Abstract
Schizophrenia (SCZ) and bipolar disorder (BD) are associated with immunological dysfunctions that have been hypothesized to lead to clinical symptomatology in particular through kynurenine pathway abnormalities. The aim of this study was thus to investigate the impact of serum kynurenine metabolite levels on diagnosis, clinical state, symptom severity and clinical course in a large French transdiagnostic cohort of SCZ and BD patients. Four patient groups (total n = 507) were included in a cross-sectional observational study: 1) hospitalized acute bipolar patients (n = 205); 2) stable bipolar outpatients (n = 116); 3) hospitalized acute schizophrenia patients (n = 111) and 4) stable schizophrenia outpatients (n = 75), in addition to healthy controls (HC) (n = 185). The quantitative determination of serum kynurenine metabolites was performed using liquid chromatography–tandem mass spectrometry.
Kynurenine levels were lower in all patients combined compared to HC while ANCOVA analyses did not reveal inter-diagnostic difference between SCZ and BD. Interestingly, hospitalized patients of both diagnostic groups combined displayed significantly lower kynurenine levels than stabilized outpatients. Psychotic symptoms were associated with lower quinaldic acid (F = 9.18, p=<.001), which is KAT-driven, whereas a longer duration of illness contributed to abnormalities in tryptophan (F = 5.41, p = .023), kynurenine (F = 16.93, p=<.001), xanthurenic acid (F = 9.34, p = .002), quinolinic acid (F = 9.18, p = .003) and picolinic acid (F = 4.15, p = .043), metabolized through the KMO-branch. These data confirm illness state rather than diagnosis to drive KP alterations in SCZ and BD. Lower levels of KP metabolites can thus be viewed as a transdiagnostic feature of SCZ and BD, independently associated with acute symptomatology and a longer duration of illness. Quinaldic acid has seldomly been investigated by previous studies and appears an important state marker in SCZ and BD. As serum samples are used in this study, it is not possible to extrapolate these findings to the brain.
Highlights
-
•
Schizophrenia and bipolar disorder are characterized by lower serum kynurenine metabolite concentrations.
-
•
Serum kynurenine metabolite aberrances are a transdiagnostic feature of bipolar and schizophrenic patients.
-
•
Quinaldic acid is an important state marker for psychotic symptoms.
-
•
Duration of illness mostly impacts xanthurenic and quinolinic acid abnormalities.
1. Introduction
Schizophrenia (SCZ) and bipolar disorder (BD) are severe mental disorders that, despite notable clinical heterogeneity, demonstrate considerable overlap in their underlying pathophysiology. On top of shared clinical symptomatology such as psychotic features in SCZ and BD type I, they share a common genetic background (Witt et al., 2017; Stahl et al., 2019; Anttila et al., 1101), brain transcriptome abnormalities (Gandal et al., 2019), structural and functional brain changes (Madre et al., 2020) and neurochemical alterations (Torrey et al., 2005) to the extent that some authors have argued SCZ and BD should be considered as part of the same clinical spectrum (Karantonis et al., 2020). Both disorders have also been associated with immunological abnormalities (Réus et al., 2015; Morrens et al., 2020a), such as blood cytokine aberrations and microglial activation (Picker et al., 2017; Haarman et al., 2014; Petrasch-Parwez et al., 2022).
A large body of evidence points towards the activation of the immune-inflammatory response system (IRS) and oxidative and nitrosative stress (O&NS) pathways in mood and psychotic disorders (Solmi et al., 2021; Maes et al., 2011). The activation of IRS is characterized by the upregulation of cell-mediated immunity, with increased levels of interleukin (IL)-6, IL-1b, interferon (IFN)-g, tumor necrosis factor (TNF)-a, soluble IL-2 receptor (sIL-2R), sCD8, …(Vasupanrajit et al., 2022) and is rebalanced by the compensatory immune-regulatory system (CIRS) (Maes and Carvalho, 2018). The O&NS pathways produce reactive oxygen and nitrogen species (ROS, RNS), damaging DNA, mitochondria, proteins and lipids (Maes et al., 2011). Moreover, mood disorders are associated with increased peripheral levels of lipopolysaccharide (LPS), which activates the innate immune system and stimulates pro- and anti-inflammatory cytokine production (Tawfik et al., 2020). These altered immune responses induce the activity of indoleamine 2,3-dioxygenase (IDO) and tryptophan dioxygenase (TDO), resulting in kynurenine pathway (KP) changes in the central nervous system (CNS) (De Picker et al., 2019; van den Ameele et al., 2018; Savitz, 2020). The KP metabolites changes are postulated as the missing link between immunological disturbances and clinical abnormalities in psychiatric illnesses (Faurbye, 1968).
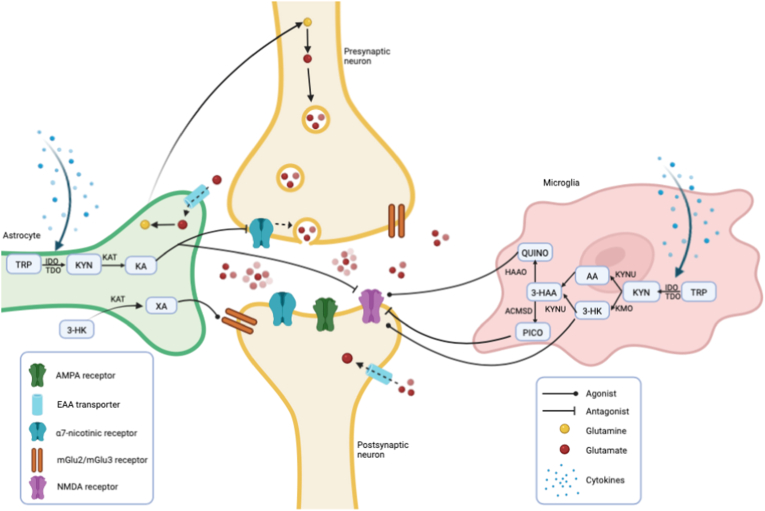
In the brain, 95% of the available essential amino acid tryptophan (TRP) is consumed by the KP, yielding kynurenine (KYN) at the expense of the neurotransmitter serotonin (5-HT). TRP is converted into KYN by the rate-limiting enzymes IDO and TDO (Badawy, 2017). Previous authors have simplified the pathway in ‘the neurotoxic kynurenine 3-monooxygenase (KMO)-branch’, where microglia produce quinolinic acid (QUINO), and ‘the neuroprotective kynurenine aminotransferases (KAT)-branch’, where astrocytes generate kynurenic acid (KA). In microglia, the enzyme KMO converts KYN to 3-hydroxy-kynurenine (3-HK), which acts as a precursor for both xanthurenic acid (XA) and 3-hydroxy anthranilic acid (3-HAA). Recently, it has been established that XA activates metabotropic glutamate receptors (mGlu2 and mGlu3), which decrease glutamate release in cortical pyramidal neurons (Fazio et al., 2015). 3-HAA is further catabolized into the N-methyl D-aspartate (NMDA) receptor agonist QUINO, acting as a potent excitotoxin through excessive glutamate release by neurons (Tavares et al., 2000, 2002). In astrocytes, KAT metabolize KYN into KA, which is further converted into quinaldic acid (QUINA). KA is an antagonist of NMDA and α7 nicotinergic receptors and as such can protect against excitotoxicity (Muller and Schwarz, 2006). However, abnormally increased brain KA may be responsible for a hypoglutaminergic state by excessive NMDA receptor antagonism, triggering psychotic and cognitive symptoms in SCZ and BD (Faurbye, 1968; Badawy, 2017; Fazio et al., 2015) (+60: Erhardt) (Albuquerque and Schwarcz, 2013; Javitt et al., 2012; Erhardt et al., 2017; Savitz et al., 2015a). The neuroactive KP metabolites directly and indirectly modulate other neurotransmissions than glutamate (see Fig. 1), such as dopamine (DA), 5-HT, noradrenaline (NA), acetylcholine (Ach) and gamma-aminobutyric acid (GABA), which are implicated in the pathophysiology of SCZ and BD (Stahl, 2018; Hilmas et al., 2001; Pérez-De La Cruz et al., 2012; Banerjee et al., 2012; Myint and Kim, 2014).
Fig. 1.
A simplified view of the influence of kynurenine metabolites on glutamatergic neurotransmission
Glutamine is converted to glutamate by glutaminase in the neuron and packaged into presynaptic vesicles, which are released into the synaptic cleft. The α7-nicotinergic receptor stimulates glutamate release by intracellular calcium influx. Glutamine is recycled by EAT transporters on astroglia, where glutamate is converted back to glutamine by glutamine synthestase and is then transported back to the neuron. In the synaptic cleft, glutamate can bind to multiple receptors, such as the NMDA, AMPA and mGlu receptors. Excessive NMDA activation causes excitotoxicity leading to neuronal cell death. TRP is converted into KYN by the rate-limiting enzymes IDO and TDO, both stimulated by proinflammatory cytokines which can easily cross the blood-brain barrier. In this figure, the kynurenine pathway is divided in the microglial branch, mostly responsible for the neurotoxin QUINO, and the astrocytic branch, generating KA. KMO is lacking in astrocytes, hence 3-HK is solely formed in microglia. 3-HK is converted to 3-HAA or travels to astrocytes, where KAT-II enzymes generate XA. XA thus depends on KMO on the formation of 3-HK, but is produced in astrocytes. QUINO and 3-HK are known as NMDA receptor agonists and as such have neurotoxic effects in the brain, whereas KA and PICO are NMDA receptor antagonists. KA also has an antagonistic effect on α7-nicotinergic receptors, thereby impeding glutamate release into the synaptic cleft. Last, XA activates mGlu2 and mGlu3 receptors, which decrease glutamate release in cortical pyramidal neurons by blocking 5-HTA receptors.
AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; EAA: excitatory amino acid; mGlu: metabotropic glutamate receptor; NMDA: N-methyl-D-aspartate; TRP: tryptophan, KYN: kynurenine; KA: kynurenic acid; QUINA: quinaldic acid; 3-HK: 3-hydroxy kynurenine; AA: anthranilic acid; XA: xanthurenic acid; 3-HAA: 3-hydroxyanthranilic acid; PICO: picolinic acid; IDO; indole-2,3-dioxygenase; TDO: tryptophan-2,3-dioxygenase; ACMSD: amino-B-carboxymuconate semialdehyde-decarboxylase; HAAO: 3-hydroxy anthranilate-3,4-dioxygenase; KAT: kynurenine aminotranspherase; KMO: kynurenine mono-oxygenase; KYNU: kynurinase; IDO: indoleamine 2,3-dioxygenase; TDO: tryptophan dioxygenase.
A recent meta-analysis revealed an overall dissociation between blood and central KP levels in SCZ (Almulla et al., 2022). Studies investigating central KP metabolites (in cerebrospinal fluid (CSF) or brain tissue) are scarce, but point towards an activation of the KP by elevated levels of mostly KYN and KA in CSF (Erhardt et al., 2001; Schwarcz et al., 2001; Miller et al., 2006). In contrast, a recent series of meta-analyses (Morrens et al., 2020b; Marx et al., 2021; Hebbrecht et al., 2021) corroborate peripheral blood concentrations of TRP, KYN and KA to be downregulated in both SCZ and BD, while inconsistency persists for KMO-driven KP metabolites to be either unaltered or downregulated (Morrens et al., 2020b; Marx et al., 2021; Skorobogatov et al., 2021). Discrepancy may partly result from the temporal dynamicity of the symptomatic state. De Picker et al. (De Picker et al., 2019) previously showed that plasma concentrations of KMO-driven metabolites 3-HK and QUINO decrease in acutely psychotic patients and normalize again after treatment-related symptomatic remission. In addition to symptom status, the chronicity of the illness may also affect KP concentrations: Fazio and colleagues (Fazio et al., 2015) reported that KMO-driven (but not KAT-driven) metabolites diverge between first-episode vs. chronically ill SZC patients. Morrens et al. (2020b) recently confirmed the impact of clinical status and age in a meta-analysis of KP metabolite concentrations in SCZ: TRP, KA and QUINO were decreased in acutely ill but not in stable patients; lower KYN and KA characterized older but not younger patients. An effect of both illness status and age has also been observed for other immunological changes in SCZ, thus arguing for acute psychosis as a separate neuro-immunological condition characterized by an increase in pro-inflammatory cytokines (Miller et al., 2011), CD4 lymphocytes (Miller et al., 2013) and microglial activation (Ottoy et al., 2018). Although fewer evidence is available for BD, state-specific immunological changes were observed in depressive phases, such as decreased QUINO and increased CRP concentrations (van den Ameele et al., 2020; Dickerson et al., 2015), and during manic episodes, such as decreased TRP and KA (Myint et al., 2007) and increased IL-1 receptor antagonist (IL-1RA), sSD4 and sSD8 (Liu et al., 2004).
Despite the existing evidence on the pathophysiological overlap between SCZ and BD and the dynamic nature of KP abnormalities in both disorders, no study has comprehensively investigated which clinical features predict KP abnormalities, and whether these predictors are shared between SCZ and BD patients. The aim of this study was to investigate the following clinical features for their impact of state-specific (acute vs. stable state; symptom severity) and illness duration-related predictors on serum KP levels in a large transdiagnostic cohort of SCZ and BD patients.
2. Materials and methods
2.1. Participants
One group of healthy controls (HC) and four patient groups were included in the current study: 1) hospitalized acute bipolar patients (aBD); 2) stable bipolar outpatients (sBD); 3) hospitalized acute schizophrenia patients (aSCZ) and 4) stable schizophrenia outpatients (sSCZ). The schizophrenia group contains mostly schizophrenia (DSM-IV codes; catatonic type: 295.20; disorganized type: 295.10; paranoid type: 295.30; undifferentiated type: 295.90) and also 29 schizo-affective disorder patients (295.70). Both bipolar type I and type II are included in the bipolar group.
From 2013 until 2019, patients with SCZ or BD who were acutely ill and hospitalized with SCZ or BD (aSCZ, aBD) were recruited in the inpatient psychiatric department, while stable SCZ and BD patients (sSCZ, sBD) were included as outpatients in the expert centers for BP or for SCZ patients (Miller et al., 2013; Ottoy et al., 2018) in the psychiatric department of Mondor Hospital (Créteil, France). These patients belong to the French cohort I-GIVE (Immunogenetic, inflammation, retro-virus, environment). All hospitalized patients and outpatients were screened and inclusion was proposed to every eligible patient. Exclusion criteria were (Witt et al., 2017): age <18 years (Stahl et al., 2019), pregnancy or breastfeeding (Anttila et al., 1101), acute infectious disease (Gandal et al., 2019), recent vaccination (<4 weeks) (Madre et al., 2020), seropositivity for HIV or hepatitis A, B and C viruses (Torrey et al., 2005), acute or chronic auto-immune or inflammatory disease (Karantonis et al., 2020), neurological illness (Réus et al., 2015), functional disorders. HCs (>18 years) were recruited at the Clinical Investigation Center (CIC) of Mondor Hospital. To be included, controls must not have a mood disorder or SCZ according to the French version of the DIGS (Diagnostic Interview for Genetic Studies) assessed by a trained psychiatrist or psychologist, must not have a known chronic inflammatory pathology. The Comité de Protection des Personnes Ile-de-France III approved the study and the research activities complied with the Helsinki Declaration. All subjects received detailed study information and gave written informed consent before inclusion.
2.2. Clinical assessment
Clinical diagnosis was established using the French version of the Structured Clinical Interview for DSM-IV (Association, 2000) while HCs were screened using the Diagnostic Interview for Genetic Studies (DIGS, French Version) (Nurnberger et al., 1994). Depressive symptom severity was scored using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) in participants with BD, while mania was assessed using the Young Mania Rating Scale (YMRS) (Young et al., 1978). Psychotic symptoms were rated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). A patient was defined as clinically stable based on the following cut-offs: MADRS<15, YMRS<8, PANSS<60. All clinical scales were used in all patient groups. Furthermore, we documented demographic variables including but not limited to age and gender, as well as patients’ clinical history (number of hospitalizations, duration of illness, number of depressive/manic/psychotic episodes and history of suicidal behavior) and in some circumstances metabolic and other health variables such as BMI, glycemia, lipids and substance use. All participants were interviewed by well-trained psychiatrists or psychologists. For inpatients, the assessment was performed as soon as possible within seven days after admission.
2.3. Measurement of serum kynurenine metabolites
Non-fasting blood samples were preferentially collected at 10 a.m. via venipuncture in gel clot (free) serum tubes (BD Vacutainer®, New Jersey, United States of America) upon clinical evaluation and immediately sent to the Biological Research repository of the Henri Mondor University Hospital for processing and storage under adequate conditions at −80 °C. Samples were shipped frozen and stored at−80 °C until analysis and were processed on ice.
KP analytes (TRP, KYN, KA, QUINA, AA, PICO, 3-HK, XA, QUINO) were measured in serum samples using liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis at Metabrain Research in Paris (SHIMADZU NEXERA2 LC system coupled to a SHIMADZU MS8050 triple quadrupole mass spectrometer). In brief, 10 μL of internal standard and 150 μL of methanol were added to 30 μL of sample. This mixture was stirred for 10 min and allowed to rest for 30min at −20 °C to support protein precipitation. After centrifugation (2250×g, 4 °C, 15min) supernatants were removed and evaporated with evaporator system (Genevac EZ-2 or HT4). Dried extracts were reconstituted in 60 μL of a mixture of Methanol (40%)/water (60%) with 0.07% of TFA. 20 μL were injected into the LC-MS/MS system and the column used is Raptor Biphenyl (100 * 3.0)mm 2.7 μm RESTEK. LC-MS/MS samples were run together with quality standard samples and compared to calibration curve ranging from 0.1 to 1000 ng/mL.
Lower detection limits varied between analysis runs, as they are dependent on sample preparation and equipment (see Supplementary Material). The laboratory staff was blinded for the analysis results. Data acquisition and processing were carried out using Labsolutions software Version 5.86.
-
4.
Statistical analysis
Because the sample size of the cohort was not determined a priori, we performed a power calculation for the ANCOVA analyses using G*power (version 3.1.9.6), which returned a 99,9% power to detect a medium sized effect (Cohen’s f = 0.25) for a total sample size of n = 692.
