Abstract
Oral squamous cell carcinoma (OSCC) is one of the common types of cancer. Its progression follows a transition from oral potentially malignant disorders (OPMDs) such as oral submucous fibrosis (OSMF). Epigenetic modifiers, especially microRNAs (miRNAs), have an appreciable role in the regulation of various carcinogenic pathways which are being used as biomarkers. miRNAs may also be helpful in the differentiation of oral submucous fibrosis from oral squamous cell carcinoma. Three miRNAs, miR-221-3p, miR133a-3p, and miR-9-5p, were found differentially expressed in many cancers in the literature search supported by our preliminary database search-based screening. The literature and our functional enrichment analysis in an earlier study have reported these miRNAs to regulate carcinogenesis at various steps. In the present study, the expression of these miRNAs was examined in 34 histopathologically confirmed OSCC, 30 OSMF, and 29 control (healthy volunteers) human samples. There was a significant downregulation of miRNA-133a-3p in OSCC compared to OSMF and controls, whereas there was up-regulation in oral submucous fibrosis compared to controls. There was no significant difference in the expression of miR-221-3p between OSCC and OSMF, but an upregulation in OSