Abstract
In this perspective, we describe how developmental improvements in youth executive function (EF) are supported by hierarchically-organized maturational changes in functional brain systems. We highlight evidence that functional brain systems are embedded within a hierarchical sensorimotor-association axis of cortical organization and that functional system development varies along this axis: systems near the associative end become more functionally segregated, while those in the middle become more integrative. Developmental changes that strengthen cortical hierarchical organization may support EF by facilitating top-down information flow and balancing within-system and between-system communication. We propose a central role for attention and control systems in the maturation of healthy EF and suggest that reduced functional system differentiation across the sensorimotor-association axis contributes to transdiagnostic EF deficits.
Keywords: Executive function, adolescence, fMRI, connectome, development, psychopathology
Executive Function is Supported by Functional Systems
Executive function (EF; see Glossary) is a broad cognitive domain that encompasses multiple subdomains including working memory, response inhibition, and set shifting. EF undergoes protracted development [1] throughout childhood and adolescence, paralleling the human brain’s protracted maturational time course. Deficits in EF during youth are associated with potentially detrimental outcomes including poorer academic performance [2], increased risk-taking behaviors [3], and reduced quality of life [4]. Moreover, EF deficits are prominent in nearly every major mental illness, suggesting that poor EF may be a transdiagnostic vulnerability factor for diverse forms of psychopathology [5]. Understanding how child and adolescent brain development either supports the emergence of EF—or is associated with risk for executive dysfunction—may inform neurodevelopmental interventions that promote healthy cognitive and psychosocial development in youth.
Building on prior work that primarily focused on individual brain regions or small subsets of regions, recent work in developmental cognitive neuroscience has begun to elucidate how large-scale functional brain organization is refined as EF develops. This work has suggested that EF relies on complex and continuous interactions within the human functional connectome, defined as the dynamic set of all functional interactions across the brain. In this review, we focus on the functional connectome of the cerebral cortex, which undergoes a markedly prolonged developmental course. The cortex’s functional connectome can be decomposed into distinct functional systems that exhibit synchronized fluctuations in cortical activity during rest and task, and that are thus understood to be functionally connected. These macroscale, functional connectivity-defined systems—including for example somatomotor, ventral attention, frontoparietal, and default mode systems—support dissociable yet overlapping cognitive and behavioral functions. As described in the following sections, studies of EF have highlighted the importance of both within-system functional connections for supporting specialized processing as well as between-system functional connections for enabling flexible communication and information integration.
The capacity for individual functional systems to make unique contributions to human faculties in general, and to EF in particular, emerges in part due to differences in their connectivity profiles and intrinsic neurobiological properties. In adulthood, such differences are most pronounced between lower-order functional systems (e.g., visual and somatomotor systems) and higher-order systems (e.g., frontoparietal control and default mode systems), with graded system variation being patterned along the brain’s sensorimotor-association (S-A) axis. The S-A axis is an evolutionarily-rooted axis of macroscale brain organization that spans continuously and hierarchically from unimodal cortices to transmodal cortices. Diverse neurobiological features exhibit clear spatial variability along this S-A axis [6], and functional system properties systematically adhere to this dominant organizational motif [7]. Understanding how functional systems are embedded within the S-A axis offers insight into both within-system specialization and between-system integrative communication—two critical components of EF. Further, understanding how the brain’s functional connectome becomes aligned with the S-A axis, when during youth this alignment occurs, and how it imbues functional systems with the capacity to execute specialized and integrative hierarchical processing may provide a new perspective on the development of EF.
Here, we review recent evidence supporting the theory that healthy EF development depends on coordinated changes in large-scale functional organization during childhood and adolescence, and we place this evidence within a hierarchical neurodevelopmental framework. We first describe how functional connectome organization coheres with the organization of the cortex’s S-A axis. Next, we detail evidence that maturational changes within and between functional systems depend on a system’s position along the S-A axis; these changes ensure that the functional connectome becomes increasingly aligned with the S-A axis and more capable of supporting mature EF with age. We further propose that middle-axis control and attention systems may have a unique role in coordinating developmental changes across the functional connectome and in supporting both hierarchical signal propagation and global, multi-system integration. Finally, we discuss the implications of diminished connectome differentiation along the S-A axis for psychopathology.
Functional Systems are Embedded within the Sensorimotor-Association Axis
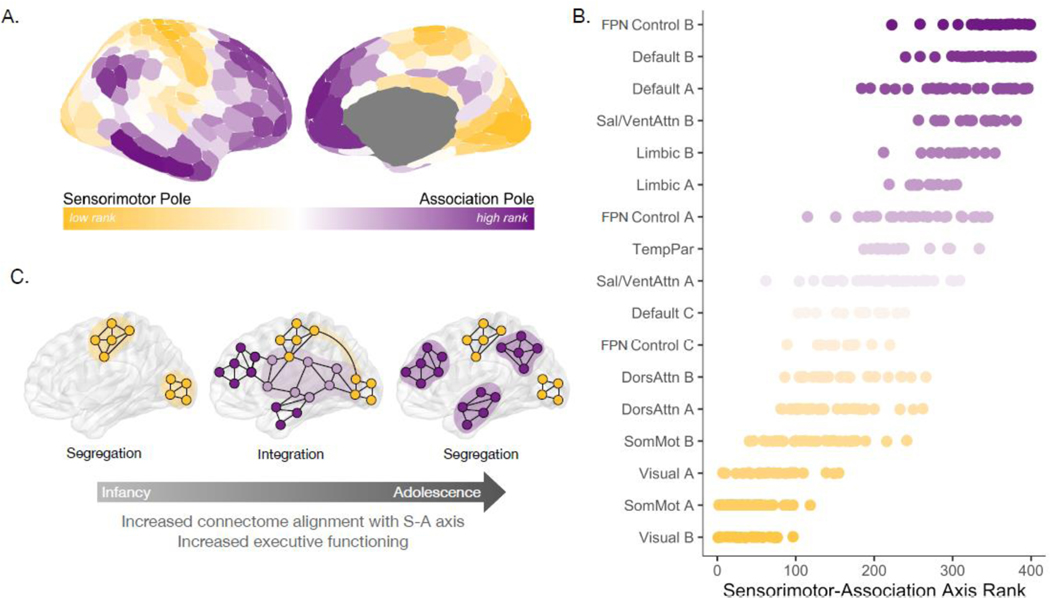
Organizational differences present across the cortical functional connectome are embedded within the cortex’s S-A axis, a dominant, large-scale axis of human brain organization that spans from primary to higher-order cortices (Figure 1A). The S-A axis captures across-cortex variability in diverse structural, metabolic, molecular, genetic, functional, and connectivity properties [6–10], and thus represents the stereotyped patterning of cortical heterogeneity along a common spatial dimension [6]. The ordering of regions within the S-A axis provides insight into which cortical areas are characterized by similar versus disparate properties; cortical regions that fall close to each other on the S-A axis exhibit similar features, yet can be located physically near or distant from each other on the cortex. Critically, cortical regions that belong to the same functional system tend to fall in nearby positions along the S-A axis (Figure 1B), demonstrating that functional systems systematically adhere to this dominant organizational pattern [7]. Visual, motor, and somatosensory systems define the sensorimotor end of the axis; dorsal attention, ventral attention, and frontoparietal control (FPN Control A and FPN C) systems tend to span the middle of the axis; whereas higher-order control subsystems (FPN Control B) along with limbic and default mode systems occupy the most associative end of the axis. Accordingly, the S-A axis is arranged as a cortical hierarchy, spanning from systems that produce perception and action, to those supporting attention and decision making, and then to systems strongly linked to socioemotional processing and internal mentation [7,11]. This organization captures a transition from concrete, extrinsic processing to abstract, intrinsic processing [7,10,11].
Figure 1. Functional connectome development proceeds along the hierarchical sensorimotor-association axis.
