Abstract
We report the case of a patient with unilateral diffuse frontotemporal epilepsy in whom we implanted a responsive neurostimulation system with leads spanning the anterior and centromedian nucleus of the thalamus. During chronic recording, ictal activity in the centromedian nucleus consistently preceded the anterior nucleus, implying a temporally organized seizure network involving the thalamus. With stimulation, the patient had resolution of focal impaired awareness seizures and secondarily generalized seizures. This report describes chronic recordings of seizure activity from multiple thalamic nuclei within a hemisphere and demonstrates the potential efficacy of closed‐loop neurostimulation of multiple thalamic nuclei to control seizures.
Introduction
Both the anterior (ANT) and centromedian (CMT) nuclei of the thalamus have been shown to be efficacious targets for neuromodulation in drug‐resistant epilepsy (DRE). 1 , 2 , 3 , 4 These trials have largely employed continuous or cycled open‐loop stimulation with current deep brain stimulation (DBS) systems, meaning no aspect of the ongoing target region activity is assessed before stimulation and stimulation is not adjusted according to that ongoing activity. Closed‐loop stimulation has also been used to target thalamic nuclei and, generally, either bilateral CMT or bilateral ANT have been targeted. 5 , 6
We present a patient with evidence of unilateral, broad fronto‐temporal onset seizures that secondarily generalize in whom we implanted a closed‐loop Responsive Neurostimulation System (RNS; Neuropace, Mountainview, CA) with leads spanning and stimulating multiple thalamic nuclei. The nuclei include the targeted ANT and CMT and the adjacent mediodorsal nucleus (MD) and pulvinar on the left side. All of these nuclei have been shown to have abnormal structural and functional connectivity in patients with epilepsy. 7 , 8 , 9 , 10 Closed‐loop systems allow for the concurrent sampling and modulation of extended thalamic networks, potentially leading to improved responses and insights into the thalamic networks involved in epilepsy. This report is the first to capture chronic recordings of seizure activity from multiple thalamic nuclei within one brain hemisphere of a patient using the RNS device.
Methods
Data collection and review were approved by the institutional review board with a waiver of informed consent. The patient was a 37‐year‐old man with cryptogenic DRE first diagnosed at 9 years of age. At that time, he presented with generalized tonic–clonic seizures (GTCs) and was prescribed carbamazepine. He was seizure free from ages 13 to 15 years but had recurrence at age 21 years, with breakthrough seizures while on medications. Valproic acid and phenytoin were also trialed without success until lamotrigine was added, which improved his seizure control. At age 30, he was started on topiramate, and a year later, he was started on levetiracetam.
At the time of the surgical evaluation, the patient had focal impaired awareness seizures (FIASs), which started with feelings of déjà vu, confusion which progressed to speech arrest and manual automatisms lasting 2–15 s and occurring 2–3 times per month. The patient also had secondary generalization of these seizures to tonic–clonic activity 2–3 times per month. Seizure triggers included stress, sleep deprivation, and missed doses of medication. The patient complained of significant deficits in memory and attention but was able to independently perform all activities of daily living.
The presurgical workup was as follows (Table 1). Magnetic resonance imaging (MRI) of the brain showed increased fluid‐attenuated inversion recovery signal in the left hippocampus, but no other focal abnormalities such as mesial temporal sclerosis, cortical dysplasia, or other lesions. Interictal positron emission tomography showed hypometabolism of bilateral temporal lobes covering mesial and lateral areas. There was 10% asymmetry in the signal, with a greater reduction on the left compared with the right.
Table 1.
Presurgical‐work up studies.
