Abstract
BACKGROUND:
The gut microbiota-dependent metabolite phenylacetylgutamine (PAGln) is both associated with atherothrombotic heart disease in humans, and mechanistically linked to cardiovascular disease (CVD) pathogenesis in animal models via modulation of adrenergic receptor signaling.
METHODS:
Here we examined both clinical and mechanistic relationships between PAGln and heart failure (HF). First, we examined associations among plasma levels of PAGln and HF, left ventricular ejection fraction (LVEF), and N-terminal pro B-type natriuretic peptide (NT-proBNP) in two independent clinical cohorts of subjects undergoing coronary angiography in tertiary referral centers (an initial discovery US Cohort, n=3256; and a validation European Cohort, n=829). Then, the impact of PAGln on cardiovascular phenotypes relevant to HF in cultured cardiomyoblasts, and in vivo were also examined.
RESULTS:
Circulating PAGln levels were dose-dependently associated with HF presence and indices of severity (reduced LVEF, elevated NT-proBNP) independent of traditional risk factors and renal function in both cohorts. Beyond these clinical associations, mechanistic studies showed both PAGln and its murine counterpart, phenylacetylglycine, directly fostered HF-relevant phenotypes including decreased cardiomyocyte sarcomere contraction, and B-type natriuretic peptide gene expression in both cultured cardiomyoblasts and murine atrial tissue.
CONCLUSION:
The present studies reveal the gut microbial metabolite PAGln is clinically and mechanistically linked to HF presence and severity. Modulating the gut microbiome in general, and PAGln production specifically, may represent a potential therapeutic target for modulating HF.
REGISTRATION:
URL: https://clinicaltrials.gov/; Unique identifier: NCT00590200 and https://drks.de/drks_web/; Unique identifier: DRKS00020915
Keywords: heart failure, human commensal, metabolism, phenylacetylglutamine, gut microbiome
INTRODUCTION
The gut microbiome is known to contribute to a number of human diseases, including cardiovascular disease (CVD), and represents an as yet underappreciated endocrine organ 1–7. The mechanisms by which the gut microbial community contributes to disease processes remain largely unknown. A critical step in addressing this knowledge gap is understanding associations between gut microbiota-generated metabolites and clinical phenotypes, as well as the activity of gut microbiota-derived metabolites, which can function as non-canonical hormones. Moreover, with a better understanding of the molecular participants involved in gut microbiome-linked diseases, we may be able to design effective therapeutic strategies to combat modern lifestyle-related ailments like obesity, diabetes, and CVD.
Using untargeted metabolomics, our lab recently identified phenylacetylglutamine (PAGln), a microbiota-derived metaorganismal metabolite associated with CVD and major adverse cardiovascular events (MACE: myocardial infarction, stroke, or death) 8. Both gain- and loss-of-function studies utilizing a combination of genetic and pharmacological tools showed that PAGln signals within host cells via G-protein coupled receptors (GPCRs), including adrenergic receptors 8. It is well established that sympathetic tone is elevated in heart failure (HF), and sustained activation of the sympathetic nervous system is thought to contribute to the poor prognosis associated with HF 9. Moreover, incorporation of beta blockers into HF treatment regimens is still recommended under the current clinical guidelines 10,11. Given the direct connection between PAGln, CVD, and adrenergic signaling, we sought to examine the clinical association between PAGln and HF, and to investigate whether PAGln may foster or modulate HF relevant phenotypes. Here we show that circulating PAGln levels track with clinical HF risk and disease severity in two independent cohorts (US and European). We expand on these clinical associations to show PAGln, and its murine counterpart PAGly, directly contribute to physiological manifestations of HF-relevant phenotypes, including B-type natriuretic peptide (BNP) induction, and indirect myocardial suppressant effects (indirect because only observed in presence of a sympathetic agonist). Taken together, our findings support the idea that the gut microbiota-generated metabolite PAGln is a candidate marker of and contributor to HF associated phenotypes.
METHODS
Data availability
There are restrictions to the availability of some of the clinical data generated in the present study because we do not have permission in our informed consent from research subjects to share data other than in summary format outside our institution. Where permissible, the datasets generated and/or analyzed during the present studies, or summary data, and all methods used to conduct the research, are available from the corresponding author upon reasonable request.
Study approval
All clinical study protocols and informed consent for human subjects complied with the Declaration of Helsinki and received ethical approval by the Cleveland Clinic Institutional Review Board, or by the ethics committee of Charité-Universitätsmedizin Berlin. Written informed consent was obtained from all subjects. All animal model studies were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Genebank - US Cohort
Plasma samples and associated clinical data were collected as part of studies at a tertiary care referral center. All subjects gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved all study protocols. Metabolomics studies were performed on sequential samples from a large and well-characterized longitudinal tissue repository with associated clinical database named GeneBank at the Cleveland Clinic: Molecular Determinants of Coronary Artery Disease (GATC). GeneBank is registered under ClinicalTrials.gov Identifier: NCT00590200. It is comprised of sequential stable subjects without evidence of acute coronary syndrome (cardiac troponin I< 0.03 ng/mL) who underwent elective diagnostic coronary angiography (cardiac catheterization or coronary computed tomography) for evaluation of CAD. All subjects had extensive clinical and longitudinal outcome data monitored, including adjudicated outcomes over the ensuing 3 to 5 years after enrollment. History of HF was detected by (a) directly asking patient by research personnel, (b) reviewing medical records for confirmation (all patients were seen by cardiologist at Cleveland Clinic before the left heart catheterization), and (c) ICD codes and adjudication by research personnel 12. NT-proBNP levels were measured in all GeneBank samples by the Preventive Research Lab (PRL) - a CAP and CLIA reference lab. Measurements for NT-ProBNP were completed using the Elecsys proBNP II STAT assay on the Roche Cobas e601 analyzer. For the present studies, we leveraged access to the previously reported PAGln levels obtained using stable isotope dilution liquid chromatography tandem mass spectrometry (LC/MS/MS) from sequential consenting subjects enrolled for whom PAGln, LVEF and NT-proBNP data were available (n=3256) 8.
LipidCardio - European Cohort
Plasma samples (n=833) were also obtained from subjects enrolled in the LipidCardio at the Charité-Universitätsmedizin Berlin: Role of lipoproteins in cardiovascular disease 13. The study was approved by the local research ethics committee (approval number: EA1/135/16) under an approved protocol registered under German Clinical Trial Register (drks.de); Identifier: DRKS00020915. All participants provided written informed consent. Patients aged 18 years and older undergoing cardiac catheterization at a single large academic center (Department of Cardiology, Campus Benjamin Franklin, Charité-Universitätsmedizin Berlin), except those with troponin-positive acute coronary syndromes (ACS), were eligible for inclusion. HF was defined as New York Heart Association (NYHA) class ≥2. NT-proBNP was measured in all samples from subjects in the European Cohort using the Elecsys proBNP II STAT assay on the Roche Cobas e601 analyzer.
LC/MS/MS analysis of PAGln in human plasma samples and PAGly in mouse plasma samples
Stable-isotope-dilution LC/MS/MS was used for quantification of PAGln in human plasma (European Cohort), and PAGly in mouse plasma, as previously described 8. Briefly, ice cold methanol containing internal standard (D5-phenylacetylglutamine) was added to the plasma samples, followed by vortexing and centrifuging (14,000 rpm; 4 °C for 15 min). The clear supernatant was transferred to a to glass vials with microinserts and submitted to LC/MS/MS analysis. LC/MS/MS analysis was performed on a chromatographic system consisting of two Shimadzu LC-30 AD pumps (Nexera X2), a CTO 20AC oven operating at 30 °C, and a SIL-30 AC-MP autosampler in tandem with a triple quadruple mass spectrometer (8050 series, Shimadzu Scientific Instruments, Inc., Columbia, MD, USA). For chromatographic separation, a Kinetex C18 column (50 mm × 2.1 mm; 2.6 μm) (Cat # 00B-4462-AN, Phenomenex, Torrance, CA) was used. Solvent A (0.1% acetic acid in water) and B (0.1% acetic acid in acetonitrile) were run using the following gradient: 0.0 min(0% B); 0.0-2.0 min (0% B); 2.0-5.0 min (0%B→20%B); 5.0-6.0 min (20%B→60%B); 6.0-7.5 min (60%B→70%B); 7.5-8.0 min (70%B→100%B); 8.0-9.5 min (100%); 9.5-10 min (100%B→0%B); 10.0-15.0 min (0% B) with flow rate of 0.4 mL/min and an injection volume of 1 μL. Electrospray ionization in the positive mode was used with multiple reaction monitoring (MRM) for detection of endogenous and stable isotope labeled internal standard. The following transitions were used: m/z 265.2→130.15 for PAGln, m/z 193.8→ 76.1 for PAGly and m/z 270.1→130.15 for D5-PAGln. D5-PAGln was used as internal standard for both PAGln and PAGly. The following ion source parameters were applied: nebulizing gas flow, 3 l/min; heating gas flow, 10 l/min; interface temperature, 300 °C; desolvation line temperature, 250 °C; heat block temperature, 400°C; and drying gas flow, 10 l/min. Limit of detection (LOD) and limit of quantification (LOQ) for PAGln were 0.010 and 0.033 μM, respectively. And LOD and LOQ for PAGly were 0.021 and 0.073 μM, respectively. Quality control samples were run with each batch of samples and inter-batch variations expressed as coefficient of variation (CV) were less than 10% for all analytes monitored. Data were collected and analyzed by LabSolution 5.91 software (Shimadzu).
