Abstract
Objective.
Adolescent girls who grow up with mothers who are depressed are themselves highly vulnerable to developing depression (i.e., “intergenerational transmission of depression”). Stressor exposure is a strong risk factor for depression, and the transmission of depression risk from mothers to daughters is partly due to mothers experiencing more stressors, increasing daughters’ stressor burden. However, research in this area has only assessed recent stressors, making the role of cumulative lifetime stressors unclear.
Method.
To address this issue, we recruited 52 dyads of mothers and adolescent daughters, of which 22 daughters were at high maternal risk for depression. Participants completed diagnostic interviews, and daughters additionally self-reported their depressive symptoms. Participants also completed the Stress and Adversity Inventory, a new-generation instrument for assessing cumulative lifetime history of acute and chronic stressors based on the contextual threat approach. We tested moderated mediation models evaluating the conditional indirect effects of mothers’ lifetime stressors on high- vs. low-risk daughters’ depressive symptoms through daughters’ lifetime stressors.
Results.
As hypothesized, mothers of high-risk (but not low-risk) adolescent daughters who reported more lifetime acute stressors had daughters who reported more lifetime acute stressors and current depressive symptoms. Moreover, this finding was driven specifically by mothers’ stressors occurring after their daughters’ births. There was also tentative evidence that high-risk daughters’ lifetime chronic stressors potentiated the impact of daughters’ acute stressors on their depressive symptoms.
Conclusion.
These findings provide new insights into how stressful contexts are transmitted intergenerationally.
Keywords: adolescence, depression, environmental influences, family history, stress
Introduction
Depression is a common psychiatric disorder that is one of the largest contributors to disability and disease burden worldwide (Friedrich, 2017; Malhi & Mann, 2018). People who are depressed are at heightened risk for numerous other psychological and somatic health problems (Greenberg et al., 2003; Hammen, 2017; National Research Council and Institute of Medicine, 2009; Slavich, 2020a). Compounding these risks, many individuals with depression do not obtain long-lasting remission from currently available treatments (Berlim & Turecki, 2007; Malhi & Mann, 2018; Verduijn et al., 2017). Furthermore, experiencing depression is one of the strongest predictors of developing future depressive episodes, with each episode further increasing the risk of experiencing subsequent depression and related health problems (Burcusa & Iacono, 2007).
Unlike many other common chronic health conditions, depression frequently first occurs early in the life course (Malhi & Mann, 2018). Early life depression can increase the total number of years of disability an individual might endure while also stymieing progress on important social, academic, and occupational goals and milestones with cascading repercussions across the lifespan. Additionally, by the second decade of life, rates of depression increase between two and threefold for girls relative to boys (Cyranowski et al., 2000; Van de Velde et al., 2010), making adolescent females an especially important group to study (see also Nolen-Hoeksema, 2001). Adolescence is also a developmental period during which broader lifelong physical and mental health trajectories begin to crystalize but are still modifiable (Patton et al., 2016; Paus et al., 2008; Sawyer et al., 2012). For these reasons, it is critical to understand the mechanisms that give rise to depression vulnerability early in life when primary or secondary prevention and intervention strategies are still possible.
Having a mother with a history of depression is a well-recognized risk factor for developing the disorder, and this is especially true for female offspring (Hammen, 2018; Hyde et al., 2008). The process through which this occurs is called the intergenerational transmission of depression (Hammen, 2017). Interestingly, although depression is moderately heritable (Levinson, 2006), genetics account for less than half of the variance in depression rates among children of mothers with a history of depression (Goodman, 2007). Instead, the transmission of depression from mother to child appears more strongly influenced by parental modeling of maladaptive beliefs, behavioral proclivities, and coping styles, as well as circumstances present in the shared family environment (Hammen, 2018).
Notably, individuals who are depressed tend to generate more stressors in their lives (i.e., stress generation; Hammen, 1991, 1992; Liu, 2013), making the family environment of a home with a mother who is depressed fertile ground for children to experience more frequent and severe stressors (Hammen, Brennan, et al., 2004). This finding is concerning due to robust evidence that exposure to major life stressors is among the most powerful proximal predictors of depression (Cohen et al., 2019; Kendler et al., 2000; Monroe et al., 2009; Slavich & Irwin, 2014). Consequently, one mechanism that could help explain the intergenerational transmission of risk for depression involves the “transmission” of stressful environments from mother to daughter (Liu, 2013).
Although some evidence exists supporting the intergenerational transmission of acute and chronic stressors, especially interpersonal stressors (e.g., Hammen et al., 2012; Hammen, Shih, et al., 2004), to our knowledge, this work has only assessed stressors occurring over the past year, with no studies assessing stressors occurring across the entire life course. This approach of assessing recent life stressors is limited as it only enables the investigation of mechanisms underlying stressor-related proximal risk for depression and may miss critical predictive information occurring more distally to the actual onset of depression, which, in turn, permits only a narrow window in which to focus intervention efforts. Furthermore, lifetime exposure to major stressors is an important predictor of risk for depression among adolescents and young adults (Turner & Lloyd, 2004), suggesting that the intergenerational transmission of lifetime stressors may provide additional predictive information about depression risk that could, in turn, inform the development of earlier intervention targets. Indeed, numerous studies have demonstrated that exposure to distal stressors occurring early in life predicts increased risk for a variety of physical and psychiatric morbidities later in life (see Miller et al., 2011; Slavich & Irwin, 2014). Moreover, theorists have also long hypothesized that the negative impact of stressors on physical and psychological health should be cumulative over time, with individuals who experience more stressors being at relatively higher risk for deleterious outcomes (Cohen et al., 2019). Consequently, studies on the intergenerational transmission of stressors related to depression risk that focus on past-year stressors are limited in that they reveal only part of the mechanistic story.
To address this issue, we used a unique, new-generation stressor assessment instrument to comprehensively measure cumulative lifetime acute and chronic stressor exposures in adolescent females at either high or low maternal risk for developing a first episode of major depression. We also assessed the lifetime stressor exposure profiles of the youths’ mothers using a version of this instrument that is specifically designed to assess lifetime stressors in adults. This methodology was notable as it enabled us to investigate the extent to which lifetime stressors among high-risk adolescent daughters accounted for associations between the mothers’ lifetime exposures to stressors and the daughters’ premorbid depressive symptoms.
To accomplish this aim, we analyzed a series of moderated mediation models comparing two groups of mother-daughter dyads categorized by the daughters’ maternal risk for depression (high- versus low-risk) as determined using diagnostic interviews. In the low-risk group, neither the mothers nor the daughters had a history of depression. In the high-risk group, mothers, but not daughters, had a history of depression. We hypothesized that more lifetime acute and chronic stressors among mothers would be associated with higher depressive symptoms among their daughters indirectly through a positive association with the number of acute and chronic stressors daughters experienced over their lifetime. Moreover, we hypothesized that this indirect association would only occur in the group of high-risk daughters. For a conceptual diagram of this model, see Figure 1.
Figure 1.