| Study | Findings |
|---|---|
| Video EEG | Left frontotemporal and centro‐frontotemporal seizure onset |
| SEEG |
Left lateral temporal, cingulate, and orbitofrontal and lateral frontal ictal activity. Interictal activity was in left orbitofrontal cortex, hippocampus, amygdala, and right amygdala. The complete montage included left amygdala, left anterior hippocampus, left posterior hippocampus, left dorsal cingulate above the genu of the corpus callosum, and left ventral cingulate, which is ventral to the genu, left orbitofrontal cortex via a mediolateral trajectory through inferior frontal lobe, left insula, right amygdala, and right hippocampus. |
| PET | Slightly increased hypometabolism on the left |
| Neuropsychological testing | No significant lateralization |
| fMRI | Language lateralization on the left side |
Long‐term video‐electroencephalography captured three seizures. The ictal onset localized to the left frontotemporal region. The patient underwent stereotactic depth electrode placement during which seven FIAS episodes were captured with rapid secondary generalization (Fig. 1). Seizure semiology included raising the right arm before the left, having a fearful face, having an ictal cry, and turning to the right before onset of tonic–clonic movement. Electrographic activity preceded the clinical onset, showing broad network activation in the left lateral temporal lobe (proximal contacts on the amygdala and hippocampal depth electrodes), cingulate cortex, orbitofrontal cortex, and inferior frontal lobe. The maximal buildup of activity occurred in the posterior lateral temporal lobe followed by cingulate, orbitofrontal cortex, and then rapidly became bisynchronous on all depth electrodes including the right leads. Interictal activity was in the left orbitofrontal cortex, left hippocampus, left amygdala, and right amygdala.
Figure 1.
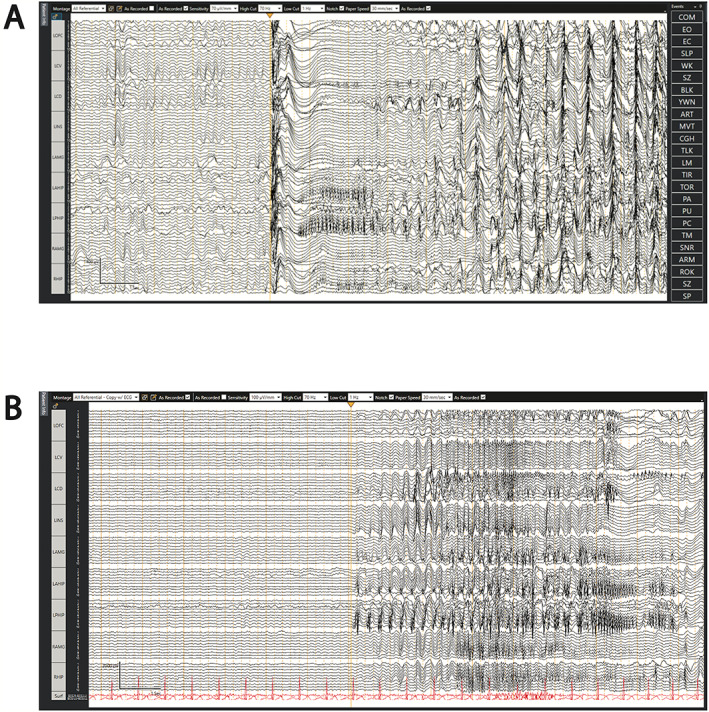
SEEG recordings showing example ictal onsets. (A) Ictal onset showing herald spike that is wide‐field on the left side marked by yellow arrow. This is followed by low‐voltage, high‐frequency activity within the left hippocampus and amygdala, which is followed again by wide‐field, rhythmic activity. (B) Example of limbic origin seizure with periodic high‐voltage spikes and sharps in the hippocampus and amygdala marked by the yellow arrow that become rhythmic periodic discharges.
Neuropsychological testing showed average overall cognitive functioning (Wechsler Adult Intelligence Scale WAIS‐II full scale IQ score 95) with slightly better performance on verbal compared with non‐verbal tasks (verbal comprehensive index score 99; perceptual reasoning index score 92). Functional MRI showed left‐sided expressive and receptive language dominance. Wada testing confirmed left‐sided language function and adequate memory reserve of the right hemisphere (3/8 items recalled, 8/8 items recognized with left injection) with functional adequacy of the left hemisphere as well (2/8 items recalled, 8/8 items recognized with right injection).
