5 mg/kg (maximum dose = 10 mg/day). IND group received nasal drops of dexmedetomidine (Neon Laboratories Ltd, Mumbai, India) (administered through a 1-ml syringe inserted 0.5 cm into nostril) at a dose of 3 µg/kg, and TRI group received oral triclofos (Syrup Pedicloryl, Dr. Reddy’s Laboratories, Hyderabad, India) at a dose of 100 mg/kg/day (maximum dose = 2000 mg/day). The drugs were administered 40 min before the MRI, and baseline vital parameters were recorded. Pulse rate, respiratory rate, and SpO2 were continuously monitored and recorded every 10 min. The lowest SpO2 was recorded; hypoxemia was considered when SpO2 is < 92%. Blood pressure was monitored at the start and the end of the procedure. Any hypotension, respiratory depression/distress, bradycardia, and hypoxemia were recorded per standard pediatric protocol. Additional adverse events like vomiting, nasal irritation, or any other complications were also recorded. All children were observed post-procedure with vitals monitoring every 10 min till discharge criteria were met. However, a qualified anesthetist was available during the procedure and in case of occurrence of any adverse event, the child was rescued and managed as per standard institute practice by the investigators who were trained pediatricians.
Rate of successful/adequate sedation was assessed using the Paediatric Sedation State Scale (PSSS) [12]. Typically, a PSSS of < 4 is considered apt to tolerate diagnostic imaging studies. Children were assessed for PSSS every 5 min from administering the drug and post-procedure every 10 min till 30 min. Vitals were recorded at the start and every 10 min till discharge criteria were met. The “time of onset of sedation” was defined as the time from administration of the study drug to the time of achievement of a PSSS of < 4. The time of administration of the drug was taken as 0. “Duration of sedation” was the time from the onset to the emergence from sedation (achievement of a PSSS of ≥ 4). Discharge criteria after MRI were according to the American College of Emergency Physician guidelines. The duration of sedation was calculated as the difference between the end and start times. MRI (1.5 Tesla, MAGNETOM Aera, SIEMENS Healthineers, Germany) was performed in children with PSSS < 4, without simultaneous addition of an intravenous sedative. If at any time during the procedure there was any movement or decrease in sedation depth (PSSS ≥ 4), rescue sedation was given as per anesthesia protocol of the institute (intravenous Ketamine). The duration of a diagnostic MRI sequence depends on the organ or body part being imaged (varies from 20 to 60 min). The peak noise level measured during the noisiest sequence of a 1.5 Tesla MRI has been shown to vary from 101 to 117 decibel (dB) [13]. Ear protectors were used in all children to reduce distraction and protect against damage by the MRI noise.
The primary outcome was the efficacy (successful sedation or sedation rate) of oral triclofos and intranasal midazolam and dexmedetomidine in completing MRI procedure. It was defined as the acquisition of an adequate MRI for confident reporting by the radiologist. In case of failure, the cause was reviewed and rescue medication was given to complete the procedure. The secondary outcomes were other efficacy parameters (the time of onset of sedation, duration of sedation), and adverse events. The outcome assessor and the statistician were blinded keeping in mind the subjectivity of PSSS score.
Statistical analysis
To detect a difference in the “success rate” of 25% in sedating the participants, a sample size of 79 is required in each group (total = 237) considering a baseline success rate of 60%. This sample size is powered to 90% with an alpha error rate of 5% adjusted to multiple comparisons by Bonferroni’s technique. The data were analyzed using SPSS version 20.0 (SPSS Inc., IBM Corporation, Chicago, USA). Continuous variables were expressed as mean (± SD) or median (± IQR), and categorical values were expressed as proportions (%). To compare between the groups: one-way ANOVA was used for continuous variables, and chi-square test was used for categorical variables. Logistic regression analysis was carried out to predict the dependent variable (successful sedation) from independent (agents used, age, sex, body weight for age) variables. A p-value of < 0.05 was considered statistically significant.
