Abstract
Dermatomyositis (DM) is one of the most common autoimmune rheumatic diseases affecting women of childbearing age. Pregnancy may lead to exacerbation of DM, especially of DM with anti-melanoma differentiation-associated gene (MDA) 5 antibody positivity, leading to a poor obstetric outcome. Here, we report consecutive pregnancies complicated by DM with anti-MDA-5 antibodies. A 32-year-old pregnant woman, gravida 3 para 1, presented with fetal growth restriction. Emergency cesarean section was performed because of non-reassuring fetal status at 28 weeks of gestation. Two days postpartum, the patient's hand eczema had worsened and she was diagnosed with DM with MDA-5 antibody positivity. Immunosuppressive therapy using corticosteroids combined with tacrolimus was immediately started, suppressing the DM symptoms. Eighteen months later, she became pregnant again but was then negative for anti-MDA-5 antibodies while continuing immunosuppressive therapy. During pregnancy, the titer of the antibody gradually increased, peaked in the second trimester and declined to near normal range through the third trimester. A male infant weighing 2418 g was delivered at 38 weeks of gestation. Our case demonstrates that controlling of DM activity using immunosuppressive treatment before and during pregnancy may be beneficial to obstetric outcomes.
Keywords: Dermatomyositis, Anti-melanoma differentiation-associated gene 5 antibody, Immunosuppressive therapy, Tacrolimus
Abbreviations: DM, dermatomyositis; anti-MDA-5, anti-melanoma differentiation-associated gene 5 antibody; FGR, fetal growth restriction; CADM, clinically amyopathic dermatomyositis; SGA, small for gestational age
Highlights
-
•
Dermatomyositis (DM) is one of the most common autoimmune rheumatic disease affecting women of childbearing age.
-
•
Pregnancy may lead to exacerbation of DM.
-
•
DM is associated with poor obstetric outcomes.
-
•
Immunosuppressive therapy before and during pregnancy may be beneficial to improve obstetric outcomes.
1. Introduction
Dermatomyositis (DM) is one of the most common autoimmune rheumatic diseases affecting women of childbearing age. DM is a spectrum of muscle myalgia and weakness, with an erythematous eczema over the extensor surface of the metacarpophalangeal (Gottron's sign) and the flexor surface of the interphalangeal joint of the fingers (inverse Gottron's sign). Pregnancy is a known exacerbating factor for this disease and the obstetric outcomes in patients with active DM are poor [1,2]. For example, fetal death, fetal growth restriction (FGR), and preterm birth have been reported in association with active DM. Previous reports suggested that DM with various myositis-specific antibodies, including anti-aminoacyl tRNA synthetases, anti-Mi-2, anti-transcriptional intermediary factor 1 and anti-melanoma differentiation-associated gene 5 (MDA-5) antibodies, could be triggered by pregnancy [3]. Patients with DM and anti-MDA-5 antibody positivity tend to develop rapidly progressive interstitial lung disease with a poor prognosis, sometimes leading to death [4,5]. Here, we report consecutive pregnancies complicated by DM with anti-MDA-5 antibody positivity, which has seldom been reported [3,6].
2. Case Presentation
A 32-year-old woman, gravida 3 para 1 (1 spontaneous abortion and 1 vaginal delivery of a male infant weighing 2945 g at 38 weeks of gestation after an uneventful pregnancy), was referred to the hospital at 9 weeks of gestation. At 28 weeks of gestation, fetal growth restriction (FGR) was identified. At 28+4 weeks of gestation, emergency cesarean section was performed because of non-reassuring fetal status due to placental dysfunction. A male infant, who was very small for gestational age (SGA) at 834 g (−2.29 standard deviation), was delivered with Apgar scores of 3 and 6 at one and five minutes, respectively. The placenta had a 12.7 × 12.3 × 1.2 cm disc weighing 210 g and diffuse intervillous fibrin deposition (Rohr's fibrin) was noted on microscopic examination (Fig. 1 a-c). She had experienced hand eczema from the second trimester, which rapidly worsened two days after delivery (Fig. 2). She was diagnosed with DM with anti-MDA-5 antibody positivity due to the characteristic macroscopic and microscopic findings of her hand and an elevation of serum anti-MDA-5 antibody titer (Fig. 3). Given her clinical features, she was thought to have developed FGR due to placental dysfunction induced by activated DM. She presented with skin lesions without muscle manifestations, leading to a diagnosis of clinically amyopathic dermatomyositis (CADM) with anti-MDA-5 antibodies. Since there were concerns that this disease could progress to severe pulmonary damage, immunosuppressive therapy using corticosteroids (2 mg/day) combined with tacrolimus (3 mg/day) was immediately started. Eight days after cesarean section, she was discharged without any complications, and her DM phenotype had been suppressed by continuing immunosuppressive therapy, with her MDA-5 antibody titer returning to normal range 9 months after delivery (Fig. 3).
