Abstract
Objective:
Bilirubin-induced neurotoxicity is mediated by the fraction of total serum bilirubin (TSB) not bound to albumin (Bf). Unbound free fatty acids (FFAu) generated from lipid emulsions compete with bilirubin for albumin binding, increasing Bf. Soy-based (IL) and soy-MCT-olive-fish oil-based (SMOF) lipid emulsions contain different fatty acids with distinct albumin binding affinities. IL increases Bf in preterm infants, but the effects of SMOF on Bf are not known. Our objective was to compare changes in TSB, Bf, FFAu, and response to phototherapy in preterm infants receiving SMOF and IL. We hypothesized that SMOF would be associated with lower Bf and better response to phototherapy than IL.
Methods:
Very preterm and low birth weight infants (< 1500 g, < 32 wks) were infused with IL (n=20) or SMOF (n=20) as prescribed by providers. Phototherapy was prescribed using the standard care practice. FFAu profiles and levels, TSB, and Bf were measured on 0, 1, 2, and 3 g/kg/day of lipid infusion and at the initiation and termination of phototherapy. TSB was analyzed in the clinical laboratory using the Diazo technique. FFAu and Bf were measured using fluorescent probes.
Results:
Escalating doses of IL and SMOF increased FFAu levels and Bf, but not TSB. Phototherapy did not significantly decrease Bf for infants receiving either lipid. IL-treated infants had higher levels of unbound linoleic acid, and SMOF-treated infants had higher unbound arachidonic, oleic, and docosahexaenoic acids.
Conclusion:
IL and SMOF both increase Bf similarly, and phototherapy does not significantly affect Bf for infants receiving them.
Keywords: Intralipid, SMOF, fatty acids, albumin, bilirubin encephalopathy
INTRODUCTION
Current standard practice is to use total serum bilirubin (TSB) to quantify the risk of adverse neurodevelopmental outcomes and determine when phototherapy or exchange transfusion is indicated (1). However, TSB has low sensitivity and specificity for predicting bilirubin-mediated neonatal encephalopathy (2). Serum concentrations of free unbound bilirubin (Bf), the fraction of bilirubin that is not bound to albumin and can cross the blood-brain barrier, predict the risk of bilirubin neurotoxicity more accurately than TSB (3–5) but have been difficult to measure. Recently, a new method for measuring Bf has been developed that enables accurate and rapid determinations of Bf concentrations (6).
Bf is proportionate to TSB under stable conditions but can diverge from it in the presence of competitors for albumin binding, such as free unbound free fatty acids (FFAu) (7, 8). Thus, Bf can be elevated relative to TSB in the presence of elevated unbound free fatty acids, which vary with nutrition (8, 9). Preterm infants receive intravenous lipid emulsions as part of parenteral nutrition (10). Previous studies have shown that soybean-derived lipid emulsion (Intralipid, IL) increases FFAu and Bf (11–13). Elevation of Bf in the presence of IL can lead to inaccurate assessment of the risk for bilirubin neurotoxicity or response to phototherapy if only TSB is used.
Recently, an alternative multicomponent lipid emulsion made up of soybean oil, MCT oil, olive oil, and fish oil (SMOF) has been used in many NICUs in the U.S. The FFAu profile of SMOF and its effect on bilirubin-albumin binding compared to IL are not known. Our primary objective was to compare the levels of Bf and FFAu, and the response of Bf to phototherapy in preterm infants receiving IL vs SMOF lipid emulsions.
METHODS
This single-center prospective pilot study was performed at the Level IV Neonatal Intensive Care Unit of Cohen Children’s Medical Center (Northwell Health, New Hyde Park, NY). Approval for this study was obtained from the Institutional Review Board for Northwell Health.
Patients
Eligible infants were very preterm and low birth weight infants born at < 32 weeks gestation and < 1500 g birth weight who survived beyond 12 hours of life and received (per standard of care practice) intravenous lipid emulsion with SMOF (Fresenius-Kabi, Uppsala, Sweden) or Intralipid (IL, Baxter Healthcare, Deerfield IL). Infants with congenital anomalies and those not expected to survive were not included. Recruitment was carried out from March 2019 through February 2020. A total of 40 infants were recruited, 20 receiving SMOF and 20 receiving IL.
