Abstract
Objective
We assessed eating disorder (ED) illness status, symptomatology, treatment access, anxiety, and depression in the first year of the COVID‐19 pandemic among individuals with a pre‐existing ED in the United States (US), the Netherlands (NL), and Sweden (SE).
Methods
Participants completed online surveys in April–July 2020, at the early stage of the pandemic, and one year later. At one‐year follow‐up, we added questions addressing retrospective changes in ED symptoms, treatment, and anxiety/depression since the start of the COVID‐19 pandemic. We present descriptive statistics and assess change in ED symptomatology, treatment, and anxiety/depression among those with an active or lingering ED.
Results
Participants (US n = 132; NL n = 219; SE n = 702) were mostly young and female with a history of anorexia nervosa (>60% in all three countries). Across countries, respondents reported impact of COVID‐19 on ED symptoms at both time points, with improvement in US and NL at one‐year follow‐up, and stable but less impact on ED symptoms in SE. Furthermore, at one‐year follow‐up, roughly half of those in treatment reported reduced treatment access and quality, and the majority of the sample reported increased anxiety and depressive mood since the start of the pandemic.
Discussion
Our findings suggest that the self‐perceived impact of COVID‐19 changed over time but remained concerning even one year after the start of the pandemic. Clinicians, community organizations, and policy makers are encouraged to address potentially changing treatment needs in the face of public health emergency events.
Public Significance
Our findings suggest that the impact of COVID‐19 on individuals with eating disorders decreased over time but remained concerning even one year after the start of the pandemic and that the impact differed across countries. Clinicians, community organizations, and policy makers are encouraged to incorporate this knowledge to address potentially changing treatment needs in the face of public health emergency events.
Keywords: coronavirus, COVID‐19, eating disorders, mental health, pandemic
1. INTRODUCTION
Individuals with a pre‐existing eating disorder (ED) are vulnerable to the many challenges posed by the COVID‐19 pandemic (Frayn et al., 2021; Rodgers et al., 2020). During the early phase of the pandemic, individuals with EDs experienced worsening symptoms such as disordered eating behaviors, stress, anxiety, and depression (Birgegård et al., 2021; Castellini et al., 2020; Chan & Chiu, 2022; Fernandez‐Aranda et al., 2020; Giel et al., 2021; Phillipou et al., 2020; Schlegl et al., 2020; Termorshuizen et al., 2020). Disruption to daily routines, lack of social support, increased media consumption with possible provoking content, more time spent in a triggering environment, and fear of contagion, combined with disruptions in treatment access and quality due to public health restrictions may all be factors aggravating ED psychopathology and impeding recovery (Birgegård et al., 2021; Rodgers et al., 2020; Termorshuizen et al., 2020; Vitagliano et al., 2021; Weissman et al., 2020). Indeed, a systematic review that identified 22 mostly cross‐sectional studies reported a deterioration in ED symptoms and general well‐being among individuals with EDs since the start of the pandemic (Monteleone et al., 2021a).
Limited evidence exists concerning the longer‐term effects of the pandemic on this population. A recent review and meta‐analysis suggested that a majority of individuals with EDs reported a worsening of symptoms during lockdown in cross‐sectional studies, but the longitudinal data did not show significant differences in ED symptoms from pre‐pandemic levels to the first lockdown, and only few studies suggested an increase in distress, primarily in individuals with anorexia nervosa (Sideli et al., 2021). An Italian clinical sample reported worsening ED and general psychopathology during initial lockdown, with the latter persisting two weeks after lockdown, whereas ED symptoms returned to initial levels (Monteleone et al., 2021b). In our Swedish study, patterns of ED symptoms and well‐being were fairly stable between early on in the pandemic and six months later, although a subsample of symptom‐free individuals reported a reemergence of ED symptoms at six months (Birgegård et al., 2021). Thus, more knowledge on how the pandemic has affected people with EDs over time in different countries is of considerable value for understanding how societal events of similar nature may affect the course of an ED, as well as service planning.
Here, we examined whether ED illness status, ED symptoms, treatment, and anxiety/depression changed from the start to one year into the COVID‐19 pandemic among individuals with a self‐reported ED. We first addressed self‐reported ED illness status at both time points. We then selected two groups to enable a focused analysis on how people with current ED symptoms—specifically those with persistent active illness—were affected over time to inform clinical approaches to potential changing treatment needs. We used data from a survey launched in April–July 2020 in the United States (US), the Netherlands (NL), and Sweden (SE) of which baseline and six‐month follow‐up data have been described elsewhere (Birgegård et al., 2021; Termorshuizen et al., 2020). The countries had distinctly different public health approaches to addressing COVID‐19. Both US (CDC, 2022) and NL (de Haas et al., 2020) had extended periods of lockdown, curfews, and shuttering of schools and businesses, whereas SE (Ludvigsson, 2020) remained largely open, albeit with recommendations about working from home, not seeing people outside the close family, and isolating if symptoms arise.
2. METHODS
2.1. Study setting and design
Participants were recruited from previous studies (US [Bulik et al., 2020; Thornton et al., 2018], SE [Thornton et al., 2018]), social media (US, NL; e.g., Facebook, Twitter, Instagram), the UNC Exchanges blog (US), the online ED community Proud2Bme (NL), and the Dutch Eating Disorder Register (NL). Individuals in the study population, i.e., individuals with a current or past self‐reported ED aged 18 years or older in the US, and aged 16 years or older in the NL and SE, were invited to complete a survey about the consequences of the COVID‐19 pandemic on general health, ED symptoms, and anxiety/depression. The survey was advertised as a study on the impact of COVID‐19 on people with a current or past ED. Although all participants from SE had verified diagnoses from the parent studies, for this study, we used self‐reported diagnosis and self‐reported symptom status to capture current symptomatology.
We recruited study participants early in the COVID‐19 pandemic and subsequently continued monthly (US, NL) or bi‐annual (SE) data collection until one year after the baseline survey. The number of participants at each follow‐up is presented in Table S1. In this article, we included baseline and one‐year follow‐up data. Baseline survey data (hereafter “T1”) were collected in the second quarter of 2020 (April 8–May 6 [US], April 17–May 15 [NL], May 27–July 2 [SE]). Enrollment was open for four (US, NL) and five (SE) weeks. The one‐year follow‐up survey (hereafter “T2”) was distributed one year later (April 8–May 28 [US]; April 26–May 21 [NL]; May 6–June 16 [SE]). For T2, in the US, individuals were recontacted during the week corresponding to their initial enrollment week the year prior and were given one month to complete the survey. In NL and SE, all participants were approached at the same time for T2 (April 26 [NL], May 6 [SE]); reminders were sent one, two, and three weeks later, and individuals had four (NL) and six (SE) weeks to complete the survey. Surveys were distributed via Qualtrics software (Qualtrics, 2005) (US and NL T2), SurveyMonkey (SurveyMonkey, 2021) (NL T1), and ConfirmIT (SE). No compensation for participation was offered at T1. At T2, US participants who completed the survey were entered into a drawing for an iPad mini. No compensation was offered in NL or SE. The original surveys were developed in English and subsequently translated to Dutch and Swedish. In US and NL follow‐up surveys, Qualtrics limited responses to one per IP address and prevented indexing (preventing search engines from finding the survey)—in SE, personal identification via electronic authentication was used (“BankID”). Ethical permission was granted by the University of North Carolina Biomedical Institutional Review Board for the United States (IRB number 20‐0964) and for Sweden by the Swedish Ethical Review Authority (Dnr 2020‐04136). The Medical Research Ethics Committee (MREC‐LDD) of Leiden University Medical Centre reviewed the study protocol and confirmed that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study and official approval of this study by METC was not required for the NL survey. Participants signed online consent in all three countries.
2.2. Measures
An overview and summary of the questions included in the T1 survey can be found in Termorshuizen et al. (2020), and in the Supplementary Material. Briefly, the survey inquired about COVID‐19 exposure, national and local COVID‐19 mitigation strategies (e.g., social distancing, quarantine), and the self‐perceived impact of COVID‐19 on ED symptoms, treatment, and anxiety/depression. The T2 survey contained identical items to T1 with additional questions about racial identity (US only), employment, insurance status (US only), COVID‐19 vaccination, and long‐term COVID‐19 symptoms. Furthermore, we added five items addressing retrospective change in ED symptoms, treatment access and quality, depressive mood, and anxiety since the start of the COVID‐19 pandemic. ED diagnosis was self‐reported, and subsequently, participants indicated their current illness status at both time points: currently an ED (“actively ill”), past ED and lingering symptoms (“lingering”), or past ED and no current symptoms (“no symptoms”). Participants also completed the Generalized Anxiety Disorder 7‐item scale (GAD‐7) (Spitzer et al., 2006) to assess self‐reported symptoms of GAD. Here, we used GAD‐7 total scores, and scores of 0–4, 5–9, 10–14, and 15–21 represent minimal, mild, moderate, and severe anxiety, respectively (Spitzer et al., 2006). Cronbach's alpha showed evidence of internal consistency in NL and SE (0.9 at T1, 0.8 at T2 [NL]; 0.9 at T1, 0.9 at T2 [SE]), but was lower in US possibly due to the smaller sample size (0.5 at T1, 0.6 at T2). The scale has shown broader evidence of reliability and validity (Donker et al., 2011; Lowe et al., 2008).
2.3. Statistical analysis
Data are presented and analyzed separately by country. The study sample comprised individuals who responded to T1 and T2 surveys, and we first present participant flow, attrition analysis, and basic descriptive statistics. In the attrition analysis, we compared responders versus non‐responders (i.e., those who did vs. those who did not respond to T2) on baseline variables age, gender, ED diagnosis, illness status, ED symptoms, treatment status, GAD‐7 total score, and self‐reported change in anxiety since the end of 2019 using chi‐squared tests (or Fisher's exact test if observed counts were <6 for categorical variables) and independent t‐tests (or Wilcoxon Rank‐Sum test if continuous variables were not normally distributed).
We then present descriptive statistics of self‐reported illness status over time, and we specified two groups for subsequent analyses: (Group 1) those reporting being actively ill with an ED or having lingering symptoms at T1, and (Group 1a—a subgroup of Group 1) those reporting being actively ill at T1 and T2. We evaluated changes within these groups in ED symptomatology, ED treatment status, and well‐being with descriptive statistics and McNemar's tests for discrete variables and paired t‐tests or Wilcoxon‐signed Rank tests for nonparametric continuous variables. Variables with a Likert scale addressing the impact of COVID‐19 on ED symptoms were dichotomized: we combined those who responded, “not at all” or “once or twice” versus “frequently” or “daily or more.” We did not perform a McNemar's test if the number of discordant pairs (i.e., if within‐individual values at T1 and T2 differ for that outcome) in a contingency table was <20, as suggested in Rosner (2016). We corrected for multiple testing with the false discovery rate (FDR) per country and report q‐values (i.e., p‐values adjusted by FDR) (Benjamini & Hochberg, 1995). We considered imputation for handling missing data, but we did not expect this to remedy bias due to the high attrition rates. We therefore handled missing data by performing pairwise deletion and conducted attrition analyses to understand selection characteristics associated with nonresponse. We thus assume data are missing completely at random. Item‐level‐missingness could be present in all countries: in US, individuals could skip questions whereas, in NL and SE, individuals could prematurely exit the survey. Analyses were performed in R version 4.0.5 (R Core Team, 2020).
3. RESULTS
3.1. Attrition analysis
We observed substantial drop‐out (US 74%; NL 57%; SE 29%) between the initial recruitment (US 510; NL 510; SE 982; total 2002) and one‐year follow‐up (US 132; NL 219; SE 702) across countries. Attrition analyses revealed no differences between responders and non‐responders except on GAD‐7 total scores in the US, where scores at T1 were significantly higher among responders than non‐responders (t[510] = −3.8, p = 1.7 × 10−4) (Table S2).
3.2. Sample descriptives
The current study sample comprised 1053 participants who responded at T1 and T2. Study participants were predominantly female and young adults (Table 1). In the US, 94% of the sample reported their race as “White” and the remainder reported “Asian,” “Native Hawaiian or Pacific Islander,” “More than one race,” or “Other.” Further, 94% had health insurance at T2; these items were not assessed in NL and SE (Table S3). In all countries, 80% or more reported a history of either anorexia nervosa, bulimia nervosa, or binge‐eating disorder (US 89%, NL 80%, SE 88%). Additionally, 52% (US)/38% (NL)/51% (SE) reported multiple lifetime ED diagnoses.
TABLE 1.
Basic descriptive statistics of age group, gender, eating disorder diagnosis, and COVID‐19 circumstances
| Participants n (%) | ||||||
|---|---|---|---|---|---|---|
| United States | Netherlands | Sweden | ||||
| N | 132 | 219 | 702 | |||
| Mean ± SD | 32.1 ± 10.4 | – | 32.3 ± 8.6 | |||
| Age | ||||||
| 16–21 years | 12 (9) | 50 (23) | 22 (3) | |||
| 22–29 years | 60 (45) | 96 (44) | 258 (37) | |||
| 30–39 years | 29 (22) | 48 (22) | 274 (39) | |||
| 40–49 years | 20 (15) | 10 (5) | 119 (17) | |||
| 50+ years | 10 (8) | 15 (7) | 29 (4) | |||
| Gender | ||||||
| Male | <5 (<5) | 6 (3) | 12 (2) | |||
| Female | 130 (98) | 213 (97) | 685 (98) | |||
| Other | 0 | 0 | <5 (<5) | |||
| Eating disorder diagnosis a | ||||||
| Anorexia nervosa | 90 (68) | 147 (67) | 455 (65) | |||
| Bulimia nervosa | 42 (32) | 43 (20) | 254 (36) | |||
| Binge‐eating disorder | 31 (24) | 20 (9) | 161 (23) | |||
| Other b | 95 (72) | 96 (44) | 402 (57) | |||
| COVID‐19 circumstances c | T1 | T2 | T1 | T2 | T1 | T2 |
| Currently quarantined | 54 (41) | <5 (<5) | 34 (16) | <5 (<5) | 47 (7) | 21 (3) |
| Working from home | 86 (65) | 50 (38) | 110 (50) | 68 (31) | 332 (47) | 351 (51) |
| Physical distancing | 130 (99) | 108 (82) | 213 (98) | 181 (83) | 554 (79) | 521 (75) |
| Has had COVID‐19 | <5 (<5) | 10 (7) | <5 (<5) | 15 (7) | 12 (2) | 113 (16) |
| Got 1st vaccination | – | 98 (74) | – | 42 (19) | – | 137 (20) |
Note: Percentages on available data are reported when data are missing. In NL, participants could only select age categories rather than reporting specific ages.
Abbreviations: NL, the Netherlands; SD, standard deviation; SE, Sweden; US, United States.
Percentages could sum to over 100% as individuals could select multiple options.
Study participants could check any of the following diagnoses, which were then combined in the category “Other” for this table: Avoidant restrictive food intake disorder/atypical anorexia nervosa/purging disorder/night‐eating syndrome/other specified feeding or eating disorder/other eating disorder.
For these items, US total n 130–132, NL total n 218–219, SE total n 694–701; total n might deviate from the total included because individuals could skip questions (US) or exit the survey prematurely (US, the NL, SE).
3.3. Illness status
In US and SE, the distribution of illness states was similar at T1 and T2, whereas in NL, more individuals reported lingering symptoms at T2 compared to T1 and fewer individuals reported being actively ill at T2 (Figure 1; percentages in left and right panels). We then analyzed whether participants maintained the same illness status over time (Figure 1; body of the figure). First, among those actively ill at T1 (Figure 1, red) in all three countries, the majority still reported an active illness at T2 and the remaining ~30% improved (i.e., reported lingering or no symptoms at T2). Second, for those with lingering symptoms at T1 (Figure 1, blue), the majority reported this status at T2, as well, and the remainder either deteriorated or improved with slightly differing patterns across countries. Lastly, among those reporting no symptoms at T1 in SE (Figure 1, green), the majority again reported no symptoms at T2 and the remaining 19% reported deterioration (US and NL numbers are not presented here due to the small sample size).
FIGURE 1.
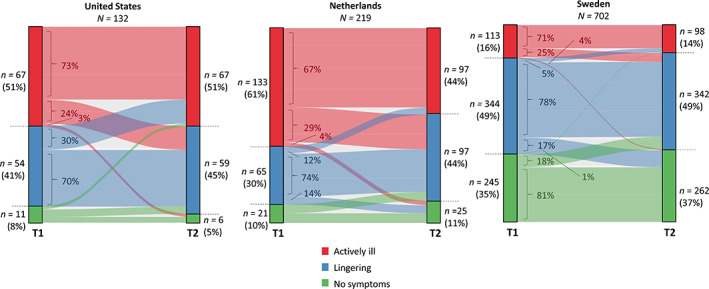
Eating disorder illness status transition. Self‐reported illness status at T1 and T2 and flow between illness states over time. The two columns—marked by T1 and T2 on the x‐axis—represent the counts and percentages of self‐reported illness states at T1 and T2, respectively. The body of the figure—with faded colors—represents shifts between illness states, with percentages indicating the proportion within each illness status reported at T1.
In the following sections we focus specifically on those reporting being actively ill or having lingering symptoms at T1 (Group 1; US n = 121; NL n = 198; SE n = 457) and a subset of Group 1 comprising those reporting being actively ill at T1 and T2 (Group 1a; US n = 49 [40%]; NL n = 89 [45%]; SE n = 80 [18%]) (descriptives in Table S4).
3.4. ED symptomatology
We observed two main patterns of change in ED symptoms due to COVID‐19‐related factors (Figure S1, Table 2). First, some respondents in all three countries self‐reported that COVID‐19‐related factors were associated with worsening of binge eating, restricting, compensatory behaviors, and anxiety about the inability to exercise at both time points. Second, in US and NL, significantly more people self‐reported restriction, compensatory behaviors, and, in the US, anxiety about the inability to exercise due to COVID‐19‐related factors at T1 compared with T2 (this pattern is visualized in Figure S1 for Group 1). This was not the case in SE, where respondents reported no difference in ED symptom engagement over time.
TABLE 2.
