Abstract
Prior studies have conflicting findings regarding the association between gastroesophageal reflux disease (GERD) and esophageal squamous cell carcinoma (ESCC). We examined this relationship in a prospective cohort in a region of high ESCC incidence. Baseline exposure data were collected from 50,045 individuals using in-person interviews at the time of cohort entry. Participants were followed until they developed cancer, died, or were lost to follow up. Participants with GERD symptoms were categorized into any GERD (heartburn or regurgitation), mixed symptoms, or heartburn alone. Multivariable Cox regression was used to assess the relationship between GERD symptom group and histologically confirmed ESCC. The model was adjusted for known risk factors for GERD and ESCC. 49,559 individuals were included in this study, of which 9,005 had GERD symptoms. Over 13.0 years of median follow up, 290 individuals were diagnosed with ESCC. We found no association between any GERD and risk of ESCC (aHR 0.90, 95% CI: 0.66–1.24, p=0.54). Similar findings were observed for the GERD symptom subtypes. Significant interactions between any GERD and sex (p=0.013) as well as tobacco smoking (p=0.028) were observed. In post-hoc analyses, GERD was associated with a decreased risk of ESCC in men (aHR 0.51, 95% CI: 0.27–0.98 p=0.04) and in smokers (aHR 0.26, 95% CI: 0.08–0.83 p=0.02). While there was little evidence for an overall association between GERD symptoms and ESCC risk, significant interactions with sex and smoking were observed. Men and smokers with GERD symptoms had a lower risk of ESCC development.
Keywords: Esophageal squamous cell carcinoma, gastroesophageal reflux, Golestan cohort study
Graphical Abstract
Introduction:
Esophageal squamous cell carcinoma (ESCC) accounts for nearly 90% of cases of esophageal cancers worldwide and has a high mortality.1, 2 In Europe, North America, and Australia, alcohol drinking and tobacco smoking account for the majority of ESCC risk.3–5 Additional environmental risk factors have been identified in high incidence regions in South America, eastern and central Asia, and southeastern Africa, including but not limited to indoor air pollution, unpiped drinking water, hot beverages, low fruit and vegetable consumption, opium smoking, and betel quid chewing.6 Nevertheless, a significant proportion of ESCC risk remains unexplained.2–4, 7, 8
Gastroesophageal reflux disease (GERD) results in increased exposure of the esophagus to highly acidic contents and bile acids, which can lead to chronic inflammation that may promote cancer development.9 While there is a clear link between GERD and increased risk of esophageal adenocarcinoma (EAC), the association between GERD and ESCC is less well studied. Some population-based studies have identified a possible association between GERD and ESCC,10–12 whereas others have found no association.13, 14 This may be due to population-level differences in non-acid fluid exposure. Esophageal exposure to non-acid fluid has been proposed as a potential contributor to the development of ESCC,15 and non-acid reflux as measured by pH-impedance monitoring has been positively associated with ESCC in small case-control studies.16, 17 Prior studies of GERD and ESCC did not differentiate between the classical subjective measures of acid and non-acid esophageal exposures, such as heartburn and regurgitation symptoms,18 respectively, or use objective measures like pH-impendence monitoring. Thus, studying GERD symptom subtypes may provide insight into the relationship between GERD and ESCC.
The Golestan Cohort Study is a well-characterized prospective study of more than 50,000 individuals in a region of high ESCC incidence and low consumption of alcohol, where tobacco use does not seem to be a major risk factor.2, 19 GERD symptom prevalence in this population is comparable to that reported in more developed populations.20 The aim of the present study was to determine whether GERD is associated with an increased risk of ESCC in this high risk region in Iran. In addition, the present study aimed to glean possible differences in the associations between ESCC risk and acid and non-acid reflux, based on GERD symptom subtypes.
Materials and Methods:
Cohort Design and Study Population
The cohort used in this study has been previously described in detail.19 Briefly, participants were recruited from urban and rural regions of the Golestan province in northeastern Iran from January 2004 to June 2008. Those who did not agree to participate, were temporary residents, or had preexisting upper gastrointestinal cancers were not enrolled.
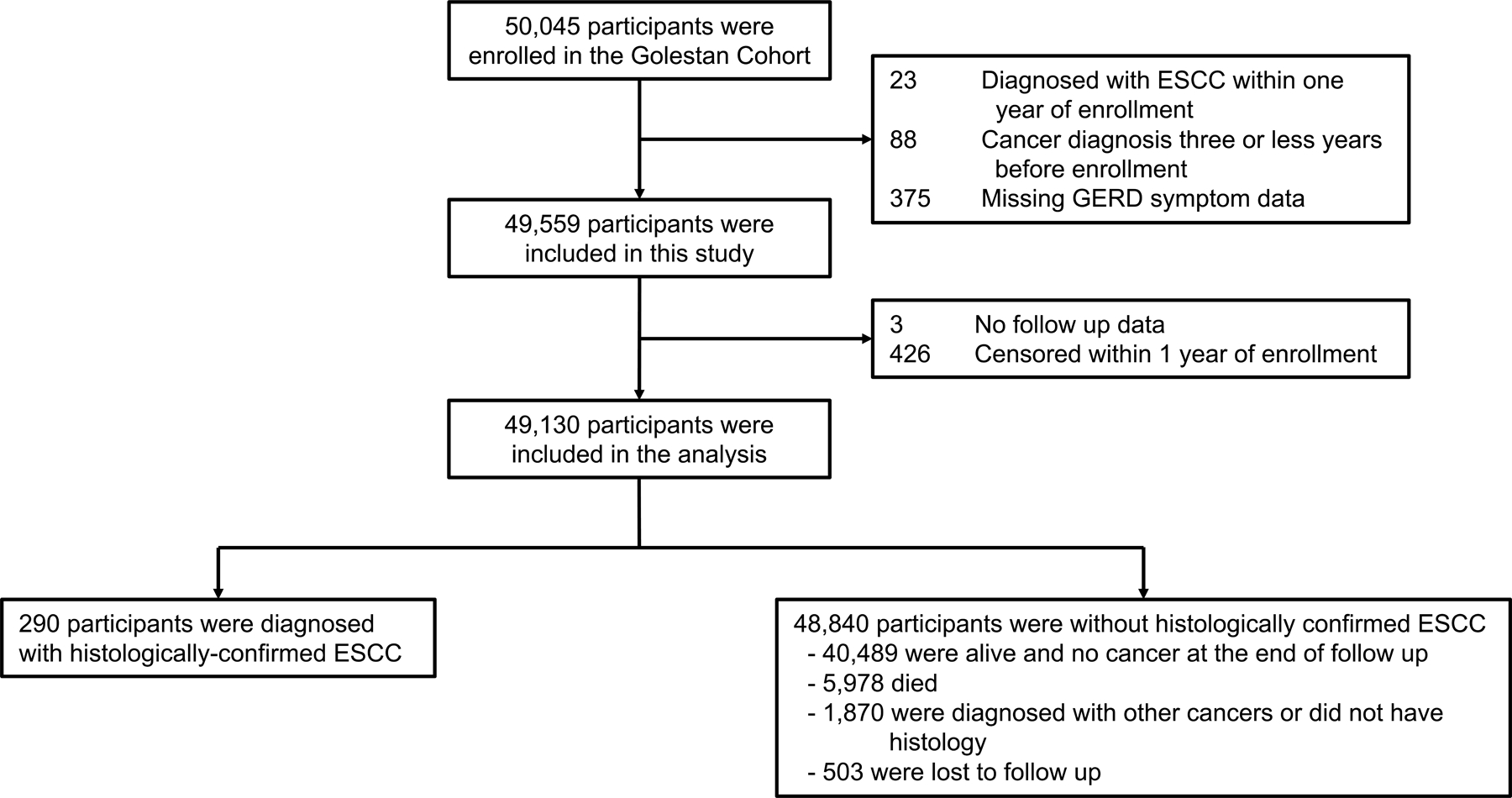
At the time of enrollment, all participants were interviewed in person by trained personnel, using validated questionnaires to obtain baseline demographic, lifestyle, socioeconomic, diet, and exposure history.21 A total of 50,045 individuals were enrolled in the Golestan Cohort, of which 49,559 were included in the current study. 23 who had been diagnosed with ESCC within one year of enrollment, 88 who had any other cancer diagnosis within three years of enrollment, and 375 who had missing GERD symptom data were excluded (Figure 1).