Only KP metabolites with less than 25% missing values were included in the analyses (TRP, KYN, KA, QUINA, AA, PICO, 3-HK, XA, QUINO). To reduce the impact of extreme outliers, a 95% winsorization of the data was performed (see Supplementary Material for means before and after winsorization), producing normal distributions for all metabolites as evaluated by visual inspection of the Q/Q plots.
All statistical analyses were performed in JMP Pro 16.0. We used crosstabulation contingency coefficients (Pearson chi-square test) for categorical demographic variables and ANOVA analyses for continuous demographic variables.
For our primary outcome, we performed ANCOVA analyses for between-group comparisons KP variables and the metabolite ratios (KYN/TRP, KA/KYN, XA/KYN and QUINO/KA) as secondary outcomes. Planned comparisons included: patients vs. controls; SCZ vs. BD patients and acute vs. stable patients with correction for age and gender. Psychotropic medication use was included as a cofactor in analysis with SCZ vs. BD and acute vs. stable patients. Correction for multiple testing was performed using False Discovery Rate (FDR).
Correlations were calculated using Spearman rank correlation coefficients. In addition, we performed a multivariate linear regression analysis, with individual KP metabolites as dependent variables.
To explore which clinical variables can be considered shared predictors of KP alterations in SCZ and BD, a multilevel stepwise regression with forward selection was used to build a hierarchical structure with predictive clinical variables: diagnostic category (HC, SCZ, BD) as level-2 factor and clinical variables as level-1 factor nested in diagnostic category. Duration of illness (DOI), age, onset age, MADRS total score, MADRS item 10 (suicidal thoughts), PANSS positive score, YMRS, gender, number of hospitalizations and of depressive/manic/psychotic episodes, history of suicide attempts as well as the interaction between DOI and symptom severity scores were included in the final model if they were statistically significant after FDR correction. Metabolic factors and information on alcohol and nicotine use were missing in more than 65% of the participants (see Supplementary Material), so these variables could not be entered in the model as potential confounders. If two clinical variables were highly correlated, only one of them was used in the regression analysis, to avoid the problem of collinearity (see results). Homoscedasticity of the residuals was evaluated by visual inspection and homogeneity of variances was assessed by Levene’s test. The output of the multivariate regression is reported as the effect estimate and the F-ratio for each significant clinical predictor per KP metabolite accompanied by the p-value.
3. Results
3.1. Demographics
We enrolled a total of 507 patients (205 aBD, 116 sBD, 111 aSCZ, 75 sSCZ) and 185 HCs in the study. Demographic variables are presented in Table 1. Significant differences in age, gender, DOI and age at onset were present between the groups. There were more male (72.6%) patients in the SCZ versus BD group (47.4%) and HCs (45.4%) and BD patients were older (M = 40.8 years, SD 14.2) compared to SCZ patients (M = 36.9 years, SD = 11.2) and controls (M = 35.3 years, SD = 13.2). Stable BD, but not sSCZ had a longer DOI (sBD, M = 19.3 years, SD = 11.9; sSCZ, (M = 14.5 years, SD = 8.7) compared to the two acute patient groups (aBD, M = 12.8 years, SD = 13,5; aSCZ = 13.7 years, SD = 11.7). In the BD stable group, only 14.9% of patients were taking psychotropic medication, which is lower than 56.0% of aBD, 93.1% of aSCZ and 84.0% of sSCZ patients. Furthermore, compared to the stable patients, the acute patients had a higher number of hospitalizations, illness episodes and reports of suicidal behavior. Notably, the acute patient groups were characterized by a heavier episode density, measured as number of hospitalizations per DOI. Stable patients had a higher education level compared to the acute group, which is most likely explained by the impact of illness severity and hospitalizations on school performance.
Table 1.
Demographic variables: mean and standard deviation.
| Controls (N = 185) | BD acute (N = 205) | BD stable (N = 116) | SCZ acute (N = 111) | SCZ stable (N = 75) | F, chi square | P value | |
|---|---|---|---|---|---|---|---|
| Gender (F,%) (N = 692) | 101 (55%) | 103 (50%) | 66 (57%) | 31 (28%) | 20 (27%) | 44.44 | <.001 |
| Age (mean, SD) (N = 692) | 35.3 (13.2) | 39.8 (15.2) | 42.4 (12.2) | 38.4 (12.5) | 34.7 (8.6) | 7.41 | <.001 |
| DOI (N = 451) | / | 12.8 (13.5) | 19.3 (11.9) | 13.7 (11.7) | 14.5 (8.7) | 7.05 | <.001 |
| Age at onset (N = 451) | / | 26.4 (10.2) | 23.3 (8.6) | 24.7 (7.0) | 20.3 (5.2) | 8.82 | <.001 |
| BMI (N = 288) | / | 27.0 (14.3) | 26.7 (6.1) | 25.2 (5.0) | 27.6 (6.5) | 2.42 | .066 |
| Education (N, %) | / | 54.84 | <.001 | ||||
|
5 (3.1) | 2 (1.9) | 3 (3.4) | 1 (4.0) | |||
|
26 (16.4) | 3 (2.8) | 19 (21.4) | 5 (20.0) | |||
|
53 (33.3) | 22 (20.4) | 43 (48.3) | 6 (24.0) | |||
|
75 (47.2) | 81 (75.0) | 24 (27.0) | 13 (52.0) | |||
| (N = 381) | |||||||
| Current psychotropic medication use (Yes, %) (N = 671) | 0 (0) | 113 (56.0) | 17 (14.9) | 95 (93.1) | 63 (84.0) | 350.24 | <.001 |
| Current antipsychotic use (Yes, %) (N = 671) | 0 (0) | 113 (58.0) | 17 (14.9) | 95 (93.1) | 63 (84.0) | 350.24 | <.001 |
| Chlorpromazine equivalent (mean, SD) (N = 610) | 0 (0) | 182.5 (267.7) | 29.3 (91.1) | 443.8 (348.3) | 360.4 (346.9) | 79.12 | <.001 |
| Current antidepressant use (Yes, %) (N = 671) | 0 (0) | 18 (9.2) | 20 (17.5) | 7 (6.9) | 26 (34.7) | 75.58 | <.001 |
| Current mood stabilizer use (Yes, %) (N = 671) | 0 (0) | 140 (71.8) | 29 (25.4) | 21 (20.6) | 12 (16.0) | 253.41 | <.001 |
| Current benzodiazepine use (Yes, %) (N = 671) | 0 (0) | 75 (38.5) | 12 (10.5) | 37 (36.3) | 12 (16.0) | 110.68 | <.001 |
| Diazepam equivalent (mean, SD) (N = 637) | 0 (0) | 7,7 (15.0) | 2,9 (17.5) | 9,6 (19.7) | 3,3 (15.4) | 10.63 | <.001 |
| YMRS (N = 453) | / | 17.4 (10.9) | 3.2 (3.8) | 13.9 (8.6) | 2.0 (3.1) | 92.80 | <.001 |
| MADRS (N = 411) | / | 16.1 (10.7) | 9.4 (9.0) | 13.0 (8.3) | 11.3 (7.1) | 10.85 | <.001 |
| PANSS_pos (N = 399) | / | 17.8 (7.7) | 8.2 (1.9) | 22.3 (5.3) | 13.2 (5.3) | 67.40 | <.001 |
| PANSS_neg (N = 561) | / | 12.6 (4.8) | 9.0 (2.5) | 20.9 (6.7) | 17.0 (7.0) | 70.53 | <.001 |
| Number of hospitalizations (N = 398) | / | 3.7 (3.9) | 1.8 (2.0) | 4.0 (3.7) | 4.2 (4.1) | 9.48 | <.001 |
| Number of depressive episodes (N = 193) | / | 2.9 (3.4) | 5.3 (4.8) | 1.1 (1.0) | 1.3 (0.8) | 10.18 | <.001 |
| Number of manic episodes (N = 209) | / | 2.6 (3.0) | 1.3 (2.3) | .7 (1.6) | 0 (0) | 6.69 | <.001 |
| Number of psychotic episodes (N = 228) | / | 0 (0) | 0 (0) | 2.9 (2.6) | 2.6 (1.8) | 70.19 | <.001 |
| History of suicide attempts (Yes, %) (N = 439) | / | 64 (36.3%) | 16 (16.3%) | 34 (35.1%) | 13 (19.1%) | 17.25 | <.001 |
| Number of suicide attempts (N = 74) | / | .8 (1.6) | .3 (1.0) | .7 (1.7) | .4 (1.0) | 3.04 | .029 |
| History of violent suicide attempts (N = 430) | / | .1 (.3) | .1 (.9) | .2 (.4) | .1 (.3) | 0.55 | .638 |
| Number of hospitalizations/DOI (N = 342) | / | 1.0 (1.8) | .1 (.2) | 1.4 (3.3) | .4 (.5) | 88.51 | <.001 |
3.2. Exploratory correlations
Spearman correlations between KP metabolites, metabolic factors and symptom severity scores in between all participants can be found in Supplementary Material.
