Abstract
In recent years, several key advances in the management of locally advanced rectal cancer have been made, including the implementation of total mesorectal excision as the standard surgical approach,; use of neoadjuvant chemoradiotherapy in selected patients with a high risk of local recurrence, and finally, adoption of organ preservation strategies, through either local excision or non-operative management in selected patients with clinical complete response following neoadjuvant chemoradiotherapy. This review aims to shed light on the role of rectal MRI in the assessment of treatment response after neoadjuvant therapy, which is especially important given the growing feasibility of non-operative management. First, an overview of current neoadjuvant therapies and response assessment based on digital rectal examination, endoscopy, and MRI will be provided. Second, the use of a high-quality restaging rectal MRI protocol will be presented. Third, a step-by-step approach to assessing treatment response on restaging rectal MRI following neoadjuvant treatment will be outlined, acknowledging challenges faced by radiologists during MRI interpretation. Finally, research related to response assessment will be discussed.
Keywords: rectal cancer, magnetic resonance imaging, neoadjuvant therapy
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most deadly cancer (1). While there is an overall downward trend in CRC incidence and mortality, there is an alarming increase in the rate of early onset CRC among adults younger than 50 years in several countries, including in the United States (2). In recent years, several key advances in the management of locally advanced rectal cancer (LARC) have been made, including the implementation of total mesorectal excision as the standard surgical approach (3), use of neoadjuvant chemoradiotherapy (CRT) in selected patients with a high risk of local recurrence (4), and finally, adoption of organ preservation strategies, through either local excision or non-operative management in selected patients with clinical complete response following neoadjuvant CRT (5,6). More recently, a new neoadjuvant strategy termed total neoadjuvant therapy (TNT), has been implemented. A meta-analysis of 15 studies concluded that the addition of TNT to the standard regimen of care improved rates of pathologic complete response (pCR), lymph node response, and resulted in more frequent tumor-free resection margins (7). Patients who received TNT had lower distal recurrence rates, improved 3-year disease-free survival, and improved overall survival. Compared with patients who undergo neoadjuvant CRT, who achieve a pCR rate of ~20% following total mesorectal excision (8), patients who undergo TNT can achieve a pCR rate of ~40% (9).
With increasing developments in neoadjuvant strategy, non-operative management will become even more relevant in years to come. Non-operative management represents a paradigm shift in the management of patients with LARC, offering the advantage of maintaining oncologic safety (wherein patients undergoing non-operative management have comparable overall survival to patients undergoing total mesorectal excision) without the risk of post-surgical adverse events which can significantly lower patients’ quality of life (e.g., acute mortality or surgical morbidity, including permanent colostomy and bowel, bladder, and sexual dysfunction) (6,10).
In this context, the accurate assessment of treatment response after neoadjuvant therapy is crucial, ultimately to predict clinical complete response and thereby allow patients to be selected for non-operative management. Digital rectal examination, endorectal ultrasound, and computed tomography (CT) were the first modalities used to assess treatment response after neoadjuvant therapy. However, due to their limited field of view (FOV), digital rectal examination and endorectal ultrasound cannot easily assess disease outside of the rectal lumen and cannot evaluate mesorectal fascia involvement. Another key limitation of these modalities is that they are highly subjective and operator-dependent (11,12). CT has low soft tissue contrast resolution and cannot adequately assess tumor invasion; thus, its main role is limited to the assessment of distant metastases (13,14). The strength of endoscopy lies in the assessment of the rectal mucosa; however, it has a limited role for the assessment of the mesorectum (15). In contradistinction, pelvic magnetic resonance imaging (MRI) of the rectum has high soft tissue contrast and high spatial resolution to assess rectal wall layers, fibrosis, and the surrounding mesorectum and extra-mesorectal structures, including the sphincter complex and lymph nodes. As a more standardized and less operator-dependent modality compared with endorectal ultrasound, MRI of the rectum allows for comparison across multiple imaging timepoints (16). Digital rectal examination, endoscopy, and MRI of the rectum are currently used as complementary tools to guide the colorectal multidisciplinary team in determining the best management strategy for patients with LARC after neoadjuvant therapy.
This review will familiarize the reader with the role of rectal MRI in the assessment of treatment response after neoadjuvant therapy, which is especially important given the increasing popularity and feasibility of non-operative management. First, an overview of current neoadjuvant therapies and response assessment based on digital rectal examination, endoscopy, and MRI will be provided. Second, the use of a high-quality restaging rectal MRI protocol will be presented. Third, a step-by-step approach to assessing treatment response on restaging rectal MRI following neoadjuvant treatment will be outlined, acknowledging challenges faced by radiologists during the interpretation of the rectal MRIs. Finally, several research directions related to response assessment will be discussed.
2. Overview of Neoadjuvant Therapy
2.1. Types of neoadjuvant therapy
Neoadjuvant therapy refers to any therapy administered prior to definitive surgical resection. Neoadjuvant CRT is characterized by concurrent fluoropyrimidine-based chemotherapy (e.g., 5-fluorouracil) as a radiosensitizer, and pelvic external beam radiation therapy (17). The standard long-course radiotherapy delivers 45–50 Gy over 25–28 fractions (each “fraction” is a one-day visit to the hospital or outpatient site), while short-course radiotherapy provides 25 Gy over 5 fractions in one week (18).
Total neoadjuvant therapy (TNT) is a relatively novel approach used in the treatment of LARC. TNT delivers both systemic chemotherapy and CRT prior to surgery. The TNT approach has shown to result in non-surgical cure in over 50% of patients in the OPRA trial (19) and pCR in approximately 40% of patients in a meta-analysis (9).There are two main types of TNT, depending on whether systemic chemotherapy is added before or after neoadjuvant CRT: induction chemotherapy followed by CRT, and consolidation chemotherapy administered after CRT.
Recently, results from several large, prospective, randomized phase III trials assessing TNT strategies have been published, such as those from RAPIDO (Rectal cancer And Pre-operative Induction Therapy Followed by Dedicated Operation) [NCT01558921], OPRA (Organ Preservation of Rectal Adenocarcinoma) [NCT02008656], and PRODIGE 23 (Partenariat de Recherche en Oncologie Digestive) [NCT01804790]:
The RAPIDO trial compared standard CRT and TNT which involved short-course radiotherapy followed by consolidation chemotherapy with CAPOX or FOLFOX before surgery. The TNT arm showed an increased pCR rate (28.4% vs 14.3%, p < 0.001) as well as a decreased rate of disease-related treatment failure (23.7% vs 30.4%, p = 0.019), predominantly due to a lower proportion of distant metastases (20).
The OPRA trial compared the 3-year disease-free survival rate between historical standard CRT, and induction and consolidation TNT strategies with FOLFOX or CAPEOX followed by surgery or organ preservation in cases of clinical complete response. The study did not reach its primary endpoint of a 10% improvement in 3-year disease-free survival (based on a 75% historical rate) in both arms but demonstrated that non-operative management was achievable in half of the patients with LARC treated with TNT, without adverse oncological outcomes (19). Additionally, the consolidation chemotherapy arm had a significantly higher rate of 3-year organ preservation than the induction arm (53% vs 41%, p = 0.01), without adverse oncological outcomes (19).
The PRODIGE 23 trial compared standard CRT versus TNT (which involved induction mFOLFIRINOX followed by CRT before surgery and followed by adjuvant therapy with mFOLFOX or capecitabine [technically not “TNT”]). The results showed increased disease- and metastasis-free survival rates among patients in the TNT arm, as well as an increased pCR rate (12% vs 28%, p < 0.001) (21).
The main advantages of TNT strategies are early treatment of micrometastases, increased patient compliance, reduced need for ileostomy, earlier closure of temporary ostomies, and increased rates of clinical complete response. However, some possible disadvantages are the potential risk of overtreatment, higher toxicity, and no definite data on optimal sequencing and type of chemotherapy and CRT regimens (9). Ongoing and future trials are needed to determine optimal patient selection and TNT strategies.
2.2. Response assessment
There is no consensus in the literature on the optimal timing of treatment response assessment, which varies depending on the trial/treatment design and treatment strategy (5). Usually, the first assessment is done at 8–12 weeks after the completion of CRT and is based on digital rectal examination, endoscopy, and MRI. The patients are classified into three groups (5), as follows:
- Clinical complete response:
- Digital rectal examination: no palpable tumor
- Endoscopy: no residual tumor with or without flat scar, and telangiectasia
- MRI: normal appearing rectal wall or only fibrosis, and no suspicious lymph nodes
- Near complete response:
- Digital rectal examination: smooth induration and/or minimal mucosal abnormalities
- Endoscopy: irregular mucosa, smooth mucosal irregularities, superficial ulcer, persistent erythema
- MRI: obvious decrease in size with predominant fibrosis and no or borderline lymph nodes
- Poor response (sometimes called incomplete response):
- Digital rectal examination: palpable tumor
- Endoscopy: visible tumor
- MRI: visible tumor and/or lack of nodal regression
Biopsy is not routinely recommended for patients with clinical complete or near complete clinical response due to the risk of false-negative results from sampling errors (5,22). Patients with clinical complete response may be offered surgery or non-operative management, while patients with near complete response may be offered additional short-term follow-up or local excision. Among patients with near complete response who undergo delayed follow-up 6–12 weeks after initial follow-up, a large proportion (90%) will evolve to have clinical complete response (23).
2.3. Organ preservation after neoadjuvant therapy
Although radical resection via total mesorectal excision is considered the standard treatment in rectal cancer, organ preservation though curative local excision and non-operative management has been increasingly accepted as an alternative option in selected patients with clinical complete or near complete response after neoadjuvant therapy. For patients selected to undergo the organ preservation pathway, carcinoembryonic antigen (CEA) testing, digital rectal examination, endoscopy, pelvic MRI, and chest and upper abdominal CT should be routinely performed for 5 years (Table 1) (5).
Table 1.
International consensus recommendations on follow-up for patients with locally advanced rectal cancer undergoing organ preservation.
| Year | DRE | CEA | Endoscopy | Pelvic MRI | Chest/Abdominal CT |
|---|---|---|---|---|---|
| 1 | 3-4 months | 3 months | 3-4 months | 3-4 months | 6-12 months |
| 2 | 3-4 months | 3 months | 3-4 months | 3-4 months | Annually |
| 3 | 6 months | 3 months | 6 months | 6 months | Annually |
| 4 | 6 months | 6 months | 6 months | 6 months | Annually |
| 5 | 6 months | 6 months | 6 months | 6 months | Annually |
Abbreviations: CEA, carcinoembryonic antigen; CT, computed tomography; DRE, digital rectal examination; MRI, magnetic resonance imaging
3. Restaging Rectal MRI Protocol
Overall, the restaging scan protocol is similar to the primary staging MRI protocol, with a few differences to allow for adequate comparison with the baseline scan. Of note, diffusion-weighted imaging (DWI) after bowel preparation is being increasingly recommended.
