Abstract
AIM:
Systematic review of epidemiological data to understand the prevalence, incidence, etiologies, and hospitalizations related to gastroparesis (GP).
METHODS:
Studies of the epidemiology of GP published in all languages, years, and countries from five databases in January 2022 using prespecified search strategies.
RESULTS:
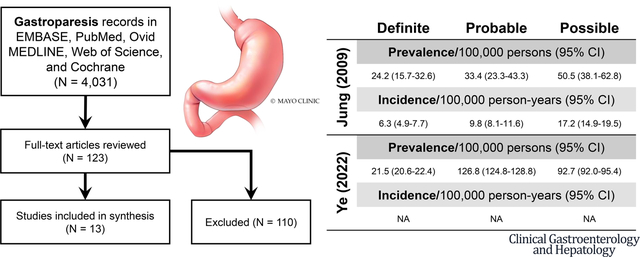
Thirteen studies (data from 1994 – 2019) were included. All but one study (from the UK) was based in USA. Prevalence of definite GP (symptoms plus delayed gastric emptying) ranged 13.8–267.7 per 100,000 adults, and incidence 1.9–6.3 per 100,000 person-years. The estimated 10-year cumulative incidence of GP in type 1 diabetes (DM) and type 2 DM was 5.2% and 1.0%, respectively. Across studies, GP was more common among females and those with DM. Rates of hospitalizations and ED visits for GP are increasing, ranging from 2- to 18-fold over approximately two decades. Mortality rates for patients with possible or definite GP were higher compared to the general population, with primary causes of death in GP being cardiovascular, respiratory failure, and malignancy. Multiple studies observed improved inpatient mortality over the mid-1990s to late-2000s. Limitations include the case identification in most studies (76.9%) utilized solely ICD codes or clinical record diagnoses; two studies (15.4%) used objective evaluation to diagnose GP. Only four studies (30.8%) used non-specialized community databases; the remaining 9 studies used inpatient, Emergency Department (ED), or disease-specific databases.
CONCLUSION:
There is a paucity of high-quality, demographically diverse, and population-based studies to accurately describe the epidemiology of GP. Future studies with valid GE measurement are needed to better characterize the epidemiology and natural history of GP.
Keywords: diabetes, idiopathic, post-surgical, gastric emptying
LAY SUMMARY
Gastroparesis is increasingly recognized, characterized by upper gastrointestinal symptoms with delayed gastric emptying. It has resulted in increasing numbers of hospitalizations and emergency department visits. This study found that it is present in up to 0.27% of the population and identifies questions for future research.
Graphical Abstract
Gastroparesis (GP) is a chronic disorder characterized by upper gastrointestinal symptoms and delayed gastric emptying in the absence of mechanical obstruction. The cardinal symptoms of GP are nausea and vomiting, although concurrent postprandial fullness, early satiation, epigastric pain, and bloating are frequently present.1–3 GP is associated with significant mortality and morbidity 4, Emergency Department (ED) visits 5, hospitalization 6, healthcare costs, and loss of work productivity.7
Delayed gastric emptying (GE) measured by scintigraphy or stable isotope breath test is a diagnostic criterion of GP along with typical symptoms and absence of obstruction. This is particularly important as the symptoms of GP and functional dyspepsia (FD) can have considerable overlap.2, 8, 9 Unfortunately, due to limited access to optimal GE scintigraphy (GES) performed at the community or population level, most studies have relied on clinical and medical record diagnoses of GP. One study demonstrated that among patients with an International Classification of Disease (ICD) or chart (medical record) diagnosis of GP, nearly 80% never underwent confirmatory testing with GES, and only 14% had undergone both esophagogastroduodenoscopy (EGD) to exclude mechanical obstruction and GE testing.10 The etiology of GP is incompletely defined in individual studies. It has been suggested that diabetes mellitus (DM) and idiopathic are two major etiologies of GP. Other causes include post-surgical (e.g. esophageal surgery, fundoplication, bariatric surgery, vagotomy), viral gastroenteritis, neuromuscular diseases (e.g. Parkinson’s disease, multiple sclerosis, amyloidosis), systemic autoimmune diseases (e.g. systemic sclerosis, systemic lupus erythematosus), and drug-induced (e.g. opioids, anticholinergics, glucagon-like peptide-1 agents). Unfortunately, the estimates of the contribution of each of these apparently minor causes of GP also varies between studies. For example, among the 401 patients in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Gastroparesis Clinical Research Consortium (GpCRC), 61% had idiopathic gastroparesis 11, but these data reflect predominantly experience at tertiary referral centers. The idiopathic nature of gastroparesis is complicated by the fact that 103/243 patients reported use of narcotics, and 83/243 reported co-morbid severe depression or anxiety though the concomitant use of central neuromodulators that may impede gastric emptying was not reported.
A search of the Cochrane database revealed no prior systematic reviews on the prevalence, incidence, risk factors, and outcomes of GP. Thus, we conducted a systematic review to evaluate the epidemiological data of GP based on population or registry studies to understand the prevalence, incidence, etiologies, and hospitalizations related to GP.
METHODS
Our systematic review was conducted with guidance from the protocol according to the checklist provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).12
Study Selection
We included studies that identified adult cases of GP using population or community-based registries, databases, or surveys over all time periods and in all languages. We identified population or community-based registries, databases, or surveys of GP among an entire population or prespecified subpopulation of patients over a defined period in a defined geographic area. Inpatient and ED patient settings were considered if they met the same criteria for population or community-based data acquisition and sample frame prespecified in their aims. International studies were also considered. Multiple case definitions for GP were permitted to obtain a comprehensive evaluation of the current documentation of the epidemiology based on published data. After initial review of the eligible publications, it transpired that a few studies utilized the Gastroparesis Cardinal Symptom Index (GCSI) for case definition.13 The GCSI is a symptom-based survey tool commonly used in GP research studies and it has been previously validated as a measure of symptom severity among patients with a diagnosis of GP.14, 15 However, it has not been validated as a diagnostic tool for GP, but rather asks about symptoms, which can be nonspecific and overlap with functional dyspepsia.16 Thus, these publications were largely excluded as not meeting the definition of GP. One publication was considered for full-text review. It was eventually excluded on the grounds that it used only GCSI which was deemed inadequate for defining cases of GP.13