3.3. Between-group differences
ANCOVA analyses confirmed significantly lower concentrations in all patients (n = 507) compared to controls (n = 185) for all investigated KP metabolites (TRP, KYN, KA, QUINA, XA and QUINO) except PICO, but revealed no significant difference between the two diagnostic groups (SCZ combined n = 186 vs. BD combined n = 321) (see Table 2a, Table 2b). Further, levels of TRP, KA, QUINA, QUINO and KYN/TRP ratio were lower in acute vs. stabilized patients, irrespective of underlying diagnosis (see Table 2a, Table 2b and Fig. 3).
Table 2a.
Group differences of kynurenine metabolites or ratios (ANCOVA) with age, gender and current psychotropic medication use as cofactors.
| Controls (n = 185) |
BD acute (n = 205) |
BD stable (n = 116) |
SCZ acute (n = 111) |
SCZ stable (n = 75) |
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F | FDR P-value | |
| TRP (uM) N = 591 | 63.22 (13.09) | 54.85 (17.03) | 58.85 (18.66) | 59.87 (17.52) | 65.66 (17.73) | 6.91 | .001 |
| KYN (uM) N = 591 | 2.43 (.55) | 2.38 (.77) | 2.39 (.74) | 2.19 (.67) | 2.36 (.66) | 3.06 | .002 |
| KA (nM) N = 591 | 68.71 (30.22) | 63.00 (33.87) | 71.86 (32.39) | 56.25 (25.59) | 64.92 (23.73) | 5.71 | <.001 |
| QUINA (nM) N = 591 | 11.43 (5.54) | 8.76 (5.40) | 12.46 (7.24) | 7.60 (3.75) | 11.22 (6.18) | 19.36 | <.001 |
| XA (nM) N = 591 | 314.96 (53.04) | 277.80 (69.39) | 287.16 (60.84) | 288.31 (61.29) | 309.41 (61.64) | 8.92 | <.001 |
| PICO (nM) N = 591 | 58.14 (20.39) | 61.99 (26.57) | 68.68 (29.75) | 64.06 (28.48) | 61.41 (26.49) | 2.48 | .052 |
| QUINO (nM) N = 442 | 101.37 (40.38) | 86.92 (52.04) | 96.49 (46.17) | 75.66 (38.60) | 91.87 (40.59) | 4.93 | .001 |
| KYN/TRP N = 692 | 0.040 (0.011) | 0.046 (0.019) | 0.043 (0.014) | 0.039 (0.017) | 0.038 (0.014) | 5.17 | <.001 |
| KA/KYN N = 692 | 29.42 (14.78) | 28.91 (19.32) | 32.40 (16.94) | 27.07 (13.19) | 29.11 (11.85) | 2.09 | .088 |
| XA/KYN N = 692 | 132.99 (28.17) | 124.88 (38.03) | 126.55 (31.30) | 139.78 (39.42) | 136.79 (29.69) | 3.07 | .022 |
| QUINO/KA N = 522 | 1.96 (1.51) | 1.78 (1.51) | 1.72 (1.53) | 1.80 (1.46) | 1.71 (1.11) | 0.51 | .729 |
Table 2b.
Group differences of kynurenine metabolites or ratios (ANCOVA) with age and gender as standard cofactors. Current psychotropic medication was used as cofactor in the ANCOVAs of SCZ-BD and acute-stable patients.
| Controls (n = 185) |
All patients (n = 507) |
Acute patients |
Stable patients |
BD |
SCZ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | F | FDR P-value | Mean (SD) | Mean (SD) | F | FDR P-value | Mean (SD) | Mean (SD) | F | FDR P-value | |
| TRP (uM) | 63.22 (13.09) | 58.46 (17.95) | 9.04 | .007 | 56.61 (17.34) | 61.52 (18.55) | 11.15 | .005 | 58.82 (59.35) | 59.35 (18.08) | .672 | .649 |
| KYN (uM) | 2.43 (.55) | 2.34 (.73) | 5.11 | .044 | 2.32 (.74) | 2.38 (.71) | 0.37 | .542 | 2.41 (.67) | 2.30 (.71) | 4.77 | .081 |
| KA (nM) | 68.71 (30.22) | 63.83 (30.88) | 5.83 | .035 | 60.63 (31.34) | 69.14 (29.42) | 6.33 | .045 | 65.71 (32.28) | 64.23 (30.23) | 0.16 | .692 |
| QUINA (nM) | 11.43 (5.54) | 9.72 (5.40) | 15.23 | .001 | 8.35 (4.91) | 11.98 (6.85) | 30.77 | .001 | 10.03 (5.62) | 10.08 (6.28) | 1.52 | .400 |
| XA (nM) | 314.96 (53.04) | 286.92 (65.30) | 26.29 | <.001 | 281.49 (66.75) | 295.90 (61.96) | 3.86 | .092 | 295.43 (64.81) | 287.72 (60.93) | 0.20 | .719 |
| PICO (nM) | 58.14 (20.39) | 63.89 (27.79) | 4.40 | .057 | 62.72 (27.23) | 65.85 (28.68) | 1.19 | .276 | 60.16 (24.00) | 66.42 (29.16) | 6.05 | .078 |
| QUINO (nM) | 101.37 (40.38) | 87.11 (46.75) | 11.31 | 0.003 | 82.83 (47.82) | 94.67 (43.96) | 5.94 | .034 | 93.87 (47.27) | 85.77 (43.58) | 1.17 | .310 |
| KYN/TRP | 0.040 (0.011) | 0.043 (0.017) | 1.90 | .231 | 0.044 (0.018) | .041 (.014) | 6.24 | .035 | 0.043 (.016) | 0.041 (.016) | 6.89 | .098 |
| KA/KYN | 29.42 (14.78) | 29.34 (16.65) | 0.07 | .788 | 28.26 (17.41) | 31.11 (15.19) | 2.00 | .248 | 29.15 (17.29) | 29.79 (15.42) | 0.27 | .833 |
| XA/KYN | 132.99 (28.17) | 130.29 (36.24) | 0.13 | .791 | 130.11 (39.11) | 130.57 (31.01) | 0.02 | .882 | 129.20 (33.98) | 133.02 (36.04) | 5.06 | .091 |
| QUINO/KA | 1.96 (1.51) | 1.76 (1.45) | 1.06 | .371 | 1.78 (1.72) | 1.72 (1.37) | .103 | .823 | 1.87 (1.76) | 1.51 (1.49) | 0.23 | .774 |
Fig. 3.
Z scores of the KP metabolites: controls versus acute patients (SCZ + BD) and stable patients (SCZ + BD).
Post-hoc Tukey tests revealed that none of the investigated KP analytes except XA (difference = 17.66; p = .032) deviated from HC levels in stable patients (SCZ + BD), whereas acute patients differed from HC for all analytes except PICO, with the largest effects for XA (difference = 32.68; p < .001) and QUINO (difference = 18.99; p < .001) (see Supplementary Material).
3.4. The association of KP and clinical variables in SCZ and BD
We explored the shared association of different clinical variables on KP alterations in SCZ and BD using hierarchical multivariate linear regression (see Table 3 and Fig. 2 for summary of the results). Nested within diagnostic category, PANSS positive score, sex, DOI, age at onset and the current use of mood stabilizers significantly contributed to the overall model. Number of hospitalizations and illness episodes, history of suicidal behavior and current suicidal thoughts (MADRS item 10) were no significant predictors. Interestingly, the PANSS positive score was predictive for decreases in QUINA and in the SCZ group also for KYN and KA, whereas depressive symptoms (MADRS) did not contribute to the overall model. Due to collinearity with PANSS positive score (r = .68; p=<.001), YMRS was excluded from this analysis.
Table 3.
Results of the multivariate forward stepwise nested linear regression analysis (with Z scores). For each metabolite separately, only the significant predictors are reported in the table.