3.1. Preparation
Spasmolytic agents such as glucagon (1 mg administered intravenously, intramuscularly, or subcutaneously) or hyoscine butylbromide (20 mg administered intravenously) are suggested to reduce artifacts caused by peristalsis particularly in upper rectal tumors (24).
A microenema 15 minutes before the scan is advised to help remove rectal air and reduce artifacts on DWI (25,26).
3.2. Protocol
An MRI scanner with a magnet strength of at least 1.5 T using a phased-array surface coil positioned such that the lower edge of the coil lies below the pubic bone is recommended. Patients should be positioned comfortably in the supine position. 2D fast spin echo (FSE) T2-weighted imaging (T2WI) without fat suppression and DWI are the two main recommended sequences. The following sequences have been proven necessary for accurate assessment (24):
T2WI axial large FOV: entire pelvis, including the aortic bifurcation to the anal margin
T2WI sagittal: from one side of the pelvic wall to the other
Oblique axial of the tumor bed: perpendicular to the tumor bed using a slice thickness equal to or less than 3 mm
Oblique coronal of the tumor bed: using a slice thickness equal to or less than 3 mm
Oblique coronal of the anal canal: for lower rectal tumors, also using a slice thickness equal to or less than 3 mm
DWI: using a b value of 800 or higher and including apparent diffusion coefficient maps (Table 2)
Table 2.
Suggested sequence parameters for DWI sequences (based on the authors’ GE magnets)
| 1.5T | 3.0T | |
|---|---|---|
| b value (s/mm2) | 0, 800 | 0, 800 |
| TR, ms | 8000 | 8000 |
| TE, ms | min | min |
| Slice thickness (mm) | 8 | 5 |
| Number of slices | 46 | 46 |
| Matrix | 128×128 | 128×128 |
| FOV (mm2) | Fit to anatomy | Fit to anatomy |
| Averages | 16 | 14 |
| Acceleration factor | 2 | 2 |
Abbreviations: FOV, field of view; min, minimum; TE, echo time; TR, repetition time
Motion artifacts can impact the imaging quality of the rectal MRI. Strategies other than spasmolytics may help, such as adapting the frequency direction as well as using postprocessing motion correction procedures (27). Comparison with the baseline scan is helpful to determine the location of the tumor and to define the best angulation through the tumor bed.
Figure 1 summarizes the techniques that are recommended (“Do’s”), those that overall are not recommended (“Don’ts”), and some that are frequently used, with growing evidence of their added value in the restaging setting (“Maybe’s”).
Figure 1.
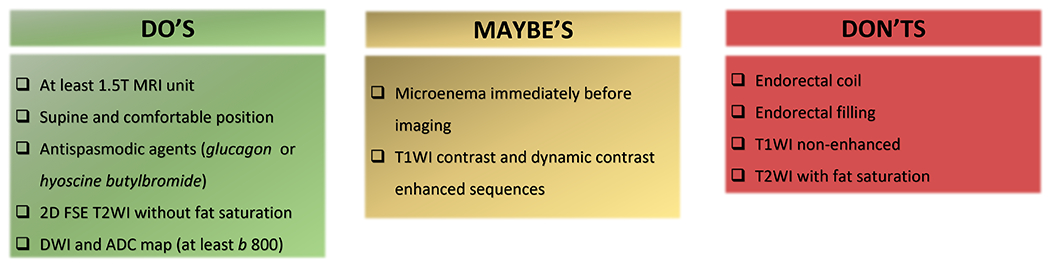
Rectal MRI techniques that are recommended (“Do’s”), those that overall are not recommended (“Don’ts”), and some that are frequently used, with growing evidence of their added value in the restaging setting (“Maybe’s”).
Overall, the following preparation and sequences have proven unnecessary: endorectal coil, endorectal filling, and T2WI with fat suppression. There is growing evidence that a microenema immediately before imaging is helpful in reducing gas-related artifacts on DWI, particularly using 3T scanners (26). The use of a gadolinium-based contrast agent is variable; of note, while a strong consensus has not been established in the literature, some institutions perform post-contrast MRI as part of their restaging rectal MRI protocol and some studies have shown good results pertaining to the use of post-contrast MRI in response assessment (28,29).
4. Step-by-Step Approach to Restaging Rectal MRI Interpretation
Figure 2 illustrates the key steps in interpreting restaging rectal MRI of the rectum, as well as the key findings to be addressed in the report (demonstrated by the mnemonic RECTAL).
Figure 2.
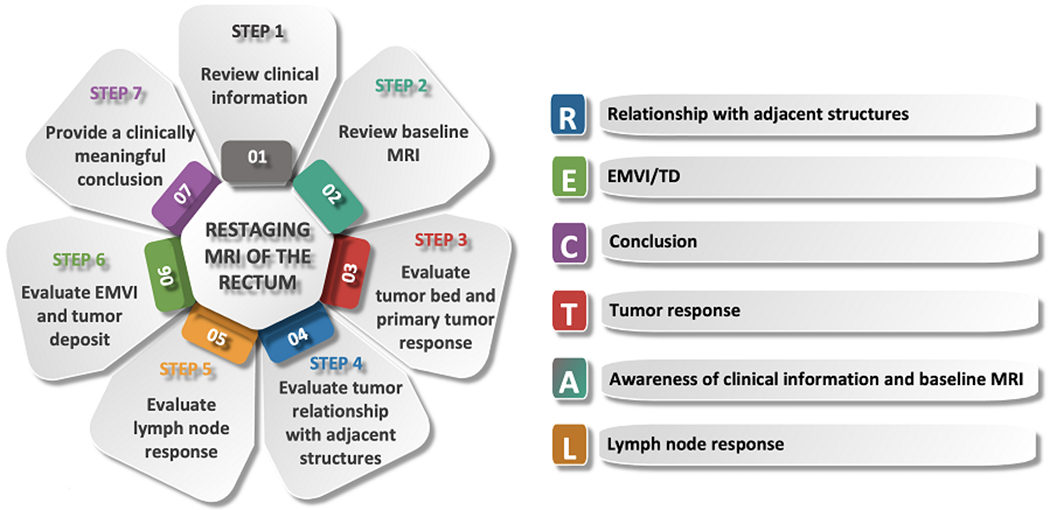
Diagram showing a step-by-step approach to interpreting restaging MRI of the rectum. The mnemonic RECTAL summarizes the key findings to be addressed in the restaging report.
4.1. STEP 1: Review clinical information
Before reporting on restaging MRI of the rectum, it is crucial to check the patient’s clinical history, such as digital rectal examination and endoscopy if performed, the type of treatment received (CRT, TNT), and the length of time since the last treatment (30). When prior clinical history is not available, it may be challenging to provide a meaningful conclusion.
As mentioned above, there is no consensus in the literature regarding the optimal timing to perform response assessment, including restaging MRI, with the timing varying depending on the type of treatment (5,31). However, increased rates of pathologic and clinical complete response have been associated with longer time intervals between restaging MRI and the end of the NAT (32,33). Overall, the optimal timing to assess response to CRT varies from 8 to 14 weeks after the completion of CRT, while the optimal timing to assess response to TNT tends to be longer, varying from 20 to 34 weeks after the completion of TNT. Notably, early response assessment is advocated by some to identify poor responders (5,31).
4.2. STEP 2: Review baseline MRI
It is advantageous to evaluate the baseline MRI scan, when available, to assess tumor location, tumor characteristics, and the presence of any mucinous components prior to the interpretation of restaging rectal MRI. After neoadjuvant therapy, the normal rectal wall adjacent to or opposite the treated tumor may become edematous and thickened, which can be misinterpreted as residual tumor (pseudotumor) by some readers (34). Thus, correlation with baseline rectal MRI is very helpful to determine the exact location of the tumor bed (Figure 3) (35).
Figure 3.
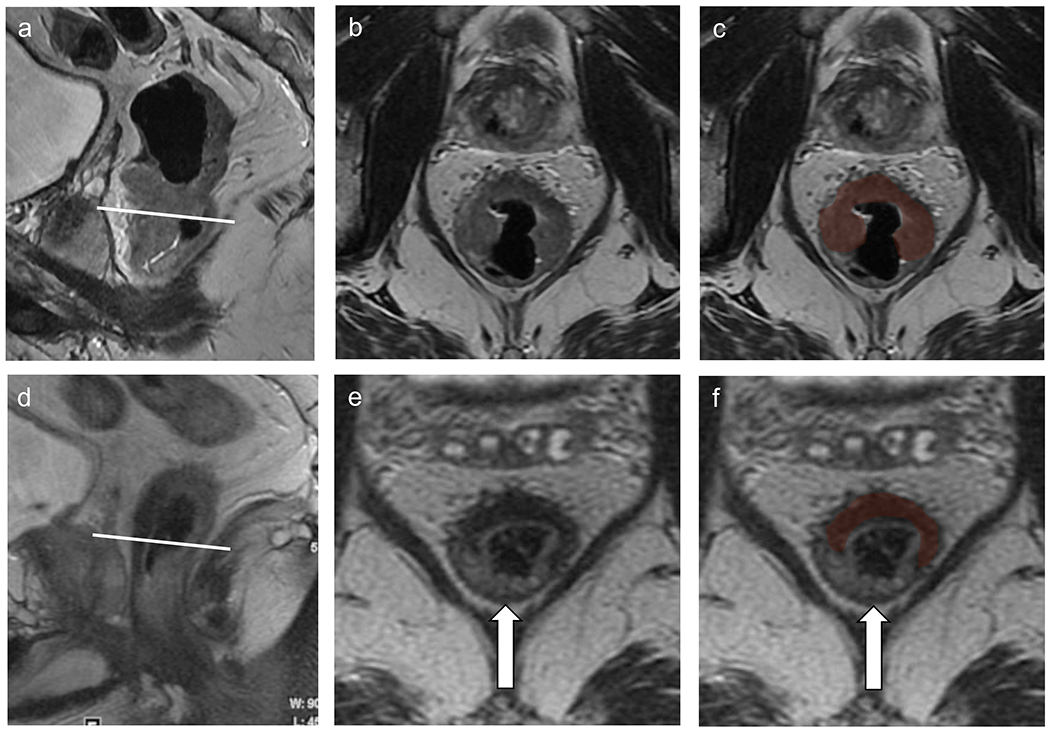
MRI of the rectum at baseline (a–c) as well as at restaging (d–f) after chemoradiation therapy. Baseline comparison allows for the delineation of the tumor bed (c and f, red shading). The opposite rectal wall shows mild submucosal edema (arrow).
