Abstract
Oral anticoagulants (OACs) are commonly used to reduce the risk of venous thromboembolism (VTE) and the risk of stroke in patients with atrial fibrillation (AF). Endorsed by the American Heart Association, American College of Cardiology, and the European Society of Cardiology, direct oral anticoagulants (DOACs) have displaced warfarin as the OAC of choice for both conditions, due to improved safety profiles, fewer drug-drug and drug-diet interactions, and lack of monitoring requirements. Despite their widespread use and improved safety over warfarin, DOAC related bleeding remains a major concern for patients. DOACs have stable pharmacokinetics and pharmacodynamics; however, variability in DOAC response is common and may be attributed to numerous factors, including patient-specific factors, concomitant medications, comorbid conditions, and genetics. While DOAC randomized controlled trials included patients of varying ages and levels of kidney function, they failed to include patients of diverse ancestries. Additionally, current evidence to support DOAC pharmacogenetic associations have primarily been derived from European and Asian individuals. Given differences in genotype frequencies and disease burden among patients of different biogeographic groups, future research must engage diverse populations to assess and quantify the impact of predictors on DOAC response. Current underrepresentation of patients from diverse racial groups does not allow for proper generalization of the influence of clinical and genetic factors in relation to DOAC variability. Herein, we discuss factors affecting DOAC response, such as age, sex, weight, kidney function, drug interactions, and pharmacogenetics, while offering a new perspective on the need for further research including frequently excluded groups.
Keywords: direct oral anticoagulant, efficacy, safety, atrial fibrillation, venous thromboembolism, pharmacogenetics, bleeding, stroke and systemic embolism, kidney function, age, gender, weight, pharmacokinetics-pharmacodynamics, drug interactions, race, clinical trials, variability, personalized medicine
Introduction:
Oral anticoagulants (OACs) are commonly used to reduce the risk of venous thromboembolism (VTE) and the risk of stroke in patients with atrial fibrillation (AF). Warfarin, introduced in 1954,1 enjoyed market monopoly as the sole OAC for almost 60 years.2 Despite its efficacy, the capricious nature of warfarin response, the multitude of drug and diet interactions influencing variability, the need for monitoring and the risk of bleeding has fueled research in search of an ideal anticoagulant. In 2004, ximelagatran, a direct thrombin inhibitor, was introduced as a potential alternative to warfarin, but it failed to attain Food and Drug Administration (FDA) approval due to increased risk of hepatotoxicity.3 However, this promising avenue of research led to the introduction of the direct oral anticoagulants (DOACs), including dabigatran (Pradaxa®; 2010), rivaroxaban (Xarelto®; 2011), apixaban (Eliquis®; 2012), and edoxaban (Savaysa®; 2015).
DOACs offer several advantages over warfarin including fixed dosing, predictable pharmacokinetics (PK) and pharmacodynamics properties, fewer food and drug interactions, lack of monitoring requirements, equal or superior efficacy, and lower risk of major hemorrhage, particularly intracranial hemorrhage (ICH).4 The American Heart Association, American College of Cardiology and the European Society of Cardiology recommend initiating new eligible patients on DOACs over warfarin,2 resulting in apixaban and rivaroxaban being among the most commonly prescribed medications in the US in 2019.5 DOAC utilization is expected to increase as the population ages and the prevalence of conditions, including AF, stroke, and VTE rises.
Despite the many advantages, bleeding remains a grave concern with DOACs as reflected in the recent trends of adverse drug related hospitalizations.6 In this review, we present the timeline of DOAC development, their efficacy and safety (versus warfarin) based on their respective clinical trials, and compare their PK and pharmacodynamics properties. We discuss factors that affect variability in DOAC response including the role of age, sex, weight, kidney function, drug interactions, and pharmacogenetics. We synthesize this evidence to identify knowledge gaps and propose research needed to personalize DOAC therapy.
Oral Anticoagulant Development Timeline:
The 21st century (Figure 1) witnessed significant advances in anticoagulation therapy, with the development of ximelagatran, the first direct thrombin inhibitor. Despite its advantages, it failed to gain FDA approval due to 9.6% of trial participants experiencing hepatotoxicity (alanine aminotransferase (ALT) levels exceeding three times normal limits).3
Figure 1.

Timeline in development of Oral Anticoagulation Drugs.
Figure 1 provides an overview of the oral anticoagulants approval timeline. Line graphs show the trend in direct oral anticoagulant and warfarin prescriptions. All approval dates represent United States approval date.
It was not until 2010 that a feasible alternative to warfarin, the direct thrombin (factor IIa) inhibitor dabigatran, was approved, followed by the factor Xa inhibitors, rivaroxaban, apixaban and edoxaban. Betrixaban, approved by the FDA in 2017 for VTE prophylaxis in acutely ill hospitalized patients, was discontinued by Portola pharmaceuticals for independent business reasons and is not available in the US market.7 Therefore it is not discussed further in this review.
Although warfarin remains the most prescribed oral anticoagulant, its prescription volumes have decreased by over 50% (from approximately 30 million in 2011 to 14.6 million in 2019).5 This trend is largely driven by increased rivaroxaban (8.7 million in 2019) and apixaban (14 million in 2019) utilization, and is expected to continue.5,8
Comparative Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin:
DOACs have similar or superior efficacy and safety in randomized clinical trials (RCTs; Table S1) when compared to warfarin dosed to maintain an international normalized ratio (INR) of 2-3. The AF RCTs primary efficacy endpoint consisted of stroke (ischemic or hemorrhagic), and systemic embolism (SSE). The primary safety endpoint was major-bleeding (MB) as defined by the International Society of Thrombosis and Hemostasis (ISTH) criteria, or MB plus non major clinically relevant bleeding in ROCKET.9–13 VTE trials efficacy endpoint consisted of VTE recurrence or related death, while the primary safety endpoint was defined as MB or MB and non-major clinically relevant bleeding for EINSTEIN and HOKUSAI trials. Non major clinically relevant bleeding was defined as overt bleeding not meeting criteria for major bleeding, but that required intervention, physician contact or led to impairment of daily live activities.14–17
For AF trials, compared to warfarin, DOACS demonstrated lower relative risks (RR; Figure 2a) of SSE ranging from 34% lower for dabigatran (150 mg), and 21% lower for rivaroxaban (20 mg), apixaban (5 mg), and edoxaban (60 mg). There was no difference in risk of SSE between warfarin and lower doses of dabigatran (110 mg) or edoxaban (30 mg).9–12
Figure 2.
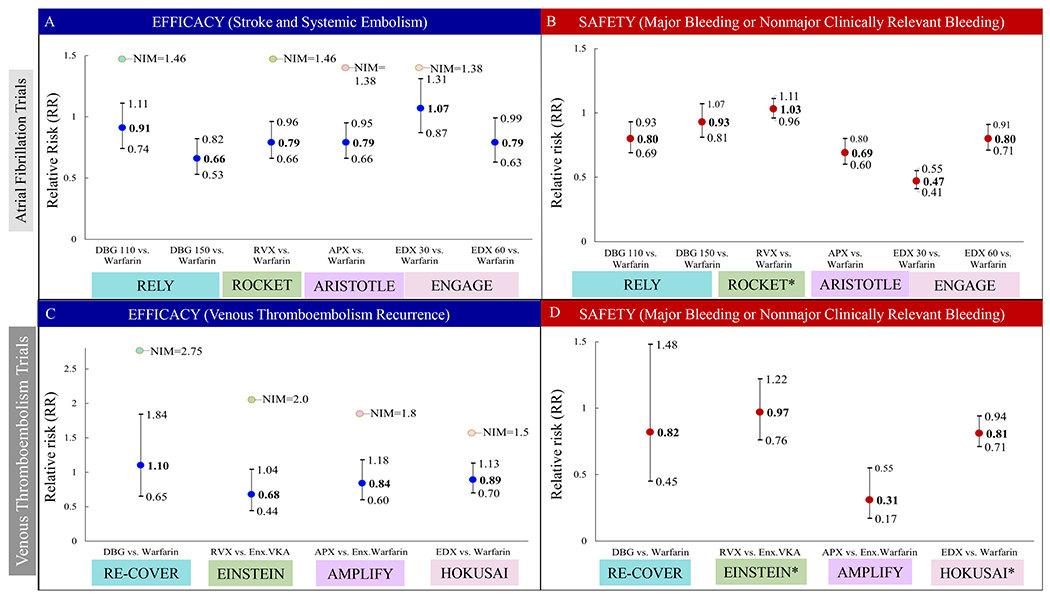
Efficacy and Safety Endpoints for Direct Oral Anticoagulants from Atrial Fibrillation and Venous Thromboembolism Randomized Clinical Trials.
• Figure 2a. Efficacy endpoint (stroke and systemic embolism) in atrial fibrillation trials as measured by relative risk. Non-inferiority margin for each represented trial is provided.
• Figure 2b. Safety endpoint for atrial fibrillation trials, described as major bleeding or major and non-major clinically relevant bleeding in the case of the ROCKET trial as measured by relative risk.
• Figure 2c. Efficacy endpoint, described as venous thromboembolism recurrence or related death for venous thromboembolism trials as measured by relative risk. Non-inferiority margin for each represented trial is provided.
