Abstract
The acute respiratory distress syndrome (ARDS) is a life-threatening condition that causes respiratory failure. Despite numerous clinical trials, there are no molecularly targeted pharmacologic therapies to prevent or treat ARDS. Drug delivery during ARDS is challenging due to the heterogenous nature of lung injury and occlusion of lung units by edema fluid and inflammation. Pulmonary drug delivery during ARDS offers several potential advantages including limiting the off-target and off-organ effects and directly targeting the damaged and inflamed lung regions. In this review we summarize recent ARDS clinical trials using both systemic and pulmonary drug delivery. We then discuss the advantages of pulmonary drug delivery and potential challenges to its implementation. Finally, we discuss the use of nanoparticle drug delivery and surfactant-based drug carriers as potential strategies for delivering therapeutics to the injured lung in ARDS.
Keywords: Acute respiratory distress syndrome (ARDS), Nanoparticles, Inhalation devices
1. Introduction
The acute respiratory distress syndrome (ARDS) results in life-threatening inflammatory lung injury following either a direct insult (e.g., bacterial or viral pneumonia) or indirect insult (e.g., sepsis, trauma). Due to coronavirus disease 2019 (COVID-19), the incidence of ARDS has been sharply increasing. The clinical manifestations of ARDS are outlined in the Berlin Definition and include acute onset of hypoxemia (PaO2/FiO2 ratio of ≤300 mmHg), non-cardiogenic pulmonary edema, and bilateral opacities on chest imaging [1,2]. Lung injury during ARDS causes the release of pro-inflammatory cytokines/chemokines that recruit neutrophils and other inflammatory cells to the alveolar space where they release toxic mediators such as proteases and reactive oxygen species. These mediators damage the alveolar epithelium and capillary endothelium [[3], [4], [5]]. This alveolar-capillary barrier dysfunction leads to further extravasation of fluid from the pulmonary vasculature into alveolar space [2]. Flooding of the alveolar space with proteinaceous fluid leads to hypoxemia and disrupts the function of surfactant which is a mixture of lipids and proteins secreted by type II alveolar epithelial cells that decreases surface tension and work of breathing. Surfactant dysfunction during ARDS further impairs lung compliance leading to increased difficulty breathing [2,6]. Despite progress in elucidating the mechanisms of lung dysfunction during ARDS, the standard of care for managing ARDS patients is supportive care with mechanical ventilation (MV). Unfortunately, MV generates physical forces that can exacerbate lung injury and lead to further lung damage. In this review we summarize recent clinical trials of systemic and inhaled therapies for ARDS patients, discuss the potential of pulmonary drug delivery for ARDS, review methods for pulmonary drug delivery in these patients, and propose potential strategies for pulmonary drug delivery in the context of ARDS.
2. Summary of recent ARDS clinical trials
2.1. Therapies targeting the inflammatory response
Excessive local and systemic inflammation is a pathophysiological hallmark of ARDS and there have been many clinical trials of anti-inflammatory therapies for ARDS [[7], [8], [9], [10]]. The most well-studied anti-inflammatory agents for ARDS are corticosteroids. Corticosteroids inhibit the action of pro-inflammatory mediators through activation of genes that drive the inflammatory response and suppression of genes that encode anti-inflammatory mediators [11,12]. Dexamethasone has been studied in patients with ARDS owing to its long biological half-life compared with other corticosteroids. In a randomized controlled trial for patients with moderate-to-severe ARDS [9] a 5-day course of 20 mg intravenous (IV) dexamethasone once daily followed by 5-days of 10 mg IV daily showed an increase in the number of ventilator-free days and a reduction in mortality. Most recently, Horby et al. conducted a study of dexamethasone for treatment of COVID-19. They found the use of a 6 mg once daily IV dose of dexamethasone reduced 28-day mortality among patients who received either MV or supplemental oxygen alone but not among those who were not hypoxemic [13]. Aspirin, a nonsteroidal anti-inflammatory drug, has been studied in patients at-risk for ARDS, as a preventative therapy. Aspirin does not reduce the incidence of ARDS within 7 days from hospital presentation while a higher number of subjects treated with aspirin were admitted to intensive care unit (ICU) [10].
Corticosteroids and aspirin dampen inflammation through effects on numerous downstream pathways. This approach lacks specificity and can lead to off-target effects, such as hypothalamic-pituitary-adrenal (HPA) axis suppression [14]. For this reason, there has been interest in using more precisely targeted anti-inflammatory therapies. One cytokine that is known to play a role in the pathophysiology of ARDS is interleukin-6 (IL-6) [15,16]. IL-6 receptor inhibitors directly block the pro-inflammatory activity of IL-6 and have been studied in several clinical trials. A recent trial published in 2022 used sarilumab (IL-6 antagonist) in hospitalized ARDS patients with COVID-19 and showed a reduction of serum IL-6 levels but did not show improvement of survival or ventilator-free days [17]. In another randomized controlled phase 3 trial, tocilizumab (IL-6 receptor inhibitor) failed to improve clinical outcomes in patients with COVID-19 related ARDS [18]. Sabbatinelli et al. subsequently showed that biomarkers in the serum may be useful in identifying COVID-19 patients who will respond to tocilizumab [19]. There are several ongoing trials studying the effects of targeting the IL-6 pathway and other inflammatory pathways but currently there are no molecularly targeted therapies that effectively dampen inflammation in non-COVID ARDS [20]. Although there have been many previous trials of anti-inflammatory therapies in ARDS, we have focused on some of the most recent ones over the past few years, and direct readers to several other excellent reviews for a more in-depth review of prior trials [21,22].
2.2. Therapies targeting barrier dysfunction and pulmonary edema
Resolution of pulmonary edema is a critical step during recovery from ARDS. As discussed above, breakdown of the alveolar-capillary barrier is the primary cause of pulmonary edema in ARDS. Pharmacotherapies that target barrier dysfunction (capillary endothelium and alveolar epithelium) have become an active area of investigation. Removal of lung edema fluid is mediated by active transport of sodium (Na) and chloride (Cl) across the basolateral surface of the alveolar epithelium, which creates an osmotic gradient for the reabsorption of water [23]. In ARDS, this active transport is impaired due to the disruption of alveolar epithelial integrity. Multiple pre-clinical studies have shown that activation of β-2 adrenergic receptors with β-2 agonists can increase Na, Cl, and fluid transport by increasing the activity of Na channels and Na/K-ATPase pump on alveolar epithelium [24]. However, clinical trials of intravenous β-2 agonists resulted in increased mortality in the treatment group [25]. Vascular endothelial leak is another important event in the development of ARDS. Interferon beta-1a (IFN-β1-a) has been shown to reduce endothelial cell permeability during lung injury by upregulating the expression of cluster of differentiation 73 (CD73) on endothelial cells. Upregulation of the CD73 enzyme can increase production of adenosine which is an endothelial stabilizing agent that can reduce endothelial cell disruption [26]. Increases in adenosine production stimulate adenosine receptor signaling that has been shown to enhance alveolar fluid clearance [27]. However, in a recent randomized controlled trial, administration of IFN-β1-a did not improve ventilator-free days and mortality for ARDS patients compared to placebo [28]. Treatment with HMG co-A reductase inhibitors (i.e. statins) can attenuate vascular leak in mouse models of ARDS and reduce cell injury in in-vitro models of ventilator-induced lung injury [29,30]. However, relatively high concentrations are required which led to higher hepatic and renal organ dysfunction in patients treated with rosuvastatin in the SAILS trial [31]. One strategy to avoid these off-target effects associated with systemic drug delivery may be the use of pulmonary drug delivery to directly target the injured lung.