Study Design
Parenteral nutrition was prescribed as per standard NICU protocols. The lipid emulsion (IL or SMOF) was selected at the discretion of the clinical team, not based on any specific management guidelines. Administration of lipid emulsion usually started at a dose of 1 g/kg/day on the first or second day of life, with a daily increase of 1 g/kg/day to a maximum of 3 g/kg/day. These decisions were guided by daily routine blood measurements approximately 8–12 hours after exposure to each new dose. Any delays in lipid dose increases were at the discretion of the clinical team, most often due to elevated triglyceride levels or changes in clinical status. Phototherapy [Natus NeoBlue LED Phototherapy (Natus Medical Inc., San Carlos, CA)] was initiated and discontinued based on routine blood measurements of TSB during the first days of life, as per standard practice guidelines.
Blood Sampling
Residual serum samples from routine blood samples during standard clinical care were collected. Serum samples were collected before initiating lipid emulsions and on each dose of lipid emulsion, 8–12 hours after beginning infusion at each dose. Samples were also collected prior to initiating and discontinuing phototherapy. All serum samples were frozen and stored at −80°C. Serum Bf, FFAu, and FFAu profiles were measured by Fluoresprobe Sciences (San Diego, CA). TSB was measured in the clinical laboratory using the standard diazo method.
Bf measurements
Bf levels were determined using the UBCheck Bf assay (6, 14). This new method measures the equilibrium Bf directly in 5μl of a serum sample in a disposable cartridge. The sample cartridge contains the Bf sensor, which is a fatty acid-binding protein labeled with a near infrared fluorophore that emits at 700 nm plus a second protein labeled with a fluorophore that emits at 820nm. Upon binding Bf, the fluorescence at 700 nm is quenched but the 820 nm fluorescence is unchanged, so the ratio of fluorescence at 700 nm/820 nm is used to calculate the Bf concentration. After adding the sample, the cartridge is placed in a custom fluorescence reader that scans the infrared fluorescence and yields the Bf concentration in 90 seconds. The UBCheck measurements were well correlated with those measured with the Arrows UB2 Analyzer, which uses the peroxidase method. UBCheck Bf levels ranged from 2.6 to 11.8 nM while the Arrows Bf levels ranged from 0.9 to 11.9 nM in the same samples. Both methods were well correlated by the Deming regression with a correlation coefficient of 0.90 (95%CI: 0.72–1.06). The UBCheck had an average Coefficient of Variation of 7% for repeated measurements. The UBCheck determines the equilibrium Bf concentration directly in a single analysis and is insensitive to substances that can interfere with the peroxidase measurement. TSB was measured using the standard diazo method in the clinical laboratory.
FFAu measurements
FFAu was measured in the plasma sample using a fluorescently labeled mutant of a fatty acid- binding protein, as described previously (15).
FFAu profiles
FFAu profiles were measured as described previously (16). Fluorescently labeled fatty acid-binding protein (FABP) mutants were used, each having different specificities and sensitivities for different long-chain FFAu, specifically arachidonic acid, myristic acid, linolenic acid, linoleic acid, oleic, palmitoleic, palmitic, steric, and docosahexaenoic acid (DHA).
Demographic and Outcome Data
The following demographic and clinical data were recorded for each patient: birth weight, gestational age, mode of delivery, Apgar scores, and extraneous sources of bilirubin such as cephalohematoma or excessive bruising noted on admission physical examination.
Statistical Analysis
Correlations between FFAu, TSB, and Bf with increasing doses of IL or SMOF were done using multiple regression analysis (linear or logistic for numerical or categorical variables, respectively), accounting for confounding variables including birth weight, gestational age, sex, and 5 minute Apgar if appropriate. Demographic variables were compared using Mann-Whitney rank-sum tests for continuous data and chi-square for categorical data. Analyses were performed using Minitab (version 14.0). Values of p < 0.05 were considered significant. Since this is a pilot study, power analysis and sample size calculation were not performed.