• Figure 2d. Safety endpoint for venous thromboembolism trials, described as major bleeding or major and non-major clinically relevant bleeding in the case of the ENSTEIN and HOKUSAI trials as measured by relative risk.
RR=Relative risk. NIM=Non-inferiority Margin. DBG 110=Dabigatran 110 milligram dose. DBG 150=Dabigatran 150 milligram dose. RVX=rivaroxaban. APX=apixaban. EDX 30= edoxaban 30 milligram dose. EDX 60= edoxaban 60 milligram dose. ENX=enoxaparin. VKA=vitamin K antagonist (Acenocoumarol or warfarin).
Compared to warfarin, major bleeding (Figure 2b) was 20% lower for dabigatran (110 mg), 31% lower for apixaban, and 20% to 53% lower for edoxaban (60 and 30 mg), respectively. There was no difference in risk of MB between warfarin and dabigatran (150 mg), or warfarin and rivaroxaban. Although not statistically significant, compared to warfarin, gastrointestinal bleeding (GIB) was higher for most DOACs; ranging from 10% (110 mg) to 50% (150 mg) higher for dabigatran, 44% higher for rivaroxaban, 11% lower for apixaban, and 23% higher for edoxaban (60 mg). GI bleed was significantly lower for low dose edoxaban (33%). DOACs consistently demonstrated significant lower risks of intracranial hemorrhage (ICH) ranging from 60 to 69% lower for dabigatran (150 and 110 mg) respectively, 33% lower for rivaroxaban, 58% lower for apixaban, and 53% to 70% lower for edoxaban (60 and 30 mg) respectively.9–12
In VTE trials, DOACs were non-inferior to warfarin (dabigatran and edoxaban) or a vitamin K antagonist plus enoxaparin (rivaroxaban and apixaban) for the established endpoints of VTE recurrence or related death. (Figure 2c) provides relative risks and non-inferiority margins for the VTE trials efficacy endpoint. The risk of MB or non-major clinically relevant bleeding (Figure 2d) was similar between dabigatran and warfarin, and between rivaroxaban and a vitamin K antagonist plus enoxaparin. Compared to warfarin, apixaban was associated with a 69% lower risk of MB, and edoxaban was associated with a 19% lower risk of MB and non-major clinically relevant bleeding.14–17 Although not significant, GIB events were 48% higher for dabigatran,14 61% lower for apixaban16 and 50% lower for edoxaban17 compared to warfarin. The risk of ICH was 50% lower for apixaban.16 Other trials had insufficient events or reported them differently. Both VTE and AF RCTs findings were based on populations primarily from European or Asian ancestries.
A meta-analysis comparing DOACs to standard therapy, comprising 6 VTE RCTs, RE-COVER I and II (dabigatran), EINSTEIN-DVT and PE (rivaroxaban), AMPLIFY (apixaban) and HOKUSAI-VTE (edoxaban), and over 27,000 patients, corroborated that DOACs were non-inferior to warfarin for recurrent VTE prevention (RR 0.91; 95% CI 0.79 to 1.06). Furthermore, the relative risk of major bleeding was 38% lower for DOACs (RR 0.62 95% CI 0.45 to 0.85).18
Real world evidence has the potential to extend RCT findings to a more generalizable population. A recently conducted meta-analysis that included 34 cohort or real-world studies and over 2 million non-valvular atrial fibrillation (NVAF) patients, primarily of European or Asian ancestry, corroborated DOACs efficacy and safety over warfarin. DOACs reduced stroke risk by 23% (HR 0.77; 95% CI 0.49 to 0.87; p<0.01), mortality risk by 29% (HR 0.71; 95% CI 0.63 to 0.81; p<0.01), major bleeding by 32% (HR 0.68; 95% CI 0.54 to 0.86; p<0.01) and ICH by 46% (HR 0.54; 95% CI 0.42 to 0.70; p<0.01). However, DOACs did not reduce the risk of GI bleeding (HR 0.79; 95% CI 0.58 to 1.06; p=0.12).19
Geller et al. evaluated anticoagulant-associated emergency department visits using the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance. They found that the percentage of all emergency department visits for oral anticoagulant bleeding that were DOAC-related increased from 2.3% in 2011 to 37.9% in 2017. Of these DOAC-related emergency department visits in 2017, 49.3% were attributed to rivaroxaban, 41.8% to apixaban, and 8.9% to dabigatran,6 with apixaban being the most commonly prescribed based on prescription volumes that year (Figure 1).
Dose and Plasma Concentration Response:
DOACs are dosed based on indication (Table 1) after additional adjustments are made based on factors such as, age, kidney function, body weight and drug interactions. In general these patient-level factors have been shown to contribute to variability in DOAC concentrations, and are discussed individually in further detail later in this review. Given the potential thromboembolic and hemorrhagic consequences associated with altered DOAC response understanding the relationship between dose, plasma concentrations, and therapeutic or impaired response is necessary for appropriate DOAC dose selection and adjustment.
Table 1.
Overview of Characteristics of Direct Oral Anticoagulants
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
|---|---|---|---|---|
| PK/PD Properties | ||||
| Pharmacologic Target | Factor IIa | Factor Xa | Factor Xa | Factor Xa |
| Bioavailability | 3-7% | 80-100% | 50% | 62% |
| Peak (hours) | 1-2 | 2-4 | 3-4 | 1-2 |
| Half-life (hours) | 12-17 | 5-9 | 12 | 10-14 |
| Renal clearance | 80% | 36% | 27% | 50% |
| Metabolic Pathways | CES1, CES2 | CYP3A4/5, CYP2J2 | CYP3A4 | CES1, CYP3A4 |
| Substrate of | P-gp | CYP3A4/5, CYP2J2, ABCG2, P-gp | CYP3A4, P-gp, ABCG2 | P-gp |
| Dosing ‡ | ||||
| NVAF | 150 mg twice daily | 20 mg daily | 5 mg twice daily | 60 mg daily |
| DVT/PE treatment | 15 mg twice daily for 21 days 20 mg daily afterwards |
10 mg twice daily for 7 days 5 mg twice daily afterwards |
||
| Renal Adjustments * | ||||
| CrCl 30-50 mL/min | NVAF:15 mg daily | 2.5 mg twice daily if ≥2 of the following: • SCR ≥1.5 • Age ≥ 80 • Weight ≤ 60 kg |
30 mg daily | |
| CrCl 15-30 mL/min |
NVAF: 75 mg twice daily DVT/PE: Not provided |
NVAF:15 mg daily ɕDVT/PE: Not Studied |
30 mg daily | |
| ESRD (CrCl <15 mL/min) | Not provided |
NVAF: Not provided DVT/PE: Avoid use |
Not recommended | |
| Other Considerations | ||||
| Take with food |
NVAF: Do not use if CrCl >95 mL/min DVT/PE: (Weight ≤60kg) 30 mg daily |
|||
| Hepatic Impairment | ||||
| Child-Pugh Class A | N/A | N/A | No dose adjustment | No dose adjustment |
| Child-Pugh Class B | N/A | Avoid use | Not provided | Not recommended |
| Child-Pugh Class C | N/A | Avoid use | Not recommended | Not recommended |
| Drug Interactions§ | ||||
| Avoid use | • P-gp inducers • P-gp inhibitors in patients with CrCl<30 mL/min (NVAF) or CrCl <50 mL/min (DVT/PE) |
• Combined P-gp and strong CYP3A4 inhibitors AND inducers • Combined P-gp and moderate CYP3A4 inhibitors when CrCl 15 to <80 mL/min |
• Combined P-gp and strong CYP3A4 inhibitors when dose 2.5 mg twice daily • Combined P-gp and strong CYP3A4 inducers |
P-gp inducers |
| Dose adjustment | 75 mg twice daily with P-gp inhibitors dronedarone or systemic ketoconazole in patients with CrCl 30-50 mL/min | Reduce 5 or 10 mg dose 50% with combined P-gp and strong CYP3A4 inhibitors | 30 mg daily with P-gp inhibitors: verapamil, quinidine, azithromycin, clarithromycin, erythromycin, oral itraconazole, or oral ketoconazole (DVT/PE) | |
| No dose adjustment | P-gp inhibitors: verapamil, amiodarone, quinidine, clarithromycin, and ticagrelor | Clarithromycin | P-gp inhibitors | |
| Reversal Agent | idarucizumab (Praxbind®) | andexanet alfa (Andexxa®) | andexanet alfa (Andexxa®) | ciraparantag (under investigation) |
Dosing/other considerations are limited to NVAF and DVT/PE treatment dosing
For renal adjustments, if NVAF or DVT/PE is not indicated, the recommended dosing is the same for both indications
For rivaroxaban, patients with CrCl < 30 mL/min were excluded from the Einstein trials. Serum concentrations are expected to be similar to those of patients with moderate renal impairment. Close observation and promptly evaluation of signs and symptoms of blood loss are recommended for patients with CrCl 15-<30 using rivaroxaban for DVT/PE treatment or reduction.
