Abstract
Global regulatory genes in Staphylococcus aureus, including agr and sar, are known to regulate the expression of multiple virulence factors, including cell wall adhesins. In the present study, the adherence of S. aureus RN6390 (wild type), RN6911 (agr), ALC136 (sar), and ALC135 (agr sar) to immobilized fibrinogen, fibronectin, von Willebrand factor (vWF), extracellular matrix (ECM), and human endothelial cells (EC) EAhy.926 was studied. Bacteria grown to postexponential phase were subjected to light oscillation (static condition) or to shear stress at 200 s−1 (flow condition) on tissue culture polystyrene plates coated with either protein ligands, ECM, or EC. Adherence of nonlabeled bacteria to immobilized ligands was measured by an image analysis system, while adherence of [3H]thymidine-labeled S. aureus to ECM and EC was measured by a β-scintillation counter. The results showed increased adherence of agr and agr sar mutants to immobilized fibrinogen and higher potential of these mutants to induce platelet aggregation in suspension, decreased adherence of sar and agr sar mutants to immobilized fibronectin and vWF as well as to ECM and EC, increased adherence of both S. aureus wild type and sar mutant to EC treated with platelet-rich plasma (PRP) compared to platelet-poor plasma (PPP) and to EC treated with PPP compared to the control, and increased adherence of S. aureus wild type to EC coated with PRP in which platelets were activated with phorbol 12-myristate 13-acetate compared to intact PRP. This finding paralleled the increased adherence to EC of activated compared to intact platelets. It is suggested that platelet-mediated S. aureus adherence to EC depends on platelet activation and the number of adherent platelets and available receptors on the platelet membrane. In conclusion, the agr locus downregulates S. aureus adherence to fibrinogen, while the sar locus upregulates S. aureus adherence to fibronectin, vWF, ECM, and EC. The effect of both agr and sar on S. aureus adherence properties develops primarily under flow conditions, which suggests different adhesion mechanisms in static and flow conditions.
The pathogenesis of Staphylococcus aureus infection depends on the expression of cell wall-associated adhesins and on the secretion of extracellular toxins. The control of expression and secretion of virulence factors is a complex process involving global regulatory systems, including agr and sar. The well-characterized agr locus encodes two divergent transcripts, RNAII and RNAIII, initiated from the P2 and P3 promoter, respectively (32). It has been shown that agr acts at the level of both transcription and translation to regulate the production of many toxins, enzymes, and cell surface proteins (29, 33). A second regulatory locus, sar, is essential for agr-dependent regulation. It is believed that agr activation is partially mediated by the binding of sar gene products to the agr promoter, stimulating the transcription of RNAII and RNAIII (11). Recent studies have shown that sar may bind to the P2 promoter region, regulating agr-mediated exoprotein production (20, 25). The activation of the sar PI promotes expression of SarA protein, a key regulatory molecule, which directly activates downstream genes associated with bacteria binding to fibrinogen, fibronectin, etc. During exponential growth of bacteria, the sar system promotes the synthesis of cell wall adhesins (e.g., fibronectin-binding proteins). At the postexponential phase, other virulence factors are governed by global regulatory systems (7). At this phase, the actual effector, RNAIII, activates the transcription of secretory protein genes (1).
Several studies have explored different receptors mediating S. aureus adherence to plasma proteins, biological surfaces, and foreign-body devices. S. aureus has been reported to specifically bind to fibrinogen, fibronectin, laminin, vitronectin, collagen, and thrombospondin (21, 22, 24, 34, 43). More recently, it has been shown that S. aureus binds directly to von Willebrand factor (vWF) (20), and this interaction is believed to be an important mechanism in attachment of bacteria to platelets and endothelial cells (EC).
Platelets have been considered to have a central role in the pathogenesis of infective endocarditis by providing an adhesive surface on the damaged endothelium for bacterial binding (12). S. aureus binds directly to platelets and causes platelet aggregation, resulting in the formation of infected vegetation (38). In experimental endocarditis, antiplatelet agents cause substantial reduction in vegetation weight (31). However, platelets internalize bacteria into vacuoles, enhancing their clearance from the circulation (30). Additionally, stimulated platelets mediate oxidative killing of the phagocysed bacteria (44). An array of microbicidal peptides have now been isolated from platelets (46). However, it has been suggested that platelet aggregation may protect bacteria from exposure to antibiotics and clearance by leukocytes (13).