RESULTS
Patient Characteristics
The study population consisted of 40 preterm infants (20 in the IL group and 20 in the SMOF group). The mean gestational ages and birth weights were not significantly different between the groups (27.5 ± 2.6 weeks and 982 ± 312 g in the IL group, vs. 27.9 ± 2.1 weeks and 1009 ± 202.9 g in the SMOF group). Exposure to medications that can displace bilirubin from albumin was similar between the groups: 15/20 infants in the IL group and 13/20 in the SMOF group received at least 1 dose of gentamicin and no infants in either group were exposed to furosemide or ibuprofen during the study period. Other demographic and clinical data are shown in Table 1.
Table 1.
Patient Characteristics
| IL (n=20) | SMOF (n=20) | p | |
|---|---|---|---|
| BW, g (mean ± SD) | 982 ± 312 | 1009 ± 202 | 0.49 |
| GA, wks (mean ± SD) | 27.5 ± 2.6 | 27.9 ± 2.1 | 0.64 |
| Sex (M/F) | 6/14 | 9/11 | 0.33 |
| Apgar - 5 min (median, quartiles) | 8 (7.5, 9) | 9 (8, 9) | 0.33 |
| Delivery (vaginal/cesarean) | 7/13 | 6/14 | 0.73 |
| Cephalohematoma (n) | 0 | 0 | 1 |
| Bruising (n) | 3 | 2 | 0.63 |
FFAu Profiles
The unbound FFA (FFAu) profiles were significantly different between IL- and SMOF-treated infants (Table 2). Specifically, the IL group had higher unbound linoleic acid levels than the SMOF group (p=0.003). SMOF-treated infants had higher levels of unbound arachidonic acid (p=.027), oleic acid (p=0.034), and docosahexaenoic acid (DHA; p=0.036).
Table 2.
Unbound free fatty acid profiles.
| FFAu | IL (n=11) (nM, mean ± SD) |
SMOF (n=10) (nM, mean ± SD) |
p |
|---|---|---|---|
| Arachidonic Acid | 0.06 ± 0.02 | 0.07 ± 0.01 | 0.027 |
| Linolenic Acid | 0.08 ± 0.07 | 0.12 ± 0.11 | 0.322 |
| Linoleic Acid | 0.24 ± 0.19 | 0.03 ± 0.05 | 0.003 |
| Oleic Acid | 0.10 ± 0.07 | 0.15 ± 0.05 | 0.034 |
| Palmitic Acid | 0.10 ± 0.05 | 0.11 ± 0.08 | 0.623 |
| Steric Acid | 0.07 ± 0.05 | 0.06 ± 0.05 | 0.435 |
| Docosahexaenoic Acid | 0.05 ± 0.03 | 0.08 ± 0.04 | 0.036 |
Bf, TSB, and FFAu Levels
Bf increased with escalating doses of lipid emulsion for infants receiving both IL (p=0.02) and SMOF (p=0.01) (Figure 1). Bf levels at each lipid dose were not different between the IL and SMOF groups. Of note, several infants had Bf levels >11 nM, which are thought to exert neurotoxicity (5), but these levels occurred similarly in both groups (p=0.34). Escalating doses of IL and SMOF did not affect levels of TSB (p=0.60 and p=0.29, respectively). FFAu levels increased in a dose-dependent manner for IL (p=0.001), but not SMOF (p=0.06) (Table 3). Of note, measurements of FFAu did not include medium-chain fatty acids, which are present in SMOF but have low binding constants for the fluorescently-labeled probe.
Figure 1.
