Abstract
Purpose
Uncertainties remain about the safety and efficacy of therapies for managing intracranial hypertension in acute brain injured (ABI) patients. This study aims to describe the therapeutical approaches used in ABI, with/without intracranial pressure (ICP) monitoring, among different pathologies and across different countries, and their association with six months mortality and neurological outcome.
Methods
A preplanned subanalysis of the SYNAPSE-ICU study, a multicentre, prospective, international, observational cohort study, describing the ICP treatment, graded according to Therapy Intensity Level (TIL) scale, in patients with ABI during the first week of intensive care unit (ICU) admission.
Results
2320 patients were included in the analysis. The median age was 55 (I-III quartiles = 39–69) years, and 800 (34.5%) were female. During the first week from ICU admission, no-basic TIL was used in 382 (16.5%) patients, mild-moderate in 1643 (70.8%), and extreme in 295 cases (eTIL, 12.7%). Patients who received eTIL were younger (median age 49 (I–III quartiles = 35–62) vs 56 (40–69) years, p < 0.001), with less cardiovascular pre-injury comorbidities (859 (44%) vs 90 (31.4%), p < 0.001), with more episodes of neuroworsening (160 (56.1%) vs 653 (33.3%), p < 0.001), and were more frequently monitored with an ICP device (221 (74.9%) vs 1037 (51.2%), p < 0.001). Considerable variability in the frequency of use and type of eTIL adopted was observed between centres and countries. At six months, patients who received no-basic TIL had an increased risk of mortality (Hazard ratio, HR = 1.612, 95% Confidence Interval, CI = 1.243–2.091, p < 0.001) compared to patients who received eTIL. No difference was observed when comparing mild-moderate TIL with eTIL (HR = 1.017, 95% CI = 0.823–1.257, p = 0.873). No significant association between the use of TIL and neurological outcome was observed.
Conclusions
During the first week of ICU admission, therapies to control high ICP are frequently used, especially mild-moderate TIL. In selected patients, the use of aggressive strategies can have a beneficial effect on six months mortality but not on neurological outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06937-1.
Keywords: Intracranial pressure, Traumatic brain injury, Therapy intensity level, Intracranial haemorrhage, Subarachnoid haemorrhage
Take-home message
| Treatments for intracranial hypertension are frequently applied in the first week of ICU management of acute brain injured patients. The use of aggressive therapies for controlling high intracranial pressure is frequent, with significant variability. The staircase approach recommended by the most recent guidelines is not followed. However, aggressive strategies applied in sicker patients can ameliorate six months of mortality but not the neurological outcome. |
Introduction
Controlling detrimental increases in intracranial pressure (ICP) is an essential component of the management of acute brain injured (ABI) patients in the intensive care unit (ICU) [1].
Many ICP-lowering treatments have been proposed [2–5]. Based on the most recent literature [6–8], current clinical algorithms suggest applying these strategies stepwise, with less aggressive and safer treatments recommended in the first instances and more aggressive ones reserved for refractory cases of intracranial hypertension [2–5]. However, considerable variability across centres and countries regarding the application of these strategies remains [9–11] because of the absence of definitive guidelines, different economic resources, and lack of a clear beneficial effect on the outcome, especially regarding more aggressive treatments such as barbiturates, hypothermia, decompressive craniectomy and hyperventilation [11, 12]. In addition, literature and clinical recommendations [3, 13] on ICP management are mainly related to traumatic brain injured (TBI) patients [14]. Limited information is available on their application in other types of ABIs, such as subarachnoid haemorrhage (SAH) [15] and intracranial haemorrhage (ICH), despite intracranial hypertension commonly occurs in these pathologies [16, 17]. The graduation of such interventions could be described with the therapy intensity level (TIL) scale, introduced more than 30 years ago and subsequently revised and validated [18–20].
Given these uncertainties, we performed a subanalysis of the SYNAPSE-ICU study [21]. The primary aim was to describe the therapeutic approaches for controlling intracranial hypertension in patients with ABI during the first week since ICU admission. Moreover, we explored the variability in the use of TILs among different pathologies (SAH, TBI and ICH), in ICP-monitored and non-monitored patients and across different countries/centres. Finally, we explored the association between the use of varying levels of TILs and clinical outcomes.
Methods
This is a pre-planned secondary analysis of the SYNAPSE-ICU study (ClinicalTrial.gov NCT03257904) [21, 22], a multicentre, prospective, international, observational cohort study whose primary aim was the evaluation of the clinical practice and management of ICP monitoring in ABIs. The SYNAPSE-ICU study included 2395 adult patients enrolled in 146 sites and 42 countries worldwide. It helped to clarify the current clinical use of ICP monitoring and treatment across different countries with different resources and its use in various types of ABIs.
The inclusion criteria of the SYNAPSE-ICU study were patients aged ≥ 18 years, with a diagnosis of ABI following TBI, ICH or SAH, and either an altered level of consciousness, defined as a Glasgow Coma Scale (GCS) eye response score of 1, no eye-opening, and a GCS motor response score ≤ 5, not obeying commands on admission to ICU, or having experienced neurological deterioration with no eye-opening and motor score decreased to 5 or less within 48 h after ICU admission. Exclusion criteria were patients not requiring ICU admission and/or being admitted for other forms of ABI. Further details on the study procedure and patients' clinical management have been published [21, 22].
The Ethical Committee approval was obtained for the SYNAPSE-ICU study at the coordinating and in all participating centres, and informed consent was obtained according to local regulations. No additional authorization from the Ethical Committee was required for this analysis.
For the present study, reported according to the Guidelines for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (Electronic Supplementary Material, ESM), we included only patients with available data on TIL on day 1 (ESM, Fig. S1).
Data collection and definitions
Data were deidentified and stored in a web-based electronic database (eCRF) and were securely held at the University of Milano-Bicocca. All the procedures complied with the European Union Regulation 2016/679 on protecting natural persons regarding personal data processing and movement.
Neuroworsening was defined as a spontaneous decrease ≥ 2 points in the GCS motor score compared with the previous examination and/or new loss of pupillary reactivity, development of pupillary asymmetry ≥ 2 mm or deterioration in neurological or computed tomography (CT) status sufficient to warrant immediate medical or surgical intervention within the first week of the ICU stay. Highly pathological CT scan was defined as Marshall classification ≥ 3 (for patients with TBI), Fisher grade ≥ 3 (for patients with SAH), or intracranial haemorrhage volume ≥ 30 mL (for patients with ICH). ICP was recorded in the eCRF as the daily highest value collected on days 1, 3, and 7 of the ICU stay.
Therapy intensity levels
Interventions to reduce intracranial hypertension were recorded according to the therapeutic intensity level (TIL) scale [20] on days 1, 3, and 7 of the ICU stay. The daily TIL scale was categorized into three therapy levels: no-basic, mild-moderate and extreme treatment (ESM, Table S1). Patients were allocated to one of these levels according to both the maximum daily and weekly TIL. The level of TIL during the week was defined as the maximum level of treatment over days 1, 3, and 7. Patients who received extreme therapies at least once on days 1, 3 and/or 7 during the week were defined as the “eTIL group”; otherwise were included in the “no-eTIL” group. Patients primarily decompressed on day 1 were classified according to the maximum TIL level without considering the primary decompression because refractory intracranial hypertension was not measured in this context. If a patient received a decompressive craniectomy on day 3, they were considered also decompressed on day 7.
Endpoints
The primary endpoint was the prevalence of TIL use in ABI in the first week since ICU admission. Secondary endpoints included mortality and Glasgow Outcome Scale Extended (GOSE) collected at a 6-month follow-up using a validated questionnaire. An unfavourable outcome was defined as a GOSE score of less than 5.
Statistical analysis
Patient’s characteristics were described by mean (standard deviation) or median (I-III quartiles) and frequencies (percentage), as appropriate.
Between-group comparisons were performed by the Mann–Whitney U test for continuous data and χ2 test for categorical data.
Variability in the practice of eTIL between countries and centres was estimated using the median odds ratio (MOR) derived by generalized linear mixed models, with country or centre included as a random effect and adjusted for relevant covariates (i.e. ICP monitoring, GCS < 8, age, pupil’s reactivity, highly pathological CT scan, neuroworsening, type of pathology and economic country level).