Because the seizure type included FIAS and secondary generalization with EEG showing wide left‐sided frontotemporal involvement, the multidisciplinary epilepsy team thought that left‐sided RNS implantation with an electrode in the ANT to address limbic network and a second electrode in the CMT to address frontal networks and the GTCs would best address the patient's pathology. ANT has been shown to treat temporal lobe epilepsy and epilepsy affecting limbic structures. 2 , 3 CMT has been shown to be effective in treating generalized epilepsy and frontal lobe epilepsy. 11 , 12 , 13
The electrodes were implanted using the ClearPoint Neuro frame system (Solana Beach, CA) under MRI guidance. A frontal, transventricular trajectory was used for the ANT lead. 14 A posterolateral trajectory was used to target the CMT electrode. The posterolateral trajectory permitted mounting of both ClearPoint frames simultaneously. The ANT was targeted directly by aiming for the mammillothalamic tract using the fast gray matter acquisition. 14 Direct targeting of the CMT was done using the white‐matter‐nulled magnetization‐prepared rapid gradient echo MRI sequence and consensus coordinates. 15 , 16 T1 inversion recovery sequence, T1 MRI with contrast and a susceptibility‐weighted MRI sequences were used to confirm that the trajectory would not injure blood vessels. For both targets, we used the DL‐330‐3.5 electrode with 30‐cm length and 3.5‐mm spacing for the thalamic nuclei. The ferrule and stimulator/battery unit (RNS‐320) were installed via a standard craniotomy. Postoperative CT confirmed that the electrode placement was at the target and that there was no intracranial hemorrhage.
Impedances for the anterior (ANT) electrode were 877–932 Ω. Impedance values from the posterolateral (CMT) electrode were 826–937 Ω. Detection parameters for both leads were as follows: A 75% threshold was used with short‐term trend 4.096 s and long‐term trend 2 min for a frequency range of 2–125 Hz; power change of 7% was used initially for detections. This was later changed to the bandpass detector with minimum frequency of 2 Hz, maximum frequency of 125 Hz, minimum amplitude of 7%, and minimum duration of 0.51 s.
The thalamic nuclei were targeted using consensus coordinates and imaging as described above. For visualization purposes, the thalamic nuclei have been isolated using the THOMAS atlas 17 and Locate Electrodes Graphical User Interface (LeGUI) software. 18 Briefly, the patient's preoperative T1 inversion recovery MRI sequence was warped to AC‐PC space and then coregistered to the THOMAS atlas using algorithms within LeGUI software (which utilizes protocols from SPM12) that our lab developed. The contact locations within the individual thalamic nuclei were identified using this method.
Results
At 10‐month follow‐up, the patient reported no secondary GTCs and no FIASs since surgery, resulting in an Engel 1B outcome. He was having one focal awareness seizures (FASs) per day; these resemble his FIASs except that he was been able to maintain consciousness, and thus these were far less debilitating compared to his prior seizures. He was maintained on lamotrigine and cenobamate without side effects.
Post‐hoc registration of the patient's post‐RNS placement CT with the preoperative MRI and the THOMAS thalamic atlas showed the anterior lead samples ANT and possibly also MD nucleus with the most distal contact (Fig. 2A). The most superior contact is within the ventricle, suggesting this electrode should have been inserted more deeply to secure all four contacts within the thalamus. Accordingly, there is no active signal from that channel (ANT3‐ANT4; Fig. 3). The posterolateral CMT lead covers CMT (CMT1‐CMT2) as well as the pulvinar (CMT3‐CMT4; Fig. 2B).
Figure 2.
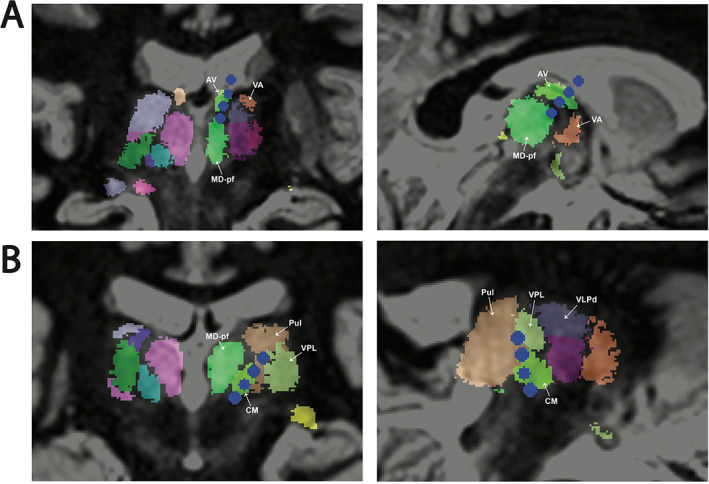
Targeted thalamic nuclei delineated in atlas space. (A) Anterior nucleus of the thalamus (ANT) was targeted with a frontal approach. The mammillothalamic tract is targeted surgically. Two in‐line views shown. (B) Centromedian nucleus of the thalamus (CMT) was targeted with a posterolateral approach using the T2 white‐matter‐null MRI sequence. Two in‐line views shown. Coordinates are as follows: reference point PC, target CM coordinates: lateral left 9.07 mm, anterior 1.55 mm, inferior 1.39 mm; direction lateral left 34.26 degrees, posterior 13.99 degrees, and trajectory length 71.18 mm. AC‐PC distance 23.79 mm. Electrode contacts are in blue. Relevant nuclei are labeled. Nuclei were derived using the THOMAS atlas 17 and LeGui software (see Methods section for details). 18 AV: anterior ventral nucleus, CM: centromedian nucleus, MD‐pf: mediodorsal‐parafascicular nucleus, Pul: pulvinar nucleus., VA: ventral anterior nucleus, VPL: ventral posterior lateral nucleus, VLPd: ventral lateral posterior dorsal group.
