Abstract
There is a significant number of Emergency Department (ED) patients with known chronic hepatitis C virus (HCV) infection who have not been treated with directly acting antivirals. We implemented a pilot ED-based linkage-to-care program to address this need and evaluated the impact of the program using the HCV Care Continuum metrics. Between March 2015 and May 2016, dedicated patient care navigators identified HCV RNA-positive patients in an urban ED and offered expedited appointments with the on-site viral hepatitis clinic. Patient demographics and care continuum outcomes were abstracted from the EMR and analyzed to determine significant factors influencing LTC and treatment initiation rates. The ED linkage-to-care program achieved a 43% linkage-to-care rate (165/384), 22% treatment rate (84/384), and 16% sustained virologic response rate (63/384). Significant associations were found between linkage-to-care and increasing age (OR = 1.03), Medicare insurance (OR = 2.21), and having a primary care physician (PCP) (OR = 4.03). For patients who were linked, the odds of initiating treatment were also positively associated with increasing age (OR=1.04) and having a PCP (OR=2.77). For patients who initiated treatment, the odds of sustained virologic response were marginally associated with having a PCP (OR=4.92).Our ED linkage-to-care program utilized care coordination to successfully link nearly half of approached HCV RNA-positive patients to care. This design can be feasibly replicated by other EDs given limited non-clinical training required for linkage-to-care staff. Adoption of similar programs in other EDs may improve the rates of LTC and treatment initiation for previously diagnosed HCV patients.
Keywords: Hepatitis C, Linkage to Care, Emergency Department, Continuity of Patient Care, Pilot Project
Introduction
Chronic hepatitis C, caused by hepatitis C virus (HCV), continues to be a major contributor to morbidity and mortality in the U.S. population,1 with an estimate of 2.4 million people living with chronic infection, and 20–30% of them progressing to liver cirrhosis in 25–30 years if untreated.2,3
In 2012, Centers for Disease Control and Prevention (CDC) first issued expanded HCV testing recommendations to include one-time testing for individuals born between 1945 and 1965 in addition to risk-based testing, subsequently expanding in 2020 to universal testing for all adults.4 Furthermore, the World Health Organization (WHO) has established goals for HCV elimination as a public health threat by 2030 with a target of 80% eligible treated.5 In spite of this, the burden of HCV persists, even with direct-acting antiviral (DAA) medications which have been demonstrated to be at least 90% of effective in cure for infection after just 8–12 weeks of treatment.6 One key reason for the shortfall is the lack of robust linkage-to-care (LTC) efforts for previously diagnosed patients.
U.S. urban emergency departments (EDs) have played an important role in identifying thousands of HCV infections via ED-based testing programs since rapid anti-HCV assays have become avaialble.7–9 Additionally, EDs have been recognized as a critical part of the care cascade with the potential to improve HCV care, given the high prevalence and that EDs care for a greater proportion of difficult-to-reach individuals such as persons who inject drugs, than other sectors of the health care system.10–12 However, a majority of previously diagnosed HCV-positive patients have not successfully treated for their chronic infection. Previous evaluations of HCV LTC programs have found that only 2–10% of RNA-positive patients initiate treatment and achieve sustained virologic response (SVR).13–17 In one of the first ED-based HCV testing programs which began following the 2012 CDC testing recommendations, Anderson et al. reported a 32% LTC rate, 8% treatment rate, and 6% SVR rate, amongst those newly diagnosed with chronic infection,13 providing a benchmark of the HCV care continuum for future evaluations in EDs. While a significant drop-off exists between both diagnosis and LTC, as well as LTC and treatment initiation, few EDs have as of yet implemented integrated HCV LTC programs.
Toward the 2030 global goal of eliminating HCV, we developed and implemented a pilot LTC program at an urban academic ED. The goal was to utilize navigators to link patients with known HCV RNA-positive to the on-site viral hepatitis clinic (VHC), as part of an ED visit for an unrelated emergent/ urgent problem. There were no systematic, structured guidelines on HCV LTC in this ED prior to implementation of this program. The referral of HCV-positive patients to care was purely based on discretion of the treating physicians, and instances of linkage were rare during the busy episodic ED care since there were no resources and infrastructure for HCV LTC. Our ED-directed patient navigation LTC program was evaluated using the HCV care continuum model, to define successes and identify ongoing gaps in progressing patients from diagnosis to SVR.13,18–20
Methods
Study Setting
The study site was an urban academic ED in Baltimore, MD with an annual census of 70,000 patients. In 2013, the seroprevalence of anti-HCV antibody (Ab) in this ED was 14%, with 69% of seropositive patients having been previously diagnosed with chronic HCV infection.9 This study was approved as quality improvement evaluation of the HCV LTC program by the School of Medicine Institutional Review Board.
