Abstract
Background:
Algorithmic application of the 2019 Society of Cardiovascular Angiography and Intervention (SCAI) shock stages effectively stratifies mortality risk for patients with cardiogenic shock (CS). However, clinician assessment of SCAI staging may differ. Moreover, the implications of the 2022 SCAI criteria update remain incompletely defined.
Methods:
The Critical Care Cardiology Trials Network (CCCTN) is a multicenter registry of cardiac intensive care units (CICUs). Between 2019–2021, participating centers (n=32) contributed at least a 2-month ‘snapshot’ of consecutive medical CICU admissions. In-hospital mortality was assessed across three separate staging methods: clinician assessment, CCCTN algorithmic application of the 2019 SCAI criteria, and a revision of the CCCTN application using the 2022 SCAI criteria.
Results:
Of 9,612 admissions, 1,340 (13.9%) presented with CS with in-hospital mortality of 35.2%. Both clinician and algorithm-based staging using the 2019 SCAI criteria identified a stepwise gradient of mortality risk (stage C-E: 19.0% to 83.7% and 14.6% to 52.2%, respectively; p-trend <0.001 for each). Clinician assignment of SCAI stages identified higher risk patients compared with algorithm-based assignment (stage D: 49.9% vs. 29.3%; stage E: 83.7% vs. 52.2%). Algorithmic application of the 2022 SCAI criteria, with incorporation of the vasoactive-inotropic score, more closely approximated clinician staging (mortality for stage C-E: 21.9% to 70.5%; p-trend <0.001).
Conclusions:
Both clinician and algorithm-based application of the 2019 SCAI stages identify a stepwise gradient of mortality risk, though clinician-staging may better allocate higher risk patients into advanced SCAI stages. Updated algorithmic staging using the 2022 SCAI criteria further refines risk stratification.
Keywords: Cardiogenic shock, risk stratification, critical care cardiology
Introduction
Cardiogenic shock (CS) is associated with high risk of in-hospital mortality, with overall estimates ranging between 30–40%.1,2 Early revascularization for the subset of patients with CS due to acute myocardial infarction (AMI) remains the only intervention rigorously demonstrated to improve outcomes.3 The difficulty in establishing a therapeutic benefit of other interventions has been partially attributed to significant heterogeneity of CS, as etiology and illness severity may influence outcomes and responsiveness to therapies.
To better account for this heterogeneity in CS presentations, the Society of Cardiovascular Angiography and Interventions (SCAI) developed a classification scheme in 2019 to categorize patients at risk for or with CS into stages of severity, with the goal of informing patient care and guiding future investigations.4 Studies using different pragmatic adaptations of these criteria in the form of data-driven algorithms have demonstrated effective risk stratification.5–11 Such algorithmic applications may be useful to facilitate generalizability, utilization in electronic health records, and integration at centers with less experience in advanced CS management. Notably, the distribution of SCAI stages and the associated mortality range within each stage has varied between these validation studies, owing in part to differences among the diverse adaptations developed for staging. Moreover, limited data exists regarding clinician application of SCAI shock staging,12,13 which may differ from these algorithmic assessments. In light of these considerations, the 2019 SCAI classification was revised in 2022 to better facilitate its accessibility and broad clinical use.14 However, the impact of this update on the application of the SCAI staging system for mortality risk stratification remains incompletely defined.
Accordingly, we sought to 1) evaluate clinician staging of shock severity based on the original 2019 SCAI criteria, 2) compare clinician staging with algorithmic staging based on an algorithmic adaptation of the original 2019 criteria and 3) assess the prognostic associations of the revised 2022 SCAI staging criteria.
Methods
Study Population and Design
The Critical Care Cardiology Trials Network (CCCTN) is an investigator-led, collaborative research network of cardiac intensive care units (CICUs) in North America coordinated by the TIMI Study Group (Boston, MA). Methods for the CCCTN Registry have been described.15 The CCCTN Registry protocol and waiver of informed consent were approved by the institutional review committees at each of the participating centers. We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
This analysis comprised of consecutive CICU admissions from a 2-month annual collection period at participating centers (n=32) between 2019–2021. In addition, several centers collected data on all consecutive admissions outside of the 2-month snapshot. The presence of shock was assessed by site investigators and categorized by type of shock as previously described.2 The current analysis was restricted to patients with either CS or mixed shock (MS; i.e., with features of both cardiogenic and distributive shock) based on clinician assessment. The etiology of CS was further subdivided into AMI- or heart failure (HF)-related CS.16
Patients were assigned a SCAI shock stage (C, D or E) using three methods. First, site investigators were asked to record the SCAI shock stage at the time of data submission using the 2019 SCAI consensus statement4, based on the patients’ clinical course restricted to the first 24 hours. Specifically, the investigators were trained to use the first 24 hours in aggregate, as opposed to a single snapshot of shock severity at a specific time point during this period, acknowledging that trajectory (e.g., failure to respond to initial therapies) is necessary for determination of SCAI stages D and E. In order to mitigate the potential for classification bias based on subsequent outcomes, investigators were trained to use the patients’ clinical course restricted only to the initial 24 hours to determine the SCAI stage. To further assess for this possibility, we also examined the pattern of reclassification between staging methods. Next, each patient was also assigned a SCAI stage using the previously reported CCCTN algorithmic adaptation of the 2019 SCAI staging criteria (Supplemental Table 1).7 Finally, a revised algorithm was developed based on the updated 2022 SCAI staging criteria,14 specifically incorporating the requirement of coma for preceding cardiac arrest, a higher lactate threshold for stratification to stage E (5 to 8 mmol/L), and use of the vasoactive-inotropic score (VIS; initial value and change at 24 hours) as a marker of escalation or de-escalation. This revised algorithm was also applied to the same population.
Statistical Analysis
Clinical and laboratory characteristics were summarized as medians (25th-75th percentiles) for continuous variables and proportions for categorical variables. The Sequential Organ Failure Assessment (SOFA) score was calculated for each patient.17 The vasoactive-inotropic score (VIS) was calculated as a weighted sum using dosing information for vasopressors and inotropes for each patient at 4-hours (4h) and 24-hours (24h) after CICU admission (Supplemental Methods).5,18,19 As there are no established thresholds for VIS or change in VIS, we evaluated the continuous relationship between in-hospital mortality and the change in VIS from 4h to 24h as a function of starting VIS at 4h using restricted cubic splines. This relationship was used to inform the absolute thresholds chosen for categorization of the 4h VIS and the change in VIS from 4h to 24h.