4.3. STEP 3: Evaluate primary tumor response
Overall assessment
Interpretation of the primary tumor response on restaging MRI may be challenging. After neoadjuvant CRT, tumors may decrease in size (36), may be replaced by fibrotic tissue, or may in a small percentage of cases completely disappear with normalization of the layered appearance of the rectal wall (37). The accuracy of post-treatment MRI is lower than that of the initial staging MRI (38), due to the difficulty in differentiating residual tumor from fibrosis or desmoplastic reaction and misinterpretation of radiation changes as residual disease. A meta-analysis by Van der Paardt et al. (39) demonstrated that using T2WI alone, restaging MRI has a low sensitivity of 50.4% albeit a good specificity of 91.2% for the detection of residual tumor. The addition of DWI significantly increases the sensitivity to 83.6%. While DWI yielded a lower specificity of 84.8%, this did not represent a significant change.
T2WI
On T2WI, scar is visualized as low signal intensity at the site of the primary tumor, whereas residual tumor demonstrates intermediate signal intensity (35). Scar tissue should follow the same distribution and shape as the primary tumor (40). Rectal wall normalization can be seen in 5% of cases and is suggestive of complete response (37).
The MRI tumor regression grade (mrTRG) scale is an adaptation of the TRG used in pathology. It consists of a 1–5 scoring system based on the percentage of residual tumor and fibrosis post neoadjuvant therapy on T2WI (Table 3) (41). It is associated with disease-free and overall survival (42) and radiologists have demonstrated good agreement when using mrTRG (κ = 0.57) (Figure 4) (43). However, there is poor association between mrTRG and pathologic TRG (44–46). MrTRG has a modest sensitivity of 74% for the detection of complete response (44). Nonetheless, this system is widely used in the UK, Canada, and some parts of the United States. Its usefulness is currently being tested in the TRIGGER trial [NCT02704520]. Modifications to this system to include DWI have been tested with some success (47), including a recent study analyzing MRI scans from the TNT trial [NCT02921256] and presented at the American Society for Radiation Oncology (ASTRO) 2021 annual meeting (48). In this study, the addition of DWI to mrTRG (add +1 to mrTRG score if DWI+, and subtract 1 if DWI−) resulted in a sensitivity of 53.5%, specificity of 70.7%, positive predictive value (PPV) of 42.1%, and negative predictive value of 79.1%. The low sensitivity and PPV in this study show the need for stronger biomarkers.
Table 3.
Summary of magnetic resonance imaging tumor regression grade (mrTRG) (43).
| Scale | mrTRG |
|---|---|
| 1 | No/minimal fibrosis visible (thin linear scar) and no tumor signal |
| 2 | Dense fibrotic scar (low signal intensity) but no macroscopic tumor signal |
| 3 | Fibrosis predominates but obvious measurable areas of tumor signal visible |
| 4 | Tumor signal predominates with little/minimal fibrosis |
| 5 | Tumor signal only: no fibrosis or increased tumor |
Figure 4.
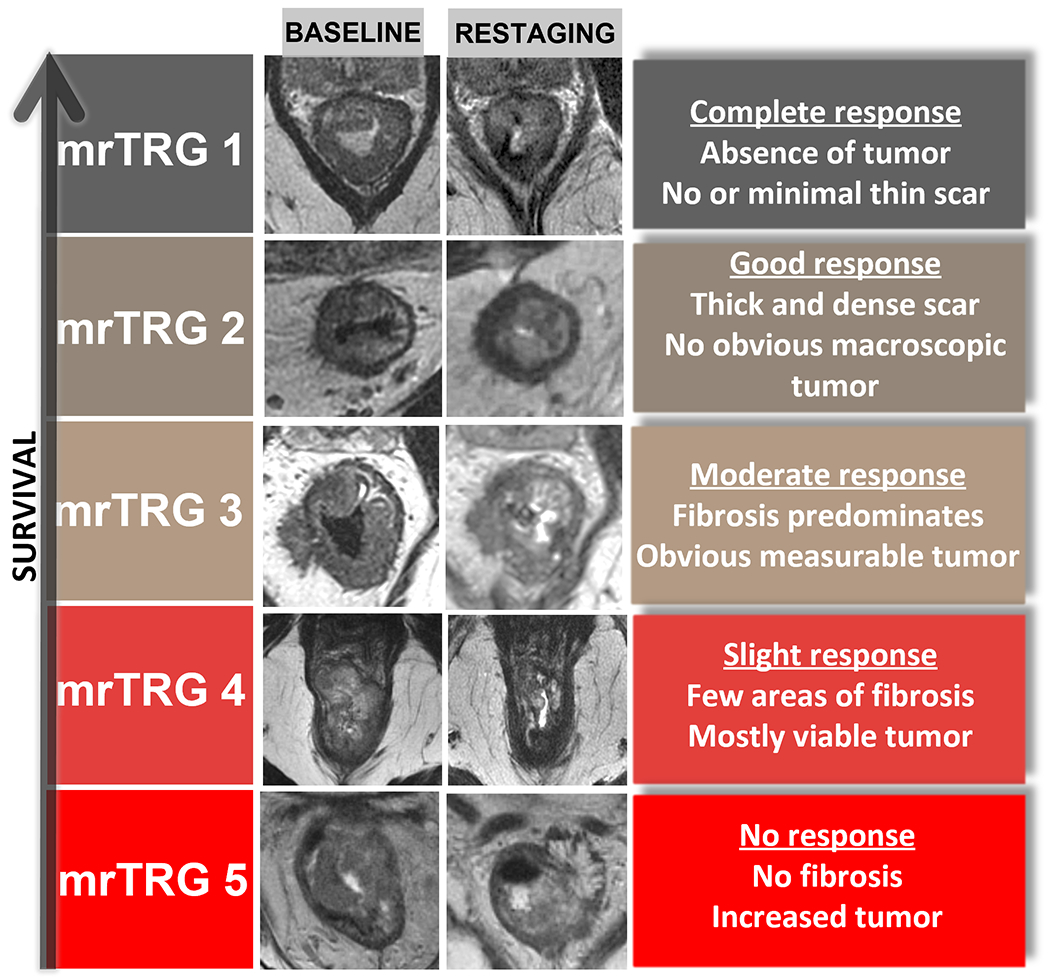
MRI tumor regression grade (mrTRG) chart demonstrates the 5-point scoring system based on the proportion of residual tumor and fibrosis after neoadjuvant therapy on T2WI.
DWI
DWI is a non-invasive technique that is sensitive to the structure of biological tissue at the microscopic level. DWI has proven to be a clinically useful, non-invasive functional imaging technique used in tumor detection, staging, and for monitoring response to treatment in a variety of tumor types. The advantages of this technique include short acquisition times, no need for intravenous administration of contrast agent, and the ability to indirectly study tissue cellularity through non-invasive probing of the diffusion of water molecules in tissue.
The motion of free water molecules without any restriction, named Brownian motion, is related to thermal kinetic energy. In biological tissues, microscopic Brownian motion detected by DWI includes microcirculation of blood in the capillary network and diffusion of water molecules, which is related to the structural components of the tissue. The degree of water diffusion in is inversely correlated to the integrity of cell membranes and tissue cellularity. Consequently, tissues with high cellular density and numerous intact cell membranes, such as viable tumor, will present restriction of motion of water.
The monoexponential diffusion model requires at least two acquisitions with different b values, one with a b value of 0 and one with a higher b value, and then ADC maps are generated. In the simplest case, the diffusion coefficient is independent of b value and described by a single exponential:
Where S(b) and S(0) are signal intensities of each voxel with and without diffusion weighting, and the quantity b, is the diffusion-sensitizing factor (commonly referred to as the b value). The apparent diffusion coefficient, ADC, is a single diffusion coefficient which describes a multitude of diffusion properties of tissue, including both diffusion and perfusion, and is assumed to be independent of the b value, i.e., D(b) = ADC = constant. The b value is a critical parameter in DWI which encodes different tissue properties into the DWI signals and controls the degree of diffusion-weighting in the image. The MR signals always decrease as b value increases. Higher b values correspond to stronger diffusion effects.
DWI has been shown to increase the diagnostic performance of tumor response assessment after neoadjuvant therapy over T2WI alone (39,49) and to improve the performance of MRI in predicting complete response (50,51). However, some factors need to be taken into account, such as DWI quality, slice thickness, and type of tumor.
Fibrosis has low cellularity and will show low signal intensity on DW images and on apparent diffusion coefficient (ADC) maps. On the other hand, residual tumor presents high cellularity and will show high signal intensity on DWI with corresponding low ADC (Figures 5 and 6). Residual tumor tends to show the same morphology as the tumor on baseline; for example, residual tumor of a prior partially circumferential or polypoid tumor will show focal restricted diffusion, and a circumferential tumor may show dispersed or circumferential foci of restricted diffusion. Keeping this in mind, a “pattern-based approach” when evaluating for residual disease increases the sensitivity to 94% and specificity to 77% in predicting complete response (40).
Figure 5.
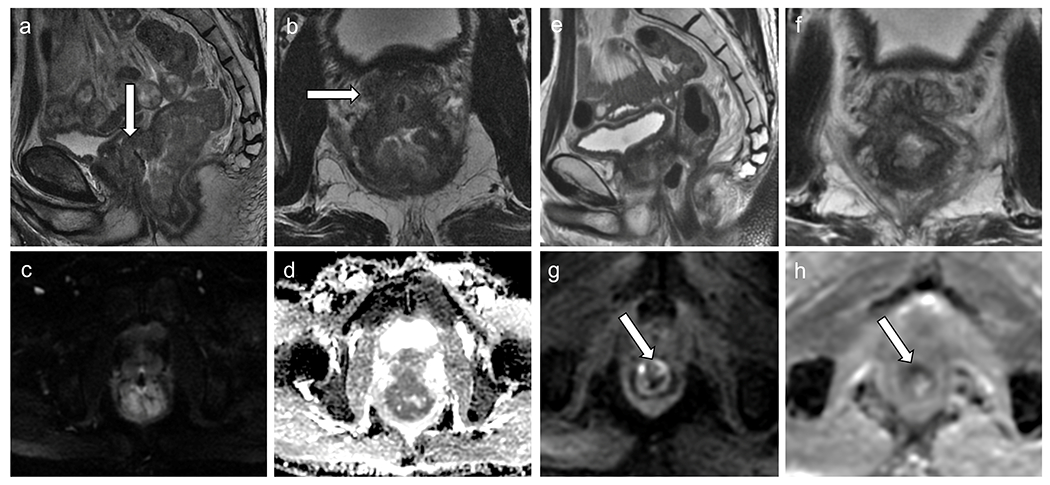
Baseline rectal MRI (a–d) shows, on T2WI, a low rectal tumor (a–b), T3N1, with diffusion restriction (c–d) infiltrating the prostate gland (white arrows). Restaging rectal MRI (e–h) demonstrates decreased rectal tumor, with few areas of fibrosis and mostly viable tumor with intermediate signal intensity on T2WI (mrTRG 4) (e–f), with areas of diffusion restriction (high signal intensity on DWI (g) and low signal intensity on ADC map (h)), inseparable from the prostate gland and seminal vesicles, consistent with incomplete response. . The patient underwent abdominoperineal resection, which demonstrated residual tumor infiltrating the prostate gland and seminal vesicles (ypT4b).
Figure 6.
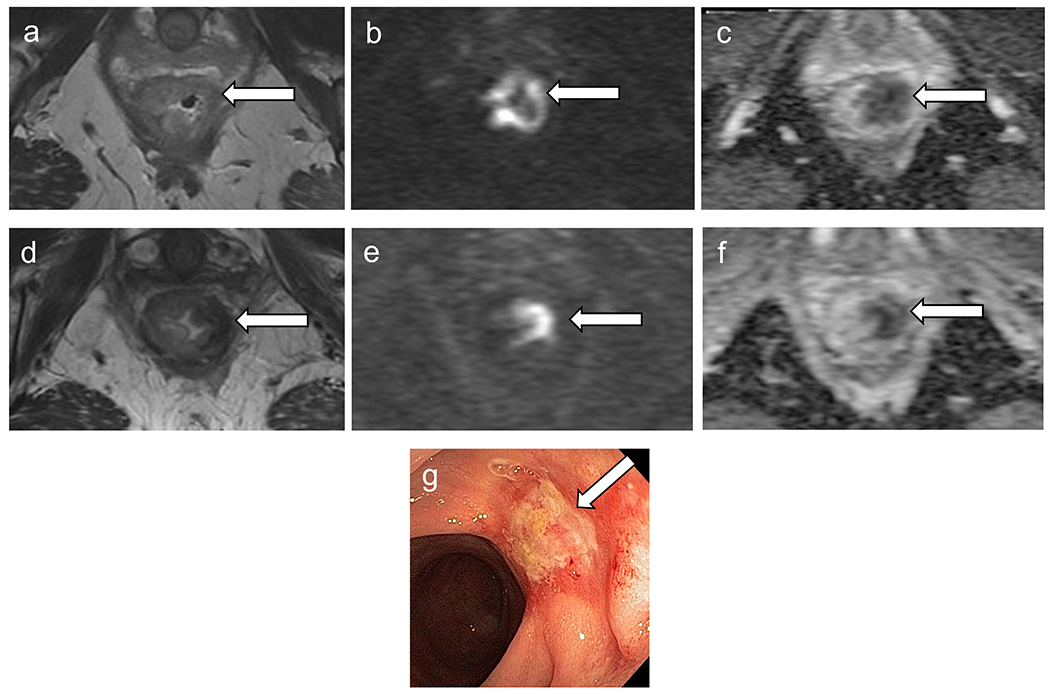
Baseline MRI (a–c) shows low rectal tumor with restriction on diffusion. Restaging MRI (d–f) demonstrates decreased size of primary tumor with thick scar and areas of intermediate signal intensity on T2 (mrTRG 3) (d) and diffusion restriction, high signal intensity on DWI (e) and low signal intensity on ADC map (f), consistent with incomplete response. (g) Restaging colonoscopy shows viable tumor. The patient underwent abdominoperineal resection and pT2N0 was confirmed.
Partial volume effects occur when the voxel intensity is dependent on multiple tissue properties within a voxel and occur more frequently when the voxel size (thickness, in-plane resolution) is large in comparison to the acquisition parameters. This is one of the limitations of DWI which tends to have lower spatial resolution compared to anatomical imaging.
The interpretation of DWI images requires training and expertise. Studies have shown moderate inter-reader agreement for DWI (κ = 0.402) (52), which mildly improves by adding T2WI (κ = 0.51–0.688) (50,52). In a recent study (6) by OPRA radiologists which involved 12 radiologists evaluating the T2WI and DWI features of 39 patients from a single surgeon’s practice, the combination of both was overall the best strategy, and yet the mean accuracy, sensitivity and specificity was only 64%, 65%, and 63%, respectively.
A study by Gollub et al. showed that most of the positive DWI findings on restaging MRI are concordant with endoscopy, with a positive predictive value (PPV) of 86%. Importantly, in 22% of discordant findings, endoscopy turned positive on subsequent follow-up within 3 months, therefore demonstrating that DWI can detect early tumor regrowth (or lack of complete regression) prior to endoscopy (53).
When interpreting DWI, it is important to be aware of several pitfalls that may mimic residual tumor (54). The most important pitfalls are as follows:
Artifacts: DWI is susceptible to various artifacts. Rectal MRI is particularly prone to susceptibility artifacts from hip prostheses and rectal air. Microenema before rectal MRI reduces rectal air, improving image quality. However, when DWI is suboptimal, this should be addressed in the report so that the multidisciplinary group can account for this in the treatment plan (55).
T2 shine-through: T2 shine-through corresponds to high signal intensity on both DWI and the ADC map. This pitfall is mainly seen secondary to the long T2 relaxation times of fluid or mucin. When it is within the lumen, a tri-radiate configuration is frequently seen (sometimes called the “Mercedes-Benz” sign) which is distinct from residual tumor which often demonstrates a nodular appearance or focal mural thickening (Figure 7). When mucin is present, MRI as of yet cannot differentiate between viable (cellular mucin) and non-viable (acellular mucin) disease, as both demonstrate high signal intensity on DWI and ADC map.
T2 dark-through: T2 dark-through corresponds to low signal intensity on both DWI and the ADC map and is related to fibrosis. Importantly, if the reader relies only on the ADC map, erroneous interpretation of residual disease can be made (Figure 7).
Figure 7.
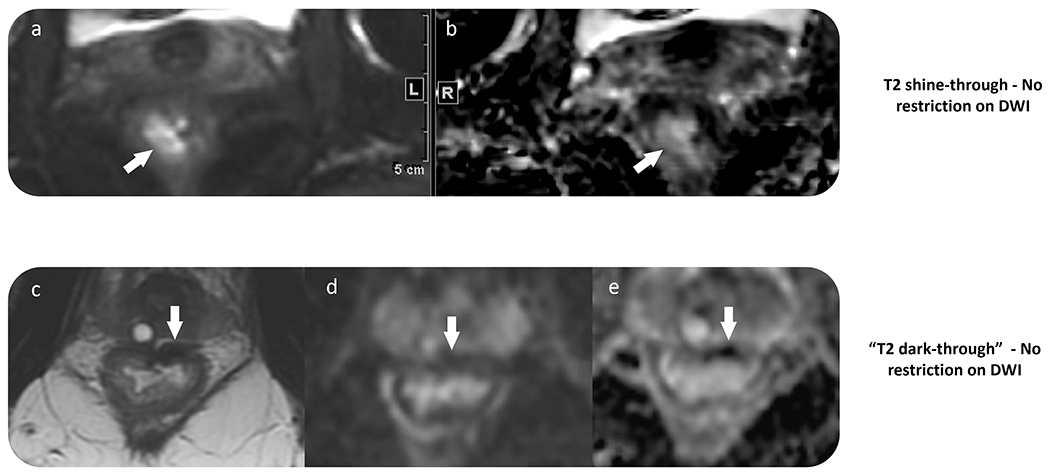
Two examples of negative DWI obtained as part of restaging rectal MRI. (a–b) T2 shine-through effect, with high signal intensity within the rectal wall on DWI and the ADC map. (c–e) T2 dark-through shows the rectal wall scar with low signal intensity on T2 imaging, DWI, and ADC due to fibrosis. Viable tumor should be considered only in cases with high signal intensity on DWI and low signal intensity on ADC map within the tumor bed. Comparison with baseline scans may also be of value.
Mucin
The presence of mucin can be seen in three different scenarios on restaging MRI post neoadjuvant therapy:
Mucinous tumor with viable disease (cellular mucin): In general, the tumor bed will show a mixture of high and intermediate signal intensity on T2WI similar to baseline MRI (35). These tumors are usually associated with poor prognosis and high risk for local recurrence (56).
Mucinous tumor without viable disease (acellular mucin): Tumors with mucinous content at baseline may develop acellular mucin post neoadjuvant therapy, which is a type of tumor response (57) with no impact on progression-free survival (58). So far, there is no reliable method for detecting acellular mucin short of complete resection.
Mucin (colloid) degeneration: Mucin degeneration consists of new mucin production in non-mucinous tumors, which is seen as high signal intensity on T2WI (35) This has been considered as post-treatment response and a sign of better prognosis (59); however, residual tumor cells may still exist and avoid radiologic detection (Figure 8).
Figure 8.
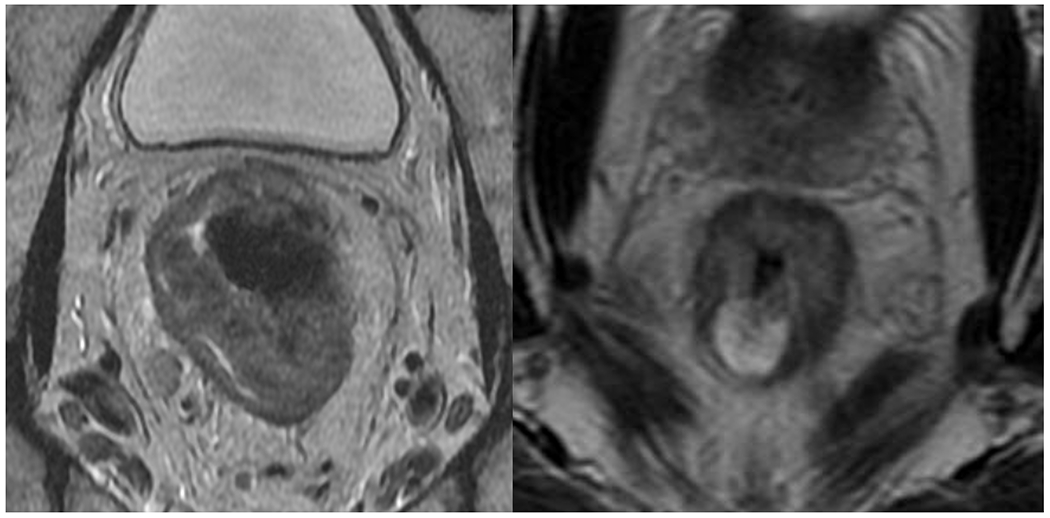
Baseline rectal MRI showing (a) a non-mucinous tumor. After neoadjuvant therapy, the patient developed (b) mucinous degeneration (black arrow). The patient underwent surgical resection and the pathology demonstrated viable tumor. Currently, MRI cannot differentiate cellular from acellular mucin.
