Abstract
Cardiotoxicity may present as (pulmonary) hypertension, acute and chronic coronary syndromes, venous thromboembolism, cardiomyopathies/heart failure, arrhythmia, valvular heart disease, peripheral arterial disease, and myocarditis. Many of these disease entities can be diagnosed by established cardiovascular diagnostic pathways. Nuclear medicine, however, has proven promising in the diagnosis of cardiomyopathies/heart failure, and peri- and myocarditis as well as arterial inflammation. This article first outlines the spectrum of cardiotoxic cancer therapies and the potential side effects. This will be complemented by the definition of cardiotoxicity using non-nuclear cardiovascular imaging (echocardiography, CMR) and biomarkers. Available nuclear imaging techniques are then presented and specific suggestions are made for their application and potential role in the diagnosis of cardiotoxicity.
Keywords: Nuclear medicine, Cancer therapy-related cardiotoxicity, Cardio-oncology, PET, SPECT, Chemotherapy, Radiotherapy, Immunotherapy
Preamble
The European Association of Nuclear Medicine (EANM) is a professional non-profit medical association that facilitates communication worldwide among individuals pursuing clinical and research excellence in nuclear medicine. The EANM was founded in 1985. This position paper is intended to assist practitioners in providing appropriate nuclear medicine care for patients. They are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care. The ultimate judgment regarding the propriety of any specific procedure or course of action must be made by medical professionals taking into account the unique circumstances of each case. Thus, there is no implication that an approach differing from this position paper, standing alone, is below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set out in the position paper when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology subsequent to publication of the position paper. The practice of medicine involves not only the science but also the art of dealing with the prevention, diagnosis, alleviation, and treatment of disease. The variety and complexity of human conditions make it impossible to always reach the most appropriate diagnosis or to predict with certainty a particular response to treatment. Therefore, it should be recognized that adherence to this position paper will not ensure an accurate diagnosis or a successful outcome. All that should be expected is that the practitioner will follow a reasonable course of action based on current knowledge, available resources, and the needs of the patient to deliver effective and safe medical care. The sole purpose of this position paper is to assist practitioners in achieving this objective. The aim of this article is to review available imaging modalities in nuclear medicine that have been validated or show promise for the assessment and prevention of cancer therapy-related cardiotoxicity (both systemic treatments and radiotherapy). The background of the respective nuclear imaging techniques will be briefly described, and the existing literature reviewed. For the clinical point of view, eligible patients and responsible therapies that cause cardiotoxicity will be summarized. Furthermore, specific application suggestions for nuclear medicine imaging for assessing cardiotoxicity are given. At this point, we would like to point out that, however, this cannot represent a guideline, since there is still not yet sufficient evidence for some of the nuclear medicine applications discussed and these very promising imaging options still need to be validated further.
Background
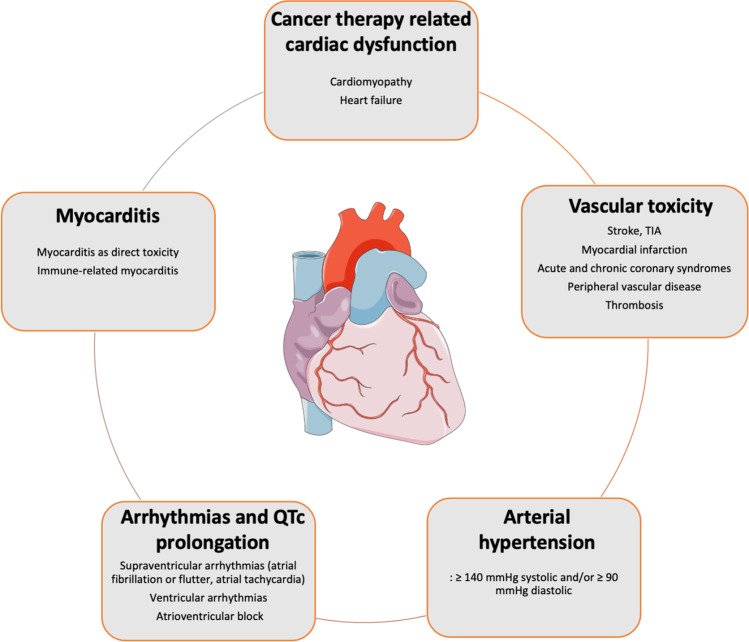
Increased survival rates in cancer patients render cardiovascular side effects of cancer therapies increasingly important for long-term morbidity and mortality. Pre-existing cardiovascular diseases, cardiovascular risk factors, genetic predisposition, previous therapies, and growing patient age are associated with an increasing risk for and may aggravate complications following cancer treatments [1, 2]. This ranges from asymptomatic, reversible changes to fulminant, life-threatening complications including heart failure, acute and chronic coronary syndromes, arrhythmias, valvular heart disease, pericardial disease, myocarditis, and thromboembolic complications (Fig. 1). The relatively new field of cardio-oncology aims to identify mechanisms that lead to cardiovascular diseases through cancer and cancer therapy, to establish appropriate diagnostic measures, and to identify the best possible therapy to reduce the burden of cardiovascular disease in cancer patients [1, 2]. The integration of a cardiologist in multidisciplinary cancer care teams to discuss treatment strategies in oncological patients has proven effective in reducing cardiovascular side effects [3]. The establishment of new, targeted cancer therapeutics may generate cardiovascular toxicity and requires close monitoring of patients. Many side effects of novel therapeutics have recently been characterized regarding incidence, mechanisms, and therapeutic approaches [3, 4]. The term cardiotoxicity refers to the various types of damage to the heart or vessels caused by chemical substances, drugs, or ionizing radiation and stem cell transplantation [5]. Table 1 lists various contemporary cancer treatment regimens and their cardiotoxic spectrum.
Fig. 1.
Main cardiovascular toxicities after cancer therapy
Table 1.
Cancer therapies and their main cardiovascular toxicities (modified according to Rassaf et al. [1], Hermann et al. [109], and Rao et al. [110])
| Therapy type | Therapy subtypes | Cancer therapy-induced dysfunction | Myocarditis/pericarditis | Arrhythmias/QT prolongation | Vascular toxicity | Hypertension |
|---|---|---|---|---|---|---|
| Conventional chemotherapies | Anthracyclines (doxorubicin, epirubicin) | ✓ | ||||
| Alkylating agents (cyclophosphamide, melphalan) | ✓ | ✓ | ✓ | |||
| Antimetabolites (5-FU, capecitabine, cytarabine) | ✓ | ✓cytarabine | ✓ | |||
| Microtubule-bonding agents (paclitaxel) | ✓ | ✓ | ||||
| Platinum based therapy (cisplatin) | ✓ | ✓ | ||||
| Antibiotic (bleomycin) | ✓ | |||||
| Immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide) | ✓ | ✓ | ✓ | |||
| Targeted agents | Proteasome inhibitors (bortezomib, carfilzomib) | ✓ | ✓ | ✓ | ||
| HDAC inhibitors (panobinostat, vorinostat) | ✓ | |||||
| CDK4/CDK6 inhibitors (ribociclib) | ✓ | |||||
| mTOR inhibitors (everolimus) | ✓ | ✓ | ✓ | ✓ | ||
| HER2 inhibitors (pertuzumab, trastuzumab, lapatinib, adotrastuzumabemtansin) | ✓ | |||||
| VEGF inhibitors (bevacizumab, ramucirumab, aflibercept, sunitinib) | ✓ | ✓bevacizumab | ✓ | ✓ | ||
| BCR-ABL1 inhibitors (dasatinib, nilotinib, ponatinib, bosutinib, imatinib) | ✓pontinib | ✓ | ✓ | ✓ | ✓ | |
| BTK inhibitors (ibrutinib, acalabrutinib, zanubrutinib) | ✓ | ✓ibrutinib | ||||
| ALK inhibitors (alectinib, ceritinib, crizotinib, brigatinib, lorlatinib) | ✓ceritinib | ✓ | ✓ | ✓brigatinib | ||
| BRAF inhibitors (dabrafenib, vemurafenib, encorafenib) | ✓ | ✓ | ✓encorafenib | ✓ | ||
| MEK inhibitors (binimetinib, cobimetinib, trametinib) | ✓ | ✓ | ✓binimetinib | ✓ | ||
| Multitarget (sorafenib, sunitinib, pazopanib, vandetanib, lenvatinib, regorafenib, cabozantinib) | ✓ | ✓ | ✓ | ✓ | ||
| EGFR inhibitors (panitumumab, necitumumab) | ✓panitumumab | ✓necitumumab | ✓panitumumab | |||
| Immunotherapies | Immune checkpoint inhibitors (ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, cemiplimab, dostarlimab) | ✓ | ✓ | ✓ | ✓ | |
| CAR-T cell therapy (tisagenlecleucel, axicabtagene cioleucel, lisocabtagene maraleucel, brexucabtagene autoleucel, idecabtagene) | ✓ | ✓ | ✓ | ✓ | ||
| Hormonal therapy | abiraterone, anastrozolem apalutamide, bicalutamide, darolutamide, enzamestane, exemestane, flutamine, letrozole, nilutamide | ✓ | ✓ | |||
| Radiation therapy | ✓ | ✓ | ✓ | ✓ | ||
Arguably, left ventricular dysfunction (cardiomyopathy) and manifest heart failure are the most concerning forms of cardiotoxicity with a profound impact on morbidity and mortality. Heart failure can manifest at an early (e.g., acute or semi-acute) or late phase (e.g., months, years to even decades after potential cardiotoxic treatment). Anthracycline chemotherapy (e.g., doxorubicin, daunorubicin) induces left ventricular dysfunction in a dose-dependent manner [1, 6]. The cardiotoxic-related complication risk is increased by pre-existing cardiovascular risk factors, genetic predisposition, manifest cardiovascular disease, or prior cancer therapy [6]. This cardiotoxic-related complication risk further increases with additional exposure to human epidermal growth factor receptor 2 (HER2) inhibitors, commonly indicated for the treatment of breast cancer [6]. Several other chemotherapeutics are associated with cardiovascular complications: alkylating agents (e.g., cyclophosphamide) can induce severe, early heart failure. Fluoropyrimidines induce endothelial injury that may lead to coronary vasospasms and acute coronary syndromes, with subsequent myocardial ischemia. Various cytotoxic cancer therapies (e.g., cisplatin) in combination with cancer-associated risk factors (i.e., the pro-thrombotic environment associated with cancer) increase arterial and venous thromboembolic complications [6, 7]. The introduction of new, targeted substances has led to new, highly specific forms of cardiovascular toxicity. For example, inhibitors of vascular endothelial growth factor signaling were found to induce arterial hypertension and arterial and venous thromboembolic complications [8]. Various tyrosine kinase inhibitors and serine threonine kinase inhibitors have been associated with left ventricular dysfunction and thromboembolic complications leading to an increased risk of pulmonary embolism [8]. Immune checkpoint inhibitors (ICI) induce severe myocarditis in 1–2% of patients, with a significant risk of major cardiovascular events and mortality [9, 10]. Other complications of the ICI therapies include pericardial disease, acute coronary syndromes, and arrhythmias at higher rates than previously expected [4, 11]. Chimeric antigen receptor (CAR)-T cell therapy is a novel autologous stem cell therapy against hematologic and solid tumors. In the acute phase following transplantation, a severe inflammatory syndrome named cytokine release syndrome (CRS) may occur. CRS but also CAR-T cell therapy may have negative effects on the cardiovascular system leading to acute heart failure and hypotension. This requires further in-depth analysis [5].