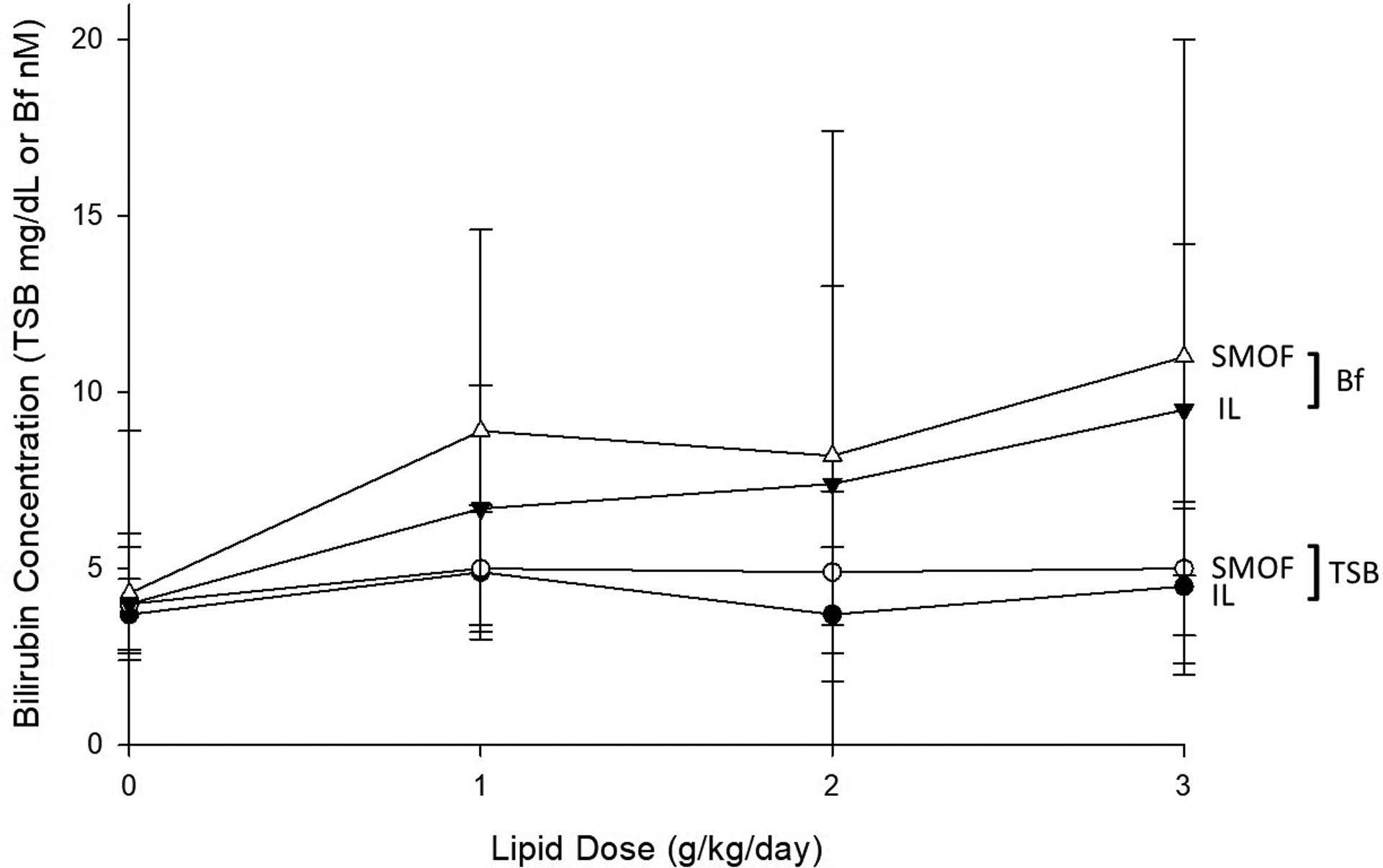
Effect of escalating doses of IL or SMOF on total serum bilirubin (TSB) and Bf (unbound bilirubin), mean ± SD
Table 3.
FFAu levels on escalating doses of IL or SMOF
| Dose (g/kg/d) | Unbound Free Fatty Acids (FFAu), nM, mean ± SD |
|
|---|---|---|
| IL n=11 |
SMOF n=10 |
|
| 0 | 2.3 ± 0.6 | 2.4 ± 0.8 |
| 1 | 5.1 ± 4.3 | 1.6 ± 0.3 |
| 2 | 9.2 ± 3.7 | 3.6 ± 3.7 |
| 3 | 29.1 ± 7.4 | 6.2 ± 3.3 |
| r | 0.83 | 0.62 |
| p | 0.001 | 0.061 |
Bf and TSB Response to Phototherapy
Phototherapy was initiated when infants were receiving 0.61 ± 0.78 and 0.70 ± 0.73 g/kg/day of IL or SMOF, respectively (p=0.63), and discontinued when they were receiving 2.32 ± 0.58 and 2.35 ± 0.59 g/kg/day, respectively (p=0.86). The average duration of phototherapy for all infants was 41.1 ± 18.5 hours. As expected, phototherapy reduced TSB. Similar statistically significant declines in Bf were not observed in response to phototherapy in either the IL group (p=0.34) or the SMOF group (p=0.12) (Table 4).
