Three years into the COVID‐19 pandemic, humanity continues to be reminded of the impact of RNA viruses on the economy and society. Discovery‐based surveys during these last 3 years have built upon decades of advances and have been reshaping knowledge of the RNA virosphere. For example, four parallel studies, exploring thousands of metatranscriptomes from diverse environments (Chen et al., 2022; Edgar et al., 2022; Neri et al., 2022; Zayed et al., 2022), have transformed our understanding of the ribovirian kingdom Orthornavirae, which harbours viruses that replicate via virus‐encoded RNA‐directed RNA polymerases (RdRps) (Figure 1A). Together, these four studies have augmented the known RNA virosphere by more than an order of magnitude, mapped orthornaviran RNA virus ecologies on a global scale, and perhaps uncovered a key missing link in our understanding of how early life evolved billions of years ago. However, a recent rarefaction analysis indicates that RNA virus discovery, orthornaviran or otherwise, is not even close to saturation (Neri et al., 2022) (Figure 1B).
FIGURE 1.
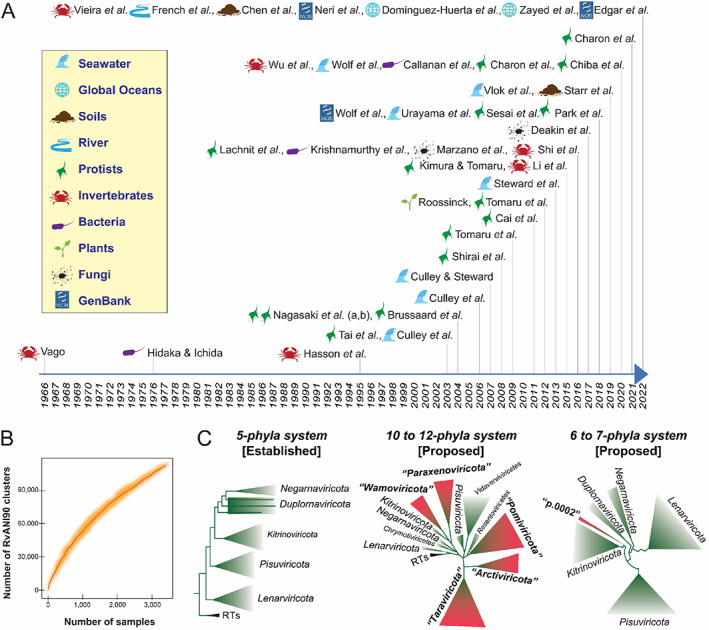
(A) Timeline of studies focusing on orthornaviran RNA viruses in biotic samples and natural environments. Relevant (non‐exhaustive) literature on orthornaviran RNA viruses derived from hosts from natural ecosystems published from the 1960s to October 2022 are listed by first‐author name. Sources of the RNA viruses are shown for each study, with icons described in the legend. (B) Rarefaction curve adapted from a previous study (Neri et al., 2022), representing the accumulation of unique clusters as a function of the number of analysed samples. (C) Global RNA‐directed RNA polymerase (RdRp) phylogenies showing established and, in quotation marks, proposed orthornaviran phyla, with reverse transcriptase (RT)‐encoding RNA viruses (ribovirian pararnavirans) as the root of the trees. Adapted, simplified figures (left, middle, and right) of the phylogenetic trees were built from datasets in three previous studies, respectively (Neri et al., 2022; Wolf et al., 2018; Zayed et al., 2022).
The COVID‐19 pandemic and these recent discoveries have catapulted RNA viruses to the forefront of virology, but researchers have been exploring Earth's RNA viruses for more than two decades. The first marine RNA virus was isolated from a portunid crab in 1966 (Vago, 1966), and the first and thus far only marine RNA prokaryotic virus was isolated a decade later (Hidaka & Ichida, 1976). This led to several decades of growing RNA virus culture collections with a focus on those that infect aquatic animals of economic interest (Lang et al., 2009), as well as those that might have ecological importance as the hypothesized causes of phytoplankton bloom termination (Lawrence et al., 2006). However, something close to magic ensued after researchers turned sequencing technologies toward uncultured virus particles and sought to explore the RNA virosphere via Sanger‐sequenced RdRp sequences (Culley et al., 2003). Despite Sanger sequencing's low throughput, this novel survey approach revealed a diverse array of highly‐divergent ‘picorna‐like’ RNA viruses that were likely infecting marine phytoplankton. A decade later, sequencing technologies had advanced sufficiently to enable the exploration of diverse environments, often via mining RdRp sequences in RNA‐seq data derived from (i) biotic samples (including holobiont, unicellular, and multicellular organisms), such as invertebrates (Li et al., 2015; Shi et al., 2016; Vieira et al., 2022b; Wu et al., 2020), vertebrates (Shi et al., 2018), plants (Roossinck, 2012; Vieira et al., 2022a), protists (Cai et al., 2012; Charon et al., 2022, 2020, 2021; Lachnit et al., 2015; Nagasaki et al., 2004; Sasai et al., 2018; Shirai et al., 2008; Tai et al., 2003; Tomaru et al. 2004, 2009, 2012), and fungi (Deakin et al., 2017; Marzano et al., 2016) and (ii) environmental samples, such as faeces (Krishnamurthy et al., 2016), sediments (Callanan et al., 2020), soils (Hillary et al., 2022; Starr et al., 2019; Wu et al., 2021), rivers (French et al., 2022), and seawater from specific sites (Culley et al., 2003, 2006, 2014; Djikeng et al., 2009; Steward et al., 2013; Urayama et al., 2018; Vlok et al., 2019; Wolf et al., 2020) and from geographic locations representing the entire global oceans (Dominguez‐Huerta et al., 2022; Zayed et al., 2022). The environments most explored for RNA viruses during these two decades are aquatic (e.g., marine, sewage, and riverine), providing us with the first insights into their ecology, evolution of their viral inhabitants, and methodological challenges associated with characterizing specific natural ecosystems (Culley, 2018; Liao et al., 2022). In all, although RNA virus taxonomy is under constant development, 20 years of metagenomic and metatranscriptomic surveys have moved the needle from a few thousand formally‐defined RNA virus species to more than 100,000 species‐rank taxa (Neri et al., 2022) that have yet to be officially recognized by the International Committee on Taxonomy of Viruses (ICTV) and a growing number of orthornaviran phyla (Neri et al., 2022; Zayed et al., 2022) (Figure 1C).
As we stare into our ‘crystal ball’, we seek clarity on how much of the orthornaviran RNA virosphere remains to be discovered by guesstimating how many distinct (i.e., species‐rank) RNA viruses exist and have existed on Earth. The number of distinct bacterial viruses of all realms was estimated to be 107–109; this conservative extrapolation was based on the projected numbers of distinct bacteria (hypothesized to be the predominant hosts of the virosphere) and assuming 10–100 distinct viruses per bacterial host (Koonin et al., 2022). In addition, the number of distinct eukaryotic viruses (of all genome configurations) was estimated to be 87 million, derived from assuming 10 distinct viruses per 8.7 million estimated distinct eukaryotic hosts (Geoghegan & Holmes, 2017). To infer the corresponding number of eukaryotic RNA viruses, we calculated a factor of 0.555 (the currently 3113 ICTV‐recognized orthornaviran species divided by the 5610 total ICTV‐recognized virus species conservatively known to harbor viruses infecting eukaryotes; ICTV, 2022a, 2022b), with some caveats: (i) potentially faulty host assignment may be included (viruses discovered in metazoans could, for instance, be viruses of metazoan bacterial fauna), (ii) DNA virus discovery is more advanced than for RNA viruses, and (iii) newly described RNA viruses are much more likely to be incompletely described (incomplete genomes, preventing their official classification) than DNA viruses. By applying the calculated factor of 0.555 to the size of the estimated eukaryotic virosphere (Geoghegan & Holmes, 2017), we obtained an estimate of 48.28 million eukaryotic orthornaviran species. Consequently, the currently 3113 eukaryotic orthornaviran species and the reported uncultivated orthornaviran RNA viruses (124,873 clusters representing taxonomic ranks from species to genus in a single study) (Neri et al., 2022) represent the very ‘tip of the iceberg’, comprising 0.006% and 0.259%, respectively, of the total eukaryotic RNA viruses on Earth.
However, the RNA virosphere may be magnitudes larger. First, the estimated eukaryotic hosts value of 8.7 million was derived from a study (Mora et al., 2011) published more than a decade before the still‐growing deluge of newly discovered microbial eukaryotes were deduced from metagenomic surveys of natural ecosystems (Behnke et al., 2011; Carradec et al., 2018; Cordier et al., 2022; de Vargas et al., 2015; Edgcomb et al., 2011; Lecroq et al., 2011; Logares et al., 2012). Analyses should be performed to see whether these newly discovered organisms fit into previous (Mora et al., 2011) and more current eukaryotic diversity predictions (Pawlowski et al., 2012; Tedersoo et al., 2022) or, as we hypothesize, whether these predictions have to be corrected, possibly by orders of magnitude. Second, the vast majority of recognized officially classified prokaryotic viruses are DNA viruses, with only relatively few RNA viruses known to infect bacteria and none known to infect archaea [with an intriguing possible exception reported in 2012 (Bolduc et al., 2012)]. However, the number of bacterial RNA viruses, assignable to lenarviricot class Leviviricetes, duplornaviricot family Cystoviridae, and pisuviricot family Picobirnaviridae, have been increasing dramatically in recent years (Boros et al., 2018; Callanan et al., 2018; Neri et al., 2022), suggesting that prokaryotic RNA viruses may not be nearly as exotic in the bacterial world as assumed. Estimates suggest the existence of 106–1012 distinct bacteria (Curtis et al., 2002; Lennon & Locey, 2016). Even if we conservatively assume that the number of orthornaviran RNA viruses that infect bacteria remains a fraction of bacterial DNA viruses, any increase of that fraction will add millions to trillions of distinct viruses to the RNA virosphere, depending on how many distinct viruses infect a given host and how many distinct hosts a particular virus may infect. For instance, calculated from ICTV metadata (ICTV, 2022a), 19.5% (889 leviviricete and vidaverviricete species) of the 4556 ICTV‐accepted bacterial virus species harbor orthornavirans, which, when applied to the estimated bacterial biosphere sizes and conservatively assuming only 10 distinct (species‐rank) virus per distinct (species‐rank) bacterium, would lead to a bacterial RNA virosphere requiring the establishment of 1.95 million and 1.95 trillion orthornaviran species, respectively; the upper bound being more than four orders of magnitude (2.2 × 104) the size of the eukaryotic RNA virosphere in our initial conservative guesstimate.
Stepping away from sheer virus numbers, we wonder whether there are sufficient data available to appropriately portray the diversity of the orthornaviran virosphere. This virosphere could be populated by numerous very closely related viruses (big but not very diverse, as evidenced by the need for few higher and many lower taxonomic ranks), few very distinct entities (small but very diverse), or anything in between. A virus diversity map would be philosophically useful to inform our understanding, for instance, of the origin of life, and practically helpful to guide efforts to characterize under‐sampled ecological niches.
Traditionally, the megataxonomic diversity of RNA viruses has been sorted into three separate blocks, represented by the groups III [double‐stranded RNA (dsRNA) viruses], IV [positive‐sense RNA (+ssRNA) viruses], and V [negative‐sense ssRNA (−ssRNA) viruses] in the historic Baltimore classification (Baltimore, 1971), which, although pragmatic, does not depict evolutionary relationships. Attempts to create a panoramic view of the virosphere have focused on creating ‘megataxa’ (ranks above order, such as classes, phyla, kingdoms, and realms), including operational taxa (such as unofficial ‘superfamilies’), using available RdRp sequences (Koonin, 1991). For example, reconstruction of phylogenetic relationships, based on nearly 5000 RdRp sequences available in GenBank in April 2017, indicated five major orthornaviran branches (Wolf et al., 2018), today officially recognized as phyla (Koonin et al., 2020). A recent study indicated the possible need to establish five additional phyla for novel orthornaviran RNA viruses infecting eukaryotes and suggested adjustments of the previously proposed evolutionary relationships of some established megataxa (Zayed et al., 2022). Shortly after, mining of more than 5000 diverse metatranscriptomes revealed the possible need to establish two different orthornaviran phyla for novel prokaryotic RNA viruses (Neri et al., 2022). In addition, both studies indicated numerous novel classes within established phyla. But, due to billions of years of evolution, the RdRp hallmark gene of orthornavirans is one of the most divergent proteins in all of biology (Koonin, 1991), making the establishment of a panoramic view very challenging.
Algorithms in current use to extract RdRp sequences from datasets essentially work using similarity thresholds (Wolf et al., 2019). If an RdRp is too diverse (for instance, ‘permuted’, that is, not following the consensus order of particular elements, or segmented), the algorithm may not recognize it as such—de facto meaning that a very diverse branch of viruses, which could constitute a novel megataxon, is overlooked. Already, we know of RdRps examples that exist and defy the RdRp alignment approach used for ribovirian classification. For instance, the RdRps of viruses belonging to families Birnaviridae, Permutotetraviridae, and Polymycoviridae clearly ‘look’ orthornaviran but are too diverse to assign them to orthornaviran taxa with current methods (Koonin et al., 2022). Numerous relatives of these viruses likely lurk in the metagenomic datasets already available and have almost certainly never been sampled.