The purpose of this study was to report the role of the agr and sar loci in the adherence properties of S. aureus RN6390 towards immobilized plasma ligands (fibrinogen, fibronectin, and vWF), subendothelial extracellular matrix (ECM), and cultured EAhy.926 EC under both static and flow conditions. The role of platelets in bacterial adherence to EC under these conditions was also examined.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strain RN6390 is a wild-type laboratory strain that maintains its hemolytic pattern; RN6911 is a derivative of RN6390 in which the agr locus has been replaced with tetM; ALC136 is an isogenic mutant of RN6390 carrying a sar mutation; and ALC135 is a derivative of RN6390 with both agr and sar mutations (4).
Preparation of bacteria.
S. aureus strains were grown overnight at 37°C on mannitol salt agar (BBL/11407), harvested in 4 ml of phosphate-buffered saline (PBS, pH 7.4), and incubated in tryptone soy broth overnight to achieve the postexponential phase. It was previously shown that these types of bacteria have comparable growth rates in broth culture (5). For radiolabeling, [methyl-3H]thymidine (1 μCi/ml) (Rotem Industries, Beer-Sheva, Israel) was added to 108 CFU of S. aureus per ml suspended in tryptone soy broth and incubated overnight at 37°C. The specific activity of the labeled S. aureus was 12,500 cpm/108 cells. Bacteria were washed three times with PBS (final volume, 3 ml) and vigorously vortexed, and the bacterial count was determined by measuring optical density (OD) at 546 nm using a standard solution (MacFarland 2 = 6 × 108 cells/ml). Bacteria were resuspended in PBS, adjusted to 109 CFU/ml, and used shortly thereafter. Under these conditions, there was no bacterial clumping.
Coating of polystyrene plates with fibrinogen, fibronectin, or vWF.
Human fibrinogen from Sigma Chemical Co (St. Louis, Mo.) was passed through a gelatin-Sepharose column to remove residual fibronectin (27). Bovine fibronectin was obtained from Biological Industries (Beit Haemek, Israel). vWF was purchased from Alexis Corp. (Laufelgingen, Switzerland). The proteins were dissolved in PBS in the presence of 0.1% bovine serum albumin (Sigma) at the following concentrations: fibrinogen, 0.5 mg/ml; fibronectin, 0.01 mg/ml; and vWF, 0.25 U/ml. Protein solution was exposed to tissue culture four-well polystyrene plates (Nunc, Roskilde, Denmark) at room temperature for 2 h. After washing with PBS containing 0.1% bovine serum albumin (BSA), plates were further incubated with PBS–1% BSA for 2 h to block the remaining free nonspecific binding sites. Coated plates were maintained at 4°C for no more than 1 week before use.
Preparation of subendothelial ECM.
ECM-coated plates were prepared as previously described (17). Briefly, bovine corneal EC were grown to confluency in four-well tissue culture polystyrene plates (Nunc, Roskilde, Denmark) and removed with 0.5% Triton X-100 and 0.1 M NH4OH solution, and the plates were extensively washed with distilled water. The wet ECM-coated plates were stored in closed bags at 4°C for up to 6 months. This ECM has been well characterized (17, 41) as being composed of collagens and proteoglycans; however, unlike vascular ECM, it does not contain vWF.
Preparation of EC.
Human EC line EAhy.926 (16), kindly provided by C. J. Edgell (Department of Pathology, University of North Carolin, Chapel Hill, N.C.), was cultured in four-well tissue culture plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), hypoxanthine-aminopterin-thymidine (HAT) supplement, and 2 mM glutamine plus 100 U of penicillin, 0.1 mg of streptomycin, and 12.5 U of nystatin per ml. Before assay, EC were gently washed twice with PBS. All experiments were performed at 37°C.
S. aureus adherence to coated polystyrene surface.
Before the experiment, the protein-coated plates were washed with PBS. S. aureus types (2 × 108 CFU per well) in PBS were incubated in the wells for 10 min at room temperature under static (light oscillation) or flow (200 s−1) conditions. Flow was created with a rotating Teflon cone, originally designed to evaluate platelet adhesion and aggregation in a Cone and Plate(let) Analyzer (42). The cone has a diameter of 14 mm, with a cone angle of 2.45°, which, according to cone-and-plate principle, induces a constant fluid shear stress over the entire plate surface. Wells were thoroughly washed with PBS and stained with May-Grünwald stain, and bacterial adherence to the surfaces was evaluated with an inverted light microscope (Olympus, Tokyo, Japan) connected to an image analysis system (Galai, Migdal Haemek, Israel).