3. Barriers to molecularly targeted therapies for ARDS
Currently there are no effective pharmacologic therapies that directly target the mechanisms of lung injury during ARDS. This is due to several factors including the complex pathophysiology of ARDS and the heterogeneous nature of lung injury. ARDS is characterized by heterogeneity at multiple levels. Physiologically, some patients with ARDS have very stiff lungs while others have relatively preserved compliance [32,33]. Another source of physiologic heterogeneity in ARDS patients is the degree of hypoxemia as measured by the ratio of the partial pressure of oxygen to the fraction of inspired oxygen (ie. P/F ratio). Some patients have severe hypoxemia requiring high levels of supplemental oxygen and prolonged ventilator support, while others have mild hypoxemia and recover more quickly [34]. A wide variety of insults can lead to ARDS including infections (i.e., pneumonia and sepsis), trauma, and toxic inhalations. Each inciting event results in distinct pathobiological changes that affects the responses to different therapies. To make things even more complicated, many patients have multiple etiologies of lung injury which makes the pathogenesis of ARDS even more complex.
In addition to physiologic and clinical heterogeneity in ARDS, there is also heterogeneity at the molecular level. One approach to addressing this molecular heterogeneity is to analyze levels of circulating biomarkers. Plasma biomarkers predict mortality in ARDS patients and can be used to phenotype patients and predict response to some therapies [35,36]. Calfee et al. used latent class analysis and found that several pro-inflammatory markers (eg. IL-6, IL-8) distinguished hyper-inflammatory and hypo-inflammatory phenotypes [33,34,37]. A secondary analysis of data from a clinical trial of simvastatin for ARDS using these phenotypes demonstrated higher survival with simvastatin treatment compared to placebo in patients with hyperinflammatory phenotype that was not identified in the original trial [38]. However, the use of these biomarkers in real-time is not yet available outside of the research setting which limits the ability to develop phenotype-specific treatment options.
4. Advantages of pulmonary delivery
One of the factors that may contribute to the lack of efficacy for drugs in the trials above is the fact that most of the drugs were delivered via the systemic circulation. Therapies delivered into the circulation are more likely to have lower concentrations in the lung potentially leading to reduced efficacy compared to pulmonary delivery. Drugs that are absorbed into the circulation via oral route often have low bioavailability which can result in low drug concentrations in the circulation and in the lung. Short time in the systemic circulation due to rapid clearance of drugs could also contribute the low pulmonary drug concentrations. Drugs circulating in the blood also have a higher chance of distributing to other uninjured organs such as the liver, kidney, or heart [39]. This may cause adverse drug effects which occur frequently in clinical trials of pharmacotherapies for ARDS. This was demonstrated in the BALTI-2 trial of intravenous salbutamol for patients with ARDS [40]. Patients treated with IV salbutamol had increased 28-day mortality compared to subjects that received placebo.
The lung is an attractive target for delivery of both pulmonary acting and systemically acting drugs. Pulmonary drug delivery is advantageous for several reasons. First, pulmonary delivery bypasses first-pass metabolism in the liver [41]. Many active drugs are metabolized in the liver and then excreted from the body, leading to only a small amount of active drug being absorbed into the circulation. Although the lungs also have metabolic enzymes, such as Cytochrome P450 (CYP450), the level of drug metabolism in the lungs is much lower than that in the liver. Therefore, pulmonary delivery greatly increases bioavailability and efficacy of the drug. Second, pulmonary drug delivery increases absorption for drugs that are not taken up efficiently via gastrointestinal (GI) tract [42]. Pulmonary delivery avoids the harsh environment in the GI tract including the acidic pH in the stomach and extracellular enzymatic degradation in the GI tract. Drugs delivered via pulmonary route are also not affected by dietary factors such as impaired absorption with certain types of nutrients. Third pulmonary drug delivery may be associated with fewer or less severe systemic adverse drug events (ADE) [42]. Typically, ADEs from drugs delivered via the pulmonary route occur in the upper respiratory tract. In contrast, systemically administered drugs may cause a wide range of ADEs in several different organ systems.
For these reasons direct delivery to the lungs can achieve high drug concentrations at the disease site with a relatively low initial dose. This may result in fewer or less severe adverse drug events (ADE) compared to drugs that are administered systemically. This effect was nicely illustrated in several studies of inhaled steroids for asthma. A randomized double-blind study conducted by Namsirikul et al. demonstrated that 400 μg of inhaled budesonide improved lung function to a greater degree than 5 mg of oral prednisolone for the treatment of moderate asthma [43]. Although no serious ADEs were observed in either group, the inhaled budesonide group had fewer systemic ADEs compared to the oral prednisolone group. In another study, Lee-Wang et al. found that 2 mg of inhaled flunisolide were as effective as 40 mg oral prednisone for treatment of severe adult asthma [44].
Finally, direct delivery to the injured lung allows rapid onset of action, leading to rapid clinical response. This is critical for trials aimed at early prevention of lung injury. The branching system of the lungs provides enormous surface area and accelerates drug absorption. A small phase II trial of inhaled budesonide and formoterol in patients at risk for ARDS demonstrated that early administration of this therapy decreased number of patients who went on to require mechanical ventilation or develop ARDS [45]. These results suggest that pulmonary delivery of therapeutics in patients at risk for ARDS may be a viable strategy.
Currently patients with ARDS and severe hypoxemia are treated with inhaled pulmonary vasodilators. Inhaled vasodilators are thought to preferentially dilate the pulmonary vasculature of well-aerated lung units to improve ventilation-perfusion matching and reduce the shunt fraction [46]. Traditionally, the vasodilator of choice has been inhaled nitric oxide (NO), which has been shown to improve oxygenation (as measured by the P/F ratio) without any improvement in mortality [47]. Inhaled prostaglandins (eg. epoprostenol) can also be used for this purpose. Inhaled prostaglandins may be preferential because they are easier to administer via the ventilator circuit and cost less [48]. Additionally, inhaled prostaglandins have been shown to have some anti-inflammatory and anti-thrombotic affects which could be useful in patients with ARDS [49,50]. Unfortunately, despite consistent data that inhaled prostaglandins and NO improve oxygenation, there is no evidence that they improve mortality, ventilator-free days, or other patient-centered outcomes [51].