ED HCV LTC Program
A pilot ED HCV LTC program was implemented during 2015 to identify best practices for linking ED HCV-infected patients to care, while scaling up integration of HCV testing in the ED. Dedicated LTC program staff consisted of students and research coordinators who were trained to review the electronic medical record (EMR), discuss HCV LTC options with patients, schedule appointments for patients, and conduct appointment reminder phone calls. During weekday 9am to 5pm coverage shifts from March 2015 until May 2016, staff utilized laboratory results or clinician notes in the EMR to identify patients with chronic HCV infection (HCV RNA-positive) who were accessing care in the ED for an unrelated urgent or emergent health concern. LTC staff approached these known positive patients and offered LTC services to those who were not currently in care. LTC services offered included meeting with a clinic case manager either the same day or within 48 hours of the ED encounter, and in the majority of cases, a subsequent establishing linkage to an on-site HCV specialty care via clinic case management. The on-site clinic was dedicated to HCV treatment with capacity for walk-in appointments 5 days per week from 8 AM to 5 PM with dedicated clinicians, nurses, case-managers and FibroScan. ED patients with no additional insurance-related requirements were often able to be seen by a clinic nurse or provider the same day as the referral. LTC staff scheduled appointments at the VHC for patients who were unable to be seen the same day, and completed reminder phone calls within 24 hours of the scheduled appointment time. While scheduling appointments with the on-site VHC was preferred, not all patients were linked to this clinic due to differences in insurance coverages and patient preferences.
Data Collection
Program staff gathered demographic (e.g. age, sex, race) and HCV relevant information [injection drug use (IDU) status, primary care physician (PCP) status, health insurance coverage] retrospectively from the EMR. Clinical data regarding scheduled appointments for liver fibrosis staging, initiation and completion of HCV treatment, and SVR status was also abstracted from the EMR to evaluate LTC and treatment.
Data Analysis
The primary outcomes of the study were the proportion of patients who achieved LTC and treatment initiation. LTC was operationally defined as any attendance to a scheduled appointment at the on-site VHC within 1 year of index encounter in the ED. Treatment initiation was defined as beginning DAA treatment within 1 year of the ED index encounter. The HCV care continuum also includes operationally defined intermediate outcomes of ‘attended fibrosis staging’ via the blood test (FibroSURE test) or transient elastography (FibroScan), ‘initiated DAA treatment’, ‘completed DAA treatment regimen’, and ‘viral load result available after completion of treatment’. The ultimate outcome of the continuum is SVR. Patients that reached fibrosis staging were also categorized into ‘mild or moderate liver fibrosis’ (fibrosis staging < F3) or ‘advanced liver fibrosis’ (fibrosis staging ≥ F3) for outcomes following fibrosis staging in the care continuum.
Demographic and relevant clinical factors were analyzed using bivariate and multivariate regression analysis. Odds ratios were calculated to determine which characteristics were significantly associated with LTC among all patients and which characteristics were significantly associated with treatment initiation among only those patients who achieved LTC. Odds ratios (OR) were determined to be significant if p < 0.05, or marginally significant if 0.05≤p<0.1, and only factors with significant or marginally significant adjusted odds ratios (adj-OR) were included in the final multivariate regression models.
Results
Population Characteristics
During the first 14 months of LTC program implementation, 384 HCV RNA-positive patients were identified and offered LTC with the on-site VHC. The mean age of these patients was 52.5 years (SD = 10.5), and 65% of the population was in the ‘baby boomer’ birth cohort (birth year 1945–1965). The majority of patients were male (66%), African American (77%), and had public insurance coverage (74%; Medicaid 53% and Medicare 21%). Of the patients in the population, 61% reported injecting drugs at some point in their lifetimes (Table 1).
Table 1:
Characteristics of 384 Urban Emergency Department Patients with Chronic HCV Infection
| Characteristics | Categories | Number (%) |
|---|---|---|
| Age (years) | Mean (± SD) | 52.5±10.5 |
| Median (IQR) | 54 (47, 59) | |
| 18–34 | 25 (7) | |
| 35–44 | 50 (13) | |
| 45–54 | 128 (33) | |
| 55–64 | 139 (36) | |
| ≥ 65 | 42 (11) | |
| Birth Cohort | Prior to 1945 | 8 (2) |
| 1945 – 1965 | 249 (65) | |
| 1966 – 1975 | 82 (21) | |
| After 1976 | 45 (12) | |
| Gender | Male | 253 (66) |
| Female | 131 (34) | |
| Race | African American | 294 (77) |
| White | 87 (23) | |
| Other | 3 (1) | |
| Health Insurance | Public - Medicaid | 202 (53) |
| Public - Medicare | 81 (21) | |
| Private | 77 (20) | |
| Self-Pay | 24 (6) | |
| Having a Primary Care Doctor | Yes | 250 (62) |
| People Who Inject Drugs | Ever | 234 (61) |
HCV Care Continuum
The LTC program successfully linked 165 (43%) of the 384 known positive patients to an appointment with the VHC. Of these 165 linked individuals, 84 (51%) received treatment, and 63 (38%) achieved SVR.
