Abstract
Background & Aims:
Nonalcoholic steatohepatitis (NASH) is prevalent in adults with obesity and can progress to cirrhosis. As a secondary analysis of prospectively-acquired data from the multicenter, randomized, placebo-controlled clinical trial Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT), we investigated the relationship between reduction in adipose tissue compartment volumes and hepatic histologic improvement.
Methods:
Adult participants in the FLINT trial with paired liver biopsies and abdominal MRI exams at baseline and end-of-treatment (72 weeks) were included (n = 76). Adipose tissue compartment volumes were obtained using magnetic resonance imaging (MRI).
Results:
Treatment and placebo groups did not differ in baseline adipose tissue volumes, or in change in adipose tissue volumes longitudinally (P = 0.107 to 0.745). Deep subcutaneous adipose tissue (dSAT) and visceral adipose tissue (VAT) volume reductions were associated with NASH histologic improvement (i.e., nonalcoholic fatty liver disease activity score [NAS] reduction of two points, at least one point from lobular inflammation or hepatocellular ballooning, and no worsening of fibrosis) (P = 0.031, and 0.030, respectively). In a stepwise logistic regression procedure, which included demographics, treatment group, baseline histology, baseline and changes in adipose tissue volumes, MRI hepatic proton density fat fraction (PDFF), and serum aminotransferases as potential predictors, reductions in dSAT and PDFF were associated with NASH histologic improvement (regression coefficient = −2.001 and −0.083, P = 0.044 and 0.033, respectively).
Conclusions:
In adults with NASH in the FLINT trial, those with greater longitudinal reductions in dSAT and potentially VAT volumes showed greater hepatic histologic improvements, independent of reductions in hepatic PDFF.
Keywords: central obesity, deep subcutaneous adipose tissue, visceral adipose tissue, liver histology
Graphical abstract
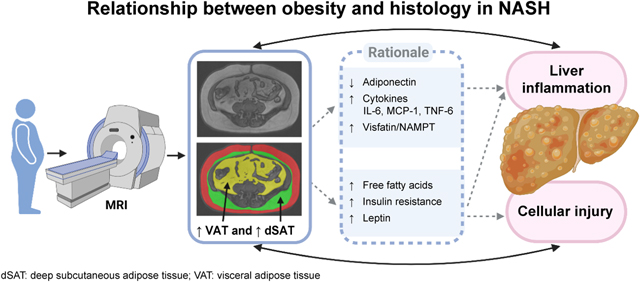
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is commonly seen in adults with obesity and can progress to cirrhosis, particularly in the subset of patients with nonalcoholic steatohepatitis (NASH) [1]. NAFLD and NASH are also associated with increased risks of cardiovascular disease and diabetes [2–5]. Although central obesity has been identified as a risk factor for obesity-related disorders including insulin resistance and cardiovascular disease [6, 7], the role of central obesity in NASH needs further clarification [8].
Once thought to be a stable energy storage depot, adipose tissue is now viewed as a diverse, hormone-secreting, metabolically active tissue [9]. Abdominal adipose tissue, especially visceral adipose tissue (VAT), is an important source of pro-inflammatory stimuli associated with inflammation and metabolic dysregulation [10–13] and may contribute to histologic alterations seen in NAFLD and NASH. Although previous studies have observed associations between increased VAT volume and NASH fibrosis stage [14], no study has examined whether a reduction in VAT volume predicts NASH histologic improvement. Further, most studies investigating the relationship between VAT volume and NASH or NAFLD have either used surrogate measures of VAT volume (i.e., anthropometrics or ultrasound), or performed only cross-sectional analysis of histologic data [14–19].
Subcutaneous adipose tissue (SAT), another important depot, is divided into two layers, superficial SAT (sSAT) and deep SAT (dSAT) [20–23] (Figure 1). Prior reports suggest that dSAT closely relates to VAT in function and may influence liver histology [20–23]. Increased macrophages and inflammatory factors were found in dSAT of NASH patients [20], and dSAT may be linked to NASH through its effect on insulin resistance [8, 24].
Fig.1. Sample adipose tissue depot segmentation.

Left panel shows 3D-reconstructed superficial subcutaneous adipose tissue (sSAT) (red, top left), deep subcutaneous adipose tissue (dSAT) (green, middle left), and visceral adipose tissue (VAT) (yellow, bottom left). Superficial and deep subcutaneous adipose tissue are divided by the fascia (indicated with white arrows) that separates the two adipose tissue depots. Right panel shows an MRI slice at L4-L5 level (top right) and segmentation of adipose tissue depots in the same colors overlaid on the MRI slice (bottom right).
A phase 2b clinical trial entitled ‘Farnesoid X Receptor (FXR) Ligand Obeticholic Acid in NASH Treatment (FLINT)’ demonstrated that administration of obeticholic acid for 72 weeks in adults resulted in NASH histologic improvement [25]. Additionally, in the subset of participants who underwent abdominal magnetic resonance imaging (MRI), a 30% reduction in hepatic proton density fat fraction (PDFF) has been established as a quantitative imaging biomarker for NASH histologic improvement [26]. In the present analysis, without intent to develop a surrogate biomarker of NASH, we aimed to determine whether longitudinal reductions in abdominal adipose tissue depot volumes in the FLINT trial were independently associated with NASH histologic improvement.
EXPERIMENTAL PROCEDURES
Protocol and design
This was a retrospective, longitudinal, secondary ancillary analysis of data acquired prospectively in the FLINT trial (termed the ‘parent study,’ NCT01265498) that was conducted by the National Institute of Diabetes and Digestive and Kidney Diseases-sponsored NASH Clinical Research Network (NASH CRN). The FLINT trial was a multicenter, double-blind, placebo-controlled, randomized clinical trial conducted at medical centers in the USA in adult participants with non-cirrhotic NASH to assess efficacy of treatment with obeticholic acid for 72 weeks. Inclusion and exclusion criteria of the FLINT trial have been reported [25]. Histology slides were scored in consensus by a panel of expert pathology investigators of the NASH CRN [25, 27].
A subset of participants in the FLINT trial underwent MRI exams at baseline and after 72 weeks of treatment [28]. The MRI exams included pulse sequences for quantifying hepatic PDFF and volumes of adipose tissue depots, namely sSAT, dSAT, and VAT. Inclusion criteria for the MRI portion of the FLINT trial were willingness and ability to complete both the baseline MRI exam (before randomization, within 90 days of baseline biopsy), and the end-of-treatment MRI exam (within 90 days of end-of-treatment biopsy) [28]. MRI exam exclusion criteria were contraindication to MRI, extreme claustrophobia, pregnancy or trying to become pregnant, weight or girth exceeding MRI scanner capability, or any conditions or circumstances that in the opinion of the clinical trial site investigator would interfere with completion of the MRI exam. Participants in the FLINT trial who had paired liver biopsy and abdominal MRI at baseline and at end-of-treatment were included in this analysis (n = 76). We investigated whether changes in adipose tissue depot volumes, namely, sSAT, dSAT, and VAT, and changes in anthropometrics including weight, body mass index (BMI), and waist circumference in the treatment and placebo groups were related to NASH histologic improvement. In this secondary ancillary study, NASH histologic improvement was defined as a composite of improvement in the NAS by ≥ 2 points, with at least 1 point improvement in the scores for ballooning or lobular inflammation, and no worsening of the fibrosis stage, a variation of the original definition which did not specify reductions in specific scores [25, 29]. We additionally investigated whether changes in adipose tissue volumes are related to change in SAF-A score (the activity part of the Steatosis, Activity, Fibrosis [SAF] scoring system that incorporates scores for ballooning and inflammation), a recently used index as an eligibility criterion in a NASH clinical trial [30].
Participants in the FLINT trial provided written informed consent at the time of enrollment. The FLINT trial, including its MRI portion and this secondary ancillary analysis were approved by an Institutional Review Board at each participating clinical trial site and complied with the Health Insurance Portability and Accountability Act.
Magnetic resonance imaging
Seven of the eight participating FLINT clinical trial sites contributed MRI data to this analysis (1.5T at three sites, 3T at four sites). At each site, only a single MRI scanner was used to minimize longitudinal instrument-dependent measurement variability. As described previously [28], the NASH CRN Radiology Coordinating Center (RCC) provided a standardized MRI protocol that included axial T1-weighted imaging through the abdomen and pelvis and axial PDFF imaging through the liver. Following acquisition, MRI exams obtained at each site were transmitted to the RCC for analysis.
Adipose tissue depots including sSAT, dSAT, and VAT (Figure 1) were semi-automatically segmented on T1-weighted images by an RCC image analyst (TID) under the supervision of an abdominal radiologist (GMC) (sliceOmatic 5.0, Tomovision, Montreal). Images were analyzed for sSAT, dSAT, and VAT volumes on six slices at the T12-L1, L1-L2, L2-L3, L3-L4, L4-L5, and L5-S1 inter-vertebral disk levels. The adipose tissue segmentation technique included threshold-based tools such as region growing (i.e., segmenting neighboring pixels of initial seed points and determining whether the pixel neighbors should be added to the region using a threshold range). Manual editing was applied when necessary. The fascia that separated sSAT and dSAT was manually traced when evident, or its location was inferred by image analysts to the best of their ability. Total sSAT, dSAT, and VAT volumes from T12-L1 to L5-S1 were calculated by interpolating between analyzed slices using the following formula:
where V was volume, Ai was the area selected on the ixh analyzed slice, hi was the interval between adjacent intervertebral disks at the ith analyzed slice, t was slice thickness, and 6 was the total number of analyzed slices [31]. The coefficient of variation for repeated acquisition (i.e., participant off table and repositioned) and analysis of adipose depots are 2.6% to 4.0% for the NASH CRN Radiology Coordinating Center [32].
Hepatic PDFF was analyzed at the RCC as previously described [28].
Statistical methods
The analysis sample was summarized overall and by parent study treatment group. Participant data were presented as the mean ± SD for continuous variables, and as numbers and percentages for categorical variables (Table 1). Treatment and placebo groups were compared on baseline characteristics and on changes over time using two-sample t-tests for continuous measures, or Fisher’s exact tests for ordinal and categorical measures. Significance of changes was evaluated with paired t-tests or Wilcoxon-Mann-Whitney tests.
Table 1.
Baseline characteristics
| Entire sample n=76 |
Treatment group n=39 |
Placebo group n=37 |
P value | |
|---|---|---|---|---|
| Male | 28(37%) | 14(36%) | 14(38%) | 1.000 |
| Race | 0.785 | |||
| Asian | 5(7%) | 2(5%) | 3(8%) | |
| Black or African American | 1(1%) | 0(0%) | 1(3%) | |
| White | 65(86%) | 34(87%) | 31(84%) | |
| Other | 5(7%) | 3(8%) | 2(5%) | |
| Hispanic | 13(17%) | 7(18%) | 6(16%) | 1.000 |
| Age (yrs) | 52.4(10.9) | 53.0(8.7) | 51.8(12.8) | 0.632 |
| Body mass index (kg/m2) | 33.7(5.1) | 34.4(5.5) | 33.0(4.7) | 0.242 |
| Weight (kg) | 95.1(16.3) | 96.1(17.8) | 93.9(14.8) | 0.560 |
| Waist circumference (cm) | 110.2(12.1) | 112.4(12.4) | 107.9(11.5) | 0.108 |
| Definite steatohepatitis | 60(79%) | 31(80%) | 29(78%) | 1.000 |
| Fibrosis stage | 18(11) | 19(11) | 17(11) | 0.491 |
| 0 | 13(17%) | 5(13%) | 8(22%) | |
| 1 | 14(18%) | 9(23%) | 5(13%) | |
| 2 | 23(30%) | 10(26%) | 13(35%) | |
| 3 | 25(34%) | 14(36%) | 11(30%) | |
| 4 | 1(1%) | 1(2%) | 0(0%) | |
| Total NAFLD activity score | 5.2(1.3) | 5.1(1.3) | 5.4(1.3) | 0.932 |
| 3 | 7(9%) | 4(10%) | 3(8%) | |
| 4 | 17(22%) | 10(26%) | 7(19%) | |
| 5 | 18(24%) | 8(21%) | 10(27%) | |
| 6 | 22(29%) | 12(31%) | 10(27%) | |
| 7 | 9(12%) | 4(10%) | 5(14%) | |
| 8 | 3(4%) | 1(3%) | 2(5%) | |
| Hepatocellular ballooning score | 1.4(0.7) | 1.4(0.8) | 1.4(0.7) | 0.812 |
| 0 | 11(14%) | 6(15%) | 5(14%) | |
| 1 | 24(32%) | 11(28%) | 13(35%) | |
| 2 | 41(54%) | 22(57%) | 19(51%) | |
| Steatosis score | 2.0(0.8) | 1.9(0.8) | 2.1(0.8) | 0.767 |
| 0 | 1(1%) | 1(3%) | 0(0%) | |
| 1 | 22(29%) | 12(31%) | 10(27%) | |
| 2 | 32(42%) | 17(43%) | 15(41%) | |
| 3 | 21(28% | 9(23%) | 12(32%) | |
| Lobular inflammation score | 1.9(0.7) | 1.8(0.7) | 1.9(0.8) | 0.795 |
| 1 | 25(33%) | 13(33%) | 12(33%) | |
| 2 | 35(46%) | 19(49%) | 16(43%) | |
| 3 | 16(21%) | 7(18%) | 9(24%) | |
| Portal inflammation score | 1.2(0.6) | 1.2(0.6) | 1.2(0.6) | 0.733 |
| 0 | 7(9%) | 4(10%) | 3(8%) | |
| 1 | 46(61%) | 22(57%) | 24(65%) | |
| 2 | 23(30%) | 13(33%) | 10(27%) | |
| Alanine aminotransferase (U/L) | 74(38) | 79(41) | 68(34) | 0.212 |
| Aspartate aminotransferase (U/L) | 54(29) | 57(33) | 50(25) | 0.298 |
| Hepatic proton density fat fraction (%) | 19(9) | 18(8) | 20(10) | 0.359 |
| Superficial subcutaneous adipose tissue (L) | 2.9(1.3) | 2.9(1.2) | 2.8(1.4) | 0.745 |
| Deep subcutaneous adipose tissue (L) | 2.3(0.8) | 2.4(0.9) | 2.3(0.8) | 0.687 |
| Visceral adipose tissue (L) | 3.4(1.3) | 3.6(13) | 3.2(1.2) | 0.177 |
Data are presented as n(%) or mean(SD); NAFLD, nonalcoholic fatty liver disease; SAT, superficial subcutaneous adipose tissue; dSAT, deep subcutaneous adipose tissue; VAT, visceral adipose tissue.
P values for comparisons between treatment and placebo groups are based on the Fisher’s Exact test for ordinal and categorical variables and the two-sample t-test for continuous variables.
Spearman’s correlations were computed between changes in weight, BMI, waist circumference, sSAT, dSAT, VAT, and hepatic PDFF, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Spearman’s correlations were also computed between changes in individual adipose depots and changes in ballooning, portal inflammation, and lobular inflammation. Relationships between changes in individual adipose depots and changes in histology were illustrated with boxplots.
A Bayesian information criterion (BIC)-based stepwise linear regression procedure was used to search for a model predicting NASH histologic improvement or SAF-A score. Of main interest in the set of potential predictors were changes in sSAT, dSAT, and VAT volumes. Additionally, the potential predictor pool included age, sex, ethnicity, treatment group, baseline weight, baseline sSAT, dSAT, and VAT volumes, baseline hepatic PDFF, baseline histology, baseline alanine aminotransferase (ALT), baseline aspartate aminotransferase (AST), weight loss, change in hepatic PDFF, change in ALT and change in AST. The procedure uses both forward selection and backward elimination to select the best small model, using penalized goodness-of-fit (i.e., BIC) to choose between competing models.
A BIC-based stepwise linear regression procedure was also used to search for a model predicting NASH histologic improvement using anthropometric measures instead of MRI measures. The potential predictor pool included weight loss, BMI change, waist circumference change, age, sex, ethnicity, treatment group, baseline weight, baseline BMI, baseline waist circumference, baseline histology, baseline ALT, baseline AST, change in ALT, and change in AST.
The anthropometric model and the MRI model were compared using chi-square test of deviances.
Two-tailed (α = 0.05) tests of significance were used.
