Abstract
Cardiovascular disease is one of the main diseases that endanger human life and health, and heart failure often occurs when the cardiovascular disease develops to the end-stage. Heart transplantation is the most effective treatment. However, there has always been a shortage of living heart organs. With the development of regenerative medicine, researchers have turned to bioprinting technology that can build tissues and organs in vitro. A large number of relevant literature on three-dimensional (3D) bioprinted hearts were searched and screened in Google Scholar. 3D bioprinting technology can accurately print biomaterials containing living cells into 3D functional living tissues, providing a feasible solution to the shortage of transplantable organs. As one of the most important organs in the human body, the research on 3D bioprinting of the heart has currently become a hot topic. This paper briefly overviews 3D bioprinting technology and the progress in bioprinting cardiac tissue. It is believed that in the future, bio-printed hearts will become a reality, making a new way of providing artificial organs for heart transplantation.
Keywords: 3D bioprinting, Cardiac, Tissue engineering, Heart transplantation
Introduction
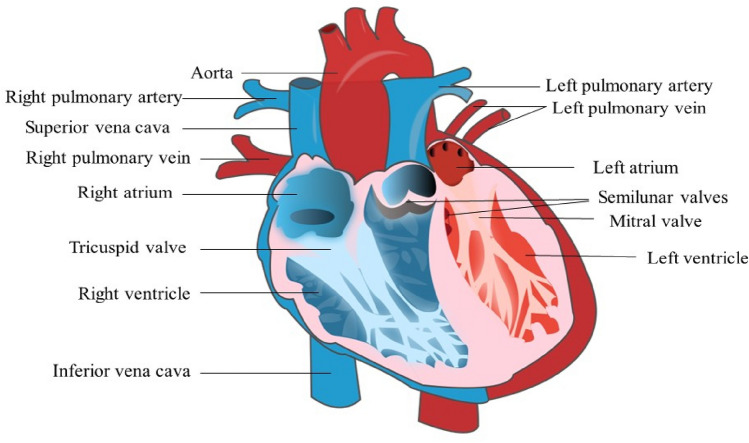
The heart is the pump of the human body which undertakes the blood circulation function, and the heart function state determines the flow rate and state of blood circulation. The human heart is mainly located in the left thoracic cavity and has a shape like that of a peach (Fig. 1). The heart contains four chambers: left atrium, right atrium, right ventricle, and left ventricle. The chambers are separated by the atrial septum and interventricular septum [1]. The diastole and contraction of the atrium and ventricle promote blood circulation throughout the body. Both ventricles connect to the great arteries of the body: the right one connects to the pulmonary artery, and the left one connects to the aorta. The right atrium mainly collects venous blood from the whole body and shoots it through the right ventricle to the lungs for gas exchange. The oxygen-rich blood returns through the pulmonary veins to the left atrium and then to the left ventricle, which shoots it to the aorta for normal metabolism and tissue and organ function.
Fig. 1.
Internal structure of the heart
Cardiovascular disease is a common heart disease that causes countless deaths every year. Studies have found that heart failure occurs in most cardiovascular diseases at the end stage, and heart organ transplantation is often considered to be the most effective treatment [2, 3]. In recent years, the demand for living organ transplantation is extensively increasing, but the number of organs has been in a state of shortage. Many people do not get a transplantable heart until the end of their lives. Even if some patients are lucky enough to receive an organ transplant, they still need to take long-term immunosuppressants to protect them from immediate rejection [4], and their postoperative life quality is also greatly damaged.
The emergence of three-dimensional (3D) printing technology provides the possibility of reconstructing organs in vitro. 3D printing technology is a technology that uses bondable materials and converts digital model files into entities through layer-by-layer printing [5], also known as "additive manufacturing". In recent years, the application of 3D printing technology in cardiac modeling areas has become commonplace with extensive uses in clinical teaching [6, 7], preoperative simulation [8–10], and doctor-patient communication [11, 12], bringing great convenience to physicians and patients. Compared to the maturity of 3D printing model technology, the use of 3D bioprinting technology to produce transplantable whole hearts in vitro is still in the laboratory research stage. 3D bioprinting is the most challenging part of 3D printing technology, which is the application of biological inks composed of biological materials and/or cells and biological factors containing cellular structures in high resolution by printing layer by layer[13, 14]. Researchers have combined biomaterials such as hydrogels and acellular extracellular matrix (ECM) with bioprinting methods such as extrusion, inkjet, and laser technology [15]. Based on biomaterials and bioprinters, cardiac tissue can be printed for cardiac repair or replacement of diseased hearts, restoring cardiac function to a certain extent [16]. At present, 3D bioprinting technology has shown great advantages in the construction of in vitro hearts due to its accuracy and flexibility, and it has become a research hotspot in this field.