5. Challenges to pulmonary drug delivery
Although pulmonary delivery has advantages over systemic delivery, it also poses some challenges. The endogenous defense mechanisms of the respiratory tract protect against pathogens and inhaled particles, but they also create barriers for drug delivery. Inhaled particles may be deposited through upper airways, conducting airways and then the alveoli as a result of impaction, sedimentation, and diffusion. Pulmonary drug delivery is also affected by particle size, and which can affect clinical efficacy. Particles >10 μm are typically deposited in the oropharynx and subsequently exhaled; particles in the 1–5 μm range can be deposited in the deep lung but are mostly phagocytosed by macrophages; particles at nanometer scale, especially ≤200 nm, are more likely to be deposited in deep lung and are less susceptible to phagocytosis [41,52]. Particle size in the lung is not static because the lung is a very humid environment. Humidity can lead to dynamic changes in the size of hydroscopic drug particles and alter their deposition in the lung [53].
5.1. Barriers in upper airways
The upper airway is comprised of the nasal and oral cavities, pharynx, and larynx. Particles administered through the nose are filtered by nasal hairs to remove particles larger than 10–15 μm in diameter. As inhaled particles pass through the nasopharynx or oropharynx, most large particles are removed by impaction on the pharynx and never reach the distal lung. In addition, the tonsils and adenoids near the impaction site provide immunologic defense against biologically active materials. Upper airways also have ciliated epithelium, which remove and reduce inhaled particles [41,54].
5.2. Barriers in conducting airways
After passing through the upper airway, particles enter the conducting airways (trachea, bronchi, and bronchioles), which are lined with ciliated epithelium interspersed with mucus secreting goblet cells. Large particles in the 2–10 μm range are removed by impaction and/or sedimentation and trapped in the mucus in the conducting airways. Mucociliary clearance is a major mechanism for removal of inhaled particles in the conducting airways. Particles trapped in the mucus are continuously moved upward toward the pharynx, and then swallowed, expectorated, or sneezed out. Inhaled drug particles are also exposed to enzymatic degradation, by enzymes such as cytochrome P450 which are found throughout the conducting airways [42,54].
5.3. Barriers in alveoli
Smaller particles <1 μm can reach the alveolar ducts and alveoli as a result of diffusion [41]. Alveolar macrophages reside at the alveolar surface and can phagocytose particles deposited there. Following phagocytosis engulfed particles may be destroyed by lysosomes or carried up the respiratory tree for mucociliary clearance. Macrophages can also transport drugs to the interstitial space for removal by the lymphatic system. Drug clearance by alveolar macrophages can reduce the efficacy of a drug delivered by inhalation. In addition, enzymes present at the alveolar surface may degrade or inactivate inhaled drug particles and decrease their efficacy. Those enzymes include proteases (eg. endopeptidase, cathepsin H) that can hydrolyze peptides and protein drugs, and drug metabolizing enzymes that can inactivate drug molecules [42]. The effect of pulmonary surfactant on drug delivery to the alveoli has been controversial. Pulmonary surfactant is secreted by type II alveolar cells and is composed of phospholipids and proteins. Surfactant is adsorbed to the air-liquid interface of the alveoli, which can potentially prevent adhesion of inhaled particles by creating a barrier or facilitate cellular uptake depending on drug solubility, charge, and local surface tension conditions [54,55]. The surfactant proteins that play a role in innate immunity (ie. SP-A and SP-D) may interact with drug molecules and function as opsonins, making them more susceptible to phagocytosis and removal by alveolar macrophages [56]. On the other hand, exogenous surfactants have been extensively studied as emulsifiers to solubilize hydrophobic drugs. Some studies have shown that drug-surfactant interactions may increase the solubility of inhaled drug, thus enhancing its bioavailability [57]. Exogenous surfactants have also been studied as carriers for drug delivery to the lungs [58,59].
6. Specific challenges to pulmonary delivery in ARDS
In ARDS, the influx of inflammatory cells, such as neutrophils and macrophages have the potential to reduce the efficacy of drugs delivered directly to the lungs. The activation of neutrophils not only results in damage to the lung, but also the release of proteolytic enzymes that can degrade protein-based drugs [60]. Increases in the number of macrophages in the alveolar space can also contribute to decreased drug efficacy due to drug clearance by phagocytosis.
Pulmonary edema is another challenge for pulmonary drug delivery in ARDS. The edema fluid in ARDS is a barrier for drug transport and absorption. In normal lungs, drug particles may be able to dissolve through a thin layer of alveolar surface lining fluid. However, diffusion may not be possible when alveoli are completely filled with fluid. The effect of pulmonary edema on drug absorption is even more significant for hydrophobic drugs, such as dexamethasone. Animal studies have shown that the absorption of dexamethasone is decreased in the presence of pulmonary edema due to a reduction in drug diffusion rate [61]. Moreover, the edema fluid contains proteins from the vascular space including proteases and albumin that can bind and inactivate drugs. Finally, lung regions occluded by edema fluid may receive less ventilation and thus the global transport of drug delivery particles to these regions will be reduced with preferential transport to non-injured aerated lung regions.
The effect of lung surfactant on pulmonary drug delivery is a topic of ongoing controversy. The structure, composition, and function of surfactant is altered in ARDS. As discussed above, the net effect of pulmonary surfactant on drug deposition within the alveoli likely varies depending on the structure and composition of the drug molecule. Pulmonary surfactant proteins can opsonize inhaled drugs which can enhance drug clearance by immune cells in the alveolar space.
7. Current methods for pulmonary drug delivery
7.1. Devices for pulmonary drug delivery
To overcome the challenges of pulmonary drug delivery and optimize clinical effectiveness of therapeutics, selection of the correct delivery system is of utmost importance. Three main types of inhalational devices are available for pulmonary drug delivery. These include pressurized metered-dose inhalers (MDI), dry powder inhalers (DPI), and nebulization. For ARDS patients that require mechanical ventilation, MDI's and nebulization are the only options that are used clinically.
7.2. Nebulized drug delivery for mechanically ventilated ARDS patients
Nebulization is the oldest and most reliable method for pulmonary delivery of aerosols in mechanically ventilated patients. Drugs delivered by nebulization are typically formulated in aqueous solutions or suspensions. These are then broken up by compressed air or ultrasonic power into small aerosol droplets that are inhaled into the lungs. There are three common types of nebulizers: 1) jet nebulizers, 2) ultrasonic wave nebulizers, and 3) vibrating mesh nebulizers. Jet nebulization uses a compressed gas (ambient air or oxygen) to break up liquid into aerosols. Ultrasonic wave nebulizers utilize high-frequency vibration of a piezoelectric element in liquid to generate aerosol droplets. Vibrating mesh nebulizers create droplets by passing the liquid through a very fine mesh/membrane with micrometer-sized holes [62].