Combined P-gp and strong CYP3A4 inhibitors: ketoconazole, itraconazole, ritonavir. Combined P-gp and strong CYP3A4 inducers: carbamazepine, phenytoin, rifampin, St. John’s Wort. Combined P-gp and moderate CYP3A4 inhibitors: erythromycin.
Abbreviations: PKPD=Pharmacokinetics/Pharmacodynamics. P-gp=P-glycoprotein. CES=Carboxylesterases. NVAF= Non Valvular Atrial Fibrillation. DVT=Deep Vein Thrombosis. PE=Pulmonary Embolism. CrCl= Creatinine Clearance. ESRD=End Stage Renal Disease. SCR=Serum Creatinine. N/A=Not Available. Mg=Milligrams.
For both dabigatran and edoxaban, low trough levels have been associated with increased ischemic stroke rates, whereas apixaban area under the curve (AUC) has not been shown to influence stroke risk. Coversely, elevated dabigatran and edoxaban steady-state trough concentrations, elevated apixaban steady-state AUC, and prolonged prothrombin time in rivaroxaban-treated patients have all been associated with increased bleeding risk. However, there is extensive variability in measured concentrations, limiting the utility of DOAC level monitoring. Median peak and trough levels have been shown to vary from 2 to 5-fold (dabigatran, apixaban) to 10 to 20-fold (rivaroxaban, edoxaban), and between individual median trough levels can vary 6 to 11-fold.20
For dabigatran, extensive PK modeling and simulation of RE-LY trial data suggested that a titration strategy based on measured plasma levels may result in improved safety and efficacy profiles compared to one size fits all dosing. Patients received dabigatran 150 mg twice daily for one week and had subsequent doses titrated based on steady state trough concentrations. Patients with plasma concentrations <90 ng/ml remained on 150 mg twice daily, while patients with concentrations from ≥90 to <140 ng/ml and ≥140 ng/ml were switched to 110 mg twice daily and 75 mg twice daily, respectively. Compared to standard 150 mg twice daily dosing, titration resulted in similar ischemic stroke/SEE events (RR 1.06; 95% CI 0.76-1.50) and significantly lower major bleeding risk (RR 0.80; 95% CI 0.66-0.97). Furthermore, approximately 55% of patients were expected to be assigned to 75 mg (25.5%) or 110 mg (29.9%) twice daily dosing.21 Although the European Medical Agency approved a plasma level diagnostic test and therapeutic range (48-200 ng/ml) due to concerns related to bleeding risk, the Food and Drug Administration does not support assessment of concentrations due to the potential for underdosing.22 Among other DOACs, lower edoxaban doses adjusted based on patient-related factors (low body weight, kidney function, etc.) resulted in lower concentrations and factor Xa activity. However, stroke and bleeding risks were similar to patients who received the full dose. Furthermore, an evaluation of apixaban levels between patients who experienced bleeding (major or major plus clinically relevant non-major) identified overlap in levels between groups. Although studies have demonstrated relationships between DOAC dose and response, and despite the availability of quantitative measures such as plasma concentration, anti-factor Xa levels (rivaroxaban, apixaban and edoxaban), ecarin thrombin time (dabigatran), or dilute thrombin time, therapeutic drug monitoring of DOACs is not commonly performed in clinical practice, complicated by pharmacokinetic variability and lack of standardized therapeutic ranges.23 Currently, the American Heart Association guidelines, suggest DOAC level monitoring could be useful in patients undergoing urgent surgical procedures, those with severe obesity, potential drug interactions, chronic kidney disease (CKD) or dialysis patients or to confirm adherence to treatment.24 While personalized approaches incorporating plasma concentrations have the potential to improve clinical outcomes, further studies are needed to evaluate the utility of these approaches in clinical care and identify causes of variability in concentrations.
Pharmacokinetics and Pharmacodynamics Properties:
Dabigatran exerts its therapeutic effect by inhibiting thrombin (factor IIa)25 while rivaroxaban, apixaban and edoxaban exert their effects by inhibiting factor Xa.26–29 Dabigatran is a prodrug, requiring bio activation by hepatic and intestinal carboxylesterases (CES 1 and CES 2).30
All DOACs are P-glycoprotein (P-gp) substrates25,27–29 but differ in their metabolism through cytochrome P450 (CYP450) enzymes. CYP3A4 is the primary CYP450 responsible for the metabolism of rivaroxaban (50%) and apixaban (20%). Conversely, edoxaban and dabigatran have minimal (<4%) and no (0%) dependence on CYP450 (CYP3A4) enzymes, respectively.31 The P-gp and CYP3A4 dependence of DOACs highlights the potential for drug interactions with P-gp and CYP3A4 inducers and inhibitors seen in clinical practice.32
Figure 3 provides an overview of DOACs PK and pharmacodynamics, while Table 1 provides dosing recommendations based on drug interactions, renal and hepatic impairment. DOACs have a quick onset of action and a shorter time to peak effects and therefore do not require bridging therapy. Unlike DOACs, warfarin therapeutic effect is delayed (24 hours) and peak effects are not achieved until 3-4 days, and may require heparin overlap until a therapeutic INR is achieved.1 DOAC’s shorter elimination half-life compared to warfarin (20-60 hours) eases the burden of preparation for procedures or surgery. While simple procedures require no interruption in DOAC therapy, high impact bleed risk procedures may require discontinuation of the DOAC for 48 hours.33 For situations where prompt action is required (e.g. life threatening emergencies like bleeding or urgent procedures), antidotes are available for the most commonly prescribed DOACs. Andexanet alfa (Andexxa®), approved in 2018, is a factor Xa decoy protein used for reversal of rivaroxaban and apixaban anticoagulation. Additionally, idarucizumab (Praxbind®), approved in 2015, is a humanized monoclonal antibody fragment indicated for dabigatran reversal for emergency procedures or life-threatening bleeding.34,35
Figure 3.

Pharmacokinetics and Pharmacodynamics of Warfarin and Direct Oral Anticoagulants.
Figure 3 represents the key processes in warfarin and Direct Oral Anticoagulant absorption, transport, coagulation factors inhibited and key genes involved in hepatic metabolism.
• Hepatic and renal involvement in their metabolism and excretion are represented in percentages.
• The effect of decreased renal function on elimination half-lives are reported for each renal function subgroup. T (1/2) =elimination half-life. h=hours. NR=Not Relevant.
• Elimination half-lives by renal function subgroups for edoxaban, apixaban and rivaroxaban are those represented in Heidbuchel et al. Warfarin and dabigatran half-lives were extracted from their respective package insert.
The Role of Age, Sex, and Weight:
Age:
The elderly population (65 and older) is expected to double by 2060.36 Older adults have a higher prevalence of chronic conditions including AF, which requires OAC therapy.37 Multiple comorbidities and a higher medication burden predisposes this population to drug interactions and adverse drug reactions.38 While DOACs have fewer interactions than warfarin, DOAC related bleeding remains a major concern in older adults. In 2017, DOAC-related bleeding accounted for 11.8% of all emergency department visits related to adverse drug events among patients ≥65 years old, whereas warfarin accounted for 18.6%.6 Alterations in DOAC concentrations have been observed among the elderly. Reduced appendicular lean mass has been associated with supratherapeutic peak and trough apixaban and rivaroxaban concentrations in patients ≥65 years old.39 Similarly, the prevalence of supratherapeutic dabigatran, rivaroxaban, and apixaban concentrations is more common among elderly patients (38.2% in ≥75 years vs 17.9% in 65-75 year old; p=0.041). Patients on apixaban were more likely to have therapeutic levels (82.9%) compared to rivaroxaban (44.3%) or dabigatran (64.3%).40
An analysis of RE-LY, found age to be the most important covariant altering dabigatran levels, with patients ≥ 75 years old exhibiting a 68% higher trough concentration compared to those < 65. This analysis also found a strong correlation between age and decreasing kidney function.41 An evaluation of predictors of high apixaban trough levels (≥ 86 ng/dL) in patients treated with low dose apixaban found that age ≥ 85 years old was associated with higher levels, in addition to female gender, creatinine clearance (CrCl) <30 mL/min, heart failure, and a history of peripheral artery disease or myocardial infarction.42
When evaluating patients 65 to 74 years of age in the clinical trials that led to DOAC approval, only apixaban was superior to warfarin in reducing both SSE (HR 0.72; 95% CI 0.54 to 0.96) and MB (HR 0.71; 95% CI 0.56 to 0.89).25,27–29 Similarly, A meta-analysis of the AF DOAC trials in this age group (n=28,135), showed dabigatran 150 mg, rivaroxaban and apixaban were superior to warfarin in SSE prevention. When compared to each other, DOACs exhibited similar rates of MB; whereas, compared to warfarin, only apixaban and edoxaban 60 mg were associated with 36% and 17% lower rates of MB, respectively. Overall, DOACs reduced ICH by 52% (HR 0.48; 95% CI 0.34 to 0.67; p<0.01) in adults ≥75 years old. However, rivaroxaban was associated with an approximately 2-fold (1.86 to 2.3) increased risk of ICH, and was the only DOAC not superior to warfarin in this aspect.43 In VTE trials, all DOACs demonstrated similar efficacy to the comparator arms. Apixaban reduced MB in those ≥65 years old compared to enoxaparin followed by warfarin, while edoxaban reduced major and clinically relevant non-major bleeding in patients < 75 years old, and was only similar to warfarin in patients 75 or older.14–17
Apart from the clinical trials, additional studies have identified differences in DOAC-related clinical outcomes for older adults. Šinigoj et al. evaluated the safety of dabigatran, rivaroxaban, or apixaban in AF patients ≥65 years old (n=2,260), and found that patients ≥85 years old had 2.5-fold increased risk of MB, 5-fold increased risk of ICH, and 2-fold increased risk of GI bleeding, compared to patients ages 65 to 74.44 A meta-analysis evaluating DOAC related bleeding risk compared to warfarin in elderly AF patients (>65 years old; n=446,042), demonstrated that apixaban and dabigatran had 40% and 21% lower MB risk, respectively. However, rivaroxaban had similar risk of MB compared warfarin.45 Perreault et al. assessed the safety and efficacy of low dose DOACs using administrative data on 22,969 elderly AF patients. Compared to apixaban, low dose dabigatran was associated with lower SEE risk, but a 2-fold increase in bleeding risk. Similarly, rivaroxaban was associated with a 58% increase in bleeding risk compared to apixaban.46
In general, benefits of anticoagulation outweigh the risks in older populations.43 For patients ≥75 years old, apixaban has the best efficacy and safety profile,43 and compared to other DOACs, plasma concentrations are less variable.40 While additional factors such as kidney function, gender, and comorbid conditions have been found to alter apixaban concentrations, the collective evidence supports the use of apixaban for AF in older adults. Additionally, implementation of DOAC monitoring in elderly patients, especially those with compromised or varying kidney function or multiple comorbidities may be beneficial; however, further studies are needed to determine therapeutic levels and their association with clinical outcomes in this patient population.