To estimate the association between the maximum TIL over the week and outcomes, we applied the Cox regression (mortality) and the logistic regression (unfavourable neurological outcome) models. All models were adjusted for ICP monitoring, GCS, age, pupils’ reactivity, highly pathological CT scan, neuroworsening and pathologies. Results were expressed as hazard ratio (HR) or odds ratio (OR) along with their 95% confidence interval (95%CI). Different sensitivity analyses were performed: (1) excluding very severe patients (defined as death within 48 h or GCS equal to 3 and both pupils unreactive), (2) considering patients with primary decompression allocated in the eTIL group instead of considering the maximum TIL level without the primary decompression, and (3) excluding patients who died after withdrawal of care. All the tests were two-sided, and the type I error was set at 0.05. The analyses were performed using R software (version 4.0.3).
Results
Characteristics of the study population
From 2395 patients enrolled in the SYNAPSE-ICU study, 75 without TIL data on day 1 were excluded, leaving 2320 patients available for this analysis (ESM, Figure S1). 1339 (57.7%) patients were admitted for TBI, 409 (17.6%) for SAH and 572 (24.7%) for ICH. The characteristics of the study cohort are presented in Table 1. The median age was 55 (I–III quartiles 39–69) years, and 800 (34.5%) were female. Of those, 1916 (85.6%) presented with GCS ≤ 8, and 744 (34%) patients had at least one unreactive pupil. Also, 409 (17.6%) patients were admitted from low-income countries.
Table 1.
Characteristics of patients, overall and stratified according to the use of extreme therapies (eTIL) over the first week since ICU admission
| Characteristics | Overall N = 2320 |
No eTIL N = 2025 (87.3%) |
eTIL N = 295 (12.7%) |
p value |
|---|---|---|---|---|
| Age (years), median (I-III quartiles) | 55 (39, 69) | 56 (40, 69) | 49.00 (35, 62) | < 0.001 |
| Female, n (%) | 800 (34.5) | 694 (34.3) | 106 (35.9) | 0.621 |
| Low-Middle income countries, n (%) | 409 (17.6) | 356 (17.6) | 53 (18) | 0.936 |
| Alcohol use, n (%) | 269 (63) | 232 (62.5) | 37 (66.1) | 0.717 |
| Drug use, n (%) | 83 (21.1) | 71 (20.7) | 12 (24) | 0.727 |
| Smoker, n (%) | 274 (66.8) | 239 (66.4) | 35 (70) | 0.728 |
| Cardiovascular history, n (%) | 949 (42.4) | 859 (44) | 90 (31.4) | < 0.001 |
| Hypertension, n (%) | 844 (89.2) | 764 (89.1) | 80 (89.9) | 0.972 |
| Neurological comorbidities, n (%) | 279 (12.5) | 243 (12.4) | 36 (12.6) | 1.000 |
| Diagnosis, n (%) | 0.014 | |||
| ICH | 572 (24.7) | 518 (25.6) | 54 (18.3) | |
| SAH | 409 (17.6) | 346 (17.1) | 63 (21.4) | |
| TBI | 1339 (57.7) | 1161 (57.3) | 178 (60.3) | |
| Pupils at admission, n (%) | 0.346 | |||
| Both reactive | 1443 (66) | 1268 (66.4) | 175 (63.2) | |
| One reactive | 260 (11.9) | 220 (11.5) | 40 (14.4) | |
| Both unreactive | 484 (22.1) | 422 (22.1) | 62 (22.4) | |
| GCS ≤ 8 at admission, n (%) | 1916 (85.6) | 1673 (85.3) | 243 (87.7) | 0.319 |
| GCS at admission, n (%) | 0.516 | |||
| 3–5 | 1164 (52) | 1019 (51.9) | 145 (52.3) | |
| 6–8 | 752 (33.6) | 654 (33.3) | 98 (35.4) | |
| > 8 | 323 (14.4) | 289 (14.7) | 34 (12.3) | |
| Neuroworsening*, n (%) | 813 (36.2) | 653 (33.3) | 160 (56.1) | < 0.001 |
| Neurosurgical intervention at day 1, n (%) | 349 (15) | 286 (14.1) | 63 (21.4) | 0.002 |
| ICPm, n (%) | 1258 (54.2) | 1037 (51.2) | 221 (74.9) | < 0.001 |
| Highly pathological CT scan, n (%) | 1494 (64.4) | 1288 (63.6) | 206 (69.8) | 0.043 |
N number, GCS Glasgow Coma Scale, ICH Intracerebral haemorrhage, SAH Subarachnoid haemorrhage, TBI Traumatic brain injury, ICPm Intracranial pressure monitoring
*Neuroworsening was defined as one or more of the following: a spontaneous decrease in the GCS motor score of 2 points or more compared with the previous examination; a new loss of pupillary reactivity, development of pupillary asymmetry ≥ 2 mm deterioration in neurological or Computed Tomography status sufficient to warrant immediate medical or surgical intervention; CT scan, defined as Marshall classification 3 or more (for patients with TBI), Fisher grade 3 or more (for patients with subarachnoid haemorrhage), or intracranial haemorrhage volume 30 mL or more (for patients with intracranial haemorrhage)
TILs on days 1, 3, and 7
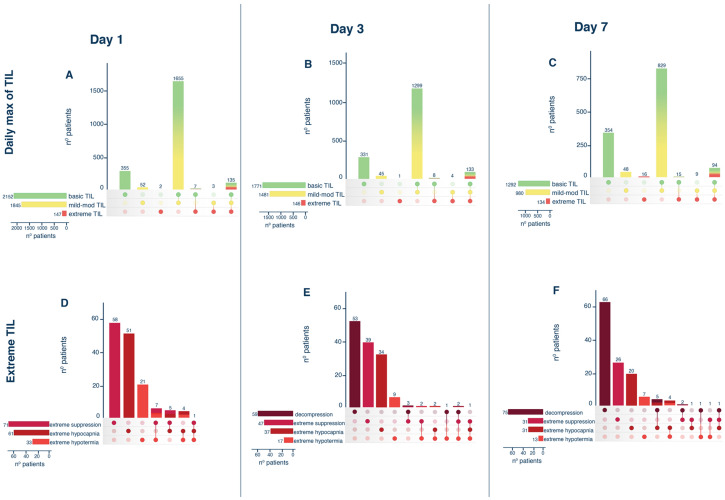
The daily combinations of each level of treatment are described in Fig. 1. On day 1 (panel A), most patients were treated with a combination of basic and mild-moderate TILs (1655 patients, 71.3%), while 147 (6.3%) patients received extreme TIL, in particular metabolic suppression with barbiturates (n = 71, 48.3%), followed by hypocapnia (51, 34.7%) and hypothermia (21, 14.3%) (panel D).
Fig. 1.
The use of TILs adopted (panel A, B, C) and the specific extreme treatment (panel D, E, F) in patients with acute brain injury at days 1, 3, and 7. In each plot, the left horizontal bars represent the numbers of patients that are treated with the specific level of TIL (panel A, B, C) or the specific eTIL (panel D, E, F); the vertical bars represent the number of subjects that received each specific combination of treatment specified by the filled dots. For instance, in panel A referring to day 1, 2152 (355 + 1655 + 7 + 135) patients received a basic TIL (horizontal green bar), among which 355 patients were treated only with basic TIL, 1665 were treated with basic and mild-moderate TIL, 7 with basic and extreme TIL and 135 were treated with basic, mild-moderate and extreme TIL on day 1 (vertical bars). Abbreviations: TIL therapy intensity level
Similarly, on days 3 and 7, most patients were treated with a combination of basic and mild-moderate TILs (1299, 66.8% on days 3 and 829, 54.5% on day 7, panel B and C), and decompressive craniectomy alone without any other treatment was the most frequent extreme TIL applied in the population (n = 53, 36.3% at day 3 and n = 66, 49.3% on day 7, panel E and F).
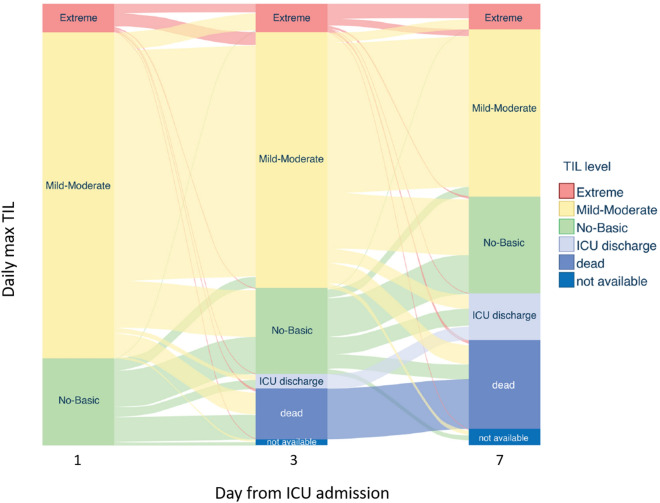
Progression over time of TILs
The dynamic of daily maximum TIL at different time points is shown in Fig. 2 and Figure S2. The alluvial plot shows that most patients treated with no-basic TIL on day 1 did not escalate their treatment level in the following days or died. Patients treated with mild-moderate TIL on day 1 had heterogeneous dynamics, with a tendency to remain within the same level. Patients who died on day 3 or 7 mainly received no-basic or mild-moderate TIL. Patients who received extreme TIL continued with this level of treatment on days 3 and 7 or progressed from mild-moderate TIL in the subsequent days; cases with a direct jump from no-basic to extreme TIL were rare and mostly from day 1 to day 3.
Fig. 2.
Alluvial plot describing the dynamic of the daily maximum therapy intensity level (TIL) and outcome (dead and ICU discharge) at days 1, 3, 7 since intensive care unit (ICU) admission. Blocks represent patient’s group according to the TIL, and stream fields between the blocks represent the patient’s transition over time
Summarizing, over the first week in ICU, 295 patients received eTIL at least once (12.7%), while 1643 patients received mild-moderate TIL (70.8%) at maximum level, and the remaining 382 received no-basic TIL (16.5%). Among the 295 eTIL patients, 93 were aggressively treated only on day 1, 35 on day 3 and 50 on day 7. Only 15 subjects were treated with extreme TIL at all time points (Figure S3).
Variability in the use of TILs among different pathologies
The use of TIL according to the different types of brain injuries on days 1, 3, and 7 is described in Table S2 and Figure S4. During the whole week, 305 SAH patients (74.6%) received mild-moderate TIL and 63 (15.4%) eTIL; in the TBI group, 932 patients (69.6%) received mild-moderate TIL and 178 (13.3%) eTIL; in the ICH group, 406 patients (71%) were treated with mild-moderate TIL and 54 (9.4%) with eTIL. Among patients who received eTIL during the week, the most frequent treatments were, in the SAH group, decompressive craniectomy, hypocapnia, and metabolic suppression (20 patients, 31.7%, each); in TBI metabolic suppression (78 patients, 43.8%) and in ICH hypocapnia, (26 patients, 48.1%).
Features of the population undergoing extreme TIL according to ICP monitoring
Table 1 compares the 295 patients who received eTIL at least once during the observation period with the others. Patients who received eTIL were younger (median age 49 vs 56 years, p < 0.001), with less cardiovascular pre-injury comorbidities (44% vs 31.4%, p < 0.001), had more frequent episodes of neuroworsening (56.1% vs 33.3%, p < 0.001), more neurosurgical interventions on day 1 (21.4% vs 14.1%, p = 0.002) and ICP monitoring inserted when compared with patients who did not receive eTIL (74.9% vs 51.2%, p < 0.001). Patients in the eTIL group had higher median values of maximum ICP on days 1, 3 and 7 (28 vs 21 mmHg, p < 0.001).
Most patients underwent mild-moderate TIL in the ICP and non-ICP groups (78.1% vs 62.1%). However, patients with ICP monitoring received more frequently eTIL (17.6% vs 7%) and less no-basic TIL (4.3% vs 30.9%) than non-monitored patients (Table S3). Regarding eTIL, the most frequent was the use of metabolic suppression in monitored patients and hypocapnia non-monitored ones (Table S3).
The use of extreme therapies across different centres and countries
The variability in eTIL use between countries and centres is shown in Figure S5. Considerable variability in the frequency of use of eTIL was observed. After adjustment for the relevant covariates, the probability of using eTIL ranged between 36 and 67% in countries and between 24 and 81% in centres (Figure S5). The median odds ratio (MOR) resulted in 1.47 and 2.26 for countries and centres, respectively. Figure S6 shows considerable variability in the choice of the specific types of eTIL.
Association between TIL and outcomes
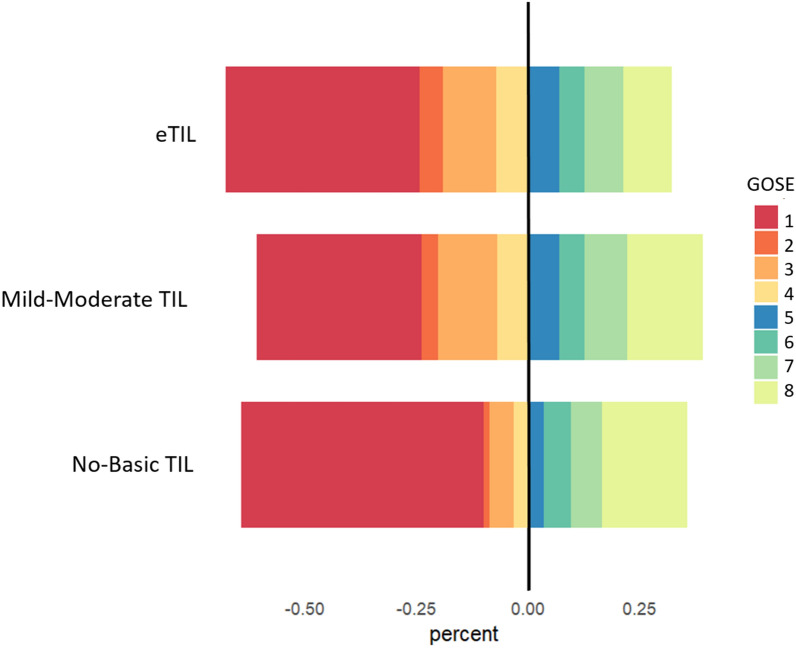
Overall, at six months, 1331 patients (62.4%) had an unfavourable neurological outcome (GOSE < 5), and 934 (40.7%) patients died. Mortality and neurological outcome according to the maximum level of treatment over the first week (days 1, 3 and 7) are presented in Fig. 3. Mortality was higher in patients in the no-basic TIL group compared to those who received mild-moderate TIL or eTIL (203, 57.3% vs 599, 40%, vs 127, 45.7%, respectively, p < 0.001). Unfavourable neurological outcome was more frequent in those patients who received eTIL compared to the other groups (190, (68.3%) eTIL, vs 907 (60.5%) mild-moderate TIL, vs 234 (65.9%) no, or basic TIL, p = 0.015).
Fig. 3.
Mortality and functional outcome at six months in the population according to different maximum TIL over the first week (considering day 1, 3, 7). GOSE Category 1. Death, 2. Vegetative state, 3. Lower severe disability, 4. Upper severe disability, 5. Lower moderate disability, 6. Upper moderate disability – some disability but can potentially return to some form of employment, 7. Lower good recovery – minor physical or mental defect, 8. Upper good recovery – full recovery
At multivariable analysis (Table 2), considering eTIL as reference, the use of no-basic TIL was independently associated with 6-month mortality (Hazard ratio, HR = 1.61, 95% CI = 1.24–2.09, p < 0.001), but the use of mild-moderate TIL was not (HR = 1.02, 95% CI = 0.83–1.26, p = 0.858, Table 2). When stratifying the analysis according to the type of brain injury, this result was mainly consistent in each pathology (Table S4). The sensitivity analysis excluding most severe patients (defined as patients with GCS = 3 and pupils both unreactive at admission or death < 48 h) obtained similar results, although the association was mitigated, especially for no-basic TIL (Table S5).
Table 2.