Therefore, the following case definitions were used for GP:
Objective evidence of delayed GE with or without typical GP symptoms
A medical record diagnosis of GP or ICD code for GP
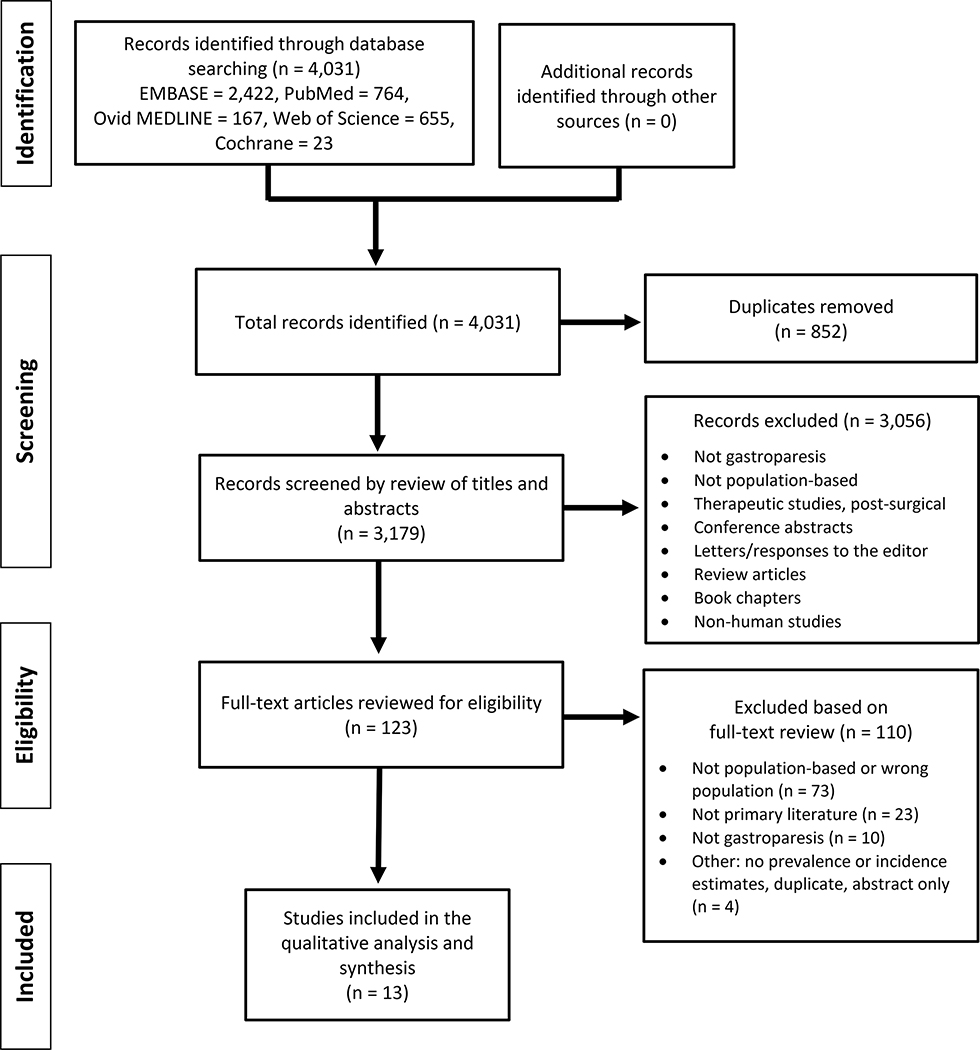
Studies were excluded if they were focused on non-human subjects, were unavailable as full-text publications, or were not original research (e.g. textbook chapters, letters, review articles). Specific reasons for exclusion were listed in the PRISMA flow diagram (Figure 1). Notably, no studies were excluded due to unavailability of the full text.
Figure 1.
PRISMA FLOW DIAGRAM
Primary and Secondary Outcomes
The primary outcomes of interest were estimates of the prevalence and incidence of GP among the general population. Secondary outcomes included the demographic characteristics (i.e. age, sex, race) of patients with GP, as well as the healthcare utilization by patients and the morbidity and mortality of these patients.
Data Sources and Detailed Search Strategy
Two authors (SD and MC) outlined a broad and comprehensive literature search strategy not limited by publication type; thus, conference proceedings, including conference abstracts and publications, were included in our search strategy. Five databases were utilized – PubMed, Ovid MEDLINE, EMBASE, Web of Science, and Cochrane. The time period of the search included all dates prior to December 31, 2021. The search strategy and terms used for each database are detailed in the Supplemental Materials.
From the resulting studies, titles and abstracts were evaluated by two independent and blinded reviewers (SD and TZ) for possible eligibility. During instances of disagreement between the two authors, a third investigator (MC) reviewed the title and abstract for eligibility. Studies considered for review by at least two investigators were deemed eligible and the full-text article was obtained and evaluated for inclusion. Studies were considered for inclusion and appraisal if at least their sampling frame or their sampling method were deemed appropriate. There were 107 unique non-English language articles. The authors reviewed the English version of the title and abstracts and determined that the vast majority were ineligible for full-text review mostly for not being population-based, not representing GP, being therapeutic/post-surgical publications, or review papers. There was one publication that attempted to address prevalence, and this was included for full-text review. It was ultimately excluded for not being population-based.17
Data Collection, Data Items, and Analysis
Data was extracted from all included studies by a single author (SD) using a standard process. Extracted information was verified by the other authors (TZ and MC). Study design data items obtained included: authors, year of publication, country of publication, language, data source, country of data source, dates of study inclusion, case definition for GP, total number of persons sampled, and funding source (if applicable). Outcome data obtained, when available, included estimates of prevalence, incidence, demographic characteristics (e.g. age, sex, race/ethnicity, comorbid conditions), and mortality. Further estimates of comorbidities, healthcare utilization, and outcomes were extracted if presented. When multiple interval estimates of summary statistics were provided, both yearly and overall estimates were extracted, and a pooled estimate was calculated. Articles in which age was presented as an ordinal variable (e.g. 10-year groupings) were identified and summary estimates were not calculated. Finally, a few included publications presented hospital admission and discharge data graphically. In these few instances, an online data digitizer, WebPlotDigitizer (version 4.5, found at https://automeris.io/WebPlotDigitizer), was utilized to provide estimates.18
Risk of Bias and Quality Assessment
Risk of bias and qualitative analysis for each included study was assessed using the Joanna Briggs Institute (JBI) Checklist for Prevalence Studies Tool (found at https://jbi.global/critical-appraisal-tools, Supplemental Table S1). 19 The JBI Checklist for Prevalence Studies Tool consists of nine items that standardize the assessment of the methodological quality of prevalence data. Key parameters include study population and sample, as well as case definition and identification. Quality assessment was completed by two independent and blinded reviewers (SD and TZ), and disagreements verified by a third author (MC). Low quality studies with a suspected high risk of bias were not necessarily excluded from the study in order to comprehensively evaluate the current state of evidence. Overall study quality was summarized, as was the overall risk of bias across studies for each component of the JBI tool. Themes between included studies were extracted, narratively described, and summarized. Given the clinical heterogeneity (e.g. populations and subpopulations) and methodological heterogeneity of sampling frames (e.g. outpatient versus inpatient, national versus regional), study designs, and reported outcomes, no formal meta-analysis was conducted. Furthermore, given that four of the 13 studies used the same database, there was a high likelihood of overlap in sample populations among included studies.
RESULTS
Database Search and Study Selection
The database searches revealed a total of 4,031 studies potentially able to address the epidemiology of GP. Removal of duplicates yielded 3,179 unique studies that were screened by review of the title and abstracts. The most common reasons for exclusion prior to full-text review included not using population or community-based databases or registries, not presenting data about prevalence or incidence, not focusing on GP, non-human studies, and not primary literature (e.g. reviews, letters, book chapters). One-hundred and twenty-three studies passed screening and the full-text articles were assessed for eligibility. Ultimately, 13 studies were included in the final analysis and qualitative synthesis (Figure 1).4–6, 10, 20–28