Statistical analyses were carried out using R 3.5.1 statistical software (R Foundation for Statistical Computing, Vienna, Austria, 2018).
RESULTS
Descriptive statistics
Baseline characteristics of the study cohort are shown in Table 1 (age range 24 to 74 yrs; 28 men and 48 women). Most participants were white (n = 65, 86%). There were 39 participants in the treatment group and 37 participants in the placebo group. Almost all participants were overweight or obese, with BMI ranging from 24.8 to 45.4 kg/m2 and weight ranging from 69 to 135 kg. sSAT volume ranged from 0.9 to 6.6 L, dSAT volume ranged from 0.9 to 4.2 L, and VAT volume ranged from 1.2 to 7.1 L. Treatment and placebo groups did not differ in baseline BMI, sSAT, dSAT, VAT, ALT, AST, histology scores, or hepatic PDFF (P = 0.177 to 0.745) (Table 1).
Mean BMI, sSAT, dSAT, and VAT volumes did not change from baseline to end-of-treatment in either group (paired t-test P = 0.062 to 0.840), and the treatment and placebo groups did not differ in changes of these parameters (P = 0.103 to 0.718). Although the treatment and placebo groups differed in changes in weight (-0.99 ± 5.19 kg, 1.36 ± 4.9 kg, P = 0.046), the change in weight in neither the treatment group (P = 0.242) nor the placebo group (P = 0.101) reached statistical significance. For individual participants, changes in adipose tissue volumes ranged from −1.1 to 1.0 L for sSAT, −0.8 to 1.1 L for dSAT, and −1.1 to 1.1 L for VAT. ALT and AST decreased in the treatment group (median, −10 U/L, Wilcoxon P < 0.001; −10 U/L, P = 0.001, respectively), but not in the placebo group (-3 U/L, Wilcoxon P = 0.152; −1 U/L, Wilcoxon P = 0.658, respectively). Hepatic PDFF decreased in the treatment group (−3.0 ± 8.6%, P = 0.036) , but not in the placebo group (−1.5 ± 9.2%, P = 0.326). However, there was no difference in hepatic PDFF reduction between treatment and placebo groups (P = 0.468). There were also no differences in lobular inflammation change, portal inflammation change, ballooning change, or fibrosis change between treatment and placebo groups (Wilcoxon P = 0.477 to 0.878). Therefore, in subsequent analyses, treatment and placebo groups were merged.
Univariate associations with histologic changes
Pairwise Spearman’s correlations between changes in weight, BMI, waist circumference, sSAT, dSAT, VAT, and hepatic PDFF ranged from 0.17 to 0.65 (P < 0.001 to 0.134) (Table 2). The Spearman’s correlation between HOMA-IR change and sSAT change, dSAT change, and VAT change were 0.06 (P = 0.609), 0.20 (P = 0.088), and 0.29 (P = 0.011).
Table 2.
Spearman’s correlations among change in anthropometrics and adipose depots (n=76)
| ΔBMI | ΔWaist circumference | ΔsSAT | ΔdSAT | ΔVAT | ΔHepatic PDFF | |
|---|---|---|---|---|---|---|
| ΔWeight | 0.88 (P<0.001) |
0.71 (P<0.001) |
0.65 (P<0.001) |
0.55 (P<0.001) |
0.62 (P<0.001) |
0.32 (P=0.006) |
| ΔBMI | - | 0.68 (P<0.001) |
0.55 (P<0.001) |
0.49 (P<0.001) |
0.65 (P<0.001) |
0.30 (P=0.008) |
| ΔWaist circumference | - | - | 0.35 (P=0.002) |
0.35 (P=0.002) |
0.53 (P=0.002) |
0.34 (P=0.003) |
| ΔsSAT | - | - | - | 0.378 (P=0.001) |
0.512 (P<0.001) |
0.306 (P=0.007) |
| ΔdSAT | - | - | - | - | 0.614 (P<0.001) |
0.174 (P=0.134) |
| ΔVAT | - | - | - | - | - | 0.363 (P=0.001) |
Δ, change; BMI, body mass index; sSAT, superficial subcutaneous adipose tissue; dSAT, deep subcutaneous adipose tissue; VAT, visceral adipose tissue; PDFF, proton density fat fraction.
As illustrated in the box plots (Figure 2A, 2B, and 2C), VAT change and dSAT change were significantly correlated with NASH histologic improvement (P = 0.031and P = 0.030, respectively), whereas sSAT change was not significantly correlated with NASH histologic improvement (P = 0.911).
Fig. 2. Boxplots showing the relationship between changes in adipose depot volumes and histologic improvement in NASH.

Relationships (A) between change in sSAT and histologic improvement in NASH (P = 0.911, Wilcoxon test); (B) change in dSAT and histologic improvement in NASH (P = 0.030, Wilcoxon test); (C) change in VAT and histologic improvement in NASH (P = 0.031, Wilcoxon test); (D) change in sSAT, dSAT and VAT for different degrees of change of lobular inflammation score (Spearman’s correlation ρ = −0.1, P = 0.399; ρ = 0.04, P = 0.737, and ρ = 0.21, P = 0.066, respectively); (E) change in sSAT, dSAT and VAT for different degrees of change of portal inflammation score (Spearman’s correlation ρ = −0.13; P = 0.265, ρ = −0.03, P = 0.789, and ρ = −0.15, P = 0.19, respectively); (F) change in sSAT, dSAT and VAT for different degrees of change of hepatocellular ballooning score (Spearman’s correlation ρ = 0.13, P = 0.252; ρ = 0.28, P = 0.015, and ρ = 0.16, P = 0.173, respectively). dSAT, deep subcutaneous adipose tissue; NASH, non-alcoholic steatohepatitis; sSAT, superficial subcutaneous adipose tissue; VAT, visceral adipose tissue.