| Overall model |
Predictors |
FDR p-value |
||||
|---|---|---|---|---|---|---|
| Z score DOI |
<.001 |
|||||
| Z-score PANSS_pos (Diag cat) |
<.001 |
|||||
| Mood stabilizer use (No = 0/Yes = 1) (Diag Cat) |
.003 |
|||||
| Sex |
.003 |
|||||
| Z score Onset age |
.030 |
|||||
| Diagnostic category (SCZ – BD – CT) |
.210 |
|||||
| For each KP metabolite | PredictorsPredictors | Df | Scaled estimate | Standard error | F ratio | P value |
| TRP |
DOI | 1 | −2.44 | 1.07 | 5.21 | .023 |
| Onset age | 1 | −2.36 | 1.06 | 4.95 | .027 | |
| Mood stabilizer use (0) | 2 | BD: 3.06 | 1.52 | 4.45 | .045 | |
| SCZ: 4.56 |
2.07 |
4.45 |
.028 |
|||
| KYN |
DOI | 1 | 0.18 | 0.04 | 16.93 | <.001 |
| Onset age | 1 | 0.09 | 0.04 | 4.79 | .030 | |
| PANSS_pos | 2 | BD: 0.01 | 0.06 | 2.51 | .910 | |
| SCZ: 0.16 |
0.07 |
2.51 |
.026 |
|||
| XA |
DOI | 1 | −11.93 | 3.90 | 9.34 | .002 |
| Mood stabilizer use (0) | 2 | BD: 16.90 | 5.55 | 6.61 | .003 | |
| SCZ: 15.08 | 7.57 | 6.61 | .048 | |||
| Sex |
1 |
−8.59 |
3.88 |
4.90 |
.028 |
|
| QUINO |
DOI | 1 | 8.74 | 2.89 | 9.18 | .003 |
| Onset Age | 1 | 6.17 | 2.87 | 4.62 | .032 | |
| Sex (F) |
1 |
8.98 |
2.87 |
9.67 |
.002 |
|
| PICO |
DOI |
1 |
3.85 |
1.89 |
4.15 |
.043 |
| KA |
Sex (F) | 1 | −4.19 | 1.81 | 5.38 | .021 |
| PANSS_pos | 2 | BD: 3.08 | 2.38 | 3.01 | .196 | |
| SCZ: 6.04 |
2.96 |
3.01 |
.042 |
|||
| QUINA | Onset age | 1 | 0.83 | 0.37 | 5.08 | .025 |
| Mood stabilizer use (0) | 2 | BD: 1.17 | 0.53 | 2.47 | .027 | |
| SCZ: 0.12 | 0.72 | 2.47 | .866 | |||
| PANSS_pos | 2 | BD: 1.55 | 0.49 | 9.19 | .001 | |
| SCZ: 1.68 | 0.61 | 9.19 | .006 | |||
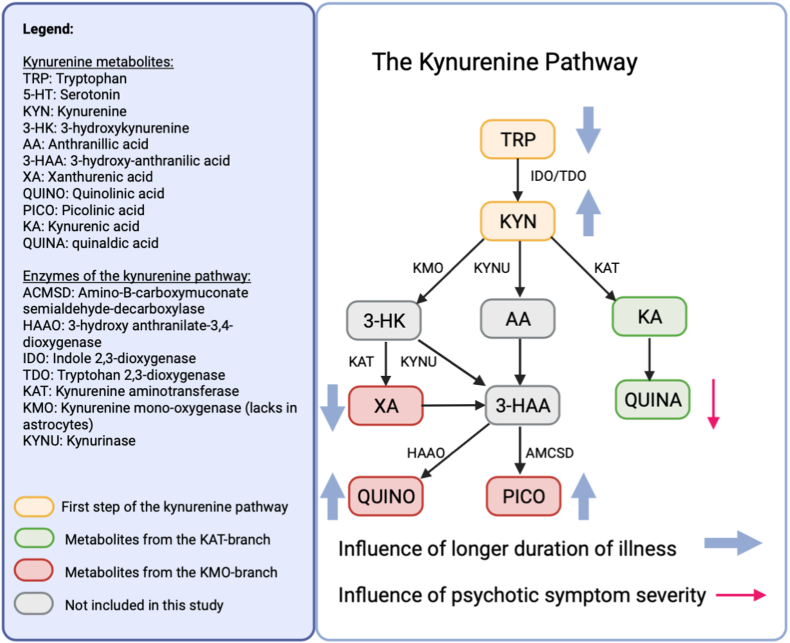
Fig. 2.
The influence of duration of illness and psychotic symptom severity on KP metabolites in SCZ and BD in our study.
Moreover, DOI contributed to increases in KYN, QUINO and PICO and to decreases in TRP and XA levels, independent of illness state, but not to the KAT-driven metabolites (KA, QUINA) (see Table 3). The effect of DOI was largest for XA (estimate = −11.93; p = .002) and QUINO (estimate = 8.74; p = .003). As age and DOI are highly correlated (r = 0.77; p=<.001), age was excluded from this analysis to avoid the problem of collinearity.
As non-specific manic symptoms like agitation, irritability and aggression impact the PANSS positive score, collinearity between YMRS and PANSS positive may be caused by this aspecific overlap in clinical presentation. To somewhat disentangle this collinearity, psychotic symptoms were alternatively defined following the factor analysis approach: the sum of items P1 delusions, G9 unusual thought content, P3 hallucinatory behavior, P6 suspiciousness and persecution, P5 grandiosity (Shafer and Dazzi, 2019). Recapitulating the previous analysis with this calculated positive symptoms factor however, gave similar results; i.e. YMRS did not contribute separately to either of the regression models.
To further explore the effect of psychotic symptomatology, the combined patient sample was stratified in low (PANSS positive score range: 7–13; n = 154), mid (PANSS positive score range: 14–20; n = 118) and high (PANSS positive score range >20; n = 127) psychotic symptom scores. We found a significant between-group difference for QUINA (F = 12.28, p < .001). Post-hoc Tukey tests further revealed significant differences for QUINA between low vs. high and low vs. mid positive PANSS scores (see Supplementary Material).
4. Discussion
To date, this is the largest study to investigate KP metabolites in SCZ and BD and the first to demonstrate state-specific and illness duration-related predictors of KP metabolite levels in these disorders. Strengths of our work are the large sample size, the inter-diagnostic approach of scrutinizing both SCZ and BD patients, a priori stratification in patient subgroups based on illness state and measurement of a multitude of KP metabolites. Especially, our study comprises the investigation of QUINA levels, which has seldomly been investigated in previous studies. Furthermore, the availability of fine-grained clinical information allowed a thorough exploration of the effect of different symptom dimensions on KP metabolites.
This study confirms previously demonstrated decreased concentrations of multiple KP analytes, consistent with an overall downregulation of the pathway, in all patients compared to controls. These peripheral findings cannot be extrapolated to the KP in the brain or CSF (Almulla et al., 2022). However, it is widely demonstrated that peripheral KP levels do represent valuable biomarkers and are associated with symptom severity (Skorobogatov et al., 2021). A plausible explanation for the peripheral downregulation of the KP in mood and psychotic disorders, lies in the involvement of the KP in the IRS and CIRS. IRS activation initially leads to the stimulation of IDO and the upregulation of the KP (Roomruangwong et al., 2020). However, IDO has anti-inflammatory and anti-oxidative effects and KYN and XA suppress T-cell proliferation. Thus, the KP has an established role in CIRS by exerting a negative feedback signal to the pro-inflammatory response (Maes et al., 2007).
Our exploratory analysis highlighted illness state, and specifically acute psychotic symptoms as well as DOI and age to mediate KP abnormalities in SCZ and BD patients. Specifically, we observed reductions in the KAT-driven metabolites (KA in SCZ and QUINA in both SCZ and BD) in psychotic states. A more complex relationship was observed between DOI and (mainly KMO-driven) KP metabolites, since longer DOI inflates KYN, QUINO and PICO levels, but is associated with a decrease in TRP and XA, irrespectively from the impact of illness state. As our study was cross-sectional in nature, more work is needed to confirm the direction of causality.
4.1. Kynurenine metabolite alterations are transdiagnostic and state-specific
Our results show that, although SCZ and BD patients differed from HC regarding TRP, KYN, KA, QUINA, XA, and QUINO levels, there were no inter-diagnostic differences between SCZ and BD patients. In contrast, acute illness state, mainly driven by psychotic symptomatology, was associated with reductions in most KP marker concentrations. Based on these findings, KP alterations in SCZ and BD should be considered as transdiagnostic and state-specific.
Several meta-analyses previously confirmed lower KP metabolite blood levels in SCZ (Morrens et al., 2020b; Marx et al., 2021), BD (Marx et al., 2021; Hebbrecht et al., 2021) and major depressive disorder (MDD) (Marx et al., 2021). Furthermore, the transdiagnostic nature of these aberrations demonstrated here is consistent with similar findings for cytokine aberrances across mood and psychotic disorders during the acute and chronic illness states (Marx et al., 2021; Goldsmith et al., 2016). Acute state was associated with an inflammatory pattern consistent with T-cell activation, characterized by elevated levels of IL-6 and TNF-α and increased expression of IL-1RA and sIL-2R) in MDD, SCZ and BD. Treatment resulted in a decrease in pro-inflammatory cytokines and an increase in anti-inflammatory cytokines. The present findings confirm KP metabolites as equally strong state dependent immunomarkers, potentially bridging these pro-inflammatory aberrances to altered neurotransmission underlying symptomatology related to acute illness. In parallel, state-independent immune changes equally persist in MDD, SCZ and BD, as stabilized outpatients also manifested increased levels of IL-6 and elevated levels of IL-1β and sIL-2R in SCZ and BD (Goldsmith et al., 2016). In our study, only XA was decreased in stabilized patients compared to controls and thus is a potential trait marker for SCZ and BD.
These transdiagnostic findings accord with the evidence for microglial activation, which is not diagnosis-specific, nor consistent in all psychiatric patients (Mondelli et al., 2017). It has been suggested that increased microglial activity is associated with severe states of psychiatric diseases, such as suicidality, rather than with a particular diagnosis (Mondelli et al., 2017).
The above adds to the hypothesis that immune changes are not diagnosis-specific and thus may reflect shared underlying pathophysiological changes in immune pathways. It would be enthralling to unravel whether the transdiagnostic nature of KP disturbances covers all forms of severe mental illness and also applies for e.g. MDD.