For the pediatric population, both dabigatran and rivaroxaban have FDA approved indications for VTE treatment and prevention. Dabigatran (dosed based on actual weight) can be used in children from 3 months to less than 12 years of age and 8 to <18 years old (after 5 days of parental anticoagulation) for VTE treatment, or for prevention of VTE recurrence if the child has previously been treated.25 Rivaroxaban can be used from birth to <18 years old, and for thromboprophylaxis in patients (≥2 years) with congenital heart disease after the Fontan procedure.27
Weight:
Patients with obesity have higher risks for AF and VTE, the main indication for DOACs. Despite potential PK variations, DOAC dose adjustments based solely on body mass index (BMI) are not recommended. Increased body weight has been associated with lower dabigatran trough concentrations. However, this finding may be related to increased renal function seen in patients with increased body weight.47 A study of rivaroxaban-treated patients (n=48) found that the maximum concentration was unaffected in patients weighing >120 kg, but was increased by 24% in patients weighing < 50 kg; however, this increase was not considered clinically significant.48 For apixaban-treated patients (n=54), apixaban exposure was 20% lower in patients with high body weight (≥120 kg) and 30% higher in patients with low body weight (≤50 kg). However, these effects were considered modest and unlikely to be clinically meaningful.49 Additionally, obese patients receiving standard doses of apixaban or rivaroxaban for VTE or AF achieved therapeutic factor Xa inhibition.50 For edoxaban, PK parameters have been shown to be consistently independent of body weight; however, patients with low body weight (≤55 kg) had lower MB rates when treated with low dose edoxaban.51 Based on data from the Hokusai trial, a dose reduction to 30 mg daily for DVT/PE is recommended in patients ≤60 kg.29
A recent meta-analysis of nine studies compared DOACs to warfarin across BMI categories, and concluded that DOACs demonstrated superior efficacy and safety profiles in underweight (BMI <18.5), normal weight (BMI 18.5 to <25), and overweight (BMI 25 to <30) patients, while being non-inferior to warfarin in obese patients (BMI ≥30).52 Recent retrospective analyses of clinical outcomes found that stroke, ICH risk, and VTE recurrence were similar among obese and non-obese DOAC-treated patients.53,54 While current evidence does not support dose adjustments based on BMI, the International Society on Thrombosis and Haemostasis (ISTH) updated the VTE guidelines in 2021 to recommend the preferential use of rivaroxaban or apixaban in obese patients.55 For AF, the 2016 guidance recommends standard DOAC dosing for patients with a BMI ≤ 40, while DOACs are not recommended in patients with BMI >40 or weight >120 kg, due to limited data. However, if DOACs are used, obtaining drug-specific levels is recommended, with a switch to a vitamin K antagonist recommended for patients with levels falling outside of the suggested therapeutic ranges.56 It is important to note that high BMI can also be seen in non-obese individuals (e.g., athletes), and due to different obesity-related mechanisms, such as inflammation and metabolic syndrome, further studies are needed before extrapolating these results to any patient with an increased BMI.
Sex:
Sex-related differences in PK parameters have been observed for numerous drugs, including DOACs. Compared to males, females have been shown to have 25% and 15% higher dabigatran and apixaban exposure, as measured by AUC, respectively.57,58 Furthermore, female sex has been independently associated with 1.2 fold higher apixaban concentrations.59 Conversely, sex has not been shown to influence rivaroxaban and edoxaban PK.60,61 With respect to clinical outcomes, in both the AF and VTE clinical trials, DOACs demonstrated similar efficacy and safety for both males and females.9–12,14–16 Edoxaban was the only DOAC with a safety profile that differed by gender, being safer than warfarin in reducing major and clinically relevant non-major bleeding in males, but demonstrating only a similar safety profile to warfarin in females.17
After considering the influences of age, weight and sex, and based on current evidence, apixaban appears to have the best efficacy and safety profile among the DOACs. For older adults, the safety profile of apixaban is particularly pronounced, reducing risks of SSE, MB and ICH.43 Additionally, it is the only DOAC with a dose adjustment based on age, with a dose reduction recommended for patients who meet ≥2 of the following criteria 1) age ≥ 80 years old, 2) weight ≤ 60 kg, or 3) serum creatinine ≥ 1.5 mg/dL.28
The Role of Kidney Function:
CKD is a common comorbidity among OACs users.62 Unlike warfarin, renal elimination accounts for a significant proportion of DOAC elimination ranging from 27% for apixaban to 80% for dabigatran25,27–29 (Figure 3, Table 1). Among DOACs, apixaban is the only DOAC approved for patients with end stage renal disease (ESRD) and hemodialysis.63
In the AF RCTs, kidney function was estimated using Cockcroft-Gault equation. Patients with low creatinine clearance (CrCl < 30 mL/min for RE-LY, ROCKET and ENGAGE and <25 mL/min for ARISTOTLE) and end stage renal disease (ESRD) were excluded.9–12 However, representation of patients with mild (CrCl 50-80 mL/min) to moderate or severe (CrCl <50ml/min) impairment was robust (42-58% and 17-21% respectively; Figure 4a), facilitating an assessment of efficacy and safety of DOACs by level of kidney function. As shown in Figure 4, the risk of SSE increases (Figure 4b; indicating a lower efficacy) and risk of hemorrhage increases (Figure 4c; indicating a lower safety) as kidney function decreases.9–12 These results highlight the need for consideration of kidney function in “treat/not treat decisions” to tailor treatment to individual patients based on the risk/benefit tradeoffs.
Figure 4.

Representation of Kidney Function Levels among Direct Oral Anticoagulant Trial Participants and Efficacy and Safety of Direct Oral Anticoagulants Stratified by Kidney Function.
• Figure 4a. Kidney function distribution in direct oral anticoagulant atrial fibrillation trials measured by Cockcroft-Gault equation. Represents direct oral anticoagulant only, excluding warfarin.
• Figure 4b. Efficacy of atrial fibrillation trials, defined as stroke and systemic embolism, stratified by kidney function and presented in event rates (% per year).
• Figure 4c. Safety of atrial fibrillation trials, defined as major bleeding, stratified by kidney function and presented in event rates (% per year).
• Figure 4d. Kidney function distribution in direct oral anticoagulants venous thromboembolism trials measured by the Cockcroft-Gault equation. Represents the distribution of kidney function for patients on both direct oral anticoagulants and warfarin. Dabigatran distribution is based on the RECOVER I trial, rivaroxaban distribution is based on EINSTEIN-DVT trial, apixaban and edoxaban distributions are based on AMPLIFY and HOKUSAI trials, respectively.
• Figure 4e. Efficacy of venous thromboembolism trials, defined as venous thromboembolism recurrence or related death, stratified by kidney function and presented in event rates (% per year). Rates represent those obtained from Fanikos et al meta-analysis of the RE-COVER II (dabigatran), EINSTEIN DVT and PE (rivaroxaban), AMPLIFY (apixaban), and HOKUSAI (edoxaban) trials.
• Figure 4f. Safety of venous thromboembolism trials defined as major bleeding or major and non-major clinically relevant bleeding, stratified by kidney function and presented in event rates (% per year). Rates represent those obtained from Fanikos et al meta-analysis of the RE-COVER II (dabigatran), EINSTEIN DVT and PE (rivaroxaban), AMPLIFY (apixaban), and HOKUSAI (edoxaban) trials. Reported apixaban rates are only for major bleeding. The overall major and clinically relevant non major bleeding was 4.3% for a mean duration of exposure of 154 days from AMPLIFY based on apixaban’s labeling.