Mortality was assessed across SCAI stages by each method using the Cochran-Armitage test-for-trend. Discrimination was quantified using the C-statistic. The results were examined for patients with CS and MS, with further stratification by etiology for patients with CS (AMI-CS or HF-CS). Sensitivity analyses were performed to assess whether preceding cardiac arrest (CA) or transfer from an outside hospital modified mortality in each stage.
Reclassification was assessed using the proportion of patients surviving and dying that were up- and down-classified by the three different approaches to assignment of SCAI CS stage. Cohen’s weighted κ was used to assess agreement across staging methods. A two-sided p-value of 0.05 was considered statistically significant. All analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Outcomes by Clinician Assigned SCAI Stage
Of 9,612 CICU admissions, 1,340 (13.9%) presented with CS and were assigned a SCAI shock stage based on clinician assessment (Table 1). Of these, 28.9% had AMI-CS and 56.8% had HF-CS. The overall CICU and in-hospital mortality rates among admissions with CS were 27.2% and 35.2%, respectively. Clinician-assigned SCAI staging identified a strong gradient of risk of death (CICU mortality: 11.2% to 77.5%; in-hospital mortality: 19.0% to 83.7%; p-trend <0.001 for each; Figure 1). This stepwise gradient of mortality risk by clinician-assigned SCAI stage was maintained across important subgroups: AMI-CS and HF-CS (Supplemental Figure 1), MS (Supplemental Figure 2), and those with and without preceding CA (Supplemental Figure 3; p-trend <0.001 for each). Discrimination by clinician-assigned SCAI shock stage was good for both CICU mortality (C-index: 0.76 [95% CI 0.74–0.79]) and in-hospital mortality (C-index 0.73 [95% CI 0.70–0.76]). Of patients with CS, 612 (45.7%) were transferred from outside hospitals with overall in-hospital mortality of 41.8%. Mortality within each clinician-assigned SCAI stage and overall discrimination was similar for transferred patients compared with the overall CS population (C-index: 0.73 [95% CI 0.70–0.76]; Supplemental Figure 4).
Table 1:
Patient Characteristics by Clinician-Assigned SCAI Shock Stage in patients with CS
| Characteristics | SCAI Stage C (N=778) | SCAI Stage D (N=433) | SCAI Stage E (N=129) | p-trend |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65 (55–74) | 66 (57–74) | 67 (60–75) | 0.27 |
| Female | 253 (32.5) | 148 (34.2) | 50 (38.8) | 0.18 |
| White | 444 (64.0) | 276 (74.4) | 68 (59.6) | 0.28 |
| BMI, kg/m2 | 27.1 (23.7–31.7) | 27.6 (23.7–32.4) | 27.9 (24.7–32.1) | 0.16 |
| Comorbidities | ||||
| Hypertension | 475 (61.1) | 258 (59.6) | 77 (59.7) | 0.63 |
| Diabetes mellitus | 300 (38.6) | 160 (37.0) | 56 (43.4) | 0.62 |
| Current smoker | 128 (16.5) | 67 (15.5) | 23 (17.8) | 0.96 |
| Chronic kidney disease | 228 (29.3) | 108 (24.9) | 30 (23.3) | 0.06 |
| Dialysis dependent | 29 (12.7) | 21 (19.4) | 8 (26.7) | 0.02 |
| Coronary artery disease | 284 (36.5) | 155 (35.8) | 40 (31.0) | 0.30 |
| Cerebrovascular disease | 69 (8.9) | 28 (6.5) | 9 (7.0) | 0.19 |
| Peripheral artery disease | 73 (9.4) | 41 (9.5) | 11 (8.5) | 0.84 |
| Prior heart failure | 436 (56.0) | 227 (52.4) | 42 (32.6) | <0.001 |
| Severe valvular disease | 129 (16.6) | 71 (16.4) | 16 (12.4) | 0.35 |
| Pulmonary hypertension | 69 (8.9) | 38 (8.8) | 9 (7.0) | 0.58 |
| Significant pulmonary disease | 99 (12.7) | 57 (13.2) | 20 (15.5) | 0.45 |
| Significant liver disease | 20 (2.6) | 6 (1.4) | 2 (1.6) | 0.20 |
| Illness Severity | ||||
| Total Day 1 SOFA Score | 7.0 (5.0–9.0) | 9.0 (6.0–12.0) | 11.0 (9.0–13.0) | <0.001 |
| IABP SHOCK-II Score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 3.0 (2.0–4.0) | <0.001 |
| Preceding cardiac arrest | 169 (21.7) | 128 (29.6) | 78 (60.5) | <0.001 |
| ICU Resource Utilization | ||||
| Mechanical ventilation | 330 (42.4) | 297 (68.6) | 118 (91.5) | <0.001 |
| Renal replacement therapy | 89 (11.4) | 107 (24.7) | 25 (19.4) | <0.001 |
| Pulmonary Artery Catheterization | 345 (44.3) | 277 (64.0) | 46 (35.7) | 0.04 |
| # of vasopressors/inotropes | 1.0 (1.0–2.0) | 2.0 (2.0–3.0) | 3.0 (2.0–4.0) | <0.001 |
| ≥2 agents | 344 (44.4) | 357 (83.0) | 111 (86.7) | <0.001 |
| VIS @ 4h | 4.0 (2.0–7.5) | 10.0 (4.0–28.5) | 33.7 (10.0–80.0) | <0.001 |
| VIS @ 24h | 3.0 (0.0–6.5) | 10.9 (3.6–24.1) | 14.0 (2.0–45.0) | <0.001 |
| Mechanical Circulatory Support | ||||
| Overall use | 219 (28.1) | 251 (58.0) | 58 (45.0) | <0.001 |
| IABP | 141 (64.4) | 173 (68.9) | 19 (32.8) | 0.005 |
| Advanced MCSa | 96 (43.8) | 129 (51.4) | 48 (82.8) | <0.001 |
| >1 MCS device | 23 (10.5) | 78 (31.1) | 18 (31.0) | <0.001 |
| Within 24h from admission | 113 (51.6) | 140 (55.8) | 42 (72.4) | 0.01 |
| Baseline Lab Indicators | ||||
| Lactate (mmol/L) | 2.8 (1.8–4.9) | 3.4 (2.1–6.5) | 8.0 (3.9–11.2) | <0.001 |
| eGFR <45 mL/min/1.732 | 439 (56.4) | 258 (59.6) | 78 (60.9) | 0.16 |
| pH ≤7.2 | 77 (12.4) | 83 (22.1) | 55 (44.0) | <0.001 |
All values represent n (%) for categorical measures and median (25–75th percentile) for continuous measures.