As radiotherapy is associated with significant cardiovascular complications, it is important to realize that approximately 35% of cancer patients undergo radiotherapy within 1 year after diagnosis [1]. Radiotherapy-related myocarditis is a known acute complication, but its incidence has decreased due to dose fractioning. Long-term complications of radiotherapy involving the heart (e.g., patients with mediastinal lymphoma, left-sided breast cancer, and lung cancer) include cardiac fibrosis and acceleration of coronary atherosclerosis, and can arise with a latency of several decades after exposure to radiation [12]. These long-term complications include coronary artery disease, valvular disease, and diastolic dysfunction. Radiation during childhood and concomitant exposure to anthracyclines is associated with a significant increased risk for cardiac complications [13].
Cardiac assessment of patients with signs suspicious of cardiotoxic origin
Patients with a predisposition for cardiovascular complications from cancer therapy require standardized cardio-oncological monitoring for an early identification and subsequent treatment of cancer therapy-related side effects [1]. Figure 2 shows the multitude of risk factors for the development of cardiotoxicity during the course of cancer and cancer treatment. The diagnostic workflow includes baseline risk assessment prior to therapy [14], monitoring for acute complications during therapy, and long-term follow-up for late cardiovascular side effects [6]. Recent position papers have therefore provided very specific recommendations for individual patients with a predisposition for cardiovascular complications and distinct cancer therapies.
Fig. 2.
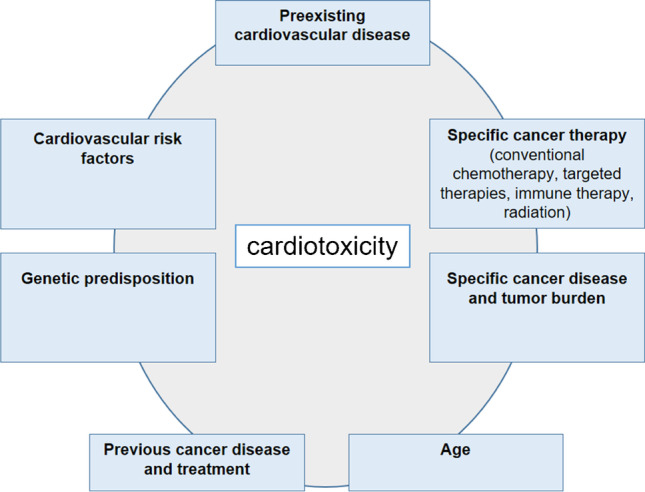
Multiple factors may contribute to an increased risk for cardiotoxicity
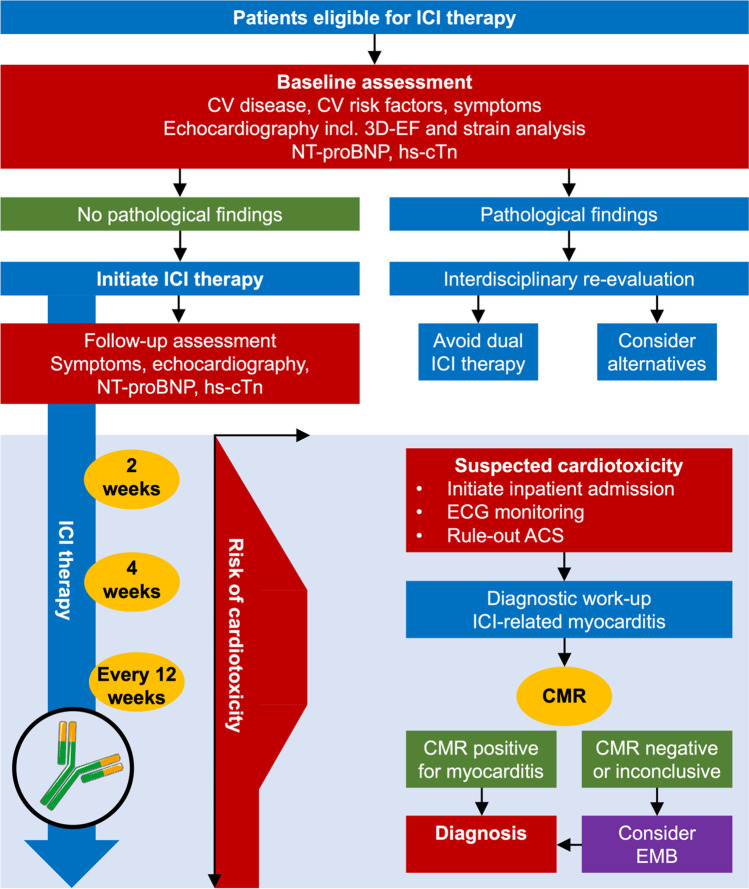
At baseline, the assessment of patients requiring cancer therapy includes the assessment of conventional cardiovascular risk factors and pre-existing cardiovascular disease that may aggravate the risk for cardiovascular toxicities [2, 14]. Pre-therapy assessment includes physical examination, electrocardiogram (ECG), cardiac biomarkers (high-sensitive troponin and N-terminal pro-brain natriuretic peptide (NT-proBNP)), and echocardiography depending on the type of therapy. The cardiovascular risk profile of the planned cancer therapy (including conventional chemotherapy, targeted therapies, immunotherapy, or radiotherapy) is evaluated in the context of the patient’s medical history and clinical findings, and may trigger intensified cardio-oncology monitoring or modification of cancer therapy in patients at high-risk to develop cardiac disease of cardiotoxic origin (particularly in those with pre-existing cardiovascular risk factors and disease). A possible, concrete approach to monitoring using nuclear imaging is discussed in the section “Approach for nuclear imaging assessment in the field of cardiotoxicity.” A proposed workflow for the surveillance of patients receiving immune checkpoint inhibitor therapy is outlined in Fig. 3.
Fig. 3.
Workflow for the surveillance of patients receiving immune checkpoint inhibitor therapy
Cardio-oncological surveillance during therapy depends on the individual patient’s risk and on the applied therapy. A general cardio-oncology visit includes a clinical examination, ECG, cardiac biomarkers, and echocardiography [6]. Of note, echocardiography is the imaging of choice for the surveillance of cancer patients when ultrasound windows are adequate. Modern, targeted therapies may require close monitoring of particular complications, e.g., QTc prolongation, atrial fibrillation, arterial hypertension, or myocarditis. In patients with suspected cardiotoxicity, further diagnostic modalities can be initiated, e.g., cardiac magnetic resonance imaging (CMR), nuclear imaging technologies, or cardiac catheterization [3, 15]. Echocardiography should include an assessment of left ventricular ejection fraction (LVEF), examination of diastolic dysfunction, and strain analysis. These measures in conjunction with cardiac biomarkers allow grading severity of cancer therapy-related cardiac dysfunction (CTRCD) into “mild,” “moderate,” and “severe” (e.g., mild CTRCD is defined as an LVEF ≥ 50% AND new relative decline in global longitudinal strain (GLS) by > 15% from baseline AND/OR new rise in cardiac biomarkers (troponin I/T > 99th percentile, BNP ≥ 35 pg/ml, NT-proBNP ≥ 125 pg/ml, Table 2) [16]. Novel parameters, e.g., for an advanced assessment of right ventricular function, are currently evaluated in clinical trials [17, 18].
Table 2.