COVID‐19‐related impact on eating disorder symptoms early on and 1 year into the pandemic
| Item In the past two weeks, I have… | Country | % “frequently” or “daily or more” | McNemar test | |||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | Conclusion | Statistics | |||||
| n (%) | n (%) | df, n | χ 2 | p | q | |||
| Group 1: Actively ill or lingering symptoms at T1 (US n = 121; NL n = 198; SE n = 457) | ||||||||
| … binged on food more | US | 18 (14) | 14 (11) | – | – | |||
| NL | 29 (15) | 26 (13) | NS | 1, 198 | .2 | .7 | .8 | |
| SE | 50 (11) | 50 (11) | NS | 1, 446 | 0 | 1 | 1 | |
| … restricted my intake more | US | 61 (50) | 25 (20) | T1 > T2 | 1, 120 | 24.5 | 7.4 × 10 −7 | 5.2 × 10 −6 |
| NL | 86 (43) | 21 (11) | T1 > T2 | 1, 198 | 56.1 | 6.8 × 10 −14 | 7.5 × 10 −13 | |
| SE | 75 (17) | 63 (14) | NS | 1, 446 | .9 | .4 | .8 | |
| … engaged more in compensatory behaviors | US | 53 (44) | 29 (24) | T1 > T2 | 1, 120 | 13.9 | 2.0 × 10 −4 | 2.8 × 10 −4 |
| NL | 79 (40) | 38 (19) | T1 > T2 | 1, 198 | 24.6 | 7.0 × 10 −7 | 1.9 × 10 −6 | |
| SE | 53 (12) | 53 (12) | NS | 1, 446 | 0 | 1 | 1 | |
| … felt anxious about not being able to exercise | US | 72 (59) | 46 (38) | T1 > T2 | 1, 120 | 14.9 | 1.0 × 10 −4 | 1.8 × 10 −4 |
| NL | – | 56 (28) | – | – | ||||
| SE | 165 (36) | 153 (34) | NS | 1, 446 | .6 | .4 | .8 | |
| Group 1a: Actively ill at both T1 and T2 (US n = 49; NL n = 89; SE n = 80) | ||||||||
| … binged on food more | US | 7 (14) | 4 (8) | – | – | |||
| NL | 15 (17) | 14 (16) | – | – | ||||
| SE | 17 (21) | 22 (28) | – | – | ||||
| … restricted my intake more | US | 35 (72) | 9 (18) | T1 > T2 | 1, 48 | 22.3 | 2.3 × 10 −6 | 8.1 × 10 −6 |
| NL | 50 (56) | 15 (17) | T1 > T2 | 1, 89 | 28.2 | 1.1 × 10 −7 | 4.0 × 10 −7 | |
| SE | 21 (26) | 21 (26) | – | – | ||||
| … engaged more in compensatory behaviors | US | 34 (70) | 13 (26) | T1 > T2 | 1, 48 | 19.0 | 1.3 × 10 −5 | 3.3 × 10 −5 |
| NL | 46 (52) | 28 (32) | T1 > T2 | 1, 89 | 8.5 | .004 | .007 | |
| SE | 20 (25) | 20 (25) | – | – | ||||
| … felt anxious about not being able to exercise | US | 33 (68) | 24 (49) | – | – | |||
| NL | – | 33 (37) | – | – | ||||
| SE | 30 (38) | 32 (40) | – | – | ||||
Note: The McNemar test was not performed (−‐) when the number of discordant pairs was <20 or due to unavailable data. Differences between two time points are indicated by a bold font (q < .05). For these items, no responses were missing in US, no responses were missing in NL, and nine were missing in SE (Group 1).
Abbreviations: NL, Netherlands; NS, nonsignificant; SE, Sweden; US, United States.
3.5. ED treatment
Here we report results from those actively ill at both time points only (Group 1a; Table 3). In the US, most individuals received online treatment at T1, and at T2 40% reported still receiving online treatment. In NL, on the other hand, the proportion of people receiving online treatment decreased significantly at T2 compared to T1, whereas the proportion receiving face‐to‐face treatment increased. In SE, proportions at both time points did not differ significantly: 10% received online care at both time points, and the majority did not receive any treatment. Also, in all three countries, the proportion of individuals not in treatment was consistently high at both time points. Last, approximately half of those in treatment in all three countries reported a decline in treatment access and/or quality since the start of the pandemic, less than 10% reported an improvement, and the remainder reported no change in treatment access and/or quality since the start of the pandemic (Figure S2).
TABLE 3.
Treatment status over time (Group 1a)
| Treatment modality | Country | T1 n (%) | T2 n (%) | McNemar test | ||||
|---|---|---|---|---|---|---|---|---|
| Conclusion | Statistics | |||||||
| df, n | χ 2 | p | q | |||||
| Group 1a: Actively ill at both T1 and T2 (US n = 49; NL n = 89; SE n = 80) | ||||||||
| Face‐to‐face | US | <5 (<5) | 12 (25) | – | – | |||
| NL | 8 (9) | 35 (39) | T2 > T1 | 1, 89 | 23.3 | 1.4 × 10 −6 | 3.1 × 10 −6 | |
| SE | 22 (28) | 23 (29) | NS | 1, 80 | 0 | 1 | 1 | |
| Online | US | 31 (63) | 19 (40) | – | – | |||
| NL | 50 (56) | 9 (10) | T1 > T2 | 1, 89 | 34 | 5.4 × 10 −9 | 3.0 × 10 −8 | |
| SE | 8 (10) | 8 (10) | – | – | ||||
| Not in treatment | US | 12 (24) | 15 (31) | – | – | |||
| NL | 27 (30) | 37 (42) | NS | 1, 89 | 3.4 | .07 | .09 | |
| SE | 46 (58) | 43 (54) | – | – | ||||
Note: We displayed McNemar test statistics if tests were performed, and “–” in case the number of discordant pairs was <20. Differences between two time points are indicated by a bold font (q < .05). For these items, no responses were missing in US, no responses were missing in NL, and no responses were missing in SE.
Abbreviations: NL, Netherlands; NS, nonsignificant; SE, Sweden; US, United States.
3.6. Anxiety/depression
Across all countries, time points, and in both illness status groups, average GAD‐7 total scores for participants fell into the “moderate” range (i.e., 10–14; Table 4). GAD‐7 total scores were significantly higher at T1 than at T2 in the US, whereas in NL, this was only true for Group 1 (those reporting being actively ill or having lingering symptoms at T1), and there were no significant differences in SE. Furthermore, across all countries, the majority self‐reported an increase in anxiety and depressive mood since the pandemic began (Figure S3) and >90% reported that retrospective increases in anxiety or depression were “somewhat” or “a lot” due to COVID‐19.
TABLE 4.
Statistics of the Generalized Anxiety Disorder 7‐item scale (GAD‐7) total score at both time points
| Country | GAD‐7 total score | Paired t‐test | |||||
|---|---|---|---|---|---|---|---|
| Conclusion | Statistics | ||||||
| T1 M ± SD | T2 M ± SD | n | t/W | p | q | ||
| Group 1: Actively ill or lingering symptoms at T1 (US n = 121; NL n = 198; SE n = 457) | |||||||
| US | 12.0 ± 5.8 | 10.7 ± 5.9 | T1 > T2 | 123 | 3 | .004 | .005 |
| NL | 11.9 ± 5.5 | 11.0 ± 5.3 | T1 > T2 | 198 | 9073.5 | .01 | .02 |
| SE | 10.9 ± 5.7 | 10.6 ± 5.7 | NS | 457 | 42,557 | .1 | .4 |
| Group 1a: Actively ill at both T1 and T2 (US n = 49; NL n = 89; SE n = 80) | |||||||
| US | 13.2 ± 5.5 | 11.3 ± 5.5 | T1 > T2 | 44 | 2.8 | .007 | .007 |
| NL | 13.3 ± 4.8 | 13.2 ± 4.9 | NS | 89 | 1414.5 | .9 | 1.0 |
| SE | 14.4 ± 4.9 | 13.7 ± 5.5 | NS | 80 | 1405.5 | .05 | .4 |
Note: The Wilcoxon‐signed rank test was performed as a nonparametric alternative for the paired t‐test if appropriate. Differences between two time points are indicated by a bold font (q < .05). For these items, 4 (Group 1) and 1 (Group 1a) responses were missing in US, 10 (Group 1) and 4 (Group 1a) responses were missing in NL, and 10 (Group 1) and 0 (Group 1a) were missing in SE.
Abbreviations: M, mean; NL, Netherlands; NS, nonsignificant; SD, standard deviation; SE, Sweden; US, United States.
4. DISCUSSION
This three‐country collaborative study suggests that the impact of COVID‐19 on individuals with an ED was substantial at the start of the pandemic and continued to adversely affect many members of this population one year later. These findings should be interpreted within the context of the timing of data collection, which began soon after the pandemic started: the results reflect self‐perceived impact in the absence of direct pre/post pandemic comparisons. In all three countries, COVID‐19‐related factors led to perceived worsening of ED symptoms, treatment access and quality, and anxiety and depression. Patterns of ED treatment suggested that the majority of NL participants transitioned back from online to face‐to‐face treatment one year into the pandemic; this pattern was less apparent in the US, where 40% still received online treatment at T2; and absent in SE, where 10% received online treatment at both time points. Our prior studies (Birgegård et al., 2021; Termorshuizen et al., 2020) and related studies (Linardon et al., 2022; Sideli et al., 2021) indicated that participants perceived that ED severity and comorbidity increased in the early phase of the pandemic from pre‐pandemic levels. This study newly suggests that the self‐perceived mental health impact of COVID‐19 on individuals in US, NL, and SE was still markedly present one year later.
4.1. Sample description and attrition
Overall, participants in this study identified as White, female, and were between the ages of 18–35. Further, across all three countries, substantial attrition was noted between T1 and T2. Part of this may be explained by the fact that participants had less free time available to complete questionnaires as COVID‐19 restrictions loosened, and/or may have experienced participant burden by having to complete the survey monthly (US, NL).
4.2. Illness status
ED illness status was fairly stable over time in the three countries, and a minority switched between illness states between the start of the pandemic and one year later. It is uncertain if and how COVID‐19 influenced this course of illness or if this reflects a natural illness course, given the absence of pre‐pandemic data. In ED samples, the longer‐term impact of COVID‐19 on mental health is not well‐characterized with one study reporting a persistent worsening of psychopathology after lockdown—based on retrospective assessments (Monteleone et al., 2021b). In general population samples, elevated mental health symptoms have been reported 5–12 months after the initial COVID‐19 peak, disproportionately affecting vulnerable populations (i.e., younger, female, low income) (Liu et al., 2021; Shi et al., 2021). Taken together, we cannot (yet) state if and how the COVID‐19 pandemic has influenced ED illness course.
4.3. ED symptomatology
Some individuals in all three countries reported marked self‐perceived impact of COVID‐19 on ED symptoms including restriction, binge eating, purging, and anxiety about exercise, at the start of and one year into the pandemic, especially early in the pandemic. Although lockdowns and other restrictions had eased one year later, the perceived impact on ED symptomatology may have arisen from new and ongoing stressors. These may include loss of loved ones to COVID‐19 death, COVID‐19 illness, economic fallout from employment furloughs and transitions, living in places severely affected by the initial COVID‐19 outbreak, unpredictable daily changes (i.e., job, school, child daycare, travel quarantines), and reduced in‐person social and family interaction, owing to remote work and schooling, vaccination status of self and others, and the vulnerability of aging family members.
4.4. ED treatment
Next, our study revealed that the initial transition to online treatment was followed by a return to face‐to‐face care in NL, which we observed to a lesser extent in the US, but not in SE. These findings fit the pattern of public health measures to limit the spread of COVID‐19, which were stricter at T1 than at T2 in US and NL, but less strict and similar over time in SE. Although our sample size did not allow us to study changes in the perception of treatment quality of online treatment over time, retrospective assessments indicate that roughly half of those in treatment reported a decline in treatment quality since the start of the pandemic. A systematic review (Monteleone et al., 2021a) suggested that transition to online therapy is often accompanied by impaired treatment quality. However, this transition can also be perceived positively (e.g., increased accessibility of treatment) and it is the most appropriate alternative if face‐to‐face therapy is not possible.
4.5. Anxiety/depression
In all three countries, average anxiety levels captured through standardized assessments were moderate at both time points but higher initially (US) or fairly stable (NL, SE). The initial higher levels of anxiety in the US could reflect the relatively rapid emergence of the pandemic and public health response that could have increased isolation and raised fears about aspects of their ED and their mental health in general. Such a pattern is supported by findings from a longitudinal nationally representative study in the United Kingdom, which showed that an increase in distress was related to waves of COVID‐19 cases (Daly & Robinson, 2022). We could also have captured a natural course of ED and anxiety levels as individuals habituated to the fears associated with the pandemic and vaccines were developed and deployed. Comparing our results with an outpatient sample of people with EDs (GAD‐7 total score M 11.6–12.4 [Weigel et al., 2019]) suggests that the anxiety levels we observed at T2 were on par with these (“pre‐pandemic”) levels, but that self‐reports at T1 were elevated, specifically among those with persistent active illness. However, we are unable to say how anxiety scores compared to pre‐pandemic levels. Other self‐report questionnaires administered at T1 and T2, designed to capture COVID‐19 impact, indicated that the majority in our study sample self‐perceived a worsening of anxiety and depressive mood due to COVID‐19 factors at both T1 and T2. This, together with consistent moderate GAD‐7 anxiety levels, suggests that the COVID‐19 pandemic may have led to higher levels of anxiety and depressive mood among individuals with a (past) ED prior to the pandemic, immediately and in the longer‐term.