Results from multivariable Cox (left) and logistic (right) models exploring the association of the maximum TIL over the first week with mortality and unfavourable neurological outcome at 6 months
| Mortality at 6 months | Unfavourable outcome at 6 months (GOSE < 5) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | OR | 95% CI | p-value | |
| TIL | ||||||
| eTIL (ref) | 1 | |||||
| Mild-moderate | 1.02 | 0.83–1.26 | 0.858 | 0.95 | 0.89–1.01 | 0.079 |
| No-basic | 1.61 | 1.24–2.09 | < 0.001 | 0.94 | 0.87–1.01 | 0.088 |
| ICPm vs no-ICPm | 0.70 | 0.61–0.82 | < 0.001 | 1.02 | 0.98–1.06 | 0.350 |
| GCS ≤ 8 vs GCS > 8 | 1.48 | 1.18–1.87 | 0.001 | 1.12 | 1.06–1.19 | < 0.001 |
| Age, year | 1.02 | 1.02–1.03 | < 0.001 | 1.01 | 1.01–1.01 | < 0.001 |
| Pupils | ||||||
| Both reactive (ref) | 1 | |||||
| One unreactive | 1.28 | 1.03–1.59 | 0.026 | 1.04 | 0.98–1.11 | 0.181 |
| Both unreactive | 2.46 | 2.10–2.87 | < 0.001 | 1.22 | 1.16–1.28 | < 0.001 |
| Highly pathological CT scan vs normal | 1.78 | 1.49–2.11 | < 0.001 | 1.09 | 1.04–1.14 | < 0.001 |
| Neuroworsening vs no | 2.68 | 2.32–3.09 | < 0.001 | 1.19 | 1.14–1.24 | < 0.001 |
| Pathologies | ||||||
| TBI (ref) | 1 | |||||
| SAH | 0.74 | 0.60–0.90 | 0.003 | 0.97 | 0.91–1.02 | 0.209 |
| ICH | 1.25 | 1.07–1.46 | 0.006 | 1.13 | 1.08–1.19 | < 0.001 |
CI Confidence Interval, CT computed tomography, GCS Glasgow Coma Scale, GOSE Glasgow Outcome Scale Extended, HR Hazard Ratio, ICH intra cranial hemorrhage, OR, Odds Ratio, , SAH subarachnoid hemorrhage, TBI traumatic brain injury, TIL Therapy Intensity level, ICPm patients monitored with intracranial pressure; Highly pathological CT scan Defined as Marshall classification 3 or more (for patients with TBI), Fisher grade 3 or more (for patients with subarachnoid haemorrhage), or intracranial haemorrhage volume 30 mL or more (for patients with intracranial haemorrhage)
No significant association of TIL groups with 6 months neurological outcome was found (no-basic TIL: OR = 0.94, 95% CI = 0.87–1.01, p = 0.088, mild-moderate TIL: Odds Ratio, OR = 0.95, 95% CI = 0.89–1.006, p = 0.079 versus eTIL as reference) (Table 2). Results were consistent when stratifying according to the type of brain injury (Table S4). Excluding most severe patients, the model showed a statistically significant association between unfavourable neurological outcome and no-basic or mild-moderate TIL comparing with eTIL (mild-moderate TIL: OR = 0.920, 95% CI = 0.857–0.987, p = 0.020; no-basic TIL: OR = 0.883, 95% CI = 0.804–0.969, p = 0.008) (Table S5).
Finally, the sensitivity analysis regarding different allocations for the primary decompression and excluding patients who died after withdrawal of care showed consistent results on mortality and unfavourable neurological outcomes (Table S5).
Discussion
The main findings of our study, including a large cohort of 2320 patients with acute brain injury, can be summarized as follows:
During the first week of ICU admission, strategies for managing intracranial hypertension, especially mild and moderate treatments, are frequently used in ABI patients.
Patients who receive extremely aggressive treatments (eTIL) are generally younger, with fewer comorbidities, more neurologically severe and more frequently undergo ICP monitoring than others.
When used, extreme TILs are consistently maintained over the whole week, from day 1 to day 7.
Large variability across centres and countries still exists regarding the use of these therapies.
eTIL use was associated with a lower risk of mortality compared to no-basic TIL (no difference compared to mild-moderate TIL). eTIL was not associated with beneficial effect on neurological outcome compared to less aggressive treatment.
This is the most extensive prospective observational study describing the specific treatments used to manage intracranial hypertension in ABIs patients over the first week of ICU stay, providing a realistic picture of the clinical practice all over the globe. The management of intracranial pressure is fundamental in neurocritical care patients to reduce secondary brain damage and improve clinical outcomes. Robust observational data and expert recommendations suggest that treating intracranial hypertension should be a cornerstone for managing these patients.
The most recent Brain Trauma Foundation Guidelines [23] and a recent consensus statement [3] suggest using a "staircase" approach [2, 25], starting with low risk–benefit profiles, which include strategies such as head elevation, optimization of systemic cardiorespiratory function [24], and then progressively escalating to more aggressive and high-risk treatments in case of refractory intracranial hypertension [3]. This approach is based on the concept that aggressive treatments “per se” can cause important complications, so their effect on the outcome is unclear [11]. Randomized controlled trials on decompressive craniectomy [6, 8] suggest that decompressive craniectomy can reduce mortality but increase the proportion of patients with poor neurological outcomes when used after medical treatment. Hypothermia, when used prophylactic or in the early phases of ICP management, can increase systemic complications and have no benefits or even worsen outcomes [7, 25]. The only randomised controlled trial available on prophylactic hyperventilation failed to demonstrate the benefits/harms of this treatment [26], and more recent data [27, 28] support the safety of its use in selected cases.
Considering the lack of definitive randomized controlled trials comparing all aggressive treatments, initiating one of these relies on the balance between risks and benefits, patients' clinical conditions and, therefore, local practice. We found that patients undergoing eTIL were more severe and younger but with better pre-injury conditions. This suggests that physicians consider applying these strategies when the benefits outweigh the risks, according to single clinician judgment. This is also reflected by the high variability observed among centres and countries regarding applying these strategies.
In addition, aggressive treatments were more often used in patients with ICP monitoring, suggesting that despite significant variability in ICP practice and treatment across centres [29], intensive treatments are mainly guided by invasive monitoring. However, there is still a consistent number of patients who receive aggressive treatment without ICP monitoring, thus highlighting the need to provide more universally accepted criteria for its use in ABIs.
We found that on days 1, 3 and 7, the most used TIL in our cohort was mild-moderate TIL, with only a relatively limited number of patients requiring extreme TIL. However, extreme TILs were applied with a similar frequency over the week. We did not observe a tendency to progressively use the staircase approach over time and systematically progress with the intensity of ICP treatment. Although surprisingly, a similar result was described by a recent study from the CENTER-TBI group [11], which suggests the need for more precise and straightforward guidelines and that the availability of monitoring and treatment tools significantly depends on local resources and practice. This is particularly important in non-TBI pathologies, such as SAH and ICH, where evidence is notably lacking, but intracranial hypertension frequently occurs [16, 30]. In fact, in our cohort, we found a considerable number of patients without traumatic pathology who received aggressive treatment of ICP, especially in the SAH cohort; as guidelines mainly refer to TBI patients, this suggests that in most cases, clinicians extrapolate the indications on TBI and apply them in other ABIs pathologies.
Finally, the multivariable analysis on 6-month mortality, considering as confounders the severity of brain injury and patients’ status, showed that aggressive treatment could reduce mortality at six months, compared to no-basic TIL. We believe that this represents a relevant result, as it suggests that even though there might be a potential harm from using high TIL therapies, monitoring and treating these patients can reduce mortality in selected patients.
The effect on neurological outcome remains unclear; no significant difference was shown in the risk of 6-month poor neurological outcome by augmenting the TIL. On the other side, the sensitivity analysis, excluding more severe patients, showed a significant association between eTIL and poor neurological outcomes favouring less aggressive treatments.
Different factors could be related to this: firstly, the scale used for its evaluation, GOSE, describes different grades of disability but does not provide more specific information on the quality of life and neurocognitive dysfunction. Secondly, several ICU and post-ICU complications can significantly influence neurological outcomes. Third, the use of aggressive treatments is reserved for more severe patients and carries some risks, thus making the selection of the patients fundamental. This is crucial as our results highlight the potentially beneficial effect on mortality but not on neurological outcomes for these patients.
Finally, although our data do not allow us to define better the importance of the appropriate timing of their application, we can speculate that early use of these therapies, before less hazardous low TIL options have failed, could expose patients to unnecessary risks, as previously suggested [11, 31].
Limitations
This study has several limitations that need to be mentioned. This is an observational study; therefore, it is impossible to draw any causality relationship between the associations found. However, our data might help to plan randomized controlled trials on this topic. In addition, the definition of TIL treatment does not strictly follow the latest evidence of the Seattle algorithm [3], where only decompressive craniectomy, hypothermia and metabolic suppression are defined as tier-three therapies. However, the SYNAPSE-ICU study and this preplanned analysis were defined before the algorithm's publication; therefore, the older classification of TIL was used. Third, despite this, being a preplanned analysis, some data are lacking, especially regarding the details of the withdrawal of care, the escalation over days in the use of TIL, data on the duration of metabolic suppression or methods of cooling to obtain hypothermia, and the lack of discrimination in the database at day one regarding primary and secondary decompressive craniectomy. Forth, as there is no universal consensus on the definition of neuroworsening, we used a predefined definition, as stated in the initial protocol of the SYNAPSE ICU study and accordingly to the National Institute of Neurological Disorders and Stroke (NINDS) common data elements (https://www.commondataelements.ninds.nih.gov), which considers neuroimaging and clinical data without considering ICP values. The NINDS common data elements try to define a standard and universally accepted tassology in neurological diseases to be used in clinical trials. This operational definition of neuroworsening is used in most neuro clinical trials.
Finally, more granular data on ICP monitoring would have made it feasible to understand the threshold of intracranial pressure and the reasoning made by the clinicians before deciding to escalate the therapy.
Conclusions
Treatments for intracranial hypertension are frequently applied in the first week of ICU management of acute brain-injured patients. Highly aggressive therapies are still significantly variable across centres and do not strictly follow the staircase approach recommended by the most recent guidelines. Aggressive strategies can benefit six months mortality but not 6-month neurological outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
The SYNAPSE-ICU investigators participated in the data collection and are non-author contributors. Argentina: (National Coordinator, Walter Videtta) Sanatorio De La Trinidad San Isidro, San Isidro, Argentina (Gustavo Domeniconi) Higa Dr Oscar Alende, Mar Del Plata, Argentina (María Estrella Giménez) Hospital Castro Rendon, Neuquen, Argentina (Mariela Fumale) Hospital El Cruce, Florencio Varela, Argentina (Edgar Daniel Amundarain) Centro Medico Integral Fitz Roy, Buenos Aires, Argentina (Matias Casanova). Australia: (National Coordinator, Michael Reade) Royal Brisbane And Women's Hospital, Brisbane, Australia (Elizabeth Hallt) Gold Coast University Hospital, Gold Coast, Australia (David Pearson) Nepean Hospital, Penrith, Australia (Ian Seppelt). Austria: (National Coordinator, Raimund Helbok) Medical University Innsbruck, Innsbruck, Austria (Raimund Helbok). Belarus: (National Coordinator, Valery Davidovich) 5th City Clinical Hospital, Minsk, Belarus (Valery Davidovich). Belgium: (National Coordinator, Geert Meyfroidt) Hopital Erasme, Bruxelles, Belgium (Ilaria Alice Crippa) University Hospitals Leuven, Leuven, Belgium (Liese Mebis) Chu Charleroi, Lodelinsart, Belgium (Patrick Biston) Az Maria Middelares, Gent, Belgium (Stijn Van De Velde) Grand Hopital De Charleroi, Charleroi, Belgium (Glorieux Denis). Brazil: (National Coordinator, Pedro Kurtz) Instituto Estadual Do Cerebro, Rio De Janeiro, Brazil (Pedro Kurtz) Das Clinicas Hospital University of Sao Paulo, Sao Paulo, Brazil (Samia Yasin Wayhs). Canada: (National Coordinator, Mypinder Sekhon) Vancouver General Hospital University of British Columbia, Vancouver, Canada (Mypinder Sekhon, Donald Griesdale) St Michael's Hospital, Toronto, Canada (Andrea Rigamonti). Chile: (National Coordinator, José Miguel Montes) Clinica Alemana De Santiago, Santiago, Chile (Rodrigo Pérez-Araos). Colombia: (National Coordinator, Jorge H Mejia-Mantilla) Fundacion Valle Del Lili, Cali, Colombia (Jorge H Mejia-Mantilla, Andrés Gempeler) Hospital San Vicente Rionegro, Rionegro, Colombia (Ray Mendoza). Croatia: (National Coordinator, Natasa Kovac) University Hospital Centre Zagreb, Zagreb, Croatia (Natasa Kovac). Cuba: (National Coordinator, Hedgar Berty Gutiérrez) Hospital Dr Miguel Enríquez, La Habana, Cuba (Hedgar Berty Gutiérrez). Czech Republic: (National Coordinator, Vera Spatenkova) St Anne's University Hospital, Brno, Czech Republic (Marek Fencl) Hospital Brno, Brno, Czech Republic (Roman Gal, Ondrej Hrdy, Kamil Vrbica) Masaryk Hospital in Usti Nad Labem, Usti Nad Labem, Czech Republic (Josef Skola, Eva Provaznikova) University Hospital Plzen, Plzen, Czech Republic (Jakub Kletecka, Pavel Lavicka) Regional Hospital Czech Republic, Liberec, Czech Republic (Vera Spatenkova). Denmark: (National Coordinator, Piergiorgio Bresil) Aalborg University Hospital, Aalborg, Denmark (Marianne Levin) Odense University Hospital, Odense, Denmark (Piergiorgio Bresil, Josefine Thomsen, Thomas Egmose Larsen, Henrik Westy Hoffmeyer, Morten Olskjaer Holm) Rigshospitalet, Copenhagen, Denmark (Jesper Borg Andersen, Birgitte Majholm, Margit Smitt, Heidi Shil Eddelien). Ecuador: (National Coordinator, Manuel Jibaja) Hospital De Especialidades Eugenio Espejo, Quito, Ecuador (Manuel Jibaja) Carlos Andrade Marin Hospital, Quito, Ecuador (Freddy Maldonado) Clinica La Merced, Quito, Ecuador (María Fernanda García). France: (National Coordinator, Karim Asehnoune) Chu Pointe-À-Pitre Abymes, Pointe-À-Pitre Cedex, France (Bertrand Pons) Hôpital Central, Nancy, France (Gérard Audibert, Manon Lucca) Chu Besancon, Besancon, France (Guillaume Besch) Grenoble University Hospital, Grenoble, France (Pierluigi Banco) Chu De Nantes, Nantes, France (Karim Asehnoune, Raphael Cinotti) Pasteur 2 Chu Nice, Nice, France (Hervé Quintard) Hôpital Lariboisière, Aphp, Paris, France (Benjamin Soyer, Anais Caillard) Caen University Hospital, Caen, France (Clement Gakuba) Bichat Claude Bernard University Hospital, Paris, France (Romain Sonneville). Germany: (National Coordinator, Stefan Wolf) Klinikum Rechts Der Isar, Munich, Germany (Kristina Fuest, Lea Albrecht, Sarah Grotheer, Sandro M Krieg, Stefan J Schaller) Charité University Medicine Berlin, Berlin, Germany (Stefan Wolf). Greece: (National Coordinator, Charikleia Vrettou) General Hospital of Chania, Chania, Greece (Eftychia Kontoudaki) Saint Savvas Hospital, Athens, Greece (Anna Efthymiou) University Hospital Larissa, Larissa, Greece (Elena Palli, Demosthenes Makris) Attikon University Hospital, Chaidari, Greece (Chrysi Diakaki) G. Papanikolaou General Hospital, Thessaloniki, Greece (Christina Iasonidou) Asklipieio Voulas General Hospital, Voula, Greece (Aikaterini Dimoula) General Hospital of Athens G. Gennimatas, Athens, Greece (Georgios Koukoulitsios) Lamia General Hospital, Lamia, Greece (George Kyriazopoulos) General Hospital of Tripolis, Tripolis, Greece (Nikolas Pantelas) Achillopoulio General Hospital, Volos, Greece (Syragoula Tsikriki) General Hospital of Kavala, Kavala, Greece (Electra Eleni Stamou) Evaggelismos General Hospital, Athens, Greece (Charikleia Vrettou, Achileas Giannopoulos) Public General Hospital Hippokratio of Thessaloniki, Thessaloniki, Greece (Eleni Mouloudi). Hong Kong: (National Coordinator, Ping Shum Hoi) Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong (Ping Shum Hoi) Tuen Mun