Abstract
Objectives
During the COVID-19 pandemic, no country with widespread community transmission has avoided outbreaks or deaths in residential aged care facilities (RACFs). As RACF residents are at high risk of morbidity and mortality from COVID-19, understanding disease severity risk factors is imperative.
Design
This retrospective cohort study aimed to compare COVID-19 disease severity (hospitalization and deaths) and associated risk factors among RACF residents in Victoria, Australia, across Delta and Omicron epidemic periods.
Settings and Participants
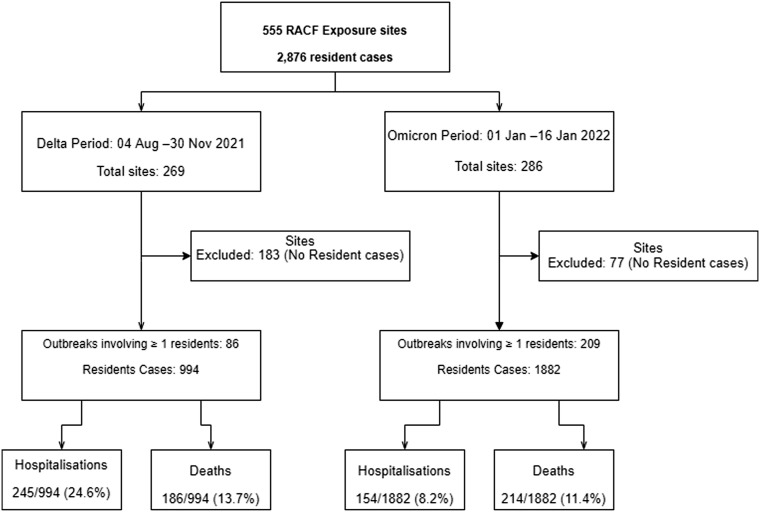
Resident case hospitalization risk (HR) and case fatality risk (CFR) were assessed using Victorian RACFs COVID-19 outbreaks data across 2 epidemic periods; Delta, 994 resident cases linked to 86 outbreaks; and Omicron, 1882 resident cases linked to 209 outbreaks.
Methods
Adjusting for outbreak-level clustering, age, sex, up-to-date vaccination status, and time since last vaccination, the odds of hospitalization and death were compared using mixed effects logistic regression.
Results
The HR and CFR was lower during the Omicron period compared with the Delta period [HR 8.2% vs 24.6%, odds ratio (OR) 0.17, 95% CI 0.11-0.26, and CFR: 11.4% vs 18.7%, OR 0.40, 95% CI 0.28-0.56]. During both periods, males had higher odds of hospitalization and odds of death; being up to date with vaccination reduced odds of hospitalization by 40% (excluding nonemergency patient transfers) and odds of death by 43%; and for each month since last vaccination, odds of hospitalization increased by 9% and odds of death by 16%.
Conclusions and Implications
This study provides empirical evidence of lower COVID-19 severity among RACF residents in the Omicron period and highlights the importance of up-to-date and timely vaccination to reduce disease severity in this cohort.
Keywords: COVID-19 severity outcomes, vaccination, residential aged care facilities
With widespread community transmission, no country has been able to avoid outbreaks or deaths due to coronavirus disease (COVID-19) in residential aged care facilities (RACFs). Aged care residents are at high risk of morbidity and mortality associated with COVID-19, and accounted for only 7% of cases but 75% of deaths in Australia in 2020.1 , 2 Understanding risk factors associated with disease severity in these cohorts is imperative.
The goals of the COVID-19 public health response in Australia evolved over time, from elimination to containment and reduction of transmission in high-risk settings. In Victoria, control strategies up to late 2021 involved highly restrictive isolation, contact tracing, quarantining, and outbreak management policies in community and high-risk settings, including RACFs.3 These measures were taken in a context of limited understanding of the pathophysiology of the severe acute respiratory syndrome coronavirus 2 (SARS CoV2), lack of antiviral treatments, low population vaccination coverage, and subsequent emergence of the more transmissible and severe Delta variant of concern (VOC).
However, increasing recognition of the harmful effects of restrictions on community mental health and well-being, particularly for the residents and workforce of aged care facilities,4 , 5 as well as increased vaccination coverage and evidence of effectiveness of vaccines against Delta VOC,6 led the Victoria government to ease restrictions toward the end of 2021. At the same time the Omicron VOC7 , 8 emerged, which resulted in the largest wave of the epidemic to date, from late December 2021 to late February 2022. This was later attributed to the apparent increased transmissibility of the Omicron infections, reduced vaccine effectiveness,9 and reduced restrictions on movement. This period was characterized by a large absolute number of deaths due to COVID-19 and a significant increase in excess mortality in older Victorians,10 despite lower clinical severity of Omicron compared to Delta.11, 12, 13
Understanding the impacts of Delta and Omicron VOCs on high-risk cohorts such as RACF residents may inform future public health responses to outbreaks when interpreted in light of prevailing pandemic restrictions and other control measures including vaccination.6 The primary aim of this study was to compare disease severity outcomes of the Delta and Omicron VOCs among residents in Victorian RACFs, by analyzing hospitalization and death across Delta- and Omicron-dominant epidemic periods, respectively.
Methods
Study Design, Participants, and Outcome Measures
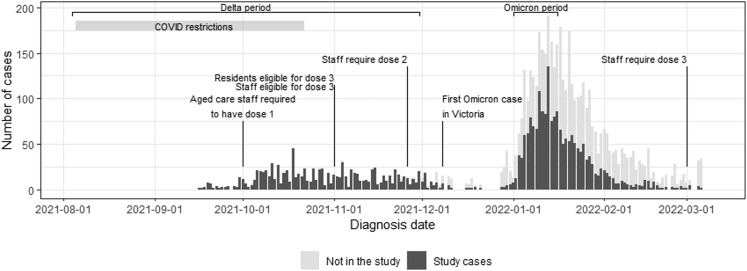
We undertook a retrospective cohort study to compare 2 main outcomes: hospitalization risk (HR) and case fatality risk (CFR) of COVID-19 among Victorian RACF residents linked to outbreaks occurring across 2 epidemic periods. These periods were characterized by their predominant SARS-CoV-2 lineage: (1) Delta VOC (B.1.617.2), August 4 to November 30, 2021; and (2) Omicron VOC (B.1.1.529), January 1 to 16, 2022 (Supplementary Figures 1 and 2). We included outbreaks declared over a shorter time frame in the Omicron period compared to the Delta to allow time for follow-up and because a sufficient number of cases required to complete the analysis had been accrued (Figure 1 ).
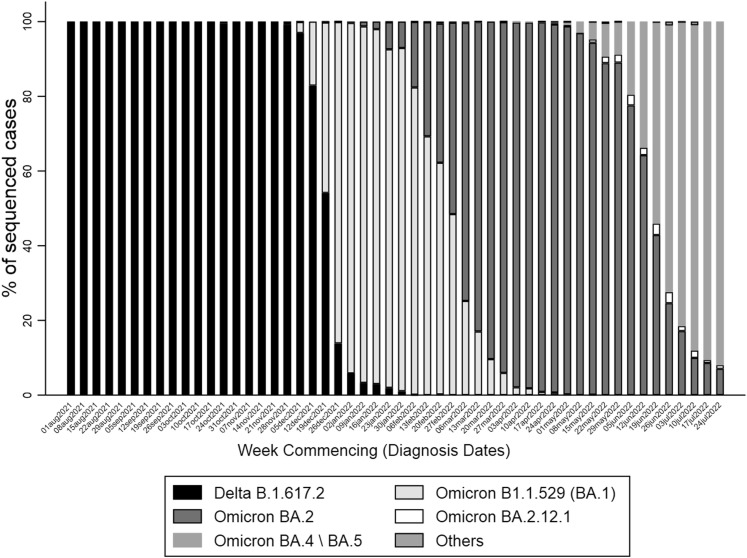
Supplementary Fig. 1.
Proportion of sequenced samples by variant of concern. “Other” category includes non-VOC, B.1.1.7, B.1.351, B.1.617.1, and Recombinant, BA.3, BA (unassigned), BA.2.75, BA.2.76 variants.
Supplementary Fig. 2.
Retrospective cohort study flow diagram.
Fig. 1.
COVID-19 resident cases linked to outbreaks declared in the Delta and Omicron epidemic periods in Victoria.
Laboratories are required to notify all polymerase chain reaction test–confirmed cases of SARS-CoV-2 to the Department of Health in Victoria (the Department). Since January 7, 2022, individuals (or their proxy) who have a COVID-19 detection by rapid antigen (RA) test must also report their positive result, and are recorded as probable cases. RACFs notify the Department of Health or their local public health unit of probable or confirmed cases at their facility. The outbreak definition has been updated throughout the pandemic, but for consistency in this analysis we considered at least 1 resident case at an RACF as constituting an outbreak.
We extracted data from the Victorian case and contact management system, for all resident cases linked to RACF outbreaks with a start date during the 2 epidemic periods of interest. We augmented the data set using enhanced data collection and linkage. For hospital admissions, an enhanced data collection tool was completed for each hospitalized case and submitted to the Department.14 We included hospital admissions for any cause among residents in one of the included outbreaks, if the date of admission occurred within 14 days of diagnosis or they were deemed COVID-19-related by the reporting hospital.
Deaths were either notified by the RACF or identified through routine data linkage with the Victorian Death Index (VDI), maintained by the Registrar of Births, Deaths and Marriages. We included deaths that occurred within a defined period of interest (up to 28 days after clearance from COVID-19 isolation) or for which COVID-19 was recorded as the primary or antecedent cause of death. Deaths were classified as either “died due to COVID” where COVID-19 was considered a primary cause of death or “died with COVID” where the person died during the period of interest from another primary cause, but where COVID-19 may have contributed. Deaths with a clearly unrelated cause (eg, trauma, motor vehicle accident, or suicide) were excluded. Vaccination status of resident cases was identified through linkage with the Australian Immunisation Register.
Statistical Analysis
We summarized descriptive statistics for outbreak and resident characteristics and compared these across the 2 epidemic periods. The crude HR was defined as the number of resident cases who were hospitalized as a proportion of the total resident cases. Similarly, the crude CFR was calculated as the proportion of total resident cases that resulted in COVID-19-related deaths. Both outcomes were also measured per outbreak and epidemic period. The median HR and CFR were calculated as the median of outbreak-specific HRs and CFRs, respectively. The duration and location of outbreaks as well as the associated resident characteristics (age and sex) were described and compared between the 2 epidemic periods. The duration of the outbreak (for outbreaks with 2 or more linked cases) was computed as the number of days from diagnosis of the first resident case to diagnosis of the last resident case linked to each outbreak.