Results
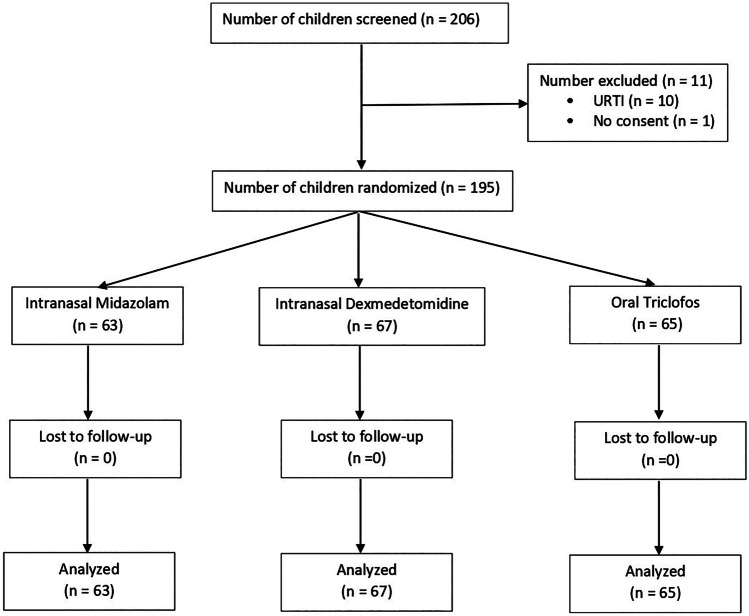
Two hundred and six children were assessed for eligibility, 195 were enrolled and completed the study. The flow of the study children is shown in Fig. 1. The baseline characteristics, MRI testing groups, and duration of MRI scan in each group are presented in Table 1.
Fig. 1.
CONSORT flow diagram—flow of study participants
Table 1.
Baseline characteristics of the study groups
| Baseline characteristics | Intranasal midazolam (n = 63) | Intranasal dexmedetomidine (n = 67) | Oral triclofos (n = 65) | p-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 4.61 (3.07) | 4 (2.63) | 4.28 (2.91) | 0.93 |
| Male (n, %) | 35 (55.5%) | 42 (62.6%) | 36 (55.4%) | 0.84 |
| Weight (kg), mean (SD) | 17.01 (8.85) | 13.68 (5.92) | 14.31(6.18) | 0.19 |
| MRI done per group | ||||
| • MRI brain | 55 (87.3) | 56 (83.7) | 56 (86.1) | 0.57 |
| • MRI abdomen | 0 (0) | 1 (1.4) | 0 (0.0) | 0.34 |
| • MRI brain and spine | 3 (4.8) | 2 (2.9) | 2 (3.1) | 0.22 |
| • MRCP | 2 (3.2) | 3 (4.4) | 2 (3.1) | 0.86 |
| • MRI hand | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0.49 |
| • MRI spine | 2 (3.2) | 3 (4.4) | 3 (4.6) | 0.27 |
| • MRI thigh | 1 (1.5) | 1 (1.4) | 2 (3.1) | 0.59 |
| Duration of MRI scan (min), mean (SD) | 37.7 (10.8) | 38.2 (10.7) | 37.9 (11) | 0.36 |
n = number
SD standard deviation, MRI magnetic resonance imaging, MRCP magnetic resonance cholangio-pancreatography
Successful sedation (defined as a PSSS of < 4 with the maintenance of the same till completion of MRI) was observed among 88.1% in the intranasal dexmedetomidine (IND) group, 56.9% in the oral triclofos (TRI) group, and 9.5% in the intranasal midazolam (INM) group. There was a statistically significant difference (p < 0.05) within and among the three groups in terms of successful sedation as shown in Table 2.
Table 2.
Primary outcome: rate of successful sedation
| Study drug | No onset of sedation | Failed sedation* (PSSS ≥ 4) | Successful sedation (PSSS ≤ 3) | Total |
|---|---|---|---|---|
| Intranasal midazolam | 4 (6.3) | 53 (84.1) | 6 (9.5) | 63 (100) |
| Intranasal dexmedetomidine | 0 (0) | 8 (11.9) | 59 (88.1) | 67 (100) |
| Oral triclofos | 0 (0) | 28 (43.07) | 37 (56.93) | 65 (100) |
| Total | 4 | 89 | 102 | 195 (100) |
All the values are provided in number (%)
PSSS paediatric sedation state scale
*Failed sedation includes the children with no sedation onset
The mean time of onset of sedation was 6.33 min in the INM group, 19.08 min in the IND group, and 25.31 min in the TRI group. The INM group showed a faster onset of sedation. The duration of sedation was 14.17 ± 12.76 min in INM group; 138 ± 81.73 in IND group; TRI group had a mean duration of sedation to be 62.31 ± 46.66 min. This showed that the IND group had prolonged sedation of over 2 h in 60% of those successfully sedated. These details are shown in Table 3.
