Abstract
Summary
Internationally, there is an osteoporosis treatment gap, which pharmacists may assist in closing. This review identifies pharmacist interventions for improving osteoporosis management and evaluates their effectiveness. Pharmacist interventions are shown to improve osteoporosis management in terms of increasing investigation and treatment commencement and osteoporosis therapy adherence.
Introduction
This review identifies pharmacist interventions for improving osteoporosis management and evaluates their effectiveness.
Methods
A literature search using PubMed, Embase, International Pharmaceutical Abstracts, and Cumulative Index to Nursing and Allied Health Literature was undertaken from database inception to June 2022. Randomised controlled trials were eligible, if they included adults diagnosed with or at risk of osteoporosis and assessed pharmacist interventions to improve osteoporosis management. Outcomes regarding investigation, treatment, adherence and patient knowledge were evaluated using qualitative analysis. The quality of included studies was assessed using the Critical Appraisal Skills Programme checklists and the Cochrane Collaboration tool to assess the risk of bias (Rob 2.0).
Results
Sixteen articles (12 different studies) with a total of 16,307 participants, published between 2005 and 2018 were included. Pharmacist interventions were classified into two categories, those targeting investigation and treatment (n = 10) and those targeting adherence (n = 2). The impact of the intervention on patient knowledge was considered by studies targeting both investigation and treatment (n = 2) and adherence (n = 1). Pharmacist interventions demonstrated benefit for all outcomes; however, the extent to which conclusions can be drawn on their effectiveness is limited by the heterogeneity of interventions employed and methodological issues identified. Patient education and counselling were identified as a cornerstone of pharmacist interventions targeting both investigation and treatment and adherence, along with the importance of pharmacist and physician collaboration.
Conclusion
Pharmacist interventions show promise for improving osteoporosis management. The potential for pharmacists to contribute to closing the osteoporosis treatment gap through undertaking population screening has been identified.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-022-06561-1.
Keywords: Interventions, Osteoporosis, Pharmacist, Systematic review
Introduction
Osteoporosis causes a significant burden to both individuals directly impacted by fractures and the wider community [1]. The physical and psychological impact of osteoporosis on individuals has been widely documented, with the most significant impact associated with hip fracture [1, 2]. Within 12 months of experiencing a hip fracture, 60% of individuals require assistance; 40% are unable to walk independently; 33% are totally dependent; and there is a 20% mortality rate [2, 3]. Whilst other fractures carry a lower mortality rate, their impact is nonetheless significant, with ongoing pain, loss of mobility, distorted body image, loss of self-esteem, depression and adverse effects on daily routine all reported [1]. At a societal level, fragility fractures and their associated disability result in a socioeconomic toll comparable to or greater than a variety of chronic non-communicable diseases, such as rheumatoid arthritis, asthma and hypertension-related heart disease [2].
As osteoporosis is a condition predominantly impacting the elderly, the global aging of the population means the burden of osteoporosis is anticipated to increase dramatically worldwide [1, 4]. Current estimates propose that one in three women and one in five men over 50 years of age will be directly impacted by osteoporosis in their remaining lifetime [1]. Fragility fractures caused by osteoporosis are largely preventable with effective treatment [1, 4]. Of the individuals who sustain a fragility fracture, 50% will experience a secondary fracture [1, 3]; despite this, an estimated 80% of individuals who experience a fragility fracture remain untreated [2]. For those in whom therapy is commenced, adherence is an ongoing issue [3, 4]. These barriers to effective management have led to repeated international calls to reduce the widely documented osteoporosis treatment gap [1, 3, 4].
Several potential reasons for this treatment gap have been proposed. A lack of public awareness, the asymptomatic nature of osteoporosis until a fracture occurs, and limited access to diagnostic methods have been identified as contributing factors to underdiagnosis [5]. Physicians placing a low level of priority on the management of osteoporosis have been shown to be a barrier to treatment commencement [6]. Potential rare adverse effects and concerns about polypharmacy have also been linked to low treatment commencement and adherence rates [5]. Fragmented health care contributes to the high proportion of individuals remaining untreated following a fragility fracture [5]. Therefore, a multifaceted approach directed at patients, health care providers and healthcare systems is necessary to reduce the osteoporosis burden [6].
Pharmacists are widely accepted as one of the most accessible health care professionals and regarded as the experts in medicines [7, 8]. Pharmacists can contribute to addressing the treatment gap in osteoporosis in several ways. Firstly, pharmacists may assist with public awareness, screening and diagnosis of osteoporosis, including identifying individuals at risk of drug-induced osteoporosis [7, 8]. Pharmacists can also help in treatment uptake, guiding the choice of agent and improving adherence [8]. Finally, pharmacists undertaking medication review can help reduce falls risk and subsequent fractures by identifying and advocating for deprescribing of medications that may contribute to increased fall risk [9, 10].
A previous systematic literature review, which included articles published up to April 2010, found pharmacist interventions increased BMD testing and calcium use in patients at risk of osteoporosis [11]. At the time of the review, there was an absence of data on pharmacist interventions for increasing osteoporosis therapy and adherence [11]. In the ensuing years, there has been an increase in available osteoporosis therapy options, with the introduction of denosumab, and more recently romosuzumab [4]. Consequently, it is anticipated there will have been an increase in the literature pertaining to pharmacist interventions and osteoporosis management. The purpose of this review is to identify and evaluate pharmacist interventions for improving the management of osteoporosis. This information will then be utilised to assist in developing strategies for pharmacists to narrow existing osteoporosis treatment gaps.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement was utilised to guide reporting of this systematic review [12]. Refer to Online Resource 1 for completed PRISMA 2020 for Abstracts and PRISMA 2020 checklists, and Online Resource 2 for the review protocol.
Literature search
Following liaison with a medical librarian, a literature search was undertaken in PubMed, Embase, International Pharmaceutical Abstracts and Cumulative Index of Nursing and Allied Health Literature from inception to June 2022. using the following keywords separated by Boolean operators: [“osteoporosis,” “osteopenia,” “bone loss,” “bone density;” “BMD,” “bone fragility,” “fracture”] AND [“pharmacist,” “pharmacy”]. Complete search strategies are available in Online Resource 3. Reference lists of articles identified for full-text review were manually searched.
Retrieved records were imported to an EndNote™ library [13], and record management was completed using the Covidence systematic review platform [14]. Following the removal of duplicates, articles were screened for eligibility by title and abstract. Articles identified as possibly relevant underwent full-text review. Screening was completed by two authors (CL and HB) with the input of the third author (KW) sought in the event of discrepancies in studies for inclusion.
Inclusion criteria
Articles were included if they met the following eligibility criteria: (1) randomised controlled trial (RCT) assessing a pharmacist intervention to improve the management of osteoporosis in an adult population with or at risk of osteoporosis; (2) full-text peer-reviewed original research, and (3) available in English language. Multidisciplinary interventions that stipulated a pharmacist’s involvement and identified their role were included.
Data extraction and analysis
Data from the eligible studies were extracted by one author (CL) and recorded utilising a standardised data extraction form. Information extracted included practice setting, participant inclusion/exclusion criteria, sample size, participant demographics, intervention details, follow-up period and outcomes. Critical Appraisal Skills Programme (CASP) checklists [15] were used to assess the quality of studies by one author (CL). The risk of bias was assessed by one author (CL) using the Cochrane Collaboration tool to assess risk of bias RoB 2.0 [16]. Where possible, published protocols and information from trial registries were obtained, especially to assess the risk of reporting bias. For risk of bias assessment purposes, the primary outcome per study methods was considered; in situations where the study stated more than one primary outcome, the risk of bias was assessed for the first outcome listed in the report. The robvis tool [17] was utilised to visually represent the risk of bias assessments.