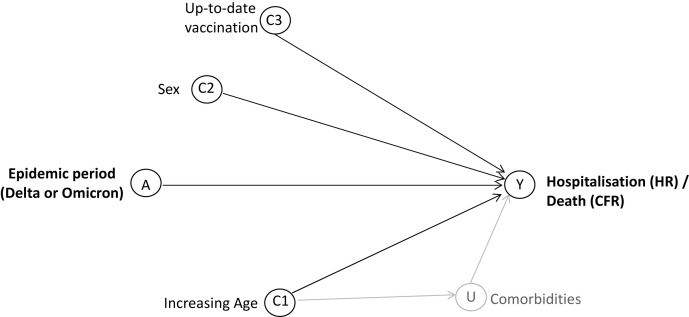
Age, sex, and vaccination status and comorbidities and frailty were identified a priori as potential risk factors for hospitalization and death (Supplementary Figure 3). Data on comorbidities and frailty were not available. However, it was assumed that a large proportion of the aged care population have comorbidities and that this proportion would be similar across the 2 epidemic periods. For each outcome, mixed effects logistic regression models were used to estimate the odds ratio (OR) of hospitalization or death. Models included a random or fixed effect to accommodate clustering by outbreak. Likelihood ratio test and the Akaike information criterion were used to assess the fit of mixed vs fixed effect models as well as mixed models with interaction terms vs those without, choosing the model with best fit. Intraclass correlation coefficients were obtained to assess the extent of clustering at the level of the outbreak.
Supplementary Fig. 3.
Directed acyclic graph of interactions between the explanatory variable of VOC epidemic period (A) and the outcomes of hospitalization/death (Y), affected by the measured covariates of age (C1), sex (C2), and up-to-date vaccination (C3) and the unmeasured covariate of comorbidities (U).
The relationship between COVID-19 vaccination and HR or CFR was examined in 2 ways: (1) as a binary variable reflecting “up-to-date” vaccine protection in each epidemic period and (2) as a continuous variable measuring time from last vaccination regardless of dose. Residents were considered up to date during the Delta period if they had received at least 2 doses ≥14 days prior to their COVID-19 diagnosis date. For the Omicron period, vaccination was considered up to date if at least 3 doses had been received ≥14 days prior to diagnosis. This approach was consistent with known reduced effectiveness of the 2-dose schedule against the Omicron variant (Supplementary Table 1).15 , 16 Cases that were diagnosed within 14 days after their last vaccination were excluded from the “time-since-last-vaccination” analysis.
The probability (and 95% confidence intervals) of HR and CFR by time since the last vaccination date were estimated from the mixed effects models and plotted using the Marginsplot function in Stata (StataCorp). In some cases, hospitalization was implemented for RACF outbreak management and not for clinical management of COVID-19 (eg, where cases’ behavior constituted a transmission risk to other residents). Therefore, a sensitivity analysis was performed that excluded residents transferred to the hospital that was mainly used for this purpose.
Ethics
The research was approved by the Barwon Health Ethics Committee (Ethics Approval Number 22/88).
Results
Residential Aged Care Outbreaks and Linked Cases
There were 86 outbreaks during the Delta period, involving 994 resident cases, and 209 outbreaks during the Omicron period, involving 1882 residents (Table 1 ). Conversely, there were fewer resident cases per outbreak during the Omicron period than the Delta period (median 4 vs 7 cases, respectively). Very large outbreaks involving 50 or more resident cases occurred in both epidemic periods. The median duration (days) was slightly longer in the Omicron than Delta period (median 14 and 11, respectively) (Table 1).
Table 1.
Characteristics of COVID-19 Outbreaks in Residential Aged Care Facilities During the Delta and Omicron Epidemic Periods
| Characteristics of Outbreaks and Residents | Delta Epidemic Period∗ (8/4/2021 to 11/30/2021) | Omicron Epidemic Period∗ (1/1/2022 to 1/16/2022) | P Value† |
|---|---|---|---|
| Number of outbreaks | |||
| Total, n | 86 | 209 | |
| Metro, n (%) | 76 (88) | 153 (73) | .005 |
| Regional, n (%) | 10 (12) | 56 (27) | .005 |
| Duration of outbreaks, d,‡ median (IQR) | 11 (7, 17) | 14 (7, 22) | .08 |
| Number of resident cases | |||
| Total | 994 | 1882 | |
| Per outbreak, median (IQR) | 7 (1, 19) | 4 (1, 13) | .26 |
| Age of resident cases, median (IQR) | 86 (80, 90) | 86 (80, 92) | .18 |
| Sex of resident cases, n (%) | |||
| Female | 597 (60.1) | 1153 (61.3) | .59 |
| Male | 394 (39.6) | 692 (36.8) | .13 |
| Not stated | 3 (0.3) | 37 (1.9) | <.001 |
| Vaccination status at notification, n (%) | |||
| Not up to date with vaccination | 91 (9.2) | 1205 (64.0) | <.001 |
| Up to date with vaccination | 658 (66.2) | 566 (30.1) | <.001 |
| Unknown vaccination status | 245 (24.7) | 111 (5.9) | <.001 |
| Time since last vaccination | |||
| Median (IQR) in months | 4.7 (3.9, 5.7) | 3.2 (1.4, 6.7) | <.001 |
| Diagnosis date of resident cases (range)§ | 9/16/2021 to 12/21/2021 | 12/28/2021 to 3/6/2022 |
IQR, interquartile range.