This paper briefly introduces the 3D bioprinting technology and the state of art related research on in vitro printing of cardiac organs, especially the research progress of bio-printed cardiac tissue in recent years.
3D bioprinting
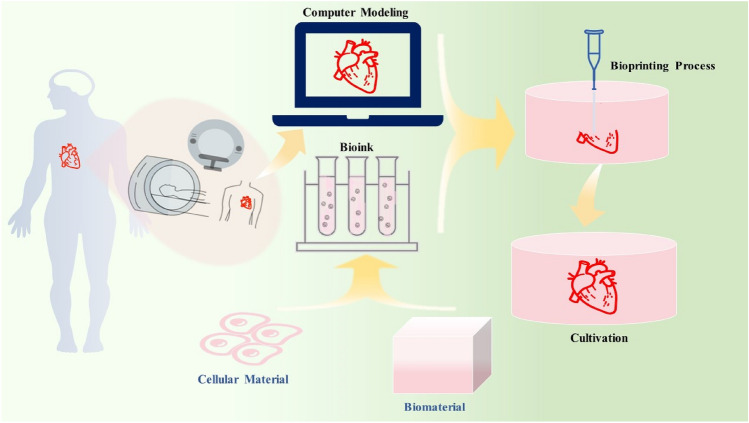
3D bioprinting technology has more advantages than traditional tissue engineering of stem cell culture and proliferation. It can print 3D tissues or organs by accurately making the internal and external structures of natural tissues [17]. It is currently the most ideal solution for tissue and organ regeneration. At present, 3D bioprinting of the heart is the focus and hot spot of current research. The process of bioprinting a heart is generally divided into 3 steps (Fig. 2): (1) design the required 3D model of the heart using computer modeling software. This 3D model can also be obtained from computed tomography (CT) or magnetic resonance imaging (MRI) [9, 18] scan; (2) prepare bioinks (mainly composed of natural or synthetic biomaterials or cell aggregates [14]), and a 3D printer simultaneously deposits cells and biomaterials in a layer-by-layer manner [18, 19]; Finally, (3) the printed heart tissue needs to be cultured, processed and analyzed [20].
Fig. 2.
3D bioprinting process
According to its working principle, 3D bioprinting technology can be roughly classified into inkjet-based bioprinting, extrusion-based bioprinting, and light-based bioprinting [21–24].
Inkjet-based bioprinting
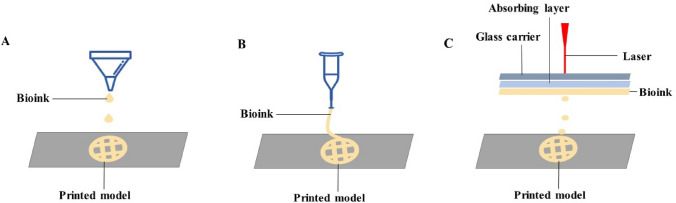
Inkjet-based bioprinting (Fig. 3A) is a non-contact forming technology. Based on data information from a computer it ejects bioinks and binder in the form of droplets to a designated position, thereby printing a target model [25]. In inkjet bioprinting, the droplet size, deposition rate, and location can be easily regulated, and inkjet bioprinting techniques can create structures with irregular shapes or complex structures, such as branched or tubular structures [26, 27]. Inkjet bioprinting, compatible with many biological materials, has the advantages of fast response speed, high molding precision, high efficiency [28], and it can maintain cell viability more than 90% [29]. However, since the bioinks used in inkjet printers must be liquid and exhibit low viscosity, the cell density of bioinks is limited [30].
Fig. 3.