As illustrated in the box plots (Figure 2D, 2E, and 2F), dSAT change was significantly correlated with ballooning change (ρ = 0.28, P = 0.015), and VAT change was correlated at a trend-level with lobular inflammation change (ρ = 0.21, P = 0.066). Adipose tissue volume changes did not significantly correlate with portal inflammation change (P = 0.190 to 0.789). sSAT change did not significantly correlate with lobular inflammation change, portal inflammation change, or ballooning change (P = 0.252 to 0.399).
Multivariable modeling of histologic changes
Summaries of the final model are presented in Table 3.
Table 3.
Regression model with NASH histologic improvement as the dependent variable.
| Independent variables | Regression coefficients (P value) |
|---|---|
| Baseline lobular inflammation score | 1.21 (P=0.011) |
| dSAT change | −2.00 (P=0.044) |
| Hepatic PDFF change | −0.08 (P=0.033) |
| Goodness of fit (deviance) test | P=0.0016 |
dSAT, deep subcutaneous adipose tissue; PDFF, proton density fat fraction.
A Bayesian information criterion-based stepwise linear regression procedure was used to search for a model predicting NASH histologic improvement. Potential predictors included age, sex, race, ethnicity, treatment group, baseline histology, baseline values and changes of weight, superficial subcutaneous adipose tissue, dSAT, visceral adipose tissue, hepatic PDFF, alanine aminotransferase and aspartate aminotransferase.
For NASH histologic improvement, the BIC-based search identified a model with baseline lobular inflammation, change in dSAT, and change in PDFF as the three predictors (Table 3). The relationship between baseline lobular inflammation and histologic improvement was positive: higher lobular inflammation at baseline was associated with histologic improvement. The relationship between change in dSAT and histologic improvement was negative: greater reduction in dSAT volume was associated with histologic improvement. Reduction in PDFF was associated with histologic improvement.
In the anthropometric model, change in waist circumference has been identified as the single predictor of NASH histologic improvement.
Histologic improvement =−1.189–0.069(waist circumference change) (Goodness of fit Chi-Square P=0.026)
The MRI model with adipose tissue depots was significantly better than the simple anthropometric model (Chi-square p-value for comparing goodness of fit = 0.006). The relationship between changes in weight, waist circumference, adipose tissue depots and NASH histologic improvement are further illustrated in Supplemental materials (Supplemental Figure 1).
For SAF-A score change, the BIC-based search identified the below model.
SAF-A score change=1.170+0.066(weight change)+0.13(AST change)–0.824(baseline lobular inflammation) (R2=0.39, P<0.001).
DISCUSSION
This secondary analysis of prospectively-collected data from the FLINT trial found that in adults with NASH, those with greater longitudinal reductions in dSAT and VAT volumes were more likely to have histologic improvement. Our results also showed that body weight change is a predictor of change in SAF-A score; there is a trend that the participants with both body weight loss and dSAT or VAT change are more likely to have histologic improvement than participants with reduction in only weight, dSAT, or VAT (Supplemental Figure 1). Reduction in sSAT volume was unrelated to histologic improvement. Although previous studies have linked central adiposity to NASH [14] and weight loss to histologic improvement [33], the present analysis suggests that reductions in abdominal adipose tissue depot volumes may correlate with histologic improvement independent of change in hepatic fat content. As there was not a difference in the reduction in dSAT or VAT between the treatment and placebo groups, dSAT and VAT volume changes at the individual level observed in the present analysis are unlikely to be an effect of obeticholic acid treatment in the subset of patients included in this analysis. Instead, these changes may be driven by diet, behavioral, or other lifestyle modifications motivated by participation in this clinical trial.
Strengths of this study include longitudinal study design, volumetric assessment of SAT and VAT rather than a single-slice approach [34], central blinded reading of histology to determine improvement in specific NASH features, and central analyses of MRI exams. As previously shown, multi-slice MRI exams collected in the FLINT trial were better suited to detect small changes in SAT and VAT volumes than single-slice protocols that have been used in other studies [34]. The present analysis also takes advantage of MRI assessment of hepatic PDFF imaging that was acquired in the parent study [28]. Since hepatic PDFF provides more precise estimation of hepatic steatosis than biopsy [35], the inclusion of PDFF in this analysis allowed investigation of the relationship between abdominal adipose tissue depots and NASH histologic improvement independent of liver fat content. In this study, we adopted a definition of NASH histologic improvement that is different from that of the parent FLINT study by requiring at least 1 point improvement in ballooning or lobular inflammation scores. This more rigorous definition aligns with regulatory guidelines as they have evolved since the FLINT study was designed to exclude patients with improvement in just steatosis or inflammation from being classified as improved [36]. Previous studies used a similar definition by requiring at least a 1 point improvement in the NAS for ballooning [29], and we further included improved lobular inflammation as lobular inflammation improvement is independently associated with fibrosis improvement [29].