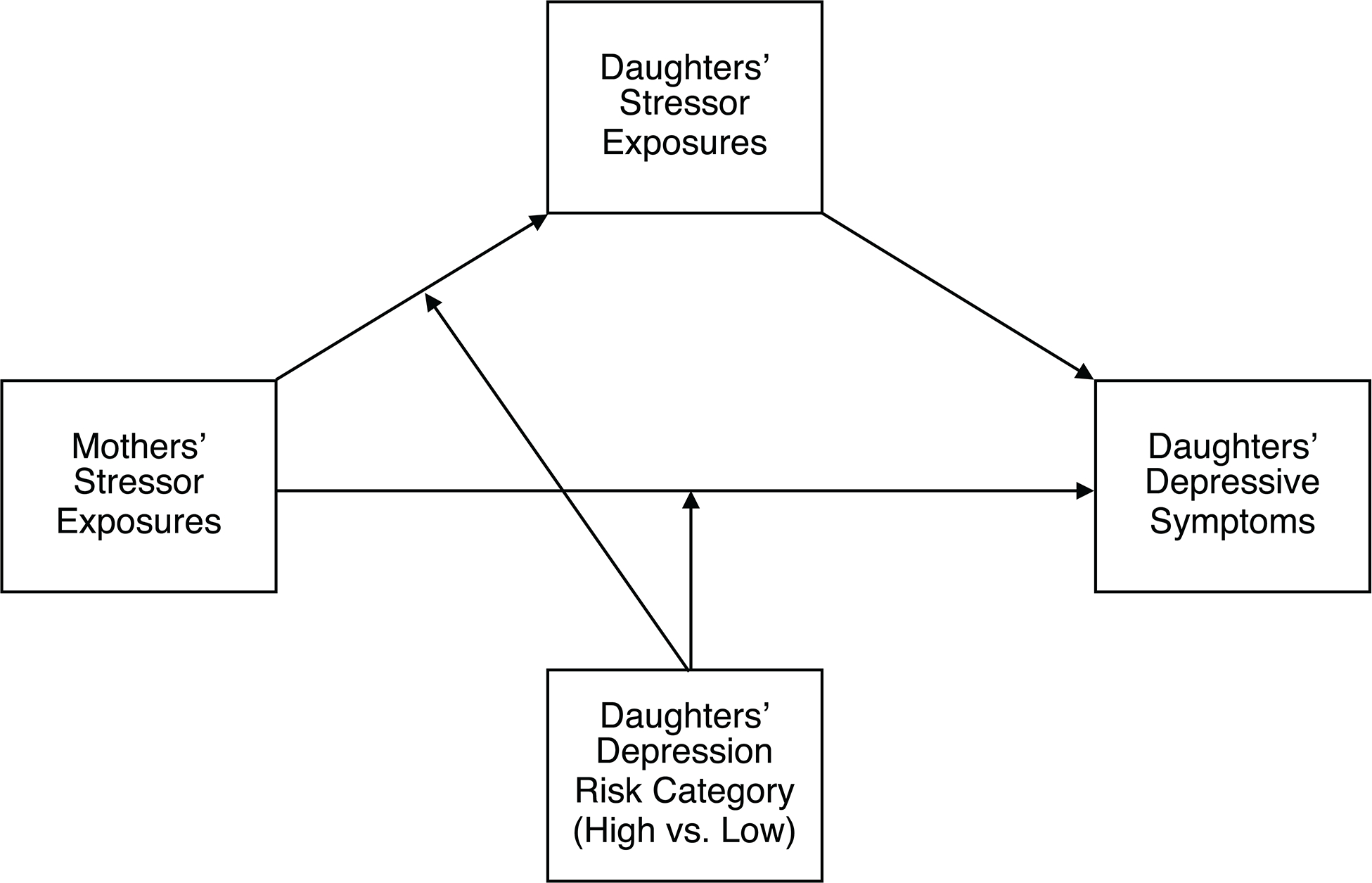
The general form of the moderated mediation models evaluated in our primary analyses. Daughters’ Stressor Exposures was the mediator, and Daughters’ Depression Risk Category (High vs. Low) was the moderator. Mothers’ Stressor Exposures included total lifetime acute stressors, total lifetime chronic stressors, acute stressors occurring before the birth of their daughters, chronic stressors occurring before the birth of their daughters, acute stressors occurring after the birth of their daughters, and chronic stressors occurring after the birth of their daughters. Daughters’ Stressor Exposures included total lifetime acute stressors and total lifetime chronic stressors. Daughters’ Depressive Symptoms included depressive symptoms assessed using the Mood and Feelings Questionnaire and depressive symptoms assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia.
In addition, we evaluated the independent contributions of mothers’ acute and chronic stressors occurring either before or after their daughters were born. Given the importance of the shared environment for transmitting the risk of depression, we hypothesized that the number of stressors mothers experienced after their daughters were born would be more strongly associated with the daughters’ depressive symptoms than the number of stressors mothers experienced before their daughters were born. Once again, we also hypothesized that this association would be unique to the group of high-risk daughters.
Finally, there is tentative evidence that chronic stressors are associated with depression in part because they can potentiate the impact of acute stressors (Hammen et al., 2009). Thus, we conducted secondary exploratory analyses to test whether the lifetime number of chronic stressors that high-risk daughters experienced further moderated the association between the lifetime number of acute stressors they experienced and their depressive symptoms. Consistent with Hammen et al. (2009), we hypothesized that the indirect association between the mothers’ lifetime acute stressors and their daughters’ depressive symptoms through the daughters’ lifetime acute stressors would be stronger among daughters reporting more versus fewer lifetime chronic stressors. As before, we hypothesized that this association would be observed only for high-risk daughters. For a conceptual diagram of this model, see Figure 2.
Figure 2.
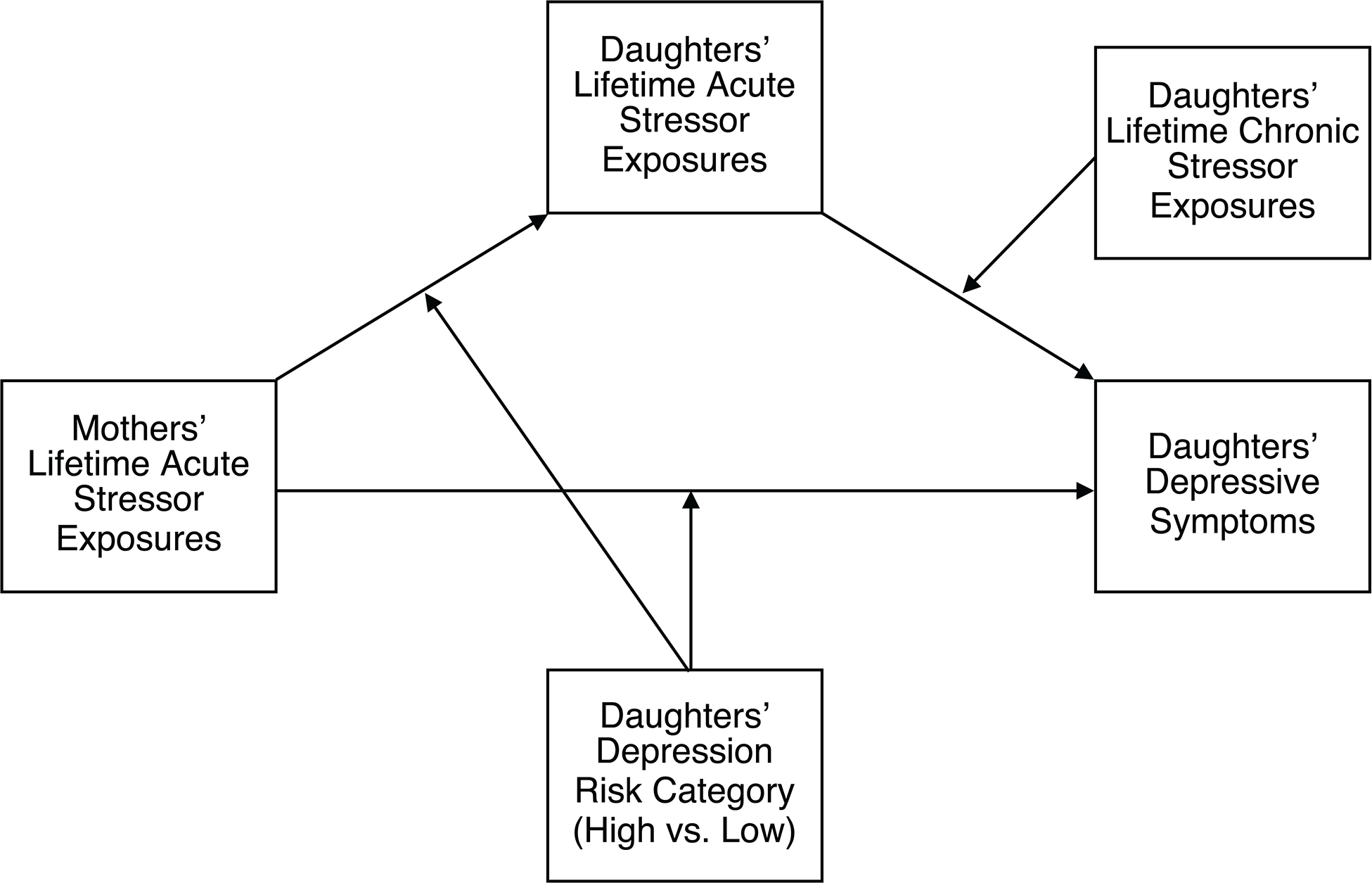
The secondary, exploratory model examining the extent to which daughters’ lifetime chronic stressor exposures additionally moderated associations between daughters’ lifetime acute stressor exposures and daughters’ depressive symptoms as assessed using either the Mood and Feelings Questionnaire or the Kiddie Schedule for Affective Disorders and Schizophrenia.