Adherence of radiolabeled S. aureus to ECM and EC.
Suspensions of 2 × 108 CFU of [3H]thymidine-labeled S. aureus types (total volume, 200 μl) were placed on either ECM- or EC-coated plates and incubated for 20 min at 37°C under static (light oscillation) or flow (200 s−1) conditions. Under these conditions, the EC monolayer always remained intact, as determined by microscopy. After incubation with S. aureus, the wells were gently washed twice with PBS. Bound cells were collected by solubilization in a 2% sodium dodecyl sulfate (SDS) solution, and radioactivity was counted in a β-scintillation counter (liquid scintillation analyzer 1600-TR; Packard).
Preparation of plasma and [3H]adenine-labeled platelets.
Peripheral vein blood was obtained from healthy volunteers who had not taken medications known to affect platelet function for at least 10 days before blood sampling. The blood was collected in polypropylene tubes (3.8% sodium citrate anticoagulant-to-blood ratio, 1:9). Centrifugation at 160 × g for 12 min produced an upper platelet-rich plasma (PRP) suspension. The upper two-thirds of this PRP was transferred to other tubes. The remaining blood was centrifuged at 1,200 × g for 12 min to produce platelet-poor plasma (PPP), which was also transferred to separate tubes. For labeling of platelets, PRP was incubated with 5 μCi of [3H]adenine (NEN, Boston, Mass.) per ml for 30 min at room temperature, followed by the addition of citric acid to a final concentration of 5 mM, centrifuged at 800 × g for 5 min, and resuspended to the original volume in autologous PPP.
Platelet adhesion experiments.
[3H]adenine-labeled platelets in autologous citrated plasma (200 μl/well, containing 2 × 107 to 4 × 107 cells; 1 × 105 to 2 × 105 cpm/well) were treated for 20 min with phorbol 12-myristate 13-acetate (PMA), prostaglandin E1/isobutylmethylxanthine (PGE1/IBMX) (all reagents from Sigma), or PBS as a control. Platelets were resuspended in autologous plasma and incubated with EC for 20 min at 37°C under static conditions. The unbound platelets were then gently washed with PBS, while bound platelets were solubilized in 2% SDS, and the radioactivity in the wells was counted in a β-scintillation counter.
S. aureus-induced platelet aggregation.
Platelet aggregation was monitored using a standard technique in which 225 μl of PRP was incubated at 37°C and stirred at 1,000 rpm in a four-channel aggregometer (PACKS-4; Helena Laboratories, Beaumont, Tex.). Aggregation was induced by adding 25 μl of S. aureus suspension in PBS (bacterium-platelet ratio, 4:1). Change in light transmission was recorded for 20 min. As a control, ADP (5 μM) was used as an inducer of platelet aggregation.
Statistical analysis.
To compare the mean ± standard deviation (SD) values of the results, an unpaired t test was used. Statistical significance was accepted for a value of P of <0.05.
RESULTS
S. aureus adherence to immobilized fibrinogen, fibronectin, and vWF.
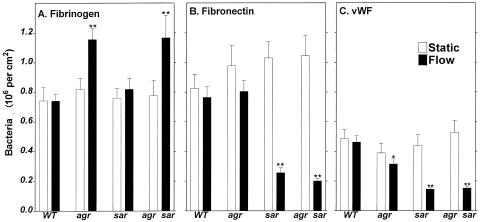
As shown in Fig. 1A, under static conditions, we found no difference in extent of binding to the immobilized fibrinogen among all four tested S. aureus types. However, under flow, there was increased adherence of both agr and agr sar mutant S. aureus types to fibrinogen (by 55 and 56%, respectively) compared to the wild type. These data suggest that the agr locus downregulates bacterial adherence to fibrinogen. Similar to adherence to fibrinogen, almost the same extent of adherence of all S. aureus types to fibronectin was demonstrated under static conditions (Fig. 1B). However, a substantial reduction (down to 25%) in adherence of both sar and agr sar types was observed, suggesting that the sar locus upregulates bacterial binding to fibronectin. Figure 1C shows similar adherence of all S. aureus types to immobilized vWF under static conditions. In contrast, under flow, substantial reduction of binding to vWF of agr, sar, and agr sar types was observed (by 32, 69, and 67%, respectively). These data suggest that both the agr and sar loci upregulate bacterial binding to vWF.