Epidemic period dates are the periods in which exposures and outbreaks were declared.
P-value from chi-squared test for categorical variables or Wilcoxon rank-sum test for continuous variables.
Duration of outbreaks only includes outbreaks with 2 or more cases; 63 outbreaks during the Delta epidemic period and 155 outbreaks in the Omicron epidemic period.
Diagnosis dates of cases linked to the outbreaks fell within a wider period outside the epidemic period dates.
There were 1.5 times more female resident cases than males during both the Delta and Omicron periods and the median age of COVID-19–positive residents was comparable in both epidemic periods (Table 1). During the Delta period, 66.2% (n = 658) of residents cases were up to date with vaccination at the time of notification, compared with 30.1% (n = 566) of COVID-19–positive residents in the Omicron period. However, 75.3% (1405) of resident cases in the Omicron period had been vaccinated with at least 2 doses at notification. The median time since the last dose (in months) was longer during the Delta period (median 4.7 months, interquartile range 3.9-5.7) compared to the Omicron period (median 3.2 months, interquartile range 1.4-6.7).
Case Hospitalization Risk
The crude HR during the Delta epidemic period was 3 times higher than in the Omicron period (24.6% and 8.2%, respectively) (Table 2 ). The median age of residents admitted to hospital and those not admitted to hospital was similar across the 2 periods (Table 2). Of the total male resident cases, a higher proportion were admitted to hospital compared to the proportion of female resident cases admitted in both the Delta and Omicron periods. The proportion of those admitted to hospital was lower in individuals who were up to date with their COVID-19 vaccine protection compared to those who were not during both periods (Table 3 ). Hospitalized residents in the Delta period had similar median time since vaccination to those in the Omicron period.
Table 2.
Demographic and Clinical Factors by Hospitalization and Deceased Status for COVID-19–Positive Residents in Residential Aged Care Facilities During the Omicron-Dominant and Delta-Dominant Epidemic Periods (Row Percentages)
| Delta Period (8/4/2021 to 11/30/2021) | Omicron Period (1/1/2022 to 1/16/2022) | P Value∗ | |
|---|---|---|---|
| Total resident cases, n | 994 | 1882 | |
| Hospitalizations | |||
| Total hospitalizations, n | 245 | 154 | |
| Crude hospitalization risk, % (95% CI) | 24.6 (22.0, 27.3) | 8.2 (7.0, 9.4) | <.001 |
| Median crude hospitalization risk per outbreak (IQR) | 25.0 (9.1, 66.7) | 0.0 (0.0, 9.1) | <.001 |
| Sex, % (n) | |||
| Women admitted to hospital | 23.3 (139) | 6.9 (80) | <.001 |
| Men admitted to hospital | 26.7 (105) | 10.4 (72) | <.001 |
| Age, y, median (IQR) | |||
| Admitted to hospital | 86 (80, 89) | 86 (81, 91) | .26 |
| No hospital admission | 87 (80, 91) | 86 (80, 92) | .53 |
| Vaccination status, % (n) | |||
| Not up to date—hospitalized | 30.8 (28) | 8.6 (104) | <.001 |
| Up to date—hospitalized | 25.1 (165) | 6.7 (38) | <.001 |
| Unknown—hospitalized | 21.2 (52) | 10.8 (12) | .02 |
| Months since last vaccination, median (IQR) | |||
| Admitted to hospital | 4.6 (2.8, 5.8) | 3.9 (2.0, 6.6) | .54 |
| No hospital admission | 4.5 (3.4, 5.5) | 2.5 (1.2, 6.6) | <.001 |
| Deaths | |||
| Total deaths, n | 186 | 214 | |
| Died from COVID-19, % (n) | 13.7 (136) | 6.8 (128) | <.001 |
| Died with COVID-19, % (n)† | 5.0 (50) | 4.6 (86) | .58 |
| Crude case fatality risk, % (95% CI) | 18.7 (16.3, 21.1) | 11.4 (9.9, 12.8) | <.001 |
| Median crude case fatality risk per outbreak, (IQR) | 12.3 (0.0, 27.8) | 0.0 (0.0, 14.3) | <.001 |
| Sex, % (n) | |||
| Women deceased | 16.3 (97) | 9.0 (104) | <.001 |
| Men deceased | 22.6 (89) | 15.9 (110) | .01 |
| Age, y, median (IQR) | |||
| Died | 88 (84, 93) | 88 (83, 93) | .52 |
| Alive | 86 (80, 90) | 86 (80, 91) | .02 |
| Vaccination status, % (n) | |||
| Not up to date—deceased | 15.4 (14) | 13.9 (167) | .69 |
| Up to date—deceased | 18.7 (123) | 6.7 (38) | <.001 |
| Unknown—deceased | 20.0 (49) | 8.1 (9) | .01 |
| Months since last vaccination, median (IQR) | |||
| Died | 4.6 (4.0, 5.7) | 5.5 (1.9, 8.3) | .02 |
| Alive | 4.5 (2.9, 5.6) | 2.2 (1.2, 6.4) | <.001 |
IQR: interquartile range.