4.6. Country differences
We found that at the start of the pandemic relatively more individuals in US and NL reported self‐perceived worsening of ED symptoms compared with SE, suggesting possible differences in COVID‐19 impact across countries. Several factors could explain the observed differences. First, we applied distinct recruitment strategies across countries, which may have resulted in capturing individuals with a more “active” ED in US and NL compared with SE. Individuals recruited via social media in US and NL could presumably be experiencing symptoms that could have increased the likelihood of visiting ED‐related social media platforms. Our recruitment strategy in SE could have identified a comparatively less actively ill population. Second, the public health response to the pandemic differed considerably across countries. At T1, many states in the US issued stay‐at‐home orders and school closures. NL had a month‐long lockdown, including the closure of all public places (schools, restaurants, gyms), and a work‐from‐home order. In contrast, SE appealed to each individual's responsibility to limit the spread of COVID‐19. Thus, stricter regulations in US and NL may have led to a more extreme initial response among those with pre‐existing EDs compared with SE, as they experienced a more sudden loss of daily structure, ability to exercise in gyms or other venues, and having to transition rapidly to online treatment as seen in our data. This interpretation is supported by a longitudinal analysis of data from 15 countries, including SE, which found that higher policy stringency was associated with poorer mental health in the general population (Aknin et al., 2022). A third factor could be the differential timing of the survey launch across countries relative to the phase of the pandemic. Specifically, the survey was deployed earlier in US and NL than SE. In US and NL, the number of COVID‐19‐related deaths was increasing rapidly at the time of the survey launch, whereas this number was decreasing in SE (World Health Organization, 2022). Thus, with the current study design we cannot conclude but only speculate that individuals with an ED may be disproportionally affected in countries with more stringent public policy restrictions.
4.7. Clinical and public health implications
Our study indicates that a public health emergency such as the COVID‐19 pandemic may have a sustained negative mental health impact on many individuals with EDs. We highlight the implications this may have both for health professionals and for the field. First, clinicians and primary care professionals are encouraged to actively monitor the mental health of patients with active or past EDs during any public health emergency. Simultaneously, attention must be paid to the impact of the emergency itself (i.e., physical illness or danger to self) and public health mitigation measures when drastic public health measures are necessary to control public health emergencies like the COVID‐19 pandemic. Bolstering resources by simplifying and clarifying routes to health care (e.g., various digital and telehealth interventions) and bringing this information to the forefront at the outset of an emergency could reduce short‐ and long‐term negative effects on the ED population mental and physical health.
Second, persisting mental health concerns a year after the onset of the pandemic and the transitions of some individuals to higher symptom groups, coupled with the broadly publicized “mental health pandemic” that has emerged (COVID‐19 Mental Disorders Collaborators, 2021), highlights the continued need for improving the workforce capacity of managing and treating EDs. Our findings suggest that although many individuals were able to access online treatment/teletherapy during the early part of the pandemic, substantial numbers of individuals in all three countries remained without care (or experienced impaired care access) both prior to the pandemic and one year later. Community‐based organizations may be able to reach individuals with impaired access to care by offering guidance and resources through online presence, social media, or outreach campaigns. We furthermore consider options such as the use of mobile apps or digital versions of treatment in stepped‐care models to improve reach when health care systems are overloaded (Rohrbach et al., 2022a, 2022b).
4.8. Limitations
Our study has several limitations. First, we used different recruitment strategies across countries. In US and NL, we mainly relied on social media advertisements, and in SE (and US, partly) we relied on participants from prior studies who had agreed to be contacted for future research. Respondents may not be representative of the entire population with EDs, may reflect different subpopulations across countries, and do not reflect the expected diagnostic distribution of a community sample. Moreover, we might have captured a population that is more active on social media in US and NL, and we would have missed individuals without internet access. Second, we relied on self‐reported ED status, diagnosis, and symptom reports. Although SE participants had confirmed ED diagnoses from the register or prior studies, the remainder self‐reported their ED and the validity of these ED diagnoses cannot be ensured. Unfortunately, the swift imperative for study design and data collection meant that diagnosis was not determined through structured diagnostic interviews. Third, the study had high attrition especially in US and NL. Although we did not observe differences on baseline variables except a higher level of anxiety among US non‐responders, the high attrition level could still have affected our results. We furthermore assumed that data were missing completely at random, but this cannot be confirmed statistically. A related limitation is that the attrition resulted in low numbers especially in US or NL subgroups, which precluded certain analyses. Fourth, due to the absence of pre‐pandemic measures of ED symptomatology and anxiety levels, it was not possible to draw causal conclusions about the effect of the COVID‐19 pandemic on our outcome variables. Assessments relied on retrospective recall since no prospective pre/post pandemic comparisons are possible, with ensuing possibility of recall bias. Last, we did not have a healthy comparison group without a lifetime ED, and thus cannot fully conclude that the observed patterns are unique for the group we studied.
4.9. Conclusion
In sum, our study indicates that the impact of COVID‐19 on individuals with an ED remained apparent one year after the start of the pandemic. Presumably, the pandemic and its accompanied public policy measures have a general negative effect on this population in terms of ED symptomatology, treatment access and quality, and comorbid symptoms such as anxiety and depressive mood. Furthermore, although not tested formally, our study did suggest some differences across the three countries that had considerably different approaches to containing COVID‐19. This study underscores the potential long‐lasting effect of a public health emergency and its related restrictions on many individuals with an ED. Future studies may focus on teasing out the specific factors that may lead to deterioration in one subgroup and/or improvement in another subgroup. Rapid dissemination of this knowledge to all involved actors is crucial: community‐based organizations, primary and general mental health care providers, as well as specialist ED clinicians can all benefit from this information. Innovations in digital care can advance the process of collaboration and dissemination, and public policy‐makers have the power to enable these innovations. We are now aware that a public health emergency may have a substantial effect on individuals with an ED. Next, we need to incorporate this knowledge into future public policies to enable an accurate response of all involved care providers. With this, we can prevent or reduce the potential persistent negative effect of a future public health emergency on individuals with an ED.