Considering the heterogeneity of studies, a qualitative approach was undertaken for analysis. Studies were classified according to the aspect of osteoporosis management being assessed and the nature of the intervention utilised. Outcomes presented are those pertaining to osteoporosis investigation, treatment, adherence and patient knowledge. The outcomes are reported using the effect measure presented by each respective study.
Results
Study selection
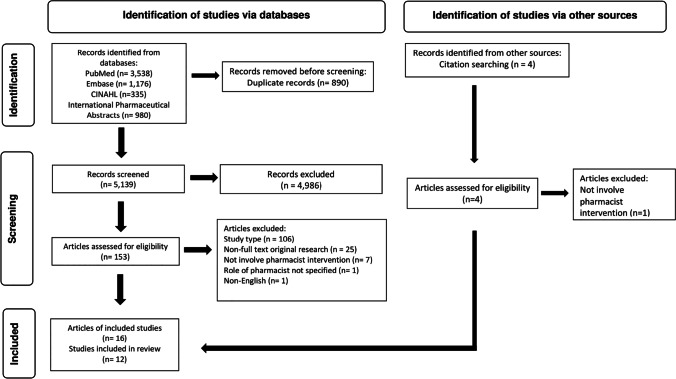
Database searches identified 6029 records, which reduced to 5139 records after the removal of duplicates. Following screening by title and abstract, 153 reports required further analysis. After full-text review, reports were excluded for not meeting the criteria for study type (n = 106), non-full text original research articles (n = 25), studies that did not involve pharmacist intervention (n = 7), studies where the role of the pharmacist was not specified (n = 1) and non-English language articles (n = 1); see Online Resource 4 for a complete list of articles excluded following full-text review. Three additional articles were identified through hand-searching of reference lists. After the exclusion of four articles that duplicated reporting of RCTs [18–21], twelve RCTs were identified for inclusion in the systematic review. The adapted PRISMA flow chart is presented in Fig. 1.
Fig. 1.
PRISMA flow diagram
One study identified in the search was excluded as a near-miss [22]. Whilst this study appeared to fulfil the inclusion criteria, the intervention was delivered by either a pharmacist or a nurse. Reporting did not differentiate the effect of pharmacist-delivered intervention from that provided by nurses, and thus the study was excluded.
Study characteristics
Study characteristics and outcomes are reported in Table 1; complete extracted results are presented in the Online Resource 5. Five of the included studies had a cluster-randomised design [23–27], and seven were randomised controlled trials [28–34]. All studies were published from 2005 onwards. Studies were predominantly conducted in the USA (n = 5) [23, 24, 28–30], with the remainder being conducted in Canada (n = 2) [25, 31], the Netherlands (n = 2) [26, 32], Australia (n = 1) [27], India (n = 1) [33] and Malaysia (n = 1) [34].
Table 1.
Study characteristics and outcomes
| Study, sample size | Inclusion criteria | Intervention/comparator | Outcomes reported | Key findings |
|---|---|---|---|---|
| Studies targeting investigation and treatment | ||||
| Interventions directed to patients only | ||||
| McDonough et al. [24], 96 participants | ≥ 18 years women or men utilising ≥ 7.5 mg/day glucocorticoid for at least 6 months |
Intervention -Education and counselling session supported by written materials -Medication review -Referral letter to physician Comparator -Usual care |
-Investigation: BMD testing -Treatment: calcium -Treatment: osteoporosis therapy |
-Greater use of calcium supplements (p-value < 0.05) -No difference in BMD testing or use of osteoporosis therapy |
| Yuksel et al. [31], 262 participants | ≥ 65 years women or men or ≥ 50–64 years women or men with 1 major risk factor for osteoporosis |
Intervention -Peripheral bone mineral density (BMD) via heel ultrasound -Education and counselling supported by written materials. Initial in-person session and follow-up telephone calls at 2 and 8 weeks -Referral to physician Comparator -Provided educational written materials -Usual care |
-Investigation: follow-up with physician per referral -Investigation: BMD testing -Treatment: calcium -Treatment: vitamin D -Treatment: osteoporosis therapy -Patient knowledge |
-Increased follow- up with physician (p-value < 0.001) -Increased BMD testing (p-value = 0.011) -Increased proportion of individuals receiving RDI of calcium (p-value = 0.011) -No difference for vitamin D, osteoporosis therapy, and patient knowledge |
| Brookhart et al. [29], 110 participants | ≥ 30 years women with no history of osteoporosis |
Intervention -Osteoporosis knowledge questionnaire administered after intervention - Peripheral BMD via heel ultrasound - Education and counselling session Comparator -As per intervention, with the questionnaire administered prior to intervention |
-Patient knowledge | -Greater patient knowledge (p-value < 0.001) |
| Sabna et al. [33], 29 participants | ≥ 20 years women with regular menses utilising antiepileptic therapy for ≥ 2 years |
Intervention -Education and counselling session -Patients with T-score <—1.0 provided 500 mg elemental calcium and 700 iU Vitamin D supplements -Central BMD via DEXA Comparator -Central BMD via DEXA -Usual care provided by the neurology department |
-Treatment: improvement in BMD | -Greater improvement in T-score at spine and femoral neck (p-value < 0.01 both) |
| Crockett et al. [27], 217 participant |
> 40 years women Or > 50 years men and No bone mineral density (BMD) test in last 2 years and No previous treatment for osteoporosis |
Intervention -Peripheral BMD via forearm ultrasound - Risk assessment questionnaire administered By a pharmacist -Counselling on results and treatment recommendations, including referral to physician if indicated Comparator -As per intervention, excluding peripheral BMD |
-Investigation: follow-up with physician per referral -Treatment: calcium -Treatment: vitamin D |
-No difference for follow-up with physician per referral -No statistical difference in calcium and vitamin D intake between groups |
| McConaha et al. [28], 87 participants |
40–65 year old postmenopausal women and Achilles T-score < -1.0 and Not receiving bisphosphonate therapy |
Intervention -FRAX administered by pharmacist - Peripheral BMD via Achilles bone densitometer -Education and counselling session -Referral letter outlining results to physician -Two-day supply of calcium citrate with vitamin D provided Comparator -As per intervention, excluding FRAX |
-Investigation: follow-up with physician per referral -Investigation: BMD testing -Treatment: calcium -Treatment: vitamin D -Treatment: osteoporosis therapy |
-Significant difference between groups for starting/increasing vitamin D supplement (p-value = 0.024) -Upward trend in both groups for follow-up with physician, BMD testing, and calcium intake, not statistically different between groups - No difference demonstrated for commencing osteoporosis therapy |
| Interventions directed to physicians | ||||
| Solomon et al. [23], 1973 participants |
≥ 65 years women or ≥ 45 years women or men with prior fracture or ≥ 45 years women or men who recently used glucocorticoids for ≥ 90 days |
Intervention -Academic detailing to primary care physician delivered by a pharmacist -Physicians provided with written materials -Physicians provided a list of patients at risk of osteoporosis -Patients received introductory letter and an automated telephone call inviting them to undergo BMD testing Comparator -Usual care |
-Investigation: BMD testing -Treatment: osteoporosis therapy -Adherencea |
-Increase in BMD testing (unadjusted p-value = 0.02) -Increase in osteoporosis therapy (unadjusted p-value = 0.03) -Secondary analysis showed no difference for adherencea |