Further complicating the quest to understand the virosphere, a recently discovered viroid‐like genomic backbone was suggested to encode an ‘ambi‐like’ virus RdRp, possibly revealing a virus that, on one hand, appears orthornaviran because of its RdRp, but, on the other hand, has many similarities with members of the other realm of RNA viruses, the Ribozyviria (non‐RdRp‐encoding hepatitis D‐like viruses) (Forgia et al., bioRxiv preprint 2022.08.21.504695) or their now numerous unclassified relatives or analogs (de la Peña et al., 2021; Edgar et al., 2022). Taken together, these findings indicate that the orthornaviran virosphere is much bigger than previously expected (i.e., has many more members that estimated). Finally, orthornavirans likely exist that have lost their RdRps and function in conjunction with other “helper” viruses (e.g., albetoviruses, aumaiviruses, papaniviruses, virtoviruses). Obviously, such viruses cannot be detected at all using RdRp screening approaches. In addition, they suggest that entire high‐ranking taxa, likely branching off at the deepest roots of the RdRp tree, will have to be established, thereby adding significant diversity. As always, in any biological taxonomy, any such additions to the overall tree may result in a revolution in the relationship of all branches to each other and, hence, likely also result in repeated dramatic taxonomic remapping. Thus, though taxonomy has at times been left for ‘dead’ (Drew, 2011), just as recent sequencing‐powered, tree‐shaking advances are transforming prokaryote taxonomy (Hugenholtz et al., 2021), parallel recent advances in our understanding of the RNA virosphere suggest that taxonomy lives on to help researchers organize life's complexity.
CONCLUDING REMARKS
As the orthornaviran virosphere is explored, challenges linked to each methodological step of the workflow will need to be addressed to bypass systematic biases and limitations. Sampling, RNA purification, library‐building, sequencing, RNA virus identification and classification (via RdRp sequence comparisons), and host inferences are currently hot topics that inspire intrepid boldness among virologists seeking fresh alternative approaches. It is hoped that the researchers in the field of RNA virus discovery, evolution, and taxonomy will collaborate with an enthusiasm that overcomes competition or acrimony to find solutions to these challenges, thereby creating best practices for phylogenetic analyses that can be adopted by the general virologist community. Standards for global analyses of RdRp sequences should be at the forefront of these efforts. In this sense, the coming RdRp summit (http://rdrp.io/) reflects the willingness of many virologists to embrace genome‐based taxonomy, avoid growing division regarding methodological options, and promote operational standards that will catapult the field forward by enabling genuine intercomparability across biomes as they are explored.
AUTHOR CONTRIBUTIONS
James M. Wainaina: Writing – review and editing (supporting). Ahmed A. Zayed: Writing – review and editing (supporting). Alexander I. Culley: Writing – review and editing (supporting). Jens H. Kuhn: Writing – review and editing (equal). Matthew Sullivan: Writing ‐ review & editing (equal). Guillermo Domínguez‐Huerta: Visualization‐Lead; Writing – original draft (Lead); Writing – review & editing (equal).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Anya Crane (Integrated Research Facility at Fort Detrick, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Fort Detrick, Frederick, MD, USA) for critically editing the manuscript. This work was supported in part through the U.S. National Science Foundation (award OCE#1829831); the U.S. Department of Energy (awards DE‐SC0020173 and DE‐SC0023307); Laulima Government Solutions, LLC, prime contract with the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) under Contract No. HHSN272201800013C. J.H.K. performed this work as an employee of Tunnell Government Services (TGS), a subcontractor of Laulima Government Solutions, LLC, under Contract No. HHSN272201800013C.