Method
Participants
Data for this report came from the Psychobiology of stress and adolescent depression (PSY SAD) study, a multimethod study designed to test hypotheses derived from the Social Signal Transduction Theory of Depression (Slavich & Irwin, 2014; Slavich & Sacher, 2019). Adolescent women who were at either high or low maternal risk for developing a first episode of major depressive disorder (MDD) and their biological mothers were recruited from throughout the greater Los Angeles, California area. Participants were recruited using community flyers, online advertisements, social media posts, school-based announcements, and word of mouth.
To be eligible, daughters had to be between 12 and 16 years old. The study focused on this age group because it is when the risk for MDD begins to increase substantially but before most individuals have experienced their first major depressive episode (Angold et al., 1998). Additionally, daughters had to be English-speaking, living with their biological mother, not be pregnant as verified with a pregnancy testing kit, not have any current or past affective disorders or recent alcohol or substance use or dependence, not have any head trauma, and not have any learning disabilities. The study also included fMRI and inflammatory biology assessments that are not relevant to the present report. Therefore, participants also had to be right-handed, have no bodily metal (except dental fillings) or other contraindications for fMRI, not be claustrophobic, not have a body mass index of ≥ 30 kg/m2, not report major sleep disturbances, tobacco use, prescription drug use, excessive caffeine use (defined as more than eight caffeinated beverages per day), and not have any current or past inflammatory diseases (O’Connor et al., 2009). There were no inclusion or exclusion criteria specific to the mothers.
A total of 52 mother-daughter dyads participated in this study, including 30 daughters at low maternal risk and 22 daughters at high maternal risk for developing a first episode of MDD. All participants provided written informed consent (mothers) and assent (daughters). The Institutional Review Board at the University of California, Los Angeles approved all study procedures. Mothers received 50 USD, and daughters received 125 USD for participating in this study. Daughters could also earn up to an additional 25 USD for participating in components of this study not relevant to the present report.
Procedures
Daughters and their biological mothers who expressed interest in the study first underwent a screening phone call that primarily involved the mothers. During this phone call, a research assistant described the study and answered questions about the study’s procedures, determined whether the daughters and their mothers would be likely to meet inclusion criteria, and scheduled a 90-minute intake session with the daughters and their mothers.
During the intake session, trained diagnostic interviewers separately screened daughters and mothers to ensure they met all inclusion criteria and did not meet any exclusion criteria and to determine the daughters’ MDD risk category. Having a mother with a history of MDD increases the risk of MDD for the daughter, especially early in adolescence, following puberty (Hammen, 2017; Hyde et al., 2008). As such, daughters’ MDD risk status was determined from standard diagnostic interviews of the daughters (Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version; K-SADS; Kaufman et al., 1997) and their mothers (Structured Clinical Interview for DSM-5–Research Version; SCID-5-RV; First et al., 2015). Daughters were categorized as high risk if they reported no current or past affective disorders but had a biological mother with a history of MDD. Daughters with no personal or maternal history of any clinical (formally Axis I) disorder were categorized as low risk. We used daughters’ depression risk status (0 = low risk, 1 = high risk) as a moderator in our analyses.
Following the diagnostic interviews, participants completed a variety of self-report questionnaires assessing sociodemographic features, psychosocial processes, and clinical symptoms. They then returned to the laboratory approximately one month later for a 3.5-hour experimental session that included fMRI-based tasks and blood draws not relevant to the present report. During this session, participants also completed a computer-administered assessment of lifetime exposure to acute and chronic stressors.
Measures
Lifetime History of Acute and Chronic Stressors
Lifetime exposure to acute and chronic stressors was assessed using the Stress and Adversity Inventory (STRAIN). Daughters completed the adolescent version of the STRAIN (Slavich et al., 2019), and mothers completed the adult version of the STRAIN (Slavich & Shields, 2018). Extensive documentation for the STRAIN is available online at https://www.strainsetup.com. In brief, the STRAIN is a new-generation instrument for assessing participants’ cumulative lifetime history of acute and chronic stressors based on the contextual threat approach (Brown & Harris, 1989; see also Cohen et al., 2016). Consistent with this framework, acute stressors are conceptualized as discrete events with a clear beginning and end, such as a spouse dying in a car accident. In contrast, chronic stressors are conceptualized as experiences that tend to be persistent or recurrent, such as experiencing ongoing financial hardship following a job loss. For an extensive discussion of this approach to conceptualizing and assessing stressors, distinctions between acute and chronic stressors, and related measurement issues, we refer readers to Monroe and Slavich (2020).
The STRAIN is designed to overcome issues with previous generations of self-report stressor inventories (for discussions, see Cohen et al., 2019; Dohrenwend, 2006; Hammen, 2016; Liu, 2013; Monroe, 2008; Monroe & Slavich, 2020). It includes many advantages of gold-standard life stress interviews such as the Life Events and Difficulties Schedule (Brown & Harris, 1989) but is easier to use and can be self-administered to reduce both participant and investigator burden (Slavich & Shields, 2018). Prior studies of both adolescents and adults spanning multiple cultural contexts have demonstrated that the STRAIN has excellent measurement properties, including very good concurrent validity, discriminant validity, predictive validity, and test-retest reliability (Cazassa et al., 2020; Slavich & Shields, 2018; Slavich et al., 2019; Sturmbauer et al., 2019).
Participants completed the STRAIN on a computer in a private testing room. The Adolescent STRAIN assesses 33 major acute and 42 major chronic stressors, and the Adult STRAIN assesses 26 major acute and 29 major chronic stressors. When participants endorsed having experienced a particular stressor, they then responded to a series of follow-up questions to determine the stressor’s frequency, exposure timing, duration, and subjective severity. The STRAIN was part of a much longer assessment battery, and some participants did not complete all the assessments due to time constraints. Consequently, six mothers did not complete the STRAIN. All 52 daughters completed the STRAIN.
We used the raw STRAIN data to compute two main STRAIN outcome variables for daughters and their mothers: (1) the total lifetime number of acute stressors and (2) the total lifetime number of chronic stressors. We also computed four additional stressor variables for mothers only: (1) the total number of acute stressors occurring before the birth of their daughter, (2) the total number of acute stressors occurring after the birth of their daughter, (3) the total number of chronic stressors occurring before the birth of their daughter, and (4) the total number of chronic stressors occurring after the birth of their daughter. To capture the temporal ordering of stressors in relation to the daughters’ births more accurately, for the stressor variables reflecting mothers’ experiences occurring before and after the daughters’ births, we did not include reported stressors that occurred during the same year that the daughters were born. As such, the number of stressors described by these variables adds up to a slightly smaller value than the total number of lifetime stressors.
The STRAIN also assesses individuals’ perceived stressor severity based on how “stressful” or “threatening” they judge each stressor to be on a scale from 1 (very slightly or not at all) to 5 (extremely). These ratings thus reflect participants’ subjective appraisals of how much the stressor impacted them. As discussed by Hammen (2016), subjective severity appraisals are confounded with depressive symptoms and related cognitive risk factors. Therefore, for the present study, we focused on the objective presence of stressors. However, for completeness of reporting, we present parallel results from models focusing on stressor severity in the Supplementary Material.