* Edoxaban ENGAGE trial distribution is represented as normal function (CrCl > 95), mild function (CrCl 50-95) and moderate function (CrCl 30-50). HOKUSAI trial reported kidney function distribution as CrCl > 50 and CrCl 30-50. DBG 150=Dabigatran 150 milligram dose. RVX=Rivaroxaban. APX=Apixaban. EDX 60=Edoxaban 60 milligram dose. AF=Atrial Fibrillation. VTE=Venous Thromboembolism.
VTE trials also excluded patients with CrCl < 30 mL/min (dabigatran, rivaroxaban, and edoxaban; < 25 or Scr >2.5 mg/dL apixaban). Although patients with mild to moderate impairment were included, a majority of patients (73%) had no kidney function impairment, and only 5-7% had moderate to severe kidney function impairment (Figure 4d).14–17 These results highlight kidney function’s influence on the efficacy (Figure 4e) and safety (Figure 4f) of DOACs in the treatment of VTE.64
Of DOACs, edoxaban is not recommended in patients with NVAF and (CrCl >95 mL/min) due to reduced efficacy.65 While edoxaban is currently the only DOAC with this recommendation, a rivaroxaban PK modeling study evaluating CrCl-based dosing, which included a broader range of CrCl values than those seen in clinical trials, suggested that this method was more precise than standard dosing. Notably, in contrast to the approved once daily dosing, the study proposed twice daily dosing for patients with CrCl ≥70 to 159 mL/min (10 mg) and patients with CrCl ≥160 mL/min (15 mg).66 This highlights the potential for reduced efficacy with higher kidney function, as seen with edoxaban. Additionally, while further studies are needed to confirm the efficacy and safety of these proposed dosing parameters, it highlights the potential for real-world data to inform personalized dosing recommendations.
While most studies have used CrCl to estimate and classify kidney function, a recent analysis67 examined AF trial data using estimated glomerular filtration rate (eGFR) to assess the influence of kidney function on the efficacy and safety endpoints. A large proportion of participants had GFR<60, (25-29% had GFR ≥45 to <60 and 9.5 to 12.6% had GFR <45). This analysis demonstrated a lower efficacy for dabigatran and warfarin in patients with kidney impairment and a lower safety for all OACs. Of all the patient specific factors known to influence DOAC concentrations, clearance, and efficacy and safety, kidney function demonstrates the most impact for the individual patient and the population of DOAC users.67,68
Drug Interactions:
Although DOACs have fewer interactions than warfarin,4 they do not have a benign drug interaction profile. Clinically relevant interactions exist with agents including P-gp and CYP3A4 inhibitors and inducers, including medications such as amiodarone, verapamil or diltiazem (Table 1).69 Given that DOAC users are frequently older, have multiple comorbidities and may be on multiple interacting medications, this may be of particular relevance to older patients.38 Furthermore, co-administration with other agents, such as antiplatelets, non-steroidal anti-inflammatory drugs (NSAIDs), and serotonin reuptake inhibitors (SSRIs) can increase bleeding risk in DOAC-treated patients.38,70,71
Inhibitors:
All DOACs are P-gp substrates25 and depend on CYP450 enzymes, primarily CYP3A4, for metabolism.27–29 Therefore interactions with strong inhibitors of P-gp and/or CYP3A4 can result in changes in DOAC concentrations and half-life. For example, amiodarone, a dual CYP3A4 and P-gp inhibitor can increase the exposure measured as AUC of dabigatran by 58%,25 edoxaban by 39.8%,72 and rivaroxaban by 36%, with a more drastic increase (86%) in patients with kidney impairment.73 Co-administration of DOACs with dual CYP3A4 and P-gp inhibitors, such as dronedarone, diltiazem, verapamil, and ritonavir,74 or CYP3A4 inhibitors, such as SSRIs,71 can also increase DOAC concentrations.32,69
Inducers:
In addition to enzyme inhibitors, interactions with P-gp and CYP3A4 inducers (e.g. rifampin, phenytoin, and carbamazepine) are equally relevant. Potent P-gp inducers, such as rifampin, have been shown to decrease rivaroxaban, apixaban and edoxaban exposure by 50%, 54% and 35%, respectively.27–29 Co-administration of DOACs and enzyme inducers can lower DOAC concentrations38 potentially predisposing the patient to thrombotic events.
Gronich et al. evaluated the influence of co-administration of DOACs and P-gp/CYP3A4 inhibitors and inducers on risk of serious bleeding, stroke and systemic embolism, and recurrent thromboembolic events. Verapamil in combination with either dabigatran or rivaroxaban, and rivaroxaban in combination with amiodarone, were all associated with an approximate 2-fold increased risk of bleeding. Use of DOACs with phenytoin, carbamazepine, valproic acid, and levetiracetam was associated with 2-fold higher risk of stroke and systemic embolism (OR 2.18 95% CI 1.55-3.10). However, further investigation is required for valproic acid and levetiracetam, as conflicting evidence exists regarding their P-gp and CYP3A4 induction potential.75
Medications that increase or decrease hemorrhage risk:
Other medication can have detrimental or protective effects when co-administrated with DOACs. Co-administration of antiplatelets and non-steroidal anti-inflammatory drugs increase the risk of hemorrhage among DOAC users.38 On the other hand, proton pump inhibitors and H2 blocker use has been associated with lower incidence of upper gastrointestinal bleeding events as well as lower hospitalization rates.76
The Role of Genetics and Ancestry:
Variation in genes involved in the activation, metabolism or transport of DOACs are in part responsible for individual variability observed in response.77 Candidate gene studies (dabigatran, apixaban, rivaroxaban, and edoxaban) and genome-wide association studies (GWAS; dabigatran) have been conducted to evaluate the influence of genetic variation on DOAC PK and clinical outcomes, including thrombosis and hemorrhage. These studies have identified variation in several key genes impacting DOAC response. Table 2 summarizes significant findings from DOAC pharmacogenetic studies, and Figure 5 highlights genotype frequencies for these significant variants across biogeographic groups.
Table 2.
Summary of Significant Findings among Direct Oral Anticoagulants Pharmacogenetic Studies Evaluating Single Variants
| Gene Variant | Drug(s) | Author (year) | Outcome(s) Evaluated | Effect Allele(s) | Direction of Effect | Group |
|---|---|---|---|---|---|---|
| ABCB1 rs1045642 | DBG | Sychev (2018) | Peak concentration and hemorrhage | TT | ↑ | Russian |
| RVX | Lähteenmäki (2021) | Thromboembolic outcomes | T | ↓ | Finnish European | |
| ABCB1 rs4148738 | DBG | Pare (2013) | Peak concentration | G | ↑ | European |
| APX | Dimatteo (2016) | Peak concentration | AA | ↓ | European | |
| APX | Lahteenmaki (2021) | Bleeding risk | A | ↓ | Finnish European | |
| ABCG2 rs2231142 | APX | Ueshima (2017) | Trough /dose ratio | AA | ↑ | Japanese |
| APX | Ueshima (2018) | Population average oral clearance | C | ↑ | Japanese | |
| APX | Gulilat (2020) | Peak and trough concentrations | C | ↑ | European | |
| CES1 rs2244613 | DBG | Pare (2013) | Trough concentration and bleeding | C | ↓ | European |
| DBG | Sychev (2020) | Trough/dose ratio | CC | ↓ | Russian | |
| DBG | Ji (2021) | Trough concentration and bleeding | A | ↑ | Chinese | |
| CES1 rs8192935 | DBG | Pare (2013) | Peak and trough concentrations | A | ↓ | European |
| DBG | Dimatteo (2016) | Trough concentrations | T | ↓ | European | |
| DBG | Liu (2021) | Max concentration divided by dose-weight ratio and half-life | GG | ↑ | Chinese | |
| DBG | Ji (2021) | Trough concentration & aPTT | C | ↑ | Chinese | |
| CYP3A5 rs776746 | APX | Ueshima (2017) | Concentration | G[*3] | ↑ | Japanese |
| APX | Ueshima (2018) | Oral clearance | AA [*1/*1] | ↑ | Japanese | |
| DBG | Zubiaur (2020) | Half-life (after stratification for pantoprazole use) | AA [*1/*1] | ↓ | Caucasian & Latin American |
Abbreviations: DBG=Dabigatran. RVX=Rivaroxaban. APX=Apixaban.
Figure 5.

Genotype Frequencies for Genes Involved in Direct Oral Anticoagulants Transport or Metabolism among populations of African ancestry (AFR), African ancestry in southwest United States (ASW), European ancestry (EUR), Utah residents with Northern and Western European Ancestry (CEU), South Asian (SAS), East Asian (EAS), American (AMR) and Mexican ancestry in Los Angeles California (MXL).