Advanced MCS includes Impella, TandemHeart, VA-ECMO, or surgical ventricular assist device.
Abbreviations: ALT = alanine aminotransferase; BMI = body mass index; eGFR = estimated glomerular filtration rate; h = hour; IABP = intra-aortic balloon pump; IABP SHOCK-II = intra-aortic balloon pump in cardiogenic shock II; MCS = mechanical circulatory support; SOFA = sequential organ failure assessment; VIS = vasoactive-inotropic score.
Figure 1: CICU and In-Hospital Mortality of Cardiogenic Shock by Clinician-Assigned SCAI Stage.
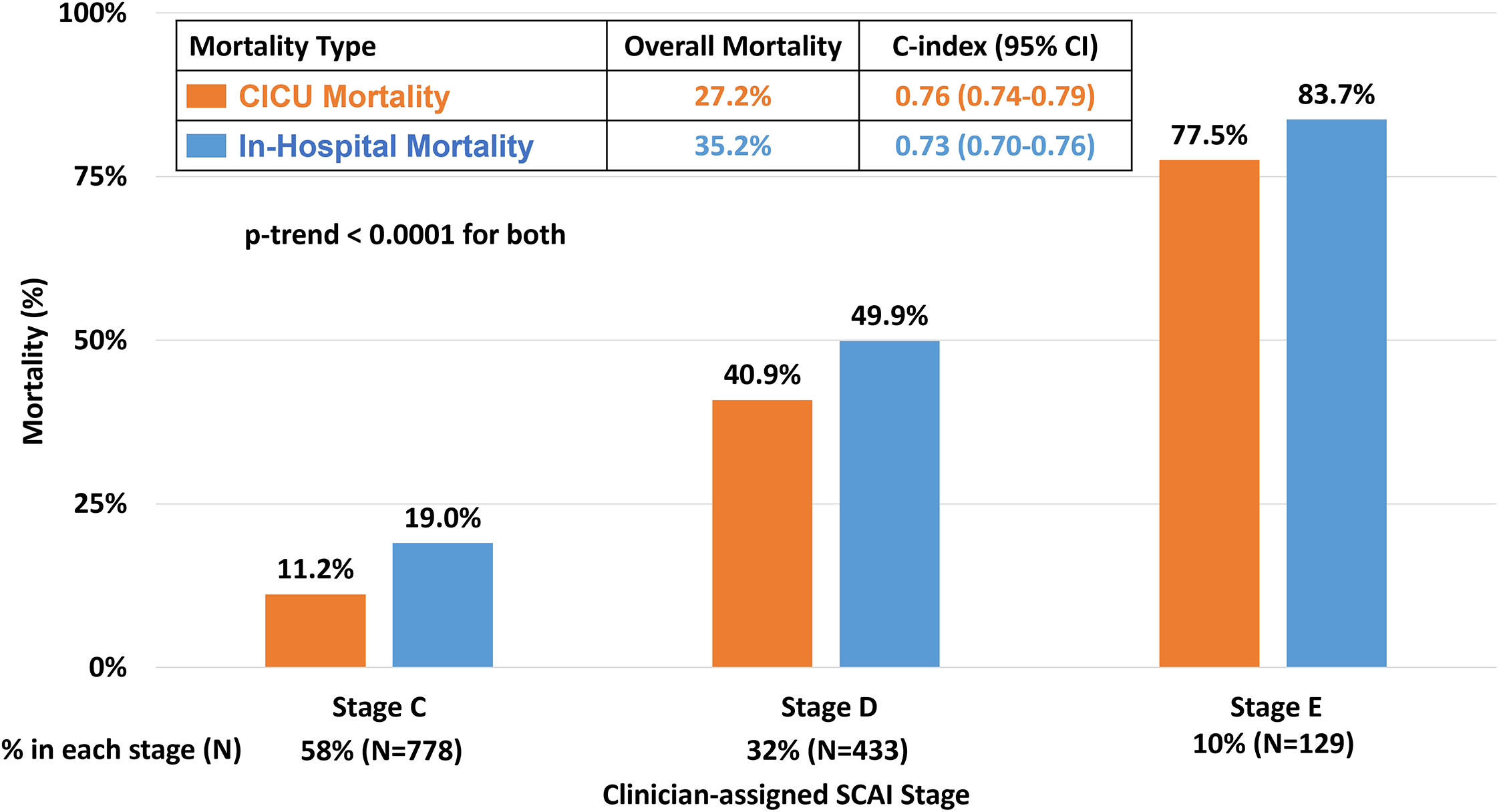
Clinician application of the 2019 SCAI shock stages identified a stepwise gradient of risk for CICU (orange) and in-hospital (blue) mortality. CICU = cardiac intensive care unit; SCAI = Society of Cardiovascular Angiography and Interventions.
Comparison of Clinician and Algorithm Assessment
Staging by the CCCTN algorithm based on the 2019 SCAI shock criteria also identified a stepwise gradient of risk for in-hospital mortality (14.6% to 52.2%; p-trend<0.001), though with poor agreement between clinician and algorithm-based staging (weighted κ = 0.20; Figure 2). However, the algorithm identified a clear gradient of mortality risk within each clinician-assigned stage (p-trend<0.001 for each; Figure 3A). For example, among patients assigned to stage C by clinicians (n=778), the data-driven algorithm identified an over 2-fold gradient of in-hospital mortality risk from 11.5% to 27.1% in algorithm-assigned stages C to E. In contrast, among patients with an algorithm-assigned stage E (n=525), clinician-assigned SCAI stage identified a range of mortality risk from 27.1% to 87.6% (p-trend <0.001 for each; Figure 3A).
Figure 2: In-Hospital Mortality of Cardiogenic Shock by SCAI Stage Across Staging Methods.