Study Characteristics
Overall study characteristics are summarized in Table 1. The studies included databases and registries containing data over the years 1994 – 2019. All but one study – which was from the United Kingdom (UK) – were based on databases or registries from the United States (USA). Only four studies (30.8%) utilized community-level databases,4, 10, 27, 28 while seven (53.8%) used inpatient or Emergency Department (ED) databases5, 6, 21, 22, 24–26 and two (15.4%) used databases or sub-registries of patients with DM.20, 23
Table 1.
Characteristics of studies for gastroparesis (GP), organized by population setting and then first author.
| Study | Data Source | Dates | Case Definition | # GP Studied | # At Risk Population | Estimates and Major Findings |
|---|---|---|---|---|---|---|
|
| ||||||
| Community | ||||||
| Jung, et al. 2009 | Rochester Epidemiology Project | 1996 – 2006 | ICD codes, objective tests, and Sx | 222 | 124,277 | Prevalence: men and women 9.6 and 37.8 (average 24.2) per 100,000 persons; Incidence: men and women 2.5 and 9.8 (average 6.3) per 100,000 person-years, respectively¥ |
| Syed, et al. 2020 | Explorys | 1999 – 2014 | ICD-9 code | 69,950 | 43,827,910 | Prevalence overall 159.6 per 100,000 persons; T1D and T2D prevalence was 4.59% and 1.31%, respectively |
| Ye, et al. 2021 (UK) | UK Clinical Practice Research Datalink | 2000 – 2016 | Chart diagnosis | 1,135 | 11,576,068 | Prevalence 13.8 per 100,000 persons; incidence 1.9 per 100,000 person-years; 39.4% idiopathic, 37.5% diabetic¥ |
| Ye, et al. 2022 (USA) | Optum Clinformatics Data Mart | 2000 – 2019 | ICD codes, objective tests, and Sx | 71,775 | 82,574,650 | Overall prevalence of GP 267.7 per 100,000; “definite” GP: 21.5 per 100,000 persons; female predominance; 57.4% diabetic, 15% post-surgical, 11.8% drug-induced, 11.3% idiopathic¥ |
| Inpatient/Emergency Department | ||||||
| Bell, et al. 2002 § | NC Hospital Discharge Database | 1998 | ICD-9 code | 1,476 | NA | Of all GP discharges, 38.9% had diabetic GP; mortality among diabetic GP 0.6%; female predominance |
| Bielefeldt 2013 | State Inpatient Database | 2007 – 2010 | ICD-9 code | NA* | NA | Fewest and greatest inpatient admissions in Utah and Maryland, respectively; mortality estimate 0.5 to 2.3 per 100,000 persons† |
| Hirsch, et al. 2019 | National ED Sample | 2006 – 2013 | ICD-9 code | 203,248 | 240 millionΩ | ED visits increased from 12.9 to 27.3 per 100,000 visits; among diabetic GP, visits increased by 148%; female predominance |
| Kichloo, et al. 2021 | 2016 – 2017 | ICD-10 code | 99,695 | > 71 millionα | 78.1% with diabetic GP; overall inpatient mortality estimate 0.25%; non-diabetic GP more common in white race | |
| Nusrat, et al. 2013 | Nationwide Inpatient Sample | 1994 – 2009 | ICD-9 code | 129,578Δ | NA | Hospitalizations for primarily GP increased 18-fold while FGIDs decreased by half; female predominance |
| Wadhwa, et al. 2017 | 1997 – 2013 | ICD-9 code | 150,532Δ | NA | Discharge diagnoses overall increased 4-fold¥; female predominance; among T1D and T2D there was 6- and 3.7-fold increases, respectively | |
| Wang, et al. 2008 | 1995 – 2004 | ICD-9 code | 60,895 | 361,768,699 | Hospitalizations with GP as primary and secondary diagnosis increased 2.5- and 2.4-fold, respectively | |
| Diabetes Mellitus | ||||||
| Aleppo, et al. 2017 | T1D Exchange Registry | 2010 – 2012 | Chart diagnosis | 340 | 7,107 | 4.8% prevalence among persons with T1D; female predominance |
| Choung, et al. 2012 | Rochester Epidemiology Project | 1996 – 2006 | ICD codes, objective tests, and Sx | 15 | 227 T1D, 360 T2D, 639 controls | Incidence of GP over 10 years: 5.2% in T1D, 1.0% in T2D, and 0.2% in controls |
Study estimates were limited to patients with concurrent diagnosis of diabetes based on the medical record.
Admissions for GP ranged from 24.3 to 117.1 per 100,000 persons (normalized to state 2010 census data).
Estimates standardized to state populations based on 2010 census data.
Number obtained by estimating values from graphical figures using the WebPlotDigitizer, version 4.5 (https://apps.automeris.io/wpd/), accessed April 10, 2022.
Estimated as NEDS captures about 30 million ED visits per year.
Exact number not provided.
Prevalence and incidence estimates were, at minimum, age-adjusted/standardized to the population.
ED, Emergency Department; FGID, functional gastrointestinal disorders; GP, gastroparesis; ICD, International Classification of Diseases; NA, not available; NC, North Caroline; Sx, symptoms; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; UK, United Kingdom; USA, United States of America.
Five studies (38.5%) did not provide information about the total sample size available from their database,5, 6, 21, 22, 25 though one of these provided yearly approximations which was used to estimate total cohort size.5 Sample sizes of studies ranged from as low as 1,226 to over 360 million.
Of the 13 studies, most (76.9%) utilized solely ICD codes or clinical record diagnoses for case identification.5, 6, 10, 20–22, 24–27 Only three studies (23.1%) used objective evaluation as part of their case definition for GP.4, 23, 28 Two of these publications differentiated GP as “possible” (only symptoms or delayed GE without symptoms), “probable” (symptoms plus food retention on barium or endoscopy study) and “definite” (typical symptoms plus delayed GE by scintigraphy).4, 28 The third study incorporated scintigraphic testing for GP as part of the case definition, but epidemiological estimates were not presented in accordance with these different criteria.23
Characteristics of Patients with Gastroparesis
Patient-level summaries are listed in Table 2. Of the patients studied, mean age at evaluation for GP ranged from 45.4 – 58.9 years. Among patients with GP, the majority were female (range: 63.7% - 76.4%) and identified as White race (range: 46.7% - 90.1%). Seven of 11 potential studies assessed the etiologies of GP. The two most common etiologies were DM and idiopathic GP, with estimates ranging from 25.3% - 78.1% and 11.3% - 49.4%, respectively. Three of the included studies classified patients as diabetic GP or non-diabetic GP and did not further subdivide the latter. There was only one study28 that estimated post-surgical GP as the second most prevalent etiology (15.0% of cases). Other data that were not consistently included or summarized were BMI, hemoglobin A1c, alcohol use, tobacco use, income, education level, and insurance coverage or status. Many of the publications utilizing inpatient or ED databases also presented data on hospital parameters, particularly length of stay (LOS), regional distribution of patients, hospital disposition, and in-hospital mortality.