FIG. 1.
Adherence of S. aureus to immobilized fibrinogen (A), fibronectin (B), and vWF (C). RN6390 wild type (WT), RN 6911 agr, ALC136 sar, and ALC135 agr sar S. aureus cells (2 × 108 CFU) were placed on polystyrene plates coated with fibrinogen (0.5 mg/ml), fibronectin (0.01 mg/ml), or vWF (0.25 U/ml) and incubated under static (light oscillation) or flow (200 s−1) conditions for 10 min at room temperature. Samples were washed, stained, and analyzed as described in Materials and Methods. Data are means ± SD for four to six determinations performed in duplicate. ∗, P < 0.01; ∗∗, P < 0.001 for mutants compared to the wild type.
S. aureus adherence to bovine ECM.
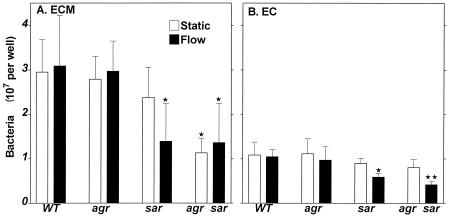
Taking into account the difficulties in applying the image analysis system for determining bacterial adherence to subendothelial ECM and EC (due to bacterial clumping upon adherence to ECM and to interference of EC with direct counting of bacteria), the subsequent experiments were performed with [3H]thymidine-treated bacteria, and the extent of binding was expressed as total count of cells per well. As shown in Fig. 2A, under static conditions, a reduction in adherence of the double mutant S. aureus to ECM was observed (down to about 50% compared to the wild type). As well, under flow, the reduced adherence of both sar and agr sar bacteria (down to about 50%) was observed. These data are in accordance with those obtained from fibronectin- and vWF-coated surface experiments.
FIG. 2.
Adherence of S. aureus to subendothelial ECM (A) and EC (B). [3H]thymidine-labeled bacteria (2 × 108 CFU) were placed on the surfaces and incubated for 20 min at 37°C under static or flow conditions. Samples were washed, and adherent bacteria were solubilized with 2% SDS. Data are means ± SD for four to six determinations performed in duplicate. ∗, P < 0.01; ∗∗, P < 0.001 for mutants compared to the wild type (WT).
S. aureus adherence to EC.
Under static conditions, no significant reduction in binding of sar and agr sar bacteria to EC monolayer was shown (Fig. 2B). However, under flow conditions, these bacteria demonstrated substantially decreased adherence (down to about 50%). Generally, these data were similar to those obtained from the ECM surface, except adherence of all S. aureus types was about 30% of that compared to ECM. Again, the difference in adherence between various S. aureus types can be observed mainly under flow conditions.
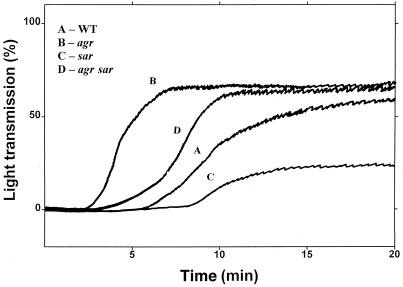
S. aureus-induced platelet aggregation.
These experiments were performed in PRP. The ability of S. aureus to aggregate platelets in the suspension phase was confirmed (Fig. 3). Taking the lag phase in aggregation traces as a parameter for evaluation of the ability of bacteria to cause platelet aggregation (39), it was found that agr and agr sar types were stronger in this action than the wild type. These data agree with those shown in Fig. 1A, which demonstrates increased adherence of the same mutants to a fibrinogen-coated surface, since fibrinogen is the main plasma ligand responsible for platelet aggregation in the suspension phase.
FIG. 3.
S. aureus-induced platelet aggregation. Wild-type (WT) and mutant bacteria suspended in PBS were added to PRP at a bacterium-platelet ratio of 4:1 in a four-channel platelet aggregometer. The change in light transmission was evaluated. Representative picture of three to four independent experiments.
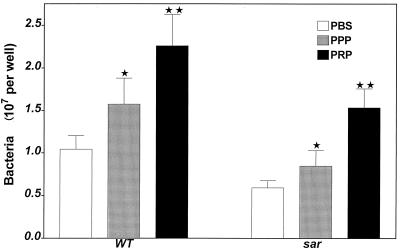
Relative role of plasma ligands and platelets in S. aureus adherence to EC.