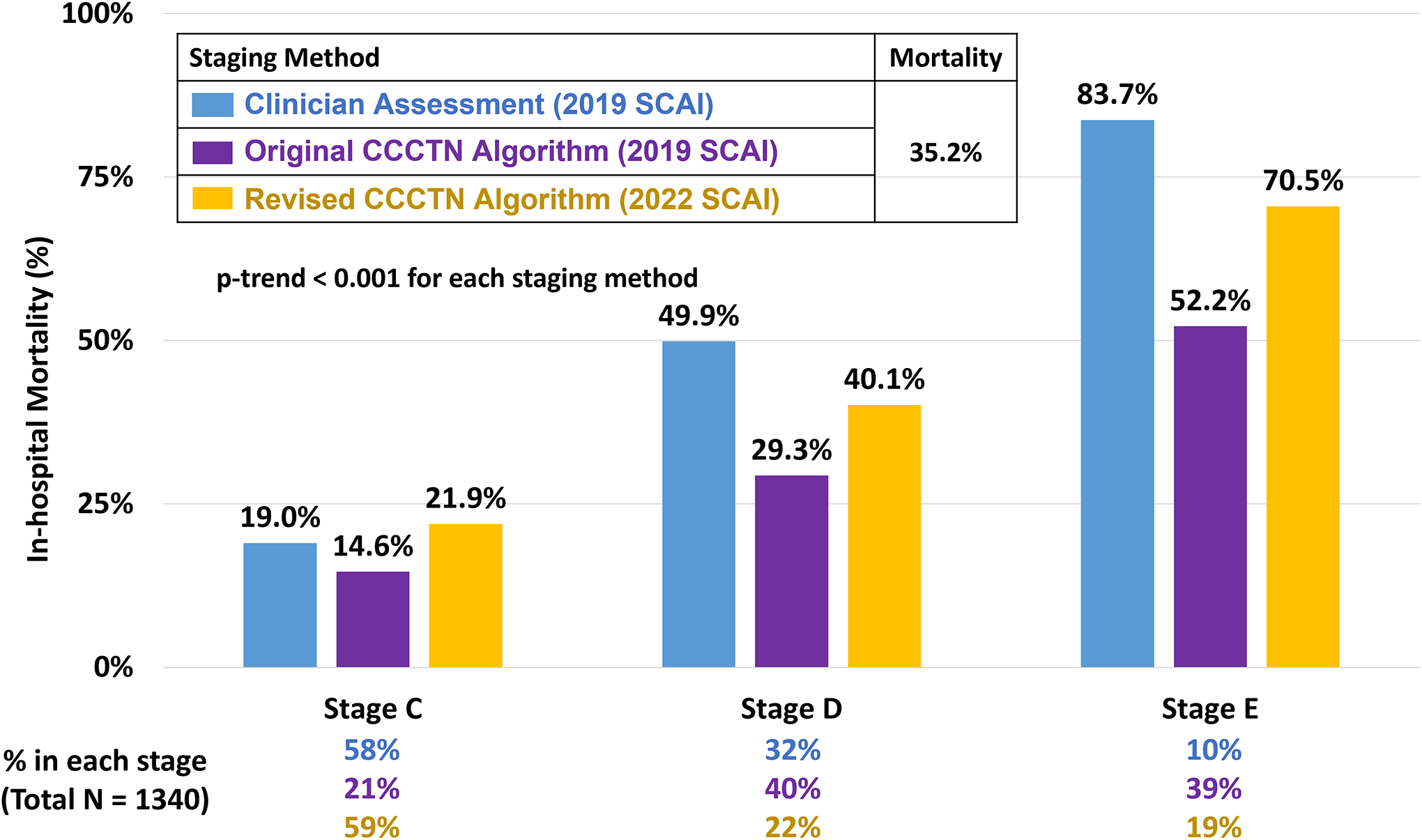
Consecutive CICU admissions with CS were assigned shock staging using all three methods: clinician assessment based on the 2019 SCAI CS stages (blue), CCCTN algorithmic application of the 2019 SCAI CS stages (purple), and CCCTN algorithmic application of the 2022 SCAI CS stages (yellow). Each staging method effectively identified a stepwise gradient of risk for in-hospital mortality (p-trend < 0.001 for each), though clinicians identified higher risk patients for allocation to the advanced SCAI stages (stages D and E). CCCTN algorithmic application of the 2022 SCAI shock stages, with incorporation of the vasoactive-inotropic score, refined original algorithm-based staging and more closely approximated clinician application. CCCTN = Critical Care Cardiology Trials Network; CS = cardiogenic shock; SCAI = Society of Cardiovascular Angiography and Interventions.
Figure 3: In-Hospital Mortality of Cardiogenic Shock by CCCTN Algorithmic Application of SCAI Staging to Clinician-Assigned 2019 SCAI Stages.
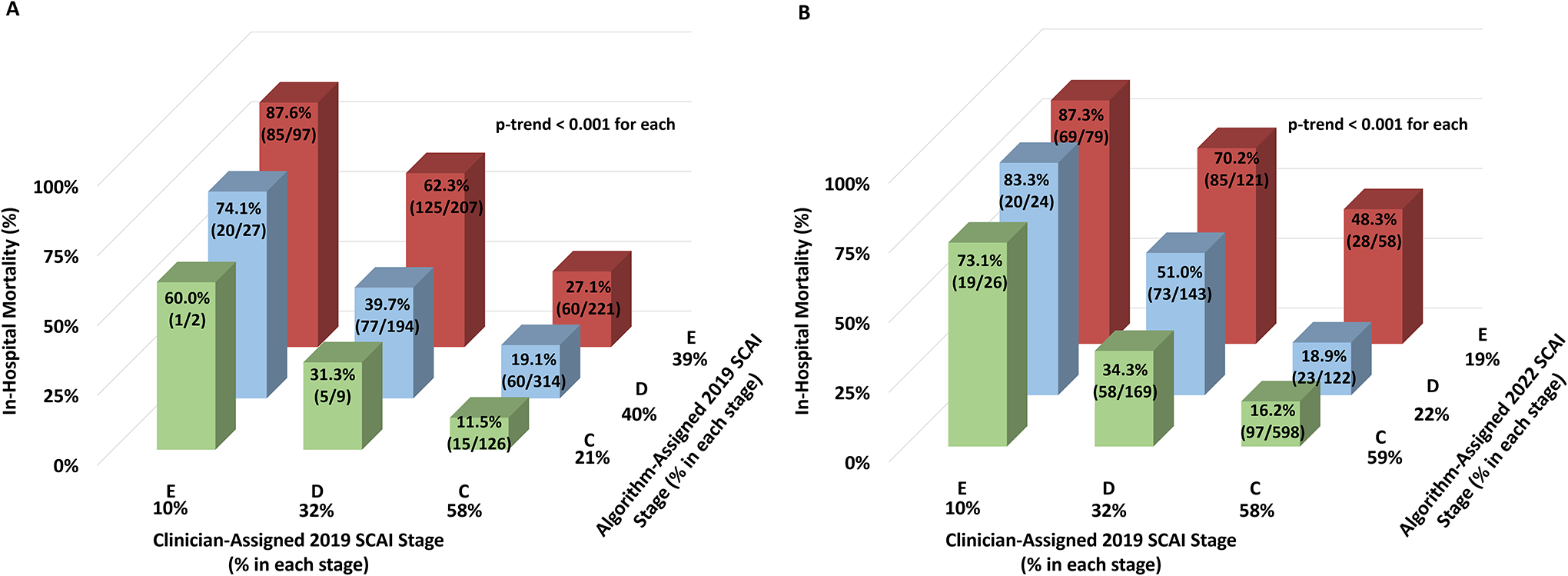
Panel A shows in-hospital mortality with CCCTN algorithmic application of the 2019 SCAI criteria for each clinician-assigned 2019 SCAI Stage. Panel B shows in-hospital mortality with CCCTN algorithmic application of the 2022 SCAI criteria for each clinician-assigned 2019 SCAI Stage. CCCTN algorithmic application of the 2019 and 2022 SCAI stages identified a gradient of risk for in-hospital mortality within each of the clinician-assigned 2019 SCAI stages. Color assignment corresponds to categories of algorithm-assigned SCAI stage. CCCTN = Critical Care Cardiology Trials Network; SCAI = Society of Cardiovascular Angiography and Interventions.