Figure 1:
Study Flow Diagram
Exposure Definitions and Assessment
GERD symptom characteristics, duration, frequency, and severity were assessed during the enrollment interview. Participants who reported neither “heartburn” nor “reflux of food from the stomach” (regurgitation) at least once a week were classified as “no GERD”, while “any GERD” was defined as having either symptom at least weekly. We defined two additional groups: those with both symptoms (mixed symptoms) and those with heartburn alone. Because there were not enough participants with regurgitation alone (n=419) to allow meaningful analyses, these individuals were included in the mixed symptom group.
Weight, height, and waist and hip circumference were measured by study personnel using standard methods. Body mass index (BMI) was calculated by dividing the weight in kilograms by the square of height in meters. BMI was categorized into underweight (< 18.5), normal range (18.5–24.9), overweight (25.0–29.9), and obese (≥ 30.0). Waist-to-hip ratio (WHR) was calculated by dividing waist circumference by hip circumference. WHR was then standardized using sex-stratified means and standard deviations. The difference between the actual and predicted number of lost teeth was calculated using a LOESS model and categorized into quartiles 22.
Socioeconomic status was categorized into quartiles based on a composite wealth score calculated using a previously described multiple correspondence analysis, which incorporates house, car, motorbike, television, refrigerator, vacuum, washing machine, and bath ownership in addition to home size.23 Indoor air pollution was defined as use of non-gas fuel without a chimney. Hot tea consumption was defined as drinking tea above 60 °C, which is the threshold at which there is an increased risk of ESCC.24 Smoking tobacco (including both hookah and cigarette) and chewed tobacco (nass) use were classified into never, former, and current use. Opium use was classified into ever or never use. Alcohol was not included as a covariate in our study due to the overall low rate of alcohol consumption in the cohort. 3.4% of participants ever used alcohol (8.0% of men and 0.1% of women) and fewer than 1% reported regular use.
Follow up and Outcome Ascertainment
Incident cancers and deaths from all causes were obtained using annual telephone surveys and monthly reviews of provincial cancer and death registries from cohort enrollment through 12/31/2019. Final case ascertainment was confirmed by linkage to the Golestan population-based cancer registry (GPCR). GPCR conforms to the highest international standards of cancer registries and has been a voting member of the International Association of Cancer Registries (IACR) since 2007.25 All clinical records were obtained and independently reviewed by two study physicians to confirm the timing and accuracy of the ESCC diagnosis. Less than 1.0% (n=503) of the cohort was lost to telephone follow up at the time of analysis. Also, in 2011–2012, a random sample of the participants (n=11,418) was re-evaluated for the baseline characteristics (the repeated measurement phase).
Statistical Analysis
To assess GERD correlates, the Chi square test was used for categorical variables and the student’s t-test was used for continuous variables. Potential GERD risk factors with a p-value less than 0.05 in the univariable analysis were included in the GERD multivariable analysis using logistic regression. These variables included age, sex, urban or rural residence, socioeconomic status, body mass index, waist-to-hip ratio, tea drinking temperature, fruit and vegetable consumption, indoor air pollution exposure, unpiped drinking water exposure, tooth loss, tobacco smoking, opiate use, and NSAID use. All variables within the GERD model were assessed for simple collinearity and multicollinearity and no meaningful collinearity was detected.
To assess the association between GERD symptoms and a diagnosis of ESCC, Cox proportional hazards regression models were used to estimate hazard ratios and 95% confidence intervals. Entry time was defined as 1 year after date of enrollment to avoid bias from reverse causation and to exclude prevalent cancers. Survival failure was defined by the date of diagnosis with histologically confirmed ESCC. Cohort participants were censored at the date of last follow up (until January 1, 2020), death from any cause, or first primary cancer diagnosis. The models were adjusted for key ESCC risk factors6, 26–28 as well as variables which changed the estimates for GERD symptoms by more than 10%. The final model included age, sex, ethnicity, socioeconomic status, BMI, tobacco smoking, and opiate use. In all models, the proportional hazards assumption was violated for ethnicity, and so all models were stratified for this.
We performed exploratory statistical analyses, testing interactions with several important variables by adding interaction terms to the Cox regression models and using the Wald test. We identified significant interactions between GERD symptoms and sex as well as tobacco smoking. There was no significant interaction between GERD symptoms and Turkmen ethnicity. Tobacco smoking was categorized as ever/never due to an inadequate number of cases when using never/former/current. We repeated the Cox regression models, stratifying by sex and smoking separately. Stratifying analyses by both sex and tobacco smoking produced unstable models due to an inadequate number of cases. Cumulative adjusted hazard curves were plotted for GERD symptoms for the overall cohort, men, and women.
We performed sensitivity analyses using variations of the primary Cox regression models. The survival analysis was repeated using GERD symptom frequency, duration, and severity as exposures of interest. Another analysis defined entry time as 2 years after cohort entry to further adjust for possible reverse causality. A third analysis included all esophageal cancer diagnoses (including those not histologically confirmed). All statistical analyses were performed using Stata/SE version 17.0 (Stata Corporation, College Station, TX).
Results:
Cohort description
The baseline characteristics of the study cohort are shown in Table 1. Of the cohort population, 57.6% were female, 79.9% lived in rural regions, and 74.3% were of Turkmen ethnicity. 18.2% reported GERD symptoms; 4.8% had heartburn alone, 0.8% had regurgitation alone, and 12.5% had both regurgitation and heartburn symptoms.