AUTHOR CONTRIBUTIONS
Jet D. Termorshuizen: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; validation; visualization; writing – original draft; writing – review and editing. Quan Sun: Conceptualization; data curation; formal analysis; investigation; methodology; software; validation; visualization; writing – review and editing. Stina Borg: Data curation; formal analysis; investigation; software; validation. Emma F. Mantilla: Conceptualization; supervision; writing – review and editing. Rachel W. Goode: Conceptualization; writing – review and editing. Christine M. Peat: Conceptualization; writing – review and editing. Laura M. Thornton: Conceptualization; methodology; writing – review and editing. Hunna Watson: Conceptualization; data curation; methodology; supervision; writing – review and editing. Eric F. van Furth: Conceptualization; resources; writing – review and editing. Andreas Birgegård: Conceptualization; resources; supervision; writing – review and editing. Cynthia M. Bulik: Conceptualization; funding acquisition; resources; supervision; writing – original draft; writing – review and editing.
CONFLICTS OF INTEREST
CM Bulik reports: Shire (grant recipient, Scientific Advisory Board member); Pearson (author, royalty recipient); Equip Health Inc. (Clinical Advisory Board); CM Peat reports: Equip Health (Clinical Advisory Board).
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
Dr. Bulik acknowledges support from National Institute of Mental Health (R01MH120170; R01MH124871; R01MH119084; R01MH118278); Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council (Vetenskapsrådet, award: 538‐2013‐8864); Lundbeck Foundation (Grant no. R276‐2018‐4581). Dr. Goode acknowledges support from National Institute of Health, National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) (K23DK129832). Drs. Peat, Goode, and Bulik acknowledge support from the Substance Abuse and Mental Health Administration (H79 SM081924).
Termorshuizen, J. D. , Sun, Q. , Borg, S. , Mantilla, E. F. , Goode, R. W. , Peat, C. M. , Thornton, L. M. , Watson, H. , van Furth, E. F. , Birgegård, A. , & Bulik, C. M. (2023). Longer‐term impact of COVID‐19 among individuals with self‐reported eating disorders in the United States, the Netherlands, and Sweden. International Journal of Eating Disorders, 56(1), 80–90. 10.1002/eat.23824
Action Editor: Ruth Striegel Weissman
Funding information Brain and Behavior Research Foundation; Lundbeckfonden, Grant/Award Number: R276‐2018‐4581; National Institute of Mental Health, Grant/Award Numbers: R01MH118278, R01MH119084, R01MH120170, R01MH124871; Substance Abuse and Mental Health Services Administration, Grant/Award Number: H79 SM081924; Vetenskapsrådet, Grant/Award Number: 538‐2013‐8864
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Aknin, L. B. , Andretti, B. , Goldszmidt, R. , Helliwell, J. F. , Petherick, A. , De Neve, J. E. , Dunn, E. W. , Fancourt, D. , Goldberg, E. , Jones, S. P. , Karadag, O. , Karam, E. , Layard, R. , Saxena, S. , Thornton, E. , Whillans, A. , & Zaki, J. (2022). Policy stringency and mental health during the COVID‐19 pandemic: A longitudinal analysis of data from 15 countries. The Lancet Public Health, 7(5), e417–e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Birgegård, A. , Abbaspour, A. , Borg, A. , Clinton, D. , Mantilla, E. F. , Savva, A. , Termorshuizen, J. D. , & Bulik, C. M. (2021). Longitudinal experiences and impact of the COVID‐19 pandemic among people with past or current eating disorders in Sweden. Eating Disorders, 1–16. 10.1080/10640266.2021.1985286 [DOI] [PubMed] [Google Scholar]
- Bulik, C. M. , Butner, J. E. , Tregarthen, J. , Thornton, L. M. , Flatt, R. E. , Smith, T. , Carroll, I. M. , Baucom, B. R. W. , & Deboeck, P. R. (2020). The binge eating genetics initiative (BEGIN): Study protocol. BMC Psychiatry, 20(1), 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini, G. , Cassioli, E. , Rossi, E. , Innocenti, M. , Gironi, V. , Sanfilippo, G. , Felciai, F. , Monteleone, A. M. , & Ricca, V. (2020). The impact of COVID‐19 epidemic on eating disorders: A longitudinal observation of pre versus post psychopathological features in a sample of patients with eating disorders and a group of healthy controls. The International Journal of Eating Disorders, 53(11), 1855–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . (2022, June 3). Guidance Documents for COVID‐19. Available from: https://www.cdc.gov/coronavirus/2019-ncov/communication/guidance-list.html?Sort=Date%3A%3Adesc.
- Chan, C. Y. , & Chiu, C. Y. (2022). Disordered eating behaviors and psychological health during the COVID‐19 pandemic. Psychology, Health & Medicine, 27(1), 249–256. [DOI] [PubMed] [Google Scholar]
- COVID‐19 Mental Disorders Collaborators . (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID‐19 pandemic. Lancet, 398(10312), 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, M. , & Robinson, E. (2022). Psychological distress associated with the second COVID‐19 wave: Prospective evidence from the UK household longitudinal study. Journal of Affective Disorders, 310, 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas, M. , Faber, R. , & Hamersma, M. (2020). How COVID‐19 and the Dutch ‘intelligent lockdown’ change activities, work and travel behaviour: Evidence from longitudinal data in the Netherlands. Transportation Research Interdisciplinary Perspectives, 6, 100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donker, T. , Van Straten, A. , Marks, I. , & Cuijpers, P. (2011). Quick and easy self‐rating of generalized anxiety disorder: Validity of the Dutch web‐based GAD‐7, GAD‐2 and GAD‐SI. Psychiatry Research, 188(1), 58–64. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Aranda, F. , Casas, M. , Claes, L. , Bryan, D. C. , Favaro, A. , Granero, R. , Gudiol, C. , Jiménez‐Murcia, S. , Karwautz, A. , Le Grange, D. , Menchón, J. M. , Tchanturia, K. , & Treasure, J. (2020). COVID‐19 and implications for eating disorders. European Eating Disorders Review, 28(3), 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn, M. , Fojtu, C. , & Juarascio, A. (2021). COVID‐19 and binge eating: Patient perceptions of eating disorder symptoms, tele‐therapy, and treatment implications. Current Psychology, 40(12), 6249–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giel, K. E. , Schurr, M. , Zipfel, S. , Junne, F. , & Schag, K. (2021). Eating behaviour and symptom trajectories in patients with a history of binge eating disorder during COVID‐19 pandemic. European Eating Disorders Review, 29(4), 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardon, J. , Messer, M. , Rodgers, R. F. , & Fuller‐Tyszkiewicz, M. (2022). A systematic scoping review of research on COVID‐19 impacts on eating disorders: A critical appraisal of the evidence and recommendations for the field. The International Journal of Eating Disorders, 55(1), 3–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Haucke, M. N. , Heinzel, S. , & Heinz, A. (2021). Long‐term impact of economic downturn and loneliness on psychological distress: Triple crises of COVID‐19 pandemic. Journal of Clinical Medicine, 10(19), 4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, B. , Decker, O. , Müller, S. , Brähler, E. , Schellberg, D. , Herzog, W. , & Herzberg, P. Y. (2008). Validation and standardization of the generalized anxiety disorder screener (GAD‐7) in the general population. Medical Care, 46(3), 266–274. [DOI] [PubMed] [Google Scholar]