Overall, clinician assessment of SCAI shock stage resulted in significant down-classification versus the algorithm-based 2019 SCAI stages, with down-classification of 742 patients (55.3% of all CS) compared with up-classification of only 64 patients (Supplemental Table 2). Discrimination for clinician staging was higher (C-index: 0.73, 95% CI 0.70–0.76]) than the algorithm (C-index: 0.67, 95% CI 0.65–0.70).
Factors Associated with Differential Staging
Patient demographics and co-morbidities were predominantly similar between corresponding clinician and algorithm-assigned SCAI stages (Supplemental Table 3). However, patients within clinician-assigned SCAI stages had greater illness severity and use of advanced ICU therapies, including advanced MCS, compared with the corresponding algorithm-assigned stage, and these differences were more pronounced with advancing SCAI stage: SOFA score (clinician vs. algorithm-assigned stage E: 11 [9–13] vs. 10 [7–12]), preceding cardiac arrest (E: 60.5% vs. 45.9%), advanced MCS (E: 82.8% vs. 60.0%); mechanical ventilation (E: 91.5% vs. 74.5%; Supplemental Table 4). In addition, lactate was higher and pH was lower in the highest clinician-assigned stages (Supplemental Table 5).
While no patients in algorithm-based stage C were treated with ≥2 vasoactive agents by definition, a similar proportion of patients in stage D were treated with ≥2 agents in each staging method (83.0% vs. 82.3%). However, those in clinician-assigned stage D had a significantly higher VIS at both time points compared with algorithm-based stage D (4h VIS: 10.0 [4.0–28.5] vs. 5.0 [2.5–14.0]; 24h VIS: 10.9 [3.6–24.1] vs. 5.0 [2.0–12.5]; p<0.001 for each; Supplemental Table 4).
Relationship between VIS and Outcome
Given those findings, we further explored the relationship between VIS and in-hospital mortality. Continuous modeling demonstrated a robust association between in-hospital mortality and both starting VIS at 4h as well as the change in VIS from 4h to 24h (Supplemental Figure 5). When examined categorically, a 20 point higher starting value for the VIS at 4h was associated with a 20–25% higher rate of in-hospital mortality, and patients with increases or decreases in VIS of ≥10 at 24 hours were associated with significantly higher or lower mortality compared to patients with stable (change <10) VIS values (p-trend <0.001 for each; C-index 0.69 [95% CI 0.66–0.72]; Figure 4). In contrast, the association between in-hospital mortality and the number of vasoactive agents or MCS devices was more variable (Supplemental Figure 6). Regardless of whether a single or multiple vasoactive agents were used, there was a gradient of risk for in-hospital mortality by starting VIS at 4h (Supplemental Figure 7).
Figure 4: In-Hospital Mortality by Initial and Change in Vasoactive-Inotropic Score.
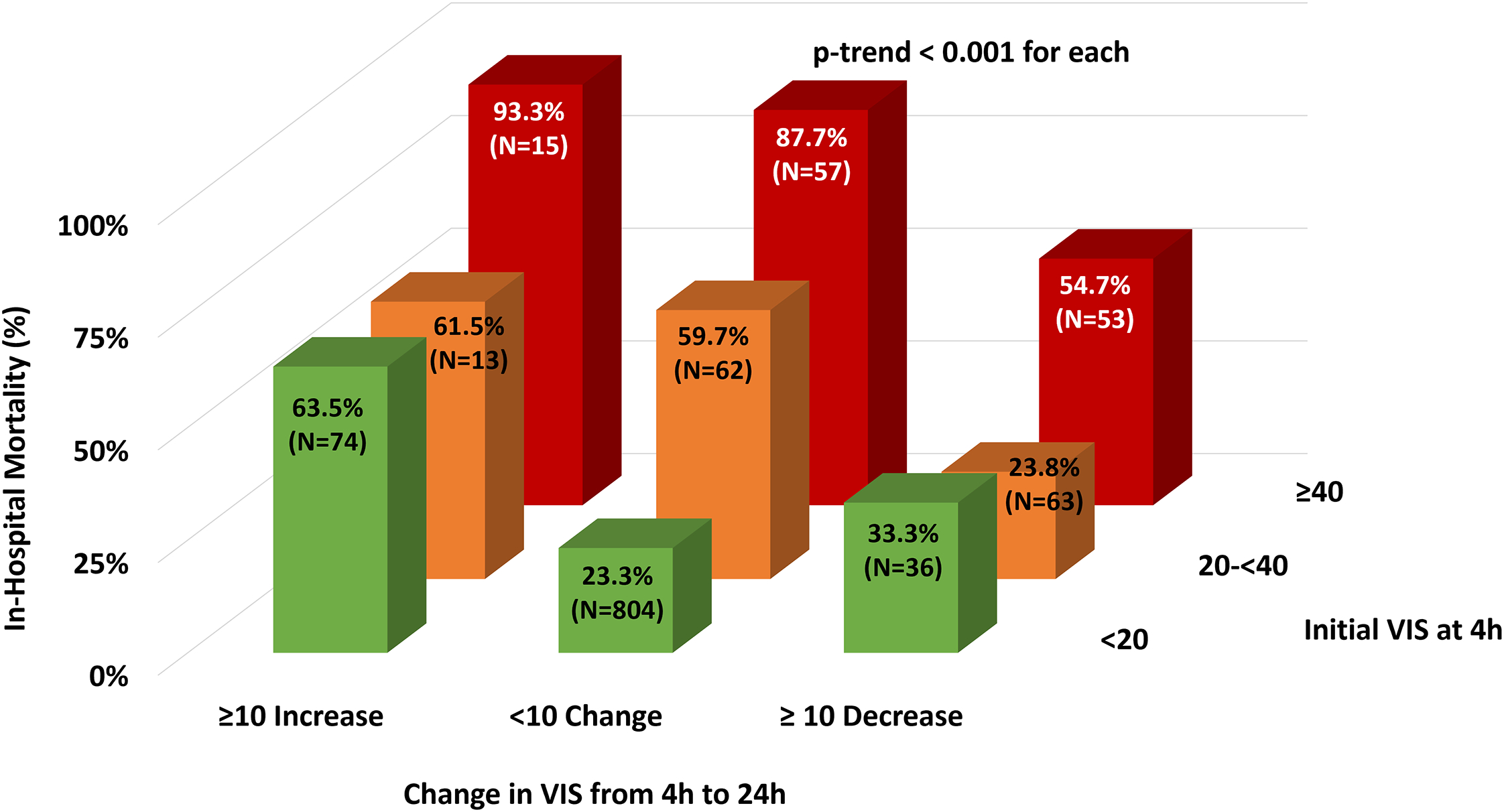
In-hospital mortality progressively increased with higher starting 4h VIS (p-trend <0.001 for each category of initial VIS at 4h). In addition, significant increases in VIS ≥10 from 4h to 24h were associated with higher in-hospital mortality across the range of starting VIS values (p-trend <0.001 for each). Color assignment corresponds to range of starting VIS. VIS = vasoactive-inotropic score, a weighted sum reflective of combined dosing of all vasopressors and inotropes; h = hours.
