Abstract
Purpose of Review-
Several clinical and large population studies indicate that women are more salt-sensitive than men, yet the precise mechanisms by which the sexually dimorphic onset manifests remains incompletely understood. Herein, we evaluate recent epidemiological data and highlight current knowledge from studies investigating sex-specific mechanisms of salt sensitive blood pressure (SSBP).
Recent Findings-
Emerging evidence indicates that women of all ethnicities are more salt-sensitive than men, at all ages both pre- and post-menopausal. However, menopause exacerbates severity and prevalence of SSBP, suggesting that female sex chromosomes predispose to and female sex hormones mitigate SSBP. Results from both human and rodent studies support the contribution of an enhanced and inappropriate activation of the aldosterone-endothelial cell mineralocorticoid receptor (ECMR) axis promoting vascular dysfunction in females. Increases in adrenal response to angiotensin II, in association with higher ECMR expression and activation of endothelial ENaC (epithelial sodium channel) in females compared to males are emerging as central players in the development of endothelial dysfunction and SSBP in females.
Keywords: Aldosterone, Mineralocorticoid receptor, Endothelium, Immune activation, Inflammation, Sex Differences
Summary-
Female sex increases the prevalence and susceptibility of SSBP and sex hormones and sex chromosome complement may exert antagonistic effects in the development of the female heightened SSBP.
Introduction
Sex differences in cardiovascular diseases (CVD) is a field of ongoing study. Until recently, the general dogma is that premenopausal women garner a favorable, sex-specific protection against CVD due in large part to the beneficial effects of female sex hormones. Years of research, predominantly in men and male animal models, feed the misconception that women are less salt-sensitive. Salt sensitivity of blood pressure (SSBP), where BP increases or decreases more than 10% in response to changes in dietary salt consumption1, is a heritable pathophysiological trait that accounts for 50–80% of essential hypertension diagnoses2–6. SSBP is present in approximately half of those with essential hypertension and contributes to resistant hypertension in most cases7. Biological sex and sex hormones, in combination with genetic predisposition, influence the mechanisms that regulate the onset and development of SSBP. Further confounding this sex-specificity is the demonstrated increases in SSBP in women following menopause. The purpose of this review is to summarize the most recent epidemiological and experimental data demonstrating that SSBP in women changes throughout the reproductive lifespan. We will summarize emerging evidence that mechanisms underlying SSBP involve sex-specific activation of the renin-angiotensin-aldosterone system (RAAS) and increased propensity for vascular dysfunction in women, whereas renal sodium retention and immune activation, which were first identified in male patients and animal models, may play lesser roles in SSBP in women than in their male counterparts.
Women Demonstrate Greater Salt Sensitivity of Blood Pressure than Men
Epidemiological data
Despite a relatively robust rodent literature indicating that male rodents are more salt-sensitive than females, a large body of evidence indicates that women are more salt-sensitive than men. SSBP is common in both men and women, however, many large population studies around the globe have revealed that SSBP is more prevalent in women. The GenSalt study, in Chinese adults, revealed higher drops (−8.07 vs. −7.05 mmHg, P=0.0004) and increases (+6.35 vs. 5.25 mmHg, P<0.0001) in systolic BP in women compared to men in response to low and high-salt dietary interventions respectively8, 9. The Dietary Approaches to Stop Hypertension (DASH) study of Black, White, and Asian Americans also indicated that a low sodium diet reduces BP in women to a greater extent than in men. The combination of the DASH diet and a low level of sodium lowered systolic BP by 6.8 mmHg in men vs. 10.5 mmHg in women (P< 0.001)10, 11. Further, analysis of the Hypertensive Pathotype (HyperPath) and the Hypertension Insulin–Resistance Study (HTN-IR) cohorts composed of individuals from the USA, France, Spain and Mexico uncovered a 30% greater salt-sensitive prevalence in women compared to men3, 12. Most notably, the results of the INTERSALT study, composed of >10,000 patients from 32 countries, revealed a significant higher association of BP and sodium excretion in women than in men2, 13. Altogether, these studies indicate that women are more salt-sensitive than men and are thereby at higher risk for developing SSBP, posing a sex-specific cardiovascular health dilemma.
Racial differences and genetic predisposition
Considering the aforementioned large population studies were conducted in multiple countries, the sex-specificity for SSBP persists across ethnicities. Studies in which race was assessed do note, however, that individuals of African descent display a higher incidence overall for SSBP regardless of sex, with ~70% prevalence compared to ~50% in White populations14, 15. Remarkably, in a population of relatively high socioeconomic status, greater increases in systolic BP with salt loading were seen in hypertensive African American versus hypertensive White women (23.0 vs 14.8 mmHg, P<0.01), while no difference in SSBP was observed between the normotensive women16. Recently, genetic studies investigated whether genetic variations in both estrogen receptor (ER) and Lysine-specific demethylase 1 (LSD1), a salt-sensitive epigenetic regulator, could contribute to this racial and sex predominance12, 17. Multivariate analyses of both the HyperPATH and Hypertension Insulin–Resistance cohorts documented that ESR2 (gene coding for ER-β) rs10144225 minor risk allele carriers had a significantly positive association with SSBP, in premenopausal women only (β = +4.4 mm Hg per risk allele, P = 0.004). The association persists after adjustment for age, body mass index, underlying disease and race, but interestingly, in individuals of African descent, the prevalence of risk allele carriers was significantly higher than that of the nonrisk allele carriers, whereas a greater number of nonrisk allele carriers was observed in individuals of European descent (P < 0.0005)12. Further analysis of the HyperPATH cohort also revealed greater SSBP in the LSD1 risk allele carriers (rs587168) than the nonrisk homozygotes African Americans and demonstrated that this difference was driven by females, especially postmenopausal women. No difference in SSBP was observed between LSD1 genotypes in individuals of European descent17. In addition to this apparent genetic predisposition, women of African descent have higher prevalence for obesity and diabetes, two major risk factors for SSBP, which may underlie some of the racial propensity for that population. While further studies are needed to elucidate the respective contribution of genetic and environmental factors for racial disparities in SSBP among women, a recent report demonstrates that a potassium intake higher than the US dietary goal (87 mmol/day) abrogates the racial difference in SBBP15, indicating that differences in diets in addition to genetic predisposition could contribute to racial differences.
Sex differences in taste for salt
Interestingly, differences in diet may also contribute to the sexual dimorphic onset of SSBP18. Remarkably, a higher proclivity for dietary salt consumption is reported in Japanese women compared to men, which likely adds and participates to the adverse effects of SSBP19. While it remains to be demonstrated whether these data are translatable to other populations, compelling evidence from experimental studies support the female higher proclivity for salt9, 20, 21 which likely originates phylogenetically in the need to preserve sodium losses during pregnancy. Salt preference may be driven by sex hormone levels, as some studies indicate that during the luteal phase of the menstrual cycle women exhibit an increased preference for salt, however, other studies posit that sodium preference is not correlated with any particular menstrual phase22–25. This is in congruence with animal studies reporting that salt preference develops with sexual maturity but also that ovariectomy does not abolish the higher female taste for salt21. A deduction from these data is that either testosterone represses salt appetite or that both female sex hormones and chromosomes influence dietary salt intake. Reports indicate that testosterone suppresses salt taste in adulthood26, 27, but also rule out, using the FCG mouse model described previously, the contribution of sex chromosome completement to the regulation of salt taste27. Nevertheless, further studies are needed to delineate not only the contribution of sex steroid hormones and sex chromosomes but also the influence of genetic predisposition28 in the regulation of salt intake in humans.