| Tso et al. [30], 6591 participants |
≥ 66 years women experiencing fracture and Not received BMD testing or osteoporosis therapy within 1 year prior of fracture |
Intervention (1) -Patient education and counselling by telephone -Written education materials sent to patients -Written materials sent to primary care physician Intervention (2) -Physician education session delivered by telephone -Written materials provided to patients and physicians as per Intervention (1) Comparator -Written materials as per Intervention (1) and (2) |
-Investigation: BMD testing -Treatment: osteoporosis therapy |
-Intervention (2) resulted in increased BMD testing compared to comparator (p-value < 0.001) and Intervention (1) (p-value = 0.001) -Intervention (2) resulted in increased proportion of participants commencing osteoporosis therapy compared to comparator (p-value = 0.019) |
| Klop et al. [32], 695 participants |
All patients utilising ≥ 675 mg prednisone equivalents (without a concomitant bisphosphonate prescription) within 180 days and ≥ 1 prescription for a glucocorticoid within 90 days |
Intervention -Pharmacists received list of patients eligible for osteoporosis prophylaxis and a copy of GIOP guideline -Pharmacists to alert physicians of identified patients Comparator - Usual care |
-Treatment: calcium or vitamin D Treatment: osteoporosis therapy |
-No difference demonstrated for calcium or vitamin D use -Increased osteoporosis therapy commencement in men (HR 2.53, 95% CI 1.11, 5.74) and patients ≥ 70 years (HR 2.88, 95% CI 1.33, 6.23) |
| Intervention involving multidisciplinary care team | ||||
| Kennedy et al. [25], 5478 participants | All residents of participating long-term care facilities |
Intervention -Interdisciplinary care leaders participated in 3 educational meetings -Action planning for quality improvement undertaken -Audit and feedback review undertaken -Written resources provided to support initiatives -Provision of osteoporosis tool kit (supplied to all long-term care facilities in the region) Comparator -Usual care - Provision of osteoporosis tool kit (supplied to all long-term care facilities in the region) |
-Treatment: calcium -Treatment: vitamin D -Treatment: osteoporosis therapy |
-Increased use of calcium (OR 1.33, 95% CI 1.01,1.74 and vitamin D (OR 1.82, 95% 1.12,2.96) - No difference demonstrated for osteoporosis therapy |
| Studies targeting adherence | ||||
| Kooij et al. [26], 571 participants | ≥ 18 years receiving first dispensing of bisphosphonate in 12 months |
Intervention -Education and counselling delivered by telephone call 7 to 21 days after dispensing Comparator -Usual care |
-Adherence | - Higher level of adherence measured by mean medication Possession Ratio modified (MPRm) as a continuous outcome (RD 10.2, 95% CI 1.98, 16.4) and refill rate ≥ 8 0% (OR 2.15, 95% CI 1.32, 3.57) |
| Lai et al. [34], 198 participants |
Post-menopausal women with osteoporosis and No history of osteoporosis therapy in the past 6 months and Prescribed alendronate or risedronate |
Intervention -Education and counselling supported by written materials. Initial in-person session and follow-up telephone calls monthly for the first 6 months, then 3 monthly till 12 months -Counselling on bone turnover markers (BTMs) at 4 and 7 months -BTMs measured at 3 and 6 months -Patient to record date each dose bisphosphonate taken and bring remaining medication to 3 monthly scheduled appointments (adherence assessment) Comparator -Usual care -BMTs measured as per intervention -Patient activities for adherence assessment as per intervention |
-Adherence -Patient knowledgeb |
- Higher level of adherence at 6 and 12 months (p-value = 0.015 and 0.047) -Higher knowledge level (p-value < 0.001)b |
The studies took place across a range of pharmacist practice settings, including community pharmacies (n = 6) [24, 26, 27, 29, 31, 32], hospital outpatient clinics (n = 3) [28, 33, 34], primary healthcare centres (n = 1) [23], mobile health clinics (n = 1) [28], long-term care facilities (n = 1) [25] and a managed care organisation (n = 1) [30]. The inclusion criteria of most studies were open to those at risk of osteoporosis, irrespective of fracture history. However, one study restricted participation to individuals who had experienced a fracture [30], and another two studies were restricted to individuals commencing osteoporosis therapy [26, 34]. The studies did not differentiate between patients with primary or secondary osteoporosis, with three exceptions, two focused on glucocorticoid-induced osteoporosis (GIOP) [24, 32] and another on antiepileptic therapy-induced osteoporosis [33].
The sample size of studies ranged from 29 [33] to 6591[30] patients, with the mean age of study patients ranging from 34 [33] to 84 [25] years. Five studies restricted inclusion to female patients [28–30, 33, 34]. In the remaining studies, the percentage of female patients ranged from 55% [32] to 92% [23]. Five studies had a follow-up time of less than 6 months [27–31], and seven studies had a follow-up time of between 6 and 12 months [23–26, 32–34].
Pharmacists employed a wide variety of interventions, which were predominantly multi-component in nature. Patient education and counselling was the most employed intervention component [24, 26–29, 31, 33, 34]; other components were fracture risk screening [27–29, 31, 33], collaboration with physicians [23, 25, 30, 32] and medication review [24]. Studies were classified into two categories, those in which pharmacist interventions were targeted towards osteoporosis investigation and treatment [23–25, 27–33] and those targeting adherence [26, 34]. The impact of the intervention on patient osteoporosis knowledge level was reported by two studies targeting investigation and treatment [29, 31] and one study targeting adherence [19].
Investigation and treatment
Pharmacist interventions intended to increase osteoporosis investigation and treatment were evaluated in ten studies [23–25, 27–33]. Interventions were classified into three types according to whom the intervention was directed to: patients only (n = 6) [24, 27–29, 31, 33], physicians (n = 3) [23, 30, 32] and an intervention involving pharmacists as part of a multidisciplinary care team (n = 1) [25]. Comparison was made with usual care in seven studies [23–25, 29, 31–33], and the remainder made comparison with an alternative intervention (n = 3) [27, 28, 30].
Investigation
Two outcomes were utilised to assess the impact of pharmacist interventions on osteoporosis investigation: follow-up with physician per pharmacist referral (n = 3) [27, 28, 31] and BMD testing (n = 5) [23, 24, 28, 30, 31].
A significant increase in the number of patients attending an osteoporosis specific-appointment following pharmacist referral was achieved in comparison to usual care (p-value < 0.001) [31]. No between group difference was shown when comparing alternative pharmacist interventions [27, 28]; however, in one study both interventions appeared to provide benefit with 55.6% of participants consulting their physician about osteoporosis [28].