In vitro H9c2 studies
H9c2(2-1) cells (American Type Culture Collection, ATCC; Manassas, VA) were seeded into 10 cm plates at 60% confluency. After seeding (24h), cells were then serum starved for 18 hours. Cells were exposed to 100μM PAGln, 100μM PAGly, or equivalent volume of vehicle control for 4 hours. Following exposure, media was removed, cells washed in ice cold PBS, and 0.5 mL RNAlater was added to the plate. Cells were removed by scrapping and stored at −20 °C for RNA isolation. Prior to isolation, 1 mL of sterile PBS was added to RNAlater samples to allow for the pelleting of cells. Cells were pelleted at 10,000xg for 3 minutes. After supernatant was removed, cells were lysed in 250 μL of TRIzol (Ambion, Inc; Austin, TX) by vortexing. Chloroform (50 μl) was added to the TRIzol homogenate and samples incubated at room temperature for 3 minute. Then, tubes were centrifuged at 10,000xg for 15 minutes at 4 °C for phase separation. The aqueous layer was removed, being careful not to disturb the interface, and mixed with 150 μL isopropyl alcohol. Samples were incubated at room temperature for 10 minutes. Tubes were centrifuged at 12,000xg for 10 minutes at 4 °C for pelleting. After the supernatant was removed, RNA pellets were washed twice with 75% ethanol by vortexing and centrifuged at 7,500xg for 5 minutes at 4 °C. Supernatant was removed and pellets allowed to air-dry for 10 minutes before resuspension in DPEC treated water. Nppb (Natriuretic Peptide Precursor B gene) expression was measured using 400 ng of total RNA using the TaqMan RNA-to-Ct 1-step kit (Applied Biosystems; Waltham, MA) on the Applied Biosystems StepOnePlus Real-Time PCR System. Rn00580641_m1 (FAM) was used for detection of Nppb and Rn01775763_g1 (VIC) was used for detection of Gapdh (glyceraldehyde-3-phosphate dehydrogenase).
Animal husbandry
C57BL6/J male mice 6-10 weeks old were purchased from Laboratory (Bar Harbor, ME) and housed in the Cleveland Clinic Biological Research Unit under strict 14:10 light:dark cycles with food and water provided ad libitum. Only male mice were used to mitigate the variable of female reproductive hormones on cardiac function.
In vivo cardiac gene expression
Animals were IP injected with pH neutralized PAGln (50 mg/kg) (n=16), PAGly (50 mg/kg) (n=16) or isotonic vehicle control (n=14). PAGln and PAGly were dissolved in normal saline and neutralized with NaOH; the vehicle control solution consisted of equivalent amount of NaOH/HCl added to the normal saline. Fifteen minutes after injection, animals were euthanized by Ketamine/Xylazine overdose (300 mg/kg + 30 mg/kg); blood was collected by cardiac puncture of the right ventricle. Right after blood collection, hearts were excised from the animal and stored in RNAlater. At a later time the entire left atria was isolated from each heart. Atria were lysed in 250 μL of TRIzol by bead beating with a single 5.0 mm zirconium oxide bead. Chloroform (50 μL) was added to the TRIzol homogenate and samples incubated at room temperature for 3 minute. Tubes were centrifuged at 10,000xg for 15 minutes at 4 °C for phase separation. The aqueous layer was removed, being careful not to disturb the interface, and mixed with 150 μL isopropyl alcohol. Samples were incubated at room temperature for 10 minutes. Tubes were centrifuged at 12,000xg for 10 minutes at 4 °C for pelleting. After the supernatant was removed, RNA pellets were washed twice with 75% ethanol by vortexing and centrifuged at 10,000xg for 8 minutes at 4 °C. Supernatant was removed and pellets allowed to air-dry for 10 minutes before resuspension in DPEC treated water. Nppb expression was measured using 1.5 μL total RNA using the TaqMan RNA-to-Ct 1-step kit (Applied Biosystems; Waltham, MA) on the Applied Biosystems StepOnePlus Real-Time PCR System. Mm00435304_g1 (FAM) was used for detection of Nppb and Mm99999915_g1 (VIC) was used for detection of Gapdh. The investigators were not blinded for these studies.
Mouse cardiomyocytes contractility studies
Adult wildtype mouse cardiomyocytes were isolated and contractility studies were performed utilizing the IonOptix System (Myopace, Milton, MA) as previously described 14,15. Briefly, myocytes were isolated and plated on a glass chamber slides which was placed on a Leica microscopic stage connected to field stimulator specifically designed for driving isolated myocytes (MyoPace, IonOptix). Cardiomyocytes were stimulated at 3 Hz and imaged with a variable field-rate camera (MyoCam, IonOptix) using edge-detection and sarcomere length technology. Myocytes were treated with 10 μM of epinephrine and cardiomyocytes contractility transients were recorded in the presence or absence of 100 μM PAGln or PAGly, and pre-incubated for 15 min, before addition of epinephrine. Single sarcomere contraction cycles were recorded with time as contractility transients. Sarcomere length (μm) and sarcomere shortening (μm) were measured considering five different scanning windows.
Statistics
Human studies
The Wilcoxon rank sum test or Student’s t-test for continuous variables and chi-square test for categorical variables were used to examine the difference between the groups. Odds ratio (OR) for binary HF and corresponding 95% confidence intervals (95% CI) according to PAGIn quartiles in the US Cohort and the European Cohort were calculated using both univariable (unadjusted) and multivariable (adjusted) logistic regression models. Logistic regression model was adjusted for traditional cardiac risk factors in a multivariable model including age, sex, smoking status, systolic blood pressure (SBP), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides (TG), high sensitive C-reactive protein (hs-CRP), diabetes and obesity (in the US Cohort). Additional adjustment for estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) is calculated on the basis of the Chronic Kidney Disease Epidemiology Collaboration 2021 CKD-EPI Creatinine equation 16. The classification of heart failure (HF), according to left ventricle ejection fraction (LVEF) (HF with preserved (HFpEF; LFEF≥50%), mildly reduced (HFmrEF; 40%<LVEF<50%) and reduced (HFrEF; LVEF≤40%) ejection fraction) was defined according to the 2022 AHA/ACC/HFSA guideline for the management of HF 17. The presence of coronary artery disease (CAD) was defined by luminal stenosis of at least 50% in any major coronary artery, any history of myocardial infarction, history of percutaneous coronary intervention and/or known coronary artery bypass graft in the US cohort. In the European Cohort, CAD was defined by a luminal reduction of a major epicardial vessel >50%.
Association between PAGln and clinical measurement including LVEF or NT-proBNP levels was statistically assessed by Kruskal-Wallis test and Spearman correlation after normality testing.
Murine and cell culture studies
Murine and cell culture gene expression were assessed by one-way ANOVA test followed by Tukey’s multiple comparison post hoc for comparing vehicle control vs PAGly and vehicle control vs PAGln. In the ionotropy experiments, Kruskal-Wallis test was used for multiple comparisons and nonparametric Mann-Whitney (M.W.) test for pairwise comparisons between vehicle control with Epi, PAGly or PAGln treatments.
All analyses were performed with RStudio-R version 4.1.2. (2021-11-01) (Vienna, Austria), Stata 17.0 (Stata Corp, College Station, TX), or GraphPad Prism 9. A P-value of <0.05 was considered statistically significant.
RESULTS
PAGln is elevated in patients with HF and independently associated with HF risk.