Although the above mucin scenarios can be observed on restaging MRI, there is no reliable imaging finding to date that can differentiate cellular from acellular mucin (Figure 8). Park et al. (60) proposed an mrTRG system dedicated to patients with mucinous adenocarcinoma based on staging and restaging MRI rectum comparison, as follows: mrTRG 1, complete response and no identifiable residual lesion; mrTRG 2, no residual non-mucinous component (intermediate signal intensity on T2WI) and only pure mucin (high signal intensity on T2WI) and/or dense fibrosis (low signal intensity on T2WI); mrTRG 3, decreased nonmucinous component and minimal on restaging MRI; mrTRG 4, moderately decreased nonmucinous component (do not meet other criteria); TRG 5, no response or increased nonmucinous tumor component. However, this was a hypothesis-generating, non-validated study with moderate inter-reader agreement on MRI interpretation and no difference in survival among the groups. Consequently, in the absence of radiologist’s ability to reliably differentiate between cellular and acellular mucin, such cases will remain in need of multidisciplinary input and discussion to determine the best individualized treatment options. Of note, MRI-based radiomics has also been investigated as tool to differentiate post-treatment residual cellular and acellular mucinous rectal neoplasms, but thus far early results show that the radiomics model has only a modest AUC (61).
4.4. STEP 4: Evaluate the relationship of the tumor with adjacent structures
The relationship between the tumor and fibrotic changes with adjacent structures is important to delineate to guide treatment planning and provide a roadmap for surgical resection. The most important structures to be assessed include: mesorectal fascia (MRF), genitourinary organs (bladder, ureters, urethra, prostate, seminal vesicles, uterus, vagina), pelvic side wall, vessels (common, internal and external iliac vessels), nerves (sciatic, sacral roots, lumbosacral trunk), the sphincter complex (levator ani, puborectalis, external sphincter, intersphincteric space, internal sphincter), and osseous structures.
After neoadjuvant therapy, it is challenging to differentiate purely fibrotic tissue from fibrosis with residual tumor cells. Circumferential resection margin (CRM) involvement is one predictor of local recurrence. On pathology, positive CRM due to MRF involvement is associated with local recurrence. MRI after neoadjuvant therapy has a low positive predictive value (around 50%) and high negative predictive value (higher than 90%) for CRM involvement (62–64). In efforts to overcome the limitations of MRI, some studies have suggested specific morphologic patterns on MRI in addition to applying the distance from the MRF (1–2 mm, threatened; < 1 mm, involved). If MRF infiltration is associated with MRF thickening, there is a higher risk of positive CRM on pathology. Additionally, DWI may play a role in predicting CRM involvement (51).
However, ultimately, MRI tends to overestimate CRM, which was recently confirmed on a whole-mount study with 94 patients. The authors demonstrated that only 18% of the margins reported as involved on MRI were positive on pathology and that an anterior location and tumor proximity to the anal verge were independently associated with reduced MRI accuracy (65).
Therefore, it is important for radiologists to be cautious when assessing CRM involvement on restaging MRI. The most common challenging scenarios are differentiating pure fibrosis from fibrosis with foci of residual disease and cellular from acellular mucin within in patients with mucinous tumor. The proper plane of imaging may be helpful to minimize overestimation of CRM involvement and the use of a certainty lexicon on the MRI reports can be helpful to avoid unnecessary radical interventions (66).
4.5. STEP 5: Evaluate lymph node response
The performance of MRI in nodal restaging after neoadjuvant therapy is better than in the primary staging setting, where negative predictive values of up to 95% have been reported for the purpose of identifying ypN0 patients (67). After neoadjuvant therapy, most lymph nodes decrease in size or even completely disappear on MRI (68).
Mesorectal, obturator, and internal iliac nodes are considered locoregional lymph nodes. Common and external iliac nodes, as well as inguinal nodes, are considered non-regional lymph nodes (M1 disease). Of note, as an exception, if the lower rectal tumor extends below the dentate line (not visible at MRI, but at about the midpoint of the anal canal), inguinal nodes are considered regional lymph nodes (69). Additionally, external iliac, internal iliac, and obturator nodes are often referred to as lateral pelvic lymph nodes. The boundaries between the lateral pelvic lymph nodes are as follows:
Internal iliac: On and medial to the main trunk of the internal iliac vessels and superior to the infrapiriformis foramen.
Obturator: Lateral to the main trunk of the internal iliac vessels and posterior to the external iliac vessels.
External iliac: Along the external iliac vessels.
Mesorectal lymph nodes
Unlike in the primary staging setting, morphological criteria are not recommended to be used in the restaging setting. For mesorectal lymph nodes, imaging features that have been most reliably associated with negative mesorectal lymph node status on pathology are the non-visualization of lymph nodes on DWI, decrease of at least 70% in node size, and lymph nodes measuring less than 0.5 cm in the short axis (70–72). In general, nodes measuring less than 0.5 cm should be considered negative. Since no reliable nodal criteria exists, after neoadjuvant therapy, nodes equal to or larger than 0.5 cm are considered metastatic, although there are false positive and false negative cases (Figure 5) (31).
Lateral pelvic lymph nodes
Lateral pelvic lymph nodes are not routinely boosted in radiation planning nor are they routinely resected in Western countries. However, patients with non-responding enlarged lateral pelvic nodal metastases have a higher risk of local, and distant recurrence (73). As such, when positive on imaging, a boost of radiotherapy and/or a lymph node dissection may be indicated to improve the clinical outcome (73).
For lateral pelvic lymph nodes, morphologic criteria have not been shown to be helpful at primary staging or restaging. As suggested by the Lateral Node Consortium, at primary staging, the cut-off is equal to or larger than 0.7 cm in the short axis (74), and at restaging MRI, although not widely accepted, the cutoff is 0.4 cm for internal iliac nodes and 0.6 cm for the obturator (73). It should be emphasized that, similar to the scenario where size cut-offs are used for mesorectal nodes at baseline and after treatment, no one-size cut-off is perfect, and trade-offs exist between sensitivity and specificity. The sizes quoted here for lateral nodes are arbitrarily suggested based on “acceptable” local recurrence risks (15%), but these risks are not zero.
4.6. STEP 6: Evaluate extramural vascular invasion and tumor deposit
Both extramural vascular invasion (EMVI) and tumor deposits are associated with worse oncological prognosis (75). Regression of EMVI after neoadjuvant therapy is associated with improved survival when compared with persistent EMVI (76).
EMVI is defined on pathology as direct tumor invasion into extramural vessels. On MRI, EMVI is characterized by prominent tubular structures containing intermediate signal intensity on T2WI emanating from the tumor region, with or without irregular contour.
Tumor deposit is defined as a tumoral lesion in the mesorectum discontinuous from the tumor without lymphoid tissue on pathology (77). On imaging, it is challenging to differentiate a tumor deposit from a lymph node; usually, tumor deposits are irregular nodules that cannot be separated from the vessels. If a tumor deposit is present, it is considered N1c disease according to the American Joint Committee on Cancer 8th edition classification.
After neoadjuvant therapy, edema, desmoplastic reaction, and inflammatory changes may reduce the ability of MRI to detect viable tumor within EMVI or within a tumor deposit. DWI may be an additional tool to improve the detection of EMVI (78). MRI, however, is particularly valuable during non-operative management, when the patient may present with tumor regrowth in the form of EMVI or in a tumor deposit which cannot be detected on endoscopy but which is readily detectable on MRI (Figure 9).
Figure 9.
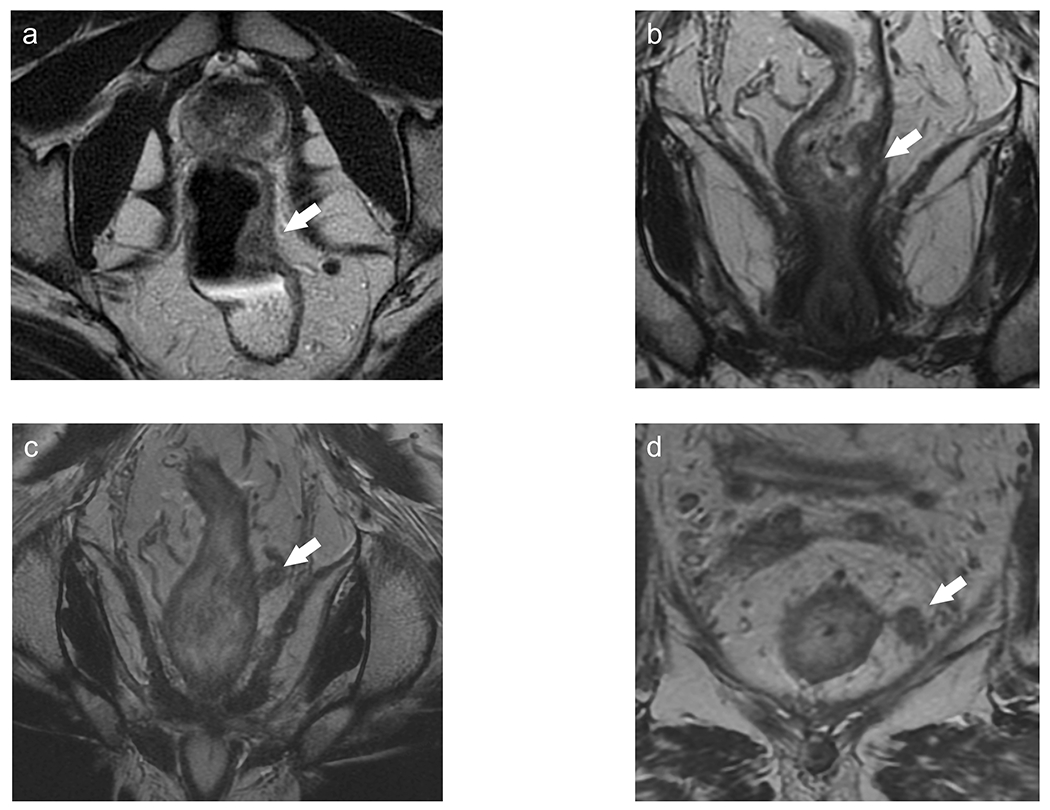
A patient with T1-2N0 lower rectal tumor at baseline (a) underwent chemoradiotherapy with clinical complete response on nonoperative management. 12 months after chemoradiotherapy, there was no evidence of tumor regrowth on MRI (b), digital rectal examination (DRE), and endoscopy. 24 months after chemoradiotherapy (d), there was new isolated EMVI, without tumor regrowth within the tumor bed on MRI, DRE, or endoscopy, consistent with locoregional regrowth.