Hospital, Tuen Mun, Hong Kong (Yan Chan Cheuk) Princess Margaret Hospital, Kwai Chung, Hong Kong (Hewa Kandamby Darshana). Hungary: (National Coordinator, Krisztián Tánczos) Peterfy Hospital and Trauma Centre, Budapest, Hungary (Gabor Nardai) University of Szeged, Szeged, Hungary (Krisztián Tánczos) Kenezy University Hospital, Debrecen, Hungary (Zoltan Szentkereszty). India: (National Coordinator, Harsh Sapra) All India Institute of Medical Sciences, Delhi, India (Deepak Gupta, Kaveri Sharma) Medanta Medicity Gurgaon, Gurgaon, India (Harsh Sapra) Artemis Hospital, Gurgaon, India (Saurabh Anand) Post Graduate Institute of Medical Education And Research Chandigarh, Chandigarh, India (Ankur Luthra, Summit Bloria, Rajeev Chauhan, Nidhi Panda) King George's Medical University, Uttar Pradesh, India (Ahmad Ozair). Indonesia: (National Coordinator, Bram Kilapong) Awal Bros Hospital, Batam, Indonesia (Bram Kilapong). Iraq: (National Coordinator, Anass Alsudani) Neurosurgery Teaching Hospital, Baghdad, Iraq (Anass Alsudani). Italy: (National Coordinator, Giuseppe Citerio) Ospedale Dell'angelo, Zelarino, Italy (Alessandra Soragni) Asst Settelaghi Varese, Varese, Italy (Alessandro Motta) Aou Modena, Modena, Italy (Andrea Marudi, Elisabetta Bertellini) Policlinico A. Gemelli, Roma, Italy (Anselmo Caricato, Camilla Gelormini, Eleonora Ioannoni, Eleonora Stival, Serena Silva) Asst Grande Ospedale Metropolitano Niguarda, Milano, Italy (Federico Pozzi) Policlinico San Martino, Genova, Italy (Iole Brunetti) Policlinico Paolo Giaccone University of Palermo, Palermo, Italy (Andrea Cortegiani) Azienda Ospedaliero Universitaria Di Parma, Parma, Italy (Edoardo Picetti) Humanitas, Rozzano, Italy (Federico Villa) Asl Toscana Centro Ospedale San Giuseppe Empoli, Empoli, Italy (Italo Calamai) Irccs Bellaria Hospital Ausl Bologna, Bologna, Italy (Maria Chiara Casadio) Ospedali Riuniti, Livorno, Italy (Maria Concetta Quartarone) Azienda Ospedaliera Universitaria Padova, Padova, Italy (Marzia Grandis) Ospedale San Gerardo, Monza, Italy (Federico Magni, Silvia Del Bianco, Claudia Bonetti) Ospedale Maggiore, Bologna, Italy (Virginia Buldini, Aimone Giugni) Asst Lariana Ospedale Sant’Anna Di Como, San Fermo Della Battaglia, Italy (Simone Maria Zerbi) Fondazione Irccs Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy (Marco Carbonara) Azienda Universitario Ospedaliera O.O.R.R. Foggia, Foggia, Italy (Antonella Cotoia, Antonio Izzi). Latvia: (National Coordinator, Olegs Sabelnikovs) Paul Stradins Clinical University Hospital, Riga, Latvia (Olegs Sabelnikovs). Libya: (National Coordinator, Muhammed Elhadi) Seoul Clinic, Tripoli, Libya (Muhammed Elhadi) Abo Selim Trauma Hospital, Tripoli, Libya (Hazem Ahmed). Mexico: (National Coordinator, Silvio Ñamendys Silva A) Fundacion Clinica Medica Sur, Mexico City, Mexico (Silvio A Ñamendys Silva) Hospital Regional De Alta Especialidad De Ixtapaluca, Ixtapaluca, Mexico (Gilberto Adrian Gasca López). Nepal: (National Coordinator, Gentle S Shrestha) Tribhuvan University Teaching Hosptial, Kathmandu, Nepal (Gentle S Shrestha) Grande International Hospital, Kathmandu, Nepal (Shirish Maskey) B&B Hospital, Kathmandu, Nepal (Tamanna Bajracharya) National Trauma Center, Kathmandu, Nepal (Khadka Nilam) Nobel Hospital, Kathmandu, Nepal (Prakash Kafle) Birat Medical College, Tankisinuwari, Nepal (Laleet Rajbanshi) Neuro Cardio & Multispecialty Hospital, Biratnagar, Nepal (Yam Bahadur Roka) Nigeria (National Coordinator, Idowu Olufemi) Lagos State University Teaching Hospital Ikeja, Lagos, Nigeria (Olufemi Idowu). Pakistan: (National Coordinator, Khan Muhammad Mukhtar) Northwest General Hospital & Research Centre, Peshawar, Pakistan (Khan Muhammad Mukhtar). Peru’: (National Coordinator, Juan Luis Pinedo Portilla) Clinica Del Pacifico, Chiclayo, Perù (Juan Luis Pinedo Portilla). Poland: (National Coordinator, Klaudyna Kojder) University Hospital No. 1, Pomeranian Medical University, Szczecin, Poland (Klaudyna Kojder). Portugal: (National Coordinator, Irene Aragao) Hospital Santo Antonio, Porto, Portugal (Irene Aragao) Centro Hospitalar e Universitario De Coimbra, Coimbra, Portugal (Ricardo Freitas, Marco Simoes) Hospital Garcia De Orta, Almada, Portugal (Dario Batista) Hospital De Braga, Braga, Portugal (Cecília Pacheco, Fátima Assunção, Luís Lencastre) Centro Hospitalar Universitário Do Algarve - Unidade De Faro, Faro, Portugal (Pedro Cavaleiro). Qatar: (National Coordinator, Mohamed Abdelaty) Hamad Medical Corporation, Doha, Qatar (Mohamed Abdelaty). Russian Federation: (National Coordinator, Alex Gritsan) Belgorod Regional Clinical Hospital of St. Ioasaph, Belgorod, Russian Federation (Sergey Khomiakov Sergey) Krasnoyarsk Regional Clinical Hospital, Krasnoyarsk, Russian Federation (Alex Gritsan, Dovbysh Nikolay). Saudi Arabia: (National Coordinator, Yaseen Arabi) King Abdulaziz Medical City, Riyadh, Saudi Arabia (Yaseen Arabi). Slovenia: (National Coordinator, Primoz Gradisek) Splosna Bolnisnica Murska Sobota, Murska Sobota, Slovenia (Petra Forjan) Izola General Hospital, Isola, Slovenia (Mara Škoti) University Medical Centre Ljubljana, Ljubljana, Slovenia (Suada Filekovic Ribaric, Primoz Gradisek, Nataša Milivojevic) Slovenj Gradec General Hospital, Slovenj Gradec, Slovenia (Sergeja Kozar). Spain: (National Coordinator, Rafael Badenes) Hospital Universitario Ramòn Y Cajal, Madrid, Spain (Aaron Blandino Ortiz) Hospital Universitario Puerta Del Mar, Cadiz, Spain (Mikel Celaya Lopez) Hospital General Universitario De Castellon, Castellon De La Plana, Spain (Laura Galarza) Hospital Universitari De Bellvitge, Hospitalet De Llobregat, Spain (Luisa Corral, Africa Lores, Ricard Soley, Laura Pariente, Pablo López Ojeda) Hospital Regional Universitario Carlos Haya, Malaga, Spain (Maria Dolores Arias Verdu) Hospital General Universitario De Ciudad Real, Ciudad Real, Spain (Luis Javier Yuste Dominguez) Complejo Hospitalario De Leon, Leon, Spain (Maria Isabel Gonzalez Perez) Hospital Germans Trias I Pujol, Badalona, Spain (Mireia Anglada) Gregorio Maranon Hospital, Madrid, Spain (Patricia Duque) Hospital Clinic Universitari De Valencia, Valencia, Spain (Ainhoa Serrano, Berta Monleon) Joan Xxiii University Hospital, Tarragona, Spain (Vanessa Blazquez). Switzerland: (National Coordinator, Mauro Oddo) Chuv Lausanne University Hospital, Lausanne, Switzerland (Samia Abed Maillard, Paola Morelli, John-Paul Miroz, Eva Favre). Tunisia: (National Coordinator, Walid Sellami) Military Hospital of Tunis, Tunis, Tunisia (Walid Sellami). United Arab Emirates (UAE): (National Coordinator, Massimo Lamperti) Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates (Jamil Dibu). United Kingdom (UK): (National Coordinator, Richard Siviter) Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom of Great Britain and Northern Ireland (Angelos Kolias) University Hospitals of North Midlands NHS Trust, Stoke on Trent Staffordshire, United Kingdom of Great Britain and Northern Ireland (Chris Thompson) NHS Greater Glasgow and Clyde, Glasgow, United Kingdom of Great Britain and Northern Ireland (Christopher Hawthorne) Salford Royal NHS Foundation Trust, Salford, United Kingdom of Great Britain and Northern Ireland (Justin Roberts) University of Oxford Hospitals NHS Foundation Trust, Oxford, United Kingdom of Great Britain and Northern Ireland (Lara Prisco) University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom of Great Britain and Northern Ireland (Roger Lightfoot). United States of America (USA): (National Coordinator, Josè I. Suarez) The Johns Hopkins University, Baltimore, United States of America (Luci Rivera-Lara) University of Massachusetts Medical School, Worcester, United States of America (Susanne Muehlschlegel) Doctors Hospital Renaissance, Edinburg, United States of America (Juan Padilla) Greenville Health System, University of South Carolina, Greenville, United States of America (Sanjeev Sivakumar) University of Texas Southwestern Medical Center, Dallas, United States of America (Daiwai Olson).