Definition of cardiovascular toxicities of cancer therapies according to the International Cardio-Oncology Society (IC-OS) consensus statement (according to Herrmann et al. [16])
| Cardiotoxicity type | Category | Severity | Diagnosis criteria |
|---|---|---|---|
| Cancer therapy-related cardiac dysfunction (CTRCD) | Asymptomatic (with or without additional biomarkers, LVEF values are based on 2D echocardiography) | Mild |
LVEF ≥ 50% AND new relative decline in GLS by > 15% from baseline AND/OR new rise in cardiac biomarkers (troponin I/T > 99th percentile, BNP ≥ 35 pg/ml, NT-proBNP ≥ 125 pg/ml) |
| Moderate |
New LVEF reduction by ≥ 10 percentage points to an LVEF of 40–49% New LVEF reduction by < 10 percentage points to an LVEF of 40–49% AND new relative decline in GLS by > 15% from baseline AND/OR new rise in cardiac biomarkers |
||
| Severe | New LVEF reduction to < 40% | ||
| Symptomatic (with LVEF and supported diagnostic biomarkers) | Mild | Mild HF symptoms, no intensification of therapy required | |
| Moderate | Need for outpatient intensification of diuretic and HF therapy | ||
| Severe | HF hospitalization | ||
| Very severe | Requiring inotropic support, mechanical circulatory support, or consideration for transplantation | ||
| Myocarditis |
Pathohistological diagnosis Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy of cardiac tissue samples or Clinical diagnosis A troponin elevation with 1 major criterion or a troponin elevation with 2 minor criteria after exclusion of acute coronary syndrome or acute infectious myocarditis based on clinical suspicion |
Severe | Hemodynamic instability, heart failure requiring non-invasive or invasive ventilation, complete or high-grade heart block, and/or no significant ventricular arrhythmia |
| Non-severe (clinically significant) | Symptomatic but hemodynamically and electrically stable, may have reduced LVEF, no features of severe disease | ||
|
Major criterion CMR diagnostic for acute myocarditis (modified Lake Louise criteria) Minor criteria •Clinical syndrome •Ventricular arrhythmia and/or new conduction system disease •Decline in cardiac function •Other immune-related adverse events •Suggestive CMR |
Smoldering (subclinical) | Incidentally diagnosed myocarditis without any clinical signs or symptoms | |
| Steroid refractory | Non-resolving or worsening myocarditis (clinical worsening or persistent troponin elevation after exclusion of other etiologies) despite high-dose methylprednisolone | ||
| Vascular toxicity | Asymptomatic | Atherosclerosis |
Coronary artery disease: new corona artery stenosis > 50% on coronary computed tomography angiogram or > 70% on coronary angiogram, or newly abnormal electrocardiogram (ECG), nuclear or echo stress test Peripheral arterial disease: new ankle-brachial index (ABI) value ≤ 0.9 is considered abnormal, with 0.7–0.9 being mildly reduced, 0.4–0.69 moderately reduced, and < 0.4 severely reduced or chance in ABI from baseline by − 0.15 Carotid artery disease: new intima-media thickness (IMT) > 0.9 mm or new plaque on carotid ultrasound, or change in IMT > 0.04/year from baseline |
| Thrombosis |
Venous thrombosis: new characteristic features and Duplex ultrasound, contrast CT, or venogram Arterial thrombosis: new characteristic features on ultrasound or angiogram, or optical coherence tomography |
||
| Abnormal vasoreactivity |
Peripheral: new flow-mediated dilation of the brachial artery (FMD) < 7.1% or reactive hyperemia index (RHI) < 2 on Endo-PAT, or change in FMD or RHI by > 50% from baseline Coronary epicardial: new coronary vasoconstriction (reduction in coronary artery diameter) in response to acetylcholine infusion Coronary microvascular: new < 50% increase in coronary blood flow in response to acetylcholine infusion, or a coronary flow reserve < 2 in response to adenosine |
||
| Symptomatic | Stroke |
2018 AHA/ASA Guidelines for the Early Management of Patients With Ischemic Stroke An Updated Definition of Stroke for the 21st Century Stroke |
|
| Transient ischemic attack | |||
| Myocardial infarction | 4th Universal Definition of MI | ||
| Acute coronary syndromes |
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation |
||
| Chronic coronary syndromes | 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) | ||
| Peripheral arterial disease | 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS) | ||
| Vasospastic angina |
2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) International standardization of diagnostic criteria for vasospastic angina |
||
| Microvascular angina |
2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) International standardization of diagnostic criteria for microvascular angina |
||
| Raynaud’s phenomenon | Meeting the diagnostic criteria of an international consensus panel of recurrent episodes bilateral blanching or tricolor change of the fingers | ||
| Hypertension |
Normal SBP ≤ 130 mmHg And DBP ≤ 80 mmHg |
Treatment threshold for hypertension before, during, and off therapy/cancer survivors |
CVD or ASCVD risk ≥ 10%: ≥ 130 mmHg systolic and/or ≥ 80 mmHg diastolic Otherwise: ≥ 140 mmHg systolic and/or ≥ 90 mmHg diastolic |
| Cancer therapy holding threshold | ≥ 180 mmHg systolic and/or ≥ 110 mmHg diastolic | ||
| Exaggerated hypertensive response | Systolic BP increase > 20 mmHg or mean arterial BP increase > 15 mmHg | ||
| Hypertensive emergency response | Very high BP elevations associated with acute hypertension-mediated organ damage (heart, retina, brain, kidneys, and large arteries), therefore requiring immediate BP reduction to limit extension or promote regression of target organ damage | ||
| QT prolongation and arrhythmias | QTc prolongation | QTcF < 480 ms | Acceptable: continue current treatment |
| QTcF 480–500 ms | Prolonging: proceed with caution; minimize other QT-prolonging medications, replete electrolytes | ||
| QTcF > 500 ms | Prolonged: stop treatment and evaluate. May require dose reduction or alternative therapy | ||
| Arrhythmias | Ventricular arrhythmia |
2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death |
|
| Ventricular tachycardia (VT), including polymorphic VT (torsades de pointes) | |||
| Ventricular fibrillation | |||
| Atrial fibrillation |
2020 ESC Guidelines for Management of Atrial Fibrillation 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation |
||
| Atrial flutter | |||
| Atrial tachycardia |
2019 ESC Guidelines on Supraventricular Tachycardia 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society |
||
| Supraventricular tachycardia | |||
| Sinus tachycardia | |||
| Sinus bradycardia | 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay | ||
| Sick sinus syndrome | |||
| Atrioventricular block first, second and third degree | |||
| Conduction disorder (disease) |
ACC American College of Cardiology, AHA American Heart Association, ASCVD atherosclerotic cardiovascular disease, ASE American Society of Echocardiography, BP blood pressure, CMR cardiac magnetic resonance, CTRCD cancer therapeutics-related cardiac dysfunction, DBP diastolic blood pressure, GLS global longitudinal strain, HF heart failure, LVEF left ventricular ejection fraction, QTcF QT interval corrected by the Fridericia formula, SBP systolic blood pressure
Moreover, other imaging modalities including CMR have been also proposed for the investigation of cancer therapy-related cardiotoxicity [19]. For anthracycline therapy, the European Society of Medical Oncologists (ESMO) has proposed a guideline which incorporates high-sensitive troponin serial measurements, and recommended echocardiography as the baseline imaging tool because it is widely available, is inexpensive, and does not require ionizing radiation [20]. Indeed, echocardiography is a widely available, low-cost technique. Nevertheless, it is a highly observer-dependent technique, and the image quality is influenced by the acoustic windows obtained. Therefore, echocardiography may not be able to capture mild changes in LVEF when evaluating cancer therapy-related cardiotoxic effects [21]. However, these may be detected with newer methods such as GLS in combination with cardiac troponins [2].
Given its accuracy and reproducibility in LVEF determination, CMR is considered by the American College of Cardiology/American Heart Association (ACC/AHA) as a method to screen for cardiotoxicity in patients undergoing cancer therapy [21]. Moreover, CMR can depict structural changes in the myocardium, including signs of edema and inflammation, prior to the LV dysfunction [22]. On the other hand, CMR disadvantages include the lower availability, higher cost, adverse reaction to gadolinium contrast agents and contraindications related to kidney failure, and the presence of metal devices (e.g., pacemakers, implantable cardiac defibrillators), as well as the lower tolerance by patients due to longer scanning times and/or claustrophobia. In these cases, nuclear imaging can be considered as a second-line imaging approach in patients with poor echogenicity or image quality [20].
The long-term follow-up of patients after completion of cancer treatment is driven by the baseline risk factors and therapy-related risk factors. Patients receiving chest radiotherapy are at high risk for late complications, particularly new-onset coronary artery disease, and require long-term follow-up [1, 2, 23]. In case of suspected cardiotoxicity, patients need to undergo specific diagnostic tests. In case of suspected coronary artery disease, patients may need additional cardiac imaging, including cardiac catheterization and/or nuclear cardiology techniques [12]. In the following sections, the different imaging approaches in nuclear medicine will be discussed for detecting cardiovascular complications related to cancer therapy in oncological patients particularly regarding its potential advantages over echocardiography and CMR.
Nuclear imaging of therapy-related cardiotoxicity in oncology patients
There are a number of nuclear medicine imaging modalities to detect cardiotoxic damage. The following paragraphs discuss the most commonly used imaging modalities or those with the highest potential from the authors’ perspective. Table 3 provides an overview of the nuclear medicine methods currently used clinically to investigate the occurrence of cardiotoxicity. The majority of the techniques evolve around the assessment of left ventricular function. Table 4 summarizes the radiation exposure from the radiotracers most commonly used in clinical settings to image cardiotoxicity.
Table 3.
Overview of clinically used nuclear medicine methods for monitoring for the occurrence of cardiotoxicity
| Modality | Parameters | Advantages | Disadvantages |
|---|---|---|---|
| ERNA |
-Ventricular volumes -Ejection fractions -Systolic and diastolic function -Wall motion -Phase analysis |
-Highly standardized -Excellent reproducibility -Observer independent -Virtually no restrictions regarding patient selection (e.g., obesity, cardiac devices) -Evaluation of both right and left ventricles |
-No assessment of myocardial perfusion |
| MPI (SPECT) |
-Left ventricular volumes -Left ventricular ejection fraction -Systolic and diastolic function -Wall motion -Phase analysis -Myocardial perfusion (qualitatively) |
-Highly standardized -Good reproducibility -Virtually no restrictions regarding patient selection (e.g., obesity, cardiac devices) |
-No assessment of right ventricle |
| MPI (PET) |
-Ventricular volumes -Ejection fractions -Systolic and diastolic function -Wall motion -Myocardial perfusion (quantitatively) |
-Highly standardized -Good reproducibility -Quantification (MBF, MFR) -Assessment of epicardial and microvascular disease -Detection of even subtle changes |
-Limited availability -Relatively expensive |
| Innervation imaging (e.g., MIBG SPECT, HED PET) |
-Distribution and integrity of sympathetic innervation -Sympathetic tone (tracer washout) -Novel tracers, e.g., receptor density |
-Changes in innervation appear before structural or functional changes of the heart | -Limited data |
| Glucose metabolism (2-[18F]FDG PET) of the myocardium |
-Regional changes in 2-[18F]FDG uptake -Quantitative parameters (SUV) |
-Changes appear before structural or functional changes of the heart -Patients often receive the examination anyways for their oncological disease |
-Many influential factors on 2-[18F]FDG uptake (not specific for cardiotoxicity) -Limited data |
ERNA equilibrium radionuclide angiography, 2-[18F]FDG 18-fluoro-deoxyglucose, HED [11C]C-metahydroxyephedrine, MBF myocardial blood flow, MFR myocardial flow reserve, MIBG [123I]-metaiodobenzylguanidine, MPI myocardial perfusion imaging, PET positron emission tomography, SPECT single-photon emission computed tomography
Table 4.
Radiation exposure from the most commonly used clinical nuclear imaging procedures
| Procedure | Radiotracer | Effective dose per MBq | Recommended activity | Resulting effective dose | References |
|---|---|---|---|---|---|
| ERNA | [99mTc]Tc-RBCs | 0.0047 mSv/MBq | 555–1110 MBq | 2.6–5.2 mSv | [24] |
| SPECT MPI | [99mTc]Tc-labeled perfusion tracers |
[99mTc]Tc-tetrofosmin (stress and rest): 0.0058 and 0.0063 mSv/MBq, respectively [99mTc]Tc-sestamibi (stress and rest): 0.0066 and 0.0070 mSv/MBq, respectively |
Depending on protocol (1-day vs. 2-day protocol, stress only, CZT vs. conventional camera etc.): 150–400 MBq (stress), 180–1200 MBq (rest) | 0.9–11.2 mSv | [29, 111] |
| PET MPI | [15O]H2O | 0.0011 mSv/MBq | 2 × 400 MBq (stress/rest) | 0.8 mSv | [36, 112] |
| 82Rb | 0.001 mSv/MBq | 2 × 10 MBq/kg (stress/rest) | 1.5 mSv | [36, 112, 113] | |
| [13N]NH3 | 0.0027 mSv/MBq | 2 × 400 MBq (stress/rest) | 1.8 mSv | [36, 112, 113] | |
| Innervation SPECT | [123I]mIBG | 0.037 mSv/MBq | 111–370 MBq | 4.1 – 13.7 mSv | [114] |
| Viability/inflammation PET | 2-[18F]FDG | 0.019 mSv/MBq | 185–555 MBq | 3.5–10.5 mSv | [112, 115, 116] |
| Vascular toxicity PET | 2-[18F]FDG | 0.019 mSv/MBq | 3–4 MBq/kg | 4–7.5 mSv | [94, 112] |
[99mTc]Tc-RBCs, [99mTc]Tc-labeled red blood cells; [123I]mIBG, [123I]-metaiodobenzylguanidine; [18F]FDG, 2-[18F]fluoro-2-deoxy-D-glucose; ERNA, equilibrium radionuclide angiography; MPI, myocardial perfusion imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography
Two/three-dimensional radionuclide angiography
Nuclear medicine techniques have the advantages of being highly examiner-independent, very reproducible, and feasible in almost all patients regardless of various patient conditions such as constitution or implanted devices which often lead to inconclusive results in other imaging modalities or when echocardiography shows poor quality and/or CMR is e.g. not-tolerated/not available. Equilibrium radionuclide angiography (ERNA) can provide valuable information regarding global and regional ventricular function (at rest and/or during stress), as well as cardiac chamber morphology. In particular, ventricular volumes, EF, ventricular wall motion, and diastolic function can be obtained and phase analysis can be performed. The most often applied technique involves the 99mTc radiolabeling of patient’s red blood cells (RBCs) [24]. For autologous RBC labeling, [99mTc]Tc-erythrocytes represent the most commonly used radiotracer. There are three methods for RBC labeling (in vivo technique, mixed in vivo/in vitro technique, in vitro technique), with different labeling efficiency (60–70%, 90%, >90%, respectively) [24]. ECG-gated blood pool planar acquisition constitutes the routine procedure in clinical practice [24]. Single-photon emission computed tomography equilibrium radionuclide angiography (SPECT-ERNA) can also be performed and allows for the assessment of right ventricular function. Cadmium-zinc-telluride (CZT) cameras allow significant reduction of the acquisition time for SPECT-ERNA, improved spatial resolution, and lower radiation exposure of patients [24, 25].