Only wild-type and sar mutant bacteria were used in this assay because the sar locus has been found to be responsible for bacterial adherence to EC. As shown in Fig. 4, treatment of EC monolayer with plasma free of platelets was followed by increased binding of both wild-type and sar mutant strains under flow compared to intact EC (51 and 43%, respectively). When EC were treated with PRP, the adherence rates increased by 154 and 159%, respectively. Thus, both plasma ligands and platelets promote S. aureus adherence to EC. The two types of bacteria similarly responded to the treatment of EC with plasma or platelets.
FIG. 4.
Adherence of S. aureus to endothelial cells treated with PBS–0.1% BSA, PPP, and PRP. Two hundred microliters of plasma was placed on the EC monolayer and incubated for 20 min at 37°C. After washing, 2 × 108 CFU of radiolabeled S. aureus (wild type [WT] and sar mutant) was added to the samples and subjected to flow for 20 min at 37°C. Samples were washed, and adherent bacteria were solubilized with 2% SDS. Data are means ± SD for four to six determinations performed in duplicate. ∗, P < 0.05; ∗∗, P < 0.01 compared to samples of EC treated with PBS–0.1% BSA.
Platelet activation modulates S. aureus adherence to EC.
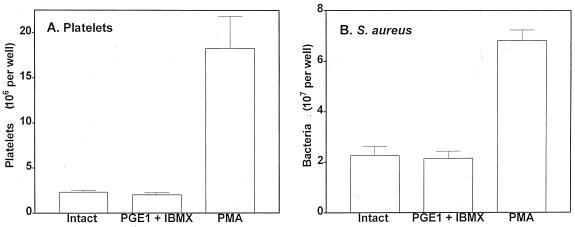
[3H]adenine-labeled platelet adherence to intact EC under flow was assayed. PGE1 and IBMX were added to PRP to inactivate platelets by increasing the intracellular cyclic AMP (cAMP) level (26). Platelet inactivation did not change the binding to EC under flow (Fig. 5). In contrast, platelet activation with PMA, an activator of protein kinase C, resulted in about a ninefold increase in platelet adherence to EC. Similarly, treatment of EC with PGE1/IBMX-inactivated platelets (nonradiolabeled) did not change [3H]thymidine-labeled S. aureus adherence to EC compared to EC treated with intact platelets. However, treatment of EC with PMA-activated platelets was followed by enhanced, up to threefold, S. aureus adherence. These data suggest that hyperexpression of various receptors on the platelet membrane upon activation, which promotes platelet-EC interaction (3), also provides binding sites for bacteria, thus mediating increased bacterial adherence.
FIG. 5.
Adherence of platelets in PRP to EC (A) and S. aureus wild type to EC treated with PRP (B). (A) [3H]adenine-labeled platelets in autologous citrated plasma (200 μl/well containing 2 × 107 to 4 × 107 cells) treated with PBS–0.1% BSA, 1 μM PMA, or PGE1/IBMX for 20 min were incubated with EC for 20 min at 37°C under static conditions. After washing and solubilization, the number of adherent platelets was measured. (B) EC monolayer was incubated for 20 min at 37°C under static conditions with PRP containing nonradiolabeled platelets treated with PBS–0.1% BSA, PMA, or PGE1/IBMX. After washing, [3H]thymidine-labeled wild-type S. aureus cells (2 × 108 CFU) were placed on the EC surface and incubated for 20 min at 37°C under flow conditions. Samples were washed, and the number of adherent bacteria was measured. Data are mean ± SD for three to five determinations performed in duplicate.
DISCUSSION
The virulence properties of S. aureus are controlled by global regulatory systems, including agr and sar. Previous studies suggest that these loci primarily regulate the expression of cell wall proteins during the exponential growth of bacteria. The secreted proteins are induced at the late phase of exponential growth and are produced at low levels in agr mutants (32). In contrast, surface proteins responsible for bacterial adhesion to host elements are produced mostly during exponential growth and in a much higher level in agr mutants (23). It has been suggested that the agr locus upregulates the expression of extracellular virulence genes, while it downregulates cell surface protein genes (1, 32). The sar locus upregulates expression of both exoproteins and cell wall adhesins (7). However, the role of these systems in virulence properties of S. aureus is probably more complex.