A) The sensorimotor-association (S-A) axis extends from primary regions at the sensorimotor pole (low hierarchical rank) to transmodal regions at the association pole (high hierarchical rank). This axis [6] captures patterns of variation across multiple cortical features, including variation in cortico-cortical connectivity architecture. B) Functional systems are organized along the S-A axis. Four hundred individual cortical regions (Schaefer400 atlas) were ranked along the S-A axis and assigned to one of 17 functional systems [95]. The scatterplot shows regional S-A axis ranks (data points) for regions that comprise each functional system; functional systems are ordered along the y-axis by median S-A axis rank. Note that at different scales, a given system (e.g., the frontoparietal network control system, denoted “FPN Control”) may be sub-divided into sub-systems that lie in different segments of the S-A axis (e.g., FPN Control B is situated at the most associative pole of the S-A axis while FPN Control A and FPN Control C are situated in the middle of the S-A axis). C) Protracted development of the functional connectome from childhood to adulthood proceeds along the S-A axis, and may be characterized by early segregation of low-rank sensorimotor systems in infancy, integration between middle axis systems and sensorimotor systems in childhood and adolescence, and prolonged segregation of high-rank association systems through early adulthood. Note that this illustration describes a characteristic pattern of functional system maturation along the S-A axis but does not illustrate the full complexity of connectome development. As such, we have illustrated time periods and systems in which there may be predominant segregation or integration but acknowledge that these changes do not play out as isolated processes.
The grouping of cortices that belong to the same functional system within a similar section of the S-A axis indicates that the functional connectome exhibits greater feature similarity within than between systems, providing insight into the neurobiological underpinnings of functional system specialization. For example, variance in gene expression [12,13] and neurotransmitter receptor expression [14] is captured by the S-A axis. As a result, the molecular environment and chemical signals acting within each system are to some extent differentiable [15]. Local, activity-related properties also vary in a graded manner between systems, including the excitation:inhibition ratio [6,16], the timescale of neural encoding [17–19], and structure-function coupling [20–22]. Traversing the S-A axis from the sensorimotor to the associative pole, levels of recurrent excitation increase, encoding timescales lengthen, and structure-function coupling decreases. These feature gradations endow transmodal associative functional systems with more prolonged, flexible, and synergistic [23] patterns of activity. Observed variation in cortical features across the S-A axis provides insight into how diverse cognitive functions emerge from small but concerted changes in neurobiological properties among functional systems.
In addition to aligning with spatial variability in molecular, cellular, and electrophysiological features, the S-A axis also captures distinct variation in cortico-cortical functional connectivity features. Regions at the sensorimotor end of the axis tend to functionally connect to local cortical areas whereas cortices in the upper third of the axis typically display a preference for longer-distance functional connections that are associated with higher physical costs and metabolic demand [24–26]. Moreover, cortices in the upper-middle of the axis generally show the most diverse and integrative connectivity profiles [27,28] due to the presence of connections that are well distributed across the cortex [29]—and thus putatively across the S-A axis. Importantly, graded changes in functional connectivity properties across the S-A axis endow functional systems with distinct forms of communication, with middle axis systems communicating over a range of distances to more diverse portions of the cortical hierarchy than systems at the poles. As described in the following sections, connectivity differences across the S-A axis develop in a protracted manner throughout childhood and adolescence as the organization of the functional connectome takes shape and EF develops. These changes within and between functional systems lead to greater alignment of the functional connectome with the S-A axis with age, creating a balance between functional system specialization and across-system communication that supports mature EF.
Functional System Segregation and Integration Along the S-A Axis
Each functional system undergoes a unique pattern of change during development, with some functional systems gaining stronger within-system connections and others developing stronger between-system connections with age (Figure 1C). Studies in adults have shown that systems at either pole of the S-A axis—i.e. sensorimotor systems and the default mode system—are characterized by a segregated pattern of functional connectivity, with substantial within-system connectivity and relatively more limited between-system connectivity [30]. In contrast, middle axis systems consistently recruited during EF tasks typically show a more integrated connectivity pattern, with greater between-system connectivity in adulthood driven by mid-distance and long-distance connections [20,25] (Figure 2A). Of note, across studies, various measures have been used to operationalize segregation and integration, as described in Box 1. The systems-level patterns of functional connectivity (and functional topography; see Box 2) seen in adulthood can be understood as the result of a developmental program that unfolds across the S-A axis.
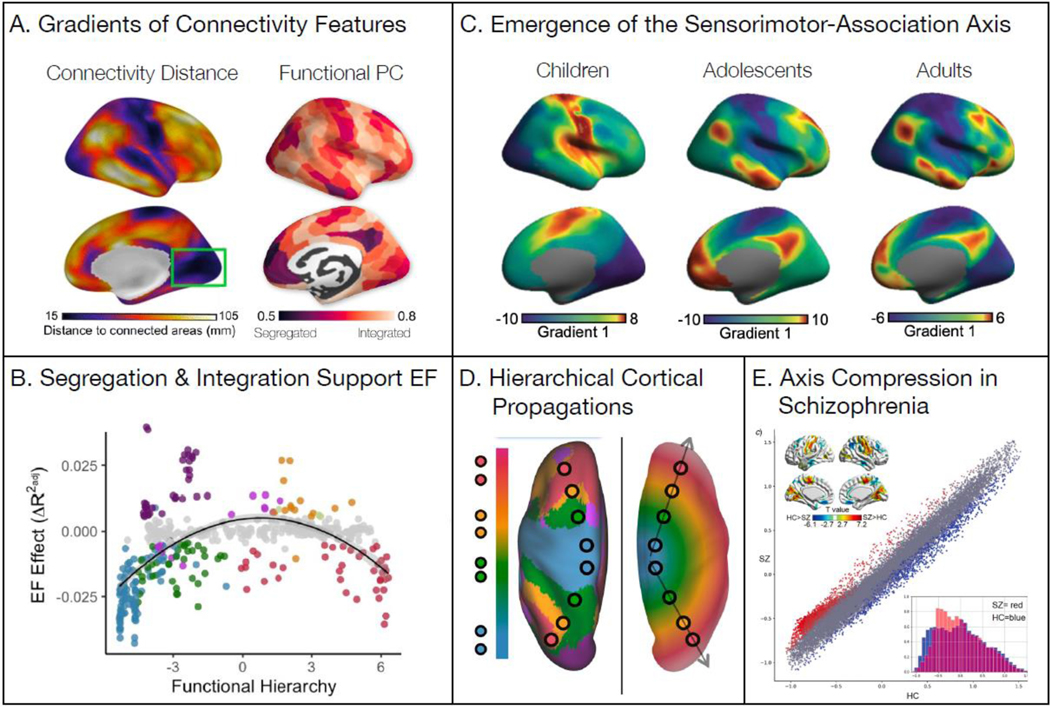
Figure 2. Outcomes of functional system organization along the S-A axis.
The organization and development of functional systems along the S-A axis is associated with improved executive function and a healthy functional connectome. A) From the functional connectivity between cortical regions, connectivity distance and participation coefficient were calculated. Studies find that both vary along the S-A axis (Images reproduced from [25] and [20]). B) Increased between-system coupling (integration; positive EF effects) at the middle of the S-A axis is positively associated with better EF, while decreased between-system coupling (segregation; negative EF effects) at the sensorimotor and association poles is associated with better EF (Reproduced from [44]). C) The principal gradient of functional connectivity becomes increasingly aligned with the S-A axis with age, shifting from a visualmotor gradient in children to the S-A axis in adolescents and adults (Reproduced from [65]). D) Cortical activity waves propagate along the principal gradient of functional connectivity. The left image shows the principal gradient of functional connectivity in canonical functional connectivity networks along the S-A axis. The right image shows how traveling waves of cortical activity may be associated with the emergence of the functional connectivity gradient through the generation of a topographic spatial gradient of time delays (phase shifts) in activity (Reproduced from [68]). E) Individuals with schizophrenia (SZ) show a compression of the S-A axis compared to healthy controls (HC). The principal gradient is depicted for SZ on the y-axis and HC on the x-axis. Significant group differences are shown in the scatterplot colors (higher in SZ: red; lower in SZ: blue) and in the brain maps in the top-left corner. Gradient compression (SZ: red; HC: blue) is highlighted in the density histograms in the bottom-right corner (Reproduced from [92]).
Box 1: Measures used to operationalize functional system segregation and integration.
Functional system segregation and integration have been operationalized using a range of statistical measures in prior work. Here, we describe the measures most frequently employed in the developmental literature, although we note that this list is not exhaustive. Many studies have used direct measures of within-system functional connectivity and between-system functional connectivity to represent segregation and integration, respectively [33, 34, 40, 44, 48, 50]. A modified connectivity measure has also been put forward [37] that measures the difference between within-system and between-system connectivity divided by within-system connectivity; values greater than zero reflect increased segregation and values lower than zero reflect diminished segregation. This measure has since been adopted in a number of studies [45, 46, 52]. Other studies have operationalized segregation as a decrease in short-range connectivity and integration as an increase in long-range connectivity [31], or have defined segregation as an increase in anti-correlations between systems (e.g., between default mode and central executive systems) [33].
Additional work has quantified segregation and integration by applying graph theory measures to functional connectivity data (for a recent review, see [51]). The participation coefficient, which quantifies the degree to which a node’s connections are non-uniformly distributed across the graph’s modules, is perhaps the most commonly used graph theoretical measure of integration [27, 28, 30, 34, 36, 45, 50, 76]. The modularity quality index (often abbreviated as simply the modularity) is commonly used as a measure of segregation; the most common index used assesses the density of connectivity within modules [45, 63]. Modularity maximization algorithms are often used to define functional systems (unlike other measures which require pre-defined systems); the modularity quality index is maximized by varying the partition of nodes into modules. Clustering coefficient, another measure of functional system segregation, assesses the degree to which a node’s neighbors in the graph are also connected to one another [43, 45]. Other studies using graph theoretical measures of functional system organization have quantified integration by a measure of efficiency [30, 53], which captures the extent to which a node’s neighborhood is connected in such a way as to facilitate relatively short paths from node to node.
Box 2: The topographic organization of functional systems varies across the S-A Axis.
Prior studies in humans using fMRI have typically utilized standardized atlases of functional systems [30,95] that assume a one-to-one correspondence between structural and functional neuroanatomy across individuals. However, multiple independent efforts using precision functional mapping techniques have demonstrated that there is important inter-individual variation in functional topography [96,101,102](Figure IA), which describes the spatial distribution of functional systems on the cortex. Notably, the greatest variability in functional topography in adults is present in the higherorder systems that are flexibly engaged by many cognitive tasks, such as the frontoparietal control systems [96,103]. Group-level atlases that fail to account for such individual variation in functional topography can alias spatial effects into the measurement of inter-regional functional connectivity [104].
An alternative to using group-level atlases is to derive personalized functional systems (also referred to as “precision functional networks”) that represent the spatial organization of functional systems in individual brains. These individually-defined systems are highly stable within individuals and predict an individual’s spatial pattern of activation on fMRI tasks [96,105]. The first study of individually-defined functional systems in youth found that inter-individual variability in functional topography was not homogeneous across the S-A axis, but rather highest in association systems [97] (Figure IB), consistent with prior work in adults. The association cortex’s functional topography was refined during development (Figure IC) and was associated with individual differences in EF (Figure ID), with the largest effects observed in frontoparietal, ventral attention, and default mode systems. Although the total spatial extent of these systems remained relatively consistent with age, local changes in system assignment revealed spatial reconfiguration: maturational changes were frequently concentrated at system boundaries, suggesting that functional system borders are refined during development.
Accumulating evidence suggests that functional systems near the associative pole of the S-A axis—especially the default mode system—undergo a protracted process of segregation, with preferential development of within-system connectivity and weakening of between-system connectivity. Notably, segregation of associative systems consistently correlates with improvements in EF [31–37]. A similar developmental pattern can be observed at the sensorimotor end of the S-A axis, though the developmental timing of sensorimotor system segregation appears to be non-linear [35,38–40]. Sensorimotor systems are apparent early in life and undergo segregation in very early developmental stages (i.e., in infancy and early childhood [41–43]). Recent evidence suggests that sensorimotor system segregation may peak in mid-childhood, followed by a decline of segregation in adolescence and early adulthood; in these later developmental stages, sensorimotor systems become more strongly inter-connected with one another [44] and with the dorsal and ventral attention systems [45]. This loss of sensorimotor system segregation in late stages of development is similarly observed in studies of aging and neurodegeneration, which have documented declines in the segregation of both sensorimotor and association systems with older age. Loss of segregation in late life has also been linked to cognitive decline [37,46–48]. Sensorimotor system segregation (and subsequent segregation loss) thus occurs heterochronously across the lifespan, leading to conflicting findings in the developmental literature depending on the age of the study population and the resolution at which these systems are defined [44]. Nonetheless, across all stages of development, sensorimotor system segregation has been linked to better executive functioning. Accordingly, segregation of functional systems at both ends of the S-A axis facilitates EF, potentially by separating stimulus-driven representations of the external environment from internally-directed cognition.
Unlike the poles of the axis, functional systems in the middle of the S-A axis may undergo a relatively greater degree of integration as they mature, developing stronger functional connections with other systems and likely facilitating between-system communication [27,49–51] in a manner that supports EF [52]. Indeed, the very functional systems that are thought to be most important for the diverse forms of cognition that facilitate EF, especially the frontoparietal control and ventral attention systems, are composed of cortices that lie at the center or upper third of the S-A axis and include regions that belong to the cortex’s diverse club [28,53,54]. In adulthood, frontoparietal control and ventral attention systems have a diverse pattern of connectivity that is spread across many other functional systems [28,54,55], and they are flexibly engaged by a wide range of cognitive tasks [56–58]. Moreover—in contrast to systems at the S-A axis poles—greater integration of ventral attention and middle-axis frontoparietal control systems is associated with better EF in youth [44](Figure 2B).
Balancing Functional System Segregation and Integration
As described above, prior studies have shown that during cortical development, functional systems display dissociable patterns of segregation versus integration across the S-A axis. Both segregation at the ends of the axis and integration in its center are associated with improvements in EF—suggesting that both within-system communication and between-system coordination support the diverse cognitive processes that enable healthy EF. Indeed, segregation supports efficient information transmission [32,59] and provides pathways for parallel computations, thus facilitating specialized processing [60]. In contrast, integration represents increased cross-system communication, thereby enabling complex combinatorial processing [28], flexibility [56], and adaptability [61], and providing the human brain with an extensive functional repertoire.
A balance between segregation and integration is therefore thought to simultaneously enable both parallel and integrative multistaged processing. In a recent large-scale study, segregation of functional systems was found to be important for processing speed and crystalized intelligence, integration was critical for general cognition, and a balance between segregation and integration was associated with improved memory [62,63]. Complementing these findings, an independent study conducted a spatiotemporal principal components analysis of fMRI activity acquired during diverse cognitive task performance and found that the primary component of cortical activity reflected a generalized task-dominant signal [64]. This signal engaged highly connected, integrative brain regions across all cognitive tasks. Subsequent components of cortical activity engaged relatively more segregated regions and were more tightly related to the demands of specific cognitive tasks. Hence, connectivity profiles that enable both functional system specialization and global system communication may allow for both general and specific aspects of EF. Of note, in all studies of functional connectivity but especially those addressing development and cognitive effects, it is critical to address ongoing challenges with data quality and reproducibility (Box 3).
Box 3: The ongoing challenge of in-scanner motion.
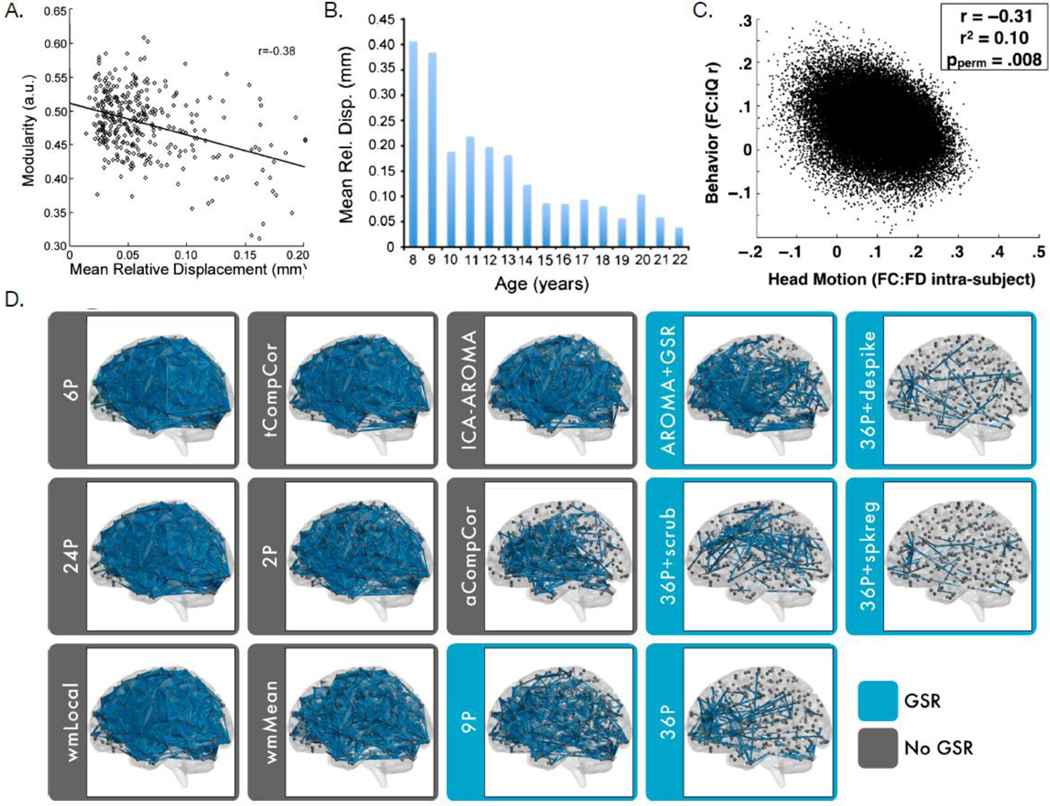
While functional connectivity provides a powerful tool to study connectome development relevant for EF, data quality related to in-scanner motion remains an important challenge that has the potential to drive observed results. In 2012, three independent studies simultaneously demonstrated that small amounts of in-scanner motion can systematically bias estimates of functional connectivity [98,106,107]. This systematic bias is particularly important for studies of development, as age is strongly related to the presence of in-scanner motion: younger children tend to move more in the scanner than older children and adults [98]. Moreover, subsequent studies have demonstrated that inscanner motion is not only related to age, but also related to diverse demographic, cognitive, and clinical variables of interest—including EF [99]. Notably, the effect size of motion on functional connectivity dwarfs the size of developmental or cognitive effects. As such, uncontrolled motion artifact has the potential to confound inference regarding both connectome development and associations with EF.
Many techniques have now been introduced that attempt to denoise fMRI data and limit the impact of in-scanner motion on estimates of functional connectivity. Benchmarking studies have established that there is substantial heterogeneity in the efficacy of denoising methods [108]; notably, even the most effective methods do not completely remove the impact of in-scanner motion. As such, it is recommended practice to use an effective denoising method and to evaluate the impact of any residual artifact as part of statistical models in hypothesis testing. Studies using these conservative procedures have demonstrated that adequately controlling for motion dramatically attenuates previously reported distance-dependent changes in functional connectivity associated with development, while age-related increases in system segregation remain robust [109,110] (Figure I).
Top-Down and Bottom-Up Cortical Propagations Traverse the S-A Axis
As each system’s functional connections are refined during youth, the functional connectome becomes increasingly aligned with the S-A axis. As a result, there is a switch in the principal axis of cortical functional connectivity organization from childhood to adulthood. Specifically, the principal gradient that explains the most variance in functional connectivity profiles in children tends to be a visual-to-motor gradient, whereas in adults the principal gradient is hierarchical, aligning with the adult gradient [7] and the S-A axis [65] (Figure 2C). This result coheres with an independent study [66] demonstrating that the brain’s hierarchical functional connectivity gradient expands during the first three decades of life, as well as with work showing that patterns of functional connectivity at the poles of the S-A axis increasingly diverge with age [44]. Thus, functional connectivity profiles of the visual and motor systems become more distinct from each other earlier in development, whereas associative system connectivity differentiates over a longer period of time.
The emergence of hierarchically organized variation in connectivity patterns along the S-A axis may engender a shift in the spatio-temporal coordination of information flow across the functional connectome, with this flow being preferentially hierarchical. Hierarchical information flow appears to be visible as directionally constrained cortical propagations of activity that traverse the S-A axis [67–69]. In a recent study of cortical activity propagations in youth [70], it was demonstrated that directional cortical propagations of BOLD activity defined by optical flow preferentially travel along the S-A axis in either a top-down (from association to sensorimotor cortices) or bottom-up (from sensorimotor to association cortices) manner. These cortical propagations may therefore represent a potential mechanism by which cortical processing makes use of a connectivity infrastructure that is hierarchically organized [67–69] (Figure 2D).
Hierarchical information processing along the S-A axis in both bottom-up and top-down directions is thought to be important for EF [11,71]. For example, bottom-up flow allows perceptual information about the external world to be communicated from specialized sensorimotor systems to association systems that influence attention and integrate cross-modal representations. Top-down input from limbic, default, and frontoparietal control systems allows actions to be aligned with goals, motivations, and self-referential representations that are typically decoupled from sensorimotor contingencies. These top-down functions such as planning and decision-making improve during development and are critical for mature EF. Strikingly, a recent, currently unpublished study found that top-down propagations of BOLD activity along the S-A axis (traversing from the association to the sensorimotor pole) increased both during EF task performance and during development [70]. Together, these findings demonstrate that as the S-A axis becomes the dominant pattern of cortical functional connectivity organization in youth, cortical activity propagates along this axis and top-down propagations become more frequent. These changes in functional connectome organization and communication may support healthy EF. Future studies may further investigate whether top-down cortical propagations along the S-A axis are central to developmental enhancements in EF (Outstanding Questions).
Outstanding Questions.
Are within-individual longitudinal changes in the alignment of the functional connectome with the S-A axis associated with improvements in EF?
How do different early life environments and experiences shape the development of the functional connectome?
What factors affect the pace and timing of functional connectome development throughout childhood and adolescence?
How does the development of the functional connectome relate to an individual’s risk for the onset of psychopathology, particularly as children transition to adolescence?
Do middle-axis attention and control systems play a causal role in coordinating the development of large-scale functional connectome organization?
Are top-down cortical propagations along the S-A axis causally related to performance on EF tasks?
Can we link developmental changes in chemical signaling within different neurotransmitter systems to developmental refinement of cortical functional connectivity?
Does the development of functional or structural connectivity between cortical and subcortical regions also follow a sensorimotor-to-association pattern?
How do individual differences in functional connectome organization relate to individual differences in other domains of cognition (e.g., social cognition)?
A Potentially Critical Role for Middle-Axis Attention and Control Systems
Current studies point to the possibility that functional systems in the middle of the S-A axis—in particular, the ventral attention and certain frontoparietal control systems (e.g., FPN Control A and FPN Control C in Figure 1B)—play an important role in driving the development of large-scale functional connectome organization and across-system communication. Two new lines of evidence lend support to this hypothesis, which may be further tested in future studies. First, the developmental switch in the principal gradient of functional connectivity from a visual-motor gradient to a gradient that aligns with the S-A axis appears to be driven in large part by connectivity changes in the ventral attention system according to a recent, currently unpublished study [72]. This finding is in line with the observation that ventral attention and frontoparietal control systems undergo substantial reorganization in their functional connectivity profiles during youth: as the ventral attention system develops, its connectivity becomes more clearly differentiated from frontoparietal and dorsal attention systems [73]. This differentiation temporally coincides with the development of adult-like attention abilities, characterized by a shift from primarily bottom-up stimulus-driven attention to more topdown goal-directed attention.