Figure 3.
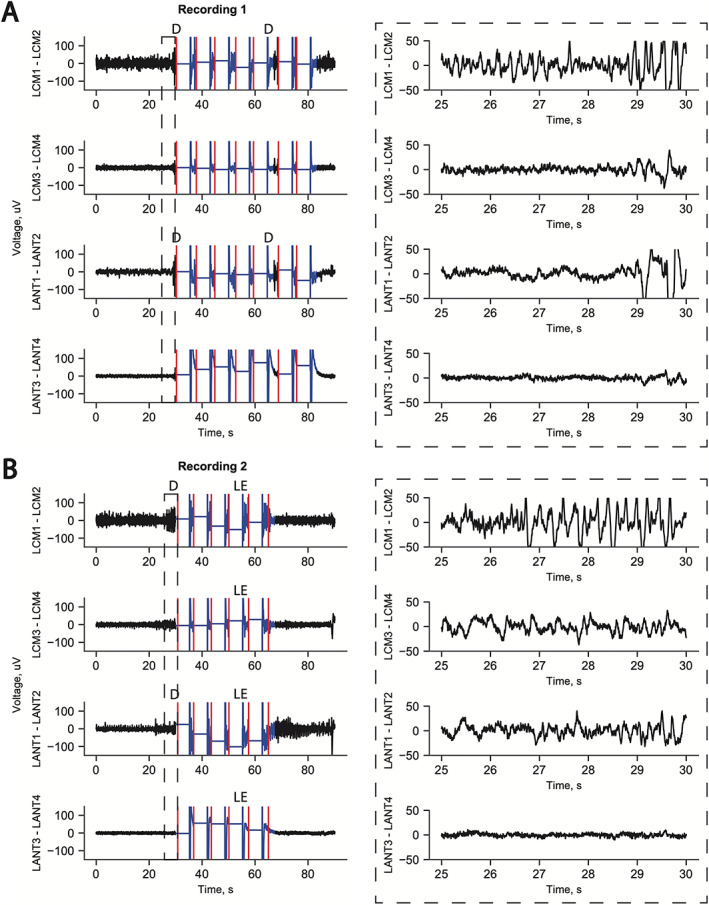
Example long‐episode ECogs from patient's RNS data. Ictal activity is consistently recorded from CMT channel 1–2 before ANT channel 1–2 in both examples. Right side insets show blown up timeline. In both examples, the seizure is aborted with stimulation across all four channels. (A) Example seizure detected first with ictal discharge recorded across the distal contact pair LCM1‐LCM2 (in CMT proper) followed by ictal activity in ANT1‐ANT2 (in MD and ANT) at 29 s and ictal activity recorded in LCM3‐LCM4 (within pulvinar) at 29.5 s. ANT3‐ANT4 (in the ventricle) is electrically quiet as expected. (B) Again, ictal activity is seen in the distal contact pair LCM1‐LCM2 (in CMT proper) followed by ictal activity in ANT1‐ANT2 (in MD and ANT) at 29 s. CMT3‐CMT4 shows ictal discharges. ANT3‐ANT4 is electrically quiet. D: detection, LE: long episode, red marks: stimulation treatment delivery.
Stimulation was delivered across all four channels on the two leads (Fig. 3). Stimulation parameters were 2.5 mA, 120 μs, 1.5 mC/cm2, 5000‐ms duration, and 100 Hz applied in five bursts with the more distal contact being the anode. The patient reported mild, non‐bothersome paresthesia in the right upper extremity at this current. Note that paresthesia may occur with CMT targeting via stimulation of the ventral posterior medial nucleus of the thalamus. This occasionally bothersome side effect occurs frequently with leads targeting canonical CMT coordinates anteriorly as well. Lead impedances have remained stable over time (500–1000 Ω). Detections and long episodes were occurring based on detections from CMT, which consistently preceded ANT, implying a consistent thalamic seizure network exists in this patient. Ictal activity was consistently recorded from CMT channels (contacts CMT1‐CMT2) followed by ANT ictal activity (contacts ANT1‐ANT2, Fig. 3). Stimulation aborted most long episodes but not all.