A Inkjet-based bioprinting; B Extrusion-based bioprinting; C Light-based bioprinting
Extrusion-based bioprinting
Extrusion-based bioprinting (Fig. 3B) is one of the most extensively used printing techniques [31]. With bioinks in the syringe, it uses the pneumatic or mechanical (plunger or rotating screw) method to deposit the bioinks on the substrate according to a predetermined trajectory and set up the target model layer by layer. Hydrogels, copolymers, and some spherical cell clusters are all available biomaterials [13, 32, 33]. The advantage of this technique is that one or more biomaterials can be used at the same time, but the disadvantage is that only high viscosity (30 to 6×104 Pas) materials can be extruded [33], which increases the possibility of nozzle clogging with cell aggregation or precipitation, and it will reduce cell viability and lower printing accuracy [34].
Light-based bioprinting
Light-based bioprinting (Fig. 3C) is not affected by the viscosity of biomaterials and is the fastest and most accurate of all printing techniques, including laser-assisted bioprinting and stereolithography [13, 30]. The advantages of light-based printing are that it can print high-viscosity materials at high resolution, there are no problems such as nozzle clogging of cells or biomaterials [28], and the mechanical stress of cells during the printing stage is low, allowing high cell viability [21]; the disadvantage is that light will damage the tissue to a certain extent and has a high cost of use [30].
Among the prevailing cardiac bioprinting, extrusion-based bioprinting is the most commonly used technology. The lower cost bioprinting technology helps people in research and development, which supports the idea that it can be used for mass production of human hearts in the future.
Bioink
Bioink is an important part of 3D bioprinting and is usually based on thermosensitive or photopolymerizable materials containing cells. As a cell carrier, bioinks can avoid mechanical damage to cells during the printing process and protect the microenvironment that is conducive to cell growth formed by biomaterials after printing [17]. Hydrogels, as well as some composite materials, are commonly used as bioinks for printing cardiac tissue. Hydrogels have good biocompatibility [13], can be easily degradable, and most of them also have cell-binding sites that are conducive to cell adhesion, proliferation, and differentiation [13, 14]. Currently, there are two main gel materials used in the 3D bioprinting of cardiac tissue: natural polymer-derived hydrogels and synthetic polymer hydrogels. Natural polymer-derived hydrogels, mainly including alginate, gelatin, collagen, fibrin, hyaluronic acid, and decellularized extracellular matrix (dECM) [35, 36], are most commonly used cardiac bioprinting. Although most natural polymers have good biocompatibility [37], the resulting printed models tend to have low mechanical strength. Synthetic polymer hydrogels mainly include polyacrylic acid derivatives, polyethylene glycol copolymers, polyvinyl alcohol [35], etc. Compared with natural polymers, synthetic polymer hydrogels have good mechanical properties and the potential for modification. However, there are still disadvantages such as poor biocompatibility and poor cell adhesion [37]. Table 1 lists some hydrogel biomaterials used in cardiac tissue engineering in recent years.
Table 1.
Use of some hydrogel biomaterials in 3D printed cardiac tissue
| Biomaterials | Cellular materials | Bioprinting technology | Printing tissue/model | References |
|---|---|---|---|---|
| Alginate | – | Extrusion-based bioprinting | Full-scale human heart model | [57] |
| Extrusion-based bioprinting | Embryonic chicken heart at day 5 | [56] | ||
| Alginate, gelatin | Primary feline adult and H1 cardiomyocytes | Inkjet-based bioprinting | Half-heart with two ventricles connected | [29] |
| Aortic root sinus smooth muscle cells (SMC) and aortic valve leaflet interstitial cells (VIC) | Extrusion-based bioprinting | Aortic valve | [42] | |
| Alginate, poly-ethylene glycol-diacrylate | Porcine aortic valve interstitial cells | Extrusion-based bioprinting | Porcine aortic valve | [41] |
| Alginate, PEG-fibrinogen | Human umbilical vein endothelial cells | Extrusion-based bioprinting | Heart tissue | [68] |
| Alginate, collagen | human stem cell-derived cardiomyocytes | Extrusion-based bioprinting | Tricuspid heart valve | [58] |
| Collagen | Human stem cell-derived cardiomyocytes | Extrusion-based bioprinting | Left ventricle | [58] |
| Human stem cell-derived cardiomyocytes | Extrusion-based bioprinting | Baby heart | [58] | |