Dominguez‐Huerta, G. , Wainaina, J.M. , Zayed, A.A. , Culley, A.I. , Kuhn, J.H. & Sullivan, M.B. (2023) The RNA virosphere: How big and diverse is it? Environmental Microbiology, 25(1), 209–215. Available from: 10.1111/1462-2920.16312
Funding information Laulima Government Solutions, Grant/Award Number: HHSN272201800013C; National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID); National Science Foundation, Grant/Award Number: OCE#1829831; U.S. Department of Energy, Grant/Award Numbers: DE‐SC0020173, DE‐SC0023307
REFERENCES
- Baltimore, D. (1971) Expression of animal virus genomes. Bacteriological Reviews, 35, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke, A. , Engel, M. , Christen, R. , Nebel, M. , Klein, R.R. & Stoeck, T. (2011) Depicting more accurate pictures of protistan community complexity using pyrosequencing of hypervariable SSU rRNA gene regions. Environmental Microbiology, 13, 340–349. [DOI] [PubMed] [Google Scholar]
- Bolduc, B. , Shaughnessy, D.P. , Wolf, Y.I. , Koonin, E.V. , Roberto, F.F. & Young, M. (2012) Identification of novel positive‐strand RNA viruses by metagenomic analysis of archaea‐dominated Yellowstone hot springs. Journal of Virology, 86, 5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros, A. , Polgár, B. , Pankovics, P. , Fenyvesi, H. , Engelmann, P. , Phan, T.G. et al. (2018) Multiple divergent picobirnaviruses with functional prokaryotic Shine‐Dalgarno ribosome binding sites present in cloacal sample of a diarrheic chicken. Virology, 525, 62–72. [DOI] [PubMed] [Google Scholar]
- Cai, G. , Myers, K. , Fry, W.E. & Hillman, B.I. (2012) A member of the virus family Narnaviridae from the plant pathogenic oomycete Phytophthora infestans . Archives of Virology, 157, 165–169. [DOI] [PubMed] [Google Scholar]
- Callanan, J. , Stockdale, S.R. , Shkoporov, A. , Draper, L.A. , Ross, R.P. & Hill, C. (2018) RNA phage biology in a metagenomic era. Viruses, 10, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan, J. , Stockdale, S.R. , Shkoporov, A. , Draper, L.A. , Ross, R.P. & Hill, C. (2020) Expansion of known ssRNA phage genomes: from tens to over a thousand. Science Advances, 6, eaay5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carradec, Q. , Pelletier, E. , Da Silva, C. , Alberti, A. , Seeleuthner, Y. , Blanc‐Mathieu, R. et al. (2018) A global ocean atlas of eukaryotic genes. Nature Communications, 9, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon, J. , Kahlke, T. , Larsson, M.E. , Abbriano, R. , Commault, A. , Burke, J. et al. (2022) Diverse RNA viruses associated with diatom, eustigmatophyte, dinoflagellate, and rhodophyte microalgae cultures. Journal of Virology, 96, e0078322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon, J. , Marcelino, V.R. , Wetherbee, R. , Verbruggen, H. & Holmes, E.C. (2020) Metatranscriptomic identification of diverse and divergent RNA viruses in green and chlorarachniophyte algae cultures. Viruses, 12, 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon, J. , Murray, S. & Holmes, E.C. (2021) Revealing RNA virus diversity and evolution in unicellular algae transcriptomes. Virus Evolution, 7, veab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.‐M. , Sadiq, S. , Tian, J.‐H. , Chen, X. , Lin, X.‐D. , Shen, J.‐J. et al. (2022) RNA viromes from terrestrial sites across China expand environmental viral diversity. Nature Microbiology, 7, 1312–1323. [DOI] [PubMed] [Google Scholar]
- Cordier, T. , Angeles, I.B. , Henry, N. , Lejzerowicz, F. , Berney, C. , Morard, R. et al. (2022) Patterns of eukaryotic diversity from the surface to the deep‐ocean sediment. Science Advances, 8, eabj9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley, A. (2018) New insight into the RNA aquatic virosphere via viromics. Virus Research, 244, 84–89. [DOI] [PubMed] [Google Scholar]
- Culley, A.I. , Lang, A.S. & Suttle, C.A. (2003) High diversity of unknown picorna‐like viruses in the sea. Nature, 424, 1054–1057. [DOI] [PubMed] [Google Scholar]
- Culley, A.I. , Lang, A.S. & Suttle, C.A. (2006) Metagenomic analysis of coastal RNA virus communities. Science, 312, 1795–1798. [DOI] [PubMed] [Google Scholar]