Table 2.
Characteristics of patients with gastroparesis, organized by population setting and first author. Data presented as mean (SD) or (%) or calculated mean/median when SD or more precise data are not provided. Two commonest etiologies included.
| Study | Age/age (SD) (years) | Female (%) | White (%) | BMI (kg/m2) | HbA1c (%) | EtOH (%) | Smokers (% current) | Etiology (%) | Other variables |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Community | |||||||||
| Jung, et al. 2009 | 47.0 (20.0)* | 76.4 | >90 | 26.2 (7.0) | - | 26.8 | 43 (33.9) | IG (49.4) DG (25.3) |
Data summarized for definite/probable groups; Marital, education, employment, insurance status |
| Syed, et al. 2020 | CBDΔ | 66.1 | 69.7 | - | - | - | - | DG (71.7) IG (28.3) |
None |
| Ye, et al. 2021 (UK) | 50.6 (20.1)* | 63.7 | 85.0 | 27.2 (8.1) | 8.2 | 582 (51.3) | 214 (18.9) | IG (39.4) DG (37.5) |
CCI, comorbidities, regional distribution |
| Ye, et al. 2022 (USA) | 58.9 (16.4)* | 68.5 | 71.7 | - | - | - | - | DG (57.4) PSG (15.0) |
Insurance status, follow-up time, GES performed, regional distribution, comorbidities |
| Inpatient/Emergency Department | |||||||||
| Bell, et al. 2002 § | CBDΔ | 65.8 | - | - | - | - | - | - | Insurance status, discharge disposition |
| Bielefeldt 2013 | - | - | - | - | - | - | - | - | Hospital admission rates (F>M), regional distribution |
| Hirsch, et al. 2019 † | 45.4 (19.4)Ω | 69.3 | - | - | - | - | - | - | Insurance status, income, region, discharge disposition |
| Kichloo, et al. 2021 | 47.3Ω | 66.9 | 46.7 | - | - | - | - | DG (78.1) Non-DG (21.9) |
CCI, comorbidities, insurance status, regional distribution, hospital outcomes |
| Nusrat, et al. 2013 | - | - | - | - | - | - | - | - | Age groups, insurance status, hospital stay, hospital charges, mortality |
| Wadhwa, et al. 2017 † | 49.6 (52.8)Ω | 71.6 | 67.5 | - | - | - | - | Non-DG (71.9) DG (28.1) |
Symptoms, CCI, insurance status, income, regional distribution |
| Wang, et al. 2008 † | 54.0 (17.5)Ω | 65.3 | - | - | - | - | - | DG (73.8) Non-DG (26.2) |
Insurance status, hospital outcomes, mortality |
| Diabetes Mellitus | |||||||||
| Aleppo, et al. 2017 | 49.4Ω | 66.2 | 90.1 | 26.3 | 8.1 | - | - | NA | Income, education, insurance status, comorbidities |
| Choung, et al. 2012 | - | - | - | - | - | - | - | NA | Predictors of GP among T1D, including age, sex, and symptoms |
Age at diagnosis.
Unable to calculate, though age categories were presented.
Study estimates were limited to patients with concurrent diagnosis of diabetes based on the medical record.
Pooled estimates using first and last year of data.
Age at study inclusion.
CBD, cannot be determined; CCI, Charlson Comorbidity Index; DG, diabetic gastroparesis; F, female; GES, gastric emptying scintigraphy; GP, gastroparesis; IG, idiopathic gastroparesis; M, male; NA, not applicable; PSG, post-surgical gastroparesis; SD, standard deviation; T1D, type 1 diabetes mellitus UK, United Kingdom; USA, United States of America.
Co-Morbidities Associated with Gastroparesis
Three studies reported the Charlson Comorbidity Index (CCI) in patients with GP,6, 24, 27 and four of them reported comorbidities.20, 24, 27, 28 Ye et al. (2021) reported 33.3%, 38.7%, 18%, and 10.0% for CCI of 0, 1–2, 3–4, and ≥5, respectively in a database in the UK.27 Wadhwa et al. reported similar results from the USA, and demonstrated these rates were comparable to the total population of hospital discharges.6 From 1997 to 2013, there was a significant increase of patients admitted for GP with CCI ≥8, from 0.69% to 3.0%.6 One study showed 59.1% of patients with diabetic GP having CCI ≥3, versus 11.0% in those with non-diabetic GP.24
There was a significant heterogeneity in the type and percentage of comorbidities reported in four studies. In the UK database, chronic pulmonary disease (27.2%) was the most prevalent comorbidities. Others included cancer (9.6%), peptic ulcer disease (8.1%), and renal disease (13.5%).27 In the USA, commonly reported comorbidities included hypertension, history of smoking, electrolyte derangements, obesity, chronic pulmonary disease, and cerebrovascular disease in both diabetic and non-diabetic GP groups.20, 24, 28 Notably, Kichloo et al. found that patients with diabetic GP had significantly higher rates of congestive heart failure (13% vs. 4.9%), chronic kidney disease (32.1% vs. 6.6%), and chronic ischemic heart disease (18.7% vs. 8.4%).24 Similarly, Ye et al. (2022) found that patients with diabetic GP had higher rates of multiple chronic diseases, including pulmonary disease, cerebrovascular disease, dementia, myocardial infarction, nephropathy, neuropathy, and retinopathy.28
Epidemiological Parameters
a. General Population Studies
Estimates of prevalence in the general population ranged from 13.8 to 267.7 per 100,000 adults.4, 10, 27, 28 The incidence rate of the general population in one study was 1.9 per 100,000 person-years, and in another study 6.3 per 100,000 person-years.4, 27 Sex-specific estimates of incidence was 2.4 and 9.8 per 100,000 person-years in males and females.4 Summaries of prevalence, incidence, and case definition is summarized for the studies using objective evaluation for GP by their case definitions (Table 3). Notably, the study to utilize the strictest case definition of GP – chart diagnosis, objective GE study, and typical symptoms – estimated the nationwide prevalence to be 21.5 per 100,000 persons.28 Unfortunately, one of the studies that used objective testing to diagnose GP did not present prevalence or incidence estimates for each specific case definition, but rather aggregated all patients that met any of the case definitions.23
Table 3.
Studies using objective measurements, with estimates based on case definition of gastroparesis, by first author.
| “Definite” | “Probable” | “Possible” | |