An increase in the proportion of patients undergoing BMD testing was achieved by a patient only directed intervention involving multiple occurrences of education and counselling (usual care 10% vs intervention 22% (relative risk (RR) 2.2, 95% confidence interval (CI) 1.2,4.1; p-value = 0.011) [31]; but not when the intervention was delivered on a single occasion [24]. In a study comparing alternative patient only interventions, whilst no difference was shown between interventions, both interventions demonstrated an upward trend for participants undergoing BMD testing, which occurred for 38.9% of patients [28]. In studies involving physician directed components combined with patient components, an increase in the proportion of patients undergoing BMD testing was achieved, both in comparison to usual care (usual care 9% vs intervention 13% unadjusted RR 1.43, 95% CI 1.06, 1.94; p-value = 0.02) [23] and alternative interventions (physician telephone call 8.1% vs patient telephone call 5.6% p-value = 0.001; physician telephone call 8.1% vs written materials alone 5.3% p-value < 0.001) [30].
Treatment
The majority of studies (n = 8) considered outcomes pertaining to treatment [23–25, 27, 28, 30, 32, 33].
The effect of the pharmacist intervention on calcium intake was reported in six studies [24, 25, 27, 28, 31, 32]. An increase in overall calcium intake was achieved by one study utilising a patient-directed intervention (p-value = 0.011) [31]. Similarly, an increase in calcium supplement use was achieved by one patient-directed intervention (p-value < 0.05) [24] and the multidisciplinary care team (odds ratio (OR) 1.33, 95% CI 1.01,1.74) [25]. However, neither the physician-directed intervention [32] nor patient only-directed interventions, making comparison with alternative interventions [27, 28], found a difference in calcium intake between groups. Although none of the interventions found a difference in calcium intake between groups both studies reported an upward trend of calcium intake, with 72.2% [28] and 79% [27] of patients increasing calcium in accordance with pharmacist advice.
Vitamin D intake was reported in five studies; of which three involved patient only-directed interventions [27, 28, 31]; one involved a physician-directed intervention [32]; and one involved a multidisciplinary care team [25]. One study, making comparison of alternative patient-directed interventions, found a between group difference for vitamin D supplementation (p-value = 0.024) [28]. In this study, both interventions achieved increases in vitamin D supplementation, with 79.2% of patients starting or increasing vitamin D supplements. Another study, making comparison of alternative patient interventions, found no between group difference, but did report 78.6% of patients across the study increased vitamin D intake in accordance with pharmacist advice [27]. An increase in vitamin D supplementation was also achieved by the multidisciplinary intervention (OR 1.82, 95% 1.12,2.96) [25]. The remaining studies did not demonstrate a difference in vitamin D intake [31, 32].
Calcium and vitamin D supplements were initiated by pharmacists in the study pertaining to anti-epileptic therapy-induced osteoporosis [33]. The provision of supplements accompanied pharmacist-delivered education and counselling and BMD testing. At 1 year, a statistically significant difference in mean deviation of T-score shifts was achieved (spine: control mean deviation (MD) 0.08 ± standard deviation (SD) 0.78 vs intervention MD 0.44 ± SD 1.65; p-value < 0.01 and femoral neck: control MD 0.05 ± SD 0.02 vs intervention MD 0.87 ± SD 0.07; p-value < 0.01). Furthermore, at 1 year, 21% of the intervention group had a BMD in normal range compared with 7% of the control group.
Pharmacist interventions that were directed to physicians were shown to increase the commencement of osteoporosis therapy [23, 30, 32]. When interventions involved physician components combined with patient components, a small increase in proportion of patients commencing therapy was achieved, both in comparison to usual care (usual care 4% vs. intervention 6% unadjusted RR 1.60, 95% CI 1.04,2.49; p-value = 0.03) [23] and written materials alone (written materials 5.3% vs. physician and patient intervention 7.0%; p-value = 0.019) [30]. The intervention delivered to physicians without a patient component did not result in an overall increase in osteoporosis therapy commencement [32]. However, a sub-analysis showed an increase in osteoporosis therapy commencement for men (usual care 5.1% vs intervention 12.8%, HR 2.53, 95% CI 1.11, 5.74) and patients 70 years of age or older (usual care 4.9% vs intervention 13.4%, HR 2.88, 95% CI 1.33, 6.23). Pharmacist interventions delivered only to patients neither demonstrate a statistical difference for osteoporosis therapy commencement [24, 28, 31], nor did the intervention involving pharmacists as part of a multidisciplinary care team [25].
Adherence
Two studies utilised pharmacist interventions to increase adherence to osteoporosis therapy [26, 34]. In both studies, the pharmacist interventions were delivered solely to patients, and comparison was made with usual care. The intensity of the pharmacist intervention varied between the two studies. One study targeting investigation and treatment also reported on adherence in a secondary analysis [20]. Of these three studies, two assessed adherence utilising dispensing record date and reported this as the medication possession ratio (MPR) [20] and medication possession ratio modified (MPRm) [26]. The MPR and MPRm are a ratio of the days of medication supplied to days in the time period being considered; hence a patient that has prescriptions filled consistently and subsequently has medication available for every day of the time period is deemed to be 100% adherent. In the third study targeting adherence, three methods for assessing adherence were used: direct reporting by patients, pill count by analysts and patient self-record [34].
In the first study targeting adherence, a statistically significant effect of the intervention was not found using the intent to treat analysis [26]. However, the intervention was not delivered to 57% of patients, for which the primary reasons were no telephone number being available and the patient not being able to be reached. Per protocol analysis demonstrated the effectiveness of this intervention in terms of MPRm expressed as a continuous outcome (risk difference (RD) 10.2, 95% CI 1.98, 16.4) and MPRm ≥ 80% (OR 2.15, 95% CI 1.32, 3.57). The second study targeting adherence found a benefit from the intervention when adherence was measured using pill count and self-recording [34]. For pill count, a difference was demonstrated at 6 months (p-value = 0.028), whilst for self-recording a difference was demonstrated at both 6 months (p-value = 0.015) and 12 months (p = 0.047).
In the secondary analysis of the study targeting investigation and treatment, which made comparison to usual care, no difference in adherence was found between groups (intervention MRP median 74% interquartile range (IQR) 19–93% vs. usual care MRP median 73%, IQR 0-–3%; p-value = 0.18) [20].
Patient knowledge
Patient osteoporosis knowledge was reported as an outcome by three studies, two targeting investigation and treatment [29, 31] and one targeting adherence [19]. Comparison was made to usual care [19, 31] or baseline knowledge [29]. Two of the three studies utilised a validated tool to assess knowledge [19, 31]; the third study developed a new tool, but this was not validated [29].
Of the two studies targeting investigation and treatment, one found the intervention resulted in an increased knowledge level (p-value < 0.001) [29]. The study targeting adherence reported a statistically significant higher knowledge score in the intervention group at 3 months (72.50 vs 62.50%), 6 months (75.0 vs 65.0%) and 12 months (78.75 vs 68.75%) (p-value < 0.001) [19].
Study quality
The quality of studies was assessed using both a risk of bias assessment and a critical appraisal checklist. In addition to the reports themselves, published protocols [35–37] and information contained in trial registries [38–41] were utilised, where possible, for these assessments. A table detailing sources utilised for risk of bias assessments and critical appraisal of each study is contained in Table 1 of the Online Resource 6.