To evaluate the contribution of both agr and sar loci in S. aureus adherence to vessel wall EC, subendothelial ECM and immobilized plasma ligands, the RN6911 (agr), ALC136 (sar), and ALC135 (agr sar) mutants of RN6390 wild–type S. aureus were used in this study. The assay was performed in parallel under both static and flow conditions, taking into account that ligand-mediated binding of bacteria to the vessel wall might differ under flow and static conditions. The results showed increased adherence of agr and agr sar mutants to immobilized fibrinogen, decreased adherence of agr, sar, and agr sar mutants to immobilized vWF, and decreased adherence of sar and agr sar mutants to immobilized fibronectin, ECM, and EC. Taken together, these data suggest that the agr locus downregulates S. aureus binding to fibrinogen and upregulates S. aureus binding to vWF. The sar locus upregulates S. aureus binding to fibronectin, vWF, ECM, and EC. These data agree with those showing upregulation of S. aureus binding to fibronectin by sar (7). Cheung et al. (10) have reported that inactivation of the sar locus in S. aureus leads to decreased expression of both fibrinogen and fibronectin-binding proteins. Moreover, in a rabbit endocarditis model, there was a significant decrease in infectivity rates for a sar mutant compared to the parental strain (10). Accordingly, mutation of the sarA gene within the sar locus resultes in decreased expression of both extracellular and cell wall proteins (9).
In the present study, both agr and agr sar mutants showed increased binding to immobilized fibrinogen. These mutant strains had a stronger potential to induce platelet aggregation where fibrinogen was the main bridging molecule in bacterium-platelet and platelet-platelet interaction. Adherence of bacteria to plasma ligands may differ in suspension and on the solid phase. Cheung et al. (5) did not observe any effect of agr on S. aureus binding to radiolabeled fibrinogen in suspension. Saravia-Otten et al. (37), using radiolabeled fibronectin in suspension, concluded that fibronectin-binding genes are negatively regulated by both agr and agr-independent mechanisms. It has also been proposed that both the agr and sar loci function within the same regulatory pathway in which the agr and sar gene products act as the repressor and the activator, respectively. Another possibility is that the sar locus acts irrespective of the functionality of the agr locus, leading to upregulation of cell wall proteins (9, 10).
These are no data in the literature regarding the role of agr and sar in S. aureus adherence to vWF. As protein A has been found to be the vWF binding protein (18) and both agr and sar negatively regulate protein A expression by S. aureus (6), it could be suggested that agr and sar mutant bacteria must show increased adherence to vWF. However, reduced adherence to vWF of both mutant S. aureus types has been found in our study. It may be that interaction of protein A with suspended or immobilized vWF differs. Furthermore, vWF has distinct binding domains for several human macromolecules, e.g., collagen, heparin, and platelet glycoproteins Ib and IIb-IIIa (35, 36). In addition, the heparin-binding domain of vWF might be the crucial region for S. aureus attachment, and both vWF and thrombospondin may recognize similar molecules on S. aureus membrane (15).
The data show that the regulation of S. aureus binding to various ligands by agr and sar is a rather complicated process. According to our data, upregulation by sar of bacterial binding to fibronectin and vWF, the important components of subendothelial ECM secreted by EC, apparently served as the main mechanism of S. aureus adherence to ECM and EC.
In our previous study, it was shown that fibrinogen and fibronectin, and not platelets, mediated S. aureus adherence to thrombin-activated EC, EC-304, under flow conditions (40). In this study, the role of plasma ligands and platelets in S. aureus adherence to resting EC EAhy.926 was evaluated (16). The sar mutant was used in parallel with the wild type, as the sar mutant was found to have a lower ability to adhere to fibronectin, vWF, ECM, and EC. It should be noted that treatment of EC with plasma free of platelets enhanced adherence of both types of S. aureus by about 50%, while about 150% enhancement was observed with PRP. Preactivation of platelets in PRP with PMA further enhanced S. aureus binding to EC, which paralleled enhanced adherence of PMA-activated platelets to EC. Unlike platelet inactivation by PGE1/IBMX, it failed to affect both platelet and bacterial adherence. Apparently, secretion of various ligands from α-granules of platelets together with hyperexpression of glycoprotein IIb-IIIa and other receptors upon platelet activation (14) causes enhanced S. aureus binding to PMA-activated platelets on the surface. These data agree with those demonstrating that S. aureus binds to platelets in a manner suggestive of a receptor-ligand interaction, which depends on the estimated number of available receptors on the platelets more than it depends on the absolute adhesive affinity of bacteria for the platelet (45).