Analysis of Factors Associated with LTC
Bivariate analysis of factors associated with LTC found significant associations for age increasing each year, baby boomer birth cohort, African American race, public health insurance coverage, having PCP, and ever IDU (Table 2). LTC rates also increased with older age groups (18–34 years: 24%, 35–44 years: 30%, 45–54 years: 38%, 55–64 years: 52%, ≥ 65 years: 55%, Cochrane-Armitage trend test: p < 0.001). In the final multivariate regression model, only age increasing each year (adj-OR = 1.03, 95% CI: 1.01, 1.05), Medicare health insurance coverage (adj-OR = 2.21, 95% CI: 1.13, 4.33; as compared to private or self-pay), and having a PCP (adj-OR = 4.03, 95% CI: 2.45, 6.61) were significantly associated with LTC (Table 3). Additionally, ever IDU (adj-OR= 0.66, 95% CI: 0.42, 1.03) was negatively marginally associated with LTC (Table 3).
Table 2:
Bivariate Analysis of Factors Associated with Linkage to HCV Care among 384 Patients with Chronic Hepatitis C in an Emergency Department-Based HCV Linkage to Care Program
| Characteristics | Category | Total No. | No. of Linked (%) | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Age | Increasing each year | 384 | 165 (43) | 1.04 (1.02, 1.06)* |
| Birth Cohort | Baby Boomer | 249 | 119 (48) | 1.77 (1.15, 2.73)* |
| Non-Baby Boomer | 135 | 46 (34) | 1.00 | |
| Sex | Male | 253 | 109 (43) | 1.01 (0.66, 1.55) |
| Female | 131 | 56 (73) | 1.00 | |
| Race | African American | 294 | 138 (47) | 2.06 (1.25, 3.42)* |
| Other | 90 | 27 (30) | 1.00 | |
| Health Insurance | Public - Medicaid | 202 | 86 (43) | 1.75 (1.05, 2.92)* |
| Public - Medicare | 81 | 49 (60) | 3.62 (1.96, 6.71)* | |
| Private and Self-Pay | 101 | 30 (30) | 1.00 | |
| Having a PCP | Yes | 250 | 137 (55) | 4.59 (2.83, 7.46)* |
| No | 134 | 28 (21) | 1.00 | |
| Injection Drug Use | Ever | 234 | 90 (38) | 0.63 (0.41, 0.95)* |
| Never | 150 | 75 (50) | 1.00 |
p<0.05
PCP: Primary Care Physician
Table 3:
Multivariate Regression Analysis of Factors Associated with Linkage to HCV Care among 384 Patients with Chronic Hepatitis C in an Emergency Department-Based HCV Linkage to Care Program
| Full Model | Final Model | ||
|---|---|---|---|
| Characteristics | Category | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Age | Increasing each year | 1.01 (0.98, 1.04) | 1.03 (1.00, 1.05)* |
| Birth Cohort | Baby Boomer | 1.21 (0.64, 2.30) | N.S. |
| Non-Baby Boomer | 1.00 | ||
| Sex | Male | 1.05 (0.65, 1.71) | N.S. |
| Female | 1.00 | ||
| Race | African American | 1.70 (0.95, 3.02)** | N.S. |
| Other | 1.00 | ||
| Health Insurance | Public – Medicaid | 1.66 (0.95, 2.89)** | 1.64 (0.95, 2.83)** |
| Public – Medicare | 2.29 (1.16, 4.54)* | 2.21 (1.13, 4.33)* | |
| Private and Self-Pay | 1.00 | 1.00 | |
| Having a PCP | Yes | 3.99 (2.41, 6.60)* | 3.94 (2.40, 6.49)* |
| No | 1.00 | 1.00 | |
| Injection Drug Use | Ever | 0.68 (0.43, 1.07)** | 0.68 (0.43, 1.06)** |
| Never | 1.00 | 1.00 |
p<0.05
0.05≤p<0.1
Analysis of Factors Associated with Initiation of DAA
For the patients who were linked to care, the only significant factors associated with treatment initiation in the bivariate analysis were age increasing each year and having PCP (Table 4). Both factors were also significant in the final multivariate regression model, with adj-OR of 1.04 (95% CI: 1.00, 1.07) for age increasing each year and adj-OR = 2.77 (95% CI: 1.13, 6.83) for having PCP. Liver fibrosis was also included as a factor in this analysis, but no significant associations were found between advanced fibrosis and treatment initiation (Table 5).