Current Depressive Symptoms
Daughters’ current depressive symptoms were assessed during the intake session using the MFQ (Costello & Angold, 1998; Wood et al., 1995), which is a self-administered, self-report instrument. Their depressive symptoms were also assessed using the K-SADS (Kaufman et al., 1997), a clinician-administered, structured diagnostic interview.
The MFQ contained 33 statements regarding how participants felt or acted during the past two weeks (e.g., “I felt miserable or unhappy,” “I blamed myself for things that weren’t my fault,” “I thought I could never be as good as other kids”). Participants indicated how accurately each statement described how they felt on a three-point scale, including 0 (not true), 1 (sometimes true), and 2 (true). We summed these scores to create an overall index of daughters’ current depressive symptoms, with higher scores indicating more symptoms. Composite reliability for this scale was excellent (ωTotal = 0.92).
The K-SADS was administered and scored by trained diagnostic interviewers. For the “present depression” component, interviewers queried participants about depressive symptoms within the past two weeks (e.g., depressed mood, anhedonia, sleep issues, cognitive disturbances). Interviewers used participant responses to code for the presence of 23 possible symptoms on a four-point scale that included 0 (no information), 1 (not present), 2 (sub-threshold), and 3 (threshold). To ensure diagnostic fidelity, senior author G.M.S oversaw weekly diagnostic training meetings and independently evaluated a random selection of cases from the high- and low-risk groups (κ = 1.00). Due to small numbers of missing data on some individual items, we averaged the scores across all available items and then multiplied these averages by 23, with higher scores indicating more symptoms.
As previously noted, having any current or past affective disorders identified using the K-SADS was a study exclusion criterion. Thus, unsurprisingly, there was a restricted range of observed K-SADS depressive symptoms (the maximum observed sample value was 64% of the maximum possible scale value). Likewise, there was also a restricted range of observed MFQ depressive symptoms. However, because the MFQ was not used to determine study eligibility, the range restriction was less dramatic than on the K-SADS (the maximum observed sample value on the MFQ was 71% of the maximum possible scale value). Therefore, the MFQ likely provided a more sensitive measure of current premorbid depressive symptoms for daughters than the K-SADS.
Covariates
For potential covariates, we considered daughters’ age, race/ethnicity, pubertal status, and subjective social status; mothers’ age, race/ethnicity, and education; family household income; and father’s education. However, including all nine variables would not have been practically feasible given the sample size and concerns about model overfitting. Therefore, we computed correlation coefficients for continuous and binary covariates and used either linear or logistic regression with dummy variables for multicategorical covariates to test for individual associations between each potential covariate and our predictor, mediator, and outcome variables, as well as depression risk status.
Not surprisingly, daughters’ ages were positively associated with their total lifetime number of acute stressors and chronic stressors, and mothers’ ages were positively associated with daughters’ total lifetime number of chronic stressors. There were no other significant associations between the covariates considered and any other study variables. As such, we only present the findings from unadjusted models. However, we note that analytic results based on models including daughters’ and mothers’ ages as covariates yielded substantively identical findings. Additionally, we present a table of associations between all study variables in the Supplementary Material.
Analytic Approach
We analyzed data using Mplus 8.6 (Muthén & Muthén, 1998–2021) to evaluate a series of moderated mediation models. Figure 1 provides a diagram showing the general form of the primary models we tested. In all models tested, the predictor variables were mothers’ stressors. The mediating variables were daughters’ stressors. The moderating variable was daughters’ depression risk status, which moderated the path from the predictor variables (i.e., mothers’ stressors) to the mediating variables (i.e., daughters’ stressors). Finally, the outcome variables were daughters’ current depressive symptoms based on either the MFQ or the K-SADS. We centered all continuous predictor and mediator variables for our analyses. Based on the results from a simulation study of various moderated mediation models by Preacher et al. (2007), using an alpha level of .05, our sample was well-powered (approximately 96%) to detect large-sized conditional indirect effects and moderately powered (approximately 63%) to detect medium-sized conditional indirect effects.
We also evaluated a secondary, exploratory model of whether daughters’ lifetime chronic stressors additionally moderated associations between daughters’ lifetime acute stressors and current depressive symptoms (see Figure 2). This model took the same general form as the model shown in Figure 1, except it added daughters’ lifetime chronic stressors as a moderator of the path from the mediating variable (i.e., lifetime acute stressors) to the outcome variable (i.e., depressive symptoms). Although we derived this model a priori based on existing theory and empirical observations (e.g., Hammen, 2016; Hammen et al., 2009), we describe the findings from this model as exploratory, given the limited sample size.
We estimated all models using full information maximum likelihood, which enabled us to retain cases with missing data (Muthén et al., 2016). To test for moderated mediation in each of the models, we calculated the index of moderated mediation (bindex), which describes the association between indirect effects and moderators (Hayes, 2015, 2018b). An index of moderated mediation that is significantly different from zero provides direct evidence that the nature of the indirect effect (i.e., through daughters’ stressors) depends on the value of the moderator (i.e., daughters’ depression risk status). When the index of moderated mediation is significant, it means that every conditional indirect effect is significantly different from every other conditional indirect effect. In models with evidence of moderated mediation, we then tested whether each of the individual conditional indirect effects (bindirect) was significantly different from zero. When describing the mediation results, we use the term “indirect effect” to be consistent with standard mediation analysis terminology and not to suggest that we inferred causation from cross-sectional analyses (for a broader discussion on using correlational data for mediation analyses, see Chapter 1, especially section 1.4, in Hayes, 2018a).
We made inferences about moderated mediation and model indirect effects using percentile bootstrapping with 100,000 resamples to construct asymmetrical 95% confidence intervals (CI95) around our parameter estimates (Biesanz et al., 2010; Hayes, 2018a). This method does not yield p-values. Mplus 8.6 does not provide users a way to set the seed used to generate initial values for bootstrapping to a specific number. As such, we used an especially large number of resamples to construct our bootstrapped confidence intervals. Doing so ensured that our model results remained stable and fully reproducible to at least three decimal places (the number of decimal places that Mplus outputs) from run to run.
Because the substantive focus of this report is the differential risk of the intergenerational transmission of lifetime stressors in predicting depressive symptoms, we do not report all the individual path results that would have historically formed the foundational basis for inferring an indirect effect using the classic causal steps approach to mediation as described by Barron and Kenny (1986). The causal steps approach to mediation requires making unnecessary assumptions about the significance of individual paths evaluated in separate models that can culminate in erroneous inferences—especially false negatives—about indirect effects (for a detailed discussion of this issue, see Chapter 4 in Hayes, 2018a). Moreover, relying on the causal steps logical framework to make inferences about mediation is no longer necessary because modern computing tools allow for the direct, quantitative evaluation of indirect effects.
Results
Preliminary and Descriptive Analyses
Demographics
The average age of the daughters was 14.96 years (SD = 1.36), and the average age of the mothers was 46.83 years (SD = 6.56). Of the daughters, 17 (32.7%) identified as White, 15 (28.8%) identified as Hispanic/Latino, 4 (7.7%) identified as Black/African American, 2 (3.8%) identified as Asian, 2 (3.8%) identified as Middle Eastern, and 12 (23.1%) identified as being multiracial. Among the 12 daughters who identified as being multiracial, 4 identified as White and Asian, 3 identified as White and Hispanic/Latino, 2 identified as Black/African American and Hispanic/Latino, 2 identified as White and Middle Eastern, and 1 identified as Middle Eastern and Native American. Of the mothers, 22 (42.3%) identified as White, 14 (26.9%) identified as Hispanic/Latino, 4 (7.7%) identified as Black/African American, 4 (7.7%) identified as Asian, 2 (3.8%) identified as Middle Eastern, 1 (1.9%) identified as Greek, 3 (5.8%) identified as being multiracial, and 2 (3.8%) declined to provide their race/ethnicity. Among the 3 mothers who identified as being multiracial, 1 identified as White and Asian, 1 identified as Black/African American and Hispanic/Latino, and 1 identified as White and Middle Eastern.