Second, middle-axis attention and frontoparietal control systems are well-positioned to facilitate hierarchical information flow along the S-A axis. These systems sit at a critical transition between cortices that carry out externally-oriented processes (e.g., perception) and cortices dedicated to internally-oriented processes (e.g., planning or self-referential thought) [7,53]. The geographic centrality of these systems may allow them to facilitate signal propagation along the S-A axis in either the top-down or bottom-up direction [7,68], thereby providing cortex-wide multi-system coordination that could support complex cognitive tasks such as those that require EF [74]. These functions are in line with the known roles of middle-axis attention systems in both stimulus-driven attention [57] and goal-directed attention [75], which inherently require bottom-up and top-down information flow.
Interestingly, increases in pupil diameter—a measure associated with increased attention—have been linked with the influence of middle-axis systems on both global functional system integration [76,77] and the hierarchical flow of activity across the S-A axis [68]. For example, increasing attentional load during a motion-tracking task led to increased functional system integration (higher participation coefficient) in a manner that directly tracked increases in pupil diameter [76]. Together, neurodevelopment and pupillometry studies provide evidence for an important role of functional systems that support attention, and thus of the ascending arousal system in particular [78], in the organization of and communication across the functional connectome. Notably, the integrative nature of ventral attention and frontoparietal control systems, while beneficial for EF, can come at a cost. On the one hand, because these systems are densely functionally interconnected with other systems, they are able to shift the functional connectome to difficult-to-reach states important for higher-order cognition [79]. On the other hand, this property also renders middle-axis systems particularly vulnerable: virtual lesion analyses have shown that disruptions to these systems could have wider-reaching effects across the functional connectome than disruptions to other systems [53]. These results collectively suggest that the development of attention and control systems might be important for the healthy development of overall EF.
Future studies may test this hypothesis in a number of ways. First, future research could test the importance of middle-axis systems for EF by examining their role in predictive models of EF in youth. We hypothesize that removing these systems from models that use connectivity features to predict EF would be associated with the largest decrements in prediction accuracy. Second, simulation studies could test the effects of removing middle-axis systems on graph theoretical measures such as global efficiency or controllability. We predict that removing these systems would result in longer path lengths and greater energy expenditure for a signal to travel between the poles of the S-A axis. Third, longitudinal studies in youth could evaluate whether the extent and rate of functional maturation of middle-axis systems (e.g., their increasing integration with lower-order and higher-order systems) are linked to the magnitude and rate of EF maturation. Fourth, neurostimulation studies could test whether stimulation of middle-axis attention and control systems lead to greater improvements on tasks requiring EF than stimulation of other functional systems. Together, such studies could corroborate existing evidence pointing toward a potentially critical role for middle-axis attention and frontoparietal control systems in EF development.
Implications for the Emergence of Psychopathology in Youth
As discussed, the functional connectome develops through coordinated patterns of functional system segregation and integration as cortical organization becomes more aligned with the S-A axis. As hierarchical variation in connectivity profiles strengthens with age, top-down cortical propagations along the S-A axis, which are prominent during EF tasks, similarly become more evident. Middle-axis attention and frontoparietal control systems may be integral to the maturational reorganization of the functional connectome and may gate or facilitate the flow of information across it. Given the importance of functional system segregation, integration, hierarchical variation, and top-down propagations for the healthy maturation of EF, it is not surprising that disruptions to this developmental progression may lead to deficits in EF.
Deficits in EF are a feature of many psychiatric conditions, including attention-deficit/hyperactivity disorder (ADHD), schizophrenia [80], major depressive disorder (MDD) [81], generalized anxiety disorder [82], and post-traumatic stress disorder [83]; EF deficits are included in the diagnostic criteria for nearly all of these disorders. Similarly to deficits seen in individual disorders, EF deficits are furthermore associated with overall psychopathology burden [5] and have been shown to predict changes in overall psychopathology in a large longitudinal sample [84]. Hence, EF deficits are a transdiagnostic risk factor for diverse forms of psychopathology [85,86]. Attention impairments in particular are a common form of EF deficits observed across disorders that can be severely debilitating [87], are associated with poorer treatment outcomes [88], and are correlated with reduced connectivity in middle-axis attention systems [89].
Similarly, nearly every major psychiatric condition has been associated with alterations in functional connectome development. Specifically, disruptions to the balance of segregation and integration are commonly observed across psychiatric disorders, including schizophrenia, bipolar disorder, and MDD [90,91]. Studies have also shown alterations in hierarchical cortical organization in individuals diagnosed with psychiatric conditions. Three independent studies have reported compression of the principal gradient of functional connectivity (e.g., a restricted gradient range) in schizophrenia [92] (Figure 2E), autism [93], and MDD [94], potentially signifying that reduced differentiation in connectivity profiles across the S-A axis is present in each of these disorders. In other words, individuals with these disorders exhibited more similar patterns of functional connectivity in sensorimotor and association systems. This pattern is indicative of less divergent functional architecture across the S-A axis, potentially due to an imbalance of functional system segregation and integration. Moreover, individuals who experienced an onset of depression during adolescence showed a greater compression of the principal functional connectivity gradient than those who were diagnosed with MDD in adulthood [94]. These findings together suggest that abnormal functional connectome organization or differentiation along the S-A axis may be associated with the onset of psychopathology during development, although further studies are needed to directly test this hypothesis (Outstanding Questions). Future studies could additionally explore the roles that positive and negative experiences play in shaping developmental alignment of the functional connectome with the S-A axis and the developmental emergence of psychiatric symptoms (Box 4).
Box 4: Impact of experiences and environments on the development of EF.
The protracted development of functional systems endows the connectome with important properties to support healthy EF. However, this prolonged period of change also renders the connectome susceptible to the impact of experiences and environments over an extended period. Given that development tends to progress along the sensorimotor-association cortical hierarchy, higher-order systems that take the longest to fully mature may be particularly vulnerable to such impacts. Prior work has shown that both positive experiences (e.g., supportive caregiving behaviors) and negative experiences (e.g., childhood abuse or neglect) can substantially impact the developing connectome and the maturation of EF.
Importantly, there exists marked specificity in which types of experiences most influence EF. For example, recent work has shown that caregiver monitoring (caregiver supervision and knowledge of the child’s whereabouts) may play a more substantial role in positively shaping cognitive development than caregiver warmth (caregiver emotional support) [111]. Additionally, in accordance with the Dimensional Model of Adversity and Psychopathology (DMAP), a model that distinguishes between dimensions of threat and deprivation [112], studies have highlighted stronger associations between resource deprivation (e.g., neglect) and cognition than between experiences of threat (e.g., abuse) and cognition [113]. In studies where both physical and emotional neglect were assessed, physical neglect more often drove associations with cognitive outcomes [114,115] while emotional neglect more often drove associations with affective psychopathology [116]. Future studies may further explore the mechanisms by which specific environments and experiences positively or negatively impact EF development.
Crucially, the pace and timing of connectome development may be governed by critical periods of developmental plasticity that progress along the S-A axis and are relevant to understanding the risk for executive dysfunction [117–120]. Early environments that are high in stress and low in cognitive enrichment may accelerate developmental changes in brain structure, potentially locking in connectome organization earlier [117]. Conversely, low-stress and high cognitive enrichment environments have been proposed to prolong time windows of association cortex plasticity that promote healthy cognition [120]. In other words, brain development may progress at a rate that is adaptive to the specific demands of each child’s environment, with higher-stress environments leading to accelerated maturation and lower-stress environments allowing for a more protracted developmental time course. Longer windows of brain plasticity, encouraged by novel positive experiences, may support adaptation to environmental demands in association cortex and prolong association system development, leading to more flexible functional connectome organization in adulthood [117]. Longitudinal studies may further our understanding of how different environments and experiences shape individual trajectories of EF development.