Table 4:
Bivariate Analysis of Factors Associated with Initiation of Hepatitis C Antiviral Treatment among 165 Patients with Chronic Hepatitis C in an Emergency Department-Based HCV Linkage to Care Program
| Characteristics | Category | Total No. | No. of Initiating Treatment (%) | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Age | Increasing each year | 165 | 84 (51) | 1.04 (1.01, 1.08)* |
| Birth Cohort | Baby Boomer | 119 | 63 (53) | 1.34 (0.68, 2.65) |
| Non-Baby Boomer | 46 | 21 (46) | 1.00 | |
| Sex | Male | 109 | 56 (51) | 1.06 (0.56, 2.01) |
| Female | 56 | 28 (50) | 1.00 | |
| Race | African American | 138 | 72 (52) | 1.36 (0.60, 3.13) |
| Other | 27 | 12 (44) | 1.00 | |
| Health Insurance | Public - Medicaid | 86 | 45 (52) | 1.44 (0.62, 3.32) |
| Public - Medicare | 49 | 26 (53) | 1.48 (0.59, 3.69) | |
| Private and Self-Pay | 30 | 13 (43) | 1.00 | |
| Having a PCP | Yes | 137 | 76 (55) | 3.12 (1.28, 7.56)* |
| No | 28 | 8 (29) | 1.00 | |
| Injection Drug Use | Ever | 90 | 43 (48) | 0.76 (0.41, 1.40) |
| Never | 75 | 41 (55) | 1.00 | |
| Liver Fibrosis† | Advanced (F3 or higher) | 69 | 40 (58) | 1.63 (0.87, 3.04) |
| Non-Advanced or Unknown | 96 | 44 (46) | 1.00 |
p<0.05
Liver fibrosis staging determined by the serology test (FibroSURE) or transient elastography (FibroScan) results
PCP: Primary Care Physician
Table 5:
Multivariate Regression Analysis of Factors Associated with Initiation of Hepatitis C Antiviral Treatment among 165 Patients with Chronic Hepatitis C in an Emergency Department-Based HCV Linkage to Care Program
| Full Model | Final Model | ||
|---|---|---|---|
| Characteristics | Category | Odds Ratio (95% CI) |
Odds Ratio (95% CI) |
| Age | Increasing each year | 1.07 (1.01, 1.13)* | 1.04 (1.00, 1.07)* |
| Birth Cohort | Baby Boomer | 0.44 (0.15, 1.31) | N.S. |
| Non-Baby Boomer | 1.00 | ||
| Sex | Male | 0.97 (0.48, 1.93) | N.S. |
| Female | 1.00 | ||
| Race | African American | 0.88 (0.35, 2.25) | N.S. |
| Other | 1.00 | ||
| Health Insurance | Public – Medicaid | 1.25 (0.51, 3.10) | N.S. |
| Public – Medicare | 0.90 (0.44, 2.49) | ||
| Private and Self-Pay | 1.00 | ||
| Having a PCP | Yes | 2.88 (1.15, 7.23)* | 2.77 (1.13, 6.83)* |
| No | 1.00 | 1.00 | |
| Injection Drug Use | Ever | 0.72 (0.37, 1.39) | N.S. |
| Never | 1.00 | ||
| Liver Fibrosis† | Advanced (F3 or higher) | 1.58 (0.81, 3.09) | N.S. |
| Non-Advanced or Unknown | 1.00 |
p<0.05
Liver fibrosis staging determined by the serology test (FibroSURE) or transient elastography (FibroScan) results
PCP: Primary Care Physician
Analysis of Factors Associated with any SVR
For the patients who had initiated treatment, the only marginally significant factors associated with any SVR in the bivariate analysis were having a PCP and ever injection drug use (Supplement Table 1). Only having a PCP was marginally significant in the final multivariate regression model, with OR of 4.92 (95% CI: 0.94, 25.68, p=0.059). (Supplement Table 2).
Discussion
Our LTC pilot program utilized intensive patient navigation via a collaborative program between the Emergency Medicine and Infectious Diseases to connect HCV RNA-positive ED patients to HCV care. This HCV LTC program was the first of its kind in the ED, with no guidelines for LTC or data collection on care continuum outcomes prior to implementation of our program. Within the integration, LTC staff linked nearly half (43%) of patients to the VHC through coordinating appointments, making reminder calls, and communicating with the VHC. Patient navigation has been shown to increase LTC for HIV patients as well as improve care processes for the treatment of chronic diseases.21–22 In our study, intensive care coordination and patient follow-up led to an overall 43% LTC rate, 22% treatment rate, and 16% SVR rate for the program.