Table 3.
Secondary outcomes: time of onset and duration of sedation
| Time onset and duration of sedation | Intranasal midazolam (n = 63) | Intranasal dexmedetomidine (n = 67) | Oral triclofos (n = 65) |
|---|---|---|---|
|
Time of onset of sedation Mean ± SD (min) |
6.33 ± 4.50 | 19.08 ± 4.32 | 25.31 ± 6.18 |
|
Duration of sedation Mean ± SD (min) |
14.17 ± 12.76 | 138.23 ± 81.73 | 62.31 ± 46.66 |
ANOVA test was done and it was found to be significant both within and in between groups (p = 0.01). To assess the robustness of the variables, post hoc Tukey’s was done
With INM as the reference group, it was found that the odds of sedation increased 4.1 times on changing from INM to IND (p < 0.01). Also, the odds of sedation increased 2.26 (p < 0.01) times on changing from INM to TRI. These are shown in Table 4.
Table 4.
Logistic regression analysis for comparing the possible factors affecting successful sedation
| Possible factors influencing successful sedation | β-coefficient | p-value |
|---|---|---|
| Trial drug/sedative agent | ||
| • Intranasal midazolam (reference group) | - | - |
| • Intranasal dexmedetomidine | 4.1 | < 0.01 |
| • Oral triclofos | 2.26 | < 0.01 |
| Age | 0.01 | 0.84 |
| Sex | 0.61 | 0.11 |
| Body weight for age | 0.93 | 0.86 |
The adverse events in the study groups were self-limited and did not require any medical intervention. In the INM group, there was nasal discomfort on instillation of the drug in 18.3%. In the IND group, bradycardia (4.6%), hypotension (10.8%), and nasal discomfort (3.1%) were observed. Rescue sedation was required in 77 children in total.
Discussion
This is the first trial comparing the efficacy of three non-injectable drugs in parallel for procedural sedation in children undergoing MRI, emphasizing a safer sedation in the absence of an anesthetist. It showed that IND (3 mcg/kg) was more successful in achieving and maintaining sedation (88.1%) till completion of MRI compared to the other two agents, INM (9.5%) and TRI (56.9%). A systematic review reported a similar sedation efficacy of 84.1% (66.7% to 98%) using IND at different dosages (1–4 mcg/kg) for procedural sedation [9].
The mean time of onset of sedation for IND in the present study was similar to that shown by previous studies, and is on an average of 20 to 40 min [2, 10, 14]. The mean duration of sedation with IND in the present study was 138.23 ± 81.73 min with prolonged sedation (> 2 h) in 60% of children. There are reports of prolonged sedation with IND with the duration of sedation varying with the amount of dose and types of procedures, the reason being unclear [9, 15, 16]. We choose the IND dose as 3 mcg/kg by taking into account of the requirement of prolonged sedation as well as high-level noise production during MRI procedure [9, 15–17].
In the present study, INM had a mean sedation onset and duration of sedation to be 6.33 (± 4.5) min and 14.17 (± 12.76), respectively. The onset of sedation was fastest with INM compared to the other 2 drugs. This mean onset of sedation and duration of maintained sedation is comparable to a previous study, where the authors used 0.5 mg/kg of INM for sedation [18]. The peak concentration of INM was observed at a mean (± SD) of 14 (± 5) min, which could explain the mechanism of sedation onset in our study group [19]. The shorter and faster action of INM can be better used for CT imaging and other short painless procedures than MRI, which requires longer motion-free period.