Table 1:
Baseline characteristics by GERD symptoms in Golestan Cohort Study
| Cohort (n = 49,559) |
No GERD (Reference) (n = 40,554) |
Any GERD (n = 9,005) |
Mixed symptoms (n = 6,618) |
Heartburn alone (n = 2,387) |
|
|---|---|---|---|---|---|
| Median age, years (IQR) | n = 49,559 50.2 (44.8–57.8) |
n = 40,554 50.1 (44.8–57.7) |
n = 9,005 50.5 (45.0–58.6)** |
n = 6,618 50.7 (45.1–58.8)** |
n = 2,387 50.0 (44.8–57.8) |
| Sex, n (%) Male Female |
21,020 (42.4%) 28,539 (57.6%) |
18,329 (45.2%) 22,225 (54.8%) |
2,691 (29.9%) 6,314 (70.1%)** |
1,845 (27.9%) 4,773 (72.1%)** |
846 (35.4%) 1,541 (64.6%)** |
| Residence, n (%) Rural Urban |
39,577 (79.9%) 9,982 (20.1%) |
32,304 (79.7%) 8,250 (20.3%) |
7,273 (80.8%) 1,732 (19.2%)* |
5,422 (81.9%) 1,196 (18.1%)** |
1,851 (77.5%) 536 (22.5%)* |
| Ethnicity, n (%) Turkmen Non-Turkmen |
36,836 (74.3%) 12,723 (25.7%) |
30,806 (76.0%) 9,748 (24.0%) |
6,030 (67.0%) 2,975 (33.0%)** |
4,406 (66.6%) 2,212 (33.4%)** |
1,624 (68.0%) 763 (32.0%)** |
| Socioeconomic status, n (%) 1st quartile (lowest) 2nd quartile 3rd quartile 4th quartile (highest) |
13,812 (27.9%) 11,040 (22.3%) 12,470 (25.2%) 12,237 (24.7%) |
10,898 (26.9%) 8,860 (21.8%) 10,219 (25.2%) 10,577 (26.1%) |
2,914 (32.4%) 2,180 (24.2%) 2,251 (25.0%) 1,660 (18.4%)** |
2,125 (32.1%) 1,638 (24.8%) 1,697 (25.6%) 1,158 (17.5%)** |
789 (33.1%) 542 (22.7%) 554 (23.2%) 502 (21.0%)** |
| BMI, n (%) < 18.5 18.5–24.9 25.0–29.9 >30 |
2,389 (4.8%) 17,732 (35.8%) 16,820 (33.9%) 12,618 (25.5%) |
1,866 (4.6%) 14,512 (35.8%) 13,945 (34.4%) 10,231 (25.2%) |
523 (5.8%) 3,220 (35.8%) 2,875 (31.9%) 2,387 (26.5%)** |
388 (5.9%) 2,352 (35.5%) 2,084 (31.5%) 1,794 (27.1%)** |
135 (5.7%) 868 (36.4%) 791 (33.1%) 593 (24.8%) |
| WHR,1 median ± IQR | n = 49,544 0.96 (0.90–1.01) |
n = 40,540 0.96 (0.90–1.01) |
n = 9,004 0.96 (0.90–1.02) |
n = 6,618 0.96 (0.90–1.02)** |
n = 2,386 0.95 (0.90–1.01)** |
| Temperature of tea consumed, n (%) < 60° C ≥ 60° C Unknown |
19,225 (38.8%) 29,730 (60.0%) 604 (1.2%) |
15,781 (38.9%) 24,316 (60.0%) 457 (1.1%) |
3,444 (38.2%) 5,414 (60.1%) 147 (1.6%)** |
2,614 (39.5%) 3,886 (58.7%) 118 (1.8%)** |
830 (34.8%) 1,528 (64.0%) 29 (1.2%)** |
| Fruit and vegetable consumption, n (%) 1st quintile (lowest) 2nd quintile 3rd quintile 4th quintile 5th quintile (highest) Unknown |
9,758 (19.7%) 9,736 (19.6%) 9,746 (19.7%) 9,730 (19.6%) 9,719 (19.6%) 870 (1.8%) |
7,423 (18.3%) 7,809 (19.3%) 7,976 (19.7%) 8,146 (20.1%) 8,380 (20.7%) 820 (2.0%) |
2,335 (25.9%) 1,927 (21.4%) 1,770 (19.7%) 1,584 (17.6%) 1,339 (14.9%) 50 (0.6%)** |
1,736 (26.2%) 1,444 (21.8%) 1,336 (20.2%) 1,147 (17.3%) 916 (13.8%) 39 (0.6%)** |
599 (25.1%) 483 (20.2%) 434 (18.2%) 437 (18.3%) 423 (17.7%) 11 (0.5%)** |
| Current exposure to indoor air pollution, n (%) No Yes Unknown |
27,863 (56.2%) 21,145 (42.7%) 551 (1.1%) |
22,881 (56.4%) 17,225 (42.5%) 448 (1.1%) |
4,982 (55.3%) 3,920 (43.5%) 103 (1.1%) |
3,733 (56.4%) 2,810 (42.5) 75 (1.1%) |
1,24 9 (52.3%) 1,110 (46.5%) 28 (1.2%) ** |
| Current exposure to unpiped water, n (%) No Yes Unknown |
41,171 (83.1%) 8,323 (16.8%) 65 (0.1%) |
33,726 (83.2%) 6,777 (16.7%) 51 (0.1%) |
7,445 (82.7%) 1,546 (17.2%) 14 (0.2%) |
5,640 (85.2%) 968 (14.6%) 10 (0.2%)** |
1,805 (75.6%) 578 (24.2%) 4 (0.2%) |
| Tooth loss, n (%) Less than predicted 1–8 excess tooth loss >9 excess tooth loss Unknown |
26,080 (52.6%) 12,029 (24.3%) 11,434 (23.1%) 16 (0.1%) |
21,294 (52.5%) 9,903 (24.4%) 9,345 (23.0%) 12 (0.1) |
4,786 (53.1%) 2,126 (23.6%) 2,089 (23.2%) 4 (0.1%) |
3,449 (52.1%) 1,621 (24.5%) 1,545 (23.3%) 3 (0.1%) |
1,337 (56.0%) 505 (21.2%) 544 (22.8%) 1 (0.1%)** |
| Ever smoked tobacco use, n (%) No Former Current |
40,537 (81.8%) 3,309 (6.7%) 5,713 (11.5%) |
33,022 (81.4%) 2,761 (6.8%) 4,771 (11.8%) |
7,515 (83.5%) 548 (6.1%) 942 (10.5%)** |
5,566 (84.1%) 398 (6.0%) 654 (9.9%)** |
1,949 (81.7%) 150 (6.3%) 288 (12.1%) |
| Median cigarette smoking, pack-years (IQR) | n = 8,505 11.7 (3.5–25.0) |
n = 7,161 11.6 (3.5–25.0) |
n = 1,344 12.0 (3.8–25.5) |
n = 935 11.3 (3.7–25.5) |
n = 409 13.0 (3.8–25.3) |
| Ever chewed tobacco use, n (%) No Former Current |
45,704 (92.2) 336 (0.7%) 3,519 (7.1) |
37,360 (92.1%) 283 (0.7%) 2,911 (7.2%) |
8,344 (92.7%) 53 (0.6%) 608 (6.8%) |
6,141 (92.8%) 32 (0.5%) 445 (6.7%) |
2,203 (92.3%) 21 (0.9%) 163 (6.8%) |
| Ever opium use, n (%) No Yes |
41,169 (83.1%) 8,390 (16.9%) |
33,960 (83.7%) 6,594 (16.3%) |
7,209 (80.1%) 1,796 (19.9%)** |
5,294 (80.0%) 1,324 (20.0%)** |
1,915 (80.2%) 472 (19.8%)** |
| GERD symptom severity, n (%) No symptoms Mild-moderate symptoms Severe symptoms |
40,558 (81.8%) 6,411 (12.9%) 2,590 (5.2%) |
40,554 (100.0%) 0 (0.0%) 0 (0.0%) |
4 (0.1%) 6,411 (71.2%) 2,590 (28.8%) |
2 (0.03%) 4,482 (67.7%) 2,134 (32.3%) |
2 (0.1%) 1,929 (80.8%) 456 (19.1%) |
| GERD symptom duration, n (%) No symptoms < 1 year 1–5 years > 5 years |
40,627 (82.0%) 1,786 (3.6%) 3,448 (7.0%) 3,698 (7.5%) |
40,554 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
73 (0.8%) 1,786 (19.8%) 3,448 (38.3%) 3,698 (41.1%) |
54 (0.8%) 1,193 (18.0%) 2,522 (38.1%) 2,849 (43.1%) |
19 (0.8%) 593 (24.8%) 926 (38.8%) 849 (35.6%) |
| Proton pump inhibitor use, n (%) No Yes |
46,321 (93.5%) 3,238 (6.5%) |