Effects of menopause, sex hormones and chromosomes
Importantly, results from the HyperPath and HTN-IR studies indicate that salt-sensitivity is more prevalent in women at all ages and reproductive stages3, 12. However, multiple clinical studies indicate that cessation of sex hormone production, particularly estradiol, associated with menopause increases the risk of SSBP29, 30. The WHO-CARDIAC study, spanning 25 countries, revealed that in 21 centers in 17 countries worldwide, menopause increased SSBP prevalence in the 2,212 women (48–56 years old) studied as assessed by a correlation of systolic BP and 24-hour sodium excretion14, 31. These data suggest that SSBP in postmenopausal women increases by two potential contributing factors: loss of female sex hormones production in combination or not with the natural process of aging. Using an unusual protocol to assess SSBP32, Shulman et al., reported that surgical induction of menopause with elective hysterectomy and oophorectomy increased SSBP in middle age women33. Concomitantly, others report that transdermal estrogen replacement therapy decreases salt-sensitivity in postmenopausal women34 suggesting that estrogens exert protective effects against SSBP despite the counterintuitive female heightened salt-sensitivity. In rat models of SSBP, notably the Dahl Salt-Sensitive (DSS) and the Spontaneously hypertensive (SHR) rat, multiple groups have investigated the mitigating role of estradiol on SSBP. Ovary-intact and actively cycling female DSS rats demonstrate only a mild SSBP phenotype compared to the males of these models whom depict greater increases in SSBP35–37, which contrasts to clinical data suggesting that reproductive-aged women are more prone to SSBP. However, these models have been utilized to demonstrate the crucial role of female sex hormones in the development of SSBP. Ovariectomy (OVX) ablates the sex difference in SSBP between male and female DSS rats36, 37. A caveat to this observation is that OVX increases BP in the absence of high salt diet in rat models35, 37, 38. In addition, high salt diet has no additive effect on OVX-mediated elevations in BP in DSS rats and reduction in salt intake is unable to restore BP in OVX DSS female rats38, which muddles conclusions of sex hormone contribution specifically in salt sensitive increases in BP in female DSS. Moreover, OVX appears to have no effects on salt sensitivity in previously hypertensive female rats (SHR)35, 37. Therefore, while female sex hormones incontestably exert protective effects on BP, the study of the contribution of female sex steroids to SSBP is confounded either by pre-existing hypertension or salt-independent effects of OVX on BP.
While menopause is associated with reduced estrogen and progesterone levels, it also involves an increase in the testosterone to estrogen ratio. Testosterone supplementation increases BP in young OVX female SHR on high salt diet39 and, seemingly congruently, suppression of endogenous androgen production by orchidectomy reduces BP in male rat models, including DSS, Sabra and Sprague-Dawley, which is restored to normal levels following testosterone supplementation40–42. However, as observed with OVX in the absence of high salt diet, testosterone supplementation in OVX female rats and orchidectomy in male rats respectively elevate and decrease BP in the absence of high salt diet as well43. This may suggest that androgens exert negative BP effects in males and females independently of salt.
Although sex steroid hormones play a key role in the control of the cardiovascular system, emerging evidence supports a role for sex chromosomes as well. The four-core genotype (FCG) mouse, in which gonadal sex is separated from the sex chromosome complement, enables comparisons of XX and XY mice with ovaries or testes. With this model, Ji et al. showed that in mice with intact ovaries/testes, angiotensin II (ANGII) induces higher elevation in BP in male compared to female mice. However, in gonadectomized FCG mice, mice with XX chromosome complement demonstrate greater BP response to ANGII irrespective of their previous priming with ovaries or testes, indicating that XX chromosome complement alone promotes increases in BP in the absence of intact sex hormone complement44. These data are in concordance with others that report similar “ying-yang” biological functions of chromosomes vs. hormones in other biological systems45. These data suggest that female sex chromosomes predispose females to a pro-hypertensive state that is rigorously regulated by sex hormones, indicating that deficits or mechanisms that negate these sex hormone effects are sex-specifically detrimental to BP control in females.
Contribution of Aldosterone to Salt Sensitivity in Women
Salt consumption directs the kidneys to balance dietary sodium by decreasing reabsorption of sodium and increasing natriuresis thereby maintaining sodium and volume homeostasis5, 46, 47. In salt-sensitive women, emerging clinical and experimental evidence indicates that females do not suppress the renin-angiotensin-aldosterone system (RAAS) as efficiently as males, thereby resulting in a sex-specific balance favoring higher RAAS activation despite high sodium intake3, 12, 17. The key RAAS pathway components involved in SSBP are ANGII and aldosterone, though by sex- and reproductive stage-specific mechanisms. Previously established sexually dimorphic RAAS activation in males involves enhanced angiotensin-converting enzyme (ACE), ANGII, and angiotensin II receptor type 1 (AT1R) axis activation which promotes vasoconstriction and increases sympathetic activation. In contrast, premenopausal women have greater activation of the angiotensin-converting enzyme 2 (ACE2), angiotensin (Ang) (1–7), mitochondrial assembly receptor (MasR), and angiotensin II receptor type 2 (AT2R) axis which usually counter ACE-ANGII-AT1R axis activation by increased vasodilation, increased nitric oxide (NO) production and inhibition of sympathetic activation48–50. Following menopause and the concomitant loss of ovarian estrogen production, women have decreased circulating Ang (1–7) and thereby lose the counter regulatory protection leading to an increased activation of the ACE-ANGII-AT1R axis35, 48. Therefore, together, these data raise the question of why females have an inadequate response to salt if they exhibit lower ANGII activation. A potential answer may be found in the fact that women produce more aldosterone than men, as reported in the Framingham Offspring Study3, 51. Consistent with this observation, Shukri et al., demonstrated that higher salt sensitivity in pre and postmenopausal women is associated with enhanced aldosterone production in response to ANGII in liberal and salt-restricted diet3, 51. Similar dysregulation of the RAAS in favor of an increased aldosterone sensitivity was also reported in salt sensitive women of African descent carriers of LSD1 (rs587168) and ESR2 (rs10144225) risk alleles12, 17. In both Balb/C and C57BL6 mice, female mice demonstrate higher sensitivity of aldosterone production in response to increases and decreases in sodium intake, respectively, overall demonstrating higher aldosterone and adrenal aldosterone synthase expression than males52, 53. Experiments in rat adrenocortical cells confirmed that females display higher adrenal response to ANGII and extend these findings by demonstrating that this involves increased adrenal aldosterone synthase (CYP11B2) expression3. Higher AT1R expression in female adrenals compared to that of males is a potential explanation for heightened aldosterone production in females53, 54. While potentially compensating for the reduced activation of the conventional RAS signaling pathway, the heightened adrenal ANGII sensitivity and ANGII-mediated aldosterone production provide a source of SSBP.
The evolutionary purpose of sex-specific increased aldosterone levels in women, independent of sodium intake, is most likely due to the physiological needs of pregnancy. In a healthy pregnancy, plasma volume expansion and the increasing demands of a growing feto-placental unit require an increase in renal blood flow (~50% increase), increased sodium retention as well as increased RAAS activation, as reviewed elsewhere55. However, while ANGII levels increase in pregnancy, aldosterone levels increase disproportionally to renin activity and plays a significant role in the sodium retention required for the late stages of fetal growth56, 57. Although pregnant women have significantly higher aldosterone levels, concurrent high elevations in progesterone levels antagonize excess activation of the mineralocorticoid receptor (MR), and in fact, mutations that excessively activate MR significantly increase BP specifically in pregnancy58. High salt intake is not a significant predictor of hypertension in pregnancy, however, aldosterone levels above that which would be normal for pregnancy are associated with gestational hypertension59. Therefore, the regulation of aldosterone by female sex is a mechanism wired to protect plasma volume expansion in pregnancy in an environment in which salt intake may be scarce. However, in women consuming westernized levels of dietary sodium and with many population risk factors (i.e. obesity, diabetes), these pro-aldosterone mechanisms predispose women to an increased risk for vascular disease and hypertension.
Endothelial Mineralocorticoid Receptor (MR) Expression and Activation are Elevated in Salt-Sensitive Women
The specificity of female sex to increase aldosterone production in the presence of elevated dietary salt consumption indicates that aldosterone-targeted therapeutics are more efficacious in women. Indeed, clinical studies indicate that MR antagonists decrease BP to a greater extent in women compared to men60–62. Experimental data also indicates that MR blockade protects female rodents from SSBP52. Our group has demonstrated that sex-specific receptor expression is responsible for the sex-specific efficacy of MR blockade. We showed that premenopausal women and cycling female mice have heightened expression of endothelial MR compared to men, which is increased in female mice and human endothelial cells via increased endothelial progesterone receptor activation63. Concomitantly, we showed in Balb/c mice, a mouse model recapitulating the sex-specific salt sensitivity developed by humans, that MR blockade restored endothelium-dependent relaxation, a measure of endothelial function, and BP in female mice52, 54. Sex-specific endothelial dysfunction developed in females in response to sodium restriction and ablated by either MR blockade or selective deletion in MR in endothelial cells further supports a heightened aldosterone-endothelial MR axis activation in female mice53, 54. Combined, these data show that salt-sensitive premenopausal females have higher aldosterone sensitivity than males and are therefore more likely to have higher activation of endothelial MR leading to vascular dysfunction and giving rise to SSBP2, 3, 52.
Salt sensitivity is associated with increased vascular resistance consecutive to endothelial dysfunction64, which may play a more significant role in females than in males. Recently, vasodysfunction was proposed as a central regulator of SSBP in that salt-sensitive individuals increased BP following excess intake of sodium not from increased renal sodium retention or increased cardiac output, but instead from the failure to adequately decrease vascular resistance65, 66. This theory is also supported by numerous experimental studies in Dahl SS rats and salt-sensitive dogs reporting an impaired ability of these animals to decrease systemic vascular resistance to offset the initial increase in pressure associated with salt intake67–69. Furthermore, comparative studies between male, intact female and OVX female DSS rats report no differences between groups in fluid and sodium balance on high salt diet (8%), indicating that sodium retention is not the contributor to the sex difference in SSBP38 in these models. Therefore, the vasculature may play a more significant role in SSBP in females, potentially due to a disruption of NO-mediated signaling. Indeed, increases in asymmetrical dimethylarginine (ADMA), a potent endogenous nitric oxide (NO) synthase inhibitor, which decreases NO bioavailability and causes abnormal or inhibited vasodilatory mechanisms is a key contributor to reduced vasomotion in salt sensitive individuals65 notably in women of African descent70. In female experimental mouse models, our recent work reports a decrease in NO bioavailability in response to endothelial MR activation in vessels from female mice exclusively52–54. Although female sex hormones, notably estrogens, enhanced NO bioavailability and vascular health30, salt sensitive females demonstrate heightened sensitivity to salt-induced decreases in vascular compliance, potentially predisposing them to SSBP.
Salt sensitive renal pressure natriuresis is blunted in females
Disruption of healthy pressure natriuresis, or the shifting of sodium retention and renal function to increase BP in response to dietary salt, was established by Guyton and colleagues predominantly in male subjects. Recent findings suggest that these mechanisms may play a lesser role in BP control in salt sensitive women. In the Balb/C mouse model of salt-sensitive hypertension, both chronic sodium intake over seven days as well as acute sodium load did not result in increased sodium retention in female mice in association with increased BP induced by high salt diet. In fact, females excreted sodium load more readily than males52. These sex differences in renal mechanisms controlling BP in response to salt may be related to differences in renal endothelin (ET-1) and NO signaling but also in renal sodium channel expressions. Within the collecting duct, ET-1 promotes sodium excretion through the activation of its endothelin B (ETB) receptor which leads to a decrease in epithelial sodium channel (ENaC) open probability71, 72. While this mechanism is common in both sexes, in females, ET-1 also activates ETA receptor which increases sodium excretion via a neuronal NO synthase (NOS1)-dependent mechanism73. Remarkably, OVX ablates this female-specific mechanism, reduces inducible and endothelial (NOS2, NOS3) NOS expression and increases ETA and ETB receptor expression in the inner medulla, while estrogen supplementation restores NOS levels. Together, these data indicate that estrogen is protective against disruptions in pressure-natriuresis in times of sodium loading, however, rendering that SSBP in estrogen-intact women likely involves extra-renal mechanisms71–75. Based on the key role of the ET-1 system in renal sodium handling, its contribution to SSBP is not surprising and highlighted by the observation that deletion in collecting duct ET-1 or its receptors results in marked salt-sensitive hypertension in male mice. However, counterintuitively, lack in functional ETB receptor alone induces SSBP in females only, suggesting that the sex-specific contribution of ETA and ETB might be altered in response to high salt consumption72, 76. While additional studies are required to investigate the contribution of the ET-1 system in salt sensitive women, these data provide additional mechanistic explanations for the female heightened ability to excrete sodium. Further supporting this point, Veiras et al. demonstrated that Sprague Dawley female rats excrete acute sodium loading more readily than males and that proximal tubule sodium reabsorption is downregulated in females compared to males77. This study also highlighted significant sex differences in proximal and distal tubule sodium transporter expression, favoring low proximal tubule reabsorption and increased distal tubule reabsorption which reduces sodium load encountering the distal collecting duct reducing ENaC reabsorption of sodium. ENaC expression and activity increases with MR activation in the distal collecting duct, which enhances sodium and water reabsorption in exchange for urinary potassium excretion78. ENaC protein expression and activity are increased by estrogen in several tissues including the kidneys79, however, counterintuitively, women and female rodents present with similar or heightened efficiency at excreting salt load compared to males52, 77, 80 even in the absence of estrogen38 which likely minimizes renal ENaC contribution to SSBP in females81. On the other hand, endothelial cell ENaC (EnNaC) membrane abundance increases in response to endothelial cell MR activation82 resulting in reduction of eNOS activity (endothelium NO synthase) and NO production and thus excessive arterial stiffness83. While this mechanism has not been described in the context of SSBP, compelling evidence indicates that aldosterone-induced endothelial MR activation increases EnNaC and promotes EnNaC-mediated endothelial and vascular stiffness in female mice84–86. Therefore, one could speculate that the aldosterone-MR-EnNAC axis activation in female vasculature predisposes to SSBP predominantly rather than shifting in the pressure natriuresis curve.