Advantages of nebulization include that it does not require a coordinated patient effort which makes it useful for patients with ARDS that often have dyspnea and increased respiratory drive [62]. Nebulization is also useful when a large dose or continuous delivery is required such as the case with some types of antibiotic therapy [54]. Owing to these advantages, nebulizers are widely used in hospitalized patients with ARDS. Nebulized medications have been studied in clinical trials of ICU patients. A recent study investigated the effect of nebulized heparin for patients with or at risk of ARDS and found that patients in the nebulized heparin group had less lung injury and were discharged earlier than those in the placebo group [63]. The LIPS-B trial demonstrated that nebulized budesonide and formoterol was effective in limiting progression to ARDS in a high-risk population. These data demonstrate that aerosol drug delivery can be effective in ARDS patients.
There are several potential limitations to the use of nebulization for pulmonary drug delivery in ARDS. The shear force and heat generated during aerosolization by jet and ultrasonic nebulizers may cause drug degradation. Aerosol droplets generated by nebulization can condense water which may increase the droplet size and affect drug deposition. In addition, the nebulization process frequently leaves residual amounts of drug after dosing which decreases the efficiency of drug delivery [54,62]. Vibrating mesh nebulization also has some specific imitations including that the drug solution or suspension may clog the micrometer-sized holes in the mesh, resulting in reduced aerosol production [62].
7.3. Inhalers for spontaneously breathing patients at risk for ARDS
Pressurized metered-dose inhalers (MDI) are the most commonly used aerosolization device for drug delivery to the lungs [64]. MDIs are commonly used to deliver bronchodilators, corticosteroids, or combinations of these drugs for airway diseases such as asthma and COPD. MDI's use a liquefied compressed gas as a propellant to generate an aerosol and reproducibly deliver multiple doses of drug. The inclusion of a propellant avoids the use of an external power supply, but may have adverse environmental effects [65]. MDIs also require proper technique to control the actuation and deliver the drug during the inspiratory phase of the respiratory cycle. Patients who fail to inhale deeply or slowly enough can have reduced drug delivery [54]. Newer breath-actuated MDIs are designed to work in synchrony with patient respiration which may overcome the problem of poor coordination [66]. Using an MDI with a spacer can reduce oropharyngeal drug deposition which can minimize adverse drug events [67].
Dry powder inhalers (DPI) are also commonly used to deliver drugs to the lungs. The dry powder is de-agglomerated and transported into the lung during inhalation [66,68]. DPIs are relatively easy to handle and do not require the use of propellant, thus avoiding the harmful environmental effects of some MDIs. Because the formulation is in powder form, the active drug is relatively stable compared to formulations for nebulization [66]. DPIs have been used in several clinical trials of COVID-19 related ARDS. A phase II clinical trial of inhaled budesonide used a DPI formulation in COVID-19 patients at risk of developing ARDS [69]. However, DPIs require high air flow for deaggregation of large particles into particles with fine sizes, and are therefore dependent on the inspiratory flow generated by the patient [70]. This can be particularly challenging in young and elderly patients or patients with respiratory distress due to ARDS [66].
Soft mist inhalers are newer, novel, and easy to handle inhaler devices. This device uses mechanical power generated by a spring to create a low velocity aerosol for inhalation [71]. It has been reported that soft mist inhalers can generate higher fraction of fine aerosols than MDIs and DPIs, thus have higher drug deposition in the lungs [72]. Although soft mist inhalers are easy to use, it requires some basic knowledge of device assembly, training is still required particularly in young children. Soft mist inhalers are more costly than other inhalers [73].
Although the use of these inhalational devices can deposit drugs into the deep lungs, each device has limitations in patients with ARDS. Many inhalers deposit less than 20% of the drugs in the lungs [74]. Drugs delivered using MDI or DPI may deposit in the oropharynx, causing adverse drug effects [75]. Therefore, it is crucial to develop more efficient and novel drug delivery systems.
8. Future strategies for pulmonary drug delivery
8.1. Nanoparticles for pulmonary drug delivery
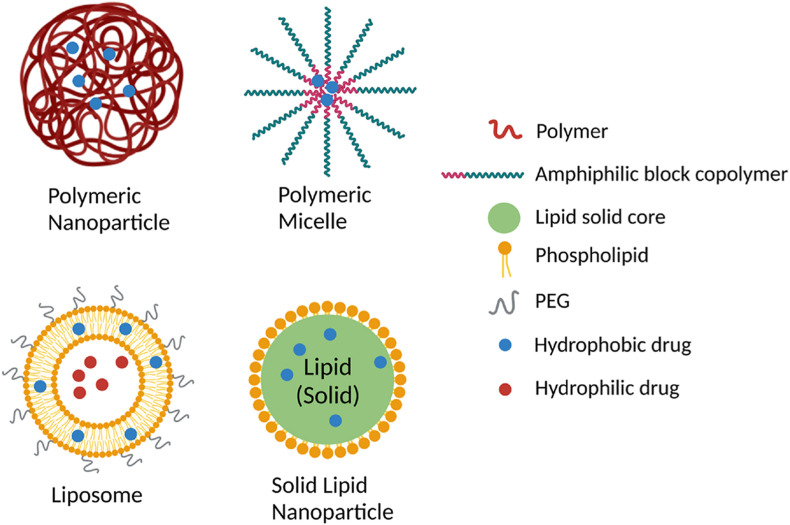
One potential strategy to enhance pulmonary drug delivery in ARDS is the use of nanoparticle delivery platforms. Different types of nanoparticles (Fig. 1 ) including liposomes, solid lipid nanoparticles, polymeric nanoparticles, and polymeric micelles have been studied in various lung diseases.
Fig. 1.
Schematic representation of polymeric nanoparticle, polymeric micelle, liposome, and solid lipid nanoparticle. Created with BioRender.com.
Nanoparticles are solid colloidal particles that can be used as drug carriers through encapsulation or adsorption of active drugs [76]. The use of nanoparticles for pulmonary drug delivery has several advantages. Nanoparticles are typically made using biodegradable and biocompatible materials, such as phospholipids, which makes them less toxic [77]. Nanoparticles are capable of dissolving hydrophobic drugs [77]. Since the pathophysiology of ARDS involves damage of alveolar epithelium and capillary endothelium, drug delivery to the deep lung is crucial. Nanoparticles can improve bioavailability via a more uniform distribution to the deep lung due to the nano-size of the particles [78]. As discussed previously, drugs delivered via inhalational route encounter several barriers. Encapsulating or conjugating drug molecules using nanoparticles has the potential to protect the drug from enzymatic degradation. To overcome drug clearance by mucus in upper and conducting airways, nanoparticles coated with mucoadhesive materials (eg. chitosan) have been designed to prolong the drug residence time [79]. However, chitosan-coated nanoparticles may be trapped in the mucus layer by hydrophobic and electrostatic interactions thereby decreasing the amount of drug that is delivered to the deep lung. A study conducted by Schuster et al. demonstrated that 100 and 200 nm sized nanoparticles coated with hydrophilic polyethylene glycol (PEG) could penetrate through respiratory mucus [80]. In addition, PEGylation has been shown to shield nanoparticles from macrophage phagocytosis and improves drug therapeutic effects [77].