We previously reported that plasma levels of PAGln are associated with cardiovascular disease (CVD) and incident major adverse cardiovascular events (myocardial infarction, stroke, or death) 8. In this same study, we showed through a variety of mechanistic investigations that PAGln signaled via adrenergic receptors. Since altered sympathetic tone is a key contributor to the development of HF 18,19, we sought to assess if systemic PAGln levels show clinical association with HF risk and severity. For initial analyses, we leveraged access to previously reported PAGln data (US Cohort; see Methods for details), in order to examine the association between PAGln levels, HF, LVEF, and NT-proBNP. Subject (n=3256) clinical characteristics, demographics, and laboratory values are provided in Table 1, and reveal a middle-aged cohort with high prevalence of CVD and related risk factors. Subjects with the adjudicated diagnosis of HF (characterized in Supplemental Table 1) had higher systemic levels of PAGln compared to non-HF subjects (P<0.0001; Fig. 1A). Further, individuals with HF without coronary artery disease (CAD) had higher levels of PAGln then control subjects (no-HF and no-CAD) (Fig. S1A) and individuals with CAD and HF had higher levels of PAGln than subjects with CAD alone (Fig. S1A). Moreover, an increased risk for HF was observed among those with higher circulating levels of PAGln (e.g. 4th versus 1st quartile), even following adjustments for traditional CVD risk factors and indices of kidney function (Fig. 1B).
Table 1.
Baseline characteristics of the participants stratified by heart failure (HF) status in the US Cohort (GeneBank) (n=3256)
| Characteristics | All participants (n=3256) | Participants without HF (n=2544) | Participants with HF (n=712) | P value |
|---|---|---|---|---|
| Age, mean ± SD, years | 62.8±10.8 | 61.9±10.8 | 66.1±10.5 | <0.001 |
| Male sex, n (%) | 2091 (64.2) | 1670 (65.6) | 421 (59.1) | <0.01 |
| Current smoking, n (%) | 435 (13.4) | 354 (13.9) | 81 (11.4) | 0.092 |
| Systolic blood pressure, mm Hg | 132.0 (119.0-146.0) | 133.0 (120.0-147.0) | 130.0 (116.0-145.0) | <0.001 |
| Diastolic blood pressure, mm Hg | 74.0 (67.0-82.0) | 76.0 (68.0-83.0) | 71.0 (63.0-80.0) | <0.001 |
| BMI, kg/m2 | 28.7 (25.6-32.5) | 28.7 (25.7-32.4) | 28.1 (25.1-32.9) | 0.20 |
| Diabetes, n (%) | 1031 (31.7) | 744 (29.2) | 287 (40.3) | <0.001 |
| CAD, n (%) | 2494 (76.7) | 1909 (75.1) | 585 (82.2) | <0.001 |
| HDL, mg/dL | 34.1 (28.2-41.1) | 34.6 (28.7-41.4) | 31.7 (26.0-39.7) | <0.001 |
| LDL, mg/dL | 96. 0(78.0-118.0) | 98.0 (80.0-119.0) | 91.0 (72.8-112.0) | <0.001 |
| TG, mg/dL | 119.0 (86.0-171.0) | 119.0 (85.0-174.0) | 118.5 (87.0-165.0) | 0.49 |
| hs-CRP, mg/L | 2.47 (1.08-6.03) | 2.22 (0.97-5.36) | 3.90 (1.57-9.01) | <0.001 |
| LVEF, % | 55.0 (45.0-60.0) | 55.0 (50.0-65.0) | 40.0 (25.0-55.0) | <0.001 |
| NT-proBNP, pg/mL | 239.3 (88.3-742.2) | 175.2 (71.8-453.5) | 895.0 (348.8-2040.2) | <0.001 |
| eGFR, mL/min/1.73 m2 | 91.2 (76.4-100.2) | 93.1 (80.6-101.3) | 79.8 (61.5-94.3) | <0.001 |
The cohort is comprised of sequential stable subjects without evidence of acute coronary syndrome (cardiac troponin I< 0.03 ng/mL) who underwent elective diagnostic coronary angiography (cardiac catheterization or coronary computed tomography) for evaluation of coronary artery disease (CAD).
Continuous data are presented as median (interquartile range or 25th percentile - 75th percentile), categorical variables are presented as %; BMI = body mass index HDL = high-density lipoprotein; LDL= low-density lipoprotein; TG = triglyceride; hs-CRP = high-sensitivity C-reactive protein; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro B-type natriuretic peptide; eGFR = estimated glomerular filtration rate.
The Wilcoxon–rank sum test or Welch two sample t-test for continuous variables and the χ2 test for categorical variables were used to determine significant difference between groups.
Estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) is calculated on the basis of the Chronic Kidney Disease Epidemiology Collaboration 2021 CKD-EPI Creatinine equation 16.
Fig. 1. The association of PAGln with heart failure (HF).
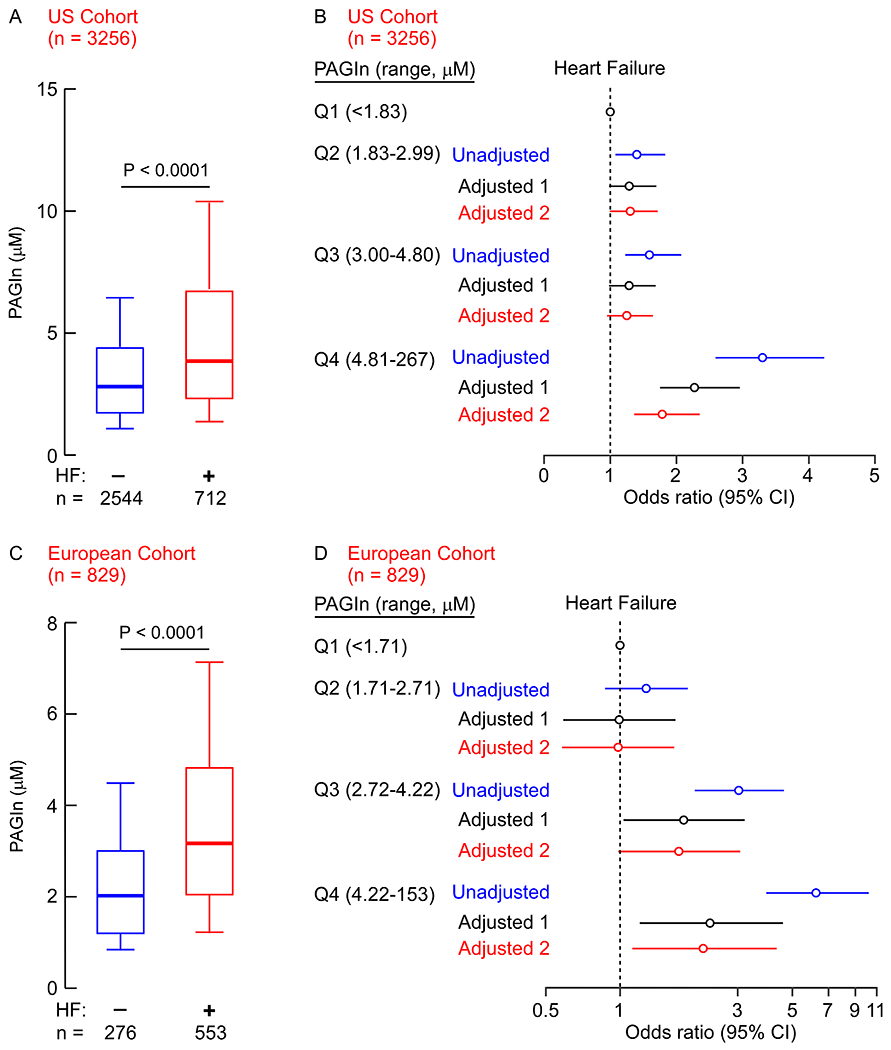
Box whisker plots of circulating PAGln levels in (A) US Cohort subjects (n=3256) or (C) European Cohort subjects (n=829) without (n=2544 and n=276, respectively) and with (n=712 and n=553, respectively) HF. Data are represented as boxplots: middle line is the median, the lower and upper hinges are the first and third quartiles, the whiskers represent 10th and 90th percentile; P values were calculated using Wilcoxon rank sum test. (B/D) Risk of HF among all test subjects according to PAGln quartile levels using a multivariable logistic regression models. Unadjusted odd ratio (blue), adjusted model 1 [age, sex, smoking status, SBP, LDL, HDL, TG, hs-CRP, diabetes and obesity (BMI≥30 kg/m2), black]; and adjusted model 2 [model 1 plus indices of renal function (eGFR≥60, or <60 mL/min per 1.73 m2), red]. Symbols represent odds ratios, and the 5%–95% confidence intervals (CI) are indicated by the line length.