|---|---|---|---|
| Jung, et al. 2009 * | Case Definitions | ||
| (A) Scintigraphic delayed GE AND (B) ≥ 3 months of typical symptoms |
Met criteria (B) AND (C) Food retention on EGD or upper GI study |
Met criteria (A) without symptoms OR Met only criteria (B) | |
| Prevalence* per 100,000 persons (95% CI) | |||
| 24.2 (15.7 – 32.6) | 33.4 (23.3 – 43.4) | 50.5 (38.1 – 62.8) | |
| Incidence* per 100,000 person-years (95% CI) | |||
| 6.3 (4.9 – 7.7) | 9.8 (8.1 – 11.6) | 17.2 (14.9 – 19.5) | |
| Ye, et al. 2022 (USA) | Case Definitions | ||
| (A) 1 inpatient OR 2 outpatient diagnoses 30 day apart AND (B) Scintigraphic delayed GE within 90 days AND (C) ≥ 3 months of typical symptoms |
Met criteria (A) and (C) OR Met criteria (A) and (B) |
Met only criteria (A) | |
| PrevalenceΔ per 100,000 persons (95% CI) | |||
| 21.5 (20.6 – 22.4) | 126.8 (124.8 – 128.8) | 93.7 (92.0 – 95.4) | |
| Incidence per 100,000 person-years (95% CI) | |||
| Not estimated | Not estimated | Not estimated | |
| Choung, et al. 2012 | Case Definitions | ||
| (A) Scintigraphic delayed GE | (B) ≥ 3 months of typical symptoms AND (C) Food retention on EGD or upper GI study |
Met criteria (B) AND (D) Physician diagnosis of gastroparesis |
|
| Prevalence§ per 100,000 persons (95% CI) | |||
| Not estimated | Not estimated | Not estimated | |
| Incidence§ per 100,000 person-years (95% CI) | |||
| Not estimated | Not estimated | Not estimated | |
Estimates are inclusive of patients that also met stricter case definitions. For example, “probable” GP estimates also included patients that met “definite” criteria; “possible” included patients that met any of the case definitions.
Standardized to age and sex of USA white population in the year 2000.
Standardized to age, sex, and geographic region.
Estimates for each method of diagnosis was not provided.
CI, confidence interval; EGD, esophagogastroduodenoscopy; GE, gastric emptying; GI, gastrointestinal; UK, United Kingdom; USA, United States of America
b. Diabetes Mellitus Studies
Among studies that used subpopulation-based databases, one estimated the 10-year cumulative incidence in patients with type 1 DM, type 2 DM, and no DM to be 5.2%, 1.0%, and 0.2%, respectively.23 Another study estimated the prevalence among those with type 1 DM to be 4.8%.20 Among studies that reported patient demographics for those with DM and GP, there was a consistent female and White predominance.20, 23
c. In-Patient and ED Databases
Other publications used inpatient or ED databases, and overall noted that the rates of hospitalizations for GP were increasing, with estimates ranging from 2.5- to 18-fold over approximately two decades.6, 24–26 Interestingly, the high and low estimates within this group used the same database – the Nationwide Inpatient Sample (NIS) – but differed notably in their case identification. The former only included those with a primary diagnosis for hospitalization, while the latter included those with GP as either a primary or secondary diagnosis.25, 26 Similarly, one study presented demographics and outcomes of patients specifically with diabetic GP that presented to the ED.21 Case definitions were those with an ED visit with primary or secondary ICD-9 code for GP with a concomitant diagnosis of DM. However, this study did not compare patient presentation, characteristics, or outcomes in the context of non-cases or a comparator group. Overall, ED visits for all GP and diabetic GP from 2006 – 2013 were estimated to increase from 12.9 to 27.3 and 4.7 to 10.5 per 100,000 ED visits, respectively.5 Unfortunately, nearly all studies involving inpatient or ED databases or registries did not present many patient-level characteristics. Those that did present patient-level data most consistently presented age, sex, and in two instances also race.5, 6, 24, 26 Of these studies, there was consistently a female predominance.5, 6, 22, 24, 26
Mortality Estimates
Six studies (46.2%) attempted to estimate the mortality associated with GP.4, 21, 24–27 Of these, only a single study4 assessed the mortality of all-cause GP in comparison to the general population. Using a community-level registry, they found the 5-year survival of those with at least possible GP to be 67% as compared to 81% among controls.4 Poorer survival was also observed among those with definite GP, and the causes of death were primarily due to cardiovascular causes followed by respiratory failure and malignancy.4 One study found correlations between the rate of mortality and Medicare status, poverty, female sex, greater number of hospitalizations, older age of admissions, and greater use of endoscopy.22 Multiple studies observed an improvement in inpatient mortality over the mid-1990s to late 2000s, for example from 0.83% to 0.13% per annual hospitalization in one study, and 2.5% to 1.2% in another study.25, 26
A few studies (23.1%) evaluated the mortality among subpopulations of GP based on etiology.4, 24, 27 In most, survival appears worse in those with diabetic GP as compared to idiopathic GP and all non-diabetic GP. From 2016 – 2017, one study estimated an elevated risk of in-hospital mortality among those with non-diabetic GP versus those with diabetic GP to be 0.30% and 0.23%, respectively.24
Assessment of Study Quality and Risk of Bias
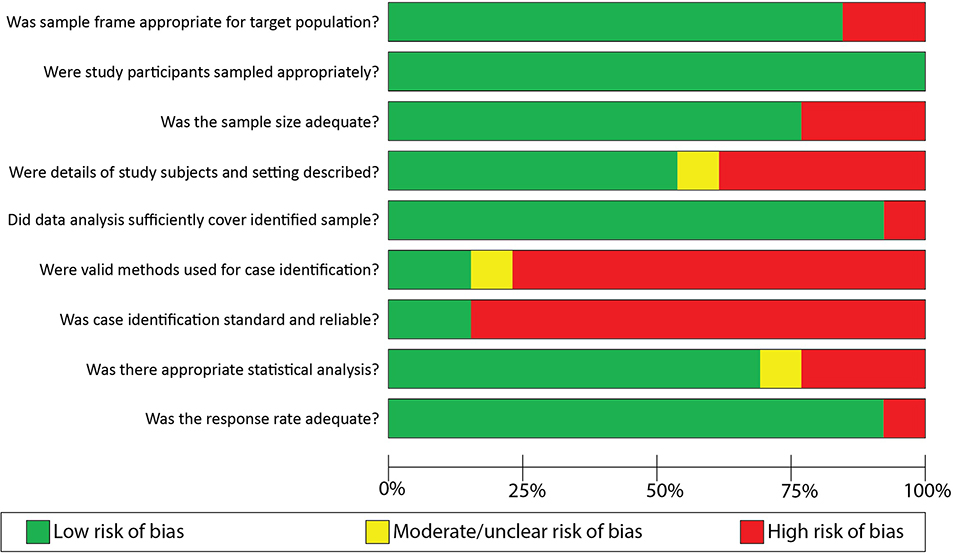
The critical appraisal and qualitative assessment, summarized in Table 4, revealed that most studies (84.6%) adequately identified a target population, a sample within that population, and recruitment method that was appropriate to the sample. Importantly, the target population was not necessarily the same between studies, as some focused on the general population, while others focused on inpatients or those with DM. A low risk of bias in the sampling frame was assigned for publications in which the population of interest was explicitly stated and agreed with the sampling methods.
Table 4.
Critical appraisal of included studies, organized by population setting and then first author*
| Study | Sample frameΔ | Sample method | Sample size | Subject & Setting | Sample coverage | Case definition† | Case reliability | Statistical analysis | Response rate§ |
|---|---|---|---|---|---|---|---|---|---|
| Responses: (+) = adequate, (?) = moderate/unclear, (−) = inadequate | |||||||||
|
| |||||||||
| Community | |||||||||
| Jung, et al. 2009 |

|

|

|

|

|

|

|

|

|
| Syed, et al. 2020 |

|

|

|

|

|

|

|

|

|
| Ye, et al. 2021 (UK) |

|

|

|

|

|

|

|

|

|
| Ye, et al. 2022 (USA) |

|

|

|

|

|

|

|

|

|
| Inpatient/Emergency Department | |||||||||
| Bell, et al. 2002 |

|

|

|

|

|

|

|

|

|
| Bielefeldt 2013 |

|

|

|

|

|

|

|

|

|
| Hirsch, et al. 2019 |

|

|

|

|

|

|

|

|

|
| Kichloo, et al. 2021 |

|

|

|

|

|

|

|

|

|
| Nusra, et al. 2013 |

|

|

|

|

|

|

|

|

|
| Wadhwa, et al. 2017 |

|

|

|

|

|

|

|

|

|
| Wang, et al. 2008 |

|

|

|

|

|

|

|

|

|
| Diabetes Mellitus | |||||||||
| Aleppo, et al. 2017 |

|

|

|

|

|

|

|

|

|
| Choung, et al. 2012 |

|

|

|

|

|

|

|

|

|
As adapted from the Joanna Briggs Institute (JBI) found at https://jbi.global/critical-appraisal-tools, accessed December 15, 2022.
Column titles correspond to questions in the JBI “Checklist for Prevalence Studies”, in numerical order.
Studies that used medical record or survey diagnoses were deemed high likelihood of misclassification and measurement bias and thus given a lower appraisal. These studies were also given the same low score for the following question of “case reliability” as chart diagnoses without objective verification are expected to be inconsistently applied.
Many of the studies (10 of 13, 76.9%) had adequate sample sizes as they considered all or nearly all adult patients at-risk for GP within their chosen national registries or databases.5, 6, 10, 20, 22, 24–28 The use of national registries or databases was considered to represent good coverage of the identified sample, and thus it was not expected that response rate would be a significant issue. As such, the response rates for most of the studies (92.3%) were deemed to be adequate. Of these national registries or databases, a total of three (23.1% of all included studies) ascertained cases from both the inpatient and ambulatory settings and thus considered all patients and subpopulations at-risk of GP.10, 27, 28 Otherwise, three studies (23.1%) did not use national registries4, 21, 23 and four studies (30.8%) did not explicitly report their total sample size or at-risk population.6, 21, 22, 25
The overwhelming majority (76.9%) used only a medical record diagnosis for the case definitions of GP, were unlikely to have a reliable case definition, and thus appraised as inadequate for both parameters.5, 6, 10, 20–22, 24–27 One study used a combination of ICD codes and objective testing for diagnosis of GP but did not provide summary estimates for those with confirmatory objective testing.23 Lastly, only a minority of studies (three of 13, 23.1%) adequately described and summarized their study subjects and study setting in detail.4, 20, 27
The risk of bias of included studies is summarized in Figure 2. Overall, we determined that there was a low risk of bias in study parameters including sampling frame, study sampling methods, population coverage, and response rates. The areas with particularly high risk of bias most notably included both case identification validity, standardization, and reliability. Both of these study parameters were deemed to be substantially undermined by the use of solely ICD diagnoses in most studies.
Figure 2.
Risk of bias of included studies, by question on JBI “Checklist for Prevalence Studies”*
* As adapted from the Joanna Briggs Institute (JBI) found at https://jbi.global/critical-appraisal-tools. Accessed December 15, 2022
DISCUSSION
This is the first and most comprehensive systematic review investigating the epidemiology of GP in the general population providing evidence regarding the prevalence and incidence of GP. There were several notable findings and themes.
First, the overall estimates of the prevalence of GP in the community was as low as 13.8 to as high as 267.7 per 100,000 persons, with the upper limit including all “possible” cases of GP from the nationwide USA Optum Clinformatics Data Mart.4, 27, 28 In reality, the true population prevalence likely lies somewhere between the extreme estimates, and closest to the two population-based studies. Thus, Jung et al.4 and Ye et al.28 estimated prevalence at respectively 24.2 and 21.5 per 100,000 persons, based on the strictest and most accurate case definition with GES.4, 28 The incidence estimates of GP also varied, though more narrowly, from 1.9 to 17.2 per 100,000 person-years.4, 27 In this case, the upper incidence limit was estimated using a community-based cohort of patients.4 Similar to prevalence, it is likely that the true population incidence is closer to that estimated for the “definite” GP group (requiring GES) reported by Jung et al., that is 6.3 per 100,000 person-years.4
Second, GP disproportionately impacts women and those of White or Caucasian races, with estimates as high as about 75% and 70–90%, respectively.4, 9, 20, 27–30 It has been suggested that the higher prevalence among females may be due to multiple factors, including sex hormones, although this has not been extensively investigated.2, 23, 29, 31 Furthermore, when known, the etiology of GP appears most often to be due to type 1 or type 2 DM. The next most prevalent classification of GP is idiopathic, with estimates ranging from 11–49% of all cases. Other known and common causes of GP from our analysis included post-surgical, drug-induced, and connective tissue and autoimmune diseases.
Third, although none of the studies compared the CCI score in patients with GP to the general outpatient population, it appeared comparable between patients admitted to hospital for GP and the total “control” inpatient population. The observed higher CCI score and prevalence of comorbidities in patients with diabetic GP compared to their non-diabetic counterparts was likely secondary to diabetes and its associated complications rather than due to GP itself.6, 20, 24, 27, 28 Overall, multiple chronic multiorgan diseases and their associated risk factors such as history of smoking, obesity, and renal failure were the most prominent.
Fourth, hospitalizations and ED visits for GP, whether as the primary or secondary diagnosis, appear to be rising.5, 6, 25, 26 The study by Nusrat et al. found that although these rates had risen, rates for FD and other unspecified functional disorders of the stomach had decreased and overall hospitalizations had only modestly increased, suggesting that the outcomes may be due to differences in diagnostic practices and awareness.25 This explanation highlights two important points. There is a need for further study since nationwide hospital admitting practices for other diseases were incompletely characterized and the rise in GP admissions was not fully accounted for by the decrease in the other diagnoses. In addition, it emphasizes the need for objective GP testing and consistent diagnostic practices.