Concluding Remarks
The enhancement of EF throughout childhood and adolescence is a hallmark of healthy development and may be important for resilience to psychopathology. EF requires both specialized, efficient processing within functional systems and extensive communication between systems, both of which are refined during protracted connectome development along a sensorimotor-association cortical axis. Emerging research suggests that the hierarchical embedding of functional system connectivity is critical for normative development and thus provides a promising theoretical framework to guide future research (see Outstanding Questions). In particular, future studies could assess the unique role of middle-axis attention and frontoparietal control systems in supporting broader EF development, characterize the impact of early life experiences on functional connectome development, study what neurochemical and signaling changes underlie connectome reorganization, and address ongoing challenges with data quality and reproducibility. Together, the studies reviewed here support a cohesive neurodevelopmental framework that may help address important open questions in developmental cognitive neuroscience research.
Figure I, Box 2: Functional topography is highly variable in association cortex and is associated with age and EF.
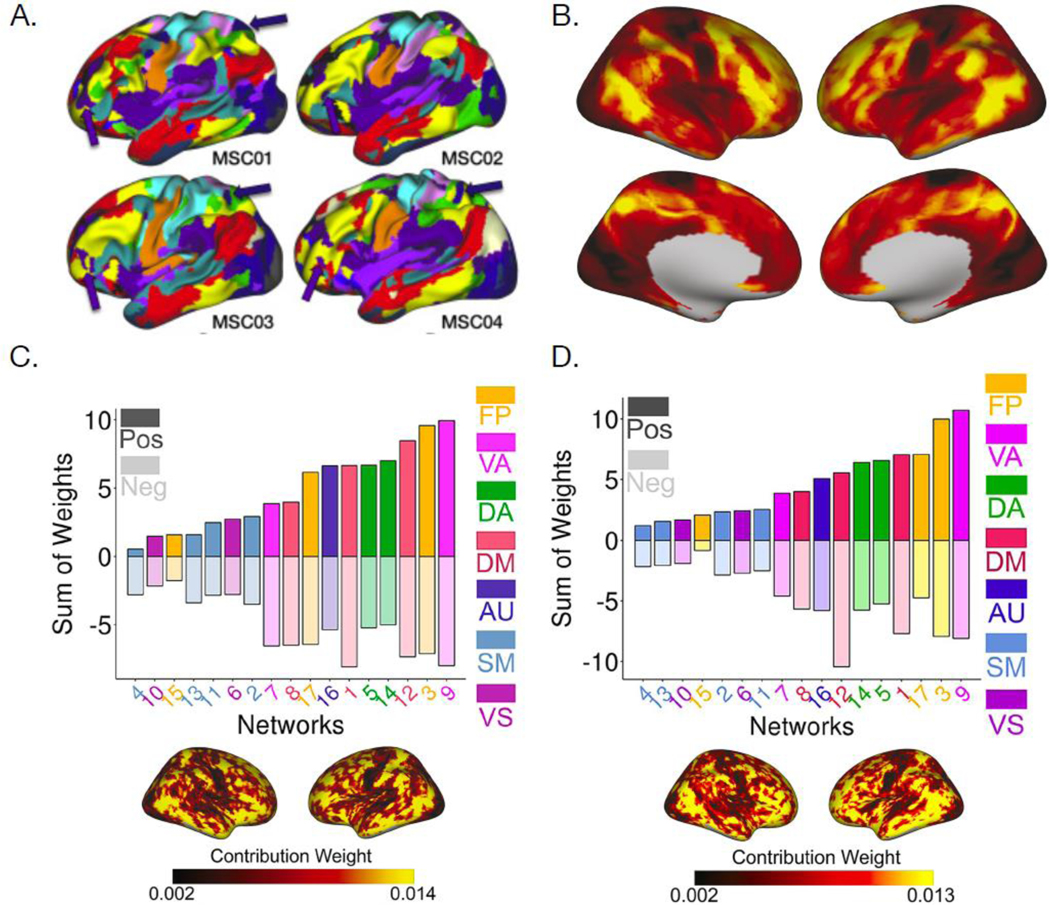
A) The spatial topography of functional brain networks is variable across individuals. Arrows indicate functional regions that were present in individually-defined functional connectomes but not present in a group-average functional connectome map (reproduced from [96]). B) Inter-individual variability in the functional topography of personalized functional brain networks in youth is highest in association cortices. Variability in the functional topography of association networks contributes most to predictive models of age (Panel C) and executive function (Panel D) (reproduced from [97]).
Figure I, Box 3. Head motion is associated with age, functional connectivity, and executive function.
A) Head motion is associated with decreased modularity, a measure of segregation (reproduced from [98]). B) Head motion decreases with age (reproduced from [98]). C) Head motion is associated with a behavioral measure of fluid intelligence (reproduced from [99]). D) Edges significantly related to subject motion in the 264-node Power network for each de-noising strategy. Strategies are ranked according to efficacy, with those that included regression of the mean global signal (blue) consistently ranked as the best performers (reproduced from [100]).
Highlights.
Executive function relies on both specialized processing within functional systems and flexible communication between functional systems
Functional systems exhibit graded differences in connectivity profiles that are embedded within a sensorimotor-association axis, a hierarchical axis of cortical organization
Functional systems at the top of the cortical hierarchy tend to become more functionally segregated during development while regions in middle-axis systems become more integrated; this balance may support healthy executive function
Middle-axis attention systems may drive large-scale functional connectome reorganization during late childhood and adolescence, facilitating hierarchical information flow and multi-system coordination
Abnormal functional system development in youth may lead to executive dysfunction, thereby increasing risk for psychopathology
Acknowledgment
This study was supported by grants from the National Institute of Health: R01MH113550 (TDS & DSB), R01MH120482 (TDS), R01MH112847 (RTS & TDS), R01EB022573 (TDS), RF1MH116920 (TDS & DSB), RF1MH121867 (TDS), R37MH125829 (TDS & DAF). ASK was supported by a Neuroengineering and Medicine T32 Fellowship from the National Institute of Neurological Disorders and Stroke (5T32NS091006-08). VJS was supported by a National Science Foundation Graduate Research Fellowship (DGE-1845298). Additional support was provided by the Penn-CHOP Lifespan Brain Institute.