| Cardiac progenitor cells/mesenchymal stem cells | Extrusion-based bioprinting | Heart patch | [17] | |
| Extracellular matrix | Cellular material isolated from fat | Extrusion-based bioprinting | Rabbit heart-sized intact heart | [63] |
| Human induced pluripotent stem cells | Extrusion-based bioprinting | Heart with chambers, ventricles, blood vessels | [61] | |
| Human bone marrow–derived mesenchymal stem cells (BM-MSCs) | Extrusion-based bioprinting | Heart patch | [69] | |
| Gelatin methacrylate, decellularized cardiac extracellular matrix | Human cardiac progenitor cell | Extrusion-based bioprinting | Heart patch | [44] |
| Gelatin methacrylate, methacryloyl-substituted recombinant human tropoelastin | Encapsulate cardiomyocytes (CMs), cardiac fibroblasts (CFs) and human umbilical vein endothelial cells | Extrusion-based bioprinting | Heart tissue | [65] |
| Gelatin, hyaluronic acid | Human cardiac-derived Progenitor cells (hCMPCs) | Extrusion-based bioprinting | Heart patch | [70] |
| Gelatin, fibrin | Human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) or CM cell lines with cardiac fibroblasts (CF) | Extrusion-based bioprinting | heart tissue | [71] |
| Fibrin-based composite hydrogel | Rat cardiomyocytes | Extrusion-based bioprinting | Heart tissue | [43] |
Bioinks take an important role in bioprinting hearts, but the development of bioinks is still not mature, and research can be directed toward discovering and developing novel bioinks, or improving and optimizing the properties of existing materials to enhance their applicability [37].
Research progress
Cardiovascular disease is the No.1 disease that endangers human life and health [38]. Since heart transplantation is limited by the number of donor organs [39], people have been actively seeking ways to construct artificial hearts in vitro. However, the chamber structure of the heart organ is complex with many muscles, connectives, nerve tissues, and complicated blood vessel distribution [40], it is very difficult to print.
Printing of partial heart tissue
Due to the complexity of the internal structure of the heart, it is still very difficult to achieve one-shot molding of the human heart [40]. Hockaday et al. printed cardiac aortic valve using porcine aortic interstitial cells and alginate-supplemented poly (ethylene glycol) diacrylate (PEG-DA) hydrogel [41]. Similarly, Duan et al. printed cardiac aortic valves using aortic root sinus smooth muscle cells (SMC) and aortic valve leaflet interstitial cells (VIC), as well as hydrogels made from alginate and gelatin [42]. Gaebel et al. use laser printed human stem cells and endothelial cells for heart regeneration [31]. Wang et al. made bioinks from primary cardiomyocytes isolated from rat hearts and printed them into centimeter-scale heart tissue with function and contraction similar to that of humans [43]. Tao and Bejleri et al. printed cardiac patches [44, 45], which were used to enhance the contractile activity of the heart of a person who had a heart attack or other injury event, and they could restore the normal ability of the damaged areas of the heart, thereby improving the overall function of the heart.
Cardiac tissue engineering generally combines cells suitable for cardiac therapy with scaffolds [46]. However, such scaffolds for tissue engineering may suffer from immune responses and reduce physical and mechanical stability [47]. Some researchers have taken an alternative approach, using a stent-free technique to print functional heart tissue, offering a new way to solve the stent problem. Atmanli et al. used the isolation of highly purified cardiomyocytes from in vitro differentiated pluripotent stem cells, the generation of cell growth surfaces with extracellular matrix protein micropatterns, and the assembly of stem cell-derived cardiomyocytes into anisotropic cardiac tissue [48]. Noguchi et al. used a "spheroid culture" scaffold-free biofabrication technique to create contractile cardiac spheroids from a mixture of rat cardiomyocytes, human dermal fibroblasts, and human coronary arteriole endothelial cells to print cardiac patches [46]. Ong et al. have developed a new method to create 3D printed cardiac tissue without biological materials. The researchers combined human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), fibroblasts (FB), and endothelial cells (ECs) to form mixed cell spheroids, and a 3D bioprinter rearranged them to form the spheroids and create the heart patch [49]. Similarly, Arai et al. also combined cardiomyocytes, endothelial cells, and fibroblasts from induced pluripotent stem cells into spheroids, and fabricated a functional tubular heart structure with a heart pump by bioprinting [50].