P value from chi-squared test for categorical variables or Wilcoxon rank-sum test for continuous variables.
Where the person died during the period of interest from another primary cause but where COVID-19 may have contributed.
Table 3.
Mixed Effects Logistic Regression of Risk Factors for Hospitalization and Death Among COVID-19–Positive Residents of Residential Aged Care
| Outcome | Explanatory Variable | Category | OR | 95% CI Lower Upper | P Value | |
|---|---|---|---|---|---|---|
| Hospitalization | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.17 | 0.11 | 0.26 | <.001 | ||
| Age, y | Age, y | 1.00 | 0.99 | 1.01 | .98 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 1.32 | 1.04 | 1.68 | .02 | ||
| Up to date protected | Not up to date (reference) | 1.00 | ||||
| Up to date with vaccination | 0.81 | 0.58 | 1.12 | .20 | ||
| Unknown status | 0.75 | 0.49 | 1.14 | .18 | ||
| Outbreak | ICC | 0.21 | 0.13 | 0.31 | ||
| Death | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.40 | 0.28 | 0.56 | <.001 | ||
| Age, y | Age, y | 1.05 | 1.04 | 1.07 | <.001 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 2.05 | 1.63 | 2.58 | <.001 | ||
| Up to date protected | Not up to date (reference) | 1.00 | ||||
| Up to date with vaccination | 0.57 | 0.42 | 0.77 | <.001 | ||
| Unknown status | 0.69 | 0.46 | 1.02 | .06 | ||
| Outbreak | ICC | 0.08 | 0.04 | 0.14 | ||
ICC, intracluster correlation coefficient.
P is for the chi-squared test.
Case Fatality Risk
A higher proportion of COVID-19–positive residents died with or due to COVID-19 in the Delta epidemic period compared to the Omicron period (CFR 18.7% and 11.4% respectively) (Table 2). Residents who died with or due to COVID-19 were slightly older than residents who did not die during their COVID-19 infection during both periods (88 vs 86 years). A higher proportion of male residents died compared to females across both time periods. The proportion of deceased residents with up-to-date vaccination was significantly lower during the omicron period compared with the Delta period (Table 2). The median time since vaccination was higher for deceased compared to alive cases in the Omicron period, but there was no difference for the Delta period (Table 2).
Independent Risk Factors for Hospitalization and Death
Multivariable Regression Model of Severity Outcomes Including Binary Vaccination Status
In the multivariable regression model, the odds of hospitalization were 83% lower during the Omicron epidemic period than during the Delta period, after adjustment for age, sex and vaccination (OR 0.17, 95% CI 0.11-0.26; Table 3).
Men had 32% greater odds of hospitalization than women, but age and vaccine protection were not significantly associated with hospitalization. There was clustering of hospitalizations at the outbreak level (intraclass correlation 0.21, 95% CI 0.13-0.31). In the sensitivity analysis excluding patient transfers to a hospital used for outbreak management, the odds of hospitalization remained reduced for the omicron period (OR 0.15, 95% CI 0.09-0.24) and were also reduced for those with up-to-date vaccination (OR 0.61, 95% CI 0.42-0.88; Supplementary Table 2).
There was a 60% decrease in the adjusted odds of death between the Delta and Omicron periods (OR 0.40, 95% CI 0.28-0.56; Table 3). The other variables in the model were also independently associated with death. The odds of death were double in men compared to women, increased by 5% for every year of age, and reduced by nearly half with up-to-date vaccination. There was minimal clustering of effects at the outbreak level (intraclass correlation 0.08, 95% CI 0.04-0.14), suggesting that facility-level factors had little influence on the death of resident cases.
Multivariable Regression Model of Severity Outcomes Including Time Between Last Vaccination and Diagnosis
When the second model was run to examine the effects of the continuous variable “time since last vaccination” on the outcomes, the adjusted OR for hospitalization between the Omicron and Delta epidemic periods was similar to the first model (OR 0.20, 95% CI 0.13-0.31). The adjusted odds of hospitalization increased by 5% for each additional month since last vaccination, although this was not statistically significant (OR 1.05, 95% CI 0.98-1.13). However, when cases admitted to the outbreak management hospital were excluded in a sensitivity analysis, there was a statistically significant increase in the odds of hospitalization of 9% per month since last vaccination (OR 1.09, 95% CI 1.02-1.18), after adjustment for age, sex, and the epidemic period in which the outbreak occurred (Delta or Omicron). Detailed results are presented in Supplementary Tables 3 and 4 and Supplementary Figure 4.
Supplementary Fig. 4.
Probability of hospitalization by time since last vaccination, excluding patients admitted to a hospital used as an alternative isolation facility. The probability of hospitalization increased by 9% for each additional unit of each month from last vaccination to diagnosis depicting an increased risk of hospitalization as immunity wanes.