4.7. STEP 7: Provide a clinically meaningful conclusion
After neoadjuvant therapy, it is important to provide a clear interpretation of the imaging findings and to classify the patient into (radiological) clinical complete response (Figure 10), near complete response, or poor/incomplete response (recently “incomplete response” has been preferred, albeit not well defined yet). In cases of viable tumor, highlighting the extra-mesorectal involved structures is relevant, since conventional total mesorectal excision will not remove them, increasing the risk of local recurrence in these cases.
Figure 10.
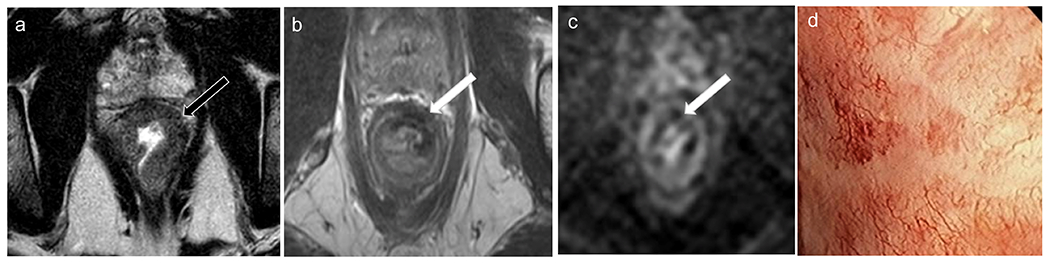
Baseline rectal MRI (a) shows on T2WI a c-shaped tumor (black arrow). Restaging MRI (b–c) shows decreased tumor with thin scar (mrTRG 1) without diffusion restriction. Endoscopy (d) shows telangiectasia and no residual tumor. The patient was classified as clinical complete response and is under nonoperative management without evidence of tumor regrowth.
For patients undergoing non-operative management after an initial diagnosis of clinical complete response, the following definitions are important:
Locoregional regrowth: detection of tumor involving either the bowel well, mesorectum, and/or pelvic organs (Figure 8)
Local regrowth: detection of tumor within the bowel only
For patients who have undergone local excision or total mesorectal excision, the following definitions are important:
Locoregional recurrence: detection of tumor involving either the bowel well, mesorectum, and/or pelvic organs
Local recurrence: detection of tumor within the bowel only (5)
5. Research Directions on Response Assessment
Over the last several years, several research initiatives have explored ways to improve the diagnostic performance of MRI in assessing tumor response after neoadjuvant therapy. Although promising, larger validation studies are awaited before the following methods can be recommended as the standard of care.
5.1. Tumor volumetry
Tumor volumetry is being evaluated as a potential tool for response assessment after neoadjuvant therapy. However, it may be difficult to differentiate residual tumor from other post-treatment changes using volumetric measures based on T2WI alone, due to fibrotic changes, inflammatory changes, and edema. Furthermore, unless automated, this tool would be cumbersome to incorporate into daily practice.
One study demonstrated that tumor volume on T2WI and its reduction correlated with the ypT stage, although MRI overestimated the volume of residual tumor (79). In another study, the authors evaluated tumor volume based on DWI (defining total lesion diffusion as the total DWI tumor volume multiplied by mean volumetric ADC), demonstrating that tumor volume based on DWI was correlated with pathological tumor regression and was comparable to DWI- and DCE-based volumetry (80).
Pooled analysis demonstrated the superiority of DWI over T2WI volumetry, wherein volumetric DWI measurements after neoadjuvant therapy were able to predict pCR with a sensitivity and an overall accuracy of 65% and 90%, respectively (81).
5.2. Functional imaging
DWI may provide functional information on the microstructure of the tumor environment through the assessment of differences in water proton mobility (82). Water diffusion characteristics depend on several factors such as cell density, vascularity, viscosity of the extracellular fluid, and cell membrane integrity. By quantifying these properties using the ADC map, DWI can be used as an imaging biomarker to predict tumoral response (83).
With regard to the qualitative, visual assessment of images, studies have investigated the use of DWI with different FOV sizes and b values as a tool for treatment response prediction. Bates et al. demonstrated similar accuracy between b800 and b1500 mm/s2 sequences, albeit there was a trend towards increased sensitivity with the b1500 mm/s2 sequence (84). Hausmann et al. demonstrated that ultra-high b value DWI (b2000 mm/s2) could be beneficial in both primary and restaging MRI (85). With regard to FOV size, some studies demonstrated improved image quality with a smaller FOV (86), while others failed to show any difference in quality (87).
With regard to model-based, quantitative assessment, mean ADC values on pre-treatment images have been shown to be negatively correlated with post-treatment tumor size, suggesting the potential of ADC to predict treatment response (88). One study also evaluated the relationship between ADC parameters and tumor volume reduction or histopathological response to predict the therapeutic response to CRT, showing that the relative change in ADC among histopathological responders was significantly higher than that among non-responders (p < 0.005) (89). However, in other studies, ADC measurements failed to accurately distinguish complete responders from partial responders, probably related to region of interest size and positioning differences for tumor ADC value calculation (90,91).
A more granular assessment of the tumor microenvironment can be extracted from DWI when multiple b values are employed. Termed intravoxel incoherent motion (IVIM), this model-based analysis reveals water diffusion and microcirculation perfusion, reflecting microvascular perfusion and diffusion of water molecules in living tissues, ultimately allowing the separation of perfusion and diffusion contributions within the image (92), with the advantage of teasing out the individual components of overlapping tissues.
IVIM using bi-exponential modeling, first proposed by Le Bihan et al. to separate perfusion and diffusion characteristics in the brain (92), has garnished substantial interest in characterizing tissues in the body. To estimate diffusion parameters with this model, the diffusion signal is measured for a large number of b values, ranging from very low to high. The effect of perfusion on the total signal is modeled by taking into account the volume fraction f of the tissue water flowing through the microvessel. According to this model, the signal attenuation is given by:
where S(b) and S(0) are the signal amplitudes at b values b and zero, respectively, D is the diffusion coefficient, f is the volume fraction of water in perfused capillaries, and D* is defined as a pseudo-diffusion coefficient, which is dependent on the mean path length and blood velocity within the capillary network.
IVIM has been applied to preoperatively assess rectal cancer treatment response (93,94), detect EMVI (95), and to serve as an early predictor of complete response a few days after the beginning of the treatment (96).
MRI spectroscopy is also being studied as a biomarker for monitoring treatment response. Some studies have suggested that a decreased choline peak is a potential biomarker of response. These results await further validation (97).
5.3. PET
Metabolic response as seen on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) can be detected earlier than a decrease in tumor volume (98). There are several FDG PET/CT predictive parameters of rectal cancer response to CRT, including visual response, maximum standardized uptake value (SUVmax), percentage SUVmax reduction, total lesion glycolysis (TLG), and metabolic tumor volume (MTV) (99).
Some studies in rectal cancer have reported that TLG and MTV are strong pre-treatment prognostic factors for cancer recurrence and cancer-related death. Patients with high TLG or MTV had a worse clinical outcome (100). Another study concluded that higher SUVmax is significantly associated with shorter survival, and that SUVmax is a useful preoperative prognostic factor (101). However, another study reported that high FDG uptake of primary mass in resectable colorectal cancer does not have a significant relationship with tumor recurrence and disease-free survival (102).
PET/MRI is a promising tool that may add anatomical resolution to the standard PET/CT. In the restaging setting after neoadjuvant therapy, the primary tumor’s metabolic characteristics can add value to deciding whether or not to pursue organ preservation (103). Concordance between PET and MRI findings in showing clinical complete response can increase the confidence of selecting a non-operative management approach. In addition, the combination of MRI with FDG-PET may improve response characterization. It has been demonstrated that the percent change in tumor SUVmax and changes in the ADC map post-CRT are predictors of outcome, although SUVmax may not reflect the heterogeneous nature of the tumor.
Volume-based PET parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been developed to measure the metabolic activity in the entire tumor mass (104). One study indicated that a higher SUV-to-ADC ratio can predict a more aggressive tumor (105), although given the tight inverse correlation between SUV and ADC, it is unclear what the additive value is of DWI and SUV. Metabolism may correlate with cellular density, although frequently these factors move inversely, while DWI correlates more with changes in fibrosis and the extracellular space volume (106).
FDG PET/MRI is also valuable in identifying distant metastases and tumor recurrence (107). In the setting of recurrent tumor, suspicious lesions on PET/MRI had a sensitivity and specificity of 94% with histopathology as the standard of reference, although no comparison to MRI or PET imaging interpretations were reported (108).
5.4. Radiomics
Radiomics is a bioinformatics tool that correlates the textural features obtained from a given region of interest or volume of interest with a specific clinical endpoint, including histology and treatment response (109,110). The textural features provide quantitative data of the image that may or may not be perceptible to human eyes (111).
With the ability to extract vast quantitative information from imaging, radiomics (110,112) is being increasingly investigated for its potential to non-invasively predict treatment response after neoadjuvant therapy in patients with LARC (113). Previous radiomic models have shown promising results based on pre- and/or post-neoadjuvant therapy MRI using either T2WI or multiparametric sequences (114,115). Radiomics has also been shown to effectively predict the status of lymph nodes as well as KRAS mutation (116) prior to neoadjuvant therapy in patients with LARC, providing a basis for clinical decision-making (117). Radiomics of the mesorectal fat has shown promising results as an important prognostic biomarker to predict pathological complete response, post-treatment T and N stages, as well as local and distant recurrence (118,119). Radiomics has also shown a potential tool to distinguishing cellular from acellular mucin (61). Although promising, the generalizability of radiomics-based results across multiple institutions using multiple different MRI vendors and sequences is still limited and further improvements are needed to implement any of the obtained data into clinical decision-making algorithms (120).