- Culley, A.I. , Mueller, J.A. , Belcaid, M. , Wood‐Charlson, E.M. , Poisson, G. & Steward, G.F. (2014) The characterization of RNA viruses in tropical seawater using targeted PCR and metagenomics. mBio, 5, e01210‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, T.P. , Sloan, W.T. & Scannell, J.W. (2002) Estimating prokaryotic diversity and its limits. Proceedings of the National Academy of Sciences of the United States of America, 99, 10494–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña, M. , Ceprián, R. , Casey, J.L. & Cervera, A. (2021) Hepatitis delta virus‐like circular RNAs from diverse metazoans encode conserved hammerhead ribozymes. Virus Evolution, 7, veab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vargas, C. , Audic, S. , Henry, N. , Decelle, J. , Mahé, F. , Logares, R. et al. (2015) Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science, 348, 1261605. [DOI] [PubMed] [Google Scholar]
- Deakin, G. , Dobbs, E. , Bennett, J.M. , Jones, I.M. , Grogan, H.M. & Burton, K.S. (2017) Multiple viral infections in Agaricus bisporus—characterisation of 18 unique RNA viruses and 8 ORFans identified by deep sequencing. Scientific Reports, 7, 2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djikeng, A. , Kuzmickas, R. , Anderson, N.G. & Spiro, D.J. (2009) Metagenomic analysis of RNA viruses in a fresh water lake. PLoS One, 4, e7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez‐Huerta, G. , Zayed, A.A. , Wainaina, J.M. , Guo, J. , Tian, F. , Pratama, A.A. et al. (2022) Diversity and ecological footprint of Global Ocean RNA viruses. Science, 376, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Drew, L.W. (2011) Are we losing the science of taxonomy? As need grows, numbers and training are failing to keep up. Bioscience, 61, 942–946. [Google Scholar]
- Edgar, R.C. , Taylor, J. , Lin, V. , Altman, T. , Barbera, P. , Meleshko, D. et al. (2022) Petabase‐scale sequence alignment catalyses viral discovery. Nature, 602, 142–147. [DOI] [PubMed] [Google Scholar]
- Edgcomb, V. , Orsi, W. , Bunge, J. , Jeon, S. , Christen, R. , Leslin, C. et al. (2011) Protistan microbial observatory in the Cariaco Basin, Caribbean. I. Pyrosequencing vs Sanger insights into species richness. The ISME Journal, 5, 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, R. , Charon, J. , Lay, C.L. , Muller, C. & Holmes, E.C. (2022) Human land use impacts viral diversity and abundance in a New Zealand river. Virus Evolution, 8, veac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan, J.L. & Holmes, E.C. (2017) Predicting virus emergence amid evolutionary noise. Open Biology, 7, 170189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka, T. & Ichida, K.‐I. (1976) Properties of a marine RNA‐containing bacteriophage. Memoirs of Faculty of Fisheries Kagoshima University, 25, 77–89. [Google Scholar]
- Hillary, L.S. , Adriaenssens, E.M. , Jones, D.L. & McDonald, J.E. (2022) RNA‐viromics reveals diverse communities of soil RNA viruses with the potential to affect grassland ecosystems across multiple trophic levels. ISME Communications, 2, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz, P. , Chuvochina, M. , Oren, A. , Parks, D.H. & Soo, R.M. (2021) Prokaryotic taxonomy and nomenclature in the age of big sequence data. The ISME Journal, 15, 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses . (2022a) The VMR: exemplar viruses for species. ICTV Virus Metadata Resourece (VMR) v3. https://ictv.global/vmr
- International Committee on Taxonomy of Viruses . (2022b) The Master Species List: a spreadsheet of current taxonomy. ICTV Master Species List v3. https://ictv.global/msl
- Koonin, E.V. (1991) The phylogeny of RNA‐dependent RNA polymerases of positive‐strand RNA viruses. The Journal of General Virology, 72(Pt 9), 2197–2206. [DOI] [PubMed] [Google Scholar]
- Koonin, E.V. , Dolja, V.V. , Krupovic, M. , Varsani, A. , Wolf, Y.I. , Yutin, N. et al. (2020) Global organization and proposed megataxonomy of the virus world. Microbiology and Molecular Biology Reviews, 84, e00061‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin, E.V. , Krupovic, M. & Dolja, V.V. (2022) The global virome: how much diversity and how many independent origins? Environmental Microbiology, 1–5. 10.1111/1462-2920.16207 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, S.R. , Janowski, A.B. , Zhao, G. , Barouch, D. & Wang, D. (2016) Hyperexpansion of RNA bacteriophage diversity. PLoS Biology, 14, e1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachnit, T. , Thomas, T. & Steinberg, P. (2015) Expanding our understanding of the seaweed holobiont: RNA viruses of the red alga Delisea pulchra . Frontiers in Microbiology, 6, 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, A.S. , Rise, M.L. , Culley, A.I. & Steward, G.F. (2009) RNA viruses in the sea. FEMS Microbiology Reviews, 33, 295–323. [DOI] [PubMed] [Google Scholar]