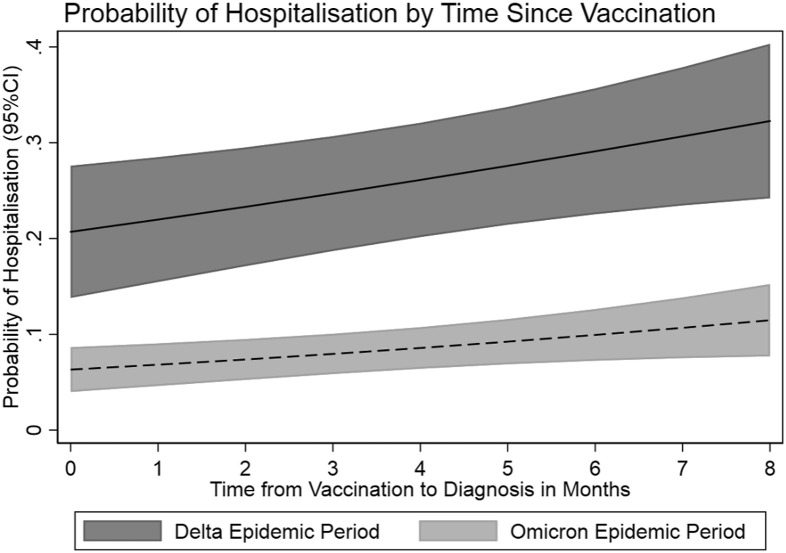
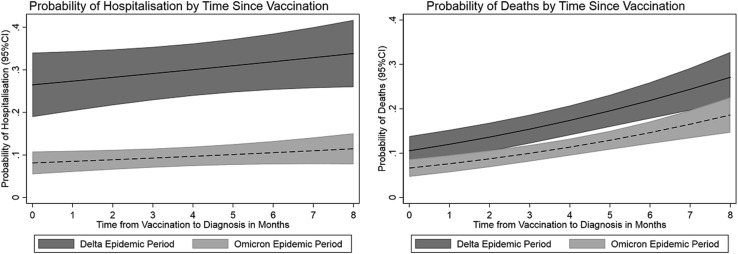
The odds of death were 41% lower in the Omicron epidemic period compared with the Delta period (OR 0.59, 95% CI 0.43-0.83) after adjustment for time since vaccination, age, sex, and epidemic period. The adjusted odds of death increased by 16% (OR 1.16, 95% CI 1.10-1.23) for each month since last vaccination. The probabilities of hospitalization and death by time since last vaccination are shown in Figure 2 .
Fig. 2.
Probability of hospitalization and death by time since last vaccination.
Discussion
We found that there was a lower risk of severe outcomes of COVID-19 (hospitalization and death) among aged care residents during the Omicron epidemic period than the Delta period in Victoria, Australia in 2021 and 2022, including after adjusting for age, sex, and vaccination status and accounting for clustering by outbreak. This is consistent with findings that Omicron is a less virulent strain than Delta, including for an older adult institutionalized population.17 , 18 The Omicron-dominant period we examined yielded more than double the number of outbreaks involving residents than the Delta-dominant period, nearly double the number of total cases, and more deaths in absolute terms, despite its much shorter duration analyzed for this study (outbreaks occurring in a 2-week period compared to 4 months). Although this is expected given the scale of community transmission in the Omicron period, it is possible that the surge in cases may have strained health services, resulting in worse patient outcomes.
A larger proportion of resident cases was deemed fully protected by vaccination during the Delta (66.2%) than Omicron (30.1%) epidemic period; that is, they had had 2 or 3 doses, respectively, at least 2 weeks prior to diagnosis (Table 1). However, a large proportion of the Omicron period cases had at least a second dose (75.3%), offering some protection against severe disease, and the time between last vaccination and diagnosis was higher in the Delta period compared to the Omicron period (4.7 vs 3.2 months), suggesting greater waning of immunity for that group.
Hospitalization was lower but not statistically different between vaccine-protected and unprotected cases in the Delta and Omicron epidemic periods. The magnitude of protection was smaller than expected based on vaccine effectiveness estimates from previous studies. For example, approximately 80% reduction in hospitalization for Delta after 5 months post second dose and for Omicron after 4 months post third dose (Supplementary Table 1).15 The effect was likely diluted by the lack of differentiation between hospitalization on clinical vs public health grounds in our data. For example, some resident cases with mild illness but higher-risk behaviors such as wandering were admitted to hospital to reduce transmission risk. This is supported by results of the sensitivity analysis excluding nonemergency patient transfers showing 39% protection against hospitalization for those who were up to date with their vaccination after adjusting for epidemic period, age, and sex.
Deaths were lower among vaccine-protected cases in the Omicron but not Delta epidemic periods (Table 2). The lack of protection against death for Delta cases is surprising and could be due to 2 doses of the vaccine being less effective against severe disease compared with 3 doses, in addition to Delta reportedly being more virulent than Omicron.18 An alternative explanation could be in part due to waning immunity among these cases; in fact, there was an increased risk of death as time since vaccination increased in both epidemic periods. The increased risk of death (Figure 2) and hospitalization (excluding nonemergency patient transfers) with increase in time since last vaccination after adjusting for epidemic period, age, and sex points to waning immunity and underscores the need for vaccine policy and guidelines around the frequency of vaccination among residents in RACFs. The risk of death did not cluster by facility, but hospitalization did, pointing to facility-level factors including nonemergency transfer of residents to hospital for transmission control.