Sex-specific Contribution of the Immune System to Salt-Sensitive Hypertension
Another organ system known to express ENaC and involved in the development of hypertension is the immune system. T-cells infiltrating the kidney and the vasculature are major contributors to hypertension by inducing vascular and renal inflammation and cytokine release promoting sodium retention and vasoconstriction thereby increasing BP87–92. While salt can act directly on T-cells and macrophages to polarize them towards a pro-inflammatory phenotype14, recent evidence introduces monocyte-derived dendritic cell (DC) activation as a potential initial step in the cascade of activation. DCs express ENaC in response to increases in extracellular Na+, which triggers a cascade of events leading to the formation of reactive oxygen species and the production of isolevuglandins (isoLGs). IsoLGs are highly reactive products from lipid oxidation whose accumulation activates T-cells, notably CD8+ T-cells. IsoLGs induce the release of inflammatory cytokines (IL-17, INF-γ) which act on the vasculature to decrease NO bioavailability and promote SSBP93–96. Interestingly, CD8+ T-cells also express MR and selective deletion of MR in T-cells prevents hypertension97 providing another source of T-cell activation and potentially another avenue for the aldosterone-MR axis to promote SSBP. While these latter mechanisms may play a crucial role in the development of hypertension in males, whether they contribute to the heightened SSBP in females remains to be demonstrated. Indeed, studies conducted in females mostly support an anti-inflammatory role of the female immune system and potentially a limited contribution to SSBP98. For instance, while adoptive transfer experiments identified male CD4+ and CD8+ T-cells as major effectors of hypertension in males99, similar experiments with female CD4+ and CD8+ T-cells support anti-hypertensive properties in this latter T-cell population100, 101. In parallel, a large body of literature established that females have more T regulatory cells (Tregs), an anti-inflammatory and anti-hypertensive subset of T-cells that suppresses immune effector function and attenuates increases in BP102–104. Lastly, further arguments in support of a protective role of the female immune system against SSBP are provided by a recent study in which deficiency of CD14, a cell surface LPS receptor for monocytes and macrophages associated with CVD, in female Dahl Salt Sensitive rats caused greater renal injury and more significant response to SSBP with increased renal infiltration of macrophages compared to males. Rescue experiments via bone marrow transplant from CD14+/+ females abrogated the effect. In addition, ovariectomy abolished the sex difference supporting the contribution of female sex steroid hormones4. Previous research has shown that estrogen upregulates and promotes increased CD14 expression in vivo in female mice and in vitro in cultured monocytes105. These findings suggest that CD14 cells protect females from SSBP via sex steroids, notably estrogen-dependent mechanisms and would likely further minimize the contribution of the immune system to the female heightened SSBP.
Microdomains of Interstitial Sodium Retention: Skin & Skeletal Muscle
Recently, studies have postulated that sub-compartments of interstitial sodium retention contribute to SSBP. The skin and skeletal muscle are microdomains for sodium retention, and contribute to immune activation in salt-sensitive populations106 where high dietary salt consumption induces increased sodium storage in skin and muscle microdomains107. Measurement of sodium disposition in the skeletal muscle and skin of animal and human tissue, with 23-sodium magnetic resonance imaging (23Na-MRI), indicates skin and muscle sodium correlates with BP in salt-sensitive patients106, 108 with increased retention in these microdomains102. In a cohort of 70 prehypertensive adults, 23Na-MRI revealed increased sodium retention in skin is associated with increased accumulation of isoLGs and responses manifested in SSBP96. Routinely, results from studies using this technique demonstrate that the specific microdomain, skin or skeletal muscle, for interstitial sodium retention following salt loading is sex-dependent. Several studies suggest that men have higher sodium storage in skin whereas women have higher storage in skeletal muscle109–111 but Braconnier et al. revealed increased muscle sodium accumulation following salt loading, regardless of sex112. Looking to recapitulate this phenotype in a rodent model, Gohar et. al. investigated how salt loading influenced skin and muscle sodium depots in Sprague Dawley rats but found no difference between sexes113. With several human studies demonstrating sex-specific compartmentalization of interstitial sodium storage, yet limited studies overall conducted using 23Na-MRI, it is difficult to fully elucidate how interstitial sodium compartments contribute to the sex-specific mechanisms of SSBP. Women are more salt-sensitive than men, and also display sex-dependent deposition of interstitial sodium, therefore, more studies are necessary to demystify how microdomain sodium accumulation contributes to SSBP in women. Additionally, it is unknown whether skeletal muscle sodium uptake is influenced by presence or absence of sex hormones or phases of menstrual/estrous cycle. Since pregnancy normally induces increased blood volume and water retention to meet the physiological demands of pregnancy, it is likely that there is an increase in interstitial sodium retention in either skin or skeletal muscle or both as an evolutionary adaptation to ensure species survival. In potential contradiction with the aforementioned findings, novel recent evidence by Rossitto et al. supports that tissue sodium excess in high salt diet conditions is systemic rather than skin-specific and similar in both sexes. Moreover, while the authors show that hypertensive patients exhibit isotonic skin sodium excess, they also demonstrate that skin sodium accumulation is unlikely to be the cause of experimental hypertension if water-independent114. Therefore, more studies are required over the full lifespan of women to elucidate if and how interstitial sodium microdomains could contribute to SSBP.
Conclusion
The most recent scientific findings illustrate that women, regardless of their ethnicity, menopausal status or age, are more salt-sensitive than men, and that, counterintuitively, female sex steroid hormones mitigate SSBP. While additional studies are still required to fully identify the underlying mechanisms of the heightened female salt sensitivity, notably to decipher the respective role of sex chromosome versus sex hormones, strong evidence from both the human and rodent literature support a role for inadequate RAAS suppression and inappropriately high aldosterone production. This, in conjunction with sex-specific increases in endothelial MR expression and EnNaC activity, likely leads to diminished NO bioavailability and vascular dysfunction promoting BP elevation, as depicted in Figure 1. While activation of both the innate and adaptative immune systems have been identified as main contributors to SSBP in males, experimental evidence would support a protective role for both CD14+ and Tregs in females. A potential new player to the sexually dimorphic onset of SSBP is the contribution of interstitial sodium microdomains, though further studies are required to fully elucidate its role.