- Ludvigsson, J. F. (2020). The first eight months of Sweden's COVID‐19 strategy and the key actions and actors that were involved. Acta Paediatrica, 109, 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone, A. M. , Cascino, G. , Barone, E. , Carfagno, M. , & Monteleone, P. (2021a). COVID‐19 pandemic and eating disorders: What can we learn about psychopathology and treatment? A systematic review. Current Psychiatry Reports, 23(12), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone, A. M. , Marciello, F. , Cascino, G. , Abbate‐Daga, G. , Anselmetti, S. , Baiano, M. , Balestrieri, M. , Barone, E. , Bertelli, S. , Carpiniello, B. , Castellini, G. , Corrivetti, G. , De Giorgi, S. , Favaro, A. , Gramaglia, C. M. , Marzola, E. , Meneguzzo, P. , Monaco, F. , Oriani, M. G. , … Monteleone, P. (2021b). The impact of COVID‐19 lockdown and of the following "re‐opening" period on specific and general psychopathology in people with eating disorders: The emergent role of internalizing symptoms. Journal of Affective Disorders, 285, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipou, A. , Meyer, D. , Neill, E. , Tan, E. J. , Toh, W. L. , Van Rheenen, T. E. , & Rossell, S. L. (2020). Eating and exercise behaviors in eating disorders and the general population during the COVID‐19 pandemic in Australia: Initial results from the COLLATE project. The International Journal of Eating Disorders, 53(7), 1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtrics . Qualtrics [2020–2021]. https://www.qualtrics.com 2005.
- R CoreTeam. (2020). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R‐project.org [Google Scholar]
- Rodgers, R. F. , Lombardo, C. , Cerolini, S. , Franko, D. L. , Omori, M. , Fuller‐Tyszkiewicz, M. , Linardon, J. , Courtet, P. , & Guillaume, S. (2020). The impact of the COVID‐19 pandemic on eating disorder risk and symptoms. The International Journal of Eating Disorders, 53(7), 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach, P. J. , Dingemans, A. E. , Spinhoven, P. , Van Ginkel, J. R. , Fokkema, M. , Wilderjans, T. F. , Bauer, S. , & Van Furth, E. F. (2022a). Effectiveness of an online self‐help program, expert‐patient support, and their combination for eating disorders: Results from a randomized controlled trial. The International Journal of Eating Disorders, 55(10), 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach, P. J. , Dingemans, A. E. , Van Furth, E. F. , Spinhoven, P. , Van Ginkel, J. R. , Bauer, S. , & Van den Akker‐van Marle, M. E. (2022b). Cost‐effectiveness of three internet‐based interventions for eating disorders: A randomized controlled trial. The International Journal of Eating Disorders, 55(8), 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner, B. (2016). Fundamentals of biostatistics. CENGAGE Learning. [Google Scholar]
- Schlegl, S. , Maier, J. , Meule, A. , & Voderholzer, U. (2020). Eating disorders in times of the COVID‐19 pandemic‐results from an online survey of patients with anorexia nervosa. The International Journal of Eating Disorders, 53(11), 1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Lu, Z. A. , Que, J. Y. , Huang, X. L. , Lu, Q. D. , Liu, L. , Zheng, Y. B. , Liu, W. J. , Ran, M. S. , Yuan, K. , Yan, W. , Sun, Y. K. , Sun, S. W. , Shi, J. , Kosten, T. , Bao, Y. P. , & Lu, L. (2021). Long‐term impact of COVID‐19 on mental health among the general public: A nationwide longitudinal study in China. International Journal of Environmental Research and Public Health, 18(16), 8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideli, L. , Lo Coco, G. , Bonfanti, R. C. , Borsarini, B. , Fortunato, L. , Sechi, C. , & Micali, N. (2021). Effects of COVID‐19 lockdown on eating disorders and obesity: A systematic review and meta‐analysis. European Eating Disorders Review, 29(6), 826–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD‐7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- SurveyMonkey, A. www.surveymonkey.com/mp/audience. 2021.
- Termorshuizen, J. D. , Watson, H. J. , Thornton, L. M. , Borg, S. , Flatt, R. E. , MacDermod, C. M. , Harper, L. E. , Furth, E. F. , Peat, C. M. , & Bulik, C. M. (2020). Early impact of COVID‐19 on individuals with self‐reported eating disorders: A survey of ~1,000 individuals in the United States and the Netherlands. The International Journal of Eating Disorders, 53(11), 1780–1790. [DOI] [PubMed] [Google Scholar]
- Thornton, L. M. , Munn‐Chernoff, M. A. , Baker, J. H. , Juréus, A. , Parker, R. , Henders, A. K. , Larsen, J. T. , Petersen, L. , Watson, H. J. , Yilmaz, Z. , Kirk, K. M. , Gordon, S. , Leppä, V. M. , Martin, F. C. , Whiteman, D. C. , Olsen, C. M. , Werge, T. M. , Pedersen, N. L. , Kaye, W. , … Bulik, C. M. (2018). The anorexia nervosa genetics initiative (ANGI): Overview and methods. Contemporary Clinical Trials, 74, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitagliano, J. A. , Jhe, G. , Milliren, C. E. , Lin, J. A. , Spigel, R. , Freizinger, M. , Woods, E. R. , Forman, S. F. , & Richmond, T. K. (2021). COVID‐19 and eating disorder and mental health concerns in patients with eating disorders. Journal of Eating Disorders, 9(1), 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, A. , Lowe, B. , & Kohlmann, S. (2019). Severity of somatic symptoms in outpatients with anorexia and bulimia nervosa. European Eating Disorders Review, 27(2), 195–204. [DOI] [PubMed] [Google Scholar]
- Weissman, R. S. , Bauer, S. , & Thomas, J. J. (2020). Access to evidence‐based care for eating disorders during the COVID‐19 crisis. The International Journal of Eating Disorders, 53(5), 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2022, June 14). WHO coronavirus (COVID‐19) dashboard . Available from: https://covid19.who.int/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