Thus, both plasma ligands and platelets mediate S. aureus binding to EC. Expression of fibronectin and vWF on the membrane of damaged EC is an important mechanism for tethering circulating platelets to EC. However, S. aureus can adhere to both fibronectin (22, 43) and vWF (20) that allowed bacteria to adhere to ligands bound to both platelets and EC. This mechanism is upregulated by the sar locus and differs from that in S. aureus-induced platelet aggregation in the conventional aggregometry assay, where plasma fibrinogen is the main ligand mediating bacterium-platelet interaction (2). The mediating role of fibrinogen in S. aureus binding to EC is also well established (8, 28, 40). As seen from this study, binding of bacteria to fibrinogen is downregulated by the agr locus, which may serve as a controlling mechanism, opposite sar, in the multifactorial regulation of adherence properties of S. aureus.
ACKNOWLEDGMENT
We thank Martha Peisachov for technical assistance.
REFERENCES
- 1.Balaban N, Novick R P. Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc Natl Acad Sci USA. 1995;92:1619–1623. doi: 10.1073/pnas.92.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer A S, Sullam P M, Ramos M, Li C, Cheung A L, Yeaman M R. Staphylococcus aureus induced platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun. 1995;63:3634–3641. doi: 10.1128/iai.63.9.3634-3641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bombeli T, Schwartz B R, Harlan J M. Adhesion of activated platelets to endothelial cells: evidence for a GPIIb-IIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), αvβ3 integrin and GPIba. J Exp Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung A L, Eberhardt K J, Chung E, Yeaman M R, Sullam P M, Ramos M, Bayer A S. Diminished virulence of sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Investig. 1994;94:1815–1822. doi: 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Eberhardt K, Heinrichs J H. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect Immun. 1997;65:2243–2249. doi: 10.1128/iai.65.6.2243-2249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Koomey J M, Butler C A, Projan A J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Krishnan M, Jaffe E A, Fischetti V A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Investig. 1991;87:2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Projan S L. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung A L, Yeaman M, Bayer A S. The role of the sar locus of Staphylococcus aureus in the induction of endocarditis in rabbits. Infect Immun. 1994;62:1719–1725. doi: 10.1128/iai.62.5.1719-1725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien Y, Manna A C, Cheung A L. sarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol. 1998;30:991–1001. doi: 10.1046/j.1365-2958.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 12.Clawson C C. The role of platelets in the pathogenesis of endocarditis. Am Heart Assoc Monogr. 1979;52:24–27. [Google Scholar]
- 13.Clawson C C, White J G. Platelet interaction with bacteria. II. Fate of bacteria. Am J Pathol. 1971;65:381–398. [PMC free article] [PubMed] [Google Scholar]
- 14.Colman R W. Receptors that activate platelets. Proc Soc Exp Biol Med. 1991;197:242–248. doi: 10.3181/00379727-197-43251. [DOI] [PubMed] [Google Scholar]
- 15.Dai-Qing L, Lundberg F, Ljungh A. Binding of von Willebrand factor by coagulase-negative staphylococci. J Med Microbiol. 2000;49:217–225. doi: 10.1099/0022-1317-49-3-217. [DOI] [PubMed] [Google Scholar]
- 16.Edgell C J, McDonald C C, Graham J B. Permanent cell line expressing factor VIII-related antigen established by hybrodization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gospodarowitz D, Greenburg G, Foidart J M, Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981;107:171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- 18.Hartleib J, Köhler N, Dickinson R B, Chhatwal G S, Sixma J J, Hartford O M, Foster T J, Peters G, Kehrel B E, Herrmann M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- 19.Heinrichs J H, Bayer M G, Cheung A L. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178:418–423. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann M, Hartleib J, Kehrel B, Montgomery R R, Sixma J J, Peters G. Interaction of von Willebrand factor with Staphylococcus aureus. J Infect Dis. 1997;176:984–991. doi: 10.1086/516502. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann M P, Suchard S J, Boxer L A, Waldvogel F A, Lew P D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991;59:279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann M, Vaudaux P, Pittet D, Auckenthaler R, Lew P D, Schumacher-Perdreau F, Peters G, Waldvogel F A. Fibronectin, fibrinogen and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 23.Janzon L, Lofdahl S, Arvidson S. Evidence for a coordinate control of alpha-toxin and protein A in Staphylococcus aureus. FEMS Microbiol Lett. 1986;33:193–198. [Google Scholar]