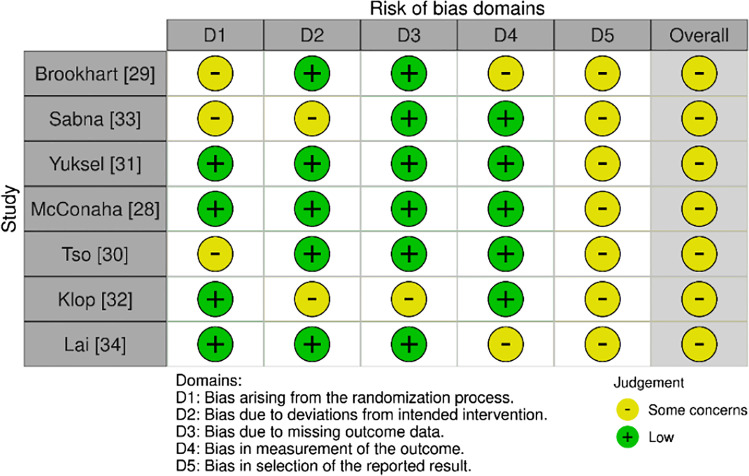
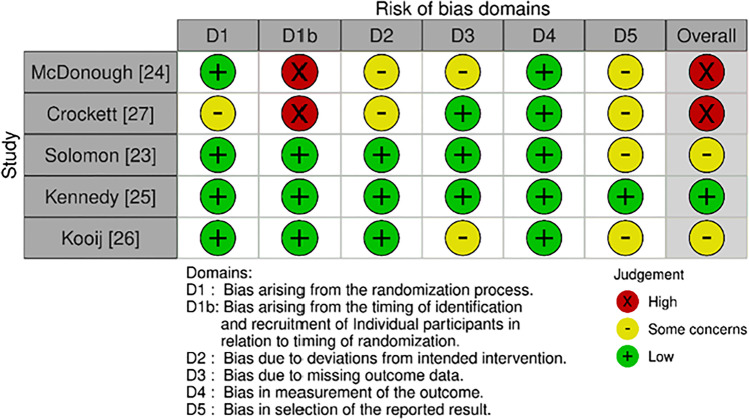
The risk of bias was assessed utilising RoB 2.0 for RCTs or cluster-RCTs as applicable (Figs. 2 and 3). Overall, one study was judged to have a low risk of bias [25], nine as having some concerns [23, 26, 28–34] and two studies had a high risk of bias [24, 27]. Eleven studies were judged to have some concerns for Domain Five, reflective of the absence of a prespecified analysis plan being available [23, 24, 26–34]. Two cluster RCTs were found to have a high risk of bias in Domain One B as clusters were aware of group allocation prior to participant recruitment [24, 27].
Fig. 2.
RoB 2.0 RCTs
Fig. 3.
RoB 2.0 Cluster RCTs
CASP checklists were utilised to assess the quality of studies. A summary of the results is available in Table 2 of Online Resource 6. Six studies identified differences in participant characteristics that may impact outcomes [23–25, 27, 31, 32], of which four performed analyses controlling for covariates [23, 25, 31, 32]. Predominantly blinding did not occur, with two exceptions; one study blinded participants [28], and another blinded the database manager and analysts [25]. Neither sustainability nor cost analysis of the intervention was considered in any study, limiting the ability to assess cost effectiveness and applicability to clinical practice. Underpowering was identified as a potential concern in three studies where the recruited sample size was lower than that proposed [24, 27, 28]. Two studies reported amendments to the planned protocol [25, 27].
Concerns are held regarding the study protocol of two studies [29, 32]. In one study, pertaining to a physician-directed intervention, there was no stipulation on how pharmacists were to implement the intervention [32]. This aspect of the study protocol raises the potential for significant inter-site variability in the intensity of the intervention utilised by pharmacists. In the second study which assessed patient knowledge, the non-validated knowledge assessment was scored by the pharmacist who was responsible for both group allocation and provision of the intervention, raising the potential for scoring to be influenced by knowledge of the intervention received [29].
In three studies, where comparison was reported to be made with usual care, there is cause to believe the care provided to the comparator group was more comprehensive than usual care and this may have resulted in undermining the effectiveness of the intervention [24, 31, 34]. In one study targeting investigation and treatment, participants in the comparator group were provided with written educational materials which the authors noted was not in keeping with the usual care provided by pharmacists [31]. In another study, with a cluster RCT design, pharmacists in both clusters received training on the intervention, raising the possibility for this training to have been implemented by pharmacists in the comparator group when interacting with patients [24]. Evidence of this was the comparable proportion of patients receiving advice from pharmacists regarding GIOP risk and the need for BMD testing. Finally, in a study targeting adherence, patients in the comparator group had a greater level of interaction with healthcare providers than is usual, due to the testing of BTMs and the means of assessing adherence employed by the study [34].
Discussion
This review sought to identify and evaluate pharmacist interventions for improving osteoporosis management. There has been an increase in the literature since the previous systematic literature review [11] was undertaken in 2011, with an additional nine studies identified [23, 25, 26, 28–30, 32–34]. In the previous literature review, all studies were directed to patients only and took place in community pharmacies [24, 27, 31]. The additional studies included in this review involved interventions delivered by pharmacists in a mix of practice settings [23, 25, 28, 30, 33, 34] and involved physician-directed components [23, 30, 32] as well as pharmacists undertaking an intervention as part of a multidisciplinary care team [25]. These additional studies not only supported the findings of the previous review to show that pharmacist interventions can increase BMD testing [23, 30] and calcium intake [25], but also showed pharmacist interventions can increase the use of vitamin D supplements [25, 28] and commencement of osteoporosis therapy [23, 30, 32]. At the time of the previous literature review, no studies were identified that evaluated the effectiveness of pharmacist interventions for adherence; this review identified three studies [20, 26, 34] that considered this, of which two [26, 34] demonstrated effectiveness.
Whilst the studies did demonstrate statistical differences, the conclusions that can be drawn from this review are impeded by the heterogeneity of interventions and patient groups and the range of methodological issues identified in quality appraisal. Comparison of the effectiveness of the interventions is also impeded by the evolving nature of osteoporosis guidelines during the timespan and the multiple jurisdictions in which the studies were conducted [4]. Three studies made comparison of alternative interventions [27, 28, 30], without the use of a control group receiving usual care further limiting evidence of effect. Whilst the upward trends in terms of investigation and treatment achieved by these studies indicate the pharmacist interventions did exhibit an effect, drawing definitive conclusions on the effectiveness of pharmacist interventions over usual care is not possible. Despite these shortcomings, pharmacist interventions show promise for improving osteoporosis management. Additionally, the studies have shed light on the components pharmacists can utilise in such an intervention. The components that have been identified for inclusion in pharmacist interventions are patient education and counselling, collaboration with physicians, fracture risk assessment and medication review.