ERNA is a useful technique for the investigation of therapy-related cardiotoxicity, which represents one of the main indications for the examination. ERNA has an excellent reproducibility (with inter- and intra-observer variability of < 5%) for the quantification of LVEF [26]. Therefore, ERNA is often performed serially in order to detect left ventricular dysfunction as an early sign of cardiotoxicity. In addition to LVEF, SPECT-ERNA also allows quantification of RVEF. To date, there are no conclusive data suggesting an additional benefit of quantifying RVEF compared with LVEF in terms of early detection of cardiotoxicity.
One very good example for the use of ERNA is cardiotoxicity from anthracyclines as e.g. used in breast cancer and hematological diseases. An algorithm for LVEF monitoring using ERNA in these patients has previously been suggested (see Table 5) [27]. Similarly, trastuzumab, a humanized monoclonal antibody, acts against the HER2/neu receptor and is known to cause cardiac dysfunction in some patients [21]. There are several protocols for the evaluation of cardiotoxicity in patients undergoing trastuzumab therapy, incorporating a baseline assessment of LVEF and subsequent serial measurements.
Table 5.
Guideline for the initiation, monitoring, and discontinuation of anthracycline chemotherapy based on the measurements of the left ventricular ejection fraction (LVEF) using equilibrium radionuclide angiography (ERNA) [27]
| Baseline ERNA | Treatment initiation | Serial monitoring (with respect to chemotherapy) | Treatment discontinuation |
|---|---|---|---|
| LVEF ≥ 50% | Yes |
•At 250–300 mg/m2 dose •At 400 mg/m2 dose if high risk* •At 450 mg/m2 dose •Prior to each dose > 450 mg/m2 |
If LVEF decreases ≥ 10% (EF units) from baseline and reaches < 50% |
| LVEF 30–50% | Yes | •Prior to each subsequent dose | If LVEF decreases ≥ 10% (EF units) from baseline, or reaches ≤ 30% |
| LVEF ≤ 30% | No | –/– | –/– |
*High-risk features: cyclophosphamide therapy, heart disease, mediastinal radiation, and/or abnormal electrocardiogram
Since cancer therapy-related cardiotoxic effects can be regarded as a life-threatening complication of an effective treatment, it is crucial to identify these patients, in order to manage the complications, or (ideally) intervene at the stage of subclinical toxicity [28]. Despite the wide use of echocardiographic techniques in this field, ERNA can offer valuable information, particularly in patients with borderline LV dysfunction, or a need for precise LVEF quantification.
Myocardial perfusion imaging
SPECT
Among different modalities to evaluate LVEF, gated myocardial perfusion imaging (MPI) offers the great advantage to evaluate in a single-session LV function and perfusion [29].
The interest in nuclear imaging procedures, particularly in MPI, to assess and monitor cardiotoxicity or to predict cardiovascular complications in cancer patients has recently increased [30, 31, 32, 33, 34]. The majority of data refers to pre-therapeutic cardiac risk assessment [30, 31] and it has been demonstrated that SPECT MPI provides incremental information over clinical risk factors in predicting increased cardiovascular morbidity and death over a 3-year follow-up period in a population of different cancer types regardless of whether chemotherapy has already been initiated [32]. It has previously been demonstrated [33] that radiotherapy-induced perfusion defects on cardiac SPECT scans initially may appear and persist 3 to 6 years post-radiotherapy in a high percentage of patients even in absence of regional wall motion abnormalities or LV function impairments. Furthermore, more severe MPI abnormalities could be detected in breast cancer patients after postoperative radiotherapy as compared to a control group [34].
As stated above, use of the recently introduced CZT cameras allows the reduction of injected activities [25, 35]. The concordance of data obtained by Anger camera and CZT method with a 70% reduction in injected dose have been recently assessed in a population of oncological patients under monitoring due to potential cardio-toxic chemotherapy [25].
SPECT MPI offers a number of advantages over echocardiography and CMR [21]. SPECT MPI allows to examine virtually every patient regardless of difficult examination conditions (e.g., obesity), presence of metal devices, or other diseases (such as kidney failure). Also, SPECT MPI has a very low intra- and interobserver variability, allows accurate assessment of regional and global wall motion, phase analysis, and ventricular volumes in a single examination without the examination protocol having to be extended and its value is validated by studies involving thousands of patients. Compared to CMR imaging in particular, SPECT MPI has the advantage of lower costs and broader availability. Disadvantages of SPECT MPI are radiation exposure of the patient, limited ability to detect structural changes (e.g., small scars, pericardial effusion, or valvular heart disease), and the inability to investigate the right ventricle. However, as mentioned above, SPECT MPI is a very valuable method if echocardiography or CMR cannot be performed, are not available, or if findings are unclear.
PET—absolute quantification of myocardial blood flow and flow reserve
Perfusion PET can be used to evaluate both the epicardial vessels and the microvascular circulation [36], the impairment of which seems to be a likely consequence of cancer therapy and in particular radiotherapy [23, 37] and may proceed cardiomyopathy and heart failure.
Direct experiences about the use of perfusion PET in the setting of cardiotoxicity assessment during cancer therapy are very limited. Perfusion PET was mainly used in small patient groups to assess whether changes in myocardial blood flow (MBF) or myocardial flow reserve (MFR) can be demonstrated in relationship with potentially cardiotoxic treatments.
In a recent study comparing MBF and MFR in a small group of women with a history of prior radiation therapy for breast cancer, no difference was found between the anterior and the posterior wall. The authors conclude that there are no direct signs of severe damage in the left anterior descending artery territory, which in theory is more exposed to radiation effects than the inferior wall [38]. Mean MFR values slightly inferior to the normal reference were observed, and since there are no major increases in the coronary artery calcium score, epicardial stenoses are unlikely and a beginning microvascular impairment could be suspected. Nevertheless, this conclusion appears questionable because a major determinant of the reduced MFR was high resting MBF values [38]. Through comparison of rest and stress [15O]H2O PET pre- versus post-radiation (2 and 8 months) therapy, a decrease in stress MBF in the majority of cases could be assessed, both considering the global values and the left anterior descending segments. Remarkably, none of the patients had a stress MBF below the diagnostic threshold for coronary artery disease [39]. In one study, rest and stress 82Rb PET in lymphoma patients before and after the first cycle of doxorubicin therapy were performed. While resting MBF remained unchanged, there was a non-significant decrease in stress MBF and a significantly lower MFR [40]. Furthermore, the authors identified a subgroup of patients with a drop in MFR of more than 20%. The authors speculated that these were patients with a low cardiotoxic threshold, supported only by older age but not by other factors [40].
In conclusion, cardiac perfusion PET may be applied for the effective detection of myocardial ischemia in the context of coronary artery disease in patients candidate to potentially cardiotoxic cancer treatments or as a late sequelae after therapy, to monitor MBF or MFR and cardiotoxic therapy or to explore mechanisms of cardiotoxicity [41].
Imaging of sympathetic innervation
The current gold standard to evaluate cardiac function in relation to cardiotoxicity due to cancer therapy is the assessment of “systolic” LVEF [2]. However, LVEF will only decrease after a critical mass of myocardial tissue has been damaged [42]. LVEF and early myocardial damage after systemic therapy correlate only weakly, as verified by endomyocardial biopsy [43, 44]. The combination of systemic therapy with radiotherapy may even worsen the burden of cardiotoxicity and has been a topic of extensive research, but it remains a major challenge to identify at-risk patients non-invasively [45]. The compensatory reserve of the myocardium enables sufficient ventricular output, even when structural damage to the myocytes started. Thus, LVEF may underestimate actual cardiac injury [42, 46]. A non-invasive approach such as cardiac innervation imaging is preferable, which accurately identifies cardiotoxicity at a subclinical stage, before decrease in LVEF occurs. Sympathetic nervous innervation imaging of the heart with PET or SPECT is thus a promising tool allowing an evaluation of the disturbed cardiac conducting innervation system. [123I]-Metaiodobenzylguanidine ([123I]mIBG) is a radiolabeled guanethidine analog, which is taken up, concentrated, and stored in the presynaptic nerve terminals of the sympathetic nervous system in a manner similar to norepinephrine. The role of the dysfunctional autonomic nervous system regulation in heart failure is well established and myocardial sympathetic imaging with [123I]mIBG now has a range of proven applications in heart failure patients [47, 48].
Functional and structural injury to myocardial adrenergic neurons may be part of the pathophysiology of cancer therapy-induced cardiotoxicity [49, 50], and assessment of the adrenergic nervous system function of the heart may therefore represent a possible tool for detection of subclinical cardiotoxicity. Despite promising results, [123I]mIBG scintigraphy has not yet found its clinical place in the early identification and monitoring of cancer therapy‐induced cardiotoxicity.
Also the role of radiotherapy in the risk of long-term cardiac disease following childhood cancer treatment is evaluated in the association with systemic therapy [51, 52]. One study [52] evaluated the long-term risk of cardiac pathology following radiotherapy and anthracycline for a childhood cancer using [123I]mIBG scintigraphy in 447 subjects. This study strongly emphasizes the need to limit heart irradiation during radiotherapy, particularly for patients with adriamycin treatment.
The fate of [123I]mIBG imaging in the setting of cardiotoxicity will depend on further investigation in prospective clinical trials, including the application of high sensitive CZT cameras.
Although PET imaging offers the advantages of being even more sensitive and the possibility of absolute quantification, there is as yet no data with established tracers such as [11C]metahydroxyephedrine ([11C]mHED) or other novel promising PET tracers including the 18F-labeled variant [18F]flubrobenguane (also known as [18F]LMI1195), a novel cardiac neuronal imaging agent with properties similar to [123I]mIBG [53], or the ligand CGP12177 for β-receptor density assessment [54].
PET/MR hybrid imaging may be of interest, allowing combined regional molecular and functional LV imaging, that could potentially detect subtle myocardial and molecular signal changes as a very early sign of cardiotoxicity [55].