Our findings add to accumulating evidence of Omicron being less virulent compared to previous VOCs. Reports of reduced risk of hospitalization and deaths among those aged ≥65 years in the general population have been published but information on severity outcomes in the more vulnerable aged care population is sparse.11, 12, 13 , 17 , 19 We found only 1 study that examined severity outcomes among RACFs in England, demonstrating a reduction in both hospitalizations and deaths during the Omicron compared to pre-Omicron periods.17 This English study demonstrated a greater reduction in hospitalizations and lower CFR (5.48% compared to 11.4% in the Omicron period). Differences in hospitalization rates may be due to the utilization of hospitalization for public health management in Australia. The higher CFR in Victoria may be due to differences in definitions of COVID-19 deaths in Australia compared to England.
Our study has a number of strengths. We demonstrate the utility of linking large public health data sets in as close to real time as possible. The linkage of state surveillance systems, including hospital and mortality data sets, allows the real-time monitoring of the impact of COVID-19 in RACFs. Both asymptomatic and symptomatic cases were included during both epidemic periods, allowing for an accurate assessment of CFR estimates compared with other studies that included only symptomatic cases.
Our analysis has some limitations. First, polymerase chain reaction testing capacity and RA testing availability were limited in early January, during our defined Omicron period, and the case definition changed on January 8, 2022, to include probable cases, detected by RA testing, and self-reported online, resulting in an initial period of underreporting of probable cases before facilities were accustomed to using the online portal. This was addressed by a manual reconciliation of case numbers with correspondence from RACFs. Additionally, there could have been lower case ascertainment due to lower sensitivity of RA tests compared to polymerase chain reaction tests during the Omicron period.20
Second, measurement error may have been introduced by using 2 different definitions of “vaccine-protected” for the 2 different epidemic periods. However, given the very low protection offered by 2 doses against infection by Omicron,21 using a 2-dose definition for both periods would have been a more biased approach.
Third, the study included aged care residents in Victoria and may not be generalized to all aged care populations or similar aged cohorts not residing in aged care settings. However, the results may be generalizable to aged care residents in the rest of Australia and other countries with similar demographic profiles and aged care systems.
Conclusions and Implications
Despite some limitations, this study delineates the real-world implications of different VOCs on COVID-19 severity among individuals in RACFs in Australia. We found that both hospitalization and death were lower for aged care residents during the Omicron period compared to the Delta period, and that this relationship remained after controlling for age, sex, and vaccination status of cases. Despite the reduced severity of outcomes during the Omicron era, both variants were associated with numerous outbreaks, some very large, and in absolute terms, the overall burden of cases and deaths was greater in the Omicron period. This study supports ongoing efforts to reduce COVID-19 incursions into RACFs and to reduce transmission by continued improvement of outbreak management and infection prevention measures. Evidence of the increase in risk of hospitalization and death with time since vaccination underscores the need for ongoing policy on the frequency of administration of booster doses for RACF residents.
Footnotes
Funding sources: This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest.
Supplementary Data
Supplementary Table 1.
Waning 2- and 3-Dose Immunity During the Delta and Omicron Waves∗
| Characteristic | Vaccine Effectiveness Against Hospitalization % (95% CI) |
|
|---|---|---|
| Delta | Omicron | |
| Any mRNA vaccine, 2 doses | 85 (84–85) | 55 (50–60) |
| <2 mo | 94 (92-96) | 71 (51-83) |
| 2-3 mo | 91 (89-92) | 65 (53-74) |
| 4 mo | 90 (89-92) | 58 (38-71) |
| ≥5 mo | 82 (82-83) | 54 (48-59) |
| Any mRNA vaccine, 3 doses | 95 (95-96) | 88 (86-90) |
| <2 mo | 96 (95-97) | 91 (88-93) |
| 2-3 mo | 93 (91-95) | 88 (85-90) |
| ≥4 mo | 76 (14-93) | 78 (67-85) |
Findings from a test-negative case control study conducted by Ferdinands et al (2022)15 using data from 8 VISION Network sites, United States. Overall, vaccine effectiveness against hospitalization was lower during the Omicron than during the Delta wave, and administration of a third dose significantly increased protection against hospital admission.
Supplementary Table 2.
Sensitivity Analysis: Mixed Effects Logistic Regression of Risk Factors for Hospitalization Among RACF Residents—Excluding a Hospital Where Patients Were Admitted for COVID-19 Outbreak Management
| Outcomes | Explanatory Variables | Categories | OR | 95% CI |
P | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Hospitalization | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.15 | 0.09 | 0.24 | <.001 | ||
| Age, y | Age, y | 1.00 | 0.98 | 1.01 | .85 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 1.31 | 1.01 | 1.70 | .04 | ||
| Up to date protected | Not up to date (reference) | 1.00 | ||||
| Up to date with vaccination | 0.61 | 0.42 | 0.88 | .01 | ||
| Unknown status | 0.60 | 0.38 | 0.95 | .03 | ||
| Outbreak | ICC | 0.23 | 0.15 | 0.33 | ||
CI, confidence interval; OR, odds ratio; RACF, residential aged care facility.
P is for the chi-squared test.
Supplementary Table 3.