Figure:
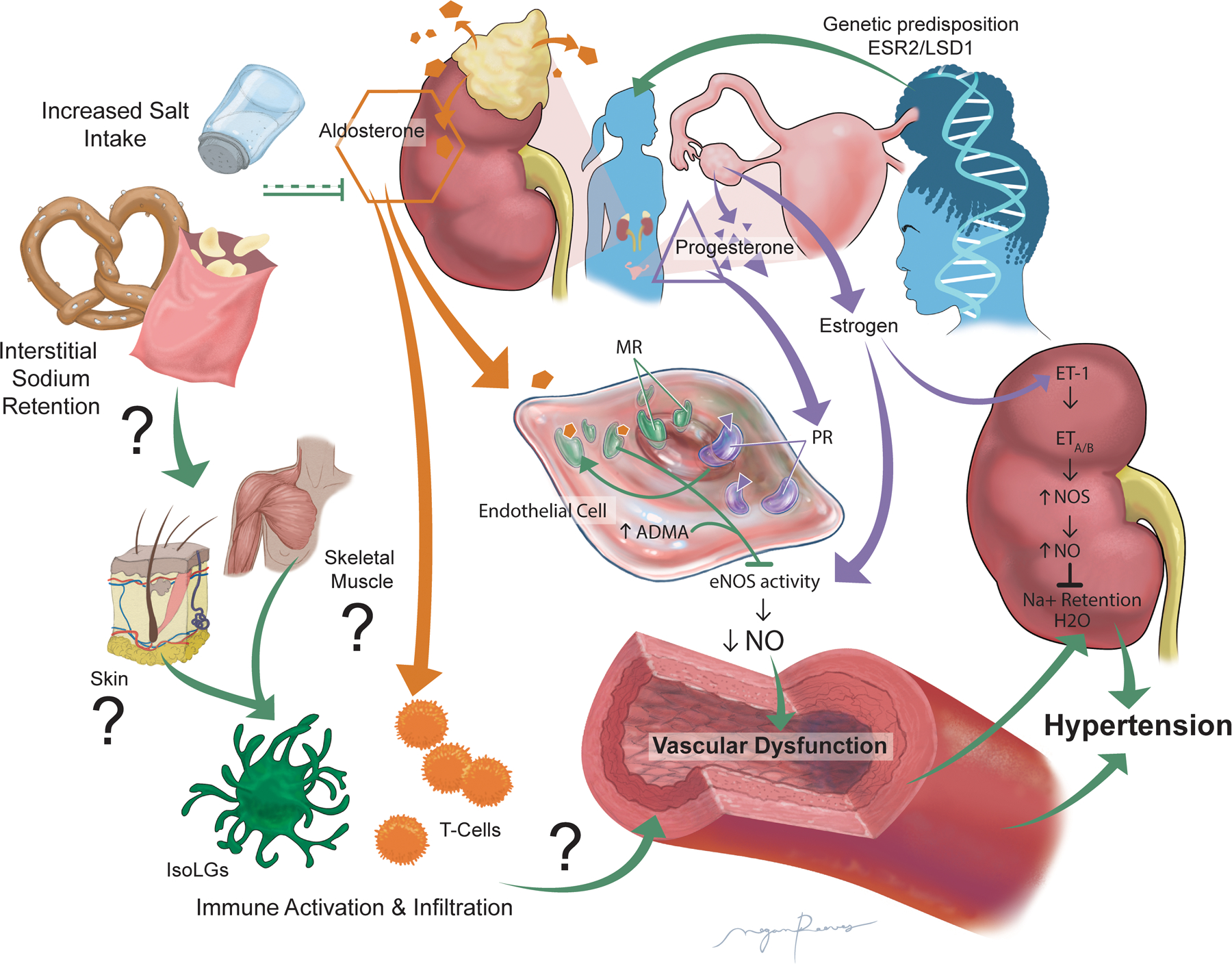
Schematic illustrating the potential mechanisms involved in the female heightened salt sensitivity of blood pressure and proposing an overactivation of the “aldosterone-endothelial cell mineralocorticoid receptor axis” leading to reduced nitric oxide production, vascular dysfunction and ultimately hypertension. Abbreviations: ADMA: asymmetrical dimethylarginine; ESR2: gene coding for estrogen receptor β; eNOS: endothelial nitric oxide synthase; ET-1: Endothelin 1; ETA: Endothelin receptor A; ETB: Endothelin receptor B; IsoLGs: isolevuglandins; LSD1: Lysine-specific demethylase 1; MR: mineralocorticoid receptor; Na+: sodium; NO: Nitric oxide; NOS: Nitric oxide synthase; PR: Progesterone receptor.
Perspectives
Despite few contradictory reports29, we estimate it reasonable, based on the strong epidemiological data presented, to state that women are more salt-sensitive than men throughout lifespan and regardless of ethnicity. However, despite numerous studies, mostly in rat models and also in menopausal women, the origin of heightened female predisposition to SSBP and the respective contribution of sex steroid hormones and chromosome complement remain rather elusive. The discrepancy in the protocol used to assess salt sensitivity32, the lack of good animal models recapitulating the human phenotype, the inconsistencies in the phenotype of the DSS female rats115 but also the recently identified alternative BP trajectories through the menopause transition116 complexify the study of the etiopathology of SSBP in humans. Moving forward, the use of standardized and validated protocol both in human117 and animal studies appears crucial. The Balb/C and FVBN mice, recently identified as spontaneously developing SSBP and recapitulating the human sex difference52, 118, may represent new tools for mechanistic studies as well as excellent background for new transgenic models. In addition, the four-core genotype mouse model36, as well as the growing transgender population, may offer new opportunities to further investigate the role of sex chromosome complement as well as both male and female sex steroids in the development of salt sensitive hypertension.