Glucose metabolism
PET allows to image changes in cellular metabolism with high sensitivity and appears therefore well suited to identify earlier stages of cardiomyocyte toxicity, before irreversible myocardial damage develops. It must be distinguished here that, on the one hand, cardiotoxic therapy may cause abnormalities in myocardial glucose metabolism, i.e., in the viability assessment of the heart. On the other hand, a myocardial inflammatory reaction may occur as a result of cancer therapy (e.g., immune checkpoint inhibitor-associated myocarditis [56]), which can be sensitively detected by 2-[18F]fluoro-2-deoxy-D-glucose (2-[18F]FDG) PET when the patient is adequately prepared (i.e., suppression of myocardial glucose metabolism).
Anthracyclines interfere with normal mitochondrial oxidative metabolism causing a shift from lipids to glucose metabolism for energy supply [57]. 2-[18F]FDG is taken up by cardiomyocytes through GLUT receptors and then phosphorylated by hexokinase resulting in its intracellular retention. 2-[18F]FDG is therefore well suited to identify the metabolic shift in the myocardium in response to anthracycline treatment. Several retrospective studies [58, 59] have found high 2-[18F]FDG uptake in the myocardium of patients treated by anthracyclines on PET, which was associated with an increased incidence of LV dysfunction during follow-up. The use of 2-[18F]FDG PET for the early identification of cardiotoxicity appears an attractive imaging approach as patients often undergo sequential 2-[18F]FDG PET examinations during the course of their oncological disease. However, increased 2-[18F]FDG uptake is not specific for anthracycline-induced cardiotoxicity as it is also observed in ischemic myocardium or in patients with elevated circulating insulin levels following sugar consumption. Further carefully conducted studies are required to confirm whether the quantification of myocardial 2-[18F]FDG uptake could be a robust early-stage predictor of cardiotoxicity-related LV dysfunction. These studies should be conducted with adequate suppression of cardiac glucose metabolism, as this increases the interpretability of 2-[18F]FDG PET/CT studies. Although no standardized guidelines are available, prolonged fasting (beyond 12 h), carbohydrate-restricted diets, fatty meals, and heparin loading have been proposed [60]. A protocol involving high-fat, low-carbohydrate diet on the day prior to scanning followed by prolonged fasting over 12 h is relatively easy to put in place in clinical routine. This can be followed by unfractionated heparin (50 UI/kg) being injected 15 min before the 2-[18F]FDG injection. The aim of this protocol is to decrease basal insulin and blood glucose levels and to increase blood free fatty acid (FFA) levels, which shift myocardial energy consumption away from glucose toward FFA. Though the bleeding risk is very low if used properly as a single dose, heparin should be avoided in patients who are already receiving anticoagulant therapy or have a history of bleeding disorders and attention should be paid to tumors at risk of bleeding and brain metastases.
Assessment of myocardial damage: [99mTc]Tc-annexin/[111In]In-antimyosin, fibroblast activation
In addition to the assessment of indirect markers of myocardial damage such as perfusion abnormalities, wall motion abnormalities, or a reduction in LVEF, nuclear medicine techniques are available for the direct assessment of myocardial damage. In this section, radiotracers that target apoptosis or necrosis will be addressed. One approach to visualize myocardial damage is to use the tracer [111In]In-antimyosin, a murine monoclonal antimyosin Fab antibody fragment. This tracer visualizes cellular necrosis because it binds to intracellular myosin and only if the sarcolemma is damaged. This tracer has been used in several studies to investigate cancer therapy-induced cardiotoxicity [61, 62, 63, 64]. For example dose-dependent cardiotoxicity of epirubicin was demonstrated [65], and more intense myocardial uptake, in terms of a higher degree of cardiotoxicity, was related to a greater impairment of LV function [65]. However, in another study examining the long-term effects of anthracyclines, both patients who had recovery of LVEF and patients with continued impaired LVEF displayed cardiac tracer accumulation [66]. Accordingly, this study questioned the value of [111In]In-antimyosin for prognostic assessment regarding the development of cardiotoxicity-related heart failure. Only limited data are available for imaging radiotherapy-induced cardiotoxicity using this tracer [67].
Phosphatidylserine is a phospholipid that is exposed on the membrane of cells undergoing apoptosis, which can be targeted using [99mTc]Tc-annexin V, a tracer that has not yet been widely used. In a doxorubicin-induced cardiotoxicity model in the rat, the feasibility of imaging with this tracer was demonstrated [68] and the degree of cardiotoxicity in histopathology was related to tracer accumulation [69, 70].
A very recent development in the field is the molecular imaging of activated fibroblasts. Activation of fibroblasts occurs in many cardiac repair and remodeling processes, such as after myocardial infarction, heart failure, and by the administration of cardiotoxic agents. Recently, 68 Ga-labeled radiotracers for targeting activated fibroblasts have been developed and have demonstrated extensive fibroblast activation in patients with acute myocardial infarction [71]. Those are quinoline-based radiotracers that function as fibroblast activation protein inhibitors (FAPIs). Initial reports indicate that fibroblast activation as a sign of damage to the myocardium by cardiotoxic agents can be detected using this tracer [72]. However, there is preliminary work indicating that fibroblast activation in the myocardium detected by PET is also associated with pre-existing cardiovascular disease or risk factors and thus is not specific for cancer therapy-induced cardiotoxic injury [73, 74].
2-[18F]FDG PET for assessment of vascular toxicity
Whereas cancer therapy-related cardiotoxicity remain the prime concern in patients suffering from cancer, the increased risk of vascular disease already posed by the cancer itself is further increased by those therapies. Vascular toxicities are the second most common cause of death in patients with cancer undergoing outpatient therapy [75].
There is a broad range of cancer therapies with a vascular toxicity risk profile, and their effects on the vessel and the related clinical spectrum are quite diverse [75]. Nowadays, sufficient data are available for conventional or targeted chemotherapy-related vascular side effects, respectively. Furthermore, with growing experience, treatment-related thromboembolism, acute vasospasm, and arterial and pulmonary hypertension as well as emerging or progressing atherosclerosis with angina and even acute myocardial infarction and stroke were recognized. It is beyond the scope of this article to illustrate the cardiovascular side effects of each respective chemotherapy protocol. However, almost all of the well-accepted conventional (like alkylating agents, antimetabolites, immunomodulatory drugs) or targeted chemotherapeutics (like proteasome inhibitors, monoclonal antibodies, VEGFR fusion molecules or multitarget kinase inhibitors) carry a relevant individual risk profile for undesired and harmful effects mentioned above [75]. Similarly, although to a lesser degree, radiotherapy-related vascular side effects have been reported, ranging from coronary vasospasm and variant angina in patients suffering from Hodgkin’s lymphoma to adverse effects of radiation on endothelial cell function and viability potentially promoting atherosclerosis [75, 76, 77, 78, 79, 80].
The timeline, in which the vascular sequelae emerge after termination of cancer therapy, is rather heterogeneous and depends on the distinct (pathological) vascular process caused by the oncological treatment. Whereas acute vasospasm frequently emerges within days to weeks and is very likely reversible, development of acute thrombosis frequently lasts weeks to months. Accelerated atherosclerosis as another side effect of cancer treatment has to be expected months to years after the therapy with a very low likelihood of reversibility.
Most of the named vascular side effects of cancer therapies can currently not be assessed by means of nuclear medicine. However, inflammatory changes of the arterial wall in the context of atherosclerosis can reliably be identified by different PET tracers, with by far the most profound experience obtained using 2-[18F]FDG. In the beginning of the twenty-first century, the first prospective milestone study in 2-[18F]FDG PET imaging of atherosclerosis was published followed by numerous publications on experimental, clinical, and methodological aspects of this topic [81, 82, 83, 84, 85, 86, 87, 88, 89]. Here, not only the correlation between 2-[18F]FDG uptake and histologically proven inflammatory changes in arterial specimen, but also with e.g. clinical cardiovascular risk factors as well as with prognostic parameters could be identified [82, 83, 84, 86, 87, 90, 91, 92, 93].
The 2016 position paper of the European Association of Nuclear Medicine on 2-[18F]FDG PET imaging of atherosclerosis [94] provides information and recommendations on methodological aspects of this non-invasive imaging approach.
To date, there are limited data on the impact of cancer therapy on vascular inflammation. In a small retrospective study of 10 patients who received radiotherapy for lymphoma, FDG PETs performed 2–7 years after therapy were analyzed. Eighty percent of patients showed higher FDG uptake on the irradiated side compared to the opposite side potentially indicating increased inflammation [95]. In a prospective study of 22 patients with head and neck cancer, FDG PET was performed before and 3 months after radiation therapy. Eighty-two percent of the patients received concurrent chemotherapy. The authors found increased FDG uptake in the carotids after cancer therapy indicating vascular inflammation [96]. In a retrospective study of 52 patients receiving anthracycline-based chemotherapy for Hodgkin lymphoma, FDG PETs were analyzed before and after chemotherapy. None of the arterial segments studied showed increased vascular FDG uptake [97]. There are conflicting data on the effect of ICI therapy on vascular inflammation in humans. While one group described increased arterial FDG uptake in both lymphoma patients [98] and melanoma patients [99] after ICI therapy in small retrospective studies, another group could not reproduce this in their melanoma patients [100]. The latter work also examined the effect of ICI therapy on arterial FDG uptake in an atherosclerotic mouse model. Again, there was no difference in vascular FDG accumulation in these animals, while at the same time, a marked increase in cytotoxic CD8+ T cells was detected in the inflammatory plaques after ICI therapy and accelerated atherosclerosis was described.
In summary, there are currently limited data on the detection of cardiotoxic effects on vessels after cancer therapy and further studies are needed to assess the utility of this imaging modality.
Future perspectives
Promising imaging approaches for future studies
As outlined in the previous sections, there are already several imaging approaches to detect cardiotoxic cardiovascular disease. Apart from those already clinically evaluated, there are other sets of radiotracers with great potential for the detection of cardiac damage caused by cardiotoxic therapies (see Table 6). In addition to the 68Ga-labeled FAPI tracer mentioned above for the detection of activated fibroblasts as a sign of cardiac damage [72], an 18F-labeled tracer for imaging sympathetic innervation is available ([18F]F-flubrobenguane) [101], the use of which is expected to be approved in the near future. However, data on the detection of cardiotoxicity are still lacking. Furthermore, there are promising tracers for imaging activated macrophages (e.g., 68Ga-labeled somatostatin receptor agonists) [102, 103] and for imaging neovascularization, with the most common tracer targeting the integrin αVβ3 [104]. Most recently, initial preclinical work attempted to use radiotracers for imaging cardiotoxicity via detection of mitochondrial damage [105, 106] or reactive oxygen species (ROS) formation [107]. Research projects assessing these promising tracers are urgently warranted.
Table 6.