We next sought to validate the observed association between circulating PAGln levels and HF risk using an independent cohort. For these analyses, we measured serum PAGln levels from subjects enrolled in a similar registry of sequential cardiology patients undergoing coronary angiography at a tertiary referral center in Europe (European Cohort; see Methods for details). Subject clinical characteristics, demographics, and laboratory values are provided in Table 2, and reveal an elderly cohort with high CVD risk factor prevalence. As observed in the US Cohort, subjects with HF in the European Cohort (characterized in Supplemental Table 1) had significantly higher serum levels of PAGln compared to those without HF (Fig. 1C). Further, individuals with HF without coronary artery disease (CAD) had higher levels of PAGln than control subjects (no-HF and no-CAD) (Fig. S1B) and individuals with CAD and HF had higher levels of PAGln than subjects with CAD alone (Fig. S1B). Moreover, circulating PAGln levels were again associated with increased risk of HF (4th vs 1st quartile), even after multivariable logistic regression adjustments for both traditional CVD risk factors and measures of kidney function (Fig. 1D). The association between elevated levels of PAGln and HF risk observed within both the US and European Cohorts also appears to hold true when individuals (combined cohort analysis) are categorized by left ventricular ejection fraction (LVEF) status (i.e. HF with preserved ejection fraction, (HFpEF) LVEF≥50; HF with mildly reduced ejection fraction, (HFmEF) 40<LVEF<50; or HF with reduced ejection fraction, HFrEF, LVEF ≤40; Fig. 2).
Table 2.
Baseline characteristics of the participants stratified by heart failure (HF) status in the European Cohort (LipidCardio) (n=829)
| Characteristics | All participants (n=829) | Participants without HF (n=276) | Participants with HF (n=553) | P value |
|---|---|---|---|---|
| Age, mean ± SD, years | 72.8±10.9 | 66.2±10.5 | 76.1±9.5 | <0.001 |
| Male sex, n (%) | 582 (70.2) | 170 (61.6) | 412 (74.3) | <0.001 |
| Smoking, n (%) | 140 (16.9) | 71 (25.7) | 69 (12.5) | <0.001 |
| Hypertension, n (%) | 667 (80.4) | 207 (75.0) | 460 (83.2) | <0.01 |
| Diabetes, n (%) | 231 (27.9) | 57 (20.7) | 174 (31.5) | <0.01 |
| CAD, n (%) | 573 (69.1) | 113 (40.9) | 460 (83.6) | <0.001 |
| HDL, mg/dL | 48.0 (39.0-60.0) | 51.0 (40.0-63.0) | 47.0 (39.0-58.0) | 0.012 |
| LDL, mg/dL | 92.0 (69.0-122.0) | 104.0 (77.0-134.0) | 87.0 (66.0-114.0) | <0.001 |
| TG, mg/dL | 118.0 (89.0-167.0) | 120.0 (91.0-166.0) | 117.0 (89.0-167.0) | 0.77 |
| hs-CRP, mg/L | 1.9 (0.8-5.1) | 1.6 (0.8-3.75) | 2.1 (0.8-5.6) | 0.059 |
| LVEF, % | 60.0 (52.0-66.0) | 65.0 (62.0-70.3) | 56.0 (48.0-63.0) | <0.001 |
| NT-proBNP, pg/mL | 313.0 (108.0-1024.0) | 126.0 (57.0-358.5) | 501.0 (173.0-1421.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 74.0 (60.3-90.7) | 84.7 (67.0-95.8) | 69.0 (56.8-85.6) | <0.001 |
The cohort is comprised of sequential stable subjects without evidence of acute coronary syndrome (cardiac troponin I< 0.03 ng/mL) who underwent elective diagnostic coronary angiography (cardiac catheterization or coronary computed tomography) for evaluation of coronary artery disease (CAD).
Continuous data are presented as median (interquartile range or 25th percentile - 75th percentile), categorical variables are presented as %; HDL = high-density lipoprotein; LDL = low-density lipoprotein; TG = triglyceride; hs-CRP = high-sensitivity C-reactive protein; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro B-type natriuretic peptide; eGFR = estimated glomerular filtration rate.
The Wilcoxon–rank sum test or Welch two sample t-test for continuous variables and the χ2 test for categorical variables were used to determine significant difference between groups.
Estimated glomerular filtration rate (eGFR) (mL/min per 1.73 m2) is calculated on the basis of the Chronic Kidney Disease Epidemiology Collaboration 2021 CKD-EPI Creatinine equation16.
Fig. 2. Box-whisker plots showing circulating PAGln levels stratified by heart failure status in the merged US and European Cohort (n=4085).
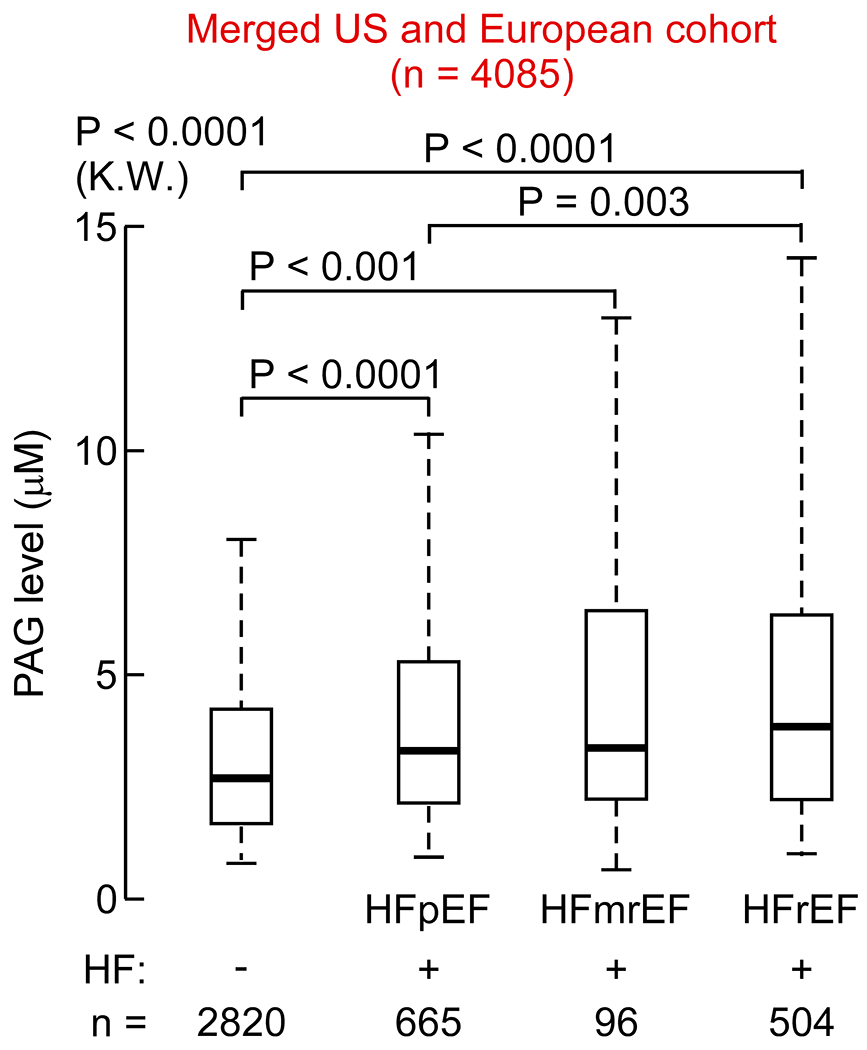
Whiskers represent 5th and 95th percentile, P values were calculated using Kruskal - Walls (K.W.) test with Dunn’s test. The classification of heart failure (HF), according to left ventricle ejection fraction (LVEF): HF with preserved ejection fraction, (HFpEF), LVEF≥50; HF with mildly reduced ejection fraction, (HFmEF), 40<LVEF<50; or HF with reduced ejection fraction, HFrEF, LVEF≤40).
Increased PAGln levels are dose-dependently associated with reduced left ventricular systolic function and subclinical indices of myocardial strain.
In both the US and European Cohorts, increasing plasma levels of PAGln were dose-dependently associated with declining LVEF (Fig. 3A, B; ρ=−0.144 (P<0.0001) and ρ=−0.185 (P<0.0001)). Similarly, increasing levels of PAGln in both the US and European Cohorts showed a dose-dependent association with increasing serum levels of N-terminal prohormone of BNP (NT-proBNP), a cardiac hormone generated in response to subclinical myocardial strain 20 (Fig. 3C,D; ρ=0.293 (P<0.0001) and ρ=0.324 (P<0.0001)). Of interest, we found that the association of PAGln with NT-proBNP remained significant in both the US and European Cohorts, when looking at the subsets of subjects with preserved left ventricular systolic function (e.g. LVEF ≥ 50; P<0.0001 (K.W.) for both cohorts, Fig. S2A,B), as well as in the subsets with normal renal function (e.g. eGFR ≥ 90 mL/min/1.73 m2; P<0.0001 and P=0.001 (K.W.) for US and EU Cohorts, respectively; Fig. S3). Moreover, an increased risk for HF was observed among those with higher circulating levels of PAGln (e.g. 4th versus 1st quartile), even following adjustments for traditional CVD risk factors, indices of kidney function and LVEF and NT-proBNP (Fig. S4).