Discussion
Akin to multitarget drug treatments, multiple‐target stimulation may be more efficacious for the treatment of DRE, which is widely considered a network disorder. 19 The ANT and CMT targets of neuromodulation have shown efficacy in randomized‐controlled trials of focal and generalized epilepsy, respectively. 2 , 3 , 4 We present a patient with evidence of unilateral broad‐onset DRE in whom a closed‐loop RNS was implanted, with leads in the ANT and CMT, which also sample the pulvinar and MD. The RNS system allows for chronic recording of ECog data. We found that ictal activity was consistently recorded first in the CMT and then ANT, and stimulation of both ANT and CMT together was related to improved seizure burden. Overall, the patient's secondarily generalized seizures were cured, and his near daily FIASs were converted to FASs that occur once per day. To our knowledge, chronic recording of ictal activity from multiple thalamic nuclei within a single hemisphere has not been reported using the RNS device. Chronic recording from neocortex and thalamus or bilateral corresponding thalamic nuclei, and/or inclusion of mesial temporal structures has been reported. 5 , 20 , 21 , 22 Burdette et al showed in a case series of patients with RNS leads targeting CMT and cortex simultaneously that seizures were detected in both cortex and CMT, and generally with lags ranging from 0–3 s with neocortex preceding CMT. 21 In another report of several patients with posterior quadrant seizures, RNS leads were placed in pulvinar and on cortex. Here seizures were recorded from thalamus and cortex with near simultaneous (<0.25 s lag) onset as well. Here we describe seizures being recorded from several thalamic nuclei with a consistent temporal order of CMT, then ANT, and finally pulvinar. This patient did not have electrodes on cortex; thus, we cannot comment on its relative timing. In terms of how effective stimulating multiple thalamic nuclei within a hemisphere might be for seizure control in someone with broad‐onset epilepsy, Alcala‐Zermeno et al compared DBS of bilateral ANT and CMT to bilateral CMT stimulation in patients with generalized epilepsy but far more seizures than our patient (mean 323 seizures per month at baseline). They report the two groups did not differ in terms of percent seizure reduction. 23 Our patient clearly had a positive effect in that he no longer had disabling FIAS with secondary generalization. He continues to have FAS, which do not have an explicit electrical correlate.
Finally, the intercontact spacing of electrodes used in commercially available closed‐loop systems precludes all four contacts from being placed within a single thalamic nucleus. 24 This presents an opportunity to devise trajectories that intentionally span multiple nuclei and offer more therapeutic options for treating refractory seizures. Because of the shape of the ANT and its relation to the MTT, the frontal and specifically transventricular (versus tranparenchymal) trajectory ostensibly affords the best outcomes. 16 , 25 Extending this trajectory inferiorly can cover the MD. Similarly, the CMT is also approached from a frontal trajectory in most trials. 11 , 12 , 15 However, a posterolateral approach to the CMT may be considered to target pulvinar (Fig. 2). The pulvinar has been implicated in refractory seizures 26 , 27 as well as temporal lobe seizures specifically. 7 , 8 , 9 , 28 , 29 Stimulation of the pulvinar may be effective at controlling seizures from posterior parietal and occipital cortex 20 as well as temporal lobe, 30 but the literature implies that pulvinar stimulation may be used for generalized epilepsy. Similarly, MD is thought to have a role in generalized 31 , 32 and temporal lobe 9 , 33 , 34 , 35 epilepsy. Stimulation controls limbic seizures in animal models 36 and demonstrates connectivity to limbic structures including piriform cortex, hippocampus, cingulate cortex, and orbitofrontal cortex. 9 , 10 , 34 , 37 The most effective stimulation location may be the dorsal area where afferent axons enter, some from the thalamic reticular nucleus as well, which is where this patient's distal contact was located. 36 , 37
Limitations
This is a report of a single patient, which cannot be used to make generalized claims about the effects of closed‐loop thalamic stimulation. The patient was not tested with stimulation of a single thalamic nucleus, so we cannot say whether the targeting of both nuclei was necessary to achieve his positive outcome. Nevertheless, the case presents a proof of principle on several fronts: Ictal activity can be recorded throughout the thalamus and, in this patient, appears to follow a consistent temporal pattern (i.e., CMT precedes pulvinar and ANT in this patient), thalamic activity can be used to trigger RNS, and finally that stimulating multiple thalamic nuclei may lead to a positive clinical outcome for patients with wide‐field ictal onset patterns that are unilateral.