38,858 (95.8%) 1,696 (4.2%) |
7,463 (82.9%) 1,542 (17.1%) |
5,411 (81.8%) 1,207 (18.2%) |
2,052 (86.0%) 335 (14.0%) |
| H2-blocker use, n (%) No Yes |
42,361 (85.5%) 7,198 (14.5%) |
36,397 (89.7%) 4,157 (10.3%) |
5,964 (66.2%) 3,041 (33.8%) |
4,403 (66.5%) 2,215 (33.5%) |
1,561 (65.4%) 826 (34.6%) |
| NSAID use, n (%) No Yes |
43,750 (88.3%) 5,809 (11.7%) |
36,063 (88.9%) 4,491 (11.1%) |
7,687 (85.4%) 1,318 (14.6%)** |
5,631 (85.1%) 987 (14.9%)** |
2,056 (86.1%) 331 (13.9%)** |
WHR – significance test compares adjusted WHR: (WHR – sex-stratified mean)/(sex-stratified standard deviation)
p<0.01
p<0.05
GERD
We performed multivariable logistic regression analyses to assess for factors associated with any GERD, mixed symptoms, and heartburn alone (Table 2). We found female sex (aOR 2.20, 95% CI: 2.07–2.34, p < 0.01), non-Turkmen ethnicity (aOR 1.53, 95% CI: 1.44–1.61, p < 0.01), and ever opium use (aOR 1.52, 95% CI: 1.42–1.63, p < 0.01) were associated with higher OR’s for GERD while fruit and vegetable consumption (5th quintile vs. 1st quintile, aOR 0.66, 95% CI: 0.61–0.72, p < 0.01) and high socioeconomic status (4th quartile vs. 1st quartile, aOR 0.67, 95% CI: 0.62–0.72, p < 0.01) were inversely associated with GERD symptoms. We did not find a significant association between BMI and any GERD, mixed symptoms, or heartburn alone but WHR was significantly associated with any GERD (aOR per SD increase=1.06; 95%CI: 1.03–1.10) and mixed symptoms (aOR per SD increase=1.09; 95%CI: 1.06–1.13). Some covariates were associated with mixed symptoms and heartburn alone in different directions. For example, urban residence was associated with increased odds of heartburn alone (aOR: 1.37, 95% CI: 1.22–1.55, p < 0.01) and decreased odds of mixed symptoms (aOR: 0.89, 95% CI: 0.82–0.96, p < 0.01). Similarly, unpiped water was associated with increased odds of heartburn alone (aOR: 1.76, 95% CI: 1.59–1.96, p < 0.01) and decreased odds of mixed symptoms (aOR: 0.86, 95% CI: 0.80–0.93, p < 0.01).
Table 2:
Multivariable logistic regression of factors associated with GERD symptoms in Golestan Cohort Study
| Any GERD | Mixed symptoms | Heartburn alone | |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Age, per 10 year increase | 1.03 (1.00–1.06)* | 1.04 (1.01–1.07)* | 1.01 (0.96–1.06) |
| Sex Male Female |
Reference 2.20 (2.07–2.34)** |
Reference 2.44 (2.28–2.62)** |
Reference 1.68 (1.51–1.87)** |
| Residence Rural Urban |
Reference 1.00 (0.93–1.07) |
Reference 0.89 (0.82–0.96)** |
Reference 1.37 (1.22–1.55)** |
| Ethnicity Turkmen Non-Turkmen |
Reference 1.53 (1.44–1.61)** |
Reference 1.56 (1.46–1.65)** |
Reference 1.47 (1.33–1.62)** |
| Socioeconomic status 1st quartile (lowest) 2nd quartile 3rd quartile 4th quartile (highest) |
Reference 0.92 (0.87–0.99) 0.88 (0.83–0.94) 0.67 (0.62–0.72)** |
Reference 0.95 (0.89–1.02) 0.92 (0.86–0.99) 0.66 (0.61–0.72)** |
Reference 0.85 (0.76–0.96) 0.78 (0.69–0.87) 0.67 (0.58–0.76) ** |
| BMI, n (%) < 18.5 18.5–24.9 25.0–29.9 > 30 |
1.10 (0.99–1.23) Reference 0.94 (0.88–1.00) 0.97 (0.90–1.04) |
1.13 (0.99–1.28) Reference 0.91 (0.84–0.97) 0.96 (0.88–1.04) |
1.04 (0.86–1.27) Reference 1.02 (0.91–1.14) 0.99 (0.86–1.13) |
| WHR, per SD | 1.06 (1.03–1.10)** | 1.09 (1.06–1.13)** | 0.98 (0.93–1.03) |
| Temperature of tea consumed < 60° C ≥ 60° C Unknown |
Reference 1.06 (1.01–1.12) 1.47 (1.21–1.80)** |
Reference(0.96–1.07) 1.56 (1.25–1.94) |
Reference 1.20 (1.10–1.31) 1.21 (0.81–1.78)** |
| Fruit and vegetable consumption 1st quintile (lowest) 2nd quintile 3rd quintile 4th quintile 5th quintile (highest) Unknown |
Reference 0.87 (0.81–0.94) 0.83 (0.77–0.89) 0.76 (0.71–0.82) 0.66 (0.61–0.72) 0.21 (0.16–0.29)** |
Reference 0.89 (0.82–0.96) 0.85 (0.79–0.93) 0.76 (0.70–0.83) 0.64 (0.58–0.70) 0.22 (0.15–0.30)** |
Reference 0.84 (0.74–0.96) 0.77 (0.68–0.88) 0.78 (0.68–0.89) 0.73 (0.64–0.84) 0.21 (0.12–0.39)** |
| Current exposure to indoor air pollution No Yes Unknown |
Reference 0.96 (0.91–1.01) 0.92 (0.74–1.14) |
Reference 0.90 (0.85–0.95) 0.86 (0.67–1.11)** |
Reference 1.16 (1.06–1.28) 1.09 (0.74–1.61)** |
| Current exposure to unpiped water No Yes Unknown |
Reference 1.07 (1.01–1.14) 0.86 (0.41–1.79) |
Reference 0.86 (0.80–0.93) 0.72 (0.31–1.69)** |
Reference 1.76 (1.59–1.96) 1.34 (0.40–4.58)** |
| Tooth loss Less than predicted 1–8 excess tooth loss >9 excess tooth loss |
Reference 0.91 (0.86–0.96) 0.97 (0.92–1.03) |
Reference 0.95 (0.89–1.02) 0.99 (0.93–1.07) |
Reference 0.78 (0.70–0.87) 0.90 (0.81–1.00)* |
| Ever smoked tobacco use No Former Current |
Reference 1.15 (1.03–1.28) 1.18 (1.08–1.29)** |
Reference 1.17 (1.03–1.33) 1.15 (1.04–1.28)** |
Reference 1.05 (0.87–1.27) 1.19 (1.03–1.39)* |
| Ever opium use No Yes |
Reference 1.52 (1.42–1.63)** |
Reference 1.58 (1.46–1.70)** |
Reference 1.40 (1.24–1.58)** |
| NSAID use No Yes |
Reference 1.33 (1.24–1.42)** |
Reference 1.34 (1.24–1.45)** |
Reference 1.27 (1.12–1.43)** |
Adjusted by age, sex, urban residency, ethnicity, socioeconomic status, waist-to-hip ratio, body mass index, hot tea consumption, fruit and vegetable consumption, unpiped water exposure, tooth loss, tobacco smoking, opiate use, and non-steroidal anti-inflammatory drug use.