Funding Sources
Support for this work was provided by NIH 1R01HL130301-01, 1R01HL155265-01 and AHA 19EIA34760167 to EJBdC, 4 R00 HL146948-03 and AHA CDA858380 to JLF and T32 HL155011-01A1 to CTB.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest
References
- 1.Kawarazaki W, Fujita T. Kidney and epigenetic mechanisms of salt-sensitive hypertension. Nat Rev Nephrol. 2021;17:350–363 [DOI] [PubMed] [Google Scholar]
- 2.Faulkner JL, Belin de Chantemele EJ. Female sex, a major risk factor for salt-sensitive hypertension. Curr Hypertens Rep. 2020;22:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, Seely EW, Adler GK, Jaffe IZ, Karas RH, Williams GH, Romero JR. Biological sex modulates the adrenal and blood pressure responses to angiotensin ii. Hypertension. 2018;71:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Keppel T, Zemaj J, Cherian-Shaw M, Gundry RL, Geurts AM, Dwinell MR, Mattson DL. Sexual dimorphic role of cd14 (cluster of differentiation 14) in salt-sensitive hypertension and renal injury. Hypertension. 2021;77:228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balafa O, Kalaitzidis RG. Salt sensitivity and hypertension. J Hum Hypertens. 2021;35:184–192 [DOI] [PubMed] [Google Scholar]
- 6.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, American Heart Association P, Public Education Committee of the Council on H, Council on Functional G, Translational B, Stroke C. Salt sensitivity of blood pressure: A scientific statement from the american heart association. Hypertension. 2016;68:e7–e46 [DOI] [PubMed] [Google Scholar]
- 7.Williams GH, Hollenberg NK. Sodium-sensitive essential hypertension: Emerging insights into an old entity. J Am Coll Nutr. 1989;8:490–494 [DOI] [PubMed] [Google Scholar]
- 8.Chen J Sodium sensitivity of blood pressure in chinese populations. Curr Hypertens Rep. 2010;12:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK, GenSalt Collaborative Research G. Gender difference in blood pressure responses to dietary sodium intervention in the gensalt study. Journal of hypertension. 2009;27:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, Group DCR. A further subgroup analysis of the effects of the dash diet and three dietary sodium levels on blood pressure: Results of the dash-sodium trial. Am J Cardiol. 2004;94:222–227 [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, Group DA-SCR. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (dash) diet. Dash-sodium collaborative research group. N Engl J Med. 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 12.Manosroi W, Tan JW, Rariy CM, Sun B, Goodarzi MO, Saxena AR, Williams JS, Pojoga LH, Lasky-Su J, Cui J, Guo X, Taylor KD, Chen YI, Xiang AH, Hsueh WA, Raffel LJ, Buchanan TA, Rotter JI, Williams GH, Seely EW. The association of estrogen receptor-beta gene variation with salt-sensitive blood pressure. J Clin Endocrinol Metab. 2017;102:4124–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott P, Dyer A, Stamler R. The intersalt study: Results for 24 hour sodium and potassium, by age and sex. Intersalt co-operative research group. J Hum Hypertens. 1989;3:323–330 [PubMed] [Google Scholar]
- 14.Sahinoz M, Elijovich F, Ertuglu LA, Ishimwe J, Pitzer A, Saleem M, Mwesigwa N, Kleyman TR, Laffer CL, Kirabo A. Salt sensitivity of blood pressure in blacks and women: A role of inflammation, oxidative stress, and epithelial na(+) channel. Antioxid Redox Signal. 2021;35:1477–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC, Jr. No evidence of racial disparities in blood pressure salt sensitivity when potassium intake exceeds levels recommended in the us dietary guidelines. Am J Physiol Heart Circ Physiol. 2021;320:H1903–H1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright JT Jr., Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–1092 [DOI] [PubMed] [Google Scholar]
- 17.Parksook WW, Heydarpour M, Gholami SK, Luther JM, Hopkins PN, Pojoga LH, Williams JS. Salt sensitivity of blood pressure and aldosterone: Interaction between the lysine-specific demethylase 1 gene, sex, and age. J Clin Endocrinol Metab. 2022;107:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tindall AM, Stallings VA. Sex differences in cardiovascular risk may be related to sex differences in diet patterns: A narrative review. Ann Hum Biol. 2021;48:517–524 [DOI] [PubMed] [Google Scholar]
- 19.Michikawa T, Nishiwaki Y, Okamura T, Asakura K, Nakano M, Takebayashi T. The taste of salt measured by a simple test and blood pressure in japanese women and men. Hypertens Res. 2009;32:399–403 [DOI] [PubMed] [Google Scholar]
- 20.Kensicki E, Dunphy G, Ely D. Estradiol increases salt intake in female normotensive and hypertensive rats. Journal of applied physiology. 2002;93:479–483 [DOI] [PubMed] [Google Scholar]
- 21.Krecek J, Novakova V, Stibral K. Sex differences in the taste preference for a salt solution in the rat. Physiol Behav. 1972;8:183–188 [DOI] [PubMed] [Google Scholar]
- 22.Alberti-Fidanza A, Fruttini D, Servili M. Gustatory and food habit changes during the menstrual cycle. Int J Vitam Nutr Res. 1998;68:149–153 [PubMed] [Google Scholar]
- 23.Frye CA, Demolar GL. Menstrual cycle and sex differences influence salt preference. Physiol Behav. 1994;55:193–197 [DOI] [PubMed] [Google Scholar]
- 24.Kanarek RB, Ryu M, Przypek J. Preferences for foods with varying levels of salt and fat differ as a function of dietary restraint and exercise but not menstrual cycle. Physiol Behav. 1995;57:821–826 [DOI] [PubMed] [Google Scholar]
- 25.Verma P, Mahajan KK, Mittal S, Ghildiyal A. Salt preference across different phases of menstrual cycle. Indian J Physiol Pharmacol. 2005;49:99–102 [PubMed] [Google Scholar]
- 26.Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci. 1992;106:172–180 [DOI] [PubMed] [Google Scholar]
- 27.Dadam FM, Caeiro XE, Cisternas CD, Macchione AF, Cambiasso MJ, Vivas L. Effect of sex chromosome complement on sodium appetite and fos-immunoreactivity induced by sodium depletion. Am J Physiol Regul Integr Comp Physiol. 2014;306:R175–184 [DOI] [PubMed] [Google Scholar]
- 28.Pilic L, Mavrommatis Y. Genetic predisposition to salt-sensitive normotension and its effects on salt taste perception and intake. Br J Nutr. 2018;120:721–731 [DOI] [PubMed] [Google Scholar]
- 29.Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. American journal of hypertension. 2004;17:994–1001 [DOI] [PubMed] [Google Scholar]
- 30.Barton M, Meyer MR. Postmenopausal hypertension: Mechanisms and therapy. Hypertension. 2009;54:11–18 [DOI] [PubMed] [Google Scholar]
- 31.Yamori Y, Liu L, Ikeda K, Mizushima S, Nara Y, Simpson FO, Diseases WHOC, Alimentary Comparison S. Different associations of blood pressure with 24-hour urinary sodium excretion among pre- and post-menopausal women. Who cardiovascular diseases and alimentary comparison (who-cardiac) study. Journal of hypertension. 2001;19:535–538 [DOI] [PubMed] [Google Scholar]
- 32.Weinberger MH. Estrogens, salt, blood pressure, and cardiovascular disease in women: How do we interpret the data? Hypertension. 2006;47:1049–1050 [DOI] [PubMed] [Google Scholar]
- 33.Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47:1168–1174 [DOI] [PubMed] [Google Scholar]
- 34.Scuteri A, Lakatta EG, Anderson DE, Fleg JL. Transdermal 17 beta-oestradiol reduces salt sensitivity of blood pressure in postmenopausal women. Journal of hypertension. 2003;21:2419–2420 [DOI] [PubMed] [Google Scholar]
- 35.Brinson KN, Rafikova O, Sullivan JC. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am J Physiol Regul Integr Comp Physiol. 2014;307:R149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng W, Ji H, Maric C, Wu X, Sandberg K. Effect of dietary sodium on estrogen regulation of blood pressure in dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol. 2008;294:H1508–1513 [DOI] [PubMed] [Google Scholar]