Author Contributions
Bornali Kundu was involved with the conception, data analysis, writing, and editing of the paper. Amir Arain was involved with the conception, data analysis and editing of the paper. Tyler Davis was involved with the data analysis of the paper. Chantel Charlebois was involved with the data analysis of the paper. John Rolston performed the surgery and was involved with the conception and editing of the paper.
Conflicts of Interest
Bornali Kundu, Chantel Charlebois, and Tyler Davis have no disclosures. Amir Arain has consulted for Neuropace. John Rolston has consulted for Medtronic, NeuroPace, and Corlieve Therapeutics.
Acknowledgements
We would like to thank Kristin Kraus for her editorial assistance.
Funding Information This project was partially funded by a grant from the National Institutes of Health (K23 1K23NS114178‐01) to John Rolston.
Funding Statement
This work was funded by National Institutes of Health grant K23 1K23NS114178‐01.
References
- 1. Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11:508‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899‐908. [DOI] [PubMed] [Google Scholar]
- 3. Salanova V, Witt T, Worth R, et al. Long‐term efficacy and safety of thalamic stimulation for drug‐resistant partial epilepsy. Neurology. 2015;84(10):1017‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dalic LJ, Warren AEL, Bulluss KJ, et al. DBS of thalamic centromedian nucleus for Lennox–Gastaut syndrome (ESTEL trial). Ann Neurol. 2022;91(2):253‐267. [DOI] [PubMed] [Google Scholar]
- 5. Roa JA, Abramova M, Fields M, et al. Responsive neurostimulation of the thalamus for the treatment of refractory epilepsy. Front Hum Neurosci. 2022;16:926337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feigen CM, Eskandar EN, Kopell BH. Responsive thalamic neurostimulation: a systematic review of a promising approach for refractory epilepsy. Front Hum Neurosci. 2022;16:910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keller SS, O'Muircheartaigh J, Traynor C, Towgood K, Barker GJ, Richardson MP. Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia. 2014;55(2):306‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willis K, Gonzalez H, Johnson G, et al. People with mesial temporal lobe epilepsy have altered thalamo‐occipital brain networks. Epilepsy Behav. 2021;115:107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jo HJ, Kenny‐Jung DL, Balzekas I, et al. Nuclei‐specific thalamic connectivity predicts seizure frequency in drug‐resistant medial temporal lobe epilepsy. NeuroImage Clin. 2019;21:101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eckert U, Metzger CD, Buchmann JE, et al. Preferential networks of the mediodorsal nucleus and centromedian‐parafascicular complex of the thalamus‐a DTI tractography study. Hum Brain Mapp. 2012;33(11):2627‐2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valentín A, García Navarrete E, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54(10):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 12. Velasco AL, Velasco F, Jiménez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox‐Gastaut syndrome. Epilepsia. 2006;47(7):1203‐1212. [DOI] [PubMed] [Google Scholar]
- 13. Yang JC, Bullinger KL, Isbaine F, et al. Centromedian thalamic deep brain stimulation for drug‐resistant epilepsy: single‐center experience. J Neurosurg. 2022;1‐10. doi: 10.3171/2022.2.JNS212237 [DOI] [PubMed] [Google Scholar]
- 14. Möttönen T, Katisko J, Haapasalo J, et al. Defining the anterior nucleus of the thalamus (ANT) as a deep brain stimulation target in refractory epilepsy: delineation using 3 T MRI and intraoperative microelectrode recording. NeuroImage Clin. 2015;7:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warren AEL, Dalic LJ, Thevathasan W, Roten A, Bulluss KJ, Archer J. Targeting the centromedian thalamic nucleus for deep brain stimulation. J Neurol Neurosurg Psychiatry. 2020;91(4):339‐349. [DOI] [PubMed] [Google Scholar]
- 16. Cukiert A, Lehtimäki K. Deep brain stimulation targeting in refractory epilepsy. Epilepsia. 2017;58:80‐84. [DOI] [PubMed] [Google Scholar]