Fig. 1.
Pathological examination of the placenta.
Macroscopic and microscopic findings of the placenta in FGR (a-c) and non-FGR (d-f) infants. Macroscopic findings of fetal (a, d) and maternal planes (b, e) of placenta. HE staining (original magnification×40) of placental tissue in FGR (c) and non-FGR (f) infants. Arrow head: fibrin deposition.
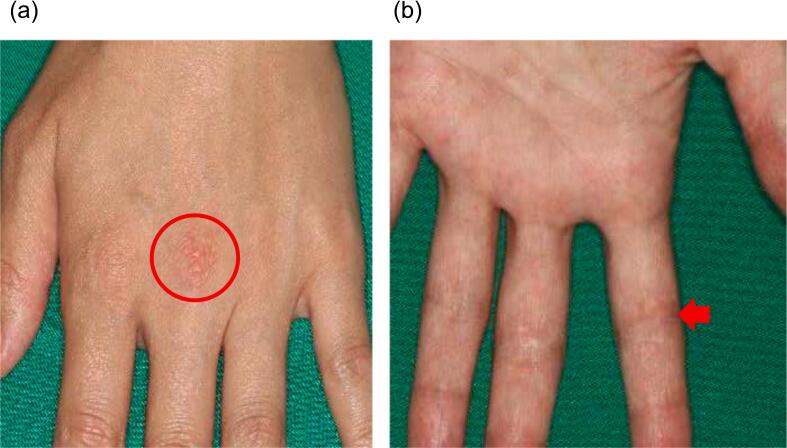
Fig. 2.
Hand eczema.
An erythematous eczema over the extensor surface of the metacarpophalangeal joint (a: Gottron's sign) and the flexor surface of interphalangeal joint of the fingers (b: inverse Gottron's sign).
Fig. 3.
Clinical course of the patient.
After starting treatment, anti-MDA5 antibody titer decreased to the normal range (below 32 index). However, anti-MDA5 antibody titer was elevated again when she became pregnant. Dotted line: 32 index.
Eighteen months after starting immunosuppressive therapy, she spontaneously conceived and was re-referred to the hospital at 9 weeks of gestation. She was negative for anti-MDA antibodies at the time of conception, but immunosuppressive treatment continued, using corticosteroids with tacrolimus. At 14 weeks of gestation, she was found to be positive for anti-MDA antibodies (65 index). The titer of the antibody gradually increased, peaked at 27 weeks of gestation (525 index) and declined to near normal range through the third trimester (Fig. 3). Her pregnancy was uneventful. Cesarean section was performed at 38+1 weeks of gestation due to the previous history of cesarean section. A male SGA-infant weighing 2418 g (−1.48 standard deviation) was delivered with Apgar scores of 8 and 8 at 1 and 5 min, respectively. The placenta had a 16.0 × 13.5 × 1.5 cm disc weighing 450 g and Rohr's fibrin was noted on microscopic examination (Fig. 1 d-f). However, the severity of fibrin deposition was markedly milder than that of the previous delivery (Fig. 1 c, f). The mother's postoperative course was uneventful.
3. Discussion
There is limited information about pregnancies complicated by DM, especially those with anti-MDA-5 antibody positivity. In our case, the fetus showed features of FGR and the patient delivered a preterm SGA infant at 29 weeks of gestation. She was diagnosed with DM based on findings of hand eczema exacerbation (Gottron's sign) and inverse Gottron's sign two days after delivery. She was successfully treated with immunosuppressive therapy using corticosteroids combined with tacrolimus. In her subsequent pregnancy, she continued this immunosuppressive therapy throughout and had a favorable obstetric outcome.