Fig. 3. PAGln tracks with reduced ejection fraction and increased circulating levels of NT-proBNP.
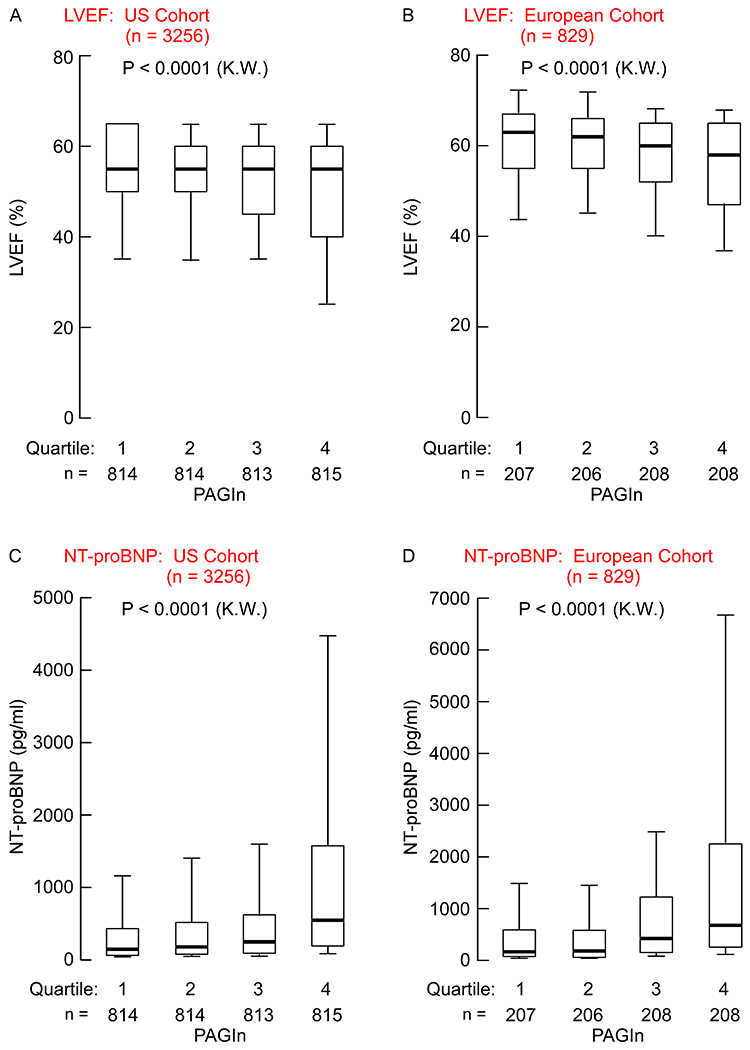
Left ventricle ejection fraction (%; LVEF) broken down by PAGln quartile in (A) US Cohort subjects (n=3256) or (B) European Cohort subjects (n=829). Circulating NT-proBNP (pg/ml) levels broken down by PAGln quartile in (C) US Cohort subjects (n=3256) and (D) Europe Cohort subjects (n=829). Data are represented as boxplots: middle line is the median, the lower and upper hinges are the first and third quartiles, the whiskers represent 10th and 90th percentile; P values were calculated using Kruskal-Wallis (K.W.) test.
PAGln, and PAGly, rapidly induce myocardial BNP gene expression.
Given the striking association observed between PAGln and NT-proBNP levels in the two clinical cohorts, we sought to determine if PAGln, or its rodent counterpart phenylacetylglycine (PAGly) 8, could directly induce BNP (Nppb) gene expression. To initially test this hypothesis in vitro, we used the immortalized rat cardiomyoblast cell line, H9c2. Following 4 h of exposure to pathophysiologically relevant levels of either PAGln or PAGly (Methods), Nppb (BNP) expression (normalized to Gapdh) was observed to be significantly increased (3.71-fold for PAGln [P=0.03], 3.66-fold for PAGly [P=0.03]) compared to vehicle treated cells (Fig. 4A). Advancing next to in vivo studies, we observed that when systemic levels of either PAGln or PAGly were acutely elevated following intraperitoneal injection (versus vehicle control), Nppb expression (normalized to Gapdh) in the left atria was significantly increased compared to vehicle treated animals (1.66- and 1.46-fold increase, P=0.01 and P=0.02, 15 min following injection of PAGln or PAGly, respectively, Fig. 4B). Intraperitoneal injection of PAGly also lead to increased Nppb expression within left ventricle (1.5 fold, P=0.001; data not shown).
Fig. 4. PAG rapidly induces BNP gene expression in vitro and in vivo.
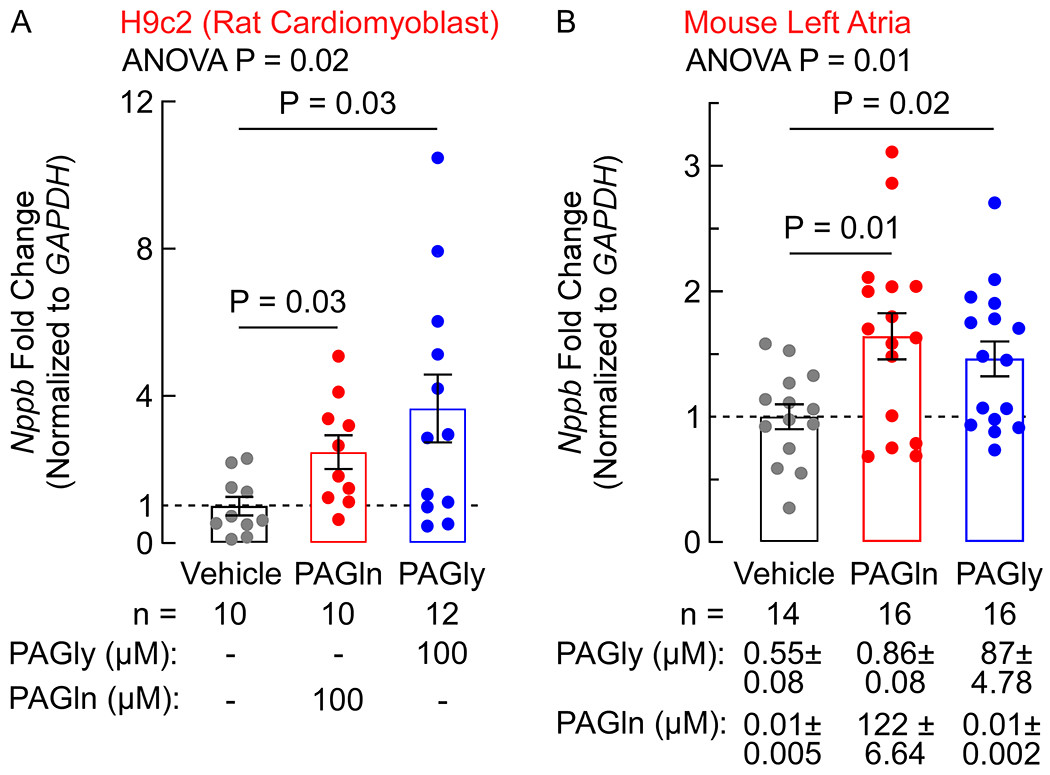
(A) Fold-change of BNP (Nppb) gene expression in H9c2 rat cardiomyoblasts, normalized to GAPDH, after 4 h exposure to 100 μM PAGln or PAGly measured in the indicated number of replicates (n=10–12 as indicated). (B) Fold-change of BNP (Nppb) gene expression in isolated left atria, normalized to GAPDH, 15 min after IP injection of PAGln (50 mg/kg), PAGly (50 mg/kg), or vehicle control measured in the indicated number of animals (n=14–16 as indicated). Circulating plasma levels of PAGln and PAGly (mean ± SEM μM) are indicated below the graph. One-way ANOVA followed by Tukey’s multiple comparison test listed. All bars represented the mean ± SEM and dots indicate single data points.
PAGln promotes a negative inotropic effect in the presence of sympathetic stimulation.
Having observed a clinical association between PAGln and reduced LVEF, we next examined the effect of PAGln or PAGly on cardiomyocyte function. When examining freshly isolated primary murine cardiomyocytes, PAGln or PAGly treatment alone did not alter the baseline shortening of cardiomyocyte sarcomere length in response to repetitive field stimulation compared to vehicle treatment (Fig. 5A–D, black vehicle versus blue line, PAGln or PAGly). Epinephrine (Epi) treatment alone resulted in significant increase in inotropic response of the primary adult murine cardiomyocytes (Fig. 5A–D, green line, Epi). However, when cardiomyocytes were incubated with both Epi and either PAGln or PAGly, the positive inotropic effect of Epi was significantly blunted, as indicated by the reduction in Epi-induced increase in contraction (sarcomere shortening) (Fig. 5A–D, red line, Epi+PAGln or Epi+PAGly). These results are consistent with PAGln (and PAGly) fostering an overall myocardial depressant effect during sympathetic stimulation.