Although not directly comparable, our LTC program’s continuum metrics slightly surpassed the care continuum rates reported by Anderson et al, 32% LTC rate, 8% treatment rate, and 6% SVR rate (See Figure 1).13 Notably, while the LTC programs analyzed by Anderson et al. utilized care coordinators and follow-up calls, our pilot focused on streamlining the referral process in the ED via direct and intensive with an on-site VHC. In both studies, having dedicated LTC coordinators was crucial to progressing patients through the stages of the care continuum. Our staff ensured continuity of care by facilitating same-day appointments, communicating daily with clinic case management, and providing phone call reminders to patients for already scheduled appointments. A distinguishing feature of our program design lies on the specific target population of patients recognized to be HCV RNA-positive as documented in the EMR. Although testing programs are crucial for identification of new cases, there is a significant unmet need for LTC programs highlighted by the lack of patients with chronic HCV who initiate treatment.10
Figure 1:
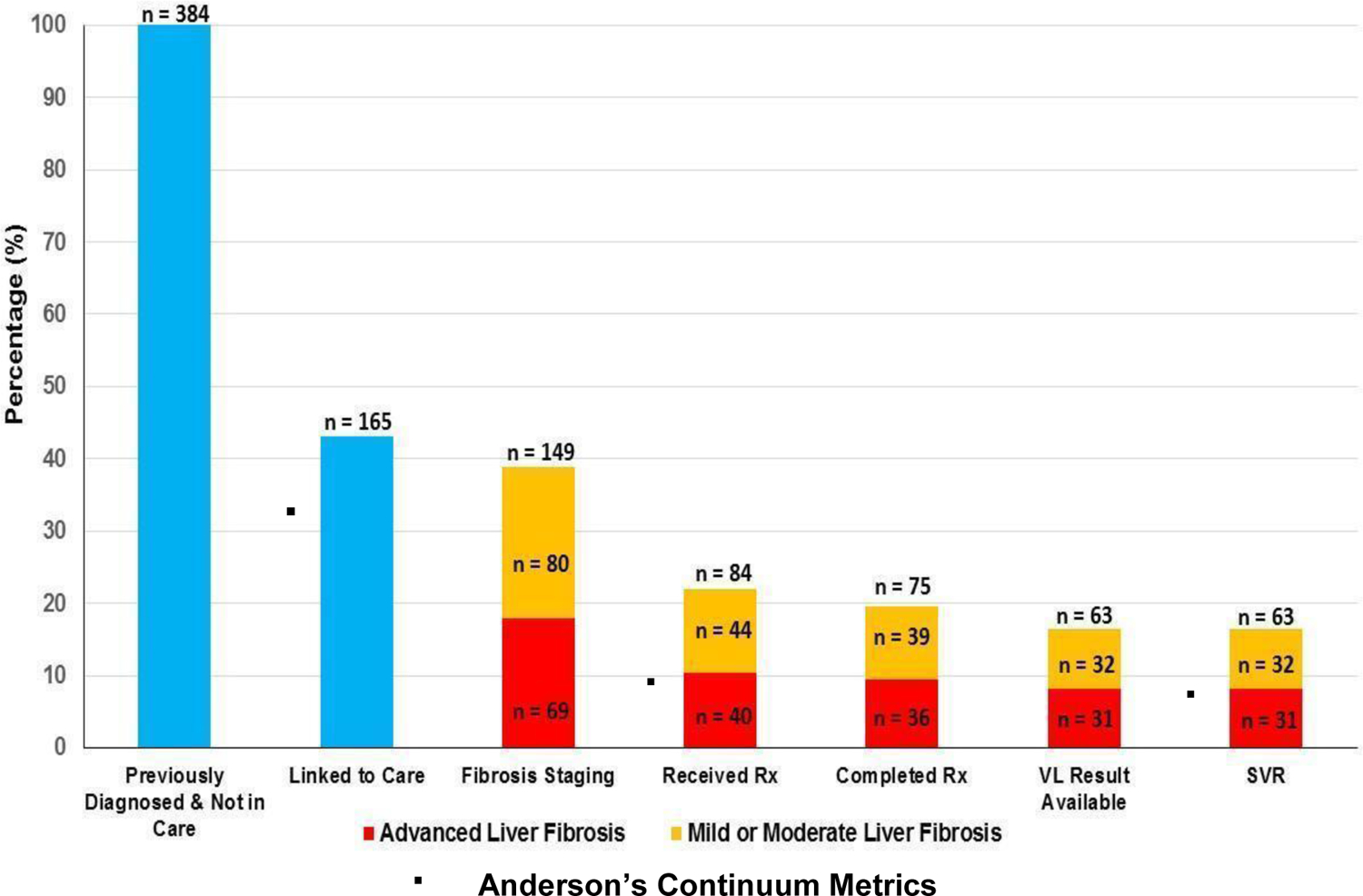
HCV Care Continuum of Emergency Department Patients with Previously Diagnosed HCV Infection & Not in Care
Additionally, our LTC program design, even piloted in 2015–2016, allows for feasible future implementation in other EDs. The minimal non-clinical training necessary for LTC navigators allows this program to be adapted for other EDs with a different staffing model and clinical resources. Existing human resources can include clinical staff, social workers, community health workers, and/or volunteer students, who can all be trained to complete the required HCV care navigation duties depending on availability of personnel and clinical culture. Furthermore, LTC programs adapting this model can exist without an integrated ED HCV testing initiative, which lowers the institutional barriers and requirements to potential implementation. Costs of implementing such a program will differ based on institutional resources and the capacity of existing ED staff to adopt LTC duties.