In this study we found that reduction in dSAT volume was associated with NASH histologic improvement, suggesting that dSAT might be related to exacerbation of NASH. Previous studies have linked increased dSAT volume to altered insulin resistance [21, 24, 37], which accelerates the development of NASH [38]. Insulin resistance promotes the accumulation of fat in the liver and increases fatty acid oxidation through the influx of serum free fatty acids, augmenting intracellular oxidative stress and hepatocellular injury that contributes to the development and progression of NASH [39, 40]. Furthermore, SAT is one of the main secretors of leptin. A previous study reported strong correlation between leptin levels and NASH, which may imply that leptin plays a role in regulating insulin levels [41]. In FLINT trial participants, who had a higher BMI than the general population, it is possible that mechanism(s) for reduction in dSAT volume led to NASH histologic improvement through improvement in insulin resistance, as increased dSAT volume associates with insulin resistance [24].
VAT change did not enter the final model of NASH histologic improvement, which might be explained by the high correlation between dSAT and VAT (Spearman correlation coefficient of 0.614, P < 0.001). Univariate correlation between VAT and histologic improvement suggests that VAT may play a role in NASH histology, consistent with previous reports on potential associations between VAT, and NAFLD and NASH [14, 15, 18, 19]. VAT might influence liver histology and contribute to the development of NASH through metabolic dysregulation and by promoting inflammation through multiple pathways [10–13]. Increased VAT volume is linked to reduced release of protective adipokines such as adiponectin, a molecule with insulin-sensitizing, anti-inflammatory, and anti-fibrotic effects [42, 43], and elevated release of pro-inflammatory adipokines such as visfatin/nicotinamide phosphoribosyltransferase (NAMPT) which are thought to promote the development of NASH [44]. Previous studies demonstrated that the I148M polymorphism of Patatin-like phospholipase domain-containing 3 (PNPLA3) is related to NAFLD development and progression to hepatic fibrosis [45, 46]. More recent studies have reported that high VAT volume also might accentuate the role of the PNPLA3 in NAFLD [47]. Finally, the genes encoding arachidonic acid, sphingolipid and glycosphingolipid metabolism are upregulated in NASH and may contribute to VAT-mediated inflammation [48].
Although the present analysis showed that VAT volume change correlated with lobular inflammation improvement, and dSAT volume change correlated with ballooning improvement, liver inflammation and hepatocellular ballooning are pathologic processes that are themselves correlated [25]. It is likely that reduction in dSAT and VAT volumes both are associated with improvements in liver inflammation and cellular injury. Future studies are needed to clarify how VAT and dSAT interact in promoting NASH pathogenesis.
Our results highlight that reduction in central obesity, specifically dSAT and VAT, may be related to NASH histologic improvement. The findings from this analysis should increase awareness of the importance of lifestyle intervention in NASH. Future studies and clinical practice may design interventions that assess the reduction of dSAT and VAT volumes as outcome measures, rather than simply weight reduction. Plausibly, reduction of dSAT and VAT volumes may benefit both NASH histologic improvement, and the mitigation of cardiovascular complications accompanying NASH.
Our analysis was limited by sample size (i.e., only one-third of the participants of the parent study consented to MRI). The small sample size in the placebo group (n=37) did not permit examination of how adipose tissue depot volume changes may influence the natural course of NASH without drug treatment. Because of the nature of the parent study, the present analysis was not designed to investigate drug effects on adipose tissue depots. Although we theorized how adipose tissue depot volumes might influence liver inflammation and cellular injury, our data cannot determine causality and we do not have adipokine (e.g., adiponectin and leptin) or inflammatory cytokine (e.g., IL-1, IL-6, and tumor necrotic factor alpha) data available. In addition, the parent study did not collect diet or exercise data, so assessments of contributions from diet and exercise on adipose tissue volume reduction and NASH histologic improvement were not possible. Larger independent studies are needed to further validate these results and to define the mechanism(s) by which changes in dSAT or VAT volumes are associated with reductions in inflammation and cell injury. Finally, our analysis sample is largely comprised of non-Hispanic Caucasian individuals. Whether our findings can be reproduced in other racial and ethnic groups remains to be determined.
In conclusion, our analyses of data acquired in the FLINT trial found that in adults with NASH, longitudinal reductions in dSAT and potentially VAT volumes were associated with histologic improvement, independent of reduction in hepatic PDFF. Reduction in sSAT volume alone did not relate to NASH histologic improvement.
Supplementary Material
Impact and implications.
Although central obesity has been identified as a risk factor for obesity-related disorders including insulin resistance and cardiovascular disease, the role of central obesity in nonalcoholic steatohepatitis (NASH) needs further clarification. Our results highlight that reduction in central obesity, specifically deep subcutaneous adipose tissue and visceral adipose tissue, may be related to NASH histologic improvement. The findings from this analysis should increase awareness of the importance of lifestyle intervention in NASH for clinical researchers and clinicians. Future studies and clinical practice may design interventions that assess the reduction of deep subcutaneous adipose tissue and visceral adipose tissue as outcome measures, rather than simply weight reduction.
Highlights.
This is a secondary analysis of a subgroup of patients who had MRI exams in the FLINT trial.
NASH patients with greater loss of deep subcutaneous adipose tissue showed greater levels of histologic improvement.
NASH patients with greater loss of visceral adipose tissue probably also had greater levels of histologic improvement.
Conflict of interest statement
Dr. Middleton reports consultation to Alimentiv, Arrowhead, Glympse, Kowa, Median, and Novo Nordisk; lab service agreements under auspices of UCSD from Alexion, AstraZeneca, Bristol-Myers Squibb, Celgene, Enanta, Galmed, Genzyme, Gilead, Guerbet, Intercept, Ionis, Janssen, Janssen, NuSirt, Organovo, Pfizer, Roche, Sanofi, Shire, Synageva, and Takeda; stockholder Pfizer; and co-founder Quantix Bio.