To date, several reports indicate that obstetric outcomes are closely associated with the DM disease activity before and/or during pregnancy [1,2,6,7]. In cases where DM has settled during pregnancy, favorable obstetric outcomes have been reported. In contrast, cases result in poor obstetric outcomes when DM activity is poorly controlled during pregnancy or when DM first presents during pregnancy. The clinical courses of our patient's pregnancies were consistent with the previous reports [1,2,6,7]. She delivered three infants: first, she had no DM and the pregnancy progressed well; second, the patient probably developed DM during pregnancy and showed severe FGR, which resulted in delivery at 28 weeks; third, she was treated with immunosuppressants before and during pregnancy to control DM activity, leading to delivery at full term. Also, in our case, pathological examination of the refractory hand eczema was performed during workup of FGR and she was diagnosed with DM associated with anti-MDA-5 antibodies. Despite its rarity, physicians should note the presence of any refractory hand eczema when faced with pregnancy complicated by FGR. In particular, women of reproductive age should be treated immediately with immunosuppressive agents based on DM activity, with consideration for possible further pregnancies.
In our case, immunosuppressive therapy had a favorable effect on placental tissue and improved obstetric outcomes in a pregnancy complicated by DM. Placental abnormalities such as massive fibrin deposition may be observed when patients with maternal collagen disease experience repeated miscarriages and/or preterm births [8,9]. It has been proposed that antibodies in autoimmune disease injure the trophoblast and disrupt the regulatory mechanism of the coagulation-fibrinolytic system in the intervillous space, resulting in synthesis of fibrinoid extracellular matrix. As a result, intervillous blood flow cannot be maintained, leading to adverse obstetric outcomes [8].
In the field of infertility, there are several reports that intensive administration of immunosuppressants such as tacrolimus after the implantation window could improve obstetric outcomes [10,11]. In the present case, FGR due to placental dysfunction was observed in a pregnancy where DM activity was poorly controlled, and histopathologic examination of the placenta revealed severe diffuse intervillous fibrin deposition, which could cause FGR and hypertensive disorder in pregnancy [8,9]. Immunosuppressive therapy likely suppressed placental fibrin deposition and improved obstetric outcomes in the subsequent pregnancy, suggesting that such active control of DM activity was essential for improving obstetric outcomes.
In most cases, pregnant patients with DM activity were initially treated with corticosteroids, and this was combined with an additional immunosuppressant including intravenous immunoglobulin, azathioprine, hydroxychloroquine, cyclosporine, and tacrolimus [2,3,12]. The autoantibody profile might allow individualized clinical presentations and treatment in DM patients [2,3,7]. Since our case appeared to represent CADM with anti-MDA-5 antibodies, there were concerns that it could progress to severe pulmonary damage. In life-threatening cases of anti-MDA-5 antibody positivity, presenting similarly to a rapidly progressive interstitial pneumonia, combination immunosuppression therapy (i.e., corticosteroids combined with another immunosuppressant) has been recommended [4,5]. Therefore, we selected corticosteroids combined with tacrolimus according to recommendations for the treatment of rapidly progressive interstitial lung disease associated with anti-MDA-5-positive dermatomyositis [4,5].
In conclusion, our case demonstrates that controlling of DM activity using immunosuppressive treatment before and during pregnancy may be beneficial to obstetric outcomes.
Acknowledgments
Contributors
Hiroyuki Goto was involved in patient care, participated in the conception of the case report, acquired and interpreted the data, and drafted the manuscript.
Kimito Kawahata was involved in patient care, was responsible for the conception of the case report, and revised the article critically for important intellectual content.
Akiko Shida was involved in patient care, was responsible for the conception of the case report, and revised the article critically for important intellectual content.
Saeko Nakagane was involved in patient care, was responsible for the conception of the case report, and revised the article critically for important intellectual content.
Hitoshi Isohata contributed to data interpretation and revised the article critically for important intellectual content.
Yu Yamazaki contributed to data interpretation and revised the article critically for important intellectual content.
Yoshihiro Yoshimura contributed to data interpretation and revised the article critically for important intellectual content.
Kyoko Hattori contributed to data interpretation and revised the article critically for important intellectual content.
Kazuki Sekiguchi contributed to data interpretation and revised the article critically for important intellectual content.
Ryuzo Ishikawa contributed to data interpretation and revised the article critically for important intellectual content.
Yoko Onishi contributed to data interpretation and revised the article critically for important intellectual content.
Yuji Kanai contributed to data interpretation and revised the article critically for important intellectual content.
Nobuya Unno contributed to data interpretation and revised the article critically for important intellectual content.
Daigo Ochiai participated in the conception of the case report, acquired and interpreted the data, drafted the manuscript and revised the article critically for important intellectual content.