Table 4.
Effects of phototherapy on TSB and Bf levels
| IL (n=20) | SMOF (n=20) | |||||
|---|---|---|---|---|---|---|
| Photo on (mean ± SD) |
Photo off (mean ± SD) |
p | Photo on (mean ± SD) |
Photo off (mean ± SD) |
p | |
| TSB (mg/dl) | 5.2 ± 2.1 | 3.1 ± 1.5 | 0.001 | 5.3 ± 1.9 | 3.6 ± 1.5 | 0.004 |
| Bf (nM/L) | 6.4 ± 3.8 | 5.2 ± 3.0 | 0.34 | 9.1 ± 6.6 | 6.3 ± 3.5 | 0.12 |
DISCUSSION
We found that escalating doses of IL and SMOF increase Bf similarly. This is consistent with recent reports of elevated Bf in the presence of other commercial and non-commercial lipid emulsions (17). Our findings suggest that the risk of bilirubin-induced neurotoxicity for infants receiving standard lipid emulsions in the U.S. may exceed that determined by conventional measurement of TSB alone.
Both IL and SMOF contain blends of free fatty acids that circulate unbound from albumin at concentrations determined by the composition of the emulsion and by their distinct albumin binding affinities. FFAu with low affinity for albumin are less likely to displace bilirubin from albumin and contribute to elevated Bf levels. SMOF contains more diverse fatty acids than IL, including medium-chain fatty acids such as capric and caprylic acids, which have with very low albumin affinities. However, SMOF also contains higher concentrations than IL of several fatty acids with relatively high albumin affinity, including oleic, steric, palmitic, and docosahexaenoic acids. We found significantly higher levels of unbound circulating free oleic and docosahexaenoic acids in infants receiving SMOF, when compared to IL. Conversely, infants receiving IL had significantly higher circulating levels of unbound free linoleic acid, which also has a high albumin binding affinity. Our finding that IL and SMOF displace bilirubin and elevate Bf comparably suggests that their composite albumin binding affinities are similar despite distinct FFAu profiles.
It is important to note that serum albumin levels are not directly predictive of Bf levels. Previous kinetic studies have shown that albumin is present in abundance under basal conditions and so is not rate-limiting for bilirubin binding. In preterm infants, bilirubin-albumin binding affinity may decrease in the first four days (18), and other lower-affinity bilirubin-binding proteins such as a-fetoprotein and apolipoprotein D are present in variable concentrations in serum (19). Albumin-bilirubin equilibrium in the vascular compartment occurs nearly instantaneously, within approximately 1 second at normal pH and temperature (20). Any effects of differences in albumin concentration on bilirubin binding are swamped by the effects of binding inhibitors in serum, such as IL and SMOF.
Although Bf is the component of circulating bilirubin most directly linked to neurotoxicity (3–5), the technical challenges of measuring it directly have prevented its inclusion in current clinical protocols for the prevention of kernicterus. Instead, TSB levels are used to determine risk levels and thresholds for treatment with phototherapy. We found that TSB, but not Bf, demonstrates a statistically significant decrease during phototherapy for infants receiving IL, which is consistent with previous reports (13, 21). Likewise, Bf did not show a statistically significant decline during phototherapy for infants receiving SMOF. Significantly elevated Bf persisted, potentially to neurotoxic levels, in several infants receiving ≥ 1 g/kg/day of either lipid emulsion. The likely explanation for this observation is that FFAu with higher affinity to albumin than bilirubin are present at high levels, increasing Bf by displacing bilirubin from binding sites. Thus, prescribing phototherapy based solely on TSB may lead to discontinuation of treatment while the risk of Bf-induced neurotoxicity is still high in infants receiving lipid infusions. Routine clinical measurements of Bf, rather than TSB, may contribute to a better risk assessment.