Dr. Fowler reports personal consultation for Epigenomics, GE, Bayer and grant support from GE, Siemens, Philips, Bayer, FNIH, Gilead, and Pfizer. Unpaid position in advisory board of Quantix Bio.
Dr. Trout received research grants from Canon Medical Systems and Siemens Medical Solutions
Dr. Bashir received funding from Carmot Therapeutics, Corcept Therapeutics, CymaBay Therapeutics, Diabetes & Endocrinology Consultants, Madrigal Pharmaceuticals, Metacrine Inc, NGM Biopharmaceuticals, Pinnacle Clinical Research, ProSciento Inc, Siemens Healthineers. Dr. Bashir has been a consultant for MedPace, ICON, Corcept.
Dr. Loomba serves on the Steering Committee of the REGENERATE Trial funded by Intercept Pharmaceuticals. In addition, he has received research grants from Siemens Diagnostics and GE. He has research collaborations with AMRA and Antaros. He serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse Bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, Prometheus, and Siemens. He is a co-founder of Liponexus, Inc.
Dr. Neuschwander-Tetri has been and advisor or consultant for Alimentiv, Allergan, Allysta, Alnylam, Amgen, Arrowhead, Axcella, Boehringer Ingelheim, BMS, Coherus, Cymabay, Enanta, Fortress, Genfit, Gilead, High Tide, HistoIndex, Innovo, Intercept, Ionis, LG Chem, Lipocine, Madrigal, Medimmune, Merck, Mirum, NGM, NovoNordisk, Novus Therapeutics, pH-Pharma, Sagimet, Target RWE, 89Bio; Stock options: HepGene; Institutional research grants: Allergan, BMS, Cirius, Enanta, Genfit, Gilead, Intercept, Madrigal, NGM
Dr. Sanyal is President of Sanyal Bio and has ownership interests in Genfit, Exhalenz, Tiziana, Inversago, Durect. He has served as a consultant to Astra Zeneca, Pfizer, Merck, Bristol Myers Squibb, Gilead, Boehringer Ingelhiem, Eli Lilly, Novo Nordisk, Hanmi, Intercept, Madrigal, Amgen, Regeneron, Blade, Genentech, Roche, Siemens, Glympse, 89Bio, Gelesis, Takeda, Salix and Malinckrodt. He gets royalties from Elsevier and Uptodate. His institution (VCU) receives grants from Pfizer, Astra Zeneca, Eli Lilly, Novo Nordisk, Hanmi, Gilead, Intercept, Boehringer Ingelhiem, Bristol Myers Squibb, Salix.
Dr. Sirlin reports grants from GE, Siemens, Philips, Bayer, Foundation of NIH, Gilead, and Pfizer (grant is to UW-Madison; UCSD is a subcontract to UW-Madison); equipment loan (ultrasound system) from GE; personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, IBM-Watson, and Pfizer; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; royalties from Wolters Kluwer for educational material outside the submitted work; honoraria to the institution from Medscape for educational material outside the submitted work; serving as Chief Medical Officer to Livivos with ownership of stock options; unpaid position in advisory board to Quantix Bio.
Dr. Lavine was an ad hoc consultant for Allergan, Gilead, Merck, Pfizer, Novartis, Intercept
No COI: Drs. Alazraki, Cunha, Delgado, Gamst, Kleiner, Ohliger, Shah, Shen, Wolfson, Zhou
Financial support statement:
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000439, UL1TR000077, UL1TR000436, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058, UL1TR000454). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. The FLINT trial was conducted by the NASH CRN and supported in part by a Collaborative Research and Development Agreement (CRADA) between NIDDK and Intercept Pharmaceuticals.
RL receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019).
The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, drafting of the manuscript, or in the decision to submit the article for publication.
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BIC
Bayesian information criterion
- BMI
body mass index
- dSAT
deep subcutaneous adipose tissue
- FXR
Farnesoid X Receptor
- FLINT
Farnesoid X Receptor (FXR) Ligand Obeticholic Acid in NASH Treatment
- MRI
magnetic resonance imaging
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- NAFLD
nonalcoholic fatty liver disease
- NAMPT
Nicotinamide phosphoribosyltransferase
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- NASH CRN
NASH Clinical Research Network
- NASH CRN RCC
NASH Clinical Research Network Radiology Coordinating Center
- PDFF
proton density fat fraction
- PNPLA3
Patatin-like phospholipase domain-containing 3
- SAF-A score
the activity part of the Steatosis, Activity, Fibrosis [SAF] scoring system that incorporates scores for ballooning and inflammation
- sSAT
superficial subcutaneous adipose tissue
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial number: NCT01265498
Data Availability Statement:
Data described in the manuscript will be made available upon request, pending the FLINT study committee’s approval.