- 37.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal at1 receptor and salt sensitivity. Hypertension. 2003;42:1157–1163 [DOI] [PubMed] [Google Scholar]
- 38.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35:484–489 [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Ely D. Testosterone increases: Sodium reabsorption, blood pressure, and renal pathology in female spontaneously hypertensive rats on a high sodium diet. Adv Pharmacol Sci 2011;2011:817835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalid M, Ilhami N, Giudicelli Y, Dausse JP. Testosterone dependence of salt-induced hypertension in sabra rats and role of renal alpha(2)-adrenoceptor subtypes. J Pharmacol Exp Ther. 2002;300:43–49 [DOI] [PubMed] [Google Scholar]
- 42.Oloyo AK, Sofola OA, Yakubu MA. Orchidectomy attenuates high-salt diet-induced increases in blood pressure, renovascular resistance, and hind limb vascular dysfunction: Role of testosterone. Clin Exp Pharmacol Physiol. 2016;43:825–833 [DOI] [PubMed] [Google Scholar]
- 43.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: Possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289:F941–948 [DOI] [PubMed] [Google Scholar]
- 44.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin ii-induced hypertension. Hypertension. 2010;55:1275–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vries GJ. Sex steroids and sex chromosomes at odds? Endocrinology. 2005;146:3277–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall JE, Guyton AC, Coleman TG, Mizelle HL, Woods LL. Regulation of arterial pressure: Role of pressure natriuresis and diuresis. Fed Proc. 1986;45:2897–2903 [PubMed] [Google Scholar]
- 47.Guyton AC, Young DB, DeClue JW, Trippodo N, Hall JE. Fluid balance, renal function, and blood pressure. Clin Nephrol. 1975;4:122–126 [PubMed] [Google Scholar]
- 48.Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep. 2013;15:71–79 [DOI] [PubMed] [Google Scholar]
- 49.Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24:687–698 [DOI] [PubMed] [Google Scholar]
- 50.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677 [DOI] [PubMed] [Google Scholar]
- 51.Kathiresan S, Larson MG, Benjamin EJ, Corey D, Murabito JM, Fox CS, Wilson PW, Rifai N, Meigs JB, Ricken G, Lifton RP, Levy D, Vasan RS. Clinical and genetic correlates of serum aldosterone in the community: The framingham heart study. Am J Hypertens. 2005;18:657–665 [DOI] [PubMed] [Google Scholar]
- 52.Faulkner JL, Harwood D, Bender L, Shrestha L, Brands MW, Morwitzer MJ, Kennard S, Antonova G, Belin de Chantemele EJ. Lack of suppression of aldosterone production leads to salt-sensitive hypertension in female but not male balb/c mice. Hypertension. 2018;72:1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faulkner JL, Lluch E, Kennard S, Antonova G, Jaffe IZ, Belin de Chantemele EJ. Selective deletion of endothelial mineralocorticoid receptor protects from vascular dysfunction in sodium-restricted female mice. Biology of sex differences. 2020;11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faulkner JL, Harwood D, Kennard S, Antonova G, Clere N, Belin de Chantemele EJ. Dietary sodium restriction sex specifically impairs endothelial function via mineralocorticoid receptor-dependent reduction in no bioavailability in balb/c mice. Am J Physiol Heart Circ Physiol. 2021;320:H211–H220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scaife PJ, Mohaupt MG. Salt, aldosterone and extrarenal na(+) - sensitive responses in pregnancy. Placenta. 2017;56:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol. 2014;306:R91–101 [DOI] [PubMed] [Google Scholar]
- 57.Bentley-Lewis R, Graves SW, Seely EW. The renin-aldosterone response to stimulation and suppression during normal pregnancy. Hypertens Pregnancy. 2005;24:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geller DS, Farhi A, Pinkerton N, Fradley M, Moritz M, Spitzer A, Meinke G, Tsai FT, Sigler PB, Lifton RP. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science. 2000;289:119–123 [DOI] [PubMed] [Google Scholar]
- 59.Birukov A, Andersen LB, Herse F, Rakova N, Kitlen G, Kyhl HB, Golic M, Haase N, Kraker K, Muller DN, Jorgensen JS, Andersen MS, Dechend R, Jensen BL. Aldosterone, salt, and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension. 2019;74:391–398 [DOI] [PubMed] [Google Scholar]
- 60.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, Poulter NR, Anglo-Scandinavian Cardiac Outcomes Trial I. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845 [DOI] [PubMed] [Google Scholar]
- 61.Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci. 2009;2:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olivieri O, Pizzolo F, Ciacciarelli A, Corrocher R, Signorelli D, Falcone S, Blengio GS. Menopause not aldosterone-to-renin ratio predicts blood pressure response to a mineralocorticoid receptor antagonist in primary care hypertensive patients. American journal of hypertension. 2008;21:976–982 [DOI] [PubMed] [Google Scholar]
- 63.Faulkner JL, Kennard S, Huby AC, Antonova G, Lu Q, Jaffe IZ, Patel VS, Fulton DJR, Belin de Chantemele EJ. Progesterone predisposes females to obesity-associated leptin-mediated endothelial dysfunction via upregulating endothelial mr (mineralocorticoid receptor) expression. Hypertension. 2019;74:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujita T, Ando K, Ogata E. Systemic and regional hemodynamics in patients with salt-sensitive hypertension. Hypertension. 1990;16:235–244 [DOI] [PubMed] [Google Scholar]
- 65.Morris RC Jr., Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133:881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1987;9:398–406 [DOI] [PubMed] [Google Scholar]
- 67.Greene AS, Yu ZY, Roman RJ, Cowley AW Jr. Role of blood volume expansion in dahl rat model of hypertension. Am J Physiol. 1990;258:H508–514 [DOI] [PubMed] [Google Scholar]
- 68.Krieger JE, Roman RJ, Cowley AW Jr. Hemodynamics and blood volume in angiotensin ii salt-dependent hypertension in dogs. Am J Physiol. 1989;257:H1402–1412 [DOI] [PubMed] [Google Scholar]
- 69.Krieger JE, Liard JF, Cowley AW Jr. Hemodynamics, fluid volume, and hormonal responses to chronic high-salt intake in dogs. Am J Physiol. 1990;259:H1629–1636 [DOI] [PubMed] [Google Scholar]
- 70.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr. Salt sensitivity in blacks: Evidence that the initial pressor effect of nacl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385 [DOI] [PubMed] [Google Scholar]
- 71.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin b receptor knockout increases enac activity. American journal of physiology. Cell physiology. 2012;302:C188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohan DE. Role of collecting duct endothelin in control of renal function and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2013;305:R659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakano D, Pollock DM. Contribution of endothelin a receptors in endothelin 1-dependent natriuresis in female rats. Hypertension. 2009;53:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gender medicine. 2008;5:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell T, De Miguel C, Gohar EY. Sex differences in redox homeostasis in renal disease. Redox Biol. 2020;31:101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in et and nos systems in etb receptor-deficient rats: Effect of a high salt diet. Hypertension. 2003;41:657–662 [DOI] [PubMed] [Google Scholar]
- 77.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol. 2017;28:3504–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mutchler SM, Kirabo A, Kleyman TR. Epithelial sodium channel and salt-sensitive hypertension. Hypertension. 2021;77:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yusef YR, Thomas W, Harvey BJ. Estrogen increases enac activity via pkcdelta signaling in renal cortical collecting duct cells. Physiol Rep. 2014;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of avp and renal sodium handling. Journal of applied physiology. 2001;91:1893–1901 [DOI] [PubMed] [Google Scholar]