Printing of the complete heart
While there have been promising results in printing functional heart tissue, researchers have also been working toward printing usable complete heart organs. Xu et al. successfully printed functional heart tissue by modifying an inkjet printer [29]. Previously, researchers demonstrated the feasibility of using inkjet printers to attach cells and biomaterials into 3D scaffolds and cellular structures [51–54]. Using a gel prepared with feline adult cardiomyocytes and H1 cardiomyocyte cell lines, sodium alginate, and gelatin as bioinks, the researchers successfully printed a contractile "half-heart" with two connected ventricles. We can observe some collapse and deformation on the surface of the printed model. This is the deformation of soft and liquid-like bioinks due to gravity and loss of printing fidelity, and due to insufficient curing speed or stiffness to maintain structural stability during printing [55].
In case of the limitations of such soft and low-viscosity bioinks in printing, Hinton et al. developed a 3D printing technique called Free-Form Reversibly Embedded Suspension Hydrogel (FRESH) [56]. The key to the technique is to deposit and embed the hydrogel being printed into a second hydrogel bath, allowing it to maintain the desired structure during printing and significantly improve print fidelity. Researchers used this method to print a fifth-day embryonic chicken heart. Using this technology, Mirdamadi et al. successfully printed the first 3D full-scale human heart model, demonstrating the capability of FRESH technology in full-scale organ fabrication [57]. In 2019, based on the work of Hinton et al., Lee et al. developed the second-generation free-form reversible intercalation suspension hydrogel (FRESH v2.0) [56, 58]. FRESH v2.0 is convenient for building scaffolds for complex tissues. With this method, researchers can remove the support gel without damaging the printed structure by simply heating the gel from room temperature to body temperature [59]. At the same time, the researchers successfully printed the left ventricle of the heart, the tri-leaflet heart valve, and a baby's heart with collagen hydrogel. The results demonstrate that FRESH technology can construct advanced tissue scaffolds for complex organ systems and provide a new method for scaffold printing.
In 2017, Cohrs et al. proposed the concept of a soft total artificial heart (sTAH), which provides a new direction for the development of artificial hearts [60]. Researchers have used 3D printing and the lost-wax casting method to create the first fully soft, beating artificial heart made of silicone elastomer. It is similar in structure to the real heart, divided into left and right ventricles, and its volume is about 1.25 times the size of the real heart. This artificial heart can only keep beating 3,000 times. The researchers also evaluated it with a hybrid simulation loop, which generated physiologically blood flow patterns and pressure signals by simulating the movements of a real heart. The experimental results show the practical potential of 3D-printed hearts and demonstrate a viable future path for artificial hearts.
One of the major obstacles to printing cardiac tissue in vitro is that cardiomyocytes rarely proliferate or migrate, making it difficult to fill tissue gaps and obtain thick layers of continuous muscle. In 2020, researchers like Kupfer from the University of Minnesota overcame this hurdle with printing using human induced pluripotent stem cells. The researchers optimized the bioinks to promote the proliferation of human induced pluripotent stem cells and the differentiation of cardiomyocytes and successfully printed a heart with chambers, ventricles, and blood vessels using FRESH technology [56, 61]. The researchers also used stem cell expansion to continuously increase the density of heart muscle cells, thus giving the heart model normal heart muscle function and the ability to beat like a human heart.
Vascularization and sustained cell survival have been longstanding challenges for in vitro organ fabrication [39, 62]. In 2019, Noor et al. took the lead in printing the world's first complete heart with cells, blood vessels, and ventricles. Cells and acellular materials isolated from patients' fat samples were made into pluripotent stem cells and hydrogels, respectively, to act as bioinks [63]. The printed heart had normal chambers and blood vessels, and the size was like that of a rabbit heart. For the time being, it could only contract and could not pump blood. This achievement made the 3D-printed heart take another step forward. In the same year, Biolife4d, a bio-company in Chicago, reported that they had printed a miniature version of the human heart, the "Mini-Heart", consisting of four chambers with blood vessels inside and the ability to conduct cardiac conduction, though only Mouse heart size, could be used in laboratory animals for cardiotoxicity screening [64]. In 2020, Lee et al. bioprinted a vascularized heart model using a highly biocompatible recombinant human tropoelastin-based model by improving the bioinks [65]. In 2022, Zhang et al. creatively transformed a six-axis robot into a biological 3D printer, which was controlled by a C++ program [66]. The researchers used the hydrophobic force between the oil-based printing environment and the water-based bioinks to ensure that the printed cells were attached to the scaffold, realizing the bio-printing on the scaffold of complex shape, and designed a repetitive "print-culture". The strategy enables the formation of a vascular network like that of in vivo organs in the printed tissue, thereby supporting the long-term survival of the printed tissue and organ. Using this method, the researchers successfully printed a heart with a network of capillaries and cardiomyocytes with a regular beating heart that survived in vitro for up to 6 months.