Glossary
- Cortical Propagations
Fluctuations of neural activity that propagate between cortical regions. These cortical propagations have been observed during both anesthesia and awake behavior
- Diverse Club
Brain regions that exhibit a diverse pattern of connectivity across many systems. Diverse club regions are often located in the frontoparietal control and dorsal and ventral attention systems, which lie in the middle of the S-A axis
- Excitation:Inhibition (E:I) Ratio
The balance between excitation and inhibition in neural circuits, often calculated as the number of excitatory-to-excitatory synapses divided by the number of inhibitory-to-excitatory synapses or as the ratio of excitatory to inhibitory neurotransmitters or neurotransmitter receptors
- Executive Function (EF)
A broad cognitive domain that encompasses multiple subdomains, including working memory, response inhibition, and set shifting
- Functional Connectome
The set of all large-scale functional interactions across the cortex; these interactions exist within and between functional systems
- Functional Systems
Networks of brain regions that exhibit synchronized fluctuations in activity and subserve dissociable yet overlapping cognitive and behavioral functions. Examples include the visual, motor, frontoparietal, ventral attention, and default mode systems
- Functional Topography
The spatial layout of functional systems on the cortical surface
- Integration
Strong functional or structural connectivity between different functional systems
- Participation Coefficient
A measurement of integration, quantifying the distribution of a given node’s connections with other communities of nodes within a graph
- Principal Gradient
The spatial axis that captures the maximum variance in functional connectivity patterns. The principal gradient of functional connectivity in adults falls along a spectrum from unimodal sensory regions to transmodal regions and thus represents the dominant pattern of organizational hierarchy of functional systems
- Segregation
Strong functional or structural connectivity within functional systems
- Sensorimotor-Association (S-A) Axis
A dominant, large-scale axis of human brain organization that describes the stereotyped patterning of cortical heterogeneity from unimodal to transmodal regions. This axis captures across-cortex variability in diverse structural, metabolic, molecular, genetic, functional, and connectivity properties. It is arranged from systems that produce perception and action to those supporting attention, memory, and decision making, and finally to systems strongly linked to emotion and internal mentation
- Structure-Function Coupling
The relationship between structural and functional properties of the functional connectome (e.g., associations between white-matter connectivity and inter-regional functional connectivity)
- Transmodal
Cortical regions involved in integrative processing of signals across multiple sensorimotor modalities
- Unimodal
Cortical regions involved in the segregated processing of signals within a single sensory modality (e.g., vision or audition)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luna B, 2009. Developmental changes in cognitive control through adolescence. Adv. Child Dev. Behav. 37, 233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortés Pascual A. et al. (2019) The Relationship Between Executive Functions and Academic Performance in Primary Education: Review and Meta-Analysis. Front. Psychol. 10, 1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamosh NA et al. (2008) Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychological science 19, 904–911 [DOI] [PubMed] [Google Scholar]
- 4.Klassen AF et al. (2004) Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics 114, e541–547 [DOI] [PubMed] [Google Scholar]
- 5.Shanmugan S. et al. (2016) Common and Dissociable Mechanisms of Executive System Dysfunction Across Psychiatric Disorders in Youth. The American journal of psychiatry 173, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sydnor VJ et al. (2021) Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 109, 2820–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margulies DS et al. (2016) Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U.S.A. 113, 12574–12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huntenburg JM et al. (2018) Large-Scale Gradients in Human Cortical Organization. Trends in Cognitive Sciences 22, 21–31 [DOI] [PubMed] [Google Scholar]
- 9.García-Cabezas MÁ et al. (2019) The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex. Brain Struct Funct 224, 985–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgetag CC et al. (2022) A natural cortical axis connecting the outside and inside of the human brain. Network Neuroscience DOI: 10.1162/netn_a_00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesulam MM (1998) From sensation to cognition. Brain 121, 1013–1052 [DOI] [PubMed] [Google Scholar]
- 12.Burt JB et al. (2018) Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci 21, 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krienen FM et al. (2016) Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proceedings of the National Academy of Sciences 113, E469–E478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulas A. et al. (2021) The natural axis of transmitter receptor distribution in the human cerebral cortex. Proceedings of the National Academy of Sciences 118, e2020574118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen JY et al. (2022). Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nature Neuroscience 10.1038/s41593-02201186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X-J (2020) Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nat Rev Neurosci 21, 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerner Y. et al. (2011) Topographic Mapping of a Hierarchy of Temporal Receptive Windows Using a Narrated Story. J. Neurosci. 31, 2906–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JD et al. (2014) A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci 17, 1661–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raut RV et al. (2020) Organization of Propagated Intrinsic Brain Activity in Individual Humans. Cerebral Cortex 30, 1716–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum GL et al. (2020) Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. U.S.A. 117, 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paquola C. et al. (2019) Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biol 17, e3000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vázquez-Rodríguez B. et al. (2019) Gradients of structure–function tethering across neocortex. Proceedings of the National Academy of Sciences 116, 21219–21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luppi AI et al. (2022) A synergistic core for human brain evolution and cognition. Nature Neuroscience 25, 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal MS et al. (2014) Aerobic Glycolysis in the Human Brain Is Associated with Development and Neotenous Gene Expression. Cell Metabolism 19, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oligschläger S. et al. (2017) Gradients of connectivity distance are anchored in primary cortex. Brain Struct Funct 222, 2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepulcre J. et al. (2010) The organization of local and distant functional connectivity in the human brain. PLoS Comput Biol 6, e1000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum GL et al. (2017) Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Current biology : CB 27, 1561–1572.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertolero MA et al. (2017) The diverse club. Nat Commun 8, 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckner RL and Krienen FM (2013) The evolution of distributed association networks in the human brain. Trends Cogn Sci 17, 648–665 [DOI] [PubMed] [Google Scholar]
- 30.Power JD et al. (2011) Functional network organization of the human brain. Neuron 72, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fair DA et al. (2007) Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America 104, 13507–13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy C. et al. (2019) Modes of operation: A topographic neural gradient supporting stimulus dependent and independent cognition. NeuroImage 186, 487–496 [DOI] [PubMed] [Google Scholar]
- 33.Sherman LE et al. (2014) Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev Cogn Neurosci 10, 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C. et al. (2020) Modular segregation of task-dependent brain networks contributes to the development of executive function in children. NeuroImage 206, 116334 [DOI] [PubMed] [Google Scholar]
- 35.Betzel RF et al. (2014) Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage 102, 345–357 [DOI] [PubMed] [Google Scholar]
- 36.Lopez KC et al. (2020) Development of Network Topology and Functional Connectivity of the Prefrontal Cortex. Cereb Cortex 30, 2489–2505 [DOI] [PubMed] [Google Scholar]
- 37.Chan MY et al. (2014) Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A 111, E4997–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wig GS (2017) Segregated Systems of Human Brain Networks. Trends in Cognitive Sciences 21, 981–996 [DOI] [PubMed] [Google Scholar]
- 39.Cao M. et al. (2017) Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cerebral Cortex 27, 1949–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu S. et al. (2015) Emergence of system roles in normative neurodevelopment. Proceedings of the National Academy of Sciences 112, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Heuvel MP et al. (2015) The Neonatal Connectome During Preterm Brain Development. Cerebral Cortex 25, 3000–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao W. et al. (2011) Temporal and Spatial Evolution of Brain Network Topology during the First Two Years of Life. PLOS ONE 6, e25278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Asis-Cruz J. et al. (2015) Functional properties of resting state networks in healthy full-term newborns. Sci Rep 5, 17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pines AR et al. (2022) Dissociable Multi-scale Patterns of Development in Personalized Brain Networks. DOI: 10.1101/2021.07.07.451458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tooley UA et al. (2022) The age of reason: Functional brain network development during childhood, Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cassady K. et al. (2019) Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geerligs L. et al. (2014) Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp 35, 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng KK et al. (2016) Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. Neuroimage 133, 321–330 [DOI] [PubMed] [Google Scholar]
- 49.Oldham S. et al. (2022) Early and late development of hub connectivity in the human brain. Curr Opin Psychol 44, 321–329 [DOI] [PubMed] [Google Scholar]