- 81.Ray EC, Pitzer A, Lam T, Jordahl A, Patel R, Ao M, Marciszyn A, Winfrey A, Barak Y, Sheng S, Kirabo A, Kleyman TR. Salt sensitivity of volume and blood pressure in a mouse with globally reduced enac gamma-subunit expression. Am J Physiol Renal Physiol. 2021;321:F705–F714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kusche-Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk-Zloy V, Schwab A, Schillers H, Oberleithner H. Aldosterone and amiloride alter enac abundance in vascular endothelium. Pflugers Arch. 2008;455:849–857 [DOI] [PubMed] [Google Scholar]
- 83.Druppel V, Kusche-Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E, Pavenstadt H, Oberleithner H, Kliche K. Long-term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J. 2013;27:3652–3659 [DOI] [PubMed] [Google Scholar]
- 84.Hill MA, Sowers JR. Mineralocorticoid antagonists and enac inhibitors in hyperaldosteronism. J Clin Hypertens (Greenwich). 2019;21:929–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res. 2016;118:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jia G, Habibi J, Aroor AR, Hill MA, Yang Y, Whaley-Connell A, Jaisser F, Sowers JR. Epithelial sodium channel in aldosterone-induced endothelium stiffness and aortic dysfunction. Hypertension. 2018;72:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dixon KB, Davies SS, Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol. 2017;312:H368–H374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. Dc isoketal-modified proteins activate t cells and promote hypertension. J Clin Invest. 2014;124:4642–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, Ma X, Yin Z, Du J. Gammadeltat cell-derived interleukin-17a via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64:305–314 [DOI] [PubMed] [Google Scholar]
- 90.Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: Roles of stat3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114:1547–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patrick DM, Van Beusecum JP, Kirabo A. The role of inflammation in hypertension: Novel concepts. Curr Opin Physiol. 2021;19:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elijovich F, Laffer CL, Sahinoz M, Pitzer A, Ferguson JF, Kirabo A. The gut microbiome, inflammation, and salt-sensitive hypertension. Curr Hypertens Rep. 2020;22:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. The Journal of experimental medicine. 2018;215:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ngwenyama N, Kirabo A, Aronovitz M, Velazquez F, Carrillo-Salinas F, Salvador AM, Nevers T, Amarnath V, Tai A, Blanton RM, Harrison DG, Alcaide P. Isolevuglandin-modified cardiac proteins drive cd4+ t-cell activation in the heart and promote cardiac dysfunction. Circulation. 2021;143:1242–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruggeri Barbaro N, Van Beusecum J, Xiao L, do Carmo L, Pitzer A, Loperena R, Foss JD, Elijovich F, Laffer CL, Montaniel KR, Galindo CL, Chen W, Ao M, Mernaugh RL, Alsouqi A, Ikizler TA, Fogo AB, Moreno H, Zhao S, Davies SS, Harrison DG, Kirabo A. Sodium activates human monocytes via the nadph oxidase and isolevuglandin formation. Cardiovasc Res. 2021;117:1358–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, Zhang WC, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen ZX, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan SZ. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res. 2017;120:1584–1597 [DOI] [PubMed] [Google Scholar]
- 98.Veiras LC, Shen JZY, Bernstein EA, Regis GC, Cao D, Okwan-Duodu D, Khan Z, Gibb DR, Dominici FP, Bernstein KE, Giani JF. Renal inflammation induces salt sensitivity in male db/db mice through dysregulation of enac. J Am Soc Nephrol. 2021;32:1131–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific t-cell regulation of angiotensin ii-dependent hypertension. Hypertension. 2014;64:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in t-lymphocyte tissue infiltration and development of angiotensin ii hypertension. Hypertension. 2014;64:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin ii-induced hypertension and vascular injury. Hypertension. 2011;57:469–476 [DOI] [PubMed] [Google Scholar]
- 103.Pollow DP Jr., Uhlorn JA, Sylvester MA, Romero-Aleshire MJ, Uhrlaub JL, Lindsey ML, Nikolich-Zugich J, Brooks HL. Menopause and foxp3(+) treg cell depletion eliminate female protection against t cell-mediated angiotensin ii hypertension. Am J Physiol Heart Circ Physiol. 2019;317:H415–H423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crislip GR, Sullivan JC. T-cell involvement in sex differences in blood pressure control. Clin Sci (Lond). 2016;130:773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface tlr4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884 [DOI] [PubMed] [Google Scholar]
- 106.Ertuglu LA, Elijovich F, Laffer CL, Kirabo A. Salt-sensitivity of blood pressure and insulin resistance. Front Physiol. 2021;12:793924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elijovich F, Kleyman TR, Laffer CL, Kirabo A. Immune mechanisms of dietary salt-induced hypertension and kidney disease: Harry goldblatt award for early career investigators 2020. Hypertension. 2021;78:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sahinoz M EF, Laffer C., Pitzer A., Ikizler T., Kirabo A. Reduction in monocyte isolevuglandins associated with high interstitial sodium mirrors salt-sensitivity of blood pressure in patients with essential hypertension. FASEB J. 2021;35 [Google Scholar]
- 109.Madhur MS, Elijovich F, Alexander MR, Pitzer A, Ishimwe J, Van Beusecum JP, Patrick DM, Smart CD, Kleyman TR, Kingery J, Peck RN, Laffer CL, Kirabo A. Hypertension: Do inflammation and immunity hold the key to solving this epidemic? Circ Res. 2021;128:908–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang P, Deger MS, Kang H, Ikizler TA, Titze J, Gore JC. Sex differences in sodium deposition in human muscle and skin. Magn Reson Imaging. 2017;36:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selvarajah V, Maki-Petaja KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB. Novel mechanism for buffering dietary salt in humans: Effects of salt loading on skin sodium, vascular endothelial growth factor c, and blood pressure. Hypertension. 2017;70:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braconnier P, Milani B, Loncle N, Lourenco JM, Brito W, Delacoste J, Maillard M, Stuber M, Burnier M, Pruijm M. Short-term changes in dietary sodium intake influence sweat sodium concentration and muscle sodium content in healthy individuals. J Hypertens. 2020;38:159–166 [DOI] [PubMed] [Google Scholar]
- 113.Gohar EY, De Miguel C, Obi IE, Daugherty EM, Hyndman KA, Becker BK, Jin C, Sedaka R, Johnston JG, Liu P, Speed JS, Mitchell T, Kriegel AJ, Pollock JS, Pollock DM. Acclimation to a high-salt diet is sex dependent. J Am Heart Assoc. 2022;11:e020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, Graham D, Touyz RM, Delles C. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmerman MA, Lindsey SH. Inconsistent blood pressure phenotype in female dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2016;311:F1391–F1392 [DOI] [PubMed] [Google Scholar]
- 116.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, El Khoudary SR. Trajectories of blood pressure in midlife women: Does menopause matter? Circulation research. 2022;130:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr. An appraisal of methods recently recommended for testing salt sensitivity of blood pressure. J Am Heart Assoc. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM. Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol. 2014;307:F901–907 [DOI] [PubMed] [Google Scholar]