p<0.01
p<0.05
GERD and ESCC
A median 13.0 (IQR: 12.0–13.9) years of follow-up were obtained through January 1, 2020. A total of 290 histologically-confirmed ESCC cases were identified during the follow-up period, comprising 92.9% of all incident histologically-confirmed esophageal cancer cases. The remaining 48,840 participants included 40,489 who were alive and cancer-free at the end of follow-up period, 5,978 who died, 1,870 who were diagnosed with another cancer or lacked histology, and 503 who were lost to follow up (Figure 1). Of those cancers without histology, 66 were esophageal tumors. The ESCC tumor characteristics are described in detail in Table S1. Individuals with ESCC and GERD symptoms were significantly more likely to have more proximal tumor location compared to those without GERD (p-value=0.035; Table S1).
The presence of GERD symptoms was not significantly associated with ESCC in either unadjusted (HR 0.89, 95% CI: 0.65–1.21, p=0.45; Table 3) or adjusted Cox regression models (aHR 0.90, 95% CI: 0.66–1.24, p=0.54; Table 3 and Figure 2A). Mixed symptoms and heartburn alone were similarly not significantly associated with ESCC. However, there was evidence of significant interaction between sex and GERD symptoms (p=0.013) as well as between tobacco smoking and GERD symptoms (p=0.028), and thus additional stratified analyses were performed. There was a significant inverse association between GERD symptoms and ESCC (aHR 0.51, 95% CI: 0.27–0.98, p=0.04) in men but not in women (aHR 1.19, 95% CI: 0.82–1.74, p=0.37; Table 3, Figure 2B, Figure 2C). There was also a significant inverse association between GERD symptoms and ESCC in tobacco smokers (aHR 0.26, 95% CI: 0.08–0.83, p=0.02) but not in non-smokers (aHR 1.09, 95% CI: 0.78–1.53, p=0.60; Table 3, Figure 2D, Figure 2E). 91.3% of smokers were male. The three cases of ESCC found in smokers with GERD symptoms had a mean of 3.4 smoking pack-years. Similar trends were seen with mixed symptoms and heartburn alone, although the associations did not achieve statistical significance.
Table 3:
ESCC Survival Analysis – GERD symptoms
| Overall | ||
|---|---|---|
| Symptoms (cases per 10,000 person-years) | Crude HR (95% CI) | aHR (95% CI) |
|
| ||
| Any GERD (5.2) |
0.89 (0.65–1.21) | 0.90 (0.66–1.24) |
| Mixed symptoms (5.2) |
0.88 (0.62–1.26) | 0.91 (0.63–1.31) |
| Heartburn alone (5.3) | 0.90 (0.51–1.57) | 0.91 (0.52–1.59) |
| Male | Female | |||
|---|---|---|---|---|
| Symptoms (cases per 10,000 person-years) | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) |
|
| ||||
| Any GERD (Male: 6.5) (Female: 4.3) |
0.50 (0.26–0.95)* | 0.51 (0.27–0.98)* | 1.29 (0.88–1.87) | 1.19 (0.82–1.74) |
| Mixed symptoms (Male: 6.6) (Female: 4.3) |
0.44 (0.20–1.01) | 0.46 (0.20–1.05) | 1.30 (0.86–1.98) | 1.19 (0.78–1.81) |
| Heartburn alone (Male: 6.8) (Female: 4.1) |
0.62 (0.23–1.68) | 0.62 (0.23–1.66) | 1.24 (0.63–2.45) | 1.18 (0.59–2.33) |
| Never Smoker | Ever Smoker | |||
|---|---|---|---|---|
| Symptoms (cases per 10,000 person-years) | Crude HR (95% CI) | aHR (95% CI) | Crude HR (95% CI) | aHR (95% CI) |
|
| ||||
| Any GERD (Non-Smoker: 5.0) (Smoker: 6.1) |
1.05 (0.76–1.46) | 1.09 (0.78–1.53) | 0.28 (0.09–0.89)* | 0.26 (0.08–0.83)* |
| Mixed symptoms (Non-Smoker: 5.0) (Smoker: 6.3) |
1.04 (0.72–1.52) | 1.10 (0.75–1.61) | 0.27 (0.07–1.10) | 0.24 (0.06–0.98) |
| Heartburn alone (Non-Smoker: 5.0) (Smoker: 6.7) |
1.08 (0.60–1.93) | 1.10 (0.61–1.97) | 0.30 (0.04–2.18) | 0.31 (0.04–2.22) |
Adjusted by age, sex, ethnicity, socioeconomic status, body mass index, tobacco smoking, and opiate use.
p<0.01
p<0.05
Adjusted by age, ethnicity, socioeconomic status, body mass index, tobacco smoking, and opiate use.
p<0.01
p<0.05
Adjusted by age, sex, ethnicity, socioeconomic status, body mass index, and opiate use.