STRAIN Variables
There were two main STRAIN outcome variables for daughters and their mothers: (1) the total lifetime number of acute stressors (for daughters, M = 12.21, SD = 6.76; for mothers, M = 14.11, SD = 8.05) and (2) the total lifetime number of chronic stressors (for daughters, M = 9.37, SD = 5.86; for mothers, M = 10.04, SD = 4.71). Expectedly, acute stressors were positively associated with chronic stressors for both daughters, r(50) = .63, p < .001, CI95 = [.43, .77], and mothers, r(44) = .44, p = .002, CI95 = [.17, .65]. Across the entire sample, mothers’ lifetime number of acute stressors were not associated with daughters’ lifetime number of acute stressors, r(44) = .18, p = .25, CI95 = [−.12, .44], nor were mothers’ lifetime number of chronic stressors associated with daughters’ lifetime number of chronic stressors, r(44) = .03, p = .87, CI95 = [−.27, .31]. However, within the high-risk group, mothers’ number of acute stressors were strongly positively associated with daughters’ number acute stressors, r(17) = .77, p < .001, CI95 = [.47 .90], though mothers’ number of chronic stressors were not associated with daughters’ number of chronic stressors, r(17) = .11, p = .66, CI95 = [−.37, .53]. Conversely, mothers’ number of acute stressors were not associated with daughters’ number of acute stressors within the low-risk group, r(25) = −.20, p = .31, CI95 = [−.54, .20], nor were mothers’ number of chronic stressors associated with daughters’ number of chronic stressors, r(25) = −.27, p = .18, CI95 = [−.58, .13].
There were four additional stressor variables for mothers only: (1) the total number of acute stressors occurring before the birth of their daughter (M = 5.94, SD = 4.82), (2) the total number of acute stressors occurring after the birth of their daughter (M = 7.61, SD = 5.88), (3) the total number of chronic stressors occurring before the birth of their daughter (M = 5.72, SD = 3.26), and (4) the total number of chronic stressors occurring after the birth of their daughter (M = 3.91, SD = 2.51). There was no significant difference in the number of acute stressors that mothers reported having occurred before versus after the birth of their daughter, t(45) = 1.60, p = .117, d = 0.31, CI95 = [−0.09, 0.70]. However, mothers reported significantly more chronic stressors having occurred before versus after the birth of their daughter, t(45) = 3.15, p = .003, d = 0.61, CI95 = [0.20, 1.03].
Daughters’ ages were positively associated with their total lifetime number of acute stressors, r(50) = .31, p = .025, CI95 = [.04, .54], and chronic stressors, r(50) = .38, p = .005, CI95 = [.12, .59], and mothers’ ages were positively associated with daughters’ total lifetime number of chronic stressors r(50) = .31, p = .027, CI95 = [.03, .53].
Finally, in comparing daughters whose mothers completed the STRAIN (n = 46) to those whose mothers did not (n = 6), there were no significant differences in Mood and Feelings Questionnaire (MFQ) depression scores, t(50) = 1.212, p = .231, K-SADS scores, t(50) = −0.905, p = .370, the total number of lifetime acute stressors, t(50) = −0.046, p = .963, the total number of lifetime chronic stressors, t(50) = 0.455, p = .651, or daughters’ depression risk group category, χ2(1) = 0.164, p = .685.
Current Depressive Symptom Variables
The mean MFQ score for daughters was 11.31 (SD = 9.66), and the mean K-SADS symptom score was 26.55 (SD = 4.87). As expected, symptom scores on the K-SADS were positively correlated with those from the MFQ, r(50) = .52, p < .001, CI95 = [.28, .69].
Mothers’ and Daughters’ Total Lifetime Number of Acute and Chronic Stressors
Acute Stressors
The index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of lifetime acute stressors through daughters’ total number of lifetime acute stressors was significant, bindex = 0.657, CI95 = [0.164, 1.178]. This finding replicated when using K-SADS depressive symptoms as the main outcome, bindex = 0.193, CI95 = [0.035, 0.395]. For low-risk daughters, there was no conditional indirect effect of mothers’ lifetime acute stressors through daughters’ lifetime acute stressors on MFQ depressive symptoms, bindirect = −0.150, CI95 = [−0.448, 0.232], or K-SADS depressive symptoms, bindirect = −0.044, CI95 = [−0.126, 0.097]. However, as hypothesized, for high-risk daughters, there was a significant, positive conditional indirect effect for both MFQ depressive symptoms, bindirect = 0.507, CI95 = [0.176, 0.875], and K-SADS depressive symptoms, bindirect = 0.149, CI95 = [0.029, 0.334].
Chronic Stressors
The index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of lifetime chronic stressors through daughters’ total number of lifetime chronic stressors was not significant, bindex = 0.389, CI95 = [−0.110, 0.994]. The index was also not significant when using K-SADS depressive symptoms as the main outcome variable, bindex = 0.132, CI95 = [−0.036, 0.430].
Mothers’ Total Lifetime Numbers of Acute & Chronic Stressors Before Versus After Daughters’ Births and Daughters’ Total Lifetime Numbers of Acute & Chronic Stressors
Acute Stressors Before Daughters’ Births
The index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of acute stressors that occurred before the birth of their daughters through daughters’ total number of lifetime acute stressors was not significant, bindex = 0.109, CI95 = [−0.271, 0.467]. The index was also not significant when evaluating K-SADS depressive symptoms as the outcome variable, bindex = 0.022, CI95 = [−0.070, 0.107].
Acute Stressors After Daughters’ Births
In contrast, the index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of acute stressors that occurred after the birth of their daughters through daughters’ total number of lifetime acute stressors was significant, bindex = 1.491, CI95 = [0.369, 2.988]. This finding replicated when using K-SADS depressive symptoms as the outcome, bindex = 0.254, CI95 = [−0.064, 0.558]. As hypothesized, for low-risk daughters, there was no conditional indirect effect of mothers’ total number of acute stressors that occurred after the birth of their daughters through daughters’ lifetime acute stressors on MFQ depressive symptoms, bindirect = −0.347, CI95 = [−1.540, 0.344], or K-SADS depressive symptoms, bindirect = −0.093, CI95 = [−0.232, 0.263]. For high-risk daughters, however, there was a significant, positive conditional indirect effect for both MFQ depressive symptoms, bindirect = 1.144, CI95 = [0.243, 1.926], and K-SADS depressive symptoms, bindirect = 0.162, CI95 = [0.018, 0.417]. Moreover, this conditional indirect effect remained significant when adjusting the model for mothers’ total number of acute stressors that occurred before the birth of their daughters, bindirect(MFQ) = 0.425, CI95 = [0.083, 0.868], bindirect(K-SADS) = 0.244, CI95 = [0.017, 0.369]. This finding further supports the hypothesis that mothers’ stressors occurring after the births of their daughters are more strongly associated with daughters’ lifetime stressors and current depressive symptoms than those occurring before the births of their daughters.
Chronic Stressors Before Daughters’ Births
The index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of chronic stressors that occurred before the birth of their daughters through daughters’ total number of lifetime chronic stressors was not significant, bindex = 0.415, CI95 = [−0.219, 1.385]. The index was also not significant when evaluating K-SADS depressive symptoms as the main outcome variable, bindex = 0.138, CI95 = [−0.082, 0.567].
Chronic Stressors After Daughters’ Births
The index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of chronic stressors that occurred after the birth of their daughters through daughters’ total number of lifetime chronic stressors was not significant, bindex = 0.362, CI95 = [−0.634, 1.786]. The index was also not significant when using K-SADS depressive symptoms as the main outcome variable, bindex = 0.129, CI95 = [−0.241, 0.708].