Mixed Effects Logistic Regression of Risk Factors (Including Time Since Last Vaccination) for Hospitalization and Death Among RACF Residents
| Outcomes | Explanatory Variables | Categories | OR | 95% CI |
P | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Hospitalization | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.20 | 0.13 | 0.32 | <.001 | ||
| Age, y | Age, y | 1.00 | 0.98 | 1.01 | .58 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 1.23 | 0.93 | 1.64 | .152 | ||
| Time from last vaccination to diagnosis (mo) | Time from last vaccination to diagnosis (mo) | 1.05 | 0.98 | 1.13 | .13 | |
| Outbreak | ICC | 0.25 | 0.13 | 0.31 | ||
| Death | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.59 | 0.43 | 0.81 | .001 | ||
| Age, y | Age, y | 1.06 | 1.04 | 1.08 | <.001 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 1.85 | 1.41 | 2.42 | <.001 | ||
| Time from last vaccination to diagnosis (mo) | Time from last vaccination to diagnosis (mo) | 1.16 | 1.10 | 1.23 | <.001 | |
| Outbreak | ICC | 0.06 | 0.16 | 0.36 | ||
CI, confidence interval; OR, odds ratio; RACF, residential aged care facility.
P is for the chi-squared test.
Supplementary Table 4.
Sensitivity Analysis: Excluding Nonemergency Patient Transfers; Mixed Effects Logistic Regression of Risk Factors (Including Time Since Last Vaccination) for Hospitalization Among RACF Residents.
| Outcomes | Explanatory Variables | Categories | OR | 95% CI |
P | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Hospitalization | Epidemic period | Delta epidemic period | 1.00 | |||
| Omicron epidemic period | 0.22 | 0.13 | 0.35 | <.001 | ||
| Age, y | Age, y | 0.99 | 0.97 | 1.01 | .25 | |
| Sex | Female (reference) | 1.00 | ||||
| Male | 1.24 | 0.91 | 1.69 | .17 | ||
| Time from last vaccination to diagnosis (mo) | Time from last vaccination to diagnosis (mo) | 1.09 | 1.02 | 1.18 | .02 | |
| Outbreak | ICC | 0.26 | 0.17 | 0.38 | ||
CI, confidence interval; OR, odds ratio; RACF, residential aged care facility.
P is for the chi-squared test.
References
- 1.Australian Institute of Health and Welfare . The first year of COVID-19 in Australia: direct and indirect health effects. AIHW; Canberra: 2021. [Google Scholar]
- 2.Department of Health - Victorian Government . Transmission and Response Epidemiology Victorian (TREVI) System. 2022. [Google Scholar]
- 3.Escandón K., Rasmussen A.L., Bogoch, et al. COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. BMC Infect Dis. 2021;21:710. doi: 10.1186/s12879-021-06357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterworth P, Schurer S, Trinh TA, et al. Effect of lockdown on mental health in Australia: evidence from a natural experiment analysing a longitudinal probability sample survey. Lancet Public Health. 2022;7:e427–e436. doi: 10.1016/S2468-2667(22)00082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krzyzaniak N., Scott A.M., Bakhit M., et al. Impact of the COVID-19 pandemic on the Australian residential aged care facility (RACF) workforce. Australian Nursing and Midwifery Federation; Melbourne, Victoria: 2021. [Google Scholar]
- 6.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartof S.Y., Slezak J.M., Puzniak L., et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022;10:689–699. doi: 10.1016/S2213-2600(22)00101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Rio C., Omer S.B., Malani P.N. Winter of Omicron—The Evolving COVID-19 Pandemic. JAMA. 2022;327:319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- 9.Feikin D.R., Higdon M.M., Abu-Raddad L.J., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Australian Beureau of Statistics . Measuring Australia’s excess mortality during the COVID-19 pandemic. 2022. https://www.abs.gov.au/articles/measuring-australias-excess-mortality-during-covid-19-pandemic Accessed February 16, 2023. [Google Scholar]
- 11.Maslo C., Friedland R., Toubkin M., et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veneti L., Bøås H., Kristoffersen A.B., et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA. 1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27:2200077. doi: 10.2807/1560-7917.ES.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward I.L., Bermingham C., Ayoubkhani D., et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022;378:e070695. doi: 10.1136/bmj-2022-070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis S.J., Cutcher Z., Brett J.A., et al. An evaluation of enhanced surveillance of hospitalised COVID-19 patients to inform the public health response in Victoria. Commun Dis Intell. 2020;44:10.33321. doi: 10.33321/cdi.2020.44.98. [DOI] [PubMed] [Google Scholar]
- 15.Ferdinands J.M., Rao S., Dixon B.E., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR (Morb Mortal Wkly Rep) 2022;71:255. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angel-Korman A., Peres E., Bryk G., et al. Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel: the case for a third vaccine dose. Clin Kidney J. 2022;15:226–234. doi: 10.1093/ckj/sfab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krutikov M., Stirrup O., Nacer-Laidi H., et al. Outcomes of SARS-CoV-2 omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Healthy Longev. 2022;3:e347–e355. doi: 10.1016/S2666-7568(22)00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki R., Yamasoba D., Kimura I., et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1. 529) and delta (B. 1.617. 2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamayoshi S., Sakai-Tagawa Y., Koga M., et al. Comparison of rapid antigen tests for COVID-19. Viruses. 2020;12:1420. doi: 10.3390/v12121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United Kingdom Health Security Agency . In: SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 34. Agency H.S., editor. Government of the United Kingdom; 2022. [Google Scholar]