Fig. 5. PAGln elicits negative ionotropic effect.
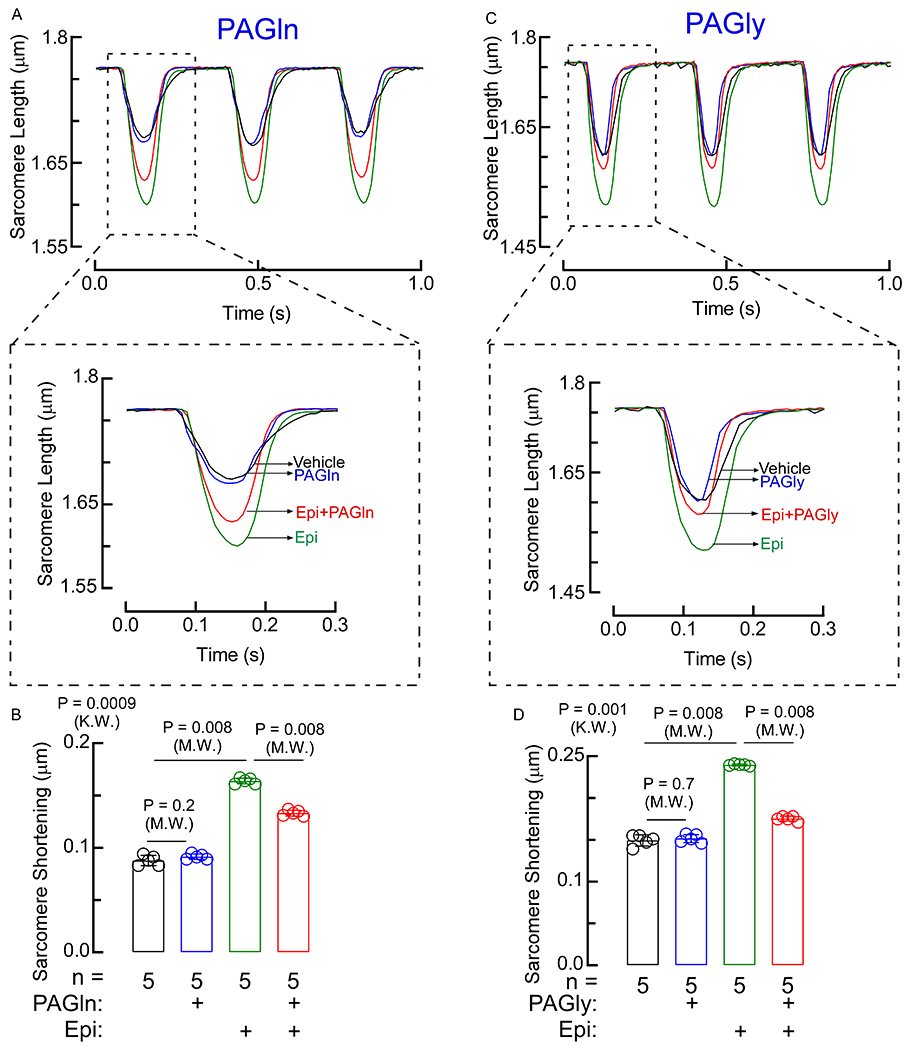
(A, C) Myograph of sarcomere contraction over ~1 second interval. Sarcomere contraction tracings of vehicle, epinephrine (Epi), PAGln, PAGly, Epi+PAGln, and Epi+PAGly were recorded over time. All tracings were trimmed so contractions were overlaid. Expanded single sarcomere contraction cycle. (B, D) Relative contraction distance represented as sarcomere shortening, was calculated considering five different scanning windows of a single sarcomere contraction cycles shown in panels A and C (n=5). Data shown represents mean ± SEM of the average maximal shortening per contraction. Nonparametric Mann-Whitney (M.W.) test was used for non-pairwise comparisons and Kruskal-Wallis (K.W.) test for multiple comparisons.
DISCUSSION
Over the past decade growing attention has been paid to a potential mechanistic link between the gut microbiome and HF development. While most studies thus far have focused either on alterations in gut microbial inhabitants in HF vs non-HF subjects 21–23, or enhanced gut leakiness that accompanies bowel wall edema, fostering systemic inflammation and potentially adverse remodeling 24, there is accumulating evidence that generation of bioactive metabolites by the intestinal microbiota can directly impact vascular and myocardial phenotypes and function 25–27. The present study adds to this latter body of evidence, and shows clinically that systemic levels of PAGln, a gut microbiota dependent metabolite, dose-dependently track both with HF risks and multiple indices of HF severity, in two independent clinical cohorts, regardless of adjustments for multiple comorbidities (Fig. 6). The association between PAGln level and HF risk held true independent of CAD status, and across the spectrum of HF phenotypes. In fact, the associations with CVD, LVEF, and NT-proBNP were also observed among the subset of subjects showing normal left ventricular systolic function and the subset with normal renal function, suggesting association between PAGln and these phenotypes occurs chronologically before clinically overt HF development or comorbidities like renal dysfunction develop.
Fig. 6. Scheme showing relationship between gut microbial PAGln pathway and heart failure.
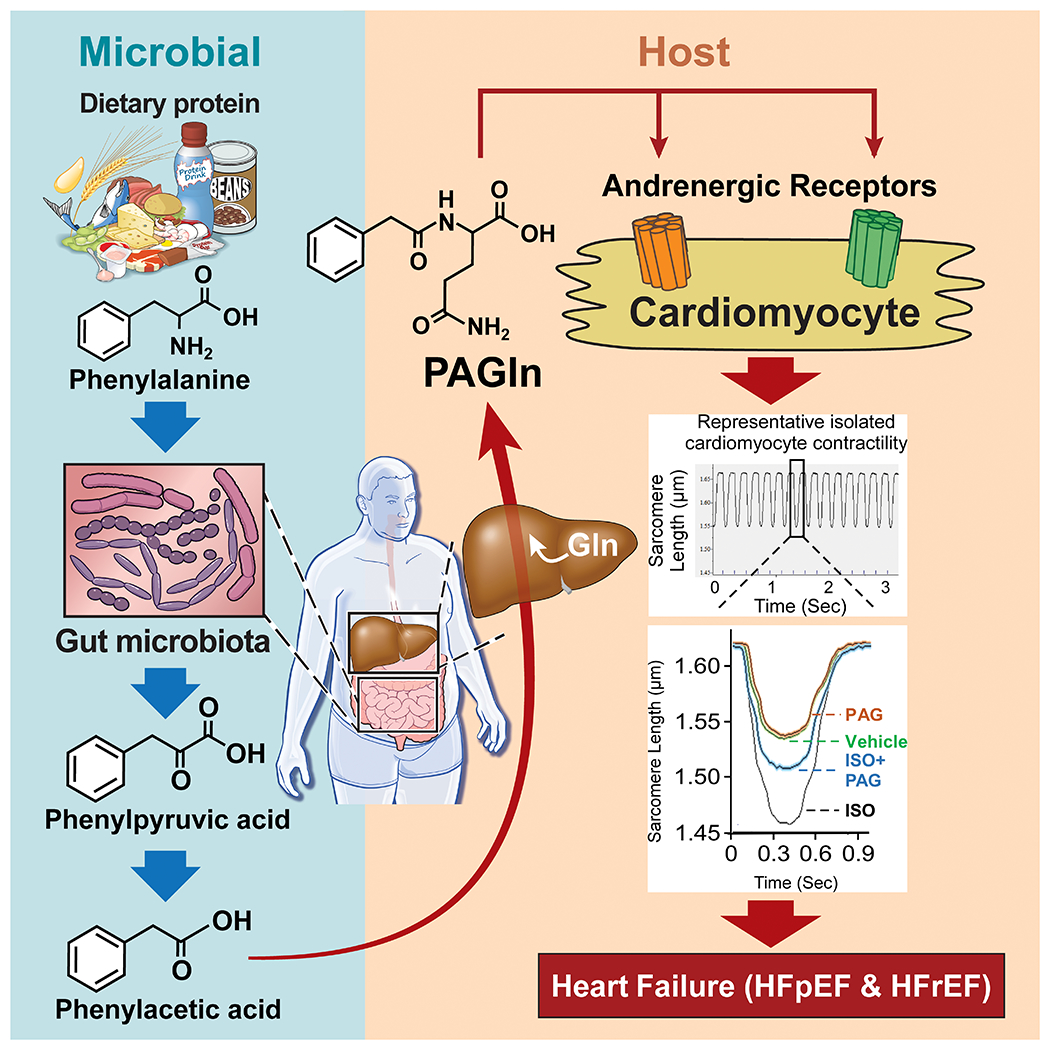
Following dietary ingestion of protein, phenylalanine is metabolized by the gut microbiome to produce phenylacetic acid. Upon absorption into the host and further conjugation, phenylacetyl glutamine (PAGln) is produced. An obligate gut-microbial metabolite, PAGln is shown to be clinically associated with heart failure presence, severity, and numerous heart failure associated phenotypes, including decreased cardiomyocyte contraction via adrenergic receptors.