REFERENCES
- [1].Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- [2].Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008;118:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Santoro N, Caprio S. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in obese adolescents: a looming marker of cardiac dysfunction. Hepatology 2014;59:372–374. [DOI] [PubMed] [Google Scholar]
- [4].Lawlor DA, Callaway M, Macdonald-Wallis C, Anderson E, Fraser A, Howe LD, et al. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab 2014;99:E410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol 2002;34:255–262. [DOI] [PubMed] [Google Scholar]
- [6].Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med 2015;163:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50–95 year olds. Diabetes Care 2006;29:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep 2019;1:312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 2000;11:327–332. [DOI] [PubMed] [Google Scholar]
- [10].Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–1013. [DOI] [PubMed] [Google Scholar]
- [11].Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis 2005;183:308–315. [DOI] [PubMed] [Google Scholar]
- [12].Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 2005;174:827–834. [DOI] [PubMed] [Google Scholar]
- [13].Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- [14].Eguchi Y, Mizuta T, Sumida Y, Ishibashi E, Kitajima Y, Isoda H, et al. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. Journal of gastroenterology 2011;46 Suppl 1:70–78. [DOI] [PubMed] [Google Scholar]
- [15].Vitturi N, Soattin M, De Stefano F, Vianello D, Zambon A, Plebani M, et al. Ultrasound, anthropometry and bioimpedance: a comparison in predicting fat deposition in non-alcoholic fatty liver disease. Eating and weight disorders : EWD 2015;20:241–247. [DOI] [PubMed] [Google Scholar]
- [16].Ercin CN, Dogru T, Genc H, Celebi G, Aslan F, Gurel H, et al. Insulin Resistance but Not Visceral Adiposity Index Is Associated with Liver Fibrosis in Nondiabetic Subjects with Nonalcoholic Fatty Liver Disease. Metabolic syndrome and related disorders 2015;13:319–325. [DOI] [PubMed] [Google Scholar]
- [17].Vongsuvanh R, George J, McLeod D, van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J Hepatol 2012;57:392–398. [DOI] [PubMed] [Google Scholar]
- [18].Choudhary NS, Duseja A, Kalra N, Das A, Dhiman RK, Chawla YK. Correlation of adipose tissue with liver histology in Asian Indian patients with nonalcoholic fatty liver disease (NAFLD). Annals of hepatology 2012;11:478–486. [PubMed] [Google Scholar]
- [19].Ha Y, Seo N, Shim JH, Kim SY, Park JA, Han S, et al. Intimate association of visceral obesity with non-alcoholic fatty liver disease in healthy Asians: A case-control study. Journal of gastroenterology and hepatology 2015;30:1666–1672. [DOI] [PubMed] [Google Scholar]
- [20].Tordjman J, Divoux A, Prifti E, Poitou C, Pelloux V, Hugol D, et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol 2012;56:1152–1158. [DOI] [PubMed] [Google Scholar]
- [21].Kim SH, Chung JH, Song SW, Jung WS, Lee YA, Kim HN. Relationship between deep subcutaneous abdominal adipose tissue and metabolic syndrome: a case control study. Diabetol Metab Syndr 2016;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15:1933–1943. [DOI] [PubMed] [Google Scholar]
- [23].Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, et al. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 2014;37:821–829. [DOI] [PubMed] [Google Scholar]
- [24].Bódis K, Jelenik T, Lundbom J, Markgraf DF, Strom A, Zaharia OP, et al. Expansion and impaired mitochondrial efficiency of deep subcutaneous adipose tissue in recent-onset type 2 diabetes. J Clin Endocrinol Metab 2019;105:e1331–e1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Loomba R, Neuschwander-Tetri BA, Sanyal A, Chalasani N, Diehl AM, Terrault N, et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in nonalcoholic steatohepatitis. Hepatology 2020;72:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, Nash Clinical Research Network. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology 2017;153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA, Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology 2019;70:522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N Engl J Med 2021;385:1547–1558. [DOI] [PubMed] [Google Scholar]
- [31].Shen W, Wang Z, Tang H, Heshka S, Punyanitya M, Zhu S, et al. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res 2003;11:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Middleton MS, Haufe W, Hooker J, Borga M, Dahlqvist Leinhard O, Romu T, et al. Quantifying Abdominal Adipose Tissue and Thigh Muscle Volume and Hepatic Proton Density Fat Fraction: Repeatability and Accuracy of an MR Imaging-based, Semiautomated Analysis Method. Radiology 2017;283:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367–378 e365; quiz e314–365. [DOI] [PubMed] [Google Scholar]
- [34].Shen W, Chen J, Gantz M, Velasquez G, Punyanitya M, Heymsfield SB. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity (Silver Spring) 2012;20:2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, Quantitative Assessment of Liver Fat by MRI-PDFF as an Endpoint in NASH Trials. Hepatology 2018;68:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Armstrong MJ, Hazlehurst JM, Hull D, Guo K, Borrows S, Yu J, et al. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes Metab 2014;16:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology 2007;132:282–293. [DOI] [PubMed] [Google Scholar]
- [39].Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 2013;48:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Al-Busafi SA, Bhat M, Wong P, Ghali P, Deschenes M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepat Res Treat 2012;2012:947575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2000;95:3584–3589. [DOI] [PubMed] [Google Scholar]
- [42].Musso G, Gambino R, Biroli G, Carello M, Faga E, Pacini G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2005;100:2438–2446. [DOI] [PubMed] [Google Scholar]
- [43].Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–451. [DOI] [PubMed] [Google Scholar]
- [44].Cordeiro A, Costa R, Andrade N, Silva C, Canabrava N, Pena MJ, et al. Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity? Clin Res Hepatol Gastroenterol 2020;44:394–402. [DOI] [PubMed] [Google Scholar]
- [45].Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1209–1217. [DOI] [PubMed] [Google Scholar]
- [46].Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Graff M, North KE, Franceschini N, Reiner AP, Feitosa M, Carr JJ, et al. PNPLA3 gene-by-visceral adipose tissue volume interaction and the pathogenesis of fatty liver disease: the NHLBI family heart study. Int J Obes (Lond) 2013;37:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shubham K, Vinay L, Vinod PK. Systems-level organization of non-alcoholic fatty liver disease progression network. Mol Biosyst 2017;13:1898–1911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request, pending the FLINT study committee’s approval.