Revised SCAI Staging Criteria
The CCCTN algorithm was revised based on the 2022 SCAI update with incorporation of changes in the VIS as a marker of escalation or de-escalation (Table 2). Application of this revised algorithm identified a strong gradient of risk for in-hospital mortality (21.9% to 70.5%; p-trend <0.001; Figure 2). The revised algorithm resulted in significant down-classification of the prior algorithm-based 2019 SCAI stage (n=595, 44.4% of all CS) with very little up-classification (n=40; Supplemental Table 6). In addition, there was greater agreement with clinician-assigned 2019 SCAI stage (weighted κ = 0.39; Supplemental Table 7), and discrimination was nominally higher compared with the 2019 algorithm (C-index 0.70, 95% CI 0.67–0.73). Application of the revised algorithm to the clinician-assigned SCAI stages identified a consistent gradient of risk across each stage (p-trend <0.001 for each; Figure 3B).
Table 2:
Revised CCCTN Adaptation Using the 2022 SCAI Staging Criteria
| SCAI Stage | SCAI Consensus Update (2022) 14 | Revised CCCTN Algorithm (2022 SCAI Adaptation) |
|
C
(“classic”) |
|
|
|
D
(“deteriorating”) |
|
|
|
E
(“extremis”) |
|
|
Low, intermediate, and high starting doses of vasoactive agents correspond to a vasoactive-inotropic score at 4 hours of <20, 20-<40 and ≥40, respectively. Escalation and de-escalation correspond to an increase or decrease in vasoactive-inotropic score ≥10 points from 4 hours to 24 hours, respectively. If vasoactive dose information is missing, a single vasoactive agent meets the vasoactive agent criterion for stage C and ≥2 vasoactive agents meet the vasoactive agent criterion for stage D and E.
Stage E lab criteria were not required if meeting vasoactive agent dosing requirements based on the vasoactive-inotropic score.
Abbreviations: CA = cardiac arrest; CCCTN = Critical Care Cardiology Trials Network; CS = cardiogenic shock; eGFR = estimated glomerular filtration rate; MCS = mechanical circulatory support; SCAI = Society of Cardiovascular Angiography and Interventions.
Discussion
In this analysis of SCAI CS staging in the CCCTN registry, we demonstrate that clinician application of the 2019 SCAI staging criteria is feasible across multiple institutions and clinicians and may improve CS risk stratification over algorithmic application although, importantly, both staging methods appear complementary. Moreover, with this novel assessment of clinician and algorithmic SCAI staging within the same population, we identified factors which may drive differential staging between methods and demonstrate elements that might be leveraged to guide further refinement of SCAI staging criteria for clinical and research applications. In particular, we demonstrate that quantifying not only the number but also the dosing of vasoactive agents may be useful. By accounting for these differences and incorporating the greater granularity provided by the 2022 SCAI staging update, a revised CCCTN algorithmic application improves risk stratification and more closely aligns with clinician assessment of risk in patients with CS.
Effective Risk Stratification across Staging Methods
Several prior studies have demonstrated effective risk stratification with pragmatic adaptations of the SCAI staging criteria,5–11 though only two studies to date have examined clinician application of SCAI staging.12,13 In the important single-center study by Baran and colleagues, prospective SCAI staging by shock team clinicians appeared to be feasible and effectively identified a gradient of mortality across stages.12 In a subsequent study from the National Cardiogenic Shock Initiative confined to patients with only AMI-CS, SCAI staging based on retrospective chart review by two independent clinicians was effective and offered consistency and reproducibility given excellent agreement between clinicians.13 Expanding on these prior studies, we demonstrate that SCAI staging may be reliably applied by clinicians across a broad range of institutions, and irrespective of the etiology of CS. Moreover, as the only study to date to assess different staging methods within the same population, we demonstrate that clinicians may better allocate higher risk patients to advanced SCAI stages D and E though both staging methods offer effective risk stratification. Importantly, we found that these staging methods are complementary. Indeed, it has been previously suggested that distinct CS phenotypes may identify lower or higher risk patients within each SCAI stage.20–22 As the algorithm relies heavily on biochemical indices of shock (e.g., pH, lactate), it is possible that algorithmic assessment more robustly identifies patients with a “hemometabolic” or “cardiometabolic” CS phenotype20,23 manifest by greater end-organ injury within each clinician-assigned SCAI stage. Importantly, these findings suggest a role for a data-driven approach to further inform clinician assessment, potentially in the form of electronic clinical decision tools to support a hybrid approach whereby clinicians assign SCAI shock stage with the help of pre-defined supporting criteria.
Differences in Clinician and Algorithmic Assessment
While both clinician and algorithmic staging based on the 2019 SCAI criteria were effective for risk stratification, there was a greater increase in mortality rate with each advancing clinician-assigned SCAI stage (C to E: 19.0% to 49.9% to 83.7%) compared with the corresponding algorithm-based stage (14.6% to 29.3% to 52.2%; Figure 2). In analyzing possible factors driving differential staging, we found marked differences in illness severity, the use of advanced ICU therapies and the aggregate dosing of vasopressors in the highest stages between classification methods.