Another appealing trait of nanoparticle is the ease of surface modification for targeted drug delivery to specific cells or tissues, which could minimize distribution of the drug to non-diseased tissues and reduce the amount drug needed. To target drugs to alveolar epithelial cells, nanoparticles can be functionalized with wheat germ agglutinin (WGA) that binds to lectin receptors on the alveolar epithelium [81]. Other groups have used nanoparticles targeting pulmonary endothelium in animal models of pulmonary diseases [82,83]. To target alveolar macrophages, anti-CD206 antibody can be conjugated to the nanoparticles to recognize mannose receptors on macrophages [84].
8.2. Polymeric nanoparticles and micelles
Polymeric nanoparticles are being studied for pulmonary drug delivery. Polymeric nanoparticles are aqueous colloidal suspensions made from polymers, such as poly (lactic-co-glycolic acid) (PLGA), polylactic acid (PLA), poly (caprolactone) (PCL) using techniques that disperse the polymers. Solvent evaporation is one method to prepare polymeric nanoparticles which requires dissolving polymers in an organic solvent [85]. Polymeric nanoparticles have been shown to have high encapsulation efficiency of therapeutic drugs and can protect drugs from degradation. However, since these type nanoparticles are made from partially synthetic polymers, they may have the potential to induce an inflammatory response. Several studies have established that these polymers are biodegradable, and they do not cause significant toxicity or lung tissue damage [86,87]. A research study by Cohen et al. evaluated the toxicity after PLGA nanoparticles delivery of hydrophilic surfactant protein D (SP-D) to mouse lungs. They found that sustained release of SP-D in mouse lungs was not associated with any toxicity and did not induce any inflammatory response [88]. Another study demonstrated that intratracheal administration of antioxidant loaded PLGA nanoparticles reduced pro-inflammatory cytokine levels in a mouse model of allergic asthma [89].
Polymeric micelles can encapsulate and protect poorly water-soluble drugs. Polymeric micelle formation occurs by spontaneous self-assembly of amphiphilic block copolymers when the concentration reaches critical micelle concentration (CMC). These micelles contain a hydrophobic polymer composed of polyesters (poly (glycolic acid (PGA)) or polyethers where lipophilic drugs are dissolved, and a hydrophilic shell composed of hydrophilic polymers such as poly (ethylene glycol) (PEG) that protect the drug from degradation [90]. However, there have not been any clinical trials that we are aware of using polymeric micelles to treat lung diseases.
8.3. Solid lipid nanoparticles (SLNs)
Solid lipid nanoparticles (SLNs) have been studied for pulmonary delivery of small molecule drugs and gene therapies. SLNs can be prepared using phospholipids or triglycerides, which are physiologic lipids that are non-toxic and suitable for pulmonary drug delivery [91,92]. A study showed that amikacin loaded SLNs delivered via pulmonary route were deposited in higher amount in rat lungs compared to kidneys, which suggests that off-target effects may be decreased using this approach [93]. Poorly water-soluble drugs can be encapsulated within the hydrophobic core or coupled to the particle. Many drugs for the treatment of ARDS are water insoluble and are often dissolved in organic solvent which may cause severe ADEs. SLNs can be used as carriers for pulmonary delivery of those water-insoluble drugs to minimize ADEs [94].
SLNs have also been investigated for pulmonary gene delivery, especially for therapeutic non-coding small RNAs, such as short interfering RNAs (siRNAs) [95] and microRNAs (miRNAs) [96]. Naked RNA oligonucleotides are highly susceptible to degradation in vitro and in vivo. SLNs can protect RNAs from degradation thereby improving their therapeutic efficacy. SLNs are considered safer delivery systems for gene delivery compared to viral vectors. One example is Patisiran, siRNA loaded lipid nanoparticles for intravenous delivery, approved by U.S. Food and Drug Administration (FDA) in 2018 for treatment of polyneuropathy caused by hereditary transthyretin-mediated amyloidosis [97]. Our recent study demonstrated that SLNs can deliver microRNAs to alveolar macrophages and mitigate lung injury during mechanical ventilation which is used for many patients with ARDS [96]. The encouraging safety and efficacy observed in preclinical studies and the clinical use of SLNs for non-pulmonary diseases suggests that SLN nanomedicines have potential for the treatment of ARDS.
8.4. Liposomes
Liposomes are an attractive option for pulmonary drug delivery because they are composed primarily of phospholipids, which are the major component of lung surfactant [98]. This makes liposomes more biocompatible. Liposomes are spherical bilayer vehicles composed of amphiphilic phospholipids. The polar heads of the phospholipids are oriented toward the aqueous core encapsulating water-soluble drugs and the hydrophobic tails form the inner region of the bilayer that where water-insoluble drugs can be loaded. This property enables the encapsulation of multiple drugs simultaneously [99]. Traditionally the thin-film hydration technique has been used for pulmonary liposome preparation. In this method, phospholipids are typically dissolved in a mixture of chloroform and methanol and then the organic solvent is removed using a rotary evaporator to form a thin film. Liposomes are formed via hydration using an aqueous buffer followed by sonication. Membrane extrusion is then performed to generate small unilamellar vesicles in the nanometer size range [100].
Because liposomes have a similar lipid composition to lung surfactant, they have been shown to be safe as a drug carrier for pulmonary delivery. Myers et al. reported that pulmonary delivery of liposomes made from hydrogenated soy phosphatidylcholine (HSPC) did not cause toxicity in mice [101]. Liposome formulations for pulmonary delivery have also been tested in healthy human subjects and found to be safe and non-toxic [102].
There are several reasons that liposomes may be the preferred platform for pulmonary drug delivery in ARDS patients. Liposomal drug delivery has been studied in pulmonary diseases such as asthma and cystic fibrosis. The first and only clinical trial of liposomes in ARDS used intravenous liposomal prostaglandin E1 and showed improvement in oxygenation but no improvement in survival or ventilation-free days [103]. Corticosteroids are well-studied anti-inflammatory agents for ARDS and the use of liposomes to deliver corticosteroids to the lung has potential to increase local drug efficacy. In a study by Hegeman et al., liposome-encapsulated dexamethasone given by IV injection attenuated ventilator-induced lung inflammation with minimal systemic side-effects in a mouse model [104]. Liposomal drugs have also been used for pulmonary delivery. Arikayce is an amikacin liposome suspension that is FDA approved for inhalational delivery to treat of Mycobacterium avium complex (MAC) in patients who do not respond to conventional treatment [105,106]. It is currently under phase III clinical development for the treatment of lung infection in cystic fibrosis [107]. The amikacin liposome formulation is primarily composed of dipalmitoylphosphatidylcholine (DPPC) and cholesterol, which is similar to lung surfactant [108]. These clinical studies showed liposomal amikacin suspension improved lung function with decreases in infection and no adverse drug events compared to placebo subjects [107,109]. These successful clinical studies provide further rationale for the development of inhalational liposome-based therapies for ARDS.