As with pharmacist interventions in other disease states [42, 43], the results of this review support pharmacists delivering osteoporosis interventions in collaboration with other health care providers. Interventions delivered to both patients and physicians were able to achieve an increase in overall osteoporosis therapy commencement [23, 30]; however, interventions delivered to patients [24, 28, 31] or health care providers [25, 32] alone did not. The success of combined patient and physician interventions likely reflects the low priority placed on osteoporosis management by both patients and physicians. Evidence shows patients are largely unaware of osteoporosis [5, 44]; however, increasing patient knowledge, as achieved by pharmacist intervention [29], translates to increased patient self-efficacy and, in turn, an increase in treatment rates [44, 45]. As pharmacists can initiate calcium and vitamin D, pharmacist interventions without physician components are able to increase their use [24, 27, 28, 31, 33]. However, with physicians responsible for the commencement of osteoporosis therapy there is a need for their involvement to increase this aspect of osteoporosis management. It is widely documented that physicians place a low level of priority on osteoporosis management and perceive several barriers to therapy commencement [5, 6, 9, 46]. Physician barriers to osteoporosis therapy commencement include a mix of clinical and systemic factors, such as uncertainty regarding therapy effectiveness [6, 9], potential adverse effects [6, 9, 46], contributing to polypharmacy [5, 9, 46], time requirements for case finding [5] and uncertainty regarding the clinical responsibility of osteoporosis management [5]. In the interventions, pharmacists addressed these issues by identifying patients for investigation and treatment and assisting the physicians to apply treatment guidelines to these patients. Hence, although pharmacist interventions to either physicians or patients alone can improve aspects of osteoporosis management, the two-pronged approach of simultaneously addressing osteoporosis management with patients and physicians appears to provide greater benefit.
Osteoporosis management guidelines recommend the performance of fracture risk assessment to guide BMD testing and treatment commencement [4, 46–48]. A systematic review undertaken by Merlijn et al. (2020) found population screening for individuals at high risk of fracture is an effective strategy for increasing treatment rates and reducing fracture rates [49]. In the studies reviewed by Merlijn et al. (2020), population screening consisted of fracture risk assessment, utilising FRAX or presence of clinical factors, with subsequent BMD testing if indicated [49]. This approach is akin to that employed in pharmacist interventions, with pharmacists performing preliminary screening of risk and identifying patients requiring BMD testing via either referral [24, 27, 28, 31] or direct collaboration [23, 30] with physicians. Given the high accessibility of pharmacists [6, 7] and their ability to incorporate fracture risk assessment in an osteoporosis intervention, the possibility of pharmacists being employed to undertake population screening for individuals at high risk of fracture should be explored.
Another component utilised in pharmacist interventions that warrants further consideration is medication review. Pharmacist-led medication review refers to a service in which pharmacists assess a patient’s medication regimen to identify and resolve medication-related issues in collaboration with physicians [50]. It has been widely recognised as an effective pharmacist intervention for both range of specific disease states and optimising medication use, including improving adherence [42, 50]. Considering this, it was surprising medication review was only employed in one of the identified studies and had limited success [24]. However, quality appraisal identified methodological concerns which may have led to the undermining of this study’s outcomes and hence impact the conclusions that can be drawn. From other literature, pharmacists undertaking medication reviews have the potential to improve osteoporosis management [8, 46, 48, 51–53]. Specifically, medication review is advocated in multiple international guidelines for reducing falls and subsequent fractures, as well as aiding in the identification and management of those at risk of drug-induced secondary osteoporosis [46, 48, 51–53]. As medication review is an established pharmacist intervention, accepted by physicians [42, 50], the potential for it being used as a framework for incorporating physician collaboration into a pharmacist osteoporosis intervention could have merit.
If the osteoporosis treatment gap is to be closed, in addition to increasing investigation and treatment, it is necessary to also improve adherence [4, 54, 55]. The need for a multifaceted approach to achieve this was demonstrated by Shu et al. (2009) who found a pharmacist intervention that increased investigation and treatment did not result in an improvement in adherence [20]. A systematic review regarding interventions to improve osteoporosis therapy adherence supported interventions that enabled individualised solutions through collaboration between patients and their health care providers [54]. This approach was utilised by both pharmacist interventions targeting adherence, with pharmacists employing patient education and counselling to reinforce the patient’s need for therapy and address patient-encountered issues with therapy [26, 34]. Although neither study involved a physician component per se, the importance of pharmacists collaborating with physicians to improve adherence was highlighted by Lai et al. (2012) in their report on issues encountered by patients [18]. Pharmacists working alone were able to resolve 41% of the osteoporosis therapy issues encountered by patients; this increased to a resolution rate of 98.5% when pharmacists liaised with physicians [18]. There is therefore evidence for pharmacists collaborating with physicians to not only deliver interventions to improve osteoporosis investigation and treatment commencement but also to support patients with adherence.
Whilst the studies in this review have shown pharmacists can improve adherence for bisphosphonates [26, 34], it is recognised low levels of adherence are problematic for other classes of osteoporosis therapy [4, 6, 56]. For example, denosumab has been shown in recent studies to be frequently abruptly ceased without successive osteoporosis therapy commenced [6, 56]. This is a significant issue, as abrupt cessation can result in the full benefit of therapy not being achieved, as well as an increased incidence of fractures [4, 6, 47, 48, 51, 52]. Further research on the pharmacist’s role in maintaining adherence for the full intended duration of therapy and for all osteoporosis therapies is needed.
The impact of the practice setting on the design and success of pharmacist interventions is also of importance. In this review, studies reported on interventions that were predominantly delivered in the community pharmacy setting. Only one study in this review was conducted in the long-term care setting where residents are reported to have a higher incidence of osteoporosis than community-dwelling individuals [9, 46]. Hence, there is a need for further research on pharmacist interventions targeting osteoporosis management in a broader range of practice settings.
Limitations
Whilst this systematic review employed rigorous methods and has been reported in keeping with PRISMA, it is recognised limitations exist, including that the review was not registered. Whilst an extensive literature search utilising a broad search strategy was undertaken, it is possible that relevant studies may not have been identified. Including only articles written in English may have resulted in relevant studies being excluded. As with all systematic reviews, the findings presented are limited by the quality of the identified studies. To this end, whilst studies were restricted to RCTs, as they are considered to provide the most reliable evidence for the effectiveness of the intervention, quality appraisal identified concerns across all studies which impeded the conclusions that can be drawn. Furthermore, the restriction of study type to RCTs may have resulted in the exclusion of studies that are more relevant to real-world clinical practice.
Finally, it is acknowledged the outcomes reported in this review represent surrogate markers for fracture reduction. However, given the efficacy of calcium, vitamin D and osteoporosis therapy has been widely established [4, 47, 48, 51, 52], it is realistic to expect increases in treatment, and adherence resulting from pharmacist interventions would translate to a reduction in fractures. Studies of larger power and longer follow-up duration would be required to demonstrate this.
Conclusion
This review has demonstrated pharmacist interventions show promise for closing the osteoporosis treatment gap. Definitive conclusions regarding effectiveness are limited by the small number of studies, utilising a wide variety of interventions and quality appraisal revealing a range of methodological issues. However, pharmacist interventions do appear to provide benefits in terms of osteoporosis investigation, treatment, adherence and patient knowledge.
Interventions tended to be multicomponent in nature, with patient education and counselling serving as the cornerstone of interventions. Pharmacist collaboration with physicians was found to be beneficial for both increasing investigation and treatment and aiding adherence. As an established means of pharmacist and physician collaboration, further research on the use of medication review as a component of pharmacist osteoporosis interventions is warranted. Additionally, pharmacists, having demonstrated their ability to utilise fracture risk assessments to improve investigation rates, may be well placed to assist in narrowing the osteoporosis treatment gap by undertaking population screening.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Catherine Laird was responsible for the study design, undertaking of the database searches, selection of articles, data extraction, and writing of the manuscript. Helen Benson assisted in the study design, selection of articles, and revision of the manuscript. Kylie Williams assisted in the selection of articles and revision of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Catherine Laird for this research was supported by an Australian Government Research Training Program (RTP) Scholarship; however, the Australian Government was not involved in the design of the study and collection, analysis, and interpretation of data or in the writing of the manuscript. Helen Benson and Kylie Williams did not receive funding to support this research.