In recent years, research on bio-printed heart tissue has been extensively reported, but a complete transplantable heart organ has not yet been constructed, which involves many aspects: First, one of the difficulties of 3D bioprinting is to maintain the survival rate and operation function of the artificial heart. To maintain the biological activity of the artificial organ, it is necessary to realize the oxygen and nutrient exchange function of the organ, of which vascularization is essential [67]. Second, in 3D bioprinted hearts, most biocompatible biomaterials and autologous cells are prevailingly used. Although it is possible to effectively reduce the rejection rate, there are still many factors that may lead to rejection [67]. Finally, the development of bioinks is still insufficient, so far, no single bioink can meet all the property requirements for cardiac tissue bioprinting, and research should be devoted to the research and development of novel bioinks [37]. More importantly, it is important to realize that 3D bioprinting-based heart regeneration technology is still in the early stage of exploration, and more translational application goals are still to be achieved through the cooperation of scientists from various fields. Looking back at every breakthrough in bio-printed heart technology in recent years, we have reason to believe that 3D-printed hearts are no longer far from being transplanted into the human body.
Conclusion
3D bioprinting technology is widely used in the medical field and has unique advantages in the field of in vitro organ reconstruction. Although the current 3D bioprinting technology is still unable to print hearts that can be used for organ transplantation, and there are bottlenecks in many aspects such as immune response, vascularization, multi-tissue printing, biomimetic structures, material mechanical properties, and cell survival, these problems are both challenges and opportunity. However, it is believed that with the exploration and research of the mechanisms behind multi-cell, multi-material, and highly complex systems, the rapid progress and modernization in 3D bioprinting technology will accelerate the development of regenerative medicine and bring revolutionary breakthroughs to modern medicine!
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir RA, McMurray JJ. Epidemiology of heart failure and left ventricular dysfunction after acute myocardial infarction. Curr Heart Fail Rep. 2006;3:175–180. doi: 10.1007/s11897-006-0019-5. [DOI] [PubMed] [Google Scholar]
- 3.Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6:409. doi: 10.21037/atm.2018.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldraich LA, Leitão SAT, Scolari FL, Marcondes-Braga FG, Bonatto MG, Munyal D, et al. A comprehensive and contemporary review on immunosuppression therapy for heart transplantation. Curr Pharm Des. 2020;26:3351–3384. doi: 10.2174/1381612826666200603130232. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 6.Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D Printing and its future directions. JACC Cardiovasc Imaging. 2017;10:171–184. doi: 10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello JP, Olivieri LJ, Krieger A, Thabit O, Marshall MB, Yoo SJ, et al. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World J Pediatr Congenit Heart Surg. 2014;5:421–426. doi: 10.1177/2150135114528721. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs S, Grunert R, Mohr FW, Falk V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg. 2008;7:6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 9.Olejnik P, Juskanic D, Patrovic L, Halaj M. First printed 3D heart model based on cardiac magnetic resonance imaging data in Slovakia. Bratisl Lek Listy. 2018;119:781–784. doi: 10.4149/BLL_2018_142. [DOI] [PubMed] [Google Scholar]
- 10.Farooqi KM, Gonzalez-Lengua C, Shenoy R, Sanz J, Nguyen K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J Pediatr Congenit Heart Surg. 2016;7:414–416. doi: 10.1177/2150135115610285. [DOI] [PubMed] [Google Scholar]
- 11.Guo HC, Wang Y, Dai J, Ren CW, Li JH, Lai YQ. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: a preliminary study. J Thorac Dis. 2018;10:867–873. doi: 10.21037/jtd.2018.01.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biglino G, Koniordou D, Gasparini M, Capelli C, Leaver LK, Khambadkone S, et al. Piloting the use of patient-specific cardiac models as a novel tool to facilitate communication during cinical consultations. Pediatr Cardiol. 2017;38:813–818. doi: 10.1007/s00246-017-1586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li BY, Meng D. Research progress of 3D bioprinting in bone tissue engineering. Chin J Prosthod. 2021. 10.19748/j.cn.kqxf.1009-3761.2021.02.014.