Despite the advantages of using nanoparticles as drug carriers for pulmonary delivery, delivery of nanoparticles with nebulizers or MDIs remains challenging. Nanoparticles may aggregate and increase in size due to the shear-induced stress during nebulization [107]. The aerosolized nanoparticles may also increase in size due to moisture within the respiratory tract [66]. A study conducted by Zhang et al. showed that lipid nanoparticle formulations increased in particle size after nebulization [110]. These limitations might affect the nanoparticle deposition in the deep lung. Therefore, future studies will be required to optimize delivery devices used for inhaled nanoparticle therapies.
8.5. Pulmonary surfactant as a carrier
Lung surfactant is composed of 90% lipids (mainly DPPC) and 10% proteins [111]. This high lipid content enables pulmonary surfactant (PS) to solubilize poorly water-soluble drugs. In addition, PS has been reported to rapidly adsorb into the air-liquid interface and spread along it to reach the alveolar region of the lung [112]. Due to these advantages, the potential use of pulmonary surfactant (PS) as a drug carrier has been explored recently. In one study, surfactant combined with beclomethasone more effectively reduced lung inflammation in an animal model of respiratory distress syndrome compared to beclomethasone alone [113]. Hidalgo et al. combined native purified PS with a large hydrophobic drug (tacrolimus) and found that pre-treatment with this mixture showed higher tacrolimus internalization in BAL cells and greater reduction of lipopolysaccharide (LPS)-induced pro-inflammation [112]. However, as discussed in above (Section 5.3), drug molecules carried by PS may interact with surfactant components, which could alter the properties and functions of both the drug and PS. An in vitro study of surfactant-antibacterial interactions conducted by Birkun demonstrated that adding amikacin or cefepime to surfactant did not affect PS properties, but colistimethate did. Furthermore, surfactant influenced the activity of cefepime and colistimethate, but not amikacin [114]. Therefore, it is important to analyze the effects of interactions between PS and drug on a case-by-case basis. The potential use of surfactant as a drug carrier in the clinical setting requires further investigation.
9. Conclusion
Prevention or treatment of ARDS has been challenging and currently there are no pharmacologic therapies that target the molecular mechanisms of lung injury. One potential reason for this lack of progress may be that most clinical trials have used systemic drug delivery. Delivery of therapeutics directly to the lung through inhalational delivery may address this limitation. However, there are many potential barriers to pulmonary drug delivery in ARDS patients. The use of nanoparticle drug delivery platforms and surfactant as a drug carrier may address some of the barriers and holds potential for use in patients with ARDS.
Funding sources
This work was partially supported by NIH R01HL142767 (JAE, SNG) and by the Eli Lilly Fellowship in Pharmaceutics at Ohio State (QF).
Data availability
No data was used for the research described in the article.
References
- 1.Force A.D.T., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Matthay M.A., et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imai Y., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay M.A., Zimmerman G.A. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005;33(4):319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppert L.A., Matthay M.A., Ware L.B. Pathogenesis of acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2019;40(1):31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenson K.E., Swenson E.R. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit. Care Clin. 2021;37(4):749–776. doi: 10.1016/j.ccc.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tongyoo S., et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit. Care. 2016;20(1):329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meduri G.U., et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 9.Villar J., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 10.Kor D.J., et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-A randomized clinical trial. JAMA. 2016;315(22):2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005;353(16):1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 12.Barnes P.J. How corticosteroids control inflammation: quintiles prize lecture 2005. Br. J. Pharmacol. 2006;148(3):245–254. doi: 10.1038/sj.bjp.0706736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group R.C., et al. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasir M., Goyal A., Sonthalia S. StatPearls. 2022. Corticosteroid adverse effects. Treasure Island (FL) [Google Scholar]
- 15.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 16.Sinha P., Bos L.D. Pathophysiology of the acute respiratory distress syndrome: insights from clinical studies. Crit. Care Clin. 2021;37(4):795–815. doi: 10.1016/j.ccc.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivapalasingam S., et al. Efficacy and safety of sarilumab in hospitalized patients with coronavirus disease 2019: a randomized clinical trial. Clin. Infect. Dis. 2022;75(1):e380–e388. doi: 10.1093/cid/ciac153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone J.H., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N. Engl. J. Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbatinelli J., et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021;193 doi: 10.1016/j.mad.2020.111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva P.L., Pelosi P., Rocco P.R.M. Personalized pharmacological therapy for ARDS: a light at the end of the tunnel. Expet Opin. Invest. Drugs. 2020;29(1):49–61. doi: 10.1080/13543784.2020.1699531. [DOI] [PubMed] [Google Scholar]
- 21.Villar J., et al. Unsuccessful and successful clinical trials in acute respiratory distress syndrome: addressing physiology-based gaps. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.774025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay M.A., McAuley D.F., Ware L.B. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir. Med. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 23.Matthay M.A., Folkesson H.G., Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82(3):569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 24.Groshaus H.E., et al. Mechanisms of beta-receptor stimulation-induced improvement of acute lung injury and pulmonary edema. Crit. Care. 2004;8(4):234–242. doi: 10.1186/cc2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Smith F., et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (Balti-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millar F.R., et al. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 27.Eckle T., et al. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118(10):3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranieri V.M., et al. Effect of intravenous interferon beta-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2020;323(8):725–733. doi: 10.1001/jama.2019.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altintas N.D., et al. Long-term simvastatin attenuates lung injury and oxidative stress in murine acute lung injury models induced by oleic Acid and endotoxin. Respir. Care. 2011;56(8):1156–1163. doi: 10.4187/respcare.00770. [DOI] [PubMed] [Google Scholar]
- 30.Higuita-Castro N., et al. Simvastatin treatment modulates mechanically-induced injury and inflammation in respiratory epithelial cells. Ann. Biomed. Eng. 2016;44(12):3632–3644. doi: 10.1007/s10439-016-1693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimaldi D., et al. Failure of statins in ARDS: the quest for the Holy Grail continues. Minerva Anestesiol. 2016;82(11):1230–1234. [PubMed] [Google Scholar]