- 17. Su JH, Thomas FT, Kasoff W, et al. Fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage. 2019;194:272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis TS, Caston RM, Philip B, et al. LeGUI: a fast and accurate graphical user interface for automated detection and anatomical localization of intracranial electrodes. Front Neurosci. 2021;15:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43(3):219‐227. [DOI] [PubMed] [Google Scholar]
- 20. Burdette D, Mirro EA, Lawrence M, Patra SE. Brain‐responsive corticothalamic stimulation in the pulvinar nucleus for the treatment of regional neocortical epilepsy: a case series. Epilepsia Open. 2021;6(3):611‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burdette DE, Haykal MA, Jarosiewicz B, et al. Brain‐responsive corticothalamic stimulation in the centromedian nucleus for the treatment of regional neocortical epilepsy. Epilepsy Behav. 2020;112:107354. [DOI] [PubMed] [Google Scholar]
- 22. Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of closed‐loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. JAMA Neurol. 2019;76(7):800‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alcala‐Zermeno JL, Gregg NM, Wirrell EC, et al. Centromedian thalamic nucleus with or without anterior thalamic nucleus deep brain stimulation for epilepsy in children and adults: a retrospective case series. Seizure. 2021;84:101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anonymous . RNS® system user manual. 2019. Accessed November 16, 2022. https://www.neuropace.com/wp‐content/uploads/2021/02/rns‐system‐manual‐300m.pdf
- 25. Lehtimäki K, Coenen VA, Gonçalves Ferreira A, et al. The surgical approach to the anterior nucleus of thalamus in patients with refractory epilepsy: experience from the international multicenter registry (MORE). Clin Neurosurg. 2019;84(1):141‐149. [DOI] [PubMed] [Google Scholar]
- 26. Ohe Y, Hayashi T, Deguchi I, et al. MRI abnormality of the pulvinar in patients with status epilepticus. J Neuroradiol. 2014;41(4):220‐226. [DOI] [PubMed] [Google Scholar]
- 27. Tan G, Li X, Niu R, et al. Functional connectivity of the thalamocortical circuit in patients with seizure relapse after antiseizure medication withdrawal. Epilepsia. 2021;62(10):2463‐2473. [DOI] [PubMed] [Google Scholar]
- 28. He X, Doucet GE, Sperling M, Sharan A, Tracy JI. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia. 2015;56(10):1571‐1579. [DOI] [PubMed] [Google Scholar]
- 29. Rosenberg DS, Mauguière F, Demarquay G, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006;47(1):98‐107. [DOI] [PubMed] [Google Scholar]
- 30. Filipescu C, Lagarde S, Lambert I, et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia. 2019;60(4):e25‐e30. [DOI] [PubMed] [Google Scholar]
- 31. Zhang CH, Sha Z, Mundahl J, et al. Thalamocortical relationship in epileptic patients with generalized spike and wave discharges–a multimodal neuroimaging study. NeuroImage Clin. 2015;9:117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young JC, Nasser HM, Casilla‐Espinosa P, et al. Multiunit cluster firing patterns of piriform cortex and mediodorsal thalamus in absence epilepsy. Epilepsy Behav. 2019;97:229‐243. [DOI] [PubMed] [Google Scholar]
- 33. Sinjab B, Martinian L, Sisodiya SM, Thom M. Regional thalamic neuropathology in patients with hippocampal sclerosis and epilepsy: a postmortem study. Epilepsia. 2013;54(12):2125‐2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dinkelacker V, Valabregue R, Thivard L, et al. Hippocampal‐thalamic wiring in medial temporal lobe epilepsy: enhanced connectivity per hippocampal voxel. Epilepsia. 2015;56(8):1217‐1226. [DOI] [PubMed] [Google Scholar]
- 35. Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 2009;29(41):13006‐13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang DX, Bertram EH. Suppressing limbic seizures by stimulating medial dorsal thalamic nucleus: factors for efficacy. Epilepsia. 2015;56(3):479‐488. [DOI] [PubMed] [Google Scholar]
- 37. Cornwall J, Phillipson OT. Afferent projections to the dorsal thalamus of the rat as shown by retrograde lectin transport‐I. The mediodorsal nucleus. Neuroscience. 1988;24(3):1035‐1049. [DOI] [PubMed] [Google Scholar]