ABCB1:
ABCB1 encodes the P-gp efflux pump, and all DOACs are P-gp substrates. ABCB1 has been the most frequently evaluated gene in DOAC candidate gene studies. These investigations have employed different methods to evaluate ABCB1, including single markers, ABCB1 haplotypes, and two gene haplotype analyses. Single marker analyses have failed to identify an association between ABCB1 rs1128503 and rs2032582, and dabigatran, rivaroxaban and apixaban PK.57,78–80 Furthermore, while the majority of studies have not found a relationship between ABCB1 rs1045642 and DOAC PK,78,80–86 one study found the TT genotype to be associated with increased peak dabigatran concentrations.87 Additionally, when clinical outcomes were evaluated, the rs1045642 T allele was associated with 58% lower risk of thromboembolic outcomes (HR 0.42; 95% CI 0.19-0.98, p=0.044) in rivaroxaban-treated patients. These inconsistencies may be due to the influence of ABCB1 haplotypes.
While studies have defined ABCB1 haplotypes differently, rs1128503 (1236 C>T), rs2032582 (2677 G>T), and rs1045642 (3435 C>T) have been the most commonly evaluated.88 Similar to the single marker analyses, this haplotype was not associated with variability in dabigatran or rivaroxaban PK.78,89 However, when this haplotype was evaluated with respect to clinical outcomes, rivaroxaban-treated patients harboring the TTT haplotype had 56% lower thromboembolic risk (HR 0.44; 95% CI 0.20-0.95;p=0.036), while patients with the CGC and TGC haplotypes had a 2.6-fold and 5.9-fold high thromboembolic risk, respectively.90 Additionally, another study found the TTT haplotype, in combination with the ABCB1 rs4148738 variant, to be associated with higher rivaroxaban levels. However, this study only included 10 patients.91 When the influence of ABCB1 rs1045642 was evaluated in combination with variants in other genes, patients with an ABCB1 rs1045642 T allele and the CES1 rs2244613 CC genotype had higher than expected peak dabigatran concentrations.87 However, ABCB1 rs1045642 in combination with CYP3A4 rs35599367 did not influence rivaroxaban peak steady state concentrations.83
ABCG2:
ABCG2 encodes breast cancer resistance protein (BCRP), an efflux transporter, and both apixaban and rivaroxaban are substrates of BCRP. ABCG2 rs2231142 has been evaluated for its influence on apixaban PK, as well as dabigatran, rivaroxaban, and apixaban clinical outcomes. ABCG2 rs2231142 has been demonstrated to influence apixaban PK. The AA genotype was associated with a significantly higher apixaban concentration/dose ratio. Additionally, patients with the C allele were shown to have higher average population oral clearance (Japanese) and peak and trough concentrations (European).59,79,80 However, ABCG2 variation was not associated with thrombosis or hemorrhage in Finnish European patients.90
CES1:
Carboxylesterases are involved in the metabolism and activation of dabigatran. Variation in CES1, encoding carboxylesterase 1, has been evaluated in relation to dabigatran, apixaban, and rivaroxaban. CES1 rs2244613 C was associated with lower exposure to the dabigatran active metabolite and lower bleeding risk in a GWAS of the RE-LY study, which primarily included patients of European descent.92 Chinese patients with the A allele had higher trough concentrations and increased bleeding risk.93 Similarly, CES1 rs8192935 has been associated with dabigatran PK in European and Chinese patients, with the A allele associated with lower peak and trough concentrations in Europeans and the C allele associated with higher trough concentrations in Chinese.85,92 While CES1 genotype frequencies vary considerably between individuals of Chinese and European ancestry, the effects of the alleles appear to be similar.
SLC22A1:
Variation in SLC22A1, encoding an organic cation transporter has been associated with dabigatran PK variability in Caucasian and Latin American patients. SLC22A1 variants, *2 (rs72552763), *3 (rs12208357), and *5 (rs34059508), were condensed into haplotypes which included 1) no variation, 2) one variant, or 3) at least 2 variants. The haplotype which included at least 2 variants was associated with higher time to max concentration and half-life compared to the haplotypes which included no or one variant.57
CYP3A4/5:
CYP3A4 and CYP3A5 variation has been evaluated in relation to dabigatran, rivaroxaban, and apixaban, PK. While CYP3A4 have not been demonstrated to influence DOAC PK, the CYP3A5 rs776746 AA (*1/*1) genotype has been shown to increase apixaban oral clearance in Japanese patients and increase dabigatran half-life in Caucasian and Latin American patients.57,80 CYP3A5*1/*1 is associated with functional CYP3A5 activity, whereas CYP3A5*3/*3, more common in individuals of European ancestry, results in non-functional CYP3A5 activity. CYP3A5*1/*1 has a significantly higher frequency in individuals of African ancestry, and therefore these patients may be at risk for decreased apixaban efficacy and/or elevated dabigatran concentrations due to increased clearance and half-life, respectively. Further studies are needed to evaluate the influence of CYP3A5 genetic variation on PK and clinical outcomes of DOACs in patients of African descent.
Other Genes:
Other genes have been evaluated, but failed to identify an association with DOAC PK or clinical outcomes. These include: ABCG2, CYP1A2. CYP2A6, CYP2C8, CYP2C9, CYP2D6, CYP4F2, and UGT1A1 in relation to dabigatran; CYP2C19 in relation to dabigatran and rivaroxaban, and SLCO1B1 in relation to dabigatran and edoxaban.77,81
Evidence to support genetic influences on DOAC PK and clinical outcomes are inconclusive. However, relevant associations have been identified. Although contradictory findings have been reported regarding genetic influences on DOAC PK and clinical outcomes, these may be attributed to differences in allele frequencies among different biogeographic groups that were included in the studies, differing haplotype definitions and assessments between studies, and the evaluation of different PK parameters. Further research is needed to determine if and how differences in genetic variants influence DOAC pharmacokinetics and/or clinical outcomes among patients of diverse ancestries. Additionally, genetic results must be interpreted with caution, as findings in one population are not neccesarily generalizable to another. Given the higher frequency of CYP3A5*1/*1 in individuals of African ancestry, the potential for reduced apixaban efficacy or elevated dabigatran concentrations should be considered, and further studies are needed to evaluate this relationship. Furthermore, given the identification of genetic variants influencing DOAC response outside of recognized drug pathways (e.g., SLC22A1-dabigatran), future studies should investigate genes outside of known metabolic pathways. While genome-wide approaches are not always feasible, prioritizing drug-related genes can allow for the identification of novel variants while preserving statistical power. Based on the current evidence, additional studies are required prior to supporting the use of genotype-guided DOAC therapy.
Race Representation in Direct Oral Anticoagulant Randomized Controlled Trials, Pharmacogenetic and Observational Studies:
Demographic changes are in perpetual motion at a local and global scale. In the upcoming decades, the United States (US) population is expected to become more racially diverse. The Black, Asian and Hispanic populations will increase exponentially, and by 2043 the country is expected to become a majority-minority nation with minorities representing up to 57% of the US population.36
Diverse populations are marked by differences in frequency of medication use and differences in disease prevalence and incidence. For example, African Americans have a 5-fold higher incidence of VTE, compared to Asians, and a 2 to 4 higher incidence of end stage renal disease, compared to whites.94,95 Despite the constant change in the national and global landscape with minority biogeographic groups increasing, the majority of patients in both AF and VTE DOAC trials were of White/European ancestry (69.9% to 94.8%), followed by Asian ancestry (2.6% to 21%). Patients of African or Hispanic ancestry were underrepresented in all major DOAC RCTs, with patients of African ancestry accounting for only 1.0% to 3.6% of the total trial population (Figure 6).9–12,14,15,17
Figure 6.
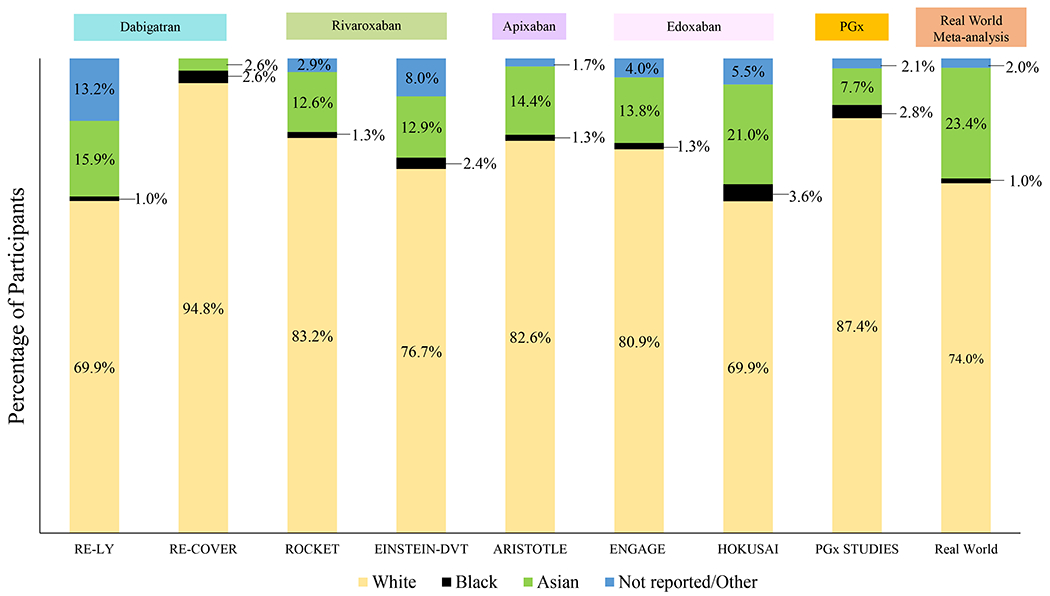
Distribution of Race among Direct Oral Anticoagulant Trials, Pharmacogenetic and Observational Studies Participants.