- Lawrence, J.E. , Brussaard, C.P.D. & Suttle, C.A. (2006) Virus‐specific responses of Heterosigma akashiwo to infection. Applied and Environmental Microbiology, 72, 7829–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecroq, B. , Lejzerowicz, F. , Bachar, D. , Christen, R. , Esling, P. , Baerlocher, L. et al. (2011) Ultra‐deep sequencing of foraminiferal microbarcodes unveils hidden richness of early monothalamous lineages in deep‐sea sediments. Proceedings of the National Academy of Sciences of the United States of America, 108, 13177–13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon, J.T. & Locey, K.J. (2016) The underestimation of global microbial diversity. mBio, 7, e01298‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C.‐X. , Shi, M. , Tian, J.‐H. , Lin, X.‐D. , Kang, Y.‐J. , Chen, L.‐J. et al. (2015) Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative‐sense RNA viruses. eLife, 4, e05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M. , Xie, Y. , Shi, M. & Cui, J. (2022) Over two decades of research on the marine RNA virosphere. iMeta, 1, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logares, R. , Audic, S. , Santini, S. , Pernice, M.C. , de Vargas, C. & Massana, R. (2012) Diversity patterns and activity of uncultured marine heterotrophic flagellates unveiled with pyrosequencing. The ISME Journal, 6, 1823–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano, S.‐Y.L. , Nelson, B.D. , Ajayi‐Oyetunde, O. , Bradley, C.A. , Hughes, T.J. , Hartman, G.L. et al. (2016) Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. Journal of Virology, 90, 6846–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora, C. , Tittensor, D.P. , Adl, S. , Simpson, A.G.B. & Worm, B. (2011) How many species are there on earth and in the ocean? PLoS Biology, 9, e1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki, K. , Tomaru, Y. , Katanozaka, N. , Shirai, Y. , Nishida, K. , Itakura, S. et al. (2004) Isolation and characterization of a novel single‐stranded RNA virus infecting the bloom‐forming diatom Rhizosolenia setigera . Applied and Environmental Microbiology, 70, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri, U. , Wolf, Y.I. , Roux, S. , Camargo, A.P. , Lee, B. , Kazlauskas, D. et al. (2022) Expansion of the global RNA virome reveals diverse clades of bacteriophages. Cell, 185(21), 4023–4037.e18. [DOI] [PubMed] [Google Scholar]
- Pawlowski, J. , Audic, S. , Adl, S. , Bass, D. , Belbahri, L. , Berney, C. et al. (2012) CBOL protist working group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS Biology, 10, e1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck, M.J. (2012) Plant virus metagenomics: biodiversity and ecology. Annual Review of Genetics, 46, 359–369. [DOI] [PubMed] [Google Scholar]
- Sasai, S. , Tamura, K. , Tojo, M. , Herrero, M.‐L. , Hoshino, T. , Ohki, S.T. et al. (2018) A novel non‐segmented double‐stranded RNA virus from an Arctic isolate of Pythium polare . Virology, 522, 234–243. [DOI] [PubMed] [Google Scholar]
- Shi, M. , Lin, X.‐D. , Chen, X. , Tian, J.‐H. , Chen, L.‐J. , Li, K. et al. (2018) The evolutionary history of vertebrate RNA viruses. Nature, 556, 197–202. [DOI] [PubMed] [Google Scholar]
- Shi, M. , Lin, X.‐D. , Tian, J.‐H. , Chen, L.‐J. , Chen, X. , Li, C.‐X. et al. (2016) Redefining the invertebrate RNA virosphere. Nature, 540, 539–543. [DOI] [PubMed] [Google Scholar]
- Shirai, Y. , Tomaru, Y. , Takao, Y. , Suzuki, H. , Nagumo, T. & Nagasaki, K. (2008) Isolation and characterization of a single‐stranded RNA virus infecting the marine planktonic diatom Chaetoceros tenuissimus Meunier. Applied and Environmental Microbiology, 74, 4022–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, E.P. , Nuccio, E.E. , Pett‐Ridge, J. , Banfield, J.F. & Firestone, M.K. (2019) Metatranscriptomic reconstruction reveals RNA viruses with the potential to shape carbon cycling in soil. Proceedings of the National Academy of Sciences of the United States of America, 116, 25900–25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward, G.F. , Culley, A.I. , Mueller, J.A. , Wood‐Charlson, E.M. , Belcaid, M. & Poisson, G. (2013) Are we missing half of the viruses in the ocean? The ISME Journal, 7, 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, V. , Lawrence, J.E. , Lang, A.S. , Chan, A.M. , Culley, A.I. & Suttle, C.A. (2003) Characterization of HaRNAV, a single‐stranded RNA virus causing lysis of Heterosigma akashiwo (Raphidophyceae). Journal of Phycology, 39, 343–352. [Google Scholar]
- Tedersoo, L. , Mikryukov, V. , Zizka, A. , Bahram, M. , Hagh‐Doust, N. , Anslan, S. et al. (2022) Global patterns in endemicity and vulnerability of soil fungi. Global Change Biology, 28, 6696–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaru, Y. , Katanozaka, N. , Nishida, K. , Shirai, Y. , Tarutani, K. , Yamaguchi, M. et al. (2004) Isolation and characterization of two distinct types of HcRNAV, a single‐stranded RNA virus infecting the bivalve‐killing microalga Heterocapsa circularisquama . Aquatic Microbial Ecology, 34, 207–218. [Google Scholar]
- Tomaru, Y. , Takao, Y. , Suzuki, H. , Nagumo, T. & Nagasaki, K. (2009) Isolation and characterization of a single‐stranded RNA virus infecting the bloom‐forming diatom Chaetoceros socialis . Applied and Environmental Microbiology, 75, 2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaru, Y. , Toyoda, K. , Kimura, K. , Hata, N. , Yoshida, M. & Nagasaki, K. (2012) First evidence for the existence of pennate diatom viruses. The ISME Journal, 6, 1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama, S.‐I. , Takaki, Y. , Nishi, S. , Yoshida‐Takashima, Y. , Deguchi, S. , Takai, K. et al. (2018) Unveiling the RNA virosphere associated with marine microorganisms. Molecular Ecology Resources, 18, 1444–1455. [DOI] [PubMed] [Google Scholar]
- Vago, C. (1966) A virus disease in crustacea. Nature, 209, 13–34. [Google Scholar]
- Vieira, A.C. , Lopes, Í.S. , Fonseca, P.L.C. , Olmo, R.P. , Bittencourt, F. , de Vasconcelos, L.M. et al. (2022a) Expanding the environmental virome: infection profile in a native rainforest tree species. Frontiers in Microbiology, 13, 874319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, P. , Subbotin, S.A. , Alkharouf, N. , Eisenback, J. & Nemchinov, L.G. (2022b) Expanding the RNA virome of nematodes and other soil‐inhabiting organisms. Virus Evolution, 8, veac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlok, M. , Lang, A.S. & Suttle, C.A. (2019) Marine RNA virus quasispecies are distributed throughout the oceans. mSphere, 4, e00157‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, Y.I. , Kazlauskas, D. , Iranzo, J. , Lucía‐Sanz, A. , Kuhn, J.H. , Krupovic, M. et al. (2019) Reply to Holmes and Duchêne, “can sequence phylogenies safely infer the origin of the global virome?”: deep phylogenetic analysis of RNA viruses is highly challenging but not meaningless. mBio, 10, e00542‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, Y.I. , Kazlauskas, D. , Iranzo, J. , Lucía‐Sanz, A. , Kuhn, J.H. , Krupovic, M. et al. (2018) Origins and evolution of the global RNA virome. mBio, 9, e02329‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, Y.I. , Silas, S. , Wang, Y. , Wu, S. , Bocek, M. , Kazlauskas, D. et al. (2020) Doubling of the known set of RNA viruses by metagenomic analysis of an aquatic virome. Nature Microbiology, 5, 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. , Davison, M.R. , Gao, Y. , Nicora, C.D. , McDermott, J.E. , Burnum‐Johnson, K.E. et al. (2021) Moisture modulates soil reservoirs of active DNA and RNA viruses. Communications Biology, 4, 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Pang, R. , Cheng, T. , Xue, L. , Zeng, H. , Lei, T. et al. (2020) Abundant and diverse RNA viruses in insects revealed by RNA‐seq analysis: ecological and evolutionary implications. mSystems, 5, e00039‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed, A.A. , Wainaina, J.M. , Dominguez‐Huerta, G. , Pelletier, E. , Guo, J. , Mohssen, M. et al. (2022) Cryptic and abundant marine viruses at the evolutionary origins of Earth's RNA virome. Science, 376, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]