Collectively, these findings support the notion that clinicians may integrate the trajectory and complexity of illness more effectively compared with algorithmic assessment based on the 2019 SCAI criteria, resulting in more distinct high-risk categories which may be preferable to guide decision-making and escalation/de-escalation of therapies.24 For example, clinicians may better discern severity of illness considering the dosing of vasopressors rather than simply the number of medications, differentiating between patients supported by two vasoactive agents at low doses vs. two vasoactive agents at maximum doses. Indeed, studies have previously demonstrated a robust association between overall treatment intensity (i.e., the number of vasoactive agents or MCS devices) and trajectory, and have leveraged this relationship for effective staging of shock severity.11 In addition, Jentzer and colleagues have also demonstrated the utility of accounting for vasoactive dosing using the VIS as a marker of escalation, using an increase in VIS at 24 hours as compared with the initial VIS as a criterion for SCAI stage D.5 Expanding on these prior findings, we demonstrate that the association between in-hospital mortality and changes in VIS varies as a function of starting VIS (Figure 4), with patients who had a higher initial VIS or a significantly rising VIS being at elevated risk of death. Moreover, we found this relationship to be robust contrasted with using only the number of vasoactive agents or MCS devices alone (Supplemental Figures 6). As expected, given these findings, incorporation of VIS into the revised algorithm resulted in greater agreement with clinician staging and a nominal improvement in discrimination. Moreover, leveraging other observed differences between staging methods (e.g., need for advanced MCS vs. IABP) may further refine algorithmic application of SCAI staging.
While the intent of the SCAI criteria is to establish a common language for categorization of CS severity with ease of applicability by bedside clinicians, our findings suggest that clinicians may intuitively account for these observed differences when applying the SCAI staging criteria, but that algorithmic application of the criteria may benefit from further granularity (e.g., specific thresholds for laboratory indicators or dosing of vasoactive agents) to refine risk stratification and facilitate consistent application in clinical research. Such efforts to refine the objective elements of CS staging criteria may be important to promote generalizability across a spectrum of health care systems and to facilitate systems-based care across “hub-and-spoke” networks for management of CS. Moreover, the development of such robust algorithms for staging severity of CS is necessary for integration into electronic health records and digital quality improvement tools as well as for research applications where prospective clinician assessment may not be available.
In addition to shock severity, assessment of mortality risk optimally incorporates additional factors such as frailty, and neurologic status in those with preceding CA, all of which may influence patient outcomes in CS and are acknowledged as risk modifiers in the updated SCAI criteria.14 Clinician-assessment of SCAI stage may incorporate these risk modifiers more effectively, whereas some of these important variables may not be systematically captured and thus remain challenging to integrate into algorithm-based assessments. Despite these inherent limitations, however, it should be noted that both the original and revised algorithmic applications were effective in identifying a gradient of mortality risk with comparable discrimination to clinician staging. As such, our findings demonstrate that the SCAI staging criteria achieve effective risk stratification regardless of the method of application, a finding that is important for epidemiological research and possible standardization across clinical trials.
Refining Algorithmic Applications of SCAI Staging Criteria
To improve the clinical utility of the staging criteria, the 2022 SCAI consensus update included many notable changes including revision of the preceding CA modifier to require coma or evidence of anoxic brain injury, an increase in the lactate threshold from 5 to 8 mmol/L as a biochemical descriptor for stage E, and an emphasis on discerning escalation and de-escalation for progression through stages.14 With respect to SCAI stages D and E, the authors of the SCAI consensus update note that these stages should reflect a failure of the patient to respond to initial therapies, often resulting in the addition of vasoactive agents or MCS devices, while acknowledging that this alone may fail to differentiate stages and prognosis for patients who require significant dose escalation of pre-existing pharmacotherapy.14
To this end, we demonstrate that incorporation of VIS may be used to help overcome this limitation, as it effectively captures dosing information and demonstrates a robust risk association with mortality. With revision of our algorithmic application based on these updated criteria (Table 2), we found that a substantial proportion of patients were down-classified compared with our initial algorithmic assessment, such that the proportion of patients in stage C increased from 21% to nearly 60%. As the result, the gradient in mortality rates across stages was more pronounced, with an increase in mortality risk for patients assigned to stage D or E based on the revised algorithm (Figure 2). Collectively, these findings suggest that the added granularity provided by the 2022 SCAI staging update may more effectively identify patients at the greatest risk of in-hospital death and facilitate uniformity of application across clinical trials and registries.
Limitations
Several limitations should be acknowledged. First, clinician assessment of SCAI staging was performed at the time of data entry and as such, knowledge of final patient outcomes may have influenced staging despite specific training to use only the clinical course in the first 24h. However, we note that there was a substantial proportion of patients who died but were down-staged by clinicians (n=249, 52.8% of patients dying; Supplemental Table 2), which argues against clinician classification being heavily influenced by final outcomes. The assessment of the data-driven algorithms in our analysis were agnostic to clinician-assessment and are not subject to this limitation. In addition, the clinical course and testing at the external referral hospital may offer incremental prognostic information. However, based on the 24h staging at participating centers, we demonstrate similar mortality rates within each clinician-assigned SCAI stage without a change in discrimination for transferred patients vs. all CS patients (Supplemental Figure 4). Second, the algorithms applied in our study represent only two potential pragmatic adaptations of the SCAI criteria. As such, other models may also be useful and perform differently.5,6,11 Third, our analysis cohort was predominantly from tertiary care academic CICUs and results may vary when applied to other settings (e.g., emergency department, catheterization laboratory, or lower acuity centers). Fourth, clinician assessment of shock stage was only captured for patients with clinical shock (i.e., with evidence of end-organ dysfunction), thus, by definition, excluding those meeting SCAI stages A and B (those at risk or with pre-shock). By constraining the range of risk, the reported discrimination and C-indices are lower and not directly comparable to prior assessments which include patients in these lower risk stages. Not addressed in this current study is the important objective of developing reliable tools for early recognition and risk stratification at the emerging and nascent stages of shock, in which it is hoped that interventions may provide the greatest benefit. Finally, our study assessed initial SCAI stage primarily using data within the first 24 hours of CICU admission. Serial assessment of SCAI staging may further improve risk stratification.11–13 However, the optimal interval over which to assess serial changes is unknown and remains an area of active investigation in emerging large registries.
Conclusion
Clinician and algorithmic application of the 2019 SCAI shock classification effectively stratifies mortality risk and appears to be complementary. However, clinician staging may more effectively integrate the trajectory and complexity of illness and identifies more distinct risk categories. Incorporation of not only the number but also the dosing of vasoactive medications, as well as the added granularity of the 2022 SCAI staging criteria update, refines algorithmic application and more closely approximates clinician-based staging.