p<0.01
p<0.05
Figure 2:
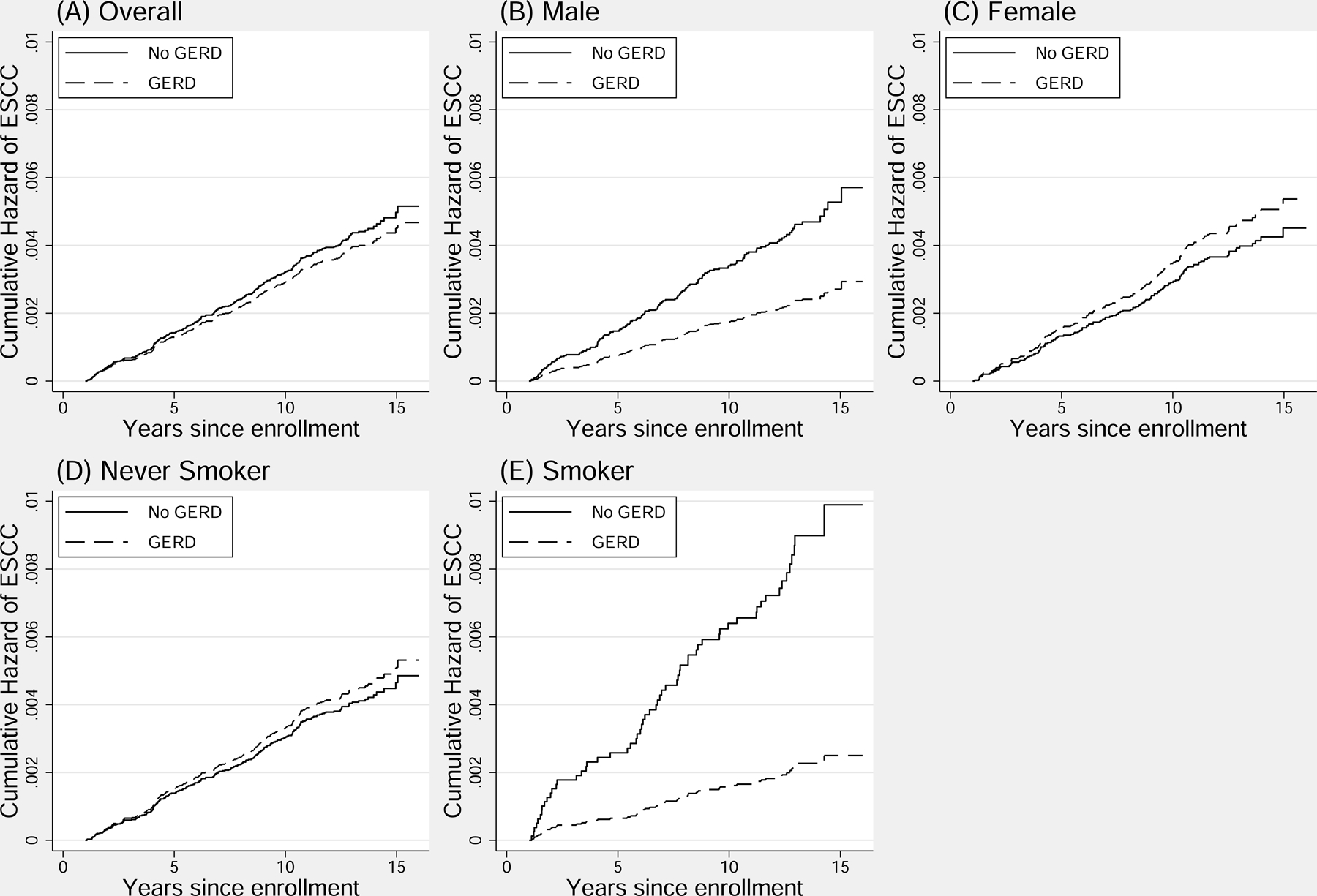
Cumulative adjusted hazard of ESCC in those with any GERD symptoms
Considering the interactions found between GERD symptoms and sex as well as tobacco smoking, post hoc analyses were conducted to determine whether there were differences in factors associated with GERD in males as opposed to females, as well as in smokers as opposed to nonsmokers; however, none were found (Table S2a and Table S2b). Analyses of the associations between GERD symptom duration, frequency, and severity with ESCC (Table S3) revealed no significant relationship between GERD symptom duration or severity and ESCC in the full cohort and all subgroups.
When excluding all ESCC diagnoses within 2 years after enrollment (Table S4) and when excluding all ESCC diagnoses within 2 years after enrollment but including all esophageal cancer tumors (Table S5), the observed associations were not meaningfully different.
The relationship between proton pump inhibitor (PPI) use and ESCC was also explored. PPI use was not significantly associated with ESCC risk in the full cohort (aHR 1.12; 95% CI 0.68–1.85) or in those with GERD (aHR 0.77; 95% CI 0.33–1.84). There was no evidence of significant interaction between PPI use and sex or tobacco smoking.
We assessed the changes in GERD symptoms among participants who were re-evaluated in 2011–2012 (repeated measurement). Of those who initially reported having GERD symptoms, 40.9% continued to report GERD on the repeat questionnaire. Men and tobacco smokers with GERD at baseline were significantly more likely to report no GERD symptoms in the follow-up (62.8% and 62.3%, respectively) compared with women (57.3%).
Discussion:
In this large, prospective study of GERD and ESCC in a population with high ESCC incidence, we found no association between GERD symptoms and risk of ESCC within the full cohort. The findings were consistent across all GERD subtypes. Interestingly, however, we found significant interactions between GERD and sex as well as GERD and tobacco smoking. GERD was associated with a 2-fold decreased risk of ESCC in men, but no significant association was observed in women. Similarly, GERD was associated with a 4-fold decreased risk of ESCC in smokers, but no significant association was observed in non-smokers. Our findings were unaltered even after controlling for known ESCC risk factors in this region. There was also a proximal shift in ESCC tumor location among cohort members who reported GERD symptoms, suggesting that gastroesophageal reflux may be exerting a biological effect on ESCC development.
We performed a cross-sectional analysis of baseline cohort data to identify risk factors associated with GERD. An earlier study using this data identified similar associations between GERD and sex.28 Prior studies in South America and in the Middle East have shown women are 40% more likely to report GERD symptoms than men.29 The initial analysis of GERD risk factors in the Golestan cohort aligns with prior studies that show ethnicity, socioeconomic status, tobacco smoking, and opium use are associated with GERD.30 We studied GERD symptoms subtypes and found novel associations that were in opposite directions for mixed symptoms and for heartburn alone. Urban residence and unpiped water were associated with significantly decreased odds of mixed symptoms and increased odds of heartburn alone.
Prior studies have had conflicting results with respect to the relationship between GERD and ESCC, either showing no association or an increased risk of ESCC in patients with GERD. A recent prospective study in the United States with a similar follow up period to our study found a 2-fold increased risk of ESCC among patients, with a population attributable risk of 17.3%.12 Of note, there were not significant sex-based differences in the association with ESCC. Three prior case-control studies in Australia, Europe, and the United States have also analyzed the relationship between reported GERD symptoms and ESCC risk.10, 13, 14 Only the study of Australian individuals found a significant relationship in the overall study population and the relationship was modified by smoking and alcohol consumption. Notably, this study found tobacco smoking increased the risk of ESCC in those with GERD symptoms, in contrast with the findings of our study. The studies of populations in the United States and Sweden found no significant association between ESCC and GERD. The reasons for the differences between these studies are not entirely clear. Though we are using GERD symptoms as a surrogate for pathological acid or non-acid reflux, prior studies have shown a poor correlation between symptoms and more objective measures of acid reflux.31–33 There are differences in the way GERD symptoms are reported across cultures, making comparisons difficult despite the establishment of a global consensus definition of GERD.34 Another possible explanation is that GERD may interact with exposures unique to high-incidence regions such as Golestan province.
The sex differences we observed could be due to differences in symptom perception and reporting between men and women, or may point to underlying behavior modifications. GERD was more common in women and nonsmokers. Men or smokers who report GERD symptoms may adjust their lifestyles in response to their symptoms in such a way that their risk of ESCC would be mitigated. GERD symptoms in both groups were more likely to resolve during the follow-up compared with women and non-smokers. Unfortunately, we were underpowered to examine the direct effects of these changes on ESCC risk. Another possibility may be that there are sex-based differences in esophageal epithelial biology, as evidenced by the increased male propensity to develop Barrett’s esophagus,35 the precursor to esophageal adenocarcinoma. There are conflicting data regarding the effect of pH and reflux on esophageal carcinogen production. In patients with Barrett’s esophagus, acid reflux was associated with increased N-nitrosamine production in the distal esophagus.36, 37 However, decreased stomach acidity is associated with increased overall N-nitrosamine production and stomach acid suppression has been associated with an increased risk of gastric cancer.38–40 Decreased esophageal pH may also influence the esophageal microbiome, possibly resulting in decreased endogenous production of carcinogens such as N-nitrosamines and acetaldehyde that have been associated with ESCC.15, 41, 42 Porphyromonas gingivalis, Fusobacterium nucleatum, and human papilloma virus are acid-sensitive microbial populations that may be linked to ESCC incidence or progression.43–49 Decreased distal esophagus pH may reduce growth of these potentially carcinogenic microbes and shift them from the distal esophagus to the mid and proximal esophagus. Further work is needed to explore these potential relationships in the Golestan cohort. Finally, there may be additional residual confounding that we have not fully controlled for in our analysis. However, given the strength of the inverse association between GERD and ESCC in males, we are not aware of factors that could be responsible for such residual confounding.
The strengths of this study include the prospective cohort design, large sample size, and long follow-up period. There was detailed collection of baseline symptoms and exposures, allowing for control of all known confounders in this population. There was also minimal loss to follow-up, and the cohort data is linked to high-quality regional cancer registry data, which has been used as a role model for cancer registries in low resource settings.25 The findings were robust, as the hazard estimates did not meaningfully change in multiple sensitivity analyses. The study did have certain limitations. We could not study the effects of changes in GERD symptoms and other covariates on ESCC risk, as these data were available for only a subset or the cohort participants. GERD was defined based on symptom assessments rather than objective measures such as pH/impedance testing or endoscopic evidence of GERD as this was not available at the time of analysis. Furthermore, we acknowledge that GERD symptom subtypes like heartburn and regurgitation have imperfect correlation to acid and non-acid reflux exposure.31–33
In conclusion, in this large prospective study from a high incidence region, GERD symptoms were not associated with ESCC risk overall. However, there were interactions between GERD symptoms and both sex and tobacco smoking, and we observed a reduced risk of ESCC in men and smokers with GERD, but not in women and nonsmokers. Further studies with endoscopic evaluation and impedance-pH testing are warranted to quantify the degree and type of reflux experienced by patients in this region. Additionally, exploration of regional variations in the relationship between GERD and ESCC may help further elucidate the carcinogenic pathways that lead to ESCC.
Supplementary Material
Novelty and Impact.
In this large, prospective study in a population with high ESCC incidence, we found no association between GERD symptoms and risk of ESCC within the full cohort, but men and tobacco smokers with GERD symptoms had a lower risk of developing ESCC. There was also a proximal shift in ESCC tumor location among cohort members who reported GERD symptoms.
Funding:
The Golestan Cohort Study was supported in part by Tehran University of Medical Sciences [grant No: 81/15]; Cancer Research UK [grant No: C20/A5860]; the Intramural Research Program of the NCI, National Institutes of Health; and various collaborative research agreements with IARC. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of abbreviations:
- aHR
adjusted hazard ratio
- BMI
body mass index
- CI
confidence interval
- ESCC
esophageal squamous cell carcinoma
- GERD
gastroesophageal reflux disease
- HR
hazard ratio
- LOESS
locally estimated scatterplot smoothing
- WHR
waist-to-hip ratio
Footnotes
Ethics Statement:
This study was approved by the institutional review boards of Columbia University Irving Medical Center, the Digestive Disease Research Center of Tehran University of Medical Sciences, the US National Cancer Institute, and the World Health Organization’s International Agency for the Research on Cancer. All study participants provided written informed consent before their enrollment into the Golestan Cohort Study.
Conflict of Interest: The authors declare no potential conflicts of interest.
Data Availability Statement:
The data that support the findings of our study are available from the corresponding author upon reasonable request and approval of the Steering Committee of the Golestan Cohort Study.
References
- 1.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–87. [DOI] [PubMed] [Google Scholar]
- 2.Sheikh M, Poustchi H, Pourshams A, Etemadi A, Islami F, Khoshnia M, Gharavi A, Hashemian M, Roshandel G, Khademi H, Zahedi M, Abedi-Ardekani B, et al. Individual and Combined Effects of Environmental Risk Factors for Esophageal Cancer Based on Results From the Golestan Cohort Study. Gastroenterology 2019;156:1416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandeya N, Olsen CM, Whiteman DC. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiology 2013;37:579–84. [DOI] [PubMed] [Google Scholar]
- 4.Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, et al. Population Attributable Risks of Esophageal and Gastric Cancers. JNCI Journal of the National Cancer Institute 2003;95:1404–13. [DOI] [PubMed] [Google Scholar]
- 5.Prabhu A, Obi KO, Rubenstein JH. The Synergistic Effects of Alcohol and Tobacco Consumption on the Risk of Esophageal Squamous Cell Carcinoma: A Meta-Analysis. American Journal of Gastroenterology 2014;109:822–27. [DOI] [PubMed] [Google Scholar]
- 6.Abnet CC, Arnold M, Wei W-Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okello S, Churchill C, Owori R, Nasasira B, Tumuhimbise C, Abonga CL, Mutiibwa D, Christiani DC, Corey KE. Population attributable fraction of Esophageal squamous cell carcinoma due to smoking and alcohol in Uganda. BMC Cancer 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JB, Fan JH, Liang H, Li J, Xiao HJ, Wei WQ, Dawsey SM, Qiao YL, Boffetta P. Attributable causes of esophageal cancer incidence and mortality in China. PLoS One 2012;7:e42281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McQuaid KR, Laine L, Fennerty MB, Souza R, Spechler SJ. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment Pharmacol Ther 2011;34:146–65. [DOI] [PubMed] [Google Scholar]
- 10.Pandeya N, Webb PM, Sadeghi S, Green AC, Whiteman DC. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut 2010;59:31–38. [DOI] [PubMed] [Google Scholar]
- 11.Lu P, Gu J, Zhang N, Sun Y, Wang J. Risk factors for precancerous lesions of esophageal squamous cell carcinoma in high-risk areas of rural China. Medicine 2020;99:e21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SM, Freedman ND, Katki HA, Matthews C, Graubard BI, Kahle LL, Abnet CC. Gastroesophageal reflux disease: A risk factor for laryngeal squamous cell carcinoma and esophageal squamous cell carcinoma in the NIH‐AARP Diet and Health Study cohort. Cancer 2021;127:1871–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrow DC, Vaughan TL, Sweeney C, Gammon MD, Chow W-H, Risch HA, Stanford JL, Hansten PD, Mayne ST, Schoenberg JB, Rotterdam H, Ahsan H, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer Cancer Causes and Control 2000;11:231–38. [DOI] [PubMed] [Google Scholar]