• Figure 6 represents race representation in each direct oral anticoagulant trial. The PGx column represents the percentage of individuals from each race included in direct oral anticoagulant pharmacogenetic studies, while the real world studies column is based on a systematic review including 34 studies and more than 2 million patients from Waranugraha et al.
• * Some of the studies included Waranugraha et al. did not include a breakdown by race. In this case, individuals from studies conducted in the United States were considered to be White, as this was the observed pattern in most observational studies conducted in the country. Race of studies conducted in other countries are also represented in this manner.
• For apixaban, AMPLIFY trial did not included a breakdown by race, therefore it is not represented in the figure.
PGx=Pharmacogenetic Studies.
DOAC pharmacogenetic studies showed similar patterns with most of the results based on individuals of European (87.4%) 59,78,82–84,86,87,90–92,96–99 or Asian ancestry (7.7%),80,85,89,93,100 with individuals from African ancestry constituting only 2.8%. Minority groups were only represented in candidate gene studies by Zubiaur et al (60% Latin-Americans),57 and Vandell et al (55% African-American; 23% Hispanic), evaluating dabigatran and edoxaban, respectively.81 As previously stated, this is particularly important when you consider biogeographical differences in genotype frequencies of variants discovered through either candidate gene analysis or GWAS that alter DOAC concentrations or clinical outcomes.
Lastly, Waranugraha et al. conducted a systematic review and meta-analysis of 34 real-world studies. Among these, individuals of White/European ancestry constituted approximately 74% of participants, followed by participants of Asian ancestry (23%) and African ancestry (1%)19 (Figure 6). Due to the underrepresentation of minority groups in trials, pharmacogenetic studies and observational studies, much of the evidence surrounding personalization of DOAC therapy has been generated in patients of European or Asian ancestry. Therefore, for patients of other ancestries, these results should be interpreted with caution. Further research is needed to evaluate the contribution of demographic, clinical, and genetic factors to alterations in DOAC concentrations and clinical outcomes among patients of diverse ancestries.
Conclusion:
DOACs are and will continue to be used as the preferred oral anticoagulants, supported by current guidelines and backed by robust evidence. A favorable efficacy and safety profile, few drug-diet interactions, convenient dosing and lack of monitoring requirements are significant advantages. However, their increasing utilization sheds light on clinical and genetic factors that influence their efficacy and safety. Of all the factors discussed in this review, kidney function affects DOAC variability the most. However, other factors such as age, body weight, sex, drug interactions and genetics hold actionable relevance and their additive effects have the potential to expose patients to higher or lower than expected DOAC concentrations.
The growing knowledge is faciliating our understanding of the interplay among clinical and genetic factors, and how these contribute to altered DOAC response. The development of algorithms incorporating these factors could help refine clinical risk prediction and allow for the personalization of anticoagulation therapy. However, in order to translate these advances to benefit all, research (clinical trials, pharmacogenetic and observational studies) must engage diverse populations to assess and quantify the impact of predictors on DOAC response, as current underrepresentation of patients from diverse racial groups constitutes a barrier for a proper personalized medicine approach.
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
Supplementary Material
Funding Information
This work was supported by NIH grants (K24HL133373 [NAL], R01HL092173 [NAL], KL2TR003097 [BHD] and T32HG008961 [LET]).
Footnotes
Declaration of Interests
The authors declared no competing interests for this work
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
Selected References:
- 1.Coumadin (Warfarin sodium) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2011. [Google Scholar]
- 2.Pundi KN et al. Direct Oral Anticoagulant Adherence of Patients With Atrial Fibrillation Transitioned from Warfarin. J Am Heart Assoc 10, e020904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurewich V Ximelagatran--promises and concerns. JAMA 293, 736–9 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Mekaj YH, Mekaj AY, Duci SB & Miftari EI New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag 11, 967–77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Top 300 Drugs of 2019. https://clincalc.com/DrugStats/Top300Drugs.aspx. Accessed 8 FEB 2022.
- 6.Geller AI et al. Emergency Visits for Oral Anticoagulant Bleeding. J Gen Intern Med 35, 371–373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevyxxa (betrixaban). Basics, Side Effects, Reviews & More. https://www.goodrx.com/bevyxxa/what-is. Accessed 1 APR 2022.
- 8.Barnes GD, Lucas E, Alexander GC & Goldberger ZD National Trends in Ambulatory Oral Anticoagulant Use. Am J Med 128, 1300–5.e2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly SJ et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361, 1139–51 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Patel MR et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365, 883–91 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Granger CB et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365, 981–92 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Giugliano RP et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369, 2093–104 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Schulman S & Kearon C Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3, 692–4 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Schulman S et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361, 2342–52 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs R et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363, 2499–510 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369, 799–808 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Büller HR et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369, 1406–15 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML & Vargas-Castrillón E Direct oral anticoagulants in the treatment of acute venous thromboembolism: A systematic review and meta-analysis. Thrombosis Research 134, 774–782 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Waranugraha Y et al. Direct comparison of non-vitamin K antagonist oral anticoagulant versus warfarin for stroke prevention in non-valvular atrial fibrillation: a systematic review and meta-analysis of real-world evidences. Egypt Heart J 73, 70 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ & Weitz JI Laboratory Monitoring of Non–Vitamin K Antagonist Oral Anticoagulant Use in Patients With Atrial Fibrillation: A Review. JAMA Cardiology 2, 566–574 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Boehringer Ingelheim. An idea for a mid to long term strategy for Pradaxa (BIPI-PRA-0028572360/Kliewer 3204854 REDACTED). <https://journals.bmj.com/sites/default/files/BMJ/dabigatran/titration_presentation.pdf> (2012). Accessed June 2, 2022.
- 22.Moore TJ, Cohen MR & Mattison DR Dabigatran, bleeding, and the regulators. Bmj 349, g4517 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Stecker E & B AW. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J Am Heart Assoc 9, e017559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.January CT et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 140, e125–e151 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Dabigatran [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2021. [Google Scholar]
- 26.Heidbuchel H et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 17, 1467–507 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Rivaroxaban [package insert]. Leverkusen, Germany: Janssen Ortho LLC; 2021. [Google Scholar]
- 28.Apixaban [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2016. [Google Scholar]
- 29.Edoxaban [package insert]. Tokyo, Japan: Daiichi Sankyo Co., LTD; 2015. [Google Scholar]
- 30.Laizure SC, Parker RB, Herring VL & Hu ZY Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis. Drug Metab Dispos 42, 201–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiggins BS, Dixon DL, Neyens RR, Page RL & Gluckman TJ Select Drug-Drug Interactions With Direct Oral Anticoagulants: JACC Review Topic of the Week. Journal of the American College of Cardiology 75, 1341–1350 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Li A, Li MK, Crowther M & Vazquez SR Drug-drug interactions with direct oral anticoagulants associated with adverse events in the real world: A systematic review. Thromb Res 194, 240–245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douketis JD et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med 179, 1469–1478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegal DM et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 373, 2413–24 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Pollack CV Jr. et al. Idarucizumab for Dabigatran Reversal. N Engl J Med 373, 511–20 (2015). [DOI] [PubMed] [Google Scholar]
- 36.U.S. Census Bureau Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now https://www.census.gov/newsroom/releases/archives/population/cb12-243.html. Accessed 7 FEB 2022.