All authors approved the final version of the paper and take full responsibility for the work.
Funding
No source of funding or any form of financial assistance supported publication of this case report.
Patient consent
The patient provided informed consent for the publication of this report and all accompanying images.
Provenance and peer review
This case report was not commissioned and was peer reviewed.
Acknowledgments
Acknowledgements
We thank the obstetrics and other healthcare personnel involved in the treatment of patients at the Department of Obstetrics and Gynecology, Kitasato University School of Medicine.
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Munira S., Christopher-Stine L. Pregnancy in myositis and scleroderma. Best Pract. Res. Clin. Obstet. Gynaecol. 2020;64:59–67. doi: 10.1016/j.bpobgyn.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Che W.I., Hellgren K., Stephansson O., Lundberg I.E., Holmqvist M. Pregnancy outcomes in women with idiopathic inflammatory myopathy, before and after diagnosis-a population-based study. Rheumatology (Oxford) 2020;59(9):2572–2580. doi: 10.1093/rheumatology/kez666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama C., Shirai T., Sato H., Fujii H., Ishii T., Harigae H. Association of various myositis-specific autoantibodies with dermatomyositis and polymyositis triggered by pregnancy. Rheumatol. Int. 2022;42(7):1271–1280. doi: 10.1007/s00296-021-04851-1. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji H., Nakashima R., Hosono Y., Imura Y., Yagita M., Yoshifuji H., Hirata S., Nojima T., Sugiyama E., Hatta K., Taguchi Y., Katayama M., Tanizawa K., Handa T., Uozumi R., Akizuki S., Murakami K., Hashimoto M., Tanaka M., Ohmura K., Mimori T. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheum. 2020;72(3):488–498. doi: 10.1002/art.41105. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Bueno F., Diaz Del Campo P., Trallero-Araguas E., Ruiz-Rodriguez J.C., Castellvi I., Rodriguez-Nieto M.J., Martinez-Becerra M.J., Sanchez-Pernaute O., Pinal-Fernandez I., Solanich X., Gono T., Gonzalez-Gay M.A., Plana M.N., Selva-O’Callaghan A., M. group Recommendations for the treatment of anti-melanoma differentiation-associated gene 5-positive dermatomyositis-associated rapidly progressive interstitial lung disease. Semin. Arthritis Rheum. 2020;50(4):776–790. doi: 10.1016/j.semarthrit.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C., Chen Y., Huang Q., Hu Q., Hong X. Case report: rapidly progressive interstitial lung disease in a pregnant patient with anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.625495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y., Yamamoto Y., Suzuki Y., Noda K., Nakajima A. Clinical and serological features and pregnancy outcomes in women with polymyositis/dermatomyositis: a case-based review. Intern. Med. 2022;61(2):143–149. doi: 10.2169/internalmedicine.7924-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornish E.F., McDonnell T., Williams D.J. Chronic inflammatory placental disorders associated with recurrent adverse pregnancy outcome. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.825075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Adnani M., Kiho L., Scheimberg I. Recurrent placental massive perivillous fibrin deposition associated with polymyositis: a case report and review of the literature. Pediatr. Dev. Pathol. 2008;11(3):226–229. doi: 10.2350/07-06-0306.1. [DOI] [PubMed] [Google Scholar]
- 10.Parhizkar F., Motavalli-Khiavi R., Aghebati-Maleki L., Parhizkar Z., Pourakbari R., Kafil H.S., Danaii S., Yousefi M. The impact of new immunological therapeutic strategies on recurrent miscarriage and recurrent implantation failure. Immunol. Lett. 2021;236:20–30. doi: 10.1016/j.imlet.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Leroy C., Rigot J.M., Leroy M., Decanter C., Le Mapihan K., Parent A.S., Le Guillou A.C., Yakoub-Agha I., Dharancy S., Noel C., Vantyghem M.C. Immunosuppressive drugs and fertility. Orphanet. J. Rare Dis. 2015;10:136. doi: 10.1186/s13023-015-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotestam Skorpen C., Hoeltzenbein M., Tincani A., Fischer-Betz R., Elefant E., Chambers C., da Silva J., Nelson-Piercy C., Cetin I., Costedoat-Chalumeau N., Dolhain R., Forger F., Khamashta M., Ruiz-Irastorza G., Zink A., Vencovsky J., Cutolo M., Caeyers N., Zumbuhl C., Ostensen M. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]