Exploratory Analysis of Daughters’ Lifetime Chronic Stressors Moderating the Association Between Daughters’ Lifetime Acute Stressors and Depressive Symptoms
Finally, we examined whether daughters’ lifetime chronic stressors moderated the association between daughters’ lifetime acute stressors and depressive symptoms (see Figure 2 for a diagram of this model). The conditional index of moderated mediation for the model predicting daughters’ MFQ depressive symptoms from mothers’ total number of lifetime acute stressors through daughters’ total number of lifetime acute stressors additionally moderated by daughters’ total number of lifetime chronic stressors was significant for daughters at the sample average of chronic stress, bindex = 0.476, CI95 = [0.081, 1.016], as well as those who were one standard deviation above the sample average of chronic stress, bindex = 0.502, CI95 = [0.050, 1.021]. However, there was only weak evidence for the conditional index of moderated mediation for daughters who were one standard deviation below the sample average of chronic stress, bindex = 0.450, CI95 = [−0.004, 1.173]. The conditional indirect effects were significant and positive among high-risk daughters who were one standard deviation below the sample average of chronic stress, bindirect = 0.347, CI95 = [0.001, 0.902], those who were at the sample average of chronic stress, bindirect = 0.367, CI95 = [0.085, 0.755], and those who were one standard deviation above the sample average of chronic stress, bindirect = 0.387, CI95 = [0.061, 0.749]. Notably, the significant conditional index of moderated mediation means that each of these conditional indirect effects differed from each other. As hypothesized, therefore, high-risk daughters who were higher in chronic stressor exposures showed the largest conditional indirect effect of acute stressors.
Conversely, the conditional indirect effects were non-significant among low-risk daughters who were one standard deviation below the sample average of chronic stress, bindirect = −0.103, CI95 = [−0.412, 0.202], those who were at the sample average of chronic stress, bindirect = −0.109, CI95 = [−0.377, 0.176], and those who were one standard deviation above the sample average of chronic stress, bindirect = −0.115, CI95 = [−0.382, 0.160]. These findings did not replicate in the model predicting daughters’ K-SADS depressive symptoms instead of MFQ depressive symptoms (all 95% confidence intervals included 0), although the point estimates followed the same descriptive pattern as those observed in the analysis of MFQ depressive symptoms. In sum, therefore, the data provide tentative support for the hypothesis that chronic stressors potentiate the impact of acute stressors on depressive symptoms (Hammen et al., 2009).
Discussion
This cross-sectional study is the first that we know of to investigate the intergenerational transmission of cumulative lifetime stressor exposure. The resulting data are consistent with the intergenerational transmission of total lifetime acute but not chronic stressors predicting premorbid depressive symptoms as measured by self-report and independent diagnostic interviews for adolescent girls at high but not low maternal risk for developing a first episode of MDD. As hypothesized, high- but not low-risk daughters whose mothers experienced more acute stressors over their lifetime reported more lifetime acute stressors themselves, which, in turn, was associated with higher depressive symptoms.
In addition, we found that, as hypothesized, mothers’ acute stressors occurring after the birth of their daughters were indirectly associated with daughters’ depressive symptoms, whereas those occurring before the birth of their daughters were not. Moreover, this indirect association persisted when additionally adjusting the model for mothers’ acute stressors occurring before the birth of their daughters. Finally, consistent with preliminary evidence reported by Hammen et al. (2009), in exploratory analyses, we found tentative support for lifetime chronic stressors among high-risk daughters moderating the association between daughters’ lifetime acute stressors and self-reported depressive symptoms, with more chronic stressors being associated with a stronger positive association between daughters’ acute stressors and self-reported depressive symptoms.
Despite the modest sample size and cross-sectional design, this study provides at least two valuable insights on how risk for MDD may transmit from mother to daughter. First, the results extend prior research on the intergenerational transmission of stressors related to depression risk that assessed stressors occurring only over the past year (e.g., Hammen et al., 2012; Hammen, Shih, et al., 2004) to include stressors occurring over the entire lifespans of both mothers and daughters. Although the depressionogenic effect of recent life stress exposure is well documented (Monroe et al., 2009), the importance of cumulative exposure to stressors over the entire lifespan continues to be understudied and poorly understood (Cohen et al., 2019; Slavich, 2019; see also Turner & Lloyd, 2004). As such, if this finding replicates in larger, prospective studies, it will have important implications for designing interventions to prevent the initial onset of MDD for at-risk youth. In particular, the findings point to the potential value of assessing lifetime stress exposure in mothers and daughters to identify those who may benefit most from primary and secondary prevention efforts to reduce the risk for depression.
Second, the finding that mothers’ acute stressors that occurred only after (and not before) the birth of their daughters were positively associated with daughters’ depressive symptoms indirectly through daughters’ lifetime acute stressors adds to the body of literature highlighting the importance of shared environmental features to the intergenerational transmission of depression risk (Hammen, 2016, 2017, 2018). Interest in understanding the genetic and epigenetic mechanisms through which mothers’ early life experiences “get under the skin” to influence their future offspring’s vulnerability to stressors and related adverse health outcomes has exploded in recent decades (Beardslee et al., 2011; Bowers & Yehuda, 2020). Although this work is still in its infancy, it has already improved our understanding of mechanisms that explain differential vulnerability to adversity, and there are still many important discoveries to be made. Nevertheless, the findings we report here converge with prior findings to provide a reminder that the distant past is not necessarily prologue. From a mechanistic standpoint, the primary social-environmental culprit of intergenerational depression risk transmission may occur during the offspring’s lifetime, not before it. From a translational standpoint, these findings may thus provide some comfort to individuals worried about the seeming biological “inevitability” of passing their depression on to their future children and suggest that interventions aimed at changing mothers’ and daughters’ environments—and their relationships with their environments—likely hold considerable currency in helping to prevent depression.
It is possible that this pre- versus post-birth maternal stressor finding could be due to methodological limitations of the STRAIN. For example, the STRAIN could better capture more recent stressor exposures than more distal ones. Contrary to this possibility, however, there was no significant difference between the number of acute stressors that mothers recalled having occurred before versus after the birth of their daughters. Moreover, mothers actually reported significantly more chronic stressors occurring before the birth of their daughters than after, further suggesting that mothers’ recall of more distal life stressors was not biased downward. Of course, definitively ruling out the possibility that the STRAIN is less sensitive to capturing more distal stressors will require a decades-long prospective study.
Contrary to hypotheses, we did not find evidence for the intergenerational transmission of chronic stressors predicting depressive symptoms. The lack of findings for chronic stressors was surprising given prior theory and research highlighting the importance of chronic stressors to depression risk (Hammen, 2016; Hammen et al., 2009). One possibility is that we were not sufficiently powered to detect a conditional indirect effect of chronic stressors. For example, in a sample of 650 women, Hammen et al. (2009) found that both acute and chronic stressors predicted the onset of depression. However, the risk associated with chronic stressors was 42% weaker than the risk associated with acute stressors. To be able to detect a conditional indirect effect 42% smaller than what we observed for acute stressors with the same power our study had for our analyses of acute stressors, we would have needed a sample size of at least 366 participants (i.e., an additional 316 participants).
It is also possible that typical real-world chronic stressors largely exert their depressionogenic effects by augmenting acute stressors’ deleterious impact. Perhaps ongoing chronic stressors deplete an individual’s ability to effectively cope with novel acute stressors, resulting in heightened vulnerability to acute stressors (i.e., a sensitization effect; Hammen et al., 2009). It is noteworthy that we found tentative support for this possibility in secondary, exploratory analyses, mirroring previously published findings by Hammen et al. (2009). Either way, future studies with large samples are needed to evaluate this issue rigorously and clarify the role of chronic stressors in the intergenerational transmission of depression risk.
This study has several limitations. First, given that this study was cross-sectional, we cannot draw causal inferences from our analyses. The association between stressors and depression is bidirectional, and there are also confounding factors that could potentially explain these findings. Although the results converge with prior studies and existing theory, future prospective studies and experimental interventions will be necessary to establish causality. Second, our sample was diverse but modest in size. As a result, our power to detect medium conditional indirect effects was moderate, and our power to detect small conditional indirect effects was low. Therefore, some of our null findings (e.g., those relating to chronic stressors) may be Type II errors. Additionally, the sample size limited our ability to include conceptually relevant covariates in our models due to concerns about model overfitting. We tested nine potentially relevant covariates and found that none were significantly associated with both our predictor and outcome variables. However, low statistical power might also have resulted in Type II errors in at least some of these analyses. Third, the STRAIN does not evaluate the role that participants play in causing the stressors they experience (i.e., stressor dependency), a key component of depression-related stress generation (Hammen, 2006). Therefore, we were unable to categorize stressors based on whether participants were responsible for causing their occurrence—something that currently remains only possible to do using intensive interviews of recent life stress that do not readily scale to assessing lifetime stressor exposures.