Our studies further show PAGln (and PAGly) can likewise foster HF relevant biological activities that may contribute to the strong underlying clinical associations observed. Indeed, the strong associations between PAGln and NT-proBNP levels may arise because of the direct ability of PAGln to elicit transcription (enhanced Nppb RNA was observed in cardiomyoblasts in culture, and within myocardial tissues in vivo following acute infusion). Moreover, acute exposure to PAGln altered sarcomere function. While exposure to PAGln alone had minimal effect, it significantly suppressed the effect of the known agonist epinephrine, demonstrating an indirect negative ionotropic effect. In all cardiac muscles, the relationship between rate and force generation depends on the abundance of proteins involved in calcium cycling between the cytosol, myofilaments and sarcoplasmic reticulum 28. Previous reports have demonstrated that changes in circulating BNP (and/or NT-proBNP) can precede clinically overt changes in left ventricle function and serve as a strong clinical prognostic indicator associated with mortality in patients without (and with) HF 29–32. While chronic sympathetic drive is thought to, in part, underlie the pathophysiology of HF 9, epinephrine elicited effects are commonly blunted in HF myocardial tissues, as indicated by reduced contractility and chronotropic responses 33. Interestingly, PAGln did not acutely alter the baseline sarcomere function in isolated murine cardiomyocytes, but PAGln altered sarcomere function by attenuating epinephrine-induced sarcomere shortening. The mechanisms of how PAGln fosters this effect are unclear, but deserve further attention in future studies.
An improved understanding of how PAGln interacts with adrenergic receptors, thereby altering sympathetic signaling, at biochemical and molecular levels, should prove informative in future investigations. Similarly, while PAGln is known to signal through adrenergic receptors, and prior animal model studies showed that the adverse atherothrombotic effects of PAGln in a murine model could be attenuated with carvedilol 8, a widely employed beta blocker in clinical practice, the role of the multiple distinct adrenergic receptors in transmitting the phenotypes observed with PAGln in cell culture, and in vivo, remains to be explored. Indeed, there are 9 distinct adrenergic receptors with differential expression on multiple cells present in myocardial and vascular tissues. Thus far, every adrenergic receptor examined (alpha 2A, 2B, B2) has shown interaction and signaling via PAGln 8. Further studies dissecting out the contribution of distinct adrenergic receptors to different PAGln effects similarly deserves attention.
This study has several limitations. Although our clinical findings were replicated in two geographically distinct cohorts and show remarkable consistency in associations and findings, these studies still require further validation. The clinical cohorts examined were enrolled at tertiary referral centers among sequential subjects undergoing coronary angiography and are notable for their high cardiovascular risk factor burden. As angiographic cohorts, they also likely show under-representation of subjects with non-ischemic cardiomyopathies. In fact, another limitation of the present studies is that neither cohort had HF with preserved ejection fraction (HFpEF) versus HF with reduced ejection fraction (HFrEF) phenotypic characterization, nor did they have ability to address the relationship between PAGln and adverse myocardial remodeling, fibrosis, or echocardiographic myocardial strain parameters. It is also worth noting that samples measured in this study represent only fasting levels at a single time point. The role of post prandial, or serial (cumulative exposure) levels of PAGln to HF phenotypes and risks remains to be seen. In addition, dietary records were not available for participants in either cohort. As a product of microbial fermentation of dietary protein (Phe), it is tempting to speculate that a diet low in protein in general, or protein Phe specifically (e.g. such as used for treatment of subjects with phenylketonuria 34–36), might lower circulating levels of PAGln. However, virtually nothing is known about dietary patterns and their impact on systemic PAGln levels. Further, there are likely multiple microbial contributors within the gut microbial community that have capacity to produce phenylacetic acid, the precursor of PAGln. The metabolic transformation catalyzed by the protein encoded by the gut microbial porA gene has previously been shown to have the capacity to convert the Phe derived metabolite phenylypyruvic acid into phenylacetic acid 8,37. However, whether other gens/enzymes and to what capacity are involved in phenylacetic acid generation in vivo is currently unknown. In general, studies looking into the interactions between gut microbes, diet and host genotype (nutrgenomics) to guide dietary interventions in the context of cardiometabolic diseases remains an understudied area 38.
While the present studies show a strong association between PAGln levels and both HF risk and numerous HF associated phenotypes, the overall impact of PAGln on HF development is not clear. Indeed, while the negative ionotropic effect fostered by PAGln observed may underlie its association with reduced LV systolic function and reduced EF, one could argue that PAGln could have an overall beneficial effect, like beta blocker therapy in HF subjects. Similarly, it is not clear if PAGln induced BNP generation via expression of Nppb (Natriuretic Peptide Precursor B gene) is an adaptive, or maladaptive process in HF. Furthermore, the observation that PAGln levels track with NT-proBNP levels in subjects with preserved left ventricular systolic ejection fraction raises the intriguing possibility of a potential contribution of PAGln, and thus gut microbiota, in HFpEF. Finally, though PAGln is a gut-microbe dependent metabolite, its generation also relies on host conjugation of phenylacetic acid within the liver. So it is feasible that impaired hepatic phenylacetic acid conjugation among heart failure patients may contribute to observed differences in circulating PAGln levels.
The present studies raise the exciting possibility that the gut microbiome may be a participant in - and thus an attractive target for - novel therapeutics in the management of HF. Prior studies looking at an alternative gut microbial metabolite, trimethylamine-N-oxide (TMAO), showed chronic exposure (at high pathophysiological levels) contribute to myocardial fibrosis, reduced LV function, and adverse remodeling; importantly, these effects were also reversed by drugs that are specifically designed to target the gut microbial pathways that generate TMAO 39. In an analogous fashion, the present study, coupled with prior studies linking PAGln to atherothrombotic adverse phenotypes both clinically and mechanistically 8, suggest that subjects with elevated PAGln levels may respond to alternative interventions – such as those targeting microbial production of PAGln (via dietary changes or small molecule non-lethal drugs) or use of broad spectrum beta blockers. Further studies to explore these and other potential interventions that can modulate PAGln levels directly or PAGln elicited effects are warranted.
This study has several limitations. Although our clinical findings were replicated in two geographically distinct cohorts and show remarkable consistency in associations and findings, these studies still require further validation. The clinical cohorts examined were enrolled at tertiary referral centers among sequential subjects undergoing coronary angiography and are notable for their high cardiovascular risk factor burden. As angiographic cohorts, they also likely show under-representation of subjects with non-ischemic cardiomyopathies. It is worth noting that we observed no significant effects on the overall association of PAGln with HF risk when including metrics of obesity (BMI) within the overall model. Moreover, in separate analyses, we have looked at the relationship between PAGln levels and obesity within the US Cohort, where BMI data is available, and noted there was an inverse association (ρ=−0.04, P=0.028), whereby subjects with higher PAGln levels tend to have lower BMI. We therefore see no evidence that the presence of obesity affects PAGln generation, nor that the relationship between PAGln and HF risk is linked to obesity.
Supplementary Material
Commentary.
WHAT IS NEW?
Circulating levels of phenylacetylglutamine (PAGln), a small molecule produced by gut microbes from dietary proteins (phenylalanine specifically), is associated with heart failure, and heart failure associated indices of severity, in clinical cohorts from both the USA and Europe
The association between PAGln levels and heart failure are independent of cardiovascular risk factors, renal function, ejection fraction, and NT-proBNP
PAGln decreases cardiomyocyte contraction in vitro
PAGln increases expression of B-type natriuretic peptide, a marker of heart failure severity, in vitro and in vivo
WHAT ARE THE CLINICAL IMPLICATIONS?
The present studies further establish the growing link between diet, gut microbiome, and heart failure (HF) risk.
Plasma levels of PAGln dose-dependently track both with heart failure risks and multiple indices of heart failure severity, independent of CAD status, and across the spectrum of HF phenotypes, in two independent clinical cohorts, regardless of adjustments for multiple comorbidities.
Measurement of PAGln levels may provide clinical prognostic value for predicting heart failure risk independent of traditional risk factors, renal function, and NT-proBNP levels.
The associations between PAGln and HF was observed among subjects showing normal left ventricular systolic function, or normal renal function.