Potential targets and promising radiotracers for cardio-oncology in the future
| Target | Involved processes | Tracers | Potential applications | Ref |
|---|---|---|---|---|
| Somatostatin receptors (SSTR) | Overexpression on macrophages | [68Ga]Ga-DOTATOC, [68Ga]Ga-DOTATATE, [68Ga]Ga-DOTANOC | Inflammatory processes (e.g., myocarditis, pericarditis, vasculitis) | [102, 103] |
| αvβ3 integrin receptor | Cell adhesion, neoangiogenesis, overexpressed on macrophages | [18F]F-galacto-RGD, [68Ga]Ga-PRGD2, [18F]F-fluciclatide | Neoangiogenesis, inflammatory processes | [104] |
| Fibroblast activation protein (FAP) | Activation of fibroblasts | Various 68Ga-labeled inhibitors of FAP (e.g., [68Ga]Ga-FAPI-04, [68Ga]Ga-FAPI-46) | Myocardial damage | [73] |
| Norepinephrine transporter (NET) | Sympathetic innervation of the heart | [18F]F-flubrobenguane | Denervation as early sign of damage to the heart | [101] |
| Mitochondrial membrane potential | Dysfunction of mitochondrial membrane | [18F]F-MitoPhos, [68Ga]Ga-Galmydar | Mitochondrial dysfunction as early sign of cardiotoxicity | [105, 106] |
| Reactive oxygen species (ROS) | Superoxide production in processes such as myocardial apoptosis or necrosis | [18F]F-DHMT | ROS generation as early sign of cardiotoxicity | (107) |
Approach for nuclear imaging assessment in the field of cardiotoxicity
Figure 4 shows a suggested approach to nuclear medicine imaging in the field of cardiotoxicity and with regard to normal values, we recommend referring to Tables 2 and 5, respectively. From the armamentarium of nuclear imaging, ERNA or SPECT MPI are particularly suitable for the investigation of pump function. If obstructive coronary artery disease is suspected, it can be evaluated by SPECT or PET MPI. This pretherapeutic assessment is used to adjust the dose of the planned therapy in case of pre-existing cardiac disease, to consider an alternative therapy regimen, or to refrain from cardiotoxic therapy completely. Furthermore, ERNA and SPECT MPI are alternative methods if echocardiography or CMR are not feasible or available. After initiation of therapy, regular assessment is necessary to detect cardiotoxicity at an early stage. In addition to reassessment of pump function by ERNA or SPECT MPI, PET MPI is particularly useful in cases of suspected microvascular disease and a new onset of this condition or a significant decrease in MBF or MFR with cancer therapy is indicative of cardiotoxicity and should lead to modification of therapy. 2-[18F]FDG PET is useful to detect altered metabolism of the myocardium or to detect cardiac inflammatory effects. Imaging of damage of myocardial innervation may also be considered as an early sign of cardiotoxicity. In particular, [68Ga]Ga-FAPI PET appears to be a potential promising application for the future, although this remains to be evaluated in studies. These very sensitive methods should be used when CMR, echocardiography, ERNA, and SPECT show no abnormalities or unclear findings and cardiotoxicity is still suspected. If PET MPI shows a decrease in MBF or MFR, or a new onset of microvascular dysfunction, this is a sign of cardiotoxicity and should lead to a modification of the therapeutic regimen. Similarly, a new abnormality on FDG PET of the heart should lead to a modification of the therapeutic regimen (dose reduction, change of therapeutic regimen, or discontinuation of therapy). After completion of therapy, reassessment of cardiac function by ERNA or SPECT MPI is reasonable to detect myocardial damage that has occurred if echocardiography or CMR are not feasible or available. Furthermore, the imaging method selected before the initiation of therapy should be chosen for follow-up for better comparability.
Fig. 4.
Suggested approach to nuclear medicine imaging in the field of cardiotoxicity
PET MPI and 2-[18F]FDG PET also seem to be useful as further diagnostics. 2-[18F]FDG PET can also be used to determine whether increased inflammatory activity in the vessels has occurred as a result of cardiotoxic therapy; however, this represents a future application, as only little data is available and the value should be further investigated. [68Ga]Ga-FAPI PET appears to be a promising future imaging modality to detect cardiotoxic cardiac injury at a very early stage. Also [68Ga]Ga-FAPI PET allows to distinguish between active fibrosis and mature scars. Therefore, [68Ga]Ga-FAPI PET can be considered when conventional imaging modalities have been unremarkable or non-conclusive and cardiotoxicity remains suspected. If cardiotoxic injury is detected, further cardiologic evaluation and initiation of therapy should follow.
Importance of interdisciplinary cooperation
The topic of cardiotoxicity is complex. The diagnosis and therapy of a cardiovascular disease due to a previous cancer therapy therefore requires a close interdisciplinary approach including cardiologists, oncologists, radiation therapists, and imaging disciplines (radiology and nuclear medicine), among others. Only this interplay of disciplines will provide optimal care for cancer patients or cancer survivors and prevent secondary diseases of the heart. This is also the reason why centers are creating innovative units as an interface between different specialties to meet this increased need for interdisciplinarity. One example of this is the clinical unit of “Nuclear Cardiology” at the University Hospital Essen [108]. Last but not least, we agree with the recommendation of international societies that the formation of the so-called cardio-oncology teams is an important pre-requisite to monitor not only current tumor patients but also recovered patients with the risk of cardiovascular complications [2]. Such cardio-oncology teams are also necessary for early detection of cardiovascular complications of novel therapies.
Conclusion
Cardio-oncology represents an important new field that should be covered by multiple specialties as part of interdisciplinary teams. Nuclear medicine can provide important insights in the early detection of impending cardiotoxicity, assist in the monitoring of cardiotoxic therapy, and may also be used as a tracking tool in the investigation of cardiotoxicity of novel therapies. While nuclear cardiology has many promising techniques available, some of which are already being used in routine clinical practice, further studies are needed to investigate the full value of nuclear medicine techniques in the care of patients treated with cardiotoxic agents.
Liability statement
This article summarizes the views of the EANM Cardiovascular Committee and the EANM Oncology & Theranostics Committee. It reflects recommendations for which the EANM cannot be held responsible. The recommendations should be taken into context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
Acknowledgements
The article was brought to the attention of the relevant EANM Committees and the National Societies of Nuclear Medicine. The comments and suggestions from the EANM Radiopharmacy and Technologists Committee are highly appreciated and have been considered for this guideline.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Cardiology.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/21/2022
A Correction to this paper has been published: 10.1007/s00259-022-06039-6
References
- 1.Rassaf T, Totzeck M, Backs J, Bokemeyer C, Hallek M, Hilfiker-Kleiner D, et al. Onco-cardiology: consensus paper of the German Cardiac Society, the German Society for Pediatric Cardiology and Congenital Heart Defects and the German Society for Hematology and Medical Oncology. Clin Res Cardiol. 2020;109(10):1197–1222. doi: 10.1007/s00392-020-01636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 3.Michel L, Schadendorf D, Rassaf T. Oncocardiology: new challenges, new opportunities. Herz. 2020;45(7):619–625. doi: 10.1007/s00059-020-04951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Totzeck M, Lutgens E, Neilan TG. Are we underestimating the potential for cardiotoxicity related to immune checkpoint inhibitors? Eur Heart J. 2021;42(16):1632–1635. doi: 10.1093/eurheartj/ehaa959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Totzeck M, Michel L, Lin Y, Herrmann J, Rassaf T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur Heart J. 2022;43(20):1928–1940. doi: 10.1093/eurheartj/ehac106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;01(280):163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. 2018;10(Suppl 35):S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6(8):e006278. doi: 10.1161/JAHA.117.006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 10.Michel L, Totzeck M, Lehmann L, Finke D. Emerging role of immune checkpoint inhibitors and their relevance for the cardiovascular system. Herz. 2020;45(7):645–651. doi: 10.1007/s00059-020-04954-8. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. 2021;42(16):1621–1631. doi: 10.1093/eurheartj/ehaa884. [DOI] [PubMed] [Google Scholar]
- 12.Mrotzek SM, Rassaf T, Totzeck M. Cardiovascular damage associated with chest irradiation. Front Cardiovasc Med. 2020;7:41. doi: 10.3389/fcvm.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddy N, Diallo S, El-Fayech C, Schwartz B, Pein F, Hawkins M, et al. Cardiac diseases following childhood cancer treatment: cohort study. Circulation. 2016;133(1):31–38. doi: 10.1161/CIRCULATIONAHA.115.016686. [DOI] [PubMed] [Google Scholar]
- 14.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löffler AI, Salerno M. Cardiac MRI for the evaluation of oncologic cardiotoxicity. J Nucl Cardiol. 2018;25(6):2148–2158. doi: 10.1007/s12350-018-1293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keramida K, Farmakis D. Right ventricular involvement in cancer therapy-related cardiotoxicity: the emerging role of strain echocardiography. Heart Fail Rev. 2021;26(5):1189–1193. doi: 10.1007/s10741-020-09938-8. [DOI] [PubMed] [Google Scholar]
- 18.Rui Zhao, Fang Shu, Chujie Zhang, Song Feiyan Xu, Yuchen Guo Ye, et al. Early detection and prediction of anthracycline-induced right ventricular cardiotoxicity by 3-dimensional echocardiography. JACC CardioOncol. 2020;2(1):13–22. doi: 10.1016/j.jaccao.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro ML, Jorge AJL, Nacif MS, de Martins WA. Early detection and monitoring of cancer chemotherapy-related left ventricular dysfunction by imaging methods. Arq Bras Cardiol. 2019;112(3):309–16. doi: 10.5935/abc.20190022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez JA, Russell RR. Cardio-oncology: the nuclear option. Curr Cardiol Rep. 2017;19(4):31. doi: 10.1007/s11886-017-0844-z. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Chung WB, Cho KI, Kim BJ, Seo JS, Park SM, et al. Diagnosis, treatment, and prevention of cardiovascular toxicity related to anti-cancer treatment in clinical practice: an opinion paper from the working group on cardio-oncology of the Korean Society of Echocardiography. J Cardiovasc Ultrasound. 2018;26(1):1–25. doi: 10.4250/jcu.2018.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. 2017;70(20):2536–2551. doi: 10.1016/j.jacc.2017.09.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell MB, Galt JR, Georgoulias P, Malhotra S, Pagnanelli R, Rischpler C, et al. SNMMI procedure standard/EANM guideline for gated equilibrium radionuclide angiography. J Nucl Med Technol. 2020;48(2):126–135. doi: 10.2967/jnmt.120.246405. [DOI] [PubMed] [Google Scholar]