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cooper C, Ferrari S (2017) IOF compendium of osteoporosis. International Osteoporosis Foundation. https://www.osteoporosis.foundation/sites/iofbonehealth/files/2019-06/2017_IOFCompendium_TR_English.pdf. Accessed 1 November 2021
- 2.International Osteoporosis Foundation (2019) Epidemiology of osteoporosis and fragility fractures. https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures. Accessed 1 November 2021
- 3.Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Willers C, Borgström F (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16(1). 10.1007/s11657-020-00871-9 [DOI] [PMC free article] [PubMed]
- 4.Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393(10169):364-376. 10.1016/s0140-6736(18)32112-3 [DOI] [PubMed]
- 5.Åkesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24(8):2135–2152. doi: 10.1007/s00198-013-2348-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naik-Panvelkar P, Norman S, Elgebaly Z, Elliott J, Pollack A, Thistlethwaite J, Weston C, Seibel MJ (2020) Osteoporosis management in Australian general practice: an analysis of current osteoporosis treatment patterns and gaps in practice. BMC Fam Pract 21(1). 10.1186/s12875-020-01103-2 [DOI] [PMC free article] [PubMed]
- 7.Kelling SE, Rondon-Begazo A, DiPietro Mager NA, Murphy BL, Bright DR. Provision of clinical preventive services by community pharmacists. Prev Chronic Dis. 2016;13:E149. doi: 10.5888/pcd13.160232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menezes MM. The osteoporosis clinical care gap: an opportunity for impact by the clinical pharmacist. J Bone Rep Recomm. 2016;2:1. doi: 10.4172/2469-6684.100017. [DOI] [Google Scholar]
- 9.Duque G, Lord SR, Mak J, Ganda K, Close JJ, Ebeling P, Papaioannou A, Inderjeeth CA. Treatment of osteoporosis in Australian residential aged care facilities: update on consensus recommendations for fracture prevention. J Am Med Dir Assoc. 2016;17(9):852–859. doi: 10.1016/j.jamda.2016.05.011ted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDerby NC, Kosari S, Bail KS, Shield AJ, Peterson G, Thorpe R, Naunton M (2020) The role of a residential aged care pharmacist: findings from a pilot study. Australas J Ageing 39(3). 10.1111/ajag.12784 [DOI] [PubMed]
- 11.Elias MN, Burden AM, Cadarette SM. The impact of pharmacist interventions on osteoporosis management: a systematic review. Osteoporos Int. 2011;22(10):2587–2596. doi: 10.1007/s00198-011-1661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, Mcdonald S, Mcguinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The EndNote Team, EndNote. 2013, Calirvate: Philadelphia, PA.
- 14.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at http://www.covidence.org. Accessed 1 Nov 2021
- 15.Critical Appraisal Skills Programme (2020) CASP (Randomised Controlled Trial) Checklist. [online] Available at: https://casp-uk.b-cdn.net/wp-content/uploads/2020/10/CASP_RCT_Checklist_PDF_Fillable_Form.pdf . Accessed 11 January 2022
- 16.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 17.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 18.Lai PSM, Chua SS, Chan SP. Pharmaceutical care issues encountered by post-menopausal osteoporotic women prescribed bisphosphonates. J Clin Pharm Ther. 2012;37(5):536–543. doi: 10.1111/j.1365-2710.2012.01335.x. [DOI] [PubMed] [Google Scholar]
- 19.Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. Int J Clin Pharm. 2013;35(4):629–637. doi: 10.1007/s11096-013-9784-x. [DOI] [PubMed] [Google Scholar]
- 20.Shu AD, Stedman MR, Polinski JM, Jan SA, Patel M, Truppo C, Breiner L, Chen YY, Weiss TW, Solomon DH (2009) Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care 15(7):417–424. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2885859/pdf/nihms204140.pdf. Accessed 15 Dec 2021 [PMC free article] [PubMed]
- 21.Yuksel N, Tsuyuki RT, Majumdar SR. Predictors of bone mineral density testing in patients at high risk of osteoporosis: secondary analyses from the OSTEOPHARM randomized trial. J Clin Densitom. 2012;15(1):61–66. doi: 10.1016/j.jocd.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Katz JN, Finkelstein JS, Polinski JM, Stedman M, Brookhart MA, Arnold M, Gauthier S, Avorn J. Osteoporosis improvement: a large-scale randomized controlled trial of patient and primary care physician education. J Bone Miner Res. 2007;22(11):1808–1815. doi: 10.1359/jbmr.070717. [DOI] [PubMed] [Google Scholar]
- 23.Solomon DH, Polinski JM, Stedman M, Truppo C, Breiner L, Egan C, Jan S, Patel M, Weiss TW, Chen Y, Brookhart MA. Improving care of patients at-risk for osteoporosis: a randomized controlled trial. J Gen Intern Med. 2007;22(3):362–367. doi: 10.1007/s11606-006-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid-induced osteoporosis. Value Health. 2005;8(1):24–31. doi: 10.1111/j.1524-4733.2005.04007.x. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy CC, Ioannidis G, Thabane L, Adachi JD, Marr S, Giangregorio LM, Morin SN, Crilly RG, Josse RG, Lohfeld L, Pickard LE, van der Horst ML, Campbell G, Stroud J, Dolovich L, Sawka AM, Jain R, Nash L, Papaioannou A. Successful knowledge translation intervention in long-term care: final results from the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Trials. 2015;16:214. doi: 10.1186/s13063-015-0720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kooij MJ, Heerdink ER, van Dijk L, van Geffen ECG, Belitser SV, Bouvy ML. Effects of telephone counseling intervention by pharmacists (TelCIP) on medication adherence; results of a cluster randomized trial. Front Pharmacol. 2016;7:269. doi: 10.3389/fphar.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crockett JA, Taylor SJ, McLeod LJ. Patient responses to an integrated service, initiated by community pharmacists, for the prevention of osteoporosis. Int J Pharm Pract. 2008;16(2):65–72. doi: 10.1211/ijpp.16.2.0003. [DOI] [Google Scholar]
- 28.McConaha JL, Berdine HJ, Skomo ML, Laux RV, Higginbotham SK, O'Neil CK. Impact of the fracture risk assessment on patient and physician behavior in osteoporosis prevention. J Pharm Pract. 2014;27(1):25–30. doi: 10.1177/0897190013503970. [DOI] [PubMed] [Google Scholar]
- 29.Brookhart AL, Brown Fountain KM, Moczygemba LR, Goode JVR (2015) Community pharmacist-provided osteoporosis screening and education: impact on patient knowledge. Innov Pharm 6(3). 10.24926/iip.v6i3.395
- 30.Tso LS, Loi D, Yi D, Stockl KM, Lew HC, Solow BK, Mosley DG. Evaluation of a nationwide pharmacist-led phone outreach program to improve osteoporosis management in older women with recently sustained fractures. J Manag Care Spec Pharm. 2015;21(9):803–810. doi: 10.18553/jmcp.2015.21.9.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Community pharmacist-initiated screening program for osteoporosis: randomized controlled trial. Osteoporos Int. 2010;21(3):391–398. doi: 10.1007/s00198-009-0977-z. [DOI] [PubMed] [Google Scholar]