- 14.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic Gastroesophageal Reflux as a Risk Factor for Esophageal Adenocarcinoma. New England Journal of Medicine 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- 15.Soroush A, Etemadi A, Abrams JA. Non-Acid Fluid Exposure and Esophageal Squamous Cell Carcinoma. Dig Dis Sci 2021. [DOI] [PubMed] [Google Scholar]
- 16.Uno K, Iijima K, Hatta W, Koike T, Abe Y, Asano N, Kusaka G, Shimosegawa T. Direct Measurement of Gastroesophageal Reflux Episodes in Patients With Squamous Cell Carcinoma by 24-h pH-Impedance Monitoring. American Journal of Gastroenterology 2011;106:1923–29. [DOI] [PubMed] [Google Scholar]
- 17.Kgomo M, Mokoena TR, Ker JA. Non-acid gastro-oesophageal reflux is associated with squamous cell carcinoma of the oesophagus. BMJ Open Gastroenterology 2017;4:e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zikos TA, Clarke JO. Non-acid Reflux: When It Matters and Approach to Management. Current Gastroenterology Reports 2020;22. [DOI] [PubMed] [Google Scholar]
- 19.Pourshams A, Khademi H, Malekshah AF, Islami F, Nouraei M, Sadjadi AR, Jafari E, Rakhshani N, Salahi R, Semnani S, Kamangar F, Abnet CC, et al. Cohort Profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. International Journal of Epidemiology 2010;39:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimian M, Nourmohammadi H, Salamati M, Hafezi Ahmadi MR, Kazemi F, Azami M. Epidemiology of gastroesophageal reflux disease in Iran: a systematic review and meta-analysis. BMC Gastroenterol 2020;20:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malekshah AF, Kimiagar M, Saadatian-Elahi M, Pourshams A, Nouraie M, Goglani G, Hoshiarrad A, Sadatsafavi M, Golestan B, Yoonesi A, Rakhshani N, Fahimi S, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. European Journal of Clinical Nutrition 2006;60:971–77. [DOI] [PubMed] [Google Scholar]
- 22.Vogtmann E, Etemadi A, Kamangar F, Islami F, Roshandel G, Poustchi H, Pourshams A, Khoshnia M, Gharravi A, Brennan PJ, Boffetta P, Dawsey SM, et al. Oral health and mortality in the Golestan Cohort Study. International Journal of Epidemiology 2017;46:2028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, Merat S, Nasseri-Moghaddam S, Semnani S, Sepehr A, Wakefield J, Møller H, et al. Socio-economic status and oesophageal cancer: results from a population-based case–control study in a high-risk area. International Journal of Epidemiology 2009;38:978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islami F, Poustchi H, Pourshams A, Khoshnia M, Gharavi A, Kamangar F, Dawsey SM, Abnet CC, Brennan P, Sheikh M, Sotoudeh M, Nikmanesh A, et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int J Cancer 2020;146:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roshandel G, Semnani S, Fazel A, Honarvar M, Taziki M, Sedaghat S, Abdolahi N, Ashaari M, Poorabbasi M, Hasanpour S, Hosseini S, Mansuri S, et al. Building cancer registries in a lower resource setting: The 10-year experience of Golestan, Northern Iran. Cancer Epidemiol 2018;52:128–33. [DOI] [PubMed] [Google Scholar]
- 26.Nirwan JS, Hasan SS, Babar Z-U-D, Conway BR, Ghori MU. Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis. Scientific Reports 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh M, Shakeri R, Poustchi H, Pourshams A, Etemadi A, Islami F, Khoshnia M, Gharavi A, Roshandel G, Khademi H, Sepanlou SG, Hashemian M, et al. Opium use and subsequent incidence of cancer: results from the Golestan Cohort Study. The Lancet Global Health 2020;8:e649–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islami F, Nasseri-Moghaddam S, Pourshams A, Poustchi H, Semnani S, Kamangar F, Etemadi A, Merat S, Khoshnia M, Dawsey SM, Pharoah PD, Brennan P, et al. Determinants of gastroesophageal reflux disease, including hookah smoking and opium use- a cross-sectional analysis of 50,000 individuals. PLoS One 2014;9:e89256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018;67:430–40. [DOI] [PubMed] [Google Scholar]
- 30.Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dent J, Vakil N, Jones R, Bytzer P, Schoning U, Halling K, Junghard O, Lind T. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 2010;59:714–21. [DOI] [PubMed] [Google Scholar]
- 32.Wang JH, Luo JY, Dong L, Gong J, Zuo AL. Composite score of reflux symptoms in diagnosis of gastroesophageal reflux disease. World J Gastroenterol 2004;10:3332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredenoord AJ, Weusten BL, Curvers WL, Timmer R, Smout AJ. Determinants of perception of heartburn and regurgitation. Gut 2006;55:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20; quiz 43. [DOI] [PubMed] [Google Scholar]
- 35.Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am 2015;44:203–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Iijima K, Scobie G, Fyfe V, McColl KE. Nitrate and nitrosative chemistry within Barrett’s oesophagus during acid reflux. Gut 2005;54:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winter JW, Paterson S, Scobie G, Wirz A, Preston T, McColl KE. N-nitrosamine generation from ingested nitrate via nitric oxide in subjects with and without gastroesophageal reflux. Gastroenterology 2007;133:164–74. [DOI] [PubMed] [Google Scholar]
- 38.Calmels S, Bereziat JC, Ohshima H, Bartsch H. Bacterial formation of N-nitroso compounds from administered precursors in the rat stomach after omeprazole-induced achlorhydria. Carcinogenesis 1991;12:435–9. [DOI] [PubMed] [Google Scholar]
- 39.Wan QY, Wu XT, Li N, Du L, Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926 386 participants. Gut 2019;68:762–64. [DOI] [PubMed] [Google Scholar]
- 40.Seo SI, Park CH, You SC, Kim JY, Lee KJ, Kim J, Kim Y, Yoo JJ, Seo WW, Lee HS, Shin WG. Association between proton pump inhibitor use and gastric cancer: a population-based cohort study using two different types of nationwide databases in Korea. Gut 2021. [DOI] [PubMed] [Google Scholar]
- 41.Yano Y, Etemadi A, Abnet CC. Microbiome and Cancers of the Esophagus: A Review. Microorganisms 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vakevainen S, Mentula S, Nuutinen H, Salmela KS, Jousimies-Somer H, Farkkila M, Salaspuro M. Ethanol-derived microbial production of carcinogenic acetaldehyde in achlorhydric atrophic gastritis. Scand J Gastroenterol 2002;37:648–55. [DOI] [PubMed] [Google Scholar]
- 43.Bradshaw DJ, Marsh PD. Analysis of pH-driven disruption of oral microbial communities in vitro. Caries Res 1998;32:456–62. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N, Schachtele CF. Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. J Dent Res 1990;69:1266–9. [DOI] [PubMed] [Google Scholar]
- 45.Teng P, Hao M. A population-based study of age-related associations between vaginal pH and the development of cervical intraepithelial neoplasia. Cancer Med 2020;9:1890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke MA, Rodriguez AC, Gage JC, Herrero R, Hildesheim A, Wacholder S, Burk R, Schiffman M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis 2012;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Shi C, Zheng J, Guo Y, Fan T, Zhao H, Jian D, Cheng X, Tang H, Ma J. Fusobacterium nucleatum predicts a high risk of metastasis for esophageal squamous cell carcinoma. BMC Microbiol 2021;21:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen MF, Lu MS, Hsieh CC, Chen WC. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma. Cell Oncol (Dordr) 2021;44:373–84. [DOI] [PubMed] [Google Scholar]
- 49.Petrelli F, De Santi G, Rampulla V, Ghidini A, Mercurio P, Mariani M, Manara M, Rausa E, Lonati V, Viti M, Luciani A, Celotti A. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2021;147:3011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request and approval of the Steering Committee of the Golestan Cohort Study.