- 25.Tissot H, Roch V, Morel O, Veran N, Perrin M, Claudin M, et al. Left ventricular ejection fraction determined with the simulation of a very low-dose CZT-SPECT protocol and an additional count-calibration on planar radionuclide angiographic data. J Nucl Cardiol. 2019;26(5):1539–1549. doi: 10.1007/s12350-019-01619-w. [DOI] [PubMed] [Google Scholar]
- 26.Sachpekidis C, Sachpekidis V, Kopp-Schneider A, Arsos G, Moralidis E. Equilibrium radionuclide angiography: intra- and inter-observer repeatability and reproducibility in the assessment of cardiac systolic and diastolic function. J Nucl Cardiol. 2021;28(4):1304–1314. doi: 10.1007/s12350-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 27.Russell RR, Alexander J, Jain D, Poornima IG, Srivastava AV, Storozynsky E, et al. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J Nucl Cardiol. 2016;23(4):856–884. doi: 10.1007/s12350-016-0538-8. [DOI] [PubMed] [Google Scholar]
- 28.Kahanda MG, Hanson CA, Patterson B, Bourque JM. Nuclear cardio-oncology: from its foundation to its future. J Nucl Cardiol. 2020;27(2):511–518. doi: 10.1007/s12350-019-01655-6. [DOI] [PubMed] [Google Scholar]
- 29.Verberne HJ, Acampa W, Anagnostopoulos C, Ballinger J, Bengel F, De Bondt P, et al. EANM procedural guidelines for radionuclide myocardial perfusion imaging with SPECT and SPECT/CT: 2015 revision. Eur J Nucl Med Mol Imaging. 2015;42(12):1929–1940. doi: 10.1007/s00259-015-3139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pryma DA, Ravizzini G, Amar D, Richards VL, Patel JB, Strauss HW. Cardiovascular risk assessment in cancer patients undergoing major surgery. J Nucl Cardiol. 2005;12(2):151–157. doi: 10.1016/j.nuclcard.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Chang K, Sarkiss M, Won KS, Swafford J, Broemeling L, Gayed I. Preoperative risk stratification using gated myocardial perfusion studies in patients with cancer. J Nucl Med. 2007;48(3):344–348. [PubMed] [Google Scholar]
- 32.Chandra S, Lenihan DJ, Wei W, Yusuf SW, Tong AT. Myocardial perfusion imaging and cardiovascular outcomes in a cancer population. Tex Heart Inst J. 2009;36(3):205–213. [PMC free article] [PubMed] [Google Scholar]
- 33.Prosnitz RG, Hubbs JL, Evans ES, Zhou SM, Yu X, Blazing MA, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110(8):1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 34.Sioka C, Exarchopoulos T, Tasiou I, Tzima E, Fotou N, Capizzello A, et al. Myocardial perfusion imaging with (99 m)Tc-tetrofosmin SPECT in breast cancer patients that received postoperative radiotherapy: a case-control study. Radiat Oncol. 2011;8(6):151. doi: 10.1186/1748-717X-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imbert L, Poussier S, Franken PR, Songy B, Verger A, Morel O, et al. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: a comprehensive analysis of phantom and human images. J Nucl Med. 2012;53(12):1897–1903. doi: 10.2967/jnumed.112.107417. [DOI] [PubMed] [Google Scholar]
- 36.Sciagrà R, Lubberink M, Hyafil F, Saraste A, Slart RHJA, Agostini D, et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2021;48(4):1040–1069. doi: 10.1007/s00259-020-05046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plana JC, Thavendiranathan P, Bucciarelli-Ducci C, Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. 2018;11(8):1173–1186. doi: 10.1016/j.jcmg.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen T, Kjær A, Lassen ML, Pedersen AN, Specht L, Aznar MC, et al. No changes in myocardial perfusion following radiation therapy of left-sided breast cancer: a positron emission tomography study. J Nucl Cardiol. 2021;28(5):1923–1932. doi: 10.1007/s12350-019-01949-9. [DOI] [PubMed] [Google Scholar]
- 39.Żyromska A, Małkowski B, Wiśniewski T, Majewska K, Reszke J, Makarewicz R. 15O–H2O PET/CT as a tool for the quantitative assessment of early post-radiotherapy changes of heart perfusion in breast carcinoma patients. Br J Radiol. 2018;91(1088):20170653. doi: 10.1259/bjr.20170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laursen AH, Elming MB, Ripa RS, Hasbak P, Kjær A, Køber L, et al. Rubidium-82 positron emission tomography for detection of acute doxorubicin-induced cardiac effects in lymphoma patients. J Nucl Cardiol. 2020;27(5):1698–1707. doi: 10.1007/s12350-018-1458-6. [DOI] [PubMed] [Google Scholar]
- 41.Biersmith MA, Tong MS, Guha A, Simonetti OP, Addison D. Multimodality cardiac imaging in the era of emerging cancer therapies. J Am Heart Assoc. 2020;9(2):e013755. doi: 10.1161/JAHA.119.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bristow MR, Mason JW, Billingham ME, Daniels JR. Dose-effect and structure-function relationships in doxorubicin cardiomyopathy. Am Heart J. 1981;102(4):709–718. doi: 10.1016/0002-8703(81)90096-X. [DOI] [PubMed] [Google Scholar]
- 43.Druck MN, Gulenchyn KY, Evans WK, Gotlieb A, Srigley JR, Bar-Shlomo BZ, et al. Radionuclide angiography and endomyocardial biopsy in the assessment of doxorubicin cardiotoxicity. Cancer. 1984;53(8):1667–1674. doi: 10.1002/1097-0142(19840415)53:8<1667::AID-CNCR2820530808>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Ewer MS, Ali MK, Mackay B, Wallace S, Valdivieso M, Legha SS, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol. 1984;2(2):112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio V, Cuomo A, Della Pepa R, Ciervo D, Cella L, Pirozzi F, et al. What is the cardiac impact of chemotherapy and subsequent radiotherapy in lymphoma patients? Antioxid Redox Signal. 2019;31(15):1166–74. doi: 10.1089/ars.2019.7842. [DOI] [PubMed] [Google Scholar]
- 46.Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10(4):391–399. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 47.Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BLF. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J. 2008;29(9):1147–1159. doi: 10.1093/eurheartj/ehn113. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55(20):2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 49.de Geus-Oei LF, Mavinkurve-Groothuis AMC, Bellersen L, Gotthardt M, Oyen WJG, Kapusta L, et al. Scintigraphic techniques for early detection of cancer treatment-induced cardiotoxicity. J Nucl Med. 2011;52(4):560–571. doi: 10.2967/jnumed.110.082784. [DOI] [PubMed] [Google Scholar]
- 50.Salm LP, Bulten BF, Van Laarhoven HWM, De Geus-Oei LF. Autonomic imaging cardiotoxicity with [123I]-MIBG: the effects of chemotherapy, monoclonal antibody therapy, and radiotherapy. In: Slart RHJA, Tio RA, Elsinga PH, Schwaiger M, editors. Autonomic innervation of the heart: role of molecular imaging. Berlin, Heidelberg: Springer; 2015. [Google Scholar]
- 51.Vallebona A. Cardiac damage following therapeutic chest irradiation. Importance, evaluation and treatment. Minerva Cardioangiol. 2000;48(3):79–87. [PubMed] [Google Scholar]
- 52.Guldner L, Haddy N, Pein F, Diallo I, Shamsaldin A, Dahan M, et al. Radiation dose and long term risk of cardiac pathology following radiotherapy and anthracyclin for a childhood cancer. Radiother Oncol. 2006;81(1):47–56. doi: 10.1016/j.radonc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Werner RA, Rischpler C, Onthank D, Lapa C, Robinson S, Samnick S, et al. Retention kinetics of the 18F-labeled sympathetic nerve PET tracer LMI1195: comparison with 11C-hydroxyephedrine and 123I-MIBG. J Nucl Med. 2015;56(9):1429–1433. doi: 10.2967/jnumed.115.158493. [DOI] [PubMed] [Google Scholar]
- 54.Kenk M, Thackeray JT, Thorn SL, Dhami K, Chow BJ, Ascah KJ, et al. Alterations of pre- and postsynaptic noradrenergic signaling in a rat model of adriamycin-induced cardiotoxicity. J Nucl Cardiol. 2010;17(2):254–263. doi: 10.1007/s12350-009-9190-x. [DOI] [PubMed] [Google Scholar]
- 55.Nensa F, Bamberg F, Rischpler C, Menezes L, Poeppel TD, la Fougère C, et al. Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM) Eur Radiol. 2018;28(10):4086–4101. doi: 10.1007/s00330-017-5008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. 2021;43(4):316–329. doi: 10.1093/eurheartj/ehab430. [DOI] [PubMed] [Google Scholar]
- 57.Bauckneht M, Ferrarazzo G, Fiz F, Morbelli S, Sarocchi M, Pastorino F, et al. Doxorubicin effect on myocardial metabolism as a prerequisite for subsequent development of cardiac toxicity: a translational 18F-FDG PET/CT observation. J Nucl Med. 2017;58(10):1638–1645. doi: 10.2967/jnumed.117.191122. [DOI] [PubMed] [Google Scholar]
- 58.Sarocchi M, Bauckneht M, Arboscello E, Capitanio S, Marini C, Morbelli S, et al. An increase in myocardial 18-fluorodeoxyglucose uptake is associated with left ventricular ejection fraction decline in Hodgkin lymphoma patients treated with anthracycline. J Transl Med. 2018;16(1):295. doi: 10.1186/s12967-018-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorla AKR, Sood A, Prakash G, Parmar M, Mittal BR. Substantial increase in myocardial FDG Uptake on interim PET/CT may be an early sign of adriamycin-induced cardiotoxicity. Clin Nucl Med. 2016;41(6):462–463. doi: 10.1097/RLU.0000000000001194. [DOI] [PubMed] [Google Scholar]
- 60.Dudoignon D, Pattison DA, Legallois D, Hicks RJ, Aide N. The utility of pharmacological and radiological interventions to optimize diagnostic information from PET/CT. Cancer Imaging. 2020;20(1):68. doi: 10.1186/s40644-020-00344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdés Olmos RA, Carrió I, Hoefnagel CA, Estorch M, ten Bokkel Huinink WW, López-Pousa J, et al. High sensitivity of radiolabelled antimyosin scintigraphy in assessing anthracycline related early myocyte damage preceding cardiac dysfunction. Nucl Med Commun. 2002;23(9):871–877. doi: 10.1097/00006231-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Estorch M, Carrió I, Berná L, Martínez-Duncker C, Alonso C, Germá JR, et al. Indium-111-antimyosin scintigraphy after doxorubicin therapy in patients with advanced breast cancer. J Nucl Med. 1990;31(12):1965–1969. [PubMed] [Google Scholar]
- 63.Carrió I, Lopez-Pousa A, Estorch M, Duncker D, Berná L, Torres G, et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J Nucl Med. 1993;34(9):1503–1507. [PubMed] [Google Scholar]
- 64.Carrió I, Estorch M, Berná L, Germá JR, Alonso C, Ojeda B, et al. Assessment of anthracycline-induced myocardial damage by quantitative indium 111 myosin-specific monoclonal antibody studies. Eur J Nucl Med. 1991;18(10):806–812. doi: 10.1007/BF00175059. [DOI] [PubMed] [Google Scholar]
- 65.Maini CL, Sciuto R, Ferraironi A, Vici P, Tofani A, Festa A, et al. Clinical relevance of radionuclide angiography and antimyosin immunoscintigraphy for risk assessment in epirubicin cardiotoxicity. J Nucl Cardiol. 1997;4(6):502–508. doi: 10.1016/S1071-3581(97)90008-8. [DOI] [PubMed] [Google Scholar]