Supplementary Material
Clinical Perspectives.
What is New?
Clinician application of the 2019 SCAI shock stages provides effective risk stratification and improves allocation of higher risk patients to advanced SCAI stages D and E compared with algorithmic data-driven assessment.
The vasoactive-inotropic score (VIS), a quantitative metric that captures the magnitude of vasoactive support, is robustly associated with in-hospital mortality and offers incremental prognostic information beyond solely the number of vasoactive agents.
Revised algorithmic application of SCAI staging, with inclusion of the VIS for discernment of escalation/de-escalation and with added granularity of the 2022 SCAI staging update, refines risk stratification and more closely aligns with clinician staging.
What are the Clinical Implications?
The SCAI staging criteria is effective regardless of the method of application (clinician vs. algorithmic) for stratification of mortality risk in cardiogenic shock.
Factors that drive differential staging between clinicians and data-driven algorithms (e.g., differences in vasoactive support) may be leveraged to further refine risk stratification of shock severity.
Use of objective quantitative parameters such as the VIS in future iterations may improve consistency of algorithmic application for clinical trials and registries.
Acknowledgements
We would like to thank the Critical Care Cardiology Trials Network executive and steering committee, the data coordinating center, and all site investigators, which are listed in the Supplement.
Sources of Funding
Dr. Patel is supported by a T32 postdoctoral training grant from the National Heart, Lung and Blood Institute (T32HL007604).
Dr. Solomon received research support from the National Institutes of Health Clinical Center intramural research funds.
Abbreviations and Acronyms
- AMI-CS
Acute myocardial infarction cardiogenic shock
- CA
Cardiac arrest
- CCCTN
Critical Care Cardiology Trials Network
- CS
Cardiogenic shock
- HF-CS
Heart failure cardiogenic shock
- MCS
Mechanical circulatory support
- SCAI
Society of Angiography and Interventions
- VIS
Vasoactive-inotropic score
Footnotes
Author Disclosures
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplemental Materials
References
- 1.Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 2.Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. 2019;12(3):e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG, et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 4.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 5.Jentzer JC, van DS, Barsness GW, et al. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J Am Coll Cardiol. 2019;74(17):2117–2128. doi: 10.1016/j.jacc.2019.07.077 [DOI] [PubMed] [Google Scholar]
- 6.Thayer KL, Zweck E, Ayouty M, et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk Among Patients With Cardiogenic Shock. Circ Heart Fail. 2020;13(9):e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawler PR, Berg DD, Park JG, et al. The Range of Cardiogenic Shock Survival by Clinical Stage: Data From the Critical Care Cardiology Trials Network Registry. Crit Care Med. 2021;49(8):1293–1302. doi: 10.1097/CCM.0000000000004948 [DOI] [PubMed] [Google Scholar]
- 8.Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96(3):E213–E219. doi: 10.1002/ccd.28707 [DOI] [PubMed] [Google Scholar]
- 9.Jentzer JC, Schrage B, Holmes DR Jr, et al. Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10(6):604–612. doi: 10.1093/ehjacc/zuaa035 [DOI] [PubMed] [Google Scholar]
- 10.Pareek N, Dworakowski R, Webb I, et al. SCAI cardiogenic shock classification after out of hospital cardiac arrest and association with outcome. Catheter Cardiovasc Interv. 2021;97(3):E288–E297. doi: 10.1002/ccd.28984 [DOI] [PubMed] [Google Scholar]
- 11.Kapur NK, Kanwar M, Sinha SS, et al. Criteria for Defining Stages of Cardiogenic Shock Severity. Journal of the American College of Cardiology. 2022;80(3):185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 12.Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter Cardiovasc Interv. 2020;96(7):1339–1347. doi: 10.1002/ccd.29319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson ID, Tagami T, Mando R, et al. SCAI shock classification in acute myocardial infarction: Insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2020;96(6):1137–1142. doi: 10.1002/ccd.29139 [DOI] [PubMed] [Google Scholar]
- 14.Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies. J Am Coll Cardiol. 2022;79(9):933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 15.Bohula EA, Katz JN, van Diepen S, et al. Demographics, Care Patterns, and Outcomes of Patients Admitted to Cardiac Intensive Care Units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019;4(9):928–935. doi: 10.1001/jamacardio.2019.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt AS, Berg DD, Bohula EA, et al. De Novo vs Acute-on-Chronic Presentations of Heart Failure-Related Cardiogenic Shock: Insights from the Critical Care Cardiology Trials Network Registry. J Card Fail. 2021;27(10):1073–1081. doi: 10.1016/j.cardfail.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc [DOI] [PubMed] [Google Scholar]
- 19.Jentzer JC, Wiley B, Bennett C, et al. Temporal Trends and Clinical Outcomes Associated with Vasopressor and Inotrope Use in The Cardiac Intensive Care Unit. Shock. 2020;53(4):452–459. doi: 10.1097/SHK.0000000000001390 [DOI] [PubMed] [Google Scholar]
- 20.Zweck E, Thayer KL, Helgestad OKL, et al. Phenotyping Cardiogenic Shock. J Am Heart Assoc. 2021;10(14):e020085. doi: 10.1161/JAHA.120.020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jentzer JC, Soussi S, Lawler PR, Kennedy JN, Kashani KB. Validation of cardiogenic shock phenotypes in a mixed cardiac intensive care unit population. Catheter Cardiovasc Interv. Published online January 25, 2022. doi: 10.1002/ccd.30103 [DOI] [PubMed] [Google Scholar]
- 22.Jentzer JC, Lawler PR, van Diepen S, et al. Systemic Inflammatory Response Syndrome Is Associated With Increased Mortality Across the Spectrum of Shock Severity in Cardiac Intensive Care Patients. Circ Cardiovasc Qual Outcomes. 2020;13(12):e006956. doi: 10.1161/CIRCOUTCOMES.120.006956 [DOI] [PubMed] [Google Scholar]
- 23.Jentzer JC, Kashani KB, Wiley BM, et al. Laboratory Markers of Acidosis and Mortality in Cardiogenic Shock: Developing a Definition of Hemometabolic Shock. Shock. 2022;57(1):31–40. doi: 10.1097/SHK.0000000000001812 [DOI] [PubMed] [Google Scholar]
- 24.Geller BJ, Sinha SS, Kapur NK, et al. Escalating and De-escalating Temporary Mechanical Circulatory Support in Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 0(0): 10.1161/CIR.0000000000001076. doi: 10.1161/CIR.0000000000001076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