Author contributions
CR: conception of the work, participation in data interpretation, drafting the manuscript, critical revision of the manuscript, final approval of the version to be published. FG, PR, SG: data analysis and verification of the data, interpretation, critical revision of the manuscript, and final approval of the version to be published. AG: participation in data interpretation, drafting, critical revision of the manuscript, and final approval of the version to be published. FST: conception of the work, critical revision of the manuscript, final approval of the version to be published. GC: conception of the work (PI), funding application, enrolment of the participants' centres, supervision of the data collection, participation in data analysis verification of the data and interpretation, revision of the manuscript, critical revision of the manuscript, final approval of the version to be published. GC is the guarantor of the entire manuscript and is responsible for the decision to submit the manuscript. All the authors have seen and approved the final text.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement. University of Milano—Bicocca is the sponsor of the SYNAPSE-ICU study. The European Society of Intensive Care Medicine (ESICM) endorsed and partially funded the study on January 31st, 2017. The ESICM contributed to the electronic Case Report Form (eCRF) design and testing. No further funding was obtained for this sub-analysis.
Data sharing statements
The data supporting the study findings are available upon reasonable request after approval of a proposal from the corresponding author (GC). Data collected for the analysis will be made available to others, including deidentified individual participant data and a data dictionary defining each field in the set. Related documents will also be available, such as the study protocol, statistical analysis plan, and informed consent form.
Declarations
Conflict of interest
GC reports grants and personal fees as a Speakers' Bureau Member and Advisory Board Member from Integra and Neuroptics, all outside the submitted work. The other authors declare no competing interests.
Ethical approval
The SYNAPSE-ICU study was approved at the sponsor site by the Ethics Committee ‘Brianza’ ASST-Monza on November 21st, 2017. It was performed according to the Helsinki Declaration and the International Conference on Harmonization for Good Clinical Practice. Since comatose patients could not provide informed consent at the time of study recruitment, each centre referred to local/national law on the lack of capacity. If the patients regained capacity at the follow-up visit, they were required to either provide informed consent to use the acute and follow-up data or refuse to participate in the research. According to the local regulations, national/local approvals at the international study sites were obtained by the National Coordinators and local PIs. For this secondary analysis, no further ethical approval was required.
Footnotes
List of study group investigators are listed in the Acknowledgement section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Citerio, Email: giuseppe.citerio@unimib.it.
the SYNAPSE-ICU Investigators:
Walter Videtta, Gustavo Domeniconi, María Estrella Giménez, Mariela Fumale, Edgar Daniel Amundarain, Matias Casanova, Michael Reade, Elizabeth Hallt, David Pearson, Ian Seppelt, Raimund Helbok, Valery Davidovich, Geert Meyfroidt, Ilaria Alice Crippa, Liese Mebis, Patrick Biston, Stijn Van De Velde, Glorieux Denis, Pedro Kurtz, Samia Yasin Wayhs, Mypinder Sekhon, Donald Griesdale, Andrea Rigamonti, José Miguel Montes, Rodrigo Pérez-Araos, Jorge H. Mejia-Mantilla, Andrés Gempeler, Ray Mendoza, Natasa Kovac, Hedgar Berty Gutiérrez, Vera Spatenkova, Marek Fencl, Roman Gal, Ondrej Hrdy, Kamil Vrbica, Josef Skola, Eva Provaznikova, Jakub Kletecka, Pavel Lavicka, Vera Spatenkova, Piergiorgio Bresil, Marianne Levin, Piergiorgio Bresil, Josefine Thomsen, Thomas Egmose Larsen, Henrik Westy Hoffmeyer, Morten Olskjaer Holm, Jesper Borg Andersen, Birgitte Majholm, Margit Smitt, Heidi Shil Eddelien, Manuel Jibaja, Freddy Maldonado, María Fernanda García, Karim Asehnoune, Bertrand Pons, Gérard Audibert, Manon Lucca, Guillaume Besch, Pierluigi Banco, Karim Asehnoune, Raphael Cinotti, Hervé Q uintard, Benjamin Soyer, Anais Caillard, Clement Gakuba, Romain Sonneville, Stefan Wolf, Kristina Fuest, Lea Albrecht, Sarah Grotheer, Sandro M. Krieg, Stefan J. Schaller, Charikleia Vrettou, Eftychia Kontoudaki, Anna Efthymiou, Elena Palli, Demosthenes Makris, Chrysi Diakaki, Christina Iasonidou, Aikaterini Dimoula, Georgios Koukoulitsios, George Kyriazopoulos, Nikolas Pantelas, Syragoula Tsikriki, Electra Eleni Stamou, Charikleia Vrettou, Achileas Giannopoulos, Eleni Mouloudi, Ping Shum Hoi, Yan Chan Cheuk, Hewa Kandamby Darshana, Krisztián Tánczos, Gabor Nardai, Zoltan Szentkereszty, Harsh Sapra, Deepak Gupta, Kaveri Sharma, Saurabh Anand, Ankur Luthra, Summit Bloria, Rajeev Chauhan, Nidhi Panda, Ahmad Ozair, Bram Kilapong, Anass Alsudani, Giuseppe Citerio, Alessandra Soragni, Alessandro Motta, Andrea Marudi, Elisabetta Bertellini, Anselmo Caricato, Camilla Gelormini, Eleonora Ioannoni, Eleonora Stival, Serena Silva, Federico Pozzi, Iole Brunetti, Andrea Cortegiani, Edoardo Picetti, Federico Villa, Italo Calamai, Maria Chiara Casadio, Maria Concetta Quartarone, Marzia Grandis, Federico Magni, Silvia Del Bianco, Claudia Bonetti, Virginia Buldini, Aimone Giugni, Simone Maria Zerbi, Marco Carbonara, Antonella Cotoia, Antonio Izzi, Olegs Sabelnikovs, Muhammed Elhadi, Hazem Ahmed, Silvio A. Ñamendys Silva, Gilberto Adrian Gasca López, Gentle S. Shrestha, Shirish Maskey, Tamanna Bajracharya, Khadka Nilam, Prakash Kafle, Laleet Rajbanshi, Yam Bahadur Roka, Olufemi Idowu, Khan Muhammad Mukhtar, Juan Luis Pinedo Portilla, Klaudyna Kojder, Irene Aragao, Ricardo Freitas, Marco Simoes, Dario Batista, Cecília Pacheco, Fátima Assunção, Luís Lencastre, Pedro Cavaleiro, Mohamed Abdelaty, Alex Gritsan, Sergey Khomiakov Sergey, Dovbysh Nikolay, Yaseen Arabi, Primoz Gradisek, Petra Forjan, Mara Škoti, Suada Filekovic Ribaric, Primoz Gradisek, Nataša Milivojevic, Sergeja Kozar, Rafael Badenes, Aaron Blandino Ortiz, Mikel Celaya Lopez, Laura Galarza, Luisa Corral, Africa Lores, Ricard Soley, Laura Pariente, Pablo López Ojeda, Maria Dolores Arias Verdu, Luis Javier Yuste Dominguez, Maria Isabel Gonzalez Perez, Mireia Anglada, Patricia Duque, Ainhoa Serrano, Berta Monleon, Vanessa Blazquez, Mauro Oddo, Samia Abed Maillard, Paola Morelli, John-Paul Miroz, Eva Favre, Walid Sellami, Massimo Lamperti, Jamil Dibu, Richard Sivities, Angelos Kolias, Chris Thompson, Christopher Hawthorne, Justin Roberts, Lara Prisco, Roger Lightfoot, Josè I. Suarez, Luci Rivera-Lara, Susanne Muehlschlegel, Juan Padilla, Sanjeev Sivakumar, and Daiwai Olson
References