- 32.Khan Y.A., Fan E., Ferguson N.D. Precision medicine and heterogeneity of treatment effect in therapies for ARDS. Chest. 2021;160(5):1729–1738. doi: 10.1016/j.chest.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha P., Calfee C.S. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr. Opin. Crit. Care. 2019;25(1):12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson J.G., Calfee C.S. ARDS subphenotypes: understanding a heterogeneous syndrome. Crit. Care. 2020;24(1):102. doi: 10.1186/s13054-020-2778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calfee C.S., et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calfee C.S., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha P., et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha P., et al. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir. Med. 2020;8(3):247–257. doi: 10.1016/S2213-2600(19)30369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., De Jesus O. StatPearls. 2022. Medication routes of administration. Treasure Island (FL) [Google Scholar]
- 40.Gates S., et al. Beta-Agonist Lung injury TrIal-2 (Balti-2): a multicentre, randomised, double-blind, placebo-controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technol. Assess. 2013;17(38):1–87. doi: 10.3310/hta17380. p. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rijt S.H., Bein T., Meiners S. Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 2014;44(3):765–774. doi: 10.1183/09031936.00212813. [DOI] [PubMed] [Google Scholar]
- 42.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Namsirikul P., et al. Comparison of inhaled budesonide with oral prednisolone at two dose-levels commonly used for the treatment of moderate asthma. Eur. Respir. J. 1989;2(4):317–324. [PubMed] [Google Scholar]
- 44.Lee-Wong M., et al. Comparison of high-dose inhaled flunisolide to systemic corticosteroids in severe adult asthma. Chest. 2002;122(4):1208–1213. doi: 10.1378/chest.122.4.1208. [DOI] [PubMed] [Google Scholar]
- 45.Festic E., et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit. Care Med. 2017;45(5):798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walmrath D., et al. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;153(3):991–996. doi: 10.1164/ajrccm.153.3.8630585. [DOI] [PubMed] [Google Scholar]
- 47.Taylor R.W., et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 48.Torbic H., et al. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J. Crit. Care. 2013;28(5):844–848. doi: 10.1016/j.jcrc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Birukova A.A., et al. Iloprost improves endothelial barrier function in lipopolysaccharide-induced lung injury. Eur. Respir. J. 2013;41(1):165–176. doi: 10.1183/09031936.00148311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose F., et al. Increased neutrophil mediator release in patients with pulmonary hypertension--suppression by inhaled iloprost. Thromb. Haemostasis. 2003;90(6):1141–1149. doi: 10.1160/TH03-03-0173. [DOI] [PubMed] [Google Scholar]
- 51.Fuller B.M., et al. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147(6):1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dailey H.L., Ghadiali S.N. Fluid-structure analysis of microparticle transport in deformable pulmonary alveoli. J. Aerosol Sci. 2007;38(3):269–288. [Google Scholar]
- 53.Haddrell A.E., et al. Pulmonary aerosol delivery and the importance of growth dynamics. Ther. Deliv. 2017;8(12):1051–1061. doi: 10.4155/tde-2017-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newman S.P. 2017. Drug Delivery to the Lungs: Challenges and Opportunities. [DOI] [PubMed] [Google Scholar]
- 55.Wang F., Liu J., Zeng H. Interactions of particulate matter and pulmonary surfactant: implications for human health. Adv. Colloid Interface Sci. 2020;284 doi: 10.1016/j.cis.2020.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murgia X., de Souza Carvalho C., Lehr C.-M. Overcoming the pulmonary barrier: new insights to improve the efficiency of inhaled therapeutics. Eur. J. Nanomed. 2014;6(3) [Google Scholar]
- 57.Balakrishnan A., et al. Surfactant-mediated dissolution: contributions of solubility enhancement and relatively low micelle diffusivity. J. Pharmaceut. Sci. 2004;93(8):2064–2075. doi: 10.1002/jps.20118. [DOI] [PubMed] [Google Scholar]
- 58.Vermehren C., et al. Lung surfactant as a drug delivery system. Int. J. Pharm. 2006;307(1):89–92. doi: 10.1016/j.ijpharm.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Haitsma J.J., Lachmann U., Lachmann B. Exogenous surfactant as a drug delivery agent. Adv. Drug Deliv. Rev. 2001;47(2–3):197–207. doi: 10.1016/s0169-409x(01)00106-5. [DOI] [PubMed] [Google Scholar]
- 60.Lai X., Tang J., ElSayed M.E.H. Recent advances in proteolytic stability for peptide, protein, and antibody drug discovery. Expet Opin. Drug Discov. 2021;16(12):1467–1482. doi: 10.1080/17460441.2021.1942837. [DOI] [PubMed] [Google Scholar]
- 61.Gardiner T.H., McAnalley B.H. Effect of lung edema on the pulmonary absorption of drugs. Life Sci. 1978;23(17–18):1827–1833. doi: 10.1016/0024-3205(78)90115-7. [DOI] [PubMed] [Google Scholar]
- 62.Martin A.R., Finlay W.H. Nebulizers for drug delivery to the lungs. Expet Opin. Drug Deliv. 2015;12(6):889–900. doi: 10.1517/17425247.2015.995087. [DOI] [PubMed] [Google Scholar]
- 63.Dixon B., et al. Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9(4):360–372. doi: 10.1016/S2213-2600(20)30470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivey J.W., Vehring R., Finlay W.H. Understanding pressurized metered dose inhaler performance. Expet Opin. Drug Deliv. 2015;12(6):901–916. doi: 10.1517/17425247.2015.984683. [DOI] [PubMed] [Google Scholar]
- 65.Myrdal P.B., Sheth P., Stein S.W. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15(2):434–455. doi: 10.1208/s12249-013-0063-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandel A., et al. Recent advances in aerosolised drug delivery. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108601. [DOI] [PubMed] [Google Scholar]
- 67.Lavorini F., Fontana G.A. Targeting drugs to the airways: the role of spacer devices. Expet Opin. Drug Deliv. 2009;6(1):91–102. doi: 10.1517/17425240802637862. [DOI] [PubMed] [Google Scholar]
- 68.Levy M.L., et al. Understanding dry powder inhalers: key technical and patient preference attributes. Adv. Ther. 2019;36(10):2547–2557. doi: 10.1007/s12325-019-01066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramakrishnan S., et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021;9(7):763–772. doi: 10.1016/S2213-2600(21)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weers J., Clark A. The impact of inspiratory flow rate on drug delivery to the lungs with dry powder inhalers. Pharm. Res. (N. Y.) 2017;34(3):507–528. doi: 10.1007/s11095-016-2050-x. [DOI] [PubMed] [Google Scholar]