The failure rate of triclofos for procedural sedation in children undergoing MRI was reported to be 5 to 20%, whereas in the present study, it was 43.1% [6]. This higher failure rate could be due to the use of a different brand of the drug, and a 1.5 Tesla MRI scanner that produces more noise than 0.5 Tesla scanners. The time of onset of sedation with triclofos was 25.31 ± 6.18 min in the present study which conforms to the usual sedation onset time reported previously [6, 20]. The sedation recovery time reported in various studies is between 60 and 120 min similar to that in our study (62.31 ± 46.66 min) [6, 20]. The sedation with oral triclofos at times can be highly variable with sedative effects for up to 24 h [20]. This wide variability in the duration of sedation might be due to the erratic gastric absorption and the unclear mechanism of action of the drug [20]. The bitter taste of oral triclofos has been attributed to be a cause of vomiting and medication loss since children refuse to take it. But in the present study, no vomiting was observed.
Although oral triclofos is a time-tested drug, its efficacy in children is variable. This can be attributed to many reasons. First, triclofos is administered with an oral syringe which requires a co-operative child. Despite the best efforts to administer the drug, some children might not receive the full dose because of spitting out or not swallowing the drug properly. In contrast, administration of IND is much more reliable, considering the smaller volume of medication and the absence of any irritant property of the drug. The effects of the drug administered via the intranasal route are thought to be a consequence of the drug traversing the nasal mucosa and entering the blood stream avoiding first-pass metabolism. The other possible action is through the nose-brain pathway wherein the drug enters the CNS directly across the nasal mucosa or through the olfactory nerves [21]. These points favor the use of IND over orally administered medications.
Dexmedetomidine acts through the same receptor (alpha-2 agonist) as that of clonidine with more potency. Hence, the cardiovascular effects are obvious in terms of bradycardia and hypotension [22]. The present study revealed similar physiologic responses of bradycardia (4.6%) and hypotension (10.8%). However, these adverse events were found to be self-limited without any requirement for intervention in accordance with two other systematic reviews reporting similar adverse event profiles using intranasal [23], as well as intravenous dexmedetomidine [17].
There is a good reason to believe that intolerance to nasal spray is unlikely to affect the usage of IND because it is tasteless, odorless, and painless. It has been reported to be non-noxious to the nasal mucosa in different studies [24, 25]. But in the present study, nasal discomfort was observed in 2 children with IND administration, which could be due to administration of the drug by a syringe and would have been best avoided with the use of a mucosal atomizer device (MAD). A notable difference with INM was the nasal irritation and burning sensation reported in various studies [26, 27], which was also observed in 18.3% of children in the present study.
It is noteworthy that most of the children in our study group completed the MRI examination successfully, with the highest success noted with IND (88.1%), without need for intravenous drug administration. A success rate of 90% with 3.28 µg/kg of IND was reported in a previous study in children undergoing electro-encephalography (EEG) [28], whereas a success rate of 87% was reported in a similar study using IND at a dose of 2.5 µg/kg [29]. In a recent systematic review, the success rate of IND was shown to vary from 30 to 100% depending on the dose of the drug and type of procedures requiring sedation [10]. However, the procedure used in the present study was MRI, and the overall success rate should have been lower in contrary to what we observed. One explanation could be that the children in our study group were younger and the dose used was higher compared to most of the other studies if we take the mean age into account. But a definite answer could only come from pharmacokinetics and pharmacodynamics study only, which was not a part of the present study. In 2016, US-FDA issued a drug safety communication mandating label changes for all anesthetic as well as sedating agents, as they may have adverse neuro-developmental outcomes in young children [30]. In animal studies, it has been shown that dexmedetomidine is neuro-protective [31]. Long-term data on humans are sparse. This again favors the use of IND as a safer alternative to benzodiazepine (midazolam) and chloral hydrate (Triclofos).
The limitations of the present study are the following. First, the desired sample size could not be achieved because of the COVID-19 pandemic. But, we could be able to enroll 82.3% of children thus maintaining the power to detect significant differences. Second, blinding of investigator and participants was not possible because of the nature of interventions. However, the outcome assessor and statistician analyzing the data were blinded.
Conclusions
In children requiring procedural sedation for MRI, intranasal dexmedetomidine provides more effective sedation compared to intranasal midazolam or oral triclofos. The adverse events were minimal, which can be successfully handled by a pediatrician.
Authors’ contributions
Conceptualization: BD, SC; methodology and carrying out of the experiment: RRD, BD, BMP, BKB; formal analysis and investigation: BD, SC, BMP, RRD; writing of initial draft: SC, RRD; review and editing: BD, BMP, BKB; guarantor: BD. All the authors have seen and approved this version for publication.