However, it is important to note that there is currently no consensus on risk stratification using Bf. While some studies have shown that Bf levels ≥ 13.6 nM strongly predict kernicterus (22), others have shown that abnormal auditory brainstem responses occur at thresholds as high as 17 nM (23) and as low as 11 nM (5). In 2018, the Japan Pediatric Society updated its guidelines to recommend standard phototherapy at a Bf threshold of 6.8 nM and intensive phototherapy at 10.2 nM for preterm infants (24), based on estimates that this would reduce the number of infants initiating phototherapy and requiring exchange transfusion (25). As the technology has advanced to allow rapid, cost-effective, and point-of-care measurement of Bf, further research will be needed to apply it to clinical management.
Our study has several limitations. Although it was performed prospectively, it was an exploratory project using residual serum samples from clinical tests. As such, the timing of sample collection relative to changes in lipid dosing was not controlled. However, compliance to NICU clinical protocols, including the timing of parenteral nutrition administration and routine morning blood sampling, was maintained throughout, so there was likely only minimal variability. Albumin levels are not routinely monitored in our NICU, so it is possible that hypoalbuminemia contributed to elevated Bf or FFAu levels in some infants. Though FFAu composition in the serum is determined primarily by the lipid infused, it may also vary based on the amount and composition of advancing enteral feeds. While feeding was not controlled for this study, standardized feeding guidelines allow only small-volume feedings during the first three days. Also, SMOF or IL was selected based on clinician preference when initiating parenteral nutrition. Although there may have been bias based on personal preference, the two groups did not differ significantly for key demographic factors. Our study was also limited by sample size, which was not calculated to avoid alpha or beta error because this study was designed as a pilot and exploratory project. It was not powered to assess long-term indices of bilirubin neurotoxicity.
In summary, we found that Bf levels are elevated and do not significantly decrease in response to phototherapy in preterm infants receiving either IL or SMOF. Moreover, up to 30% of such infants may be exposed to neurotoxic levels of Bf. These effects are similar for IL and SMOF despite their markedly different fatty acid compositions, which apparently did not affect their composite albumin binding affinity. More extensive studies will be needed to determine whether the effects of SMOF on Bf levels are gestational-age dependent, as has been shown for IL (13, 21).
FUNDING
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R44HD080412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- TSB
total serum bilirubin
- Bf
unbound free bilirubin
- FFAu
unbound free fatty acids
- IL
soy-based lipid emulsion
- SMOF
soy-MCT-olive-fish oil-based lipid emulsion
Footnotes
DISCLOSURE OF INTERESTS
AK is the founder of and partner in Fluoresprobe Sciences. AH is Director of Research at Fluoresprobe Sciences, which pays his salary. The authors declare no other conflicts of interest.
REFERENCES
- 1.Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J Pediatr. 2013;162(3):477–82.e1. Epub 2012/10/05. doi: 10.1016/j.jpeds.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. N Engl J Med. 2013;369(21):2021–30. Epub 2013/11/22. doi: 10.1056/NEJMra1308124. [DOI] [PubMed] [Google Scholar]
- 3.Cashore WJ, Oh W. Unbound bilirubin and kernicterus in low-birth-weight infants. Pediatrics. 1982;69(4):481–5. Epub 1982/04/01. [PubMed] [Google Scholar]
- 4.Funato M, Tamai H, Shimada S, Nakamura H. Vigintiphobia, unbound bilirubin, and auditory brainstem responses. Pediatrics. 1994;93(1):50–3. Epub 1994/01/01. [PubMed] [Google Scholar]
- 5.Amin SB, Ahlfors C, Orlando MS, Dalzell LE, Merle KS, Guillet R. Bilirubin and serial auditory brainstem responses in premature infants. Pediatrics. 2001;107(4):664–70. Epub 2001/05/23. doi: 10.1542/peds.107.4.664. [DOI] [PubMed] [Google Scholar]