Significant associations between LTC and age, insurance status, and PCP status were observed in this study. Age and PCP status were also significantly associated with odds of initiation of DAA treatment. Most prior studies on age and LTC have reported older age as positively associated with LTC.23–25 This observed association may be explained by the increased severity of symptoms leading to urgency in care or health literacy and readiness of the older age group. Older patients are at increased risk of complications from chronic HCV and are more likely to have higher rates of cirrhosis and liver fibrosis.26–28 A recent ED-based study on predictors of HCV LTC found that older patients were significantly more likely to be linked to care and attributed this finding to increased rates of HCV complications for older patients.23 Even though it does not entirely reverse HCV-related liver fibrosis, completing treatment and achieving SVR reduces the incidence of hepatocellular carcinoma, hepatic decompensation, and liver-related mortality, which can also serve as an incentive for the older age patient cohort.27 As a patient’s age increases, they are more likely to experience complications related to chronic HCV and may be more inclined to pursue DAA treatment to limit their odds of developing potentially life-threatening conditions.
The factor that most improved odds of both linkage and treatment was having a PCP. Patients with a PCP were 4 times more likely to be linked, and approximately 3 times more likely to initiate treatment. Having a PCP was also marginally associated with SVR for patients that had initiated treatment. Primary care coverage has been tied to better health outcomes due to longitudinally and care coordination, and seeing a PCP can mitigate the negative health impact of poor economic circumstances.29 Previous study has shown that Baby Boomers with access to primary care have significantly lower odds of failure to HCV LTC compared to those without.30 By building consistent relationships, PCPs can help develop both trust and motivation in patients to continue medical guidance and attend follow-up appointments. One study evaluating HCV LTC across hospital departments found higher LTC percentages for outpatient sites compared to ED and inpatient sites.31 Additionally, PCPs can serve a role in interrupting the waiting paradigm that dominates the healthcare system by promoting greater control over self-management of chronic diseases such as HCV.32 If notified of their patient’s linkage to the VHC, PCPs might also serve as advocates for initiating treatment and can continually support adherence throughout the 8–12 week DAA regimen. Future emphasis on HCV medication education for PCPs could solidify their role in improving patients’ initiation to treatment and complete fulfillment. Future studies should also include further exploration of the social determinants of having a PCP in this population and address drivers and barriers.
Patients with Medicaid and Medicare insurance coverage were more likely to be linked to the VHC but were not significantly more likely to initiate treatment. The reason for this variation between LTC rates of privately and publicly insured patients is unclear, however the presence of treatment coverage restrictions remains a barrier to initiation of treatment and SVR for many patients with both types of insurance coverage. Liver damage and sobriety restrictions often limit coverage of DAA treatment to patients with some level of liver fibrosis who can pass alcohol and substance use screening.33–35 During the study period, Maryland Medicaid Fee-For-Service (FFS) and Managed Care Organizations both had these restrictions in place,36 and in 2014 Maryland had the lowest percentage of Medicaid FFS spending on DAA medication Sofosbuvir when compared to 40 other states.29 Maryland Medicaid has since expanded HCV treatment coverage by loosening restrictions to allow access to treatment regardless of liver fibrosis and substance use. However, significant restrictions remain that require pre-authorization and chronic HCV documentation to receive HCV treatment.37 Until these restrictions are removed, barriers to DAA treatment access will persist and many patients with public insurance coverage may be unable to initiate treatment.
There are several notable limitations of our study. Our study included only patients whose diagnosis of chronic HCV was evident in the EMR, and this convenient sampling procedure may have excluded some RNA-positive patients with a diagnosis unknown to our institution and limit sample size to fully evaluate the program. Due to our focus on RNA-positive patients, we did not gather data on the total number of patients who were anti-HCV positive and the proportion of these patients who obtained positive or negative RNA results. LTC staff were not on duty during off hours and the weekend and there are likely to be many HCV-positive patients who were not approached by staff. The urban, academic study site also reduces generalizability, as the availability of funding and personnel resources for implementing an LTC program may be far greater compared to other EDs. However, as noted above, creative approaches using alternative human resources may be leveraged. Furthermore, the presence of an on-site VHC that collaborated with this LTC program may have contributed to the observed rates of both LTC and initiation of DAA treatment. Due to the absence of a pre-existing LTC program, we were also unable to directly compare our observed rates of LTC, treatment, and SVR to baseline data. Additional patient characteristics, such as mental health, history of non-injection substance use, and reason for ED visit, may have an impact on HCV care continuum metrics but were not analyzed in this study. Lastly, since outcomes were gathered via retrospective chart review, we were unable to collect data on which LTC services were most effective at facilitating treatment, time from positive RNA to ED visit, time to linkage, and reasons for loss to follow-up.