- 71.Dalby R.N., Eicher J., Zierenberg B. Development of respimat((R)) soft mist inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl) 2011;4:145–155. doi: 10.2147/MDER.S7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson P. Use of respimat soft mist inhaler in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2006;1(3):251–259. doi: 10.2147/copd.2006.1.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwanaga T., et al. The respimat((R)) soft mist inhaler: implications of drug delivery characteristics for patients. Clin. Drug Invest. 2019;39(11):1021–1030. doi: 10.1007/s40261-019-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dugernier J., et al. Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit. Care. 2017;21(1):264. doi: 10.1186/s13054-017-1844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ding L., et al. A quality by design framework for capsule-based dry powder inhalers. Pharmaceutics. 2021;13(8) doi: 10.3390/pharmaceutics13081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sung J.C., Pulliam B.L., Edwards D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Prasanna P., et al. Nanotherapeutics in the treatment of acute respiratory distress syndrome. Life Sci. 2021;276 doi: 10.1016/j.lfs.2021.119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iyer R., Hsia C.C., Nguyen K.T. Nano-therapeutics for the lung: state-of-the-art and future perspectives. Curr. Pharmaceut. Des. 2015;21(36):5233–5244. doi: 10.2174/1381612821666150923095742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murata M., et al. Pulmonary delivery of elcatonin using surface-modified liposomes to improve systemic absorption: polyvinyl alcohol with a hydrophobic anchor and chitosan oligosaccharide as effective surface modifiers. Eur. J. Pharm. Biopharm. 2012;80(2):340–346. doi: 10.1016/j.ejpb.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 80.Schuster B.S., et al. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34(13):3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma A., Sharma S., Khuller G.K. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother. 2004;54(4):761–766. doi: 10.1093/jac/dkh411. [DOI] [PubMed] [Google Scholar]
- 82.Brenner J.S., et al. Endothelial nanomedicine for the treatment of pulmonary disease. Expet Opin. Drug Deliv. 2015;12(2):239–261. doi: 10.1517/17425247.2015.961418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parhiz H., et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Contr. Release. 2018;291:106–115. doi: 10.1016/j.jconrel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feinberg H., et al. Structural analysis of carbohydrate binding by the macrophage mannose receptor CD206. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zielinska A., et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16) doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grabowski N., et al. Toxicity of surface-modified PLGA nanoparticles toward lung alveolar epithelial cells. Int. J. Pharm. 2013;454(2):686–694. doi: 10.1016/j.ijpharm.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 87.Hara K., et al. Histological examination of PLGA nanospheres for intratracheal drug administration. Int. J. Pharm. 2008;356(1–2):267–273. doi: 10.1016/j.ijpharm.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 88.Attias Cohen S., et al. SP-D loaded PLGA nanoparticles as drug delivery system for prevention and treatment of premature infant's lung diseases. Int. J. Pharm. 2020;585 doi: 10.1016/j.ijpharm.2020.119387. [DOI] [PubMed] [Google Scholar]
- 89.Yoo D., et al. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int. J. Pharm. 2013;450(1–2):87–94. doi: 10.1016/j.ijpharm.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 90.Ghezzi M., et al. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J. Contr. Release. 2021;332:312–336. doi: 10.1016/j.jconrel.2021.02.031. [DOI] [PubMed] [Google Scholar]
- 91.Nassimi M., et al. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010;75(2):107–116. doi: 10.1016/j.ejpb.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Paranjpe M., et al. In vitro and ex vivo toxicological testing of sildenafil-loaded solid lipid nanoparticles. Inhal. Toxicol. 2013;25(9):536–543. doi: 10.3109/08958378.2013.810315. [DOI] [PubMed] [Google Scholar]
- 93.Varshosaz J., et al. Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/136859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esmaeili M., et al. Budesonide-loaded solid lipid nanoparticles for pulmonary delivery: preparation, optimization, and aerodynamic behavior. Artif. Cell Nanomed. Biotechnol. 2016;44(8):1964–1971. doi: 10.3109/21691401.2015.1129614. [DOI] [PubMed] [Google Scholar]
- 95.Wang J.L., et al. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021;596 doi: 10.1016/j.ijpharm.2021.120215. [DOI] [PubMed] [Google Scholar]
- 96.Bobba C.M., et al. Nanoparticle delivery of microRNA-146a regulates mechanotransduction in lung macrophages and mitigates injury during mechanical ventilation. Nat. Commun. 2021;12(1):289. doi: 10.1038/s41467-020-20449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akinc A., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14(12):1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 98.Rudokas M., et al. Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications. Med. Princ. Pract. 2016;25(Suppl 2):60–72. doi: 10.1159/000445116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nisini R., et al. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018;9:155. doi: 10.3389/fimmu.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sturm L., Poklar Ulrih N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021;22(12) doi: 10.3390/ijms22126547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Myers M.A., et al. Pulmonary effects of chronic exposure to liposome aerosols in mice. Exp. Lung Res. 1993;19(1):1–19. doi: 10.3109/01902149309071077. [DOI] [PubMed] [Google Scholar]
- 102.Elhissi A.M.A., et al. In: Novel Antimicrobial Agents and Strategies. Phoenix D.A., et al., editors. Wiley-VCH Verlag GmbH & Co. KGaA.; 2015. New delivery systems – liposomes for pulmonary delivery of antibacterial drugs; pp. 387–406. [Google Scholar]
- 103.Abraham E., et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit. Care Med. 1999;27(8):1478–1485. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 104.Hegeman M.A., et al. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br. J. Pharmacol. 2011;163(5):1048–1058. doi: 10.1111/j.1476-5381.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Griffith D.E., et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am. J. Respir. Crit. Care Med. 2018;198(12):1559–1569. doi: 10.1164/rccm.201807-1318OC. [DOI] [PubMed] [Google Scholar]
- 106.Winthrop K.L., et al. Amikacin liposome inhalation suspension for Mycobacterium avium complex lung disease: a 12-month open-label extension clinical trial. Ann Am Thorac Soc. 2021;18(7):1147–1157. doi: 10.1513/AnnalsATS.202008-925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waters V., Ratjen F. Inhaled liposomal amikacin. Expet Rev. Respir. Med. 2014;8(4):401–409. doi: 10.1586/17476348.2014.918507. [DOI] [PubMed] [Google Scholar]
- 108.Shirley M. Amikacin liposome inhalation suspension: a review in Mycobacterium avium complex lung disease. Drugs. 2019;79(5):555–562. doi: 10.1007/s40265-019-01095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clancy J.P., et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax. 2013;68(9):818–825. doi: 10.1136/thoraxjnl-2012-202230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang H., et al. Aerosolizable lipid nanoparticles for pulmonary delivery of mRNA through design of experiments. Pharmaceutics. 2020;12(11) doi: 10.3390/pharmaceutics12111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang S., et al. The role of pulmonary surfactants in the treatment of acute respiratory distress syndrome in COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.698905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hidalgo A., et al. Pulmonary surfactant and drug delivery: vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Contr. Release. 2021;329:205–222. doi: 10.1016/j.jconrel.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dani C., et al. Natural surfactant combined with beclomethasone decreases lung inflammation in the preterm lamb. Respiration. 2011;82(4):369–376. doi: 10.1159/000328928. [DOI] [PubMed] [Google Scholar]
- 114.Birkun A. Exogenous pulmonary surfactant as a vehicle for antimicrobials: assessment of surfactant-antibacterial interactions in vitro. Sci. Tech. Rep. 2014;2014 doi: 10.1155/2014/930318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.