- 24.Liang O D, Flock J-I, Wadstrom T. Evidence that the heparin-binding consensus sequence of vitronectin is recognised by Staphylococcus aureus. J Biochem. 1994;116:457–463. doi: 10.1093/oxfordjournals.jbchem.a124546. [DOI] [PubMed] [Google Scholar]
- 25.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurice D H, Haslam R J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990;37:671–681. [PubMed] [Google Scholar]
- 27.McDevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, McIntire L, Foster T, Hook M. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem. 1997;247:416–424. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 28.Moreillon P, Entenza J M, Francioli P, McDevitt D, Foster T J, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morfeldt E, Yaylor D, von Gabain A, Arvidson S. Activation of alpha-toxin translation in Staphylococcus aureus by the transencoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Movat H Z, Weiser W I, Glynn M F, Mustard J F. Platelet phagocytosis and aggregation. J Cell Biol. 1965;27:531–543. doi: 10.1083/jcb.27.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolau D P, Freeman C D, Nightingale C H, Quintiliani R, Coe C J, Maderazo E G, Cooper B W. Reduction of bacterial titers by low-dose aspirin in experimental aortic valve endocarditis. Infect Immun. 1993;61:1593–1595. doi: 10.1128/iai.61.4.1593-1595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3977. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 34.Roden M, Flock J I. Evidence for three different fibrinogen-binding proteins with unique properties from Staphylococcus aureus strain. Microb Pathog. 1992;12:289–298. doi: 10.1016/0882-4010(92)90047-r. [DOI] [PubMed] [Google Scholar]
- 35.Ruggeri Z M, Ware J. The structure and function of von Willebrand factor. Thromb Haemostasis. 1992;67:594–599. [PubMed] [Google Scholar]
- 36.Sadler J E. Von Willebrand factor. J Biol Chem. 1991;266:22777–22780. [PubMed] [Google Scholar]
- 37.Saravia-Otten P, Müller H-P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheld W M, Valone J A, Sande M A. Bacterial adherence in the pathogenesis of endocarditis: interaction of bacterial dextran, platelets and fibrin. J Clin Investig. 1978;68:1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenkman B, Rubinstein E, Dardik R, Tamarin I, Savion N, Varon D. Activated platelets mediate Staphylococcus aureus deposition on subendothelial extracellular matrix: the role of glycoprotein Ib. Platelets. 1999;10:36–44. doi: 10.1080/09537109976338. [DOI] [PubMed] [Google Scholar]
- 40.Shenkman B, Rubinstein E, Tamarin I, Dardik R, Savion N, Varon D. Staphylococcus aureus adherence to thrombin-treated endothelial cells is mediated by fibrinogen but not by platelets. J Lab Clin Med. 2000;135:43–51. doi: 10.1016/s0022-2143(00)70019-9. [DOI] [PubMed] [Google Scholar]
- 41.Tseng S C G, Savion N, Gospodarowicz D, Stern R. Characterization of collagens synthesized by cultured bovine corneal endothelial cells. J Biol Chem. 1981;256:3361–3365. [PubMed] [Google Scholar]
- 42.Varon D, Dardik R, Shenkman B, Kotev-Emeth S, Farzame N, Tamarin I, Savion N. A new method for quantitative analysis of whole blood platelet interaction with extracellular matrix under flow conditions. Thromb Res. 1997;85:283–294. doi: 10.1016/s0049-3848(97)00014-5. [DOI] [PubMed] [Google Scholar]
- 43.Vercellotti M, McCarthy J B, Lindholm P, Peterson P K, Jacob H S, Furcht L T. Extracellular matrix proteins (fibronectin, laminin and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985;120:13–21. [PMC free article] [PubMed] [Google Scholar]
- 44.Yeaman M R, Bayer A S. Antimicrobial peptides from platelets. Drug Resist Updates. 1999;2:116–126. doi: 10.1054/drup.1999.0069. [DOI] [PubMed] [Google Scholar]
- 45.Yeaman M R, Sullam P M, Dazin P E, Norman D C, Bayer A S. Characterization of Staphylococcus aureus-platelet interaction by quantitative flow cytometry. J Infect Dis. 1992;166:65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]
- 46.Yeaman M R, Tang Y-Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidial proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]