The availability of curative treatment and augmentation of testing procedures for HCV has provided the potential for immense progress in achieving the WHO 2030 HCV elimination goals. Our ED HCV LTC pilot program highlights the strength of care coordination in ensuring patients achieve later stages of the HCV care continuum. This program design may also be adapted beyond the ED to improve LTC in other settings with high HCV prevalence, such as county jails. Additionally, combining an LTC program with opt-out HCV testing may increase impact when possible. Analysis of factors contributing to linkage and treatment in this study also uncovers potential interventional approaches to improve HCV care continuum matrices. Adoption of similar LTC program designs in a variety of settings may decrease the burden of HCV for the ultimate goal of global HCV elimination.
Supplementary Material
Funding:
This work was supported by the National Institute on Drug Abuse (R01DA048063 to D. L. T. and K24DA034621 to M. S. S.) at the National Institutes of Health, Maryland Department of Health (to R. E. R.) and the Gilead Sciences, Inc.’s FOCUS program (to R. E. R., Y.-H. H.).
Footnotes
Declaration of interests
We declare no competing interests.
Ethics committee approval
The Johns Hopkins University School of Medicine Institutional Review Board approved this quality assurance/quality improvement project.
References
- 1.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated With Hepatitis C Virus in the United States, 2003–2013. Clin Infect Dis. 2016. May 15;62(10):1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology. 2019;69(3):1020–1031. doi: 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Toy M, Hang Pham TT, So S. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010–2019. PLoS One. 2020. Sep 18;15(9):e0239393. doi: 10.1371/journal.pone.0239393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redfield RR, Bunnell R, Greenspan A, et al. CDC Recommendations for Hepatitis C Screening Among Adults – United States 2020. Morbidity and Mortality Weekly Report Recommendations and Reports Centers for Disease Control and Prevention. Vol 69.; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. May 2016. https://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/.
- 6.Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71(2):686–721. doi: 10.1002/hep.31060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelen G, Green G, Purcell R, et al. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med 1992; 326(21): 1399–404. [DOI] [PubMed] [Google Scholar]
- 8.Brillman J, Crandall C, Florence C, Jacobs J. Prevalence and risk factors associated with hepatitis C in ED patients. Am J Emerg Med 2002; 20(5): 476–80. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh Y, Rothman R, Laeyendecker O, et al. Evaluation of the Centers for Disease Control and Prevention Recommendations for Hepatitis C Virus Testing in an Urban Emergency Department. Clin Infect Dis 2016; 62(9): 1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N, Stein J, Hsia RY, Maselli JH, Gonzales R. Trends and characteristics of US emergency department visits, 1997–2007. JAMA - J Am Med Assoc. 2010;304(6):664–670. doi: 10.1001/jama.2010.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes KV, Gordon JA, Lowe RA. Preventive care in the emergency department, part i: clinical preventive services—are they relevant to emergency medicine? Acad Emerg Med. 2000; 7:1036–41. 22. [DOI] [PubMed] [Google Scholar]
- 12.Palepu A, Tyndall MW, Leon H, et al. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001; 165:415–20 [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson ES, Galbraith JW, Deering LJ, et al. Continuum of care for hepatitis C virus among patients diagnosed in the emergency department setting. Clin Infect Dis. 2017;64(11):1540–1546. doi: 10.1093/cid/cix163 [DOI] [PubMed] [Google Scholar]
- 14.Norton BL, Southern WN, Steinman M, et al. No Differences in Achieving Hepatitis C Virus Care Milestones Between Patients Identified by Birth Cohort or Risk-Based Screening. Clin Gastroenterol Hepatol. 2016;14(9):1356–1360. doi: 10.1016/j.cgh.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas MC, Rodriguez CV., Redd J, Sloane DA, Winston BJ, Loftus BC. Streamlining screening to treatment: The Hepatitis C cascade of care at Kaiser Permanente Mid-Atlantic States. Clin Infect Dis. 2016;62(10):1290–1296. doi: 10.1093/cid/ciw086 [DOI] [PubMed] [Google Scholar]
- 16.Hawks L, Norton BL, Cunningham CO, Fox AD. The Hepatitis C virus treatment cascade at an urban postincarceration transitions clinic. J Viral Hepat. 2016;23(6):473–478. doi: 10.1111/jvh.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akyar E, Seneca KH, Akyar S, Schofield N, Schwartz MP, Nahass RG. Linkage to care for suburban heroin users with hepatitis C virus infection, New Jersey, USA. Emerg Infect Dis. 2016;22(5):907–909. doi: 10.3201/eid2205.151980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier MM, Ross DB, Chartier M, Belperio PS, Backus LI. Cascade of care for hepatitis C virus infection within the US veterans health administration. Am J Public Health. 2016;106(2):353–358. doi: 10.2105/AJPH.2015.302927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viner K, Kuncio D, Newbern EC, Johnson CC. The continuum of hepatitis C testing and care. Hepatology. 2015;61(3):783–789. doi: 10.1002/hep.27584 [DOI] [PubMed] [Google Scholar]