Source of Funding:
This work is supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (P01 HL147823, R01HL103866, R01HL126827) and the Foundation Leducq (17CVD01). K.A.R. was supported in part by training grant T32HL134622 from the National Heart, Lung, and Blood Institute (NHLBI) of the NIH. M.W. was supported by an award from the Deutsche Forschungsgemeinschaft (WI 5229/1-1). Mass spectrometry studies were performed on instrumentation housed in a facility supported in part through a Shimadzu Center of Excellence award.
Disclosures:
Dr. Hazen reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, being a paid consultant formerly for Procter & Gamble and currently with Zehna Therapeutics. He also reports having received research funds from Procter & Gamble, Zehna Therapeutics and Roche Diagnostics, and being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, a wholly owned subsidiary of Quest Diagnostics, Procter & Gamble and Zehna therapeutics. Jennifer Buffa reports having received royalty payments from Proctor & Gamble. Dr. Tang reports being a consultant for Sequana Medical A.G., Owkin Inc, Relypsa Inc, and PreCardiac Inc, having received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation - all unrelated to the subject and contents of this paper. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Nonstandard Abbreviations and Acronyms:
- BNP
B-type natriuretic peptide
- CAD
coronary artery disease
- CI
confidence intervals
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- Epi
Epinephrine
- GPCR
G-protein coupled receptor
- HDL
high-density lipoprotein cholesterol
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- hs-CRP
high sensitive C-reactive protein
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- LDL
low-density lipoprotein cholesterol
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiovascular events (myocardial infarction, stroke, or death)
- MRM
multiple reaction monitoring
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- OR
Odds ratio
- PAGln
phenylacetylgutamine
- PAGly
phenylacetylglycine
- SBP
systolic blood pressure
- TG
triglycerides
- TMAO
trimethylamine N-oxide
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162–4165. doi: 10.1172/JCI78366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JM, Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. 2015;66:343–359. doi: 10.1146/annurev-med-060513-093205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenneman AC, Rampanelli E, Yin YS, Ames J, Blaser MJ, Fliers E, Nieuwdorp M. Gut microbiota and metabolites in the pathogenesis of endocrine disease. Biochem Soc Trans. 2020;48:915–931. doi: 10.1042/BST20190686 [DOI] [PubMed] [Google Scholar]
- 5.Scott AJ, Alexander JL, Merrifield CA, Cunningham D, Jobin C, Brown R, Alverdy J, O’Keefe SJ, Gaskins HR, Teare J, et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut. 2019;68:1624–1632. doi: 10.1136/gutjnl-2019-318556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WHW, Backhed F, Landmesser U, Hazen SL. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, Cajka T, Mohan ML, Li L, Wu Y, et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell. 2020;180:862–877 e822. doi: 10.1016/j.cell.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303 [DOI] [PubMed] [Google Scholar]
- 10.Bozkurt B, Hershberger RE, Butler J, Grady KL, Heidenreich PA, Isler ML, Kirklin JK, Weintraub WS. 2021 ACC/AHA Key Data Elements and Definitions for Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circ Cardiovasc Qual Outcomes. 2021;14:e000102. doi: 10.1161/HCQ.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 12.Hammadah M, Brennan ML, Wu Y, Hazen SL, Tang WH. Usefulness of Relative Hypochromia in Risk Stratification for Nonanemic Patients With Chronic Heart Failure. Am J Cardiol. 2016;117:1299–1304. doi: 10.1016/j.amjcard.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.König M, Joshi S, Leistner DM, Landmesser U, Sinning D, Steinhagen-Thiessen E, Demuth I. Cohort profile: role of lipoproteins in cardiovascular disease-the LipidCardio study. BMJ Open. 2019;9:e030097. doi: 10.1136/bmjopen-2019-030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Wu J, Bai Y, Zhao X, Liu L. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp. 2014;87:e1357. doi: 10.3791/51357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasudevan NT, Mohan ML, Gupta MK, Hussain AK, Naga Prasad SV. Inhibition of protein phosphatase 2A activity by PI3Kgamma regulates beta-adrenergic receptor function. Mol Cell. 2011;41:636–648. doi: 10.1016/j.molcel.2011.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 18.Tang WH, Francis GS. Neurohormonal upregulation in heart failure. Heart Fail Clin. 2005;1:1–9. doi: 10.1016/j.hfc.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, D’Arrigo G, Pisano A, Bolignano D, Mallamaci F, Dell’Oro R, Quarti-Trevano F, Seravalle G, Mancia G, Zoccali C. Sympathetic neural overdrive in congestive heart failure and its correlates: systematic reviews and meta-analysis. J Hypertens. 2019;37:1746–1756. doi: 10.1097/HJH.0000000000002093 [DOI] [PubMed] [Google Scholar]
- 20.Heil B, Tang WH. Biomarkers: Their potential in the diagnosis and treatment of heart failure. Cleve Clin J Med. 2015;82:S28–35. doi: 10.3949/ccjm.82.s2.05 [DOI] [PubMed] [Google Scholar]
- 21.Beale AL, O’Donnell JA, Nakai ME, Nanayakkara S, Vizi D, Carter K, Dean E, Ribeiro RV, Yiallourou S, Carrington MJ, et al. The Gut Microbiome of Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2021;10:e020654. doi: 10.1161/JAHA.120.020654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez-Calabres E, Ortega-Hernandez A, Modrego J, Gomez-Gordo R, Caro-Vadillo A, Rodriguez-Bobada C, Gonzalez P, Gomez-Garre D. Gut Microbiota Profile Identifies Transition From Compensated Cardiac Hypertrophy to Heart Failure in Hypertensive Rats. Hypertension. 2020;76:1545–1554. doi: 10.1161/HYPERTENSIONAHA.120.15123 [DOI] [PubMed] [Google Scholar]
- 23.Mayerhofer CCK, Kummen M, Holm K, Broch K, Awoyemi A, Vestad B, Storm-Larsen C, Seljeflot I, Ueland T, Bohov P, et al. Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Fail. 2020;7:456–466. doi: 10.1002/ehf2.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2016;9:e002314. doi: 10.1161/CIRCHEARTFAILURE.115.002314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal B, Halvorsen B, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi: 10.1111/joim.12328 [DOI] [PubMed] [Google Scholar]
- 28.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 29.York MK, Gupta DK, Reynolds CF, Farber-Eger E, Wells QS, Bachmann KN, Xu M, Harrell FE Jr., Wang TJ. B-Type Natriuretic Peptide Levels and Mortality in Patients With and Without Heart Failure. J Am Coll Cardiol. 2018;71:2079–2088. doi: 10.1016/j.jacc.2018.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Apple FS, Ravkilde J, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–109. doi: 10.1161/CIRCULATIONAHA.107.185267 [DOI] [PubMed] [Google Scholar]
- 31.Grodin JL, Liebo MJ, Butler J, Metra M, Felker GM, Hernandez AF, Voors AA, McMurray JJ, Armstrong PW, O’Connor C, et al. Prognostic Implications of Changes in Amino-Terminal Pro-B-Type Natriuretic Peptide in Acute Decompensated Heart Failure: Insights From ASCEND-HF. J Card Fail. 2019;25:703–711. doi: 10.1016/j.cardfail.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 32.Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, Storrow AB, Christenson RH, Committee N. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical utilization of cardiac biomarker testing in heart failure. Clin Biochem. 2008;41:210–221. doi: 10.1016/j.clinbiochem.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firman S, Witard OC, O’Keeffe M, Ramachandran R. Dietary protein and protein substitute requirements in adults with phenylketonuria: A review of the clinical guidelines. Clin Nutr. 2021;40:702–709. doi: 10.1016/j.clnu.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 35.MacDonald A, van Wegberg AMJ, Ahring K, Beblo S, Belanger-Quintana A, Burlina A, Campistol J, Coskun T, Feillet F, Gizewska M, et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15:171. doi: 10.1186/s13023-020-01391-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, Burlina A, Campistol J, Feillet F, Gizewska M, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson JF, Allayee H, Gerszten RE, Ideraabdullah F, Kris-Etherton PM, Ordovas JM, Rimm EB, Wang TJ, Bennett BJ, American Heart Association Council on Functional G, et al. Nutrigenomics, the Microbiome, and Gene-Environment Interactions: New Directions in Cardiovascular Disease Research, Prevention, and Treatment: A Scientific Statement From the American Heart Association. Circ Cardiovasc Genet. 2016;9:291–313. doi: 10.1161/HCG.0000000000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. doi: 10.1038/s41591-018-0128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are restrictions to the availability of some of the clinical data generated in the present study because we do not have permission in our informed consent from research subjects to share data other than in summary format outside our institution. Where permissible, the datasets generated and/or analyzed during the present studies, or summary data, and all methods used to conduct the research, are available from the corresponding author upon reasonable request.