Data availability
All the data are provided in the paper. Any other data related to the study may be available from the corresponding author on request.
Declarations
Ethical approval
The study was approved from ethical perspective by AIIMS Bhubaneswar Institute Ethics Committee (IEC). The committee follows Indian Council Medical Research (ICMR) Guideline for conduction of research. The approval number for this study is IEC/AIIMSBBSR/PG Thesis/2018–19/40.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnes S, Yaster M, Kudchadkar SR. Pediatric sedation management. Pediatr Rev. 2016;37:203–212. doi: 10.1542/pir.2014-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Dalvi NP, Tendolkar BA. Comparison between intranasal dexmedetomidine and intranasal midazolam as premedication for brain magnetic resonance imaging in pediatric patients: a prospective randomized double blind trial. J Anaesthesiol Clin Pharmacol. 2017;33:236–240. doi: 10.4103/joacp.JOACP_204_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Largo-Pineda CE, Arenas-Correa ID, Ángel-González GJ, Vélez-Arango JM, Calvo- Betancur VD, Arango-Zapata AN. Adverse events in paediatric patients taken to magnetic resonance imaging under sedation or anaesthesia. Rev Colomb Anestesiol. 2017;45:8–14. doi: 10.1016/j.rca.2016.09.004. [DOI] [Google Scholar]
- 4.Mason KP. Sedation trends in the 21st century: the transition to dexmedetomidine for radiological imaging studies. Paediatr Anaesth. 2010;20:265–272. doi: 10.1111/j.1460-9592.2009.03224.x. [DOI] [PubMed] [Google Scholar]
- 5.Subramaniam P, Girish Babu K, Lakhotia D. Evaluation of nitrous oxide-oxygen and triclofos sodium as conscious sedative agents. J Indian Soc Pedod Prev Dent. 2017;35:156–161. doi: 10.4103/JISPPD.JISPPD_82_16. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Wang Z, Song X, Fan Y, Tian H, Li B. Comparison of rescue techniques for failed chloral hydrate sedation for magnetic resonance imaging scans–additional chloral hydrate vs intranasal dexmedetomidine. Paediatr Anaesth. 2016;26:273–279. doi: 10.1111/pan.12824. [DOI] [PubMed] [Google Scholar]
- 7.Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 8.Mandell GA, Cooper JA, Majd M, Shalaby-Rana EI, Gordon I. Procedure guideline for pediatric sedation in nuclear medicine. Society of Nuclear Medicine. J Nucl Med. 1997;38:1640–1643. [PubMed] [Google Scholar]
- 9.Poonai N, Spohn J, Vandermeer B, Ali S, Bhatt M, Hendrikx S, et al. Intranasal dexmedetomidine for procedural distress in children: a systematic review. Pediatrics. 2020;145:e20191623. doi: 10.1542/peds.2019-1623. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang R, Meng HY, Wang MX, Du SZ. Efficacy and safety of intranasal dexmedetomidine premedication for children undergoing CT or magnetic resonance imaging: a systematic review and meta-analysis. Zhonghua Er Ke Za Zhi. 2020;58:314–318. doi: 10.3760/cma.j.cn112140-20191224-00830. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Anaesthesiologists physical status classification. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3106380/. Accessed 28 Jul 2022 [DOI] [PMC free article] [PubMed]
- 12.Validation of the Pediatric Sedation State Scale | American Academy of Pediatrics. https://pediatrics.aappublications.org/content/139/5/e20162897. Accessed 7 Aug 2022
- 13.Hattori Y, Fukatsu H, Ishigaki T. Measurement and evaluation of the acoustic noise of a 3 Tesla MR scanner. Nagoya J Med Sci. 2007;69:23–28. [PubMed] [Google Scholar]
- 14.Cozzi G, Monasta L, Maximova N, Poropat F, Magnolato A, Sbisà E, et al. Combination of intranasal dexmedetomidine and oral midazolam as sedation for pediatric MRI. Paediatr Anaesth. 2017;27:976–977. doi: 10.1111/pan.13202. [DOI] [PubMed] [Google Scholar]