- 20.Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The treatment cascade for chronic hepatitis C virus infection in the United States: A systematic review and meta-analysis. PLoS One. 2014;9(7). doi: 10.1371/journal.pone.0101554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno Y, Higa DH, Leighton CA, Roland KB, Deluca JB, Koenig LJ. Is HIV patient navigation associated with HIV care continuum outcomes?. AIDS. 2018;32(17):2557–2571. doi: 10.1097/QAD.0000000000001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBrien KA, Ivers N, Barnieh L, et al. Patient navigators for people with chronic disease: A systematic review. PLoS One. 2018;13(2):e0191980. Published 2018 Feb 20. doi: 10.1371/journal.pone.0191980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackwell JA, Rodgers JB, Franco RA, Cofield SS, Walter LA, Galbraith JW, Hess EP. Predictors of linkage to care for a nontargeted emergency department hepatitis C screening program. Am J Emerg Med. 2020. Jul;38(7):1396–1401. doi: 10.1016/j.ajem.2019.11.034. Epub 2019 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin M, Kramer J, White D, Cao Y, Tavakoli-Tabasi S, Madu S, Smith D, Asch SM, El-Serag HB, Kanwal F. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017. Nov;46(10):992–1000. doi: 10.1111/apt.14328. Epub 2017 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young KL, Huang W, Horsburgh CR, Linas BP, Assoumou SA. Eighteen- to 30-year-olds more likely to link to hepatitis C virus care: an opportunity to decrease transmission. J Viral Hepat. 2016. Apr;23(4):274–81. doi: 10.1111/jvh.12489. Epub 2015 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid M, Price JC, Tien PC. Hepatitis C Virus Infection in the Older Patient. Infect Dis Clin North Am. 2017;31(4):827–838. doi: 10.1016/j.idc.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naggie S. Hepatitis C Virus, Inflammation, and Cellular Aging: Turning Back Time. Top Antivir Med. 2017;25(1):3–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Su F, Beste LA, Green PK, Berry K, Ioannou GN. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17 487 patients. Eur J Gastroenterol Hepatol. 2017;29(6):686–693. doi: 10.1097/MEG.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L. The Impact of Primary Care: A Focused Review. Scientifica (Cairo). 2012;2012:1–22. doi: 10.6064/2012/432892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco RA, Overton ET, Tamhane AR, Forsythe JM, Rodgers JB, Schexnayder JK, Guthrie D, Thogaripally S, Zinski A, Saag MS, Mugavero MJ, Wang HE, Galbraith JW. Characterizing Failure to Establish Hepatitis C Care of Baby Boomers Diagnosed in the Emergency Department. Open Forum Infect Dis. 2016. Oct 4;3(4):ofw211. doi: 10.1093/ofid/ofw211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calner P, Sperring H, Ruiz-Mercado G, Miller NS, Andry C, Battisti L, et al. (2019) HCV screening, linkage to care, and treatment patterns at different sites across one academic medical center. PLoS ONE 14(7): e0218388. 10.1371/journal.pone.0218388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciocco G. Dal paradigma dell’attesa a quello dell’iniziativa. La strada per costruire il secondo pilastro della sanità [From waiting to pro-active health care. The main road to build the second pillar of health system]. Ann Ig. 2007. Nov-Dec;19(6):551–7. Italian. [PubMed] [Google Scholar]
- 33.Gowda C, Lott S, Grigorian M, et al. Absolute Insurer Denial of Direct-Acting Antiviral Therapy for Hepatitis C: A National Specialty Pharmacy Cohort Study. Open Forum Infect Dis. 2018;5(6):ofy076. Published 2018 Jun 7. doi: 10.1093/ofid/ofy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao JM, Fischer MA. Restrictions of Hepatitis C Treatment for Substance-Using Medicaid Patients: Cost Versus Ethics. Am J Public Health. 2017;107(6):893–899. doi: 10.2105/AJPH.2017.303748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CY, Ross-Degnan D, Zhang F, et al. Cost burden of hepatitis C virus treatment in commercially insured patients. Am J Manag Care. 2019;25(12):e379–e387. Published 2019 Dec 1. [PubMed] [Google Scholar]
- 36.National Viral Hepatitis Roundtable and Center for Health Law and Policy Innovation of Harvard Law School. Maryland Hepatitis C: State of Medicaid Access Report Card. 2019. https://stateofhepc.org/wp-content/themes/infinite-child/reports/HCV_Report_Maryland.pdf
- 37.National Viral Hepatitis Roundtable and Center for Health Law and Policy Innovation of Harvard Law School. Maryland Hepatitis C: State of Medicaid Access Report Card. 2022. https://stateofhepc.org/states/maryland/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