- 6.Hegyi T, Chefitz D, Weller A, Huber A, Carayannopoulos M, Kleinfeld A. Unbound bilirubin measurements in term and late-preterm infants. J Matern Fetal Neonatal Med. 2020:1–7. Epub 20200504. doi: 10.1080/14767058.2020.1761318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richieri GV, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32(29):7574–80. Epub 1993/07/27. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- 8.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16(3):165–79. Epub 1975/05/01. [PubMed] [Google Scholar]
- 9.Martin CR, Ling PR, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8(5). Epub 2016/05/18. doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salama GS, Kaabneh MA, Almasaeed MN, Alquran M. Intravenous lipids for preterm infants: a review. Clin Med Insights Pediatr. 2015;9:25–36. Epub 2015/02/24. doi: 10.4137/CMPed.S21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruben S, Kleinfeld AM, Richeiri GV, Hiatt M, Hegyi T. Serum levels of unbound free fatty acids. II: The effect of intralipid administration in premature infants. J Am Coll Nutr. 1997;16(1):85–7. Epub 1997/02/01. doi: 10.1080/07315724.1997.10718654. [DOI] [PubMed] [Google Scholar]
- 12.Ahlfors CE, Wennberg RP, Ostrow JD, Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem. 2009;55(7):1288–99. Epub 2009/05/09. doi: 10.1373/clinchem.2008.121269. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi T, Kleinfeld A, Huber A, Weinberger B, Memon N, Shih WJ, et al. Effects of Soybean Lipid Infusion on Unbound Free Fatty Acids and Unbound Bilirubin in Preterm Infants. J Pediatr. 2017;184:45–50.e1. Epub 2017/01/22. doi: 10.1016/j.jpeds.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinfeld A, inventorOne step methods, kits, and systems for the measurement of concentrations of unbound bilirubin in biological fluids patent PCT/US2029/048264.
- 15.Huber AH, Kampf JP, Kwan T, Zhu B, Kleinfeld AM. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45(48):14263–74. Epub 2006/11/30. doi: 10.1021/bi060703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber AH, Kleinfeld AM. Unbound free fatty acid profiles in human plasma and the unexpected absence of unbound palmitoleate. J Lipid Res. 2017;58(3):578–85. Epub 2017/01/14. doi: 10.1194/jlr.M074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satar M, Şimşek H, Özlü F, Tuli A, Alparslan MM, Mert MK, et al. The effect of different intravenous lipids on free bilirubin levels in premature infants. Eur J Clin Nutr. 2021. Epub 20211201. doi: 10.1038/s41430-021-01049-3. [DOI] [PubMed] [Google Scholar]
- 18.Hulzebos CV, Dijk PH. Bilirubin-albumin binding, bilirubin/albumin ratios, and free bilirubin levels: where do we stand? Semin Perinatol. 2014;38(7):412–21. Epub 20141007. doi: 10.1053/j.semperi.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Ostrow JD, Pascolo L, Shapiro SM, Tiribelli C. New concepts in bilirubin encephalopathy. Eur J Clin Invest. 2003;33(11):988–97. doi: 10.1046/j.1365-2362.2003.01261.x. [DOI] [PubMed] [Google Scholar]
- 20.Amin SB, Lamola AA. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin Perinatol. 2011;35(3):134–40. doi: 10.1053/j.semperi.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegyi T, Kleinfeld A, Huber A, Weinberger B, Memon N, Carayannopoulos M, et al. Unbound bilirubin levels in phototherapy-treated preterm infants receiving soy-based lipid emulsion. Pediatr Int. 2020;62(12):1357–63. Epub 2020/06/15. doi: 10.1111/ped.14346. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura H, Yonetani M, Uetani Y, Funato M, Lee Y. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatr Jpn. 1992;34(6):642–7. Epub 1992/12/01. doi: 10.1111/j.1442-200x.1992.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee YK, Daito Y, Katayama Y, Minami H, Negishi H. The significance of measurement of serum unbound bilirubin concentrations in high-risk infants. Pediatr Int. 2009;51(6):795–9. Epub 2009/05/08. doi: 10.1111/j.1442-200X.2009.02878.x. [DOI] [PubMed] [Google Scholar]
- 24.Morioka I Hyperbilirubinemia in preterm infants in Japan: New treatment criteria. Pediatr Int. 2018;60(8):684–90. Epub 2018/06/16. doi: 10.1111/ped.13635. [DOI] [PubMed] [Google Scholar]
- 25.Morioka I, Nakamura H. Treatment criteria for infants with hyperbilirubinemia in Japan. Semin Perinatol. 2021;45(1):151352. Epub 20201202. doi: 10.1016/j.semperi.2020.151352. [DOI] [PubMed] [Google Scholar]