- 14.Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6:915–946. doi: 10.1039/C7BM00765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato B, Wisser G, Agrawal DK, Wood T, Thankam FG. 3D bioprinting of cardiac tissue: current challenges and perspectives. J Mater Sci Mater Med. 2021;32:54. doi: 10.1007/s10856-021-06520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonzo M, AnilKumar S, Roman B, Tasnim N, Joddar B. 3D Bioprinting of cardiac tissue and cardiac stem cell therapy. Transl Res. 2019;211:64–83. doi: 10.1016/j.trsl.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang J, Park HJ, Kim SW, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Xia Z, Jin S, Ye K. Tissue and organ 3D bioprinting. SLAS Technol. 2018;23:301–314. doi: 10.1177/2472630318760515. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen AM, Yoo JJ, Atala A. Solid organ bioprinting: Strategies to achieve organ function. Chem Rev. 2020;120:11093–10127. doi: 10.1021/acs.chemrev.0c00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly AC, Prendergast ME, Hughes AJ, Burdick JA. Bioprinting for the biologist. Cell. 2021;184:18–32. doi: 10.1016/j.cell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal T, Fortunato GM, Hann SY, Ayan B, Vajanthri KY, Presutti D, et al. Recent advances in bioprinting technologies for engineering cardiac tissue. Mater Sci Eng C Mater Biol Appl. 2021;124:112057. doi: 10.1016/j.msec.2021.112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan ZW, Li SF, Li A, Zhang F, Zhao WD, Li JY, et al. Research progress in application of 3D bio-printing technology in tissue engineering and organ transplantation. J Jilin University (Med Edn) 2019;45:197–201. [Google Scholar]
- 23.Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2006;14:271. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CW, Zhang M, Yan LL, Xia PB, Liu NN, Li DJ, et al. Application of hydrogel-based 3D bioprinting in histological engineering. China Rubber/Plastics Technol Equip. 2022;48:37–42. [Google Scholar]
- 26.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cell. 2007;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 27.Xu C, Christensen K, Zhang Z, Huang Y, Fu J, Markwald RR. Predictive compensation-enabled horizontal inkjet printing of alginate tubular constructs. Manuf Lett. 2013;1:28–32. doi: 10.1016/j.mfglet.2013.09.003. [DOI] [Google Scholar]
- 28.Liu F, Liu C, Chen Q, Ao Q, Tian X, Fan J, et al. Progress in organ 3D bioprinting. Int J Bioprint. 2008;4:128. doi: 10.18063/IJB.v4i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu T, Baicu C, Aho M, Zile M, Boland T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabrication. 2009;1:035001. doi: 10.1088/1758-5082/1/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering. 2019;5:777–794. doi: 10.1016/j.eng.2019.03.009. [DOI] [Google Scholar]
- 31.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32:9218–30. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 32.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 33.Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM. Bioengineering cardiac constructs using 3D printing. J 3D Print Med. 2017;1:123–139. doi: 10.2217/3dp-2016-0009. [DOI] [Google Scholar]
- 34.Cui H, Nowicki M, Fisher JP, Zhang LG. 3D bioprinting for organ regeneration. Adv Healthc Mater. 2017;6:1601118. [DOI] [PMC free article] [PubMed]
- 35.Liu N, Ye X, Yao B, Zhao M, Wu P, Liu G, et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact Mater. 2021;6:1388–1401. doi: 10.1016/j.bioactmat.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejleri D, Davis ME. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater. 2019;8:e1801217. doi: 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parak A, Pradeep P, du Toit LC, Kumar P, Choonara YE, Pillay V. Functionalizing bioinks for 3D bioprinting applications. Drug Discov Today. 2019;24:198–205. doi: 10.1016/j.drudis.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 39.Vijayavenkataraman S, Yan WC, Lu WF, Wang CH, Fuh JYH. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296–332. doi: 10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Wu CY, Wu JH, Wu ZR, Li XG, Huang JJ, Chen MJ. New progress of biological 3D printing technology. J Mech Eng. 2021;57:114–132. doi: 10.3901/JME.2021.05.114. [DOI] [Google Scholar]