5.5. Adaptive risk trials
Considering the increasing number of neoadjuvant therapy strategies and the broad spectrum of possible toxicities, there is an urgent need for adaptive risk trials aiming at personalized treatment of LARC based on individual patient and tumor characteristics. Rectal MRI has demonstrated the potential to add value to such treatment algorithms.
6. Conclusion
Rectal MRI plays a key role in the assessment of treatment response after neoadjuvant therapy and its role will be even more important, in light of the increasing adoption of non-operative management as a viable alternative. Radiologists will continue to add value through their awareness of the types and rationale of the various neoadjuvant treatment approaches, current and best practices in the performance of a high-quality MRI protocol, and a stepwise systematic MRI rectum review, as well as through their critical input during multidisciplinary discussions. These fundamental steps will promote personalized treatment plans with the ultimate goal of improved outcomes.
Grant Support:
NH - This research was funded in part through the NIH/NCI Cancer Center Support Grant (P30 CA008748)
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Kasi PM, Shahjehan F, Cochuyt JJ, Li Z, Colibaseanu DT, Merchea A. Rising Proportion of Young Individuals With Rectal and Colon Cancer. Clin Colorectal Cancer 2019;18:e87–e95. [DOI] [PubMed] [Google Scholar]
- 3.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–1482. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 5.Fokas E, Appelt A, Glynne-Jones R, et al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol 2021;18:805–816. [DOI] [PubMed] [Google Scholar]
- 6.Yuval JB, Garcia-Aguilar J. Watch-and-wait Management for Rectal Cancer After Clinical Complete Response to Neoadjuvant Therapy. Adv Surg 2021;55:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong JC, Soucisse M, Michael M, et al. Total Neoadjuvant Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Metaanalysis of Oncological and Operative Outcomes. Ann Surg Oncol 2021;28:7476–7486. [DOI] [PubMed] [Google Scholar]
- 8.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 9.Kasi A, Abbasi S, Handa S, et al. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Rashid F, Liberman AS, Charlebois P, et al. The impact of bowel dysfunction on health-related quality of life after rectal cancer surgery: a systematic review. Tech Coloproctol 2022. [DOI] [PubMed] [Google Scholar]
- 11.Brown G, Davies S, Williams GT, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer 2004;91:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akasu T, Kondo H, Moriya Y, et al. Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg 2000;24:1061–1068. [DOI] [PubMed] [Google Scholar]
- 13.Vliegen R, Dresen R, Beets G, et al. The accuracy of Multi-detector row CT for the assessment of tumor invasion of the mesorectal fascia in primary rectal cancer. Abdom Imaging 2008;33:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maizlin ZV, Brown JA, So G, et al. Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer? Dis Colon Rectum 2010;53:308–314. [DOI] [PubMed] [Google Scholar]
- 15.Duldulao MP, Lee W, Streja L, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 2013;56:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas M, Dijkhoff RAP, Beets-Tan R. Rectal Cancer: Assessing Response to Neoadjuvant Therapy. Magn Reson Imaging Clin N Am 2020;28:117–126. [DOI] [PubMed] [Google Scholar]
- 17.NCCN. NCCN Guidelines for Rectal Cancer. 2022. [Google Scholar]
- 18.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol 2022:JCO2200032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 21.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–715. [DOI] [PubMed] [Google Scholar]
- 22.Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol 2015;22:3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hupkens BJP, Maas M, Martens MH, et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann Surg Oncol 2018;25:197–203. [DOI] [PubMed] [Google Scholar]
- 24.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Griethuysen JJM, Bus EM, Hauptmann M, et al. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - Effect of applying a micro-enema to improve image quality. Eur J Radiol 2018;99:131–137. [DOI] [PubMed] [Google Scholar]
- 26.Jayaprakasam VS, Javed-Tayyab S, Gangai N, et al. Does microenema administration improve the quality of DWI sequences in rectal MRI? Abdom Radiol (NY) 2021;46:858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu M, Mulder HT, Baron P, et al. Correction of motion-induced susceptibility artifacts and B. Magn Reson Med 2020;84:2495–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou HH, Yu J, Wei Y, Wu JF, Xu Q. Response to neoadjuvant chemoradiotherapy for locally advanced rectum cancer: Texture analysis of dynamic contrast-enhanced MRI. J Magn Reson Imaging 2019;49:885–893. [DOI] [PubMed] [Google Scholar]
- 29.Corines MJ, Nougaret S, Weiser MR, Khan M, Gollub MJ. Gadolinium-Based Contrast Agent During Pelvic MRI: Contribution to Patient Management in Rectal Cancer. Dis Colon Rectum 2018;61:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019;39:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg 2016;263:458–464. [DOI] [PubMed] [Google Scholar]
- 33.Kaytan-Saglam E, Balik E, Saglam S, et al. Delayed versus immediate surgery following short-course neoadjuvant radiotherapy in resectable (T3N0/N+) rectal cancer. J Cancer Res Clin Oncol 2017;143:1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol 2022:101739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel UB, Blomqvist LK, Taylor F, et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience. AJR Am J Roentgenol 2012;199:W486–495. [DOI] [PubMed] [Google Scholar]
- 36.Martens MH, van Heeswijk MM, van den Broek JJ, et al. Prospective, Multicenter Validation Study of Magnetic Resonance Volumetry for Response Assessment After Preoperative Chemoradiation in Rectal Cancer: Can the Results in the Literature be Reproduced? Int J Radiat Oncol Biol Phys 2015;93:1005–1014. [DOI] [PubMed] [Google Scholar]
- 37.Dresen RC, Beets GL, Rutten HJ, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 2009;252:71–80. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 2009;253:116–125. [DOI] [PubMed] [Google Scholar]
- 39.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 2013;269:101–112. [DOI] [PubMed] [Google Scholar]
- 40.Lambregts DMJ, Delli Pizzi A, Lahaye MJ, et al. A Pattern-Based Approach Combining Tumor Morphology on MRI With Distinct Signal Patterns on Diffusion-Weighted Imaging to Assess Response of Rectal Tumors After Chemoradiotherapy. Dis Colon Rectum 2018;61:328–337. [DOI] [PubMed] [Google Scholar]
- 41.Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752–760. [DOI] [PubMed] [Google Scholar]
- 42.Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753–3760. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui MR, Gormly KL, Bhoday J, et al. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG). Clin Radiol 2016;71:854–862. [DOI] [PubMed] [Google Scholar]
- 44.Sclafani F, Brown G, Cunningham D, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer 2017;117:1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahas SC, Nahas CSR, Cama GM, et al. Diagnostic performance of magnetic resonance to assess treatment response after neoadjuvant therapy in patients with locally advanced rectal cancer. Abdom Radiol (NY) 2019. [DOI] [PubMed] [Google Scholar]
- 46.Achilli P, Magistro C, Abd El Aziz MA, et al. Modest agreement between magnetic resonance and pathological tumor regression after neoadjuvant therapy for rectal cancer in the real world. Int J Cancer 2022;151:120–127. [DOI] [PubMed] [Google Scholar]
- 47.Lee MA, Cho SH, Seo AN, et al. Modified 3-Point MRI-Based Tumor Regression Grade Incorporating DWI for Locally Advanced Rectal Cancer. AJR Am J Roentgenol 2017;209:1247–1255. [DOI] [PubMed] [Google Scholar]
- 48.Hall WA, Li J, You YN, et al. Prospective Validation of the Magnetic Resonance Tumor Regression Grade (MR-TRG) and Correlation With Pathologic Endpoints Score in NRG Oncology GI002. International Journal of Radiation Oncology*Biology*Physics 2021;111:S37. [Google Scholar]
- 49.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633–4640. [DOI] [PubMed] [Google Scholar]
- 50.Lambregts DM, Vandecaveye V, Barbaro B, et al. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 2011;18:2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology 2011;260:771–780. [DOI] [PubMed] [Google Scholar]
- 52.Chandramohan A, Siddiqi UM, Mittal R, et al. Diffusion weighted imaging improves diagnostic ability of MRI for determining complete response to neoadjuvant therapy in locally advanced rectal cancer. Eur J Radiol Open 2020;7:100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gollub MJ, Das JP, Bates DDB, et al. Rectal cancer with complete endoscopic response after neoadjuvant therapy: what is the meaning of a positive MRI? Eur Radiol 2021;31:4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambregts DMJ, van Heeswijk MM, Delli Pizzi A, et al. Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching. Eur Radiol 2017;27:4445–4454. [DOI] [PubMed] [Google Scholar]
- 55.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Correction to: Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol 2004;204:183–192. [DOI] [PubMed] [Google Scholar]
- 57.Compton CC. Key issues in reporting common cancer specimens: problems in pathologic staging of colon cancer. Arch Pathol Lab Med 2006;130:318–324. [DOI] [PubMed] [Google Scholar]
- 58.Shia J, McManus M, Guillem JG, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol 2011;35:127–134. [DOI] [PubMed] [Google Scholar]
- 59.Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol 2005;29:602–606. [DOI] [PubMed] [Google Scholar]
- 60.Park SH, Lim JS, Lee J, et al. Rectal Mucinous Adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy-A Hypothesis-generating Study. Radiology 2017;285:124–133. [DOI] [PubMed] [Google Scholar]
- 61.Gollub MJ, Fox M, Horvat N, et al. Humans cannot distinguish mucinous rectal cancer from acellular mucin post-treatment: can computers? A multi-institutional pilot study of MRI radiomics. European Congress of Radiology. Vienna, Austria: European Society of Radiology; 2020. [Google Scholar]
- 62.Vliegen RF, Beets GL, Lammering G, et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: accuracy of MR imaging for prediction. Radiology 2008;246:454–462. [DOI] [PubMed] [Google Scholar]
- 63.Group MS. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006;333:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni T, Gollins S, Maw A, Hobson P, Byrne R, Widdowson D. Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis 2008;10:479–489. [DOI] [PubMed] [Google Scholar]