- 37.Wolf PA, Abbott RD & Kannel WB Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–8 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Foerster KI, Hermann S, Mikus G & Haefeli WE Drug-Drug Interactions with Direct Oral Anticoagulants. Clin Pharmacokinet 59, 967–980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendayan M et al. Muscle Mass and Direct Oral Anticoagulant Activity in Older Adults With Atrial Fibrillation. J Am Geriatr Soc 69, 1012–1018 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Kampouraki E, Avery P, Biss T, Wynne H & Kamali F Assessment of exposure to direct oral anticoagulants in elderly hospitalised patients. Br J Haematol 195, 790–801 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Reilly PA et al. The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). Journal of the American College of Cardiology 63, 321–328 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Suzuki S, Yamashita T, Akao M & Okumura K Predictors for a high apixaban level in elderly patients with atrial fibrillation prescribed reduced dose of apixaban. Eur J Clin Pharmacol 77, 1757–1758 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Malik AH, Yandrapalli S, Aronow WS, Panza JA & Cooper HA Meta-Analysis of Direct-Acting Oral Anticoagulants Compared With Warfarin in Patients >75 Years of Age. Am J Cardiol 123, 2051–2057 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Šinigoj P, Vene N, Košmelj K & Mavri A Risk of major bleeding in elderly patients with atrial fibrillation on direct oral anticoagulants: real world experience. Int J Clin Pharm 42, 445–452 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Lobraico-Fernandez J, Baksh S & Nemec E Elderly Bleeding Risk of Direct Oral Anticoagulants in Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Cohort Studies. Drugs R D 19, 235–245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perreault S et al. Comparative Effectiveness and Safety of Low-Dose Oral Anticoagulants in Patients With Atrial Fibrillation. Front Pharmacol 12, 812018 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borst JM et al. Body weight is negatively associated with direct oral anticoagulant trough concentrations in dabigatran and apixaban users. Br J Haematol 191, 941–944 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Kubitza D, Becka M, Zuehlsdorf M & Mueck W Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol 47, 218–26 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Upreti VV et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol 76, 908–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin AC et al. Direct Oral Anticoagulant Concentrations in Obese and High Body Weight Patients: A Cohort Study. Thromb Haemost 121, 224–233 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Boriani G et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation at the Extremes of Body Weight: An Analysis from the ENGAGE AF-TIMI 48 Trial. Thromb Haemost 121, 140–149 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Ma J & Zhu W Efficacy and Safety of Direct Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation Across BMI Categories: A Systematic Review and Meta-Analysis. Am J Cardiovasc Drugs 20, 51–60 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Younis M et al. The Use of Direct Oral Anticoagulants in the Management of Venous Thromboembolism in Patients With Obesity. Cureus 12, e10006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplan RM et al. Efficacy and Safety of Direct Oral Anticoagulants for Atrial Fibrillation Across Body Mass Index Categories. J Am Heart Assoc 9, e017383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin KA et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost 19, 1874–1882 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Martin K et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 14, 1308–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zubiaur P et al. Effect of Sex, Use of Pantoprazole and Polymorphisms in SLC22A1, ABCB1, CES1, CYP3A5 and CYP2D6 on the Pharmacokinetics and Safety of Dabigatran. Adv Ther 37, 3537–3550 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Frost CE et al. Effects of age and sex on the single-dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet 54, 651–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gulilat M et al. Drug interactions and pharmacogenetic factors contribute to variation in apixaban concentration in atrial fibrillation patients in routine care. J Thromb Thrombolysis 49, 294–303 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Kubitza D, Becka M, Roth A & Mueck W The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban--an oral, direct Factor Xa inhibitor. J Clin Pharmacol 53, 249–55 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Parasrampuria DA & Truitt KE Pharmacokinetics and Pharmacodynamics of Edoxaban, a Non-Vitamin K Antagonist Oral Anticoagulant that Inhibits Clotting Factor Xa. Clin Pharmacokinet 55, 641–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnocavallo M et al. Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy. J Clin Med 10(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol 56, 628–36 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Fanikos J, Burnett AE, Mahan CE & Dobesh PP Renal Function and Direct Oral Anticoagulant Treatment for Venous Thromboembolism. Am J Med 130, 1137–1143 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Mega JL et al. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385, 2280–7 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Konicki R et al. Rivaroxaban Precision Dosing Strategy for Real-World Atrial Fibrillation Patients. Clin Transl Sci 13, 777–784 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Limdi NA et al. Thromboembolic and Hemorrhagic Outcomes in the Direct Oral Anticoagulant Trials Across the Spectrum of Kidney Function. Clin Pharmacol Ther 109, 1593–1605 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terrier J et al. Population pharmacokinetic models for direct oral anticoagulants: a systematic review and clinical appraisal using exposure simulation. Clin Pharmacol Ther (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill K et al. Amiodarone, Verapamil, or Diltiazem Use With Direct Oral Anticoagulants and the Risk of Hemorrhage in Older Adults. CJC Open 4, 315–323 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis BH et al. Risk Factors for Major Hemorrhage Among Patients Receiving Dabigatran Across the Spectrum of CKD Not Requiring Dialysis Therapy. Am J Kidney Dis 78, 151–153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spina E, Barbieri MA, Cicala G, Bruno A & de Leon J Clinically relevant drug interactions between newer antidepressants and oral anticoagulants. Expert Opin Drug Metab Toxicol 16, 31–44 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Mendell J et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs 13, 331–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheong EJY, Goh JJN, Hong Y, Kojodjojo P & Chan ECY Rivaroxaban With and Without Amiodarone in Renal Impairment. Journal of the American College of Cardiology 71, 1395–1397 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Lambert CT et al. HIV, highly active antiretroviral therapy and the heart: a cellular to epidemiological review. HIV Med 17, 411–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gronich N, Stein N & Muszkat M Association Between Use of Pharmacokinetic-Interacting Drugs and Effectiveness and Safety of Direct Acting Oral Anticoagulants: Nested Case-Control Study. Clin Pharmacol Ther 110, 1526–1536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ray WA et al. Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy With Hospitalization for Upper Gastrointestinal Tract Bleeding. Jama 320, 2221–2230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raymond J et al. Pharmacogenetics of Direct Oral Anticoagulants: A Systematic Review. J Pers Med 11(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gouin-Thibault I et al. Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J Thromb Haemost 15, 273–283 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Ueshima S et al. Impact of ABCB1, ABCG2, and CYP3A5 polymorphisms on plasma trough concentrations of apixaban in Japanese patients with atrial fibrillation. Pharmacogenet Genomics 27, 329–336 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Ueshima S et al. Population pharmacokinetics and pharmacogenomics of apixaban in Japanese adult patients with atrial fibrillation. Br J Clin Pharmacol 84, 1301–1312 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vandell AG et al. An integrated pharmacokinetic/pharmacogenomic analysis of ABCB1 and SLCO1B1 polymorphisms on edoxaban exposure. Pharmacogenomics J 18, 153–159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kryukov AV et al. Influence of ABCB1 and CYP3A5 gene polymorphisms on pharmacokinetics of apixaban in patients with atrial fibrillation and acute stroke. Pharmgenomics Pers Med 11, 43–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sychev D et al. Effect of CYP3A4, CYP3A5, ABCB1 Gene Polymorphisms on Rivaroxaban Pharmacokinetics in Patients Undergoing Total Hip and Knee Replacement Surgery. High Blood Press Cardiovasc Prev 26, 413–420 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Roşian AN et al. Interindividual Variability of Apixaban Plasma Concentrations: Influence of Clinical and Genetic Factors in a Real-Life Cohort of Atrial Fibrillation Patients. Genes (Basel) 11(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji Q et al. The impact of ABCB1 and CES1 polymorphisms on dabigatran pharmacokinetics and pharmacodynamics in patients with atrial fibrillation. Br J Clin Pharmacol 87, 2247–2255 (2021). [DOI] [PubMed] [Google Scholar]
- 86.Zdovc J et al. Downregulation of ABCB1 gene in patients with total hip or knee arthroplasty influences pharmacokinetics of rivaroxaban: a population pharmacokinetic-pharmacodynamic study. Eur J Clin Pharmacol 75, 817–824 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Sychev DA et al. The impact of ABCB1 (rs1045642 and rs4148738) and CES1 (rs2244613) gene polymorphisms on dabigatran equilibrium peak concentration in patients after total knee arthroplasty. Pharmgenomics Pers Med 11, 127–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroetz DL et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics and Genomics 13(2003). [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa J et al. Impact of gene polymorphisms in drug-metabolizing enzymes and transporters on trough concentrations of rivaroxaban in patients with atrial fibrillation. Basic Clin Pharmacol Toxicol 128, 297–304 (2021). [DOI] [PubMed] [Google Scholar]
- 90.Lähteenmäki J et al. Pharmacogenetics of Bleeding and Thromboembolic Events in Direct Oral Anticoagulant Users. Clin Pharmacol Ther 110, 768–776 (2021). [DOI] [PubMed] [Google Scholar]
- 91.Sennesael AL et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thromb J 16, 28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paré G et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 127, 1404–12 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Liu Y et al. The Impact of ABCB1 and CES1 Polymorphisms on Dabigatran Pharmacokinetics in Healthy Chinese Subjects. Pharmgenomics Pers Med 14, 477–485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zakai NA & McClure LA Racial differences in venous thromboembolism. J Thromb Haemost 9, 1877–82 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Umeukeje EM & Young BA Genetics and ESKD Disparities in African Americans. Am J Kidney Dis 74, 811–821 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sychev DA et al. CYP2C19*17 May Increase the Risk of Death Among Patients with an Acute Coronary Syndrome and Non-Valvular Atrial Fibrillation Who Receive Clopidogrel and Rivaroxaban. Pharmgenomics Pers Med 13, 29–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roşian AN et al. An Exploratory Association Analysis of ABCB(1) rs1045642 and ABCB(1) rs4148738 with Non-Major Bleeding Risk in Atrial Fibrillation Patients Treated with Dabigatran or Apixaban. J Pers Med 10(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dimatteo C et al. ABCB1 SNP rs4148738 modulation of apixaban interindividual variability. Thromb Res 145, 24–6 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Sychev DA et al. Genetic determinants of dabigatran safety (CES1 gene rs2244613 polymorphism) in the Russian population: multi-ethnic analysis. Mol Biol Rep 46, 2761–2769 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Chen M, Chen H & Wang F Influence of ABCB1 Gene Polymorphism on Rivaroxaban Blood Concentration and Hemorrhagic Events in Patients With Atrial Fibrillation. Front Pharmacol 12, 639854 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