- 41.Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Lee SJ, Cheng HJ, Yoo JJ, Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bejleri D, Streeter BW, Nachlas ALY, Brown ME, Gaetani R, Christman KL, et al. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv Healthc Mater. 2018;7:e1800672. doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao ZW, Mohamed M, Jacot JG, Birla RK. Bioengineering cardiac tissue constructs with adult rat cardiomyocytes. ASAIO J. 2018;64:e105–e14. doi: 10.1097/MAT.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noguchi R, Nakayama K, Itoh M, Kamohara K, Furukawa K, Oyama JI, et al. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transplant. 2016;35:137–145. doi: 10.1016/j.healun.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–2074. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atmanli A, Domian IJ. Generation of aligned functional myocardial tissue through microcontact printing. J Vis Exp. 2013 doi: 10.3791/50288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ong CS, Fukunishi T, Zhang H, Huang CY, Nashed A, Blazeski A, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017;7:4566. doi: 10.1038/s41598-017-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arai K, Murata D, Verissimo AR, Mukae Y, Itoh M, Nakamura A, et al. Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS One. 2018;13:e0209162. doi: 10.1371/journal.pone.0209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 52.Varghese D, Deshpande M, Xu T, Kesari P, Ohri S, Boland T. Advances in tissue engineering: cell printing. J Thorac Cardiovasc Surg. 2005;129:470–472. doi: 10.1016/j.jtcvs.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 53.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 54.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–99. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Shiwarski DJ, Hudson AR, Tashman JW, Feinberg AW. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021;5:010904. doi: 10.1063/5.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirdamadi E, Tashman JW, Shiwarski DJ, Palchesko RN, Feinberg AW. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater Sci Eng. 2020;6:6453–6459. doi: 10.1021/acsbiomaterials.0c01133. [DOI] [PubMed] [Google Scholar]
- 58.Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 59.Thilmany J. 6 advances in 3D bioprinting of living tissue. ASME. 2020. https://www.asme.org/topics-resources/content/6-advances-in-3d-bioprinting-of-living-tissue
- 60.Cohrs NH, Petrou A, Loepfe M, Yliruka M, Schumacher CM, Kohll AX, et al. A soft total artificial heart-first concept evaluation on a hybrid mock circulation. Artif Organs. 2017;41:948–958. doi: 10.1111/aor.12956. [DOI] [PubMed] [Google Scholar]
- 61.Kupfer ME, Lin WH, Ravikumar V, Qiu K, Wang L, Gao L, et al. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted Chambered Organoid. Circ Res. 2020;127:207–224. doi: 10.1161/CIRCRESAHA.119.316155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34:422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci (Weinh) 2019;6:1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Have a heart – make a difference for animals too. BIOLIFE4D. 2019. https://biolife4d.com/about/have-a-heart-make-a-difference-for-animals-too/
- 65.Lee S, Sani ES, Spencer AR, Guan Y, Weiss AS, Annabi N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv Mater. 2020;32:e2003915. doi: 10.1002/adma.202003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Z, Wu C, Dai C, Shi Q, Fang G, Xie D, et al. A multi-axis robot-based bioprinting system supporting natural cell function preservation and cardiac tissue fabrication. Bioactive Materials. 2022;18:138–150. doi: 10.1016/j.bioactmat.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang WH. Frontier hotspots and research progress on 3D bioprinting in organ reconstruction. Organ Transl. 2022;13:161.
- 68.Maiullari F, Costantini M, Milan M, Pace V, Chirivi M, Maiullari S, et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep. 2018;8:13532. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park BW, Jung SH, Das S, Lee SM, Park JH, Kim H, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020;6:eaay6994-eaay. [DOI] [PMC free article] [PubMed]
- 70.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Anil Kumar S, Alonzo M, Allen SC, Abelseth L, Thakur V, Akimoto J, et al. A visible light-cross-linkable, fibrin-gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater Sci Eng. 2019;5:4551–4563. doi: 10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]