- 65.Yuval JB, Thompson HM, Firat C, et al. MRI at Restaging After Neoadjuvant Therapy for Rectal Cancer Overestimates Circumferential Resection Margin Proximity as Determined by Comparison With Whole-Mount Pathology. Dis Colon Rectum 2022;65:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panicek DM, Hricak H. How Sure Are You, Doctor? A Standardized Lexicon to Describe the Radiologist’s Level of Certainty. AJR Am J Roentgenol 2016;207:2–3. [DOI] [PubMed] [Google Scholar]
- 67.Lahaye MJ, Beets GL, Engelen SM, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology 2009;252:81–91. [DOI] [PubMed] [Google Scholar]
- 68.Heijnen LA, Maas M, Beets-Tan RG, et al. Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging? Int J Colorectal Dis 2016;31:1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lambregts DMJ, Bogveradze N, Blomqvist LK, et al. Current controversies in TNM for the radiological staging of rectal cancer and how to deal with them: results of a global online survey and multidisciplinary expert consensus. Eur Radiol 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. European Radiology 2013;23:2522–2531. [DOI] [PubMed] [Google Scholar]
- 71.Ogura A, Konishi T, Beets GL, et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg 2019:e192172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogura A, Konishi T, Cunningham C, et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol 2019;37:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schaap DP, Boogerd LSF, Konishi T, et al. Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg 2021;108:205–213. [DOI] [PubMed] [Google Scholar]
- 74.Ogura A, Konishi T, Beets GL, et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg 2019;154:e192172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoue A, Sheedy SP, Heiken JP, et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging 2021;12:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan JJ, Carten RV, Babiker A, Abulafi M, Lord AC, Brown G. Prognostic Importance of MRI-Detected Extramural Venous Invasion in Rectal Cancer: A Literature Review and Systematic Meta-Analysis. Int J Radiat Oncol Biol Phys 2021;111:385–394. [DOI] [PubMed] [Google Scholar]
- 77.Nagtegaal ID, Knijn N, Hugen N, et al. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol 2017;35:1119–1127. [DOI] [PubMed] [Google Scholar]
- 78.Fornell-Perez R, Vivas-Escalona V, Aranda-Sanchez J, et al. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: the role of diffusion-weighted imaging. Radiol Med 2020;125:522–530. [DOI] [PubMed] [Google Scholar]
- 79.Torkzad MR, Lindholm J, Martling A, Cedermark B, Glimelius B, Blomqvist L. MRI after preoperative radiotherapy for rectal cancer; correlation with histopathology and the role of volumetry. Eur Radiol 2007;17:1566–1573. [DOI] [PubMed] [Google Scholar]
- 80.Gollub MJ, Hotker AM, Woo KM, Mazaheri Y, Gonen M. Quantitating whole lesion tumor biology in rectal cancer MRI: taking a lesson from FDG-PET tumor metrics. Abdom Radiol (NY) 2018;43:1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joye I, Deroose CM, Vandecaveye V, Haustermans K. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 2014;113:158–165. [DOI] [PubMed] [Google Scholar]
- 82.Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI--a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol 2008;5:220–233. [DOI] [PubMed] [Google Scholar]
- 83.Seierstad T, Røe K, Olsen DR. Noninvasive monitoring of radiation-induced treatment response using proton magnetic resonance spectroscopy and diffusion-weighted magnetic resonance imaging in a colorectal tumor model. Radiother Oncol 2007;85:187–194. [DOI] [PubMed] [Google Scholar]
- 84.Bates DDB, Golia Pernicka JS, Fuqua JL, et al. Diagnostic accuracy of b800 and b1500 DWI-MRI of the pelvis to detect residual rectal adenocarcinoma: a multi-reader study. Abdom Radiol (NY) 2020;45:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hausmann D, Liu J, Budjan J, et al. Image Quality Assessment of 2D versus 3D T2WI and Evaluation of Ultra-high b-Value (b=2,000 mm/s(2)) DWI for Response Assessment in Rectal Cancer. Anticancer Res 2018;38:969–978. [DOI] [PubMed] [Google Scholar]
- 86.Jang S, Lee JM, Yoon JH, Bae JS. Reduced field-of-view versus full field-of-view diffusion-weighted imaging for the evaluation of complete response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Abdom Radiol (NY) 2021;46:1468–1477. [DOI] [PubMed] [Google Scholar]
- 87.Attenberger UI, Tavakoli A, Stocker D, et al. Reduced and standard field-of-view diffusion weighted imaging in patients with rectal cancer at 3 T-Comparison of image quality and apparent diffusion coefficient measurements. Eur J Radiol 2020;131:109257. [DOI] [PubMed] [Google Scholar]
- 88.Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002;360:307–308. [DOI] [PubMed] [Google Scholar]
- 89.Jung SH, Heo SH, Kim JW, et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging 2012;35:110–116. [DOI] [PubMed] [Google Scholar]
- 90.Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011;260:734–743. [DOI] [PubMed] [Google Scholar]
- 91.Lambregts DM, Beets GL, Maas M, et al. Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 2011;21:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 1992;27:171–178. [DOI] [PubMed] [Google Scholar]
- 93.Sun Y, Hu P, Wang J, et al. Radiomic features of pretreatment MRI could identify T stage in patients with rectal cancer: Preliminary findings. J Magn Reson Imaging 2018. [DOI] [PubMed] [Google Scholar]
- 94.Zhao L, Liang M, Yang Y, Zhao X, Zhang H. Histogram models based on intravoxel incoherent motion diffusion-weighted imaging to predict nodal staging of rectal cancer. Eur J Radiol 2021;142:109869. [DOI] [PubMed] [Google Scholar]
- 95.Gao F, Shi B, Wang P, et al. The Value of Intravoxel Incoherent Motion Diffusion-Weighted Magnetic Resonance Imaging Combined With Texture Analysis of Evaluating the Extramural Vascular Invasion in Rectal Adenocarcinoma. Front Oncol 2022;12:813138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L, Zhou G, Rao S, Zeng M. Early changes in intravoxel incoherent motion MRI parameters can potentially predict response to chemoradiotherapy in rectal cancer: An animal study. Magn Reson Imaging 2021;78:52–57. [DOI] [PubMed] [Google Scholar]
- 97.Kim MJ, Lee SJ, Lee JH, et al. Detection of rectal cancer and response to concurrent chemoradiotherapy by proton magnetic resonance spectroscopy. Magn Reson Imaging 2012;30:848–853. [DOI] [PubMed] [Google Scholar]
- 98.Avallone A, Aloj L, Caracò C, et al. Early FDG PET response assessment of preoperative radiochemotherapy in locally advanced rectal cancer: correlation with long-term outcome. Eur J Nucl Med Mol Imaging 2012;39:1848–1857. [DOI] [PubMed] [Google Scholar]
- 99.Capirci C, Rubello D, Pasini F, et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys 2009;74:1461–1469. [DOI] [PubMed] [Google Scholar]
- 100.Choi BW, Kang S, Bae SU, et al. Prognostic value of metabolic parameters on. Sci Rep 2021;11:12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi D, Cai G, Peng J, et al. The preoperative SUVmax for (18)F-FDG uptake predicts survival in patients with colorectal cancer. BMC Cancer 2015;15:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JE, Kim SW, Kim JS, et al. Prognostic value of 18-fluorodeoxyglucose positron emission tomography-computed tomography in resectable colorectal cancer. World J Gastroenterol 2012;18:5072–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crimì F, Valeggia S, Baffoni L, et al. [18F]FDG PET/MRI in rectal cancer. Ann Nucl Med 2021;35:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avallone A, Aloj L, Pecori B, et al. F-FDG PET/CT Is an Early Predictor of Pathologic Tumor Response and Survival After Preoperative Radiochemotherapy with Bevacizumab in High-Risk Locally Advanced Rectal Cancer. J Nucl Med 2019;60:1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rakheja R, Chandarana H, DeMello L, et al. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. AJR Am J Roentgenol 2013;201:1115–1119. [DOI] [PubMed] [Google Scholar]
- 106.Cerny M, Dunet V, Rebecchini C, et al. Response of locally advanced rectal cancer (LARC) to radiochemotherapy: DW-MRI and multiparametric PET/CT in correlation with histopathology. Nuklearmedizin 2019;58:28–38. [DOI] [PubMed] [Google Scholar]
- 107.Avci GG, Aral IP. The role of MRI and 18F-FDG PET/CT with respect to evaluation of pathological response in the rectal cancer patients after neoadjuvant chemoradiotherapy. Indian J Cancer 2021. [DOI] [PubMed] [Google Scholar]
- 108.Plodeck V, Rahbari NN, Weitz J, et al. FDG-PET/MRI in patients with pelvic recurrence of rectal cancer: first clinical experiences. Eur Radiol 2019;29:422–428. [DOI] [PubMed] [Google Scholar]
- 109.Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017;37:1483–1503. [DOI] [PubMed] [Google Scholar]
- 110.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haralick RM SK, Dinstein IH. Textural features for image classification. IEEE Transactions on Systems Man and Cybernetics: SMC; 1973. p. 610–621. [Google Scholar]
- 112.Horvat N, Miranda J, El Homsi M, et al. A primer on texture analysis in abdominal radiology. Abdom Radiol (NY) 2021. [DOI] [PubMed] [Google Scholar]
- 113.Miranda J, Tan GXV, Fernandes MC, et al. Rectal MRI radiomics for predicting pathological complete response: Where we are. Clin Imaging 2022;82:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nie K, Shi L, Chen Q, et al. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin Cancer Res 2016;22:5256–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Liu X, Hu B, Gao Y, Chen J, Li J. Development and validation of an MRI-based radiomic nomogram to distinguish between good and poor responders in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. Abdom Radiol (NY) 2021;46:1805–1815. [DOI] [PubMed] [Google Scholar]
- 116.Oh JE, Kim MJ, Lee J, et al. Magnetic Resonance-Based Texture Analysis Differentiating KRAS Mutation Status in Rectal Cancer. Cancer Res Treat 2020;52:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou X, Yi Y, Liu Z, et al. Radiomics-Based Preoperative Prediction of Lymph Node Status Following Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Front Oncol 2020;10:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jayaprakasam VS, Paroder V, Gibbs P, et al. MRI radiomics features of mesorectal fat can predict response to neoadjuvant chemoradiation therapy and tumor recurrence in patients with locally advanced rectal cancer. Eur Radiol 2022;32:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shaish H, Aukerman A, Vanguri R, et al. Radiomics of MRI for pretreatment prediction of pathologic complete response, tumor regression grade, and neoadjuvant rectal score in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation: an international multicenter study. Eur Radiol 2020;30:6263–6273. [DOI] [PubMed] [Google Scholar]
- 120.Miranda J, Tan GXV, Fernandes MC, et al. Rectal MRI radiomics for predicting pathological complete response: Where we are. Clin Imaging 2022;82:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]