- 15.Behrle N, Birisci E, Anderson J, Schroeder S, Dalabih A. Intranasal dexmedetomidine as a sedative for pediatric procedural sedation. J Pediatr Pharmacol Ther. 2017;22:4–8. doi: 10.5863/1551-6776-22.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baier NM, Mendez SS, Kimm D, Velazquez AE, Schroeder AR. Intranasal dexmedetomidine: an effective sedative agent for electroencephalogram and auditory brain response testing. Paediatr Anaesth. 2016;26:280–285. doi: 10.1111/pan.12851. [DOI] [PubMed] [Google Scholar]
- 17.Tug A, Hanci A, Turk HS, Aybey F, Isil CT, Sayin P, et al. Comparison of two different intranasal doses of dexmedetomidine in children for magnetic resonance imaging sedation. Paediatr Drugs. 2015;17:479–485. doi: 10.1007/s40272-015-0145-1. [DOI] [PubMed] [Google Scholar]
- 18.Chokshi AA, Patel VR, Chauhan PR, Patel DJ, Chadha IA, Ramani MN. Evaluation of intranasal Midazolam spray as a sedative in pediatric patients for radiological imaging procedures. Anesth Essays Res. 2013;7:189–193. doi: 10.4103/0259-1162.118954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoester PD, Jonker DM, van der Hoeven RTM, Vermeij TAC, Edelbroek PM, Brekelmans GJ, et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53:501–507. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung SM. Drug selection for sedation and general anesthesia in children undergoing ambulatory magnetic resonance imaging. Yeungnam Univ J Med. 2020;37:159–168. doi: 10.12701/yujm.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds J, Rogers A, Capehart S, Manyang P, Watcha MF. Retrospective comparison of intranasal dexmedetomidine and oral chloral hydrate for sedated auditory brainstem response exams. Hosp Pediatr. 2016;6:166–171. doi: 10.1542/hpeds.2015-0152. [DOI] [PubMed] [Google Scholar]
- 22.Sulton C, Kamat P, Mallory M, Reynolds J. The use of intranasal dexmedetomidine and midazolam for sedated magnetic resonance imaging in children: a report from the Pediatric Sedation Research Consortium. Pediatr Emerg Care. 2020;36:138–142. doi: 10.1097/PEC.0000000000001199. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Shin WJ, Park S, Ahn HS, Oh JH. The sedative effects of the intranasal administration of dexmedetomidine in children undergoing surgeries compared to other sedation methods: a systematic review and meta-analysis. J Clin Anesth. 2017;38:33–39. doi: 10.1016/j.jclinane.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67:825–831. doi: 10.1007/s00228-011-1002-y. [DOI] [PubMed] [Google Scholar]
- 25.Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LHY. A double-blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105:374–380. doi: 10.1213/01.ane.0000269488.06546.7c. [DOI] [PubMed] [Google Scholar]
- 26.Fantacci C, Fabrizio GC, Ferrara P, Franceschi F, Chiaretti A. Intranasal drug administration for procedural sedation in children admitted to pediatric Emergency Room. Eur Rev Med Pharmacol Sci. 2018;22:217–222. doi: 10.26355/eurrev_201801_14120. [DOI] [PubMed] [Google Scholar]
- 27.Kogan A, Katz J, Efrat R, Eidelman LA. Premedication with midazolam in young children: a comparison of four routes of administration. Paediatr Anaesth. 2002;12:685–689. doi: 10.1046/j.1460-9592.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Sun M, Zhang J, Tian Q, Yu Q, Liu Y, et al. Determination of the 90% effective dose of intranasal dexmedetomidine for sedation during electroencephalography in children. Acta Anaesthesiol Scand. 2019;63:847–852. doi: 10.1111/aas.13372. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Yang F, Ye M, Liu H, Zhang J, Tian Q, et al. Intranasal dexmedetomidine is an effective sedative agent for electroencephalography in children. BMC Anesthesiol. 2020;20:61. doi: 10.1186/s12871-020-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. FDA, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and. Accessed 20 Aug 2022
- 31.Koo E, Oshodi T, Meschter C, Ebrahimnejad A, Dong G. Neurotoxic effects of dexmedetomidine in fetal cynomolgus monkey brains. J Toxicol Sci. 2014;39:251–262. doi: 10.2131/jts.39.251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are provided in the paper. Any other data related to the study may be available from the corresponding author on request.