Fourth, there is a growing body of evidence that interpersonal stressors—especially those that threaten social safety—may both be particularly transmittable and depressionogenic (Hammen, 2017; Kendler et al., 2003; Slavich, 2020b; Slavich & Irwin, 2014). The present study was not powered to examine the comparative “heritability” of individual stressors or stressor categories. Therefore, future studies that administer the STRAIN to much larger numbers of mothers and daughters will be necessary for evaluating whether specific stressors are more likely to transmit across generations in a way that subsequently increases depression risk. Fifth, the purpose of assessing mothers’ depression history was to categorize daughters into high- and low-risk groups. Because any maternal history of MDD was sufficient to characterize the daughter as high-risk, we did not code for other more nuanced details related to mothers’ depression histories, nor did we collate extensive information about affective comorbidities. Therefore, we were unable to evaluate whether any specific features of maternal depression or related affective comorbidities contributed more strongly to the association between the intergenerational transmission of stressors and daughters’ depressive symptoms. Additional research is thus needed to address these questions. Finally, there were several exclusion criteria for daughters in this study. Most of these exclusion criteria related to components of the study that were not reported on here (e.g., fMRI and immunological assessments). These exclusion criteria may limit the generalizability of our results (e.g., young females without major inflammatory diseases), and future studies are needed to examine these associations in other populations.
Despite these limitations, this study also has several notable strengths. First, we determined mothers’ and daughters’ mental health histories and daughters’ depression risk status using gold-standard diagnostic interviews, and we employed a multimethod (self-report and clinician-assessed) approach to assessing depressive symptoms. Second, we assessed lifetime stressor exposure using the STRAIN, a new generation stressor assessment instrument based on the contextual threat approach to measuring exposure to acute and chronic stressors, but that assesses stressors occurring over the entire lifetime (Slavich & Shields, 2018; Slavich et al., 2019). Third, we used state-of-the-science analytical methods to evaluate our models, increasing our power to detect moderated mediation while preserving Type I error rates.
In conclusion, the present data showed that mothers’ lifetime acute stressors are indirectly and positively associated with higher depressive symptoms among daughters at high risk for a first episode of MDD through increased lifetime acute stressors among the high-risk daughters. Moreover, this finding was driven by mothers’ acute stressors occurring after the birth of their daughter and not by those occurring before the birth of their daughter. There was also tentative evidence that high-risk daughters’ lifetime chronic stressors might further amplify the negative impact of their lifetime acute stressors on depressive symptoms. Additional research is needed to replicate these findings using prospective study designs as well as to examine the social-environmental, cognitive, and biological processes that might underlie the transmission of lifetime stressor exposure from mothers to daughters (Slavich, 2020b). In the interim, these findings provide important new evidence for how stressful contexts may be transmitted from mothers to daughters, especially in at-risk families. These data also suggest the possible utility of screening for and mitigating stressor exposure as a potential strategy for reducing the risk for depression and related disorders in adolescent females (Polick et al., 2021; Valderhaug & Slavich, 2020).
Supplementary Material
Acknowledgments
This research was supported by a Society in Science–Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and National Institutes of Health grant K08 MH103443 to GMS. These organizations were not involved in designing or conducting the study; collecting, managing, analyzing, or interpreting the data; preparing, reviewing, or approving the article; or deciding to submit the article for publication. The authors declare no conflicts of interest with respect to their authorship or the publication of this article.
Contributor Information
Michael L. M. Murphy, Department of Psychological Sciences, Texas Tech University
Stassja Sichko, Department of Psychology, University of California, Los Angeles.
Theresa Q. Bui, Tulane University School of Medicine
Mark R. Libowitz, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles
Grant S. Shields, Department of Psychological Science, University of Arkansas, Fayetteville
George M. Slavich, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles.
References
- Angold A, Costello EJ, & Worthman CM (1998). Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine, 28(1), 51–61. 10.1017/s003329179700593x [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Gladstone TRG, & O’Connor EE (2011). Transmission and prevention of mood disorders among children of affectively ill parents: A review. Journal of the American Academy of Child and Adolescent Psychiatry, 50(11), 1098–1109. 10.1016/j.jaac.2011.07.020 [DOI] [PubMed] [Google Scholar]
- Berlim MT, & Turecki G (2007). Definition, assessment, and staging of treatment-resistant refractory major depression: A review of current concepts and methods. The Canadian Journal of Psychiatry, 52(1), 46–54. 10.1177/070674370705200108 [DOI] [PubMed] [Google Scholar]
- Biesanz JC, Falk CF, & Savalei V (2010). Assessing mediational models: Testing and interval estimation for indirect effects. Multivariate Behavioral Research, 45, 661–701. 10.1080/00273171.2010.498292 [DOI] [PubMed] [Google Scholar]
- Bowers ME, & Yehuda R (2020). Intergenerational transmission of stress vulnerability and resilience. In Chen A (Ed.), Stress resilience: Molecular and behavioral aspects (pp. 257–267). Academic Press. 10.1016/b978-0-12-813983-7.00017-3 [DOI] [Google Scholar]
- Brown GW, & Harris TO (1989). Life events and illness. Guilford Press. [Google Scholar]
- Burcusa SL, & Iacono WG (2007). Risk for recurrence in depression. Clinical Psychology Review, 27(8), 959–985. 10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazassa MJ, Oliveira MDS, Spahr CM, Shields GS, & Slavich GM (2020). The Stress and Adversity Inventory for Adults (Adult STRAIN) in Brazilian Portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Frontiers in Psychology, 10, Article 3083. 10.3389/fpsyg.2019.03083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gianaros PJ, & Manuck SB (2016). A stage model of stress and disease. Perspectives on Psychological Science, 11(4), 456–463. 10.1177/1745691616646305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Murphy MLM, & Prather AA (2019). Ten surprising facts about stressful life events and disease risk. Annual Review of Psychology, 70, 577–597. 10.1146/annurev-psych-010418-102857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, & Angold A (1998). Scales to assess child and adolescent depression: Checklists, screens, and nets. Journal of the American Academy of Child and Adolescent Psychiatry, 27(6), 726–737. 10.1097/00004583-198811000-00011 [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, & Shear K (2000). Adolescent onset of the gender difference in lifetime rates of major depression. Archives of General Psychiatry, 57, 21–27. 10.1001/archpsyc.57.1.21 [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP (2006). Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin, 132(3), 477–495. 10.1037/0033-2909.132.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured Clinical Interview for DSM-5 – Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- Friedrich MJ (2017). Depression is the leading cause of disability around the world. JAMA, 317(15), 1517. 10.1001/jama.2017.3826 [DOI] [PubMed] [Google Scholar]
- Goodman SH (2007). Depression in mothers. Annual Review of Clinical Psychology, 3, 107–135. 10.1146/annurev.clinpsy.3.022806.091401 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, & Corey-Lisle PK (2003). The economic burden of depression in the United States: How did it change between 1990 and 2000? Journal of Clinical Psychiatry, 64, 1465–1475. [DOI] [PubMed] [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology, 100(4), 555–561. 10.1037/0021-843X.100.4.555 [DOI] [PubMed] [Google Scholar]
- Hammen C (1992). Life events and depression: The plot thickens. American Journal of Community Psychology, 20(2), 179–193. 10.1007/bf00940835 [DOI] [PubMed] [Google Scholar]
- Hammen C (2006). Stress generation in depression: Reflections on origins, research, and future directions. Journal of Clinical Psychology, 62, 1065–1082. 10.1002/jclp.20293 [DOI] [PubMed] [Google Scholar]