- 50.Marek S. et al. (2015) The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol 13, e1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grayson DS and Fair DA (2017) Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage 160, 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen JR and D’Esposito M. (2016) The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J Neurosci 36, 12083–12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rawls E. et al. (2022) The resting-state causal human connectome is characterized by hub connectivity of executive and attentional networks. Neuroimage 255, 119211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertolero MA et al. (2015) The modular and integrative functional architecture of the human brain. Proceedings of the National Academy of Sciences 112, E6798–E6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guimerà R. and Amaral LAN (2005) Cartography of complex networks: modules and universal roles. J Stat Mech 2005, nihpa35573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo BTT et al. (2015) Functional Specialization and Flexibility in Human Association Cortex. Cereb Cortex 25, 3654–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dosenbach NUF et al. (2006) A Core System for the Implementation of Task Sets. Neuron 50, 799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dosenbach NUF et al. (2008) A dual-networks architecture of top-down control. Trends in Cognitive Sciences 12, 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y. et al. (2017) Features of spatial and functional segregation and integration of the primate connectome revealed by trade-off between wiring cost and efficiency. PLOS Computational Biology 13, e1005776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Power JD et al. (2010) The Development of Human Functional Brain Networks. Neuron 67, 735–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashtan N. and Alon U. (2005) Spontaneous evolution of modularity and network motifs. Proc. Natl. Acad. Sci. U.S.A. 102, 13773–13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H. et al. (2021) Functional Connectivity Predicts Individual Development of Inhibitory Control during Adolescence. Cerebral Cortex 31, 2686–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens AA et al. (2012) Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS One 7, e30468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shine JM et al. (2019) Human cognition involves the dynamic integration of neural activity and neuromodulatory systems. Nat Neurosci 22, 289–296 [DOI] [PubMed] [Google Scholar]
- 65.Dong H-M et al. (2021) Shifting gradients of macroscale cortical organization mark the transition from childhood to adolescence. Proc. Natl. Acad. Sci. U.S.A. 118, e2024448118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nenning K-H et al. (2020) Joint embedding: A scalable alignment to compare individuals in a connectivity space. NeuroImage 222, 117232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu Y. et al. (2021) Brain Activity Fluctuations Propagate as Waves Traversing the Cortical Hierarchy. Cerebral Cortex 31, 3986–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raut RV et al. (2021) Global waves synchronize the brain’s functional systems with fluctuating arousal. Science Advances 7, eabf2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yousefi B. and Keilholz S. (2021) Propagating patterns of intrinsic activity along macroscale gradients coordinate functional connections across the whole brain. NeuroImage 231, 117827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pines A. et al. (2022) Development of Top-Down Cortical Propagations in Youth. bioRxiv, 2022.06.14.496175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones EG and Powell TPS (1970) An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain 93, 793–820 [DOI] [PubMed] [Google Scholar]
- 72.Dong H-M et al. (2022) Reduced ventral attention network connectivity is linked to the accelerated maturation of adult-like cortical organization in childhood. bioRxiv, 2022.04.12.488101 [Google Scholar]
- 73.Tooley UA et al. (2022) Functional brain network community structure in childhood: Unfinished territories and fuzzy boundaries. Neuroimage 247, 118843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sepulcre J. et al. (2012) Stepwise Connectivity of the Modal Cortex Reveals the Multimodal Organization of the Human Brain. J. Neurosci. 32, 10649–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corbetta M. and Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience 3, 201–215 [DOI] [PubMed] [Google Scholar]
- 76.Wainstein G. et al. (2021) The ascending arousal system promotes optimal performance through mesoscale network integration in a visuospatial attentional task. Netw Neurosci 5, 890–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shine JM et al. (2016) The Dynamics of Functional Brain Networks: Integrated Network States during Cognitive Task Performance. Neuron 92, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shine JM (2019) Neuromodulatory Influences on Integration and Segregation in the Brain. Trends Cogn Sci 23, 572–583 [DOI] [PubMed] [Google Scholar]
- 79.Gu S. et al. (2015) Controllability of structural brain networks. Nature communications 6, 8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kataoka Y. et al. (2020) Differences in executive function among patients with schizophrenia, their unaffected first-degree relatives and healthy participants. Int J Neuropsychopharmacol DOI: 10.1093/ijnp/pyaa052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keller AS et al. (2019) Paying attention to attention in depression. Translational Psychiatry 9, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller AS et al. (2021) Spatial attention impairments are characterized by specific electroencephalographic correlates and partially mediate the association between early life stress and anxiety. Cognitive, Affective, & Behavioral Neuroscience DOI: 10.31234/osf.io/ge4wb [DOI] [PubMed] [Google Scholar]
- 83.Blair KS et al. (2013) Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychological Medicine 43, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romer AL and Pizzagalli DA (2021) Is executive dysfunction a risk marker or consequence of psychopathology? A test of executive function as a prospective predictor and outcome of general psychopathology in the adolescent brain cognitive development study®. Dev Cogn Neurosci 51, 100994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder HR et al. (2015) Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol 6, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McTeague LM et al. (2016) Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res 83, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jaeger J. et al. (2006) Neurocognitive deficits and disability in major depressive disorder. Psychiatry Research 145, 39–48 [DOI] [PubMed] [Google Scholar]
- 88.Majer M. et al. (2004) Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychological Medicine 34, 1453–1463 [DOI] [PubMed] [Google Scholar]
- 89.Keller AS et al. (2019) Deep phenotyping of attention impairments and the ‘Inattention Biotype’ in Major Depressive Disorder. Psychological Medicine DOI: 10.1017/s0033291719002290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duan J. et al. (2019) Dynamic changes of functional segregation and integration in vulnerability and resilience to schizophrenia. Human Brain Mapping 40, 2200–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo Z. et al. (2021) Shared and specific dynamics of brain segregation and integration in bipolar disorder and major depressive disorder: A resting-state functional magnetic resonance imaging study. J Affect Disord 280, 279–286 [DOI] [PubMed] [Google Scholar]
- 92.Dong D. et al. (2021) Compressed sensorimotor-to-transmodal hierarchical organization in schizophrenia. Psychol Med DOI: 10.1017/S0033291721002129 [DOI] [PubMed] [Google Scholar]
- 93.Hong S-J et al. (2019) Atypical functional connectome hierarchy in autism. Nat Commun 10, 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xia M. et al. (2022) Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes. Mol Psychiatry 27, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 95.Yeo BTT et al. (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon EM et al. (2017) Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cui Z. et al. (2020) Individual Variation in Functional Topography of Association Networks in Youth. Neuron 106, 340–353.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satterthwaite TD et al. (2012) Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage 60, 623–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siegel JS et al. (2017) Data quality influences observed links between functional connectivity and behavior. Cerebral Cortex 27, 4492–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ciric R. et al. (2017) Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage 154, 174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laumann TO et al. (2015) Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron 87, 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glasser MF et al. (2016) A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kong R. et al. (2019) Spatial Topography of Individual-Specific Cortical Networks Predicts Human Cognition, Personality, and Emotion. Cerebral cortex (New York, N.Y. : 1991) 29, 2533–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bijsterbosch JD et al. (2019) The relationship between spatial configuration and functional connectivity of brain regions revisited. eLife 8, e44890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tavor I. et al. (2016) Task-free MRI predicts individual differences in brain activity during task performance. Science (New York, N.Y.) 352, 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Power JD et al. (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Dijk KRA et al. (2012) The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciric R. et al. (2018) Mitigating head motion artifact in functional connectivity MRI. Nature Protocols 13, 2801–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Satterthwaite TD et al. (2013) Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. NeuroImage 83, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fair DA et al. (2012) Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in systems neuroscience 6, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keller AS et al. (2022) Caregiver monitoring, but not caregiver warmth, is associated with general cognition in two large sub-samples of youth. Developmental Science, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheridan MA and McLaughlin KA (2014) Dimensions of early experience and neural development: deprivation and threat. Trends in cognitive sciences 18, 580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Machlin L. et al. (2019) Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Frontiers in Behavioral Neuroscience 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dannehl K. et al. (2017) Childhood adversity and cognitive functioning in patients with major depression. Child Abuse and Neglect 70, 247–254 [DOI] [PubMed] [Google Scholar]
- 115.Vaskinn A. et al. (2021) Childhood trauma, social cognition and schizophrenia: Specific association between physical neglect and cognitive theory of mind in homicide offenders. Psychiatry Research 303, [DOI] [PubMed] [Google Scholar]
- 116.Grummitt LR et al. (2022) Associations of childhood emotional and physical neglect with mental health and substance use in young adults. Australian and New Zealand Journal of Psychiatry 56, 365–375 [DOI] [PubMed] [Google Scholar]
- 117.Tooley UA et al. (2021) Environmental influences on the pace of brain development. Nature reviews. Neuroscience 22, 372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roubinov D. et al. (2021) Change of pace: How developmental tempo varies to accommodate failed provision of early needs. Neuroscience and Biobehavioral Reviews 131, 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Larsen B. and Luna B. (2018) Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94, 179–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sydnor VJ et al. (2022) Intrinsic Activity Develops Along a Sensorimotor-Association Cortical Axis in Youth, bioRxiv, 2022.08.15.503994 [DOI] [PMC free article] [PubMed] [Google Scholar]