- 66.Nousiainen T, Vanninen E, Jantunen E, Remes J, Kuikka J, Hartikainen J. Anthracycline-induced cardiomyopathy: long-term effects on myocardial cell integrity, cardiac adrenergic innervation and fatty acid uptake. Clin Physiol. 2001;21(1):123–128. doi: 10.1046/j.1365-2281.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 67.Peñafiel Ramírez A, Pardo Masferrer J, Peña VC. Unexpected accumulation of 111In-antimyosin in the chest in an area corresponding to the radiation field of a squamous cell lung carcinoma. Rev Esp Med Nucl. 1999;18(3):204–208. [PubMed] [Google Scholar]
- 68.Bennink RJ, van den Hoff MJ, van Hemert FJ, de Bruin KM, Spijkerboer AL, Vanderheyden JL, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med. 2004;45(5):842–848. [PubMed] [Google Scholar]
- 69.Panjrath GS, Jain D. Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging. J Nucl Cardiol. 2006;13(3):415–426. doi: 10.1016/j.nuclcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol. 2007;49(25):2457–2464. doi: 10.1016/j.jacc.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 71.Diekmann J, Koenig T, Zwadlo C, Derlin T, Neuser J, Thackeray JT, et al. Molecular imaging identifies fibroblast activation beyond the infarct region after acute myocardial infarction. J Am Coll Cardiol. 2021;77(14):1835–1837. doi: 10.1016/j.jacc.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J. 2020;41(9):1060. doi: 10.1093/eurheartj/ehz736. [DOI] [PubMed] [Google Scholar]
- 73.Siebermair J, Köhler MI, Kupusovic J, Nekolla SG, Kessler L, Ferdinandus J, et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol. 2021;28(3):812–821. doi: 10.1007/s12350-020-02307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13(9):e010628. doi: 10.1161/CIRCIMAGING.120.010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020;17(8):503–522. doi: 10.1038/s41569-020-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller DD, Waters DD, Dangoisse V, David PR. Symptomatic coronary artery spasm following radiotherapy for Hodgkin’s disease. Chest. 1983;83(2):284–285. doi: 10.1378/chest.83.2.284. [DOI] [PubMed] [Google Scholar]
- 77.Yahalom J, Hasin Y, Fuks Z. Acute myocardial infarction with normal coronary arteriogram after mantle field radiation therapy for Hodgkin’s disease. Cancer. 1983;52(4):637–641. doi: 10.1002/1097-0142(19830815)52:4<637::AID-CNCR2820520411>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 78.Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290(21):2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 79.Patel DA, Kochanski J, Suen AW, Fajardo LF, Hancock SL, Knox SJ. Clinical manifestations of noncoronary atherosclerotic vascular disease after moderate dose irradiation. Cancer. 2006;106(3):718–725. doi: 10.1002/cncr.21636. [DOI] [PubMed] [Google Scholar]
- 80.Venkatesulu BP, Mahadevan LS, Aliru ML, Yang X, Bodd MH, Singh PK, et al. Radiation-induced endothelial vascular injury: a review of possible mechanisms. JACC Basic Transl Sci. 2018;3(4):563–572. doi: 10.1016/j.jacbts.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. doi: 10.1161/01.CIR.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 82.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 83.Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JHF, Calcagno C, et al. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging. 2011;4(11):1195–1205. doi: 10.1016/j.jcmg.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bucerius J, Mani V, Moncrieff C, Rudd JHF, Machac J, Fuster V, et al. Impact of noninsulin-dependent type 2 diabetes on carotid wall 18F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol. 2012;59(23):2080–2088. doi: 10.1016/j.jacc.2011.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bucerius J, Mani V, Moncrieff C, Machac J, Fuster V, Farkouh ME, et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging. 2014;41(2):369–383. doi: 10.1007/s00259-013-2569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myers KS, Rudd JHF, Hailman EP, Bolognese JA, Burke J, Pinto CA, et al. Correlation between arterial FDG uptake and biomarkers in peripheral artery disease. JACC Cardiovasc Imaging. 2012;5(1):38–45. doi: 10.1016/j.jcmg.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudd JHF, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2(2):107–115. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudd JHF, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49(6):871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 89.Rudd JHF, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50(9):892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 90.Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71(5):709–718. doi: 10.1002/ana.23553. [DOI] [PubMed] [Google Scholar]
- 91.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Cho SG, Park KS, Kim J, Kang SR, Kwon SY, Seon HJ, et al. Prediction of coronary artery calcium progression by FDG uptake of large arteries in asymptomatic individuals. Eur J Nucl Med Mol Imaging. 2017;44(1):129–140. doi: 10.1007/s00259-016-3523-1. [DOI] [PubMed] [Google Scholar]
- 93.Moon SH, Cho YS, Noh TS, Choi JY, Kim BT, Lee KH. Carotid FDG uptake improves prediction of future cardiovascular events in asymptomatic individuals. JACC Cardiovasc Imaging. 2015;8(8):949–956. doi: 10.1016/j.jcmg.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Bucerius J, Hyafil F, Verberne HJ, Slart RHJA, Lindner O, Sciagra R, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–792. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ripa RS, Hag AM, Knudsen A, Loft A, Specht L, Kjær A. (18)F-FDG PET imaging in detection of radiation-induced vascular disease in lymphoma survivors. Am J Nucl Med Mol Imaging. 2015;5(4):408–415. [PMC free article] [PubMed] [Google Scholar]
- 96.Chen X, Zheng Y, Tatsuoka C, Muzic RF, Okoye CC, O’Donnell JK, et al. Chemoradiotherapy-related carotid artery inflammation in head and neck cancer patients quantified by [18F]FDG PET/CT. Oral Oncol. 2019;1(93):101–106. doi: 10.1016/j.oraloncology.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Lawal IO, Orunmuyi AT, Popoola GO, Lengana T, Mokoala KMG, Ankrah AO, et al. FDG PET/CT for evaluating systemic arterial inflammation induced by anthracycline-based chemotherapy of Hodgkin lymphoma: a retrospective cohort study. Medicine. 2020;99(48):e23259. doi: 10.1097/MD.0000000000023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calabretta R, Staber PB, Kornauth C, Lu X, Binder P, Pichler V, et al. Immune checkpoint inhibitor therapy induces inflammatory activity in the large arteries of lymphoma patients under 50 years of age. Biology (Basel) 2021;10(11):1206. doi: 10.3390/biology10111206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calabretta R, Hoeller C, Pichler V, Mitterhauser M, Karanikas G, Haug A, et al. Immune checkpoint inhibitor therapy induces inflammatory activity in large arteries. Circulation. 2020;142(24):2396–2398. doi: 10.1161/CIRCULATIONAHA.120.048708. [DOI] [PubMed] [Google Scholar]
- 100.Poels K, van Leent MMT, Boutros C, Tissot H, Roy S, Meerwaldt AE, et al. Immune checkpoint inhibitor therapy aggravates T cell–driven plaque inflammation in atherosclerosis. JAC CCardioOncol. 2020;2(4):599–610. doi: 10.1016/j.jaccao.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zelt JGE, Britt D, Mair BA, Rotstein BH, Quigley S, Walter O, et al. Regional distribution of fluorine-18-flubrobenguane and carbon-11-hydroxyephedrine for cardiac PET imaging of sympathetic innervation. JACC Cardiovasc Imaging. 2021;14(7):1425–1436. doi: 10.1016/j.jcmg.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 102.Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail. 2018;5(2):249–261. doi: 10.1002/ehf2.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - a comparison to cardiac MRI. Int J Cardiol. 2015;1(194):44–49. doi: 10.1016/j.ijcard.2015.05.073. [DOI] [PubMed] [Google Scholar]
- 104.Makowski MR, Rischpler C, Ebersberger U, Keithahn A, Kasel M, Hoffmann E, et al. Multiparametric PET and MRI of myocardial damage after myocardial infarction: correlation of integrin αvβ3 expression and myocardial blood flow. Eur J Nucl Med Mol Imaging. 2021;48(4):1070–1080. doi: 10.1007/s00259-020-05034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCluskey SP, Haslop A, Coello C, Gunn RN, Tate EW, Southworth R, et al. Imaging of chemotherapy-induced acute cardiotoxicity with 18F-labeled lipophilic cations. J Nucl Med. 2019;60(12):1750–1756. doi: 10.2967/jnumed.119.226787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sivapackiam J, Kabra S, Speidel S, Sharma M, Laforest R, Salter A, et al. 68Ga-Galmydar: a PET imaging tracer for noninvasive detection of doxorubicin-induced cardiotoxicity. PLoS ONE. 2019;14(5):e0215579. doi: 10.1371/journal.pone.0215579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boutagy NE, Wu J, Cai Z, Zhang W, Booth CJ, Kyriakides TC, et al. In vivo reactive oxygen species detection with a novel positron emission tomography tracer, 18F-DHMT, allows for early detection of anthracycline-induced cardiotoxicity in rodents. JACC Basic Transl Sci. 2018;3(3):378–90. doi: 10.1016/j.jacbts.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rassaf T, Heusch G. The West German Heart and Vascular Center at University Medicine Essen. Eur Heart J. 2021;42(10):963–964. doi: 10.1093/eurheartj/ehaa980. [DOI] [PubMed] [Google Scholar]
- 109.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17(8):474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rao VU, Reeves DJ, Chugh AR, O’Quinn R, Fradley MG, Raghavendra M, et al. Clinical approach to cardiovascular toxicity of oral antineoplastic agents: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(21):2693–2716. doi: 10.1016/j.jacc.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hyafil F, Gimelli A, Slart RHJA, Georgoulias P, Rischpler C, Lubberink M, et al. EANM procedural guidelines for myocardial perfusion scintigraphy using cardiac-centered gamma cameras. Eur J Hybrid Imaging. 2019;2(3):11. doi: 10.1186/s41824-019-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mattsson S, Johansson L, Leide Svegborn S, Liniecki J, Noßke D, Riklund KÅ, et al. ICRP publication 128: radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. Ann ICRP. 2015;44(2 Supp):7–321. doi: 10.1177/0146645314558019. [DOI] [PubMed] [Google Scholar]
- 113.Case JA, deKemp RA, Slomka PJ, Smith MF, Heller GV, Cerqueira MD. Status of cardiovascular PET radiation exposure and strategies for reduction: an Information Statement from the Cardiovascular PET Task Force. J Nucl Cardiol. 2017;24(4):1427–1439. doi: 10.1007/s12350-017-0897-9. [DOI] [PubMed] [Google Scholar]
- 114.Flotats A, Carrió I, Agostini D, Le Guludec D, Marcassa C, Schäfers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging. 2010;37(9):1802–1812. doi: 10.1007/s00259-010-1491-4. [DOI] [PubMed] [Google Scholar]
- 115.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 116.Slart RHJA, Glaudemans AWJM, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J Nucl Cardiol. 2018;25(1):298–319. doi: 10.1007/s12350-017-1043-4. [DOI] [PubMed] [Google Scholar]