4.2. KP aberrations in the KAT branch are mainly driven by acute illness state
Decreased KP metabolite concentrations appear to be more pronounced in acutely ill patients. This effect was most apparent for KYN and the metabolites from the KAT branch, KA and QUINA. This finding is strengthened by the meta-analysis of Morrens et al. (Morrens et al., 2020b; Mondelli et al., 2017), which showed decreased TRP, KA and QUINO only in studies including acute SCZ patients, and not in studies on stabilized peers. The state dependency of these KP deviations is in line with the moderating effect of symptom severity on pro-inflammatory cytokines (increased IL1−β and IL-6), CIRS products such as TGF-b and other peripheral immune markers such as IL-8, IL1RA, TNF-α, CCL-2 (Miller et al., 2011), increased autoantibody concentrations (Ezeoke et al., 2013) and blood lymphocyte abnormalities (increased CD4/CD8 ratio) (Miller et al., 2013), that were independent of pharmacological treatment. A recent imaging study found that acute, untreated psychosis in SCZ patients (n = 21) was associated with astrocyte dysfunction, evidenced by reduced uptake of myo-inositol in the anterior cingulate cortex, and consequent glutamate changes. Interestingly, gradual normalization of myo-inositol levels was concomitant with decreasing psychotic symptom severity, arguing for dynamic astrocyte changes in the acute state of SCZ (Jeon et al., 2021).
Although studies examining peripheral values consequently find lower KA values in psychotic and mood disorders, CSF and post-mortem brain studies show elevated levels of KA in SCZ and BD (Erhardt et al., 2001; Schwarcz et al., 2001; Miller et al., 2006; Olsson et al., 2010). It is important to note that KA does not pass the BBB under normal conditions, which explains the discrepancy with elevated central levels (Fukui et al., 1991). KA is thought to be involved in the pathophysiology of SCZ by mediating glutamatergic, dopaminergic, and cholinergic neurotransmission causing psychotic and cognitive symptoms (Erhardt et al., 2017).
In our analysis, psychotropic medication use and psychotic symptoms are both associated with lower levels of KP metabolites. However, in a cross-sectional observational study, it is not possible to entangle the effects of psychotropic medication and psychiatric symptoms on KP levels. A recent meta-analysis demonstrated that medicated SCZ patients have higher KYN levels compared to controls (Cao et al., 2021). Previous longitudinal studies have shown that antipsychotic treatment partially reverses KP aberrances (De Picker et al., 2019; Myint et al., 2011; Kim et al., 2009). However, it is unclear whether KP levels normalize because of direct medication effects or because of the diminution of psychotic symptoms.
4.3. The impact of duration of illness is more complex
Longer DOI seems to aggravate altered KP metabolism in all patients, independent of diagnosis or illness state. Thus, psychotic and mood disorders seem to affect these immune markers differentially in the short and the long term. Apparently, these abnormalities present themselves in KMO-driven metabolites, as mostly XA and QUINO, but not KAT metabolite levels (KA, QUINA) are found deviant.
Available literature on the association between DOI and peripheral KP markers in SCZ is scarce. Negative correlations between serum KA and DOI were previously reported in a small sample of SCZ patients (n = 51; r = -0.327, p < .05), which is not in line with our results (Szymona et al., 2017). Barry and colleagues (Barry et al., 2009) reported a positive correlation between the KYN/TRP ratio in plasma and DOI (n = 34; r = 0.36; p = .04), whereas this association was not confirmed by others (Kindler et al., 2020).
Of note, as age and DOI were highly intercorrelated (r = 0.77; p=<.001), age as confounder was left out of the analyses. Nonetheless, an age-dependency of microglial activity in psychotic patients potentially underlying KMO-driven KP changes was recently described in two studies, while the effect of duration of illness was not significant (Ottoy et al., 2018; Picker et al., 2019). Furthermore, age-related immune-induced and oxidative damage are aggravated in psychotic and mood patients compared to healthy controls, which has led to the accelerated aging hypothesis in SCZ and mood disorders (Morrens et al., 2020a; van den Ameele et al., 2018; Savitz, 2020). A recent study in mice and human brains found that microglia in the aging brain show impaired phagocytosis and play a significant role in age-related neuro-inflammation by secreting elevated levels of reactive oxygen species (ROS) and cytokines, such as IL-6 and TNF-α (Marschallinger et al., 2020).
4.4. Limitations
The current study has some limitations. The observational nature and cross-sectional design of the study preclude causal inferences. Large-scale follow-up studies could establish the relationship between acute symptom severity and abnormalities in the KP more precisely. Second, although efforts were made to control for known moderators like gender, other factors such as BMI, smoking or alcohol use, known to impact the KP, could not be controlled for in the current sample due to missing values. However, all correlations between metabolic factors and KP metabolites were either low (r < 0.30) or non-significant. Also, BMI did not differ between the groups (see demographics table). Another source of important missing data is the lack of symptom severity scales in the control group. Third, although disturbances in the KP are mostly investigated in plasma/serum, it is unclear to what extent these peripheral measurements reflect brain concentrations (Erhardt et al., 2001; Cao et al., 2021) (Skorobogatov et al., 2021; Fukui et al., 1991). KP metabolite measurements are preferentially investigated in the CSF through lumbar punctures, but this technique is painful and invasive and not common practice in clinical psychiatry. Last, although there is an important overlap in the symptomatology of mania and psychosis, the possible interference of manic symptoms on the acute state effects in this study cannot be completely ruled out. However, items of the PANSS related to resistance or excitement, which is an important symptom of mania, did not contribute to the model in the presence of psychotic symptom items, which underlines the hypothesis that psychosis is the main driver for acute state effects.
4.5. Methodological issues in KP research
Bioanalytical inconsistencies are a common phenomenon in the research field of KP metabolism and may considerably affect KP metabolite quantification (Young and Anderson, 2010; Coppens et al., 2022). Here, metabolite concentrations were determined by liquid chromatography - mass spectrometry (LC-MS/MS), which is considered the gold standard for KP metabolite measurement but can be sensitive to quality issues. Illustratively, blood levels of QUINO and some other KP markers often border on the lower detection limits of these techniques; thereby contributing to inter-study discrepancies (Young and Anderson, 2010). Moreover, methodological differences across studies (e.g. plasma vs. serum, storage conditions, blood vs. brain/CSF type of chromatographic technology …) resulted in varying concentration ranges (Coppens et al., 2022).
Compared to other KP metabolites, research on the KMO-branch has yielded even more diverging results, illustrated by increased (Young et al., 2016; Ravikumar et al., 2000), unaltered (van den Ameele et al., 2020; Brundin et al., 2016; Savitz et al., 2015b) and - in line with the current study - decreased (Tawfik et al., 2020; Marschallinger et al., 2020; Young and Anderson, 2010) (Fazio et al., 2015; Castillo et al., 2020; Liu et al., 2018) QUINO blood levels. These different results possibly explain the overall nonsignificant changes in MDD, BD and SCZ resulting from meta-analyses (Marx et al., 2021; Hebbrecht et al., 2021).
5. Conclusion
This large-scale study confirms that state-specific rather than diagnosis-specific effects determine KP alterations in SCZ and BD. Moreover, aberrations in KP metabolites not only appear to be a transdiagnostic feature of SCZ and BD patients, but also seem independently associated with acute psychotic symptoms and a longer duration of illness. This suggests that the divergences across studies may reflect the dynamic nature of these abnormalities related to the illness course. QUINA has seldomly been investigated by previous studies and appears an important state marker in SCZ and BD. Future studies should take these clinical factors into account when interpreting KP abnormalities in psychiatric patients. Clinical trials on staging could provide more insight in the relationship between duration of illness and KP metabolites. Longitudinal studies in medicated and unmedicated stable patients are useful to gain insight in the effects of psychotropic medication on the KP. A large-scale longitudinal study with both peripheral and CSF samples is desirable to validate the current results.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The I-GIVE study was funded by Agence Nationale de la Recherche (I-GIVE ANR-13-SAMA-0004-01), INSERM (Institut National de la Santé et de la Recherche Médicale) and Fondation FondaMental, in France. We express our gratitude to the patients who participated to this study, the clinical teams from the University department of Psychiatry and Addictology of Henri Mondor hospital (DMU IMPACT and FHU ADAPT), as well as the team from the Biobank of Henri Mondor Hospital (AP-HP) who stored the biological samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2022.100584.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Albuquerque E.X., Schwarcz R. Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: facts and challenges [Internet] Biochem. Pharmacol. 2013;85:1027–1032. doi: 10.1016/j.bcp.2012.12.014. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almulla A.F., Vasupanrajit A., Tunvirachaisakul C., Al-Hakeim H.K., Solmi M., Verkerk R., et al. The tryptophan catabolite or kynurenine pathway in schizophrenia: meta-analysis reveals dissociations between central, serum, and plasma compartments. Mol. Psychiatr.[Internet] 2022;27(9):3679–3691. doi: 10.1038/s41380-022-01552-4. Available from: [DOI] [PubMed] [Google Scholar]
- van den Ameele S., Fuchs D., Coppens V., de Boer P., Timmers M., Sabbe B., et al. Markers of inflammation and monoamine metabolism indicate accelerated aging in bipolar disorder [internet] Front. Psychiatr. 2018;9 doi: 10.3389/fpsyt.2018.00250. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ameele S., van Nuijs A.L., Lai F.Y., Schuermans J., Verkerk R., van Diermen L., et al. A mood state-specific interaction between kynurenine metabolism and inflammation is present in bipolar disorder. Bipolar Disord. 2020;22(1):59–69. doi: 10.1111/bdi.12814. Available from: [DOI] [PubMed] [Google Scholar]
- Anttila V, Bulik-Sullivan B, Finucane H, Walters R, Bras J, Duncan L, et al.. Anal.Shared Heritability Common. Disord.Brain. Available from:: 10.1101/048991. [DOI]
- Association A.P. American psychiatric association. Diagnostic and statistical manual of mental disorders, fourth edition. Text Revis.(DSM-IV-TR) 2000 doi: 10.1176/appi.books.9780890423349. Available from: [DOI] [Google Scholar]
- Badawy A.A.B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 2017;10 doi: 10.1177/1178646917691938. 1178646917691938. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J., Alkondon M., Pereira E.F.R., Albuquerque E.X. Regulation of GABAergic Inputs to CA1 Pyramidal Neurons by Nicotinic Receptors and Kynurenic Acid [Internet] J. Pharmacol. Exp. Therapeut. 2012;341:500–509. doi: 10.1124/jpet.111.189860. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry S., Clarke G., Scully P., Dinan T.G. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation [Internet] J. Psychopharmacol. 2009;23:287–294. doi: 10.1177/0269881108089583. Available from: [DOI] [PubMed] [Google Scholar]
- Brundin L., Sellgren C.M., Lim C.K., Grit J., Pålsson E., Landén M., et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl. Psychiatry. 2016;6(8):e865. doi: 10.1038/tp.2016.133. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Chen Y., Ren Z., Pan Z., McIntyre R.S., Wang D. Dysregulation of kynurenine pathway and potential dynamic changes of kynurenine in schizophrenia: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. [Internet] 2021;123:203–214. doi: 10.1016/j.neubiorev.2021.01.018. Available from: [DOI] [PubMed] [Google Scholar]
- Castillo M.F.R., Murata S., Schwarz M., Schütze G., Moll N., Martin B., et al. Corrigendum to “celecoxib augmentation of escitalopram in treatment-resistant bipolar depression and the effects on quinolinic acid” [neurology, psychiatry and brain research 32 (2019) june 22–29] [internet] Neurol. Psychiatr. Brain Res. 2020;36:98–100. doi: 10.1016/j.npbr.2019.10.005. Available from: [DOI] [Google Scholar]
- Coppens V., Verkerk R., Morrens M. Tracking trycat: a critical appraisal of kynurenine pathway quantifications in blood. Front Pharmacol. 2022;13:825948. doi: 10.3389/fphar.2022.825948. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F., Katsafanas E., Schweinfurth L.A.B., Savage C.L.G., Stallings C., Origoni A., et al. Immune alterations in acute bipolar depression [Internet] Acta Psychiatr. Scand. 2015;132:204–210. doi: 10.1111/acps.12451. Available from: [DOI] [PubMed] [Google Scholar]
- Erhardt S., Blennow K., Nordin C., Skogh E., Lindström L.H., Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313(1–2):96–98. doi: 10.1016/s0304-3940(01)02242-x. Available from: [DOI] [PubMed] [Google Scholar]
- Erhardt S., Schwieler L., Imbeault S., Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder [Internet] Neuropharmacology. 2017;112:297–306. doi: 10.1016/j.neuropharm.2016.05.020. Available from: [DOI] [PubMed] [Google Scholar]
- Ezeoke A., Mellor A., Buckley P., Miller B. A systematic, quantitative review of blood autoantibodies in schizophrenia. Schizophr. Res. 2013;150(1):245–251. doi: 10.1016/j.schres.2013.07.029. Available from: [DOI] [PubMed] [Google Scholar]
- Faurbye A. The role of amines in the etiology of schizophrenia. Compr. Psychiatry. 1968;9(2):155–177. doi: 10.1016/s0010-440x(68)80051-3. Available from: [DOI] [PubMed] [Google Scholar]
- Fazio F., Lionetto L., Curto M., Iacovelli L., Cavallari M., Zappulla C., et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci. Rep. 2015;5:17799. doi: 10.1038/srep17799. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S., Schwarcz R., Rapoport S.I., Takada Y., Smith Q.R. Blood?Brain barrier transport of kynurenines: implications for brain synthesis and metabolism [internet] J. Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. Available from: [DOI] [PubMed] [Google Scholar]
- Gandal M., Zhang P., Walker R., Won H., Hadjimichael E., van Bakel H., et al. SA75LARGE-SCALE TRANSCRIPTOME-WIDE characterization of asd, schizophrenia, and bipolar disorder [internet] Eur. Neuropsychopharmacol. 2019;29:S1228. doi: 10.1016/j.euroneuro.2018.08.297. Available from: [DOI] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatr.[Internet] 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarman B.C.M., Haarman B.C., Riemersma-Van der Lek R., de Groot J.C., Ruhé H.G., Klein H.C., et al. Neuroinflammation in bipolar disorder – a [11C]-(R)-PK11195 positron emission tomography study [Internet] Brain Behav. Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. Available from: [DOI] [PubMed] [Google Scholar]
- Hebbrecht K., Skorobogatov K., Giltay E.J., Coppens V., De Picker L., Morrens M. Tryptophan catabolites in bipolar disorder: a meta-analysis. Front. Immunol. 2021;12:667179. doi: 10.3389/fimmu.2021.667179. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmas C., Pereira E.F., Alkondon M., Rassoulpour A., Schwarcz R., Albuquerque E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21(19):7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D.C., Zukin S.R., Heresco-Levy U., Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia [internet] Schizophr. Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon P., Mackinley M., Théberge J., Palaniyappan L. The trajectory of putative astroglial dysfunction in first episode schizophrenia: a longitudinal 7-Tesla MRS study [Internet] Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01773-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantonis J.A., Rossell S.L., Carruthers S.P., Sumner P., Hughes M., Green M.J., et al. Cognitive validation of cross-diagnostic cognitive subgroups on the schizophrenia-bipolar spectrum. J. Affect Disord. 2020;266:710–721. doi: 10.1016/j.jad.2020.01.123. Available from: [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. Available from: [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Myint A.M., Verkerk R., Scharpe S., Steinbusch H., Leonard B. Cytokine changes and tryptophan metabolites in medication-naïve and medication-free schizophrenic patients [internet] Neuropsychobiology. 2009;59:123–129. doi: 10.1159/000213565. Available from: [DOI] [PubMed] [Google Scholar]
- Kindler J., Lim C.K., Weickert C.S., Boerrigter D., Galletly C., Liu D., et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatr.[Internet] 2020;25(11):2860–2872. doi: 10.1038/s41380-019-0401-9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.C., Yang Y.Y., Chou Y.M., Chen K.P., Shen W.W., Leu S.J. Immunologic variables in acute mania of bipolar disorder. J. Neuroimmunol. 2004;150(1–2):116–122. doi: 10.1016/j.jneuroim.2004.01.006. Available from: [DOI] [PubMed] [Google Scholar]
- Liu H., Ding L., Zhang H., Mellor D., Wu H., Zhao D., et al. The Metabolic Factor Kynurenic Acid of Kynurenine Pathway Predicts Major Depressive Disorder [Internet] Front. Psychiatr. 2018;9 doi: 10.3389/fpsyt.2018.00552. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madre M., Canales-Rodríguez E.J., Fuentes-Claramonte P., Alonso-Lana S., Salgado-Pineda P., Guerrero-Pedraza A., et al. Structural abnormality in schizophrenia versus bipolar disorder: a whole brain cortical thickness, surface area, volume and gyrification analyses. Neuroimage Clin. 2020;25:102131. doi: 10.1016/j.nicl.2019.102131. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Carvalho A.F. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 2018;55(12):8885–8903. doi: 10.1007/s12035-018-1016-x. Available from: [DOI] [PubMed] [Google Scholar]
- Maes M., Mihaylova I., Ruyter M.D., Kubera M., Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the Ido pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol. Lett. 2007;28(6):826–831. https://www.ncbi.nlm.nih.gov/pubmed/18063923 Available from: [PubMed] [Google Scholar]
- Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness [Internet] Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. Available from: [DOI] [PubMed] [Google Scholar]
- Marschallinger J., Iram T., Zardeneta M., Lee S.E., Lehallier B., Haney M.S., et al. Author Correction: lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain [Internet] Nat. Neurosci. 2020;23 doi: 10.1038/s41593-020-0595-9. 294–294. Available from: [DOI] [PubMed] [Google Scholar]
- Marx W., McGuinness A.J., Rocks T., Ruusunen A., Cleminson J., Walker A.J., et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol. Psychiatr.[Internet] 2021;26(8):4158–4178. doi: 10.1038/s41380-020-00951-9. Available from: [DOI] [PubMed] [Google Scholar]
- Miller C.L., Llenos I.C., Dulay J.R., Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. Available from: [DOI] [PubMed] [Google Scholar]
- Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.J., Gassama B., Sebastian D., Buckley P., Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatry. 2013;73(10):993–999. doi: 10.1016/j.biopsych.2012.09.007. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Vernon A.C., Turkheimer F., Dazzan P., Pariante C.M. Brain microglia in psychiatric disorders. Lancet. Psychiatry. 2017;4(7):563–572. doi: 10.1016/S2215-0366(17)30101-3. Available from: [DOI] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. Available from: [DOI] [PubMed] [Google Scholar]
- Morrens M., Coppens V., Walther S. Do immune dysregulations and oxidative damage drive mood and psychotic disorders? Neuropsychobiology. 2020;79(4–5):251–254. doi: 10.1159/000496622. Available from: [DOI] [PubMed] [Google Scholar]
- Morrens M., De Picker L., Kampen J.K., Coppens V. Blood-based kynurenine pathway alterations in schizophrenia spectrum disorders: a meta-analysis. Schizophr. Res. 2020;223:43–52. doi: 10.1016/j.schres.2020.09.007. Available from: [DOI] [PubMed] [Google Scholar]
- Muller N., Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10(2):131–148. doi: 10.1007/BF03033242. Available from: [DOI] [PubMed] [Google Scholar]
- Myint A.M., Kim Y.K. Network beyond Ido in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog. Neuropsychopharmacol.Biol. Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. Available from: [DOI] [PubMed] [Google Scholar]
- Myint A.M., Kim Y.K., Verkerk R., Park S.H., Scharpé S., Steinbusch H.W.M., et al. Tryptophan breakdown pathway in bipolar mania. J. Affect Disord. 2007;102(1–3):65–72. doi: 10.1016/j.jad.2006.12.008. Available from: [DOI] [PubMed] [Google Scholar]
- Myint A.M., Schwarz M.J., Verkerk R., Mueller H.H., Zach J., Scharpé S., et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients [Internet] Brain Behav. Immun. 2011;25:1576–1581. doi: 10.1016/j.bbi.2011.05.005. Available from. [DOI] [PubMed] [Google Scholar]
- Nurnberger J.I., Jr., Blehar M.C., Kaufmann C.A., York-Cooler C., Simpson S.G., Harkavy-Friedman J., et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch. Gen. Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–4. Available from: [DOI] [PubMed] [Google Scholar]
- Olsson S.K., Samuelsson M., Saetre P., Lindström L., Jönsson E.G., Nordin C., et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatr. Neurosci. 2010;35(3):195–199. doi: 10.1503/jpn.090180. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoy J., De Picker L., Verhaeghe J., Deleye S., Wyffels L., Kosten L., et al. F-PBR111 PET imaging in healthy controls and schizophrenia: test-retest reproducibility and quantification of neuroinflammation. J. Nucl. Med.. 2018;59(8):1267–1274. doi: 10.2967/jnumed.117.203315. Available from: [DOI] [PubMed] [Google Scholar]
- Pérez-De La Cruz V., Carrillo-Mora P., Santamaría A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012;5:1–8. doi: 10.4137/IJTR.S8158. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasch-Parwez E., Schöbel A., Benali A., Moinfar Z., Förster E., Brüne M., et al. Correction to: lateralization of increased density of Iba1-immunopositive microglial cells in the anterior midcingulate cortex of schizophrenia and bipolar disorder. Eur. Arch. Psychiatry. Clin. Neurosci . 2022;272(1):171. doi: 10.1007/s00406-021-01339-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L.J.D., De Picker L.J., Morrens M., Chance S.A., Boche D. Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review [internet] Front. Psychiatr. 2017;8 doi: 10.3389/fpsyt.2017.00238. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Picker L., Fransen E., Coppens V., Timmers M., de Boer P., Oberacher H., et al. Immune and neuroendocrine trait and state markers in psychotic illness: decreased kynurenines marking psychotic exacerbations. Front. Immunol. 2019;10:2971. doi: 10.3389/fimmu.2019.02971. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L.D., De Picker L., Ottoy J., Verhaeghe J., Deleye S., Wyffels L., et al. State-associated changes in longitudinal [18F]-PBR111 TSPO PET imaging of psychosis patients: evidence for the accelerated ageing hypothesis? Inside Internet. 2019;77:46–54. doi: 10.1016/j.bbi.2018.11.318. Brain, Behavior, and Immunity. Available from: [DOI] [PubMed] [Google Scholar]
- Ravikumar A., Deepadevi K.V., Arun P., Manojkumar V., Kurup P.A. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol. India. 2000;48(3):231–238. https://www.ncbi.nlm.nih.gov/pubmed/11025626 Available from: [PubMed] [Google Scholar]
- Réus G.Z., Fries G.R., Stertz L., Badawy M., Passos I.C., Barichello T., et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders [Internet] Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. Available from: [DOI] [PubMed] [Google Scholar]
- Roomruangwong C., Noto C., Kanchanatawan B., Anderson G., Kubera M., Carvalho A.F., et al. The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol. Neurobiol. 2020;57(2):778–797. doi: 10.1007/s12035-019-01737-z. Available from: [DOI] [PubMed] [Google Scholar]
- Savitz J. The kynurenine pathway: a finger in every pie [Internet] Mol. Psychiatr. 2020;25:131–147. doi: 10.1038/s41380-019-0414-4. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J., Drevets W.C., Smith C.M., Victor T.A., Wurfel B.E., Bellgowan P.S.F., et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder [internet] Neuropsychopharmacology. 2015;40:463–471. doi: 10.1038/npp.2014.194. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J., Dantzer R., Meier T.B., Wurfel B.E., Victor T.A., McIntosh S.A., et al. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. 2015;62:54–58. doi: 10.1016/j.psyneuen.2015.07.609. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R., Rassoulpour A., Wu H.Q., Medoff D., Tamminga C.A., Roberts R.C. Increased cortical kynurenate content in schizophrenia [Internet] Biol. Psychiatr. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. Available from: [DOI] [PubMed] [Google Scholar]
- Shafer A., Dazzi F. Meta-analysis of the positive and negative syndrome scale (PANSS) factor structure. J. Psychiatr. Res. 2019;115:113–120. doi: 10.1016/j.jpsychires.2019.05.008. Available from: [DOI] [PubMed] [Google Scholar]
- Skorobogatov K., De Picker L., Verkerk R., Coppens V., Leboyer M., Müller N., et al. Brain versus blood: a systematic review on the concordance between peripheral and central kynurenine pathway measures in psychiatric disorders [internet] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.716980. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M., Suresh Sharma M., Osimo E.F., Fornaro M., Bortolato B., Croatto G., et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: a meta-analysis of mean differences and variability. Brain Behav. Immun. 2021;97:193–203. doi: 10.1016/j.bbi.2021.07.014. Available from: [DOI] [PubMed] [Google Scholar]
- Stahl S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate [Internet] CNS Spectr. 2018;23:187–191. doi: 10.1017/s1092852918001013. Available from: [DOI] [PubMed] [Google Scholar]
- Stahl E.A., Breen G., Forstner A.J., McQuillin A., Ripke S., Trubetskoy V., et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019;51(5):793–803. doi: 10.1038/s41588-019-0397-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymona K., Zdzisińska B., Karakuła-Juchnowicz H., Kocki T., Kandefer-Szerszeń M., Flis M., et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32(1):17–26. doi: 10.1007/s12640-017-9714-0. Available from: [DOI] [PubMed] [Google Scholar]
- Tavares R.G., Tasca C.I., Santos C.E.S., Wajner M., Souza D.O., Dutra-Filho C.S. Quinolinic acid inhibits glutamate uptake into synaptic vesicles from rat brain [Internet] Neuroreport. 2000;11:249–254. doi: 10.1097/00001756-200002070-00005. Available from: [DOI] [PubMed] [Google Scholar]
- Tavares R.G., Tasca C.I., Santos C.E.S., Alves L.B., Porciúncula L.O., Emanuelli T., et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002;40(7):621–627. doi: 10.1016/s0197-0186(01)00133-4. Available from: [DOI] [PubMed] [Google Scholar]
- Tawfik D.M., Lankelma J.M., Vachot L., Cerrato E., Pachot A., Joost Wiersinga W., et al. Comparison of host immune responses to LPS in human using an immune profiling panel, in vivo endotoxemia versus ex vivo stimulation [Internet] Sci. Rep. 2020;10 doi: 10.1038/s41598-020-66695-2. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey E.F., Barci B.M., Webster M.J., Bartko J.J., Meador-Woodruff J.H., Knable M.B. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol. Psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. Available from: [DOI] [PubMed] [Google Scholar]
- Vasupanrajit A., Jirakran K., Tunvirachaisakul C., Solmi M., Maes M. Inflammation and nitro-oxidative stress in current suicidal attempts and current suicidal ideation: a systematic review and meta-analysis. Mol. Psychiatr.[Internet] 2022;27(3):1350–1361. doi: 10.1038/s41380-021-01407-4. Available from: [DOI] [PubMed] [Google Scholar]
- Witt S.H., Streit F., Jungkunz M., Frank J., Awasthi S., Reinbold C.S., et al. Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. Transl. Psychiatry. 2017;7(6):e1155. doi: 10.1038/tp.2017.115. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S.N., Anderson G.M. Bioanalytical inaccuracy: a threat to the integrity and efficiency of research [Internet] J. Psychiatr. Neurosci. 2010;35:3–6. doi: 10.1503/jpn.090171. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. Available from: [DOI] [PubMed] [Google Scholar]
- Young K.D., Drevets W.C., Dantzer R., Teague T.K., Bodurka J., Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav. Immun. 2016;56:335–342. doi: 10.1016/j.bbi.2016.04.007. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.