- Hammen C (2016). Depression and stressful environments: Identifying gaps in conceptualization and measurement. Anxiety, Stress, & Coping, 29(4), 335–351. 10.1080/10615806.2015.1134788 [DOI] [PubMed] [Google Scholar]
- Hammen C (2018). Risk factors for depression: An autobiographical review. Annual Review of Clinical Psychology, 14, 1–28. 10.1146/annurev-clinpsy-050817084811 [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, & Shih JH (2004). Family discord and stress predictors of depression and other disorders in adolescent children of depressed and nondepressed women. Journal of the American Academy of Child and Adolescent Psychiatry, 43(8), 994–1002. 10.1097/01.chi.0000127588.57468.f6 [DOI] [PubMed] [Google Scholar]
- Hammen C, Hazel NA, Brennan PA, & Najman J (2012). Intergenerational transmission and continuity of stress and depression: Depressed women and their offspring in 20 years of follow-up. Psychological Medicine, 42(5), 931–942. 10.1017/s0033291711001978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Kim EY, Eberhart NK, & Brennan PA (2009). Chronic and acute stress and the prediction of major depression in women. Depression and Anxiety, 26(8), 718–723. 10.1002/da.20571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Shih JH, & Brennan PA (2004). Intergenerational transmission of depression: Test of an interpersonal stress model in a community sample. Journal of Consulting and Clinical Psychology, 72(3), 511–522. 10.1037/0022-006x.72.3.511 [DOI] [PubMed] [Google Scholar]
- Hammen CL (2017). Maternal depression and the intergenerational transmission of depression. In Cohen NL (Ed.), Public Health Perspectives on Depressive Disorders (pp. 147–170). Johns Hopkins University Press. [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50(1), 1–22. 10.1080/00273171.2014.962683 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018a). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (2nd ed.). The Guilford Press. [Google Scholar]
- Hayes AF (2018b). Partial, conditional, and moderated moderated mediation: Quantification, inference, and interpretation. Communication Monographs, 85(1), 4–40. 10.1080/03637751.2017.1352100 [DOI] [Google Scholar]
- Hyde JS, Mezulis AH, & Abramson LY (2008). The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review, 115(2), 291–313. 10.1037/0033-295X.115.2.291 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, & Prescott CA (2003). Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry, 60, 789–796. 10.1001/archpsyc.60.8.789 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, & Gardner CO (2000). Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. American Journal of Psychiatry, 157, 1243–1251. 10.1176/appi.ajp.157.8.1243 [DOI] [PubMed] [Google Scholar]
- Levinson DF (2006). The genetics of depression: A review. Biological Psychiatry, 60(2), 84–92. 10.1016/j.biopsych.2005.08.024 [DOI] [PubMed] [Google Scholar]
- Liu RT (2013). Stress generation: Future directions and clinical implications. Clinical Psychology Review, 33(3), 406–416. 10.1016/j.cpr.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Malhi GS, & Mann JJ (2018). Depression. Lancet, 392(10161), 2299–2312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM (2008). Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology, 4, 33–52. 10.1146/annurev.clinpsy.4.022007.141207 [DOI] [PubMed] [Google Scholar]
- Monroe SM, & Slavich GM (2020). Major life events: A review of conceptual, definitional, measurement issues, and practices. In Harkness KL & Hayden EP (Eds.), The Oxford Handbook of Stress and Mental Health (pp. 7–26). Oxford University Press. 10.1093/oxfordhb/9780190681777.013.1 [DOI] [Google Scholar]
- Monroe SM, Slavich GM, & Georgiades K (2009). The social environment and life stress in depression. In Gotlib IH & Hammen CL (Eds.), Handbook of depression (2nd ed., pp. 340–360). Guilford Press. [Google Scholar]
- Muthén BO, Muthén LK, & Asparouhov T (2016). Regression and Mediation Analysis Using Mplus. Muthén & Muthén. [Google Scholar]
- Muthén LK, & Muthén BO (1998–2021). Mplus user’s guide (8th ed.). Muthén & Muthén. [Google Scholar]
- National Research Council and Institute of Medicine. (2009). Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. The National Academies Press. [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2001). Gender differences in depression. Current Directions in Psychological Science, 10(5), 173–176. [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, & Irwin MR (2009). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behavior and Immunity, 23, 887–897. 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, Arora M, Azzopardi P, Baldwin W, Bonell C, Kakuma R, Kennedy E, Mahon J, McGovern T, Mokdad AH, Patel V, Petroni S, Reavley N, Taiwo K, Waldfogel J, Wickremarathne D, Barroso C, Bhutta Z, Fatusi AO, Mattoo A, Diers J, Fang J, Ferguson J, Ssewamala F, & Viner RM (2016). Our future: A Lancet commission on adolescent health and wellbeing. Lancet, 387, 2423–2478. 10.1016/S0140-6736(16)00579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, & Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence. Nature Reviews Neuroscience, 9, 947–957. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polick CS, Polick SR, Stoddard SA, Braley TJ, & Slavich GM (2021). The importance of assessing life stress exposure in multiple sclerosis: A case report. Multiple Sclerosis and Related Disorders, 54, 103145. 10.1016/j.msard.2021.103145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, & Hayes AF (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42(1), 185–227. 10.1080/00273170701341316 [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, & Patton GC (2012). Adolescence: A foundation for future health. Lancet, 379, 1630–1640. 10.1016/s0140-6736(12)60072-5 [DOI] [PubMed] [Google Scholar]
- Slavich GM (2019). Stressnology: The primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain, Behavior, and Immunity, 75, 3–5. 10.1016/j.bbi.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2020a). Psychoneuroimmunology of stress and mental health. In Harkness KL & Hayden EP (Eds.), The Oxford handbook of stress and mental health (pp. 519–546). Oxford University Press. 10.1093/oxfordhb/9780190681777.013.24 [DOI] [Google Scholar]
- Slavich GM (2020b). Social Safety Theory: A biologically based evolutionary perspective on life stress, health, and behavior. Annual Review of Clinical Psychology, 16, 265–295. 10.1146/annurev-clinpsy-032816045159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Sacher J (2019). Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology, 236(10), 3063–3079. 10.1007/s00213-019-05326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the Stress and Adversity Inventory for adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17–27. 10.1097/psy.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Stewart JG, Esposito EC, Shields GS, & Auerbach RP (2019). The Stress and Adversity Inventory for Adolescents (Adolescent STRAIN): Associations with mental and physical health, risky behaviors, and psychiatric diagnoses in youth seeking treatment. Journal of Child Psychology and Psychiatry, 60(9), 998–1009. 10.1111/jcpp.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmbauer SC, Shields GS, Hetzel EL, Rohleder N, & Slavich GM (2019). The Stress and Adversity Inventory for Adults (Adult STRAIN) in German: An overview and initial validation. PLOS ONE, 15(5), e0216419. 10.1371/journal.pone.0216419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, & Lloyd DA (2004). Stress burden and the lifetime incidence of psychiatric disorder in young adults: Racial and ethnic contrasts. Archives of General Psychiatry, 61(5), 481–488. 10.1001/archpsyc.61.5.481 [DOI] [PubMed] [Google Scholar]
- Valderhaug TG, & Slavich GM (2020). Assessing life stress: A critical priority in obesity research and treatment. Obesity, 28, 1571–1573. 10.1002/OBY.22911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde S, Bracke P, & Levecque K (2010). Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Social Science & Medicine, 71(2), 305–313. 10.1016/j.socscimed.2010.03.035 [DOI] [PubMed] [Google Scholar]
- Verduijn J, Verhoeven JE, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman ATF, & Penninx BWJH (2017). Reconsidering the prognosis of major depressive disorder across diagnostic boundaries: Full recovery is the exception rather than the rule. BMC Medicine, 15(1), 215. 10.1186/s12916-017-0972-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A, Kroll L, Moore A, & Harrington R (1995). Properties of the Mood and Feelings Questionnaire in adolescent psychiatric outpatients: A research note. Journal of Child Psychology and Psychiatry, 36, 327–334. 10.1111/j.1469-7610.1995.tb01828.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