- 1.Steyerberg EW, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18:923–934. doi: 10.1016/s1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- 2.Robba C, Citerio G. How I manage intracranial hypertension. Crit Care. 2019;23:243. doi: 10.1186/s13054-019-2529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intens Care Med. 2019;45:1783–1794. doi: 10.1007/s00134-019-05805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyfroidt G, Bouzat P, Casaer MP, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intens Care Med. 2022;48:649–666. doi: 10.1007/s00134-022-06702-4. [DOI] [PubMed] [Google Scholar]
- 5.Hawryluk GWJ, Citerio G, Hutchinson P, et al. Intracranial pressure: current perspectives on physiology and monitoring. Intens Care Med. 2022 doi: 10.1007/s00134-022-06786-y. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of Decompressive craniectomy for traumatic intracranial hypertension. New Engl J Medicine. 2016;375:1119–1130. doi: 10.1056/nejmoa1605215. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DJ, Nichol AD, Bailey M, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: The polar randomized clinical trial. JAMA. 2018;320:2211. doi: 10.1001/jama.2018.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. New Engl J Medicine. 2011;364:1493–1502. doi: 10.1056/nejmoa1102077. [DOI] [PubMed] [Google Scholar]
- 9.Cnossen MC, Polinder S, Andriessen TM, et al. Causes and consequences of treatment variation in moderate and severe traumatic brain injury. Crit Care Med. 2017;45:660–669. doi: 10.1097/ccm.0000000000002263. [DOI] [PubMed] [Google Scholar]
- 10.Cnossen MC, Huijben JA, van der Jagt M, et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: a survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit Care. 2017;21:233. doi: 10.1186/s13054-017-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijben JA, Dixit A, Stocchetti N, et al. Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit Care. 2021;25:78. doi: 10.1186/s13054-020-03370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robba C, Iannuzzi F, Taccone FS (2021) Tier-three therapies for refractory intracranial hypertension in adult head trauma. Minerva Anestesiol 87:1359–1366. 10.23736/s0375-9393.21.15827-4 [DOI] [PubMed]
- 13.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC) Intens Care Med. 2020;46:919–929. doi: 10.1007/s00134-019-05900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maas AIR, Menon DK, Manley GT, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21:1004–1060. doi: 10.1016/s1474-4422(22)00309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citerio G, Gaini SM, Tomei G, Stocchetti N, et al. Management of 350 aneurysmal subarachnoid hemorrhages in 22 Italian neurosurgical centers. Intensive Care Med. 2007;33:1580–1586. doi: 10.1007/s00134-007-0700-5. [DOI] [PubMed] [Google Scholar]
- 16.Dallagiacoma S, Robba C, Graziano F, et al. Intracranial pressure monitoring in patients with spontaneous intracerebral hemorrhage. Neurology. 2022;99:e98–e108. doi: 10.1212/wnl.0000000000200568. [DOI] [PubMed] [Google Scholar]
- 17.Baggiani M, Graziano F, Rebora P et al (2022) Intracranial pressure monitoring practice, treatment, and effect on outcome in aneurysmal subarachnoid hemorrhage. Neurocrit Care 1–11. 10.1007/s12028-022-01651-8 [DOI] [PubMed]
- 18.Maset AL, Marmarou A, Ward JD, et al. Pressure-volume index in head injury. J Neurosurg. 1987;67:832–840. doi: 10.3171/jns.1987.67.6.0832. [DOI] [PubMed] [Google Scholar]
- 19.Shore PM, Hand LL, Roy L, et al (2006) Reliability and validity of the pediatric intensity level of therapy (PILOT) scale: A measure of the use of intracranial pressure–directed therapies. Crit Care Med 34:1981–1987 10.1097/01.ccm.0000220765.22184.ed [DOI] [PubMed]
- 20.Zuercher P, Groen JL, Aries MJH, et al. Reliability and validity of the therapy intensity level scale: analysis of clinimetric properties of a novel approach to assess management of intracranial pressure in traumatic brain injury. J Neurotraum. 2016;33:1768–1774. doi: 10.1089/neu.2015.4266. [DOI] [PubMed] [Google Scholar]
- 21.Robba C, Graziano F, Rebora P, et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol. 2021;20:548–558. doi: 10.1016/s1474-4422(21)00138-1. [DOI] [PubMed] [Google Scholar]
- 22.Citerio G, Prisco L, Oddo M, et al. International prospective observational study on intracranial pressure in intensive care (ICU): the SYNAPSE-ICU study protocol. BMJ Open. 2019;9:e026552. doi: 10.1136/bmjopen-2018-026552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney N, Totten AM, O’Reilly C, et al (2016) Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80:6–15. 10.1227/neu.0000000000001432 [DOI] [PubMed]
- 24.Robba C, Badenes R, Battaglini D, et al. Ventilatory settings in the initial 72 h and their association with outcome in out-of-hospital cardiac arrest patients: a preplanned secondary analysis of the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2) trial. Intens Care Med. 2022 doi: 10.1007/s00134-022-06756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews PJD, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. New Engl J Medicine. 2015;373:2403–2412. doi: 10.1056/nejmoa1507581. [DOI] [PubMed] [Google Scholar]
- 26.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 27.Citerio G, Robba C, Rebora P, et al. Management of arterial partial pressure of carbon dioxide in the first week after traumatic brain injury: results from the CENTER-TBI study. Intens Care Med. 2021;47:961–973. doi: 10.1007/s00134-021-06470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diringer MN, Yundt K, Videen TO, et al. No reduction in cerebral metabolism as a result of early moderate hyperventilation following severe traumatic brain injury. J Neurosurg. 2000;92:7–13. doi: 10.3171/jns.2000.92.1.0007. [DOI] [PubMed] [Google Scholar]
- 29.Huijben JA, Volovici V, Cnossen MC, et al. Variation in general supportive and preventive intensive care management of traumatic brain injury: a survey in 66 neurotrauma centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. Crit Care. 2018;22:90. doi: 10.1186/s13054-018-2000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoerle T, Lombardo A, Colombo A, et al. Intracranial pressure after subarachnoid hemorrhage. Crit Care Med. 2015;43:168–176. doi: 10.1097/ccm.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 31.Kolias AG, Adams H, Timofeev IS, et al. Evaluation of outcomes among patients with traumatic intracranial hypertension treated with decompressive craniectomy vs standard medical care at 24 months. Jama Neurol. 2022 doi: 10.1001/jamaneurol.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the study findings are available upon reasonable request after approval of a proposal from the corresponding author (GC). Data collected for the analysis will be made available to others, including deidentified individual participant data and a data dictionary defining each field in the set. Related documents will also be available, such as the study protocol, statistical analysis plan, and informed consent form.