- 32.Klop C, de Vries F, Vinks T, Kooij MJ, van Staa TP, Bijlsma JW, Egberts AC, Bouvy ML. Increase in prophylaxis of glucocorticoid-induced osteoporosis by pharmacist feedback: a randomised controlled trial. Osteoporos Int. 2014;25(1):385–392. doi: 10.1007/s00198-013-2562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabna M, Thomas A. Impact of pharmaceutical care on bone health in epileptic women taking antiepileptic drugs: an interventional trial. Int Res J Phar. 2018;9(7):173–176. doi: 10.7897/2230-8407.097144. [DOI] [Google Scholar]
- 34.Lai PS, Chua SS, Chew YY, Chan SP. Effects of pharmaceutical care on adherence and persistence to bisphosphonates in postmenopausal osteoporotic women. J Clin Pharm Ther. 2011;36(5):557–567. doi: 10.1111/j.1365-2710.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy CC, Ioannidis G, Giangregorio LM, Adachi JD, Thabane L, Morin SN, Crilly RG, Marr S, Josse RG, Lohfeld L, Pickard LE, King S, van der Horst ML, Campbell G, Stroud J, Dolovich L, Sawka AM, Jain R, Nash L, Papaioannou A. An interdisciplinary knowledge translation intervention in long-term care: study protocol for the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Implement Sci. 2012;7:48. doi: 10.1186/1748-5908-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kooy MJ, van Geffen EC, Heerdink ER, van Dijk L, Bouvy ML. Effects of a TELephone counselling intervention by pharmacist (TelCIP) on medication adherence, patient beliefs and satisfaction with information for patients starting treatment: study protocol for a cluster randomized controlled trial. BMC Health Serv Res. 2014;14:219. doi: 10.1186/1472-6963-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Design of a randomized trial of a community pharmacists’ initiated screening and intervention program for osteoporosis. Can Pharm J. 2006;139:50–51. doi: 10.1177/171516350613900205. [DOI] [Google Scholar]
- 38.Berdine HJ (2012) Impact of the FRAX Assessment on physician and patient treatment behaviour. Identification number NCT01572766. Retrieved from. https://clinicaltrials.gov/ct2/show/study/NCT01572766?term=pharmacist&cond=osteoporosis&draw=2&rank=2. Accessed 21 Jan 2022
- 39.Kooij MJ (2012) Effects of telephone counselling intervention by pharmacist on medication for patients starting treatment: a cluster randomized controlled trial. Identification number NTR3237. Retrieved from. https://www.trialregister.nl/trial/3089. Accessed 21 Jan 2022
- 40.Papaioannou A (2011) An interdisciplinary knowledge translation intervention in long-term care: the vitamin D and osteoporosis study (ViDOS) pilot cluster randomized controlled trial. Identification number NCT01398527. Retrieved from. https://clinicaltrials.gov/ct2/show/NCT01398527?term=NCT01398527&cond=osteoporosis&draw=2&rank=1. Accessed 21 Jan 2022
- 41.Thomas A, Chander JSJU, Sabna M (2015) Impact of pharmacist led intervention study (PLIS)- trial on improving bone health of epileptic women. Identification number CTRI/2015/08/006103. Retrieved from. http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=11737&EncHid=&modid=&compid=%27,%2711737det%27. Accessed 21 Jan 2022
- 42.Brown D, Portlock J, Rutter P. Review of services provided by pharmacies that promote healthy living. Int J Clin Pharmacy. 2012;34(3):399–409. doi: 10.1007/s11096-012-9634-2. [DOI] [PubMed] [Google Scholar]
- 43.Buss V, Deeks LS, Shield A, Kosari S, Naunton M. Analytical quality and effectiveness of point-of-care testing in community pharmacies: a systematic literature review. Res Social Adm Pharm. 2019;15(5):483–495. doi: 10.1016/j.sapharm.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Majumdar SR, McAlister FA, Johnson JA, Weir DL, Bellerose D, Hanley DA, Russell AS, Rowe BH. Critical impact of patient knowledge and bone density testing on starting osteoporosis treatment after fragility fracture: secondary analyses from two controlled trials. Osteoporos Int. 2014;25(9):2173–2179. doi: 10.1007/s00198-014-2728-z. [DOI] [PubMed] [Google Scholar]
- 45.Gai QY, Lv H, Li YP, Fu QM, Li P. Education intervention for older adults with osteoporosis: a systematic review. Osteoporos Int. 2020;31(4):625–635. doi: 10.1007/s00198-019-05166-5. [DOI] [PubMed] [Google Scholar]
- 46.Duque G, Iuliano S, Close JCT, Fatima M, Ganda K, Bird S, Kirk B, Levidiotis M, Said CM, Papaioannou A, Inderjeeth CA (2022) Prevention of osteoporotic fractures in residential aged care: updated consensus recommendations. J Am Med Dir Assoc Jan 21:S1525–8610(22)00001–9. 10.1016/j.jamda.2021.12.041. Epub ahead of print. PMID: 35074360. [DOI] [PubMed]
- 47.Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinolgists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 48.Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, McCloskey E, Moss K, Parker J, Paskins Z, Poole K, Reid DM, Stone M, Thomson J, Vine N, Compston J. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2022;17:58. doi: 10.1007/s11657-022-01061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM. Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int. 2020;31(2):251–257. doi: 10.1007/s00198-019-05226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jokanovic N, Tan EC, Sudhakaran S, Kirkpatrick CM, Dooley MJ, Ryan-Atwood TE, Bell JS. Pharmacist-led medication review in community settings: an overview of systematic reviews. Res Social Adm Pharm. 2017;13(4):661–685. doi: 10.1016/j.sapharm.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.College TRA, of General Practitioners and Osteoporosis Australia, Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. 2. East Melbourne, Vic: RACGP; 2017. [Google Scholar]
- 53.Montero-Odasso MM, Kamkar N, Pieruccini-Faria F, Osman A, Sarquis-Adamson Y, Close J, Hogan DB, Hunter SW, Kenny RA, Lipsitz LA, Lord SR, Madden KM, Petrovic M, Ryg J, Speechley M, Sultana M, Tan MP, van der Velde N, Verghese J, Masud T, Task Force on Global Guidelines for Falls in Older Adults (2021) Evaluation of clinical practice guidelines on fall prevention and management for older adults: a systematic review. JAMA Netw Open 4(12): e2138911. 10.1001/jamanetworkopen.2021.38911 [DOI] [PMC free article] [PubMed]
- 54.Cornelissen D, de Kunder S, Si L, Reginster JY, Evers S, Boonen A, Hiligsman M; on behalf of the European Society for Clinical and Economic Aspect of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESDCEO) interventions to improve adherence to anti-osteoporosis medications: an updated systematic review. Osteoporos Int. 2020;31:1645–1669. doi: 10.1007/s00198-020-05378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiligsmann M, Salas M, Hughes DA, Manias E, Gwadry-Sridhar FH, Linck P, Cowell W. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–2918. doi: 10.1007/s00198-013-2364-z. [DOI] [PubMed] [Google Scholar]
- 56.Witteveen J, Flaes SB, Mullenders P, van den Broek H, Lems W, Groeneveld I (2021) Successive antiosteoporosis treatment after denosumab in the years 2011 till 2017. Bone Rep 14 (Supplement): 100981. 10.1016/j.bonr.2021.100981
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.