Fifth, in our review, the diagnosis of GP appears to confer an elevated mortality risk, particularly among those with diabetic GP.4, 24, 27 The major causes of death appear to be those related to cardiovascular diseases, respiratory failure, and malignancy, though mortality rates from these causes was not directly compared to the general population to assess a baseline level of disease burden.4 Furthermore, the specific mechanisms by which GP results in poorer survival has still not been evaluated. Fortunately, based on inpatient records, the rate of death per annual hospitalization appears to be improving.25, 26 We did not identify any studies that have adequately estimated mortality among the general population of patients with GP for nearly 20 years, which suggests this could be another avenue of investigation.
Methodological patterns were also noticed between included studies. The vast majority of the studies were from USA databases and registries. This suggests that the data regarding world or different country prevalence of GP is incomplete and that robust estimates outside of the USA and UK are essentially nonexistent. As such, the true international burden and impact of GP remains to be determined. Furthermore, of those studies included, most used either medical record or ICD diagnoses for case identification. Because their use of ICD codes is not among the diagnostic criteria for GP and it is expected to be inconsistent across providers and regions, the diagnosis is suspected to be at high risk of misclassification and measurement biases. Furthermore, the directionality of bias is unclear, as the diagnostic and documenting practices regarding the diagnosis of GP are likely not uniform and comprehensive. It is also conceivable that varying physician training and differential access to GES may result in one patient being diagnosed with GP in one region and FD in another despite similar features. This is exemplified by the relative prevalence of gastroparesis in the different countries of the UK where marked differences were recorded between Scotland and England, and even between regions such as Scotland compared to London and the South East region.27 Similarly, imprecision was observed between studies attempting to estimate hospitalizations and healthcare utilization. The use of chart or medical record diagnoses likely also contributed to wide variations in estimates of GP etiologies. For example, estimates of GP due to DM in different studies were as low as 25% to as high as nearly 80%.4, 10, 24 It was for this reason that most of the included studies were deemed to be inadequate regarding both case definition and case reliability during our critical appraisal of the literature.
There are significant deficiencies intrinsic to our analysis. Thus, it is unfortunate that our review of the literature documented the crucial lack of consistent, patient-level data to comprehensively characterize the epidemiology of GP, with significant heterogeneity in data acquisition, target population, and study design. Furthermore, most studies have utilized inpatient databases which do not consistently provide comprehensive demographic data and risk factors such as BMI, glycemic control, and alcohol and tobacco use. Among the demographic data, a very important index which is not sufficiently documented is the association between weight loss and GP. Multiple studies, including those by Sarnelli et al. and Parkman et al., have found no consistent association between delayed GE, GP and weight loss.9, 11, 32, 33 Similarly, although DM is a risk factor for GP, the relative risk from type 1 versus type 2 DM remains uncertain based on our systematic review. Additionally, there were not enough epidemiological studies to adequately address social determinants of health, comorbidities, quality of life measures, and the role of mental health and psychiatric comorbidities, all of which have been assessed in non-population-based studies.7, 34–36 Lastly, although many of the studies utilizing hospitalization records were appropriately explicit regarding the scope and aims (i.e. assessing the burden of GP hospitalizations and ED visits), it is important to emphasize that most health care utilization takes place in the outpatient setting, including for GP.37
Strengths and Limitations
Our study has been conducted with rigorous methodology congruent with the expectations of the systematic review process. Only studies that utilized population-based methods were included to minimize the risk of sampling bias and maximize inferences. The study compiled data collected from a cohort of more than 700 million patients.
However, there are a number of critical limitations. First, despite strict inclusion criteria of studies utilizing population-level data, there remained a significant amount of heterogeneity between the studies, including the study setting. For this reason, a meta-analysis was not conducted. There was a paucity of case definitions relying on objective measurements of GE which led to imprecise overall estimates of the prevalence and incidence of GP. It is conceivable that there was overlap among studies using similar databases or registries over similar timeframes. For example, four of the studies utilized the same NIS database over the periods 1994 – 2013 and 2016 – 2017.6, 24–26 Three of these utilized data specifically from the years 1997 – 2004.6, 25, 26 Furthermore, it is possible that more severe cases of GP could have resulted in repeat hospitalizations or ED visits and thus may have been counted multiple times. The relatively small number of population or community-based studies limits inferences to the general population. Given the data collected in the included studies, our systematic review was unable to generate conclusions regarding risk factors and comorbidities in the general population, as this was inconsistently reported.
Conclusion
Despite rising numbers of hospitalizations and ED visits for GP, there is a paucity of high-quality, demographically diverse, and population-based studies to accurately describe the epidemiology and risk factors of GP, particularly at the patient level. More studies, particularly with objective case definitions requiring GE measurements, are needed to better characterize the epidemiology and natural history of GP.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND:
Despite rising numbers of hospitalizations and emergency department visits for gastroparesis, the epidemiology of gastroparesis based on community prevalence remains incomplete.
FINDINGS:
The prevalence of gastroparesis in the general population ranged from 13.8 to 267.7 per 100,000 adults, and incidence from 1.9 to 6.3 per 100,000 person-years, being commonest in diabetes and idiopathic forms. Only four studies (30.8%) used community databases, only two (15.4%) reported estimates using rigorous case definitions, and there was a paucity of high-quality, demographically diverse, and population-based studies.
IMPLICATIONS FOR PATIENT CARE:
Given rising numbers of hospitalizations and ED visits, future studies with valid GE measurement are needed to better characterize the epidemiology and natural history of GP.
Acknowledgements:
The authors thank Cindy Stanislav for exceptional secretarial assistance and Larry Prokop for outstanding librarianship and advice with the search strategy.
Grant support:
This work was supported by grants to Michael Camilleri from National Institutes of Health (R01-DK122280 and R01-DK125680).
Abbreviations:
- CCI
Charlson Comorbidity Index
- DG
diabetic gastroparesis
- DM
diabetes mellitus
- ED
Emergency Department
- FGID
functional gastrointestinal disorders
- GCSI
Gastroparesis Cardinal Symptoms Index
- GP
gastroparesis
- ICD
International Classification of Diseases
- IG
idiopathic gastroparesis
- NEDS
National Emergency Department Sample
- NIS
Nationwide Inpatient Sample
- PSG
post-surgical gastroparesis
- T1D
type 1 diabetes mellitus
- T2D
type 2 diabetes mellitus
- UK
United Kingdom
- USA
United States of America
Footnotes
Disclosures: The authors have no competing interests or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data transparency statement:
Original data is available upon request directed to the corresponding author.
REFERENCES
- 1.Schol J, Wauters L, Dickman R, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. Neurogastroenterol Motil. 2021;33(8):e14237. Epub 2021/08/17. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. Epub 2018/11/06. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37; quiz 8. Epub 2012/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33. Epub 2009/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch W, Nee J, Ballou S, et al. Emergency Department Burden of Gastroparesis in the United States, 2006 to 2013. J Clin Gastroenterol. 2019;53(2):109–13. Epub 2017/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhwa V, Mehta D, Jobanputra Y, et al. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23(24):4428–36. Epub 2017/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacy BE, Crowell MD, Mathis C, et al. Gastroparesis: Quality of Life and Health Care Utilization. J Clin Gastroenterol. 2018;52(1):20–4. Epub 2016/10/25. [DOI] [PubMed] [Google Scholar]
- 8.Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63(12):1972–8. Epub 2014/09/28. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Locke GR 3rd, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55(7):933–9. Epub 2005/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and Diagnosis of Gastroparesis in the United States: A Population-based Study. J Clin Gastroenterol. 2020;54(1):50–4. Epub 2019/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–15. Epub 2010/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Epub 2021/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LK, Xu J, Freedman BI, et al. Symptoms Suggestive of Gastroparesis in a Community-Based Cohort of European Americans and African Americans with Type 2 Diabetes Mellitus. Dig Dis Sci. 2020;65(8):2321–30. Epub 2019/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18(1):141–50. Epub 2003/07/10. [DOI] [PubMed] [Google Scholar]
- 15.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13(4):833–44. Epub 2004/05/08. [DOI] [PubMed] [Google Scholar]
- 16.Pasricha PJ, Grover M, Yates KP, et al. Functional Dyspepsia and Gastroparesis in Tertiary Care are Interchangeable Syndromes With Common Clinical and Pathologic Features. Gastroenterology. 2021;160(6):2006–17. Epub 2021/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomi S, Plazinska M, Zagorowicz E, et al. [Gastric emptying disorders in diabetes mellitus]. Polskie Archiwum Medycyny Wewnetrznej. 2002;108(3):879–86. [PubMed] [Google Scholar]
- 18.Rohatgi A WebPlotDigitizer. 2021. [April 10, 2022]; 4.5:[Available from: https://automeris.io/WebPlotDigitizer. [Google Scholar]
- 19.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–53. Epub 2015/09/01. [DOI] [PubMed] [Google Scholar]
- 20.Aleppo G, Calhoun P, Foster NC, et al. Reported gastroparesis in adults with type 1 diabetes (T1D) from the T1D Exchange clinic registry. J Diabetes Complications. 2017;31(12):1669–73. Epub 2017/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell RA, Jones-Vessey K, Summerson JH. Hospitalizations and outcomes for diabetic gastroparesis in North Carolina. South Med J. 2002;95(11):1297–9. Epub 2003/01/24. [PubMed] [Google Scholar]
- 22.Bielefeldt K Regional differences in healthcare delivery for gastroparesis. Dig Dis Sci. 2013;58(10):2789–98. Epub 2013/03/26. [DOI] [PubMed] [Google Scholar]
- 23.Choung RS, Locke GR 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107(1):82–8. Epub 2011/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kichloo A, Dahiya DS, Wani F, et al. Diabetic and Non-Diabetic Gastroparesis: A Retrospective Comparative Outcome Study From the Nationwide Inpatient Sample. Gastroenterology Res. 2021;14(1):21–30. Epub 2021/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nusrat S, Bielefeldt K. Gastroparesis on the rise: incidence vs awareness? Neurogastroenterol Motil 2013;25(1):16–22. Epub 2012/09/04. [DOI] [PubMed] [Google Scholar]
- 26.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103(2):313–22. Epub 2007/12/01. [DOI] [PubMed] [Google Scholar]
- 27.Ye Y, Jiang B, Manne S, et al. Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut. 2021;70(4):644–53. Epub 2020/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Y, Yin Y, Huh SY, et al. Epidemiology, Etiology, and Treatment of Gastroparesis: Real-World Evidence From a Large US National Claims Database. Gastroenterology. 2022;162(1):109–21 e5. Epub 2021/10/09. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24(12):1076–e562. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97(3):604–11. Epub 2002/04/02. [DOI] [PubMed] [Google Scholar]
- 31.Ravella K, Al-Hendy A, Sharan C, et al. Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function. Dig Dis Sci. 2013;58(6):1507–15. Epub 2013/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarnelli G, Caenepeel P, Geypens B, et al. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98(4):783–8. Epub 2003/05/10. [DOI] [PubMed] [Google Scholar]
- 33.Perri F, Clemente R, Festa V, et al. Patterns of symptoms in functional dyspepsia: role of Helicobacter pylori infection and delayed gastric emptying. Am J Gastroenterol. 1998;93(11):2082–8. Epub 1998/11/20. [DOI] [PubMed] [Google Scholar]
- 34.Hasler WL, Parkman HP, Wilson LA, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol. 2010;105(11):2357–67. Epub 2010/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jehangir A, Parkman HP. Chronic opioids in gastroparesis: Relationship with gastrointestinal symptoms, healthcare utilization and employment. World J Gastroenterol. 2017;23(40):7310–20. Epub 2017/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D, Ramsey FV, Norton WF, et al. The Burdens, Concerns, and Quality of Life of Patients with Gastroparesis. Dig Dis Sci. 2017;62(4):879–93. Epub 2017/01/23. [DOI] [PubMed] [Google Scholar]
- 37.2019 Health Care Cost and Utilization Report. Health Care Cost Institute, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data is available upon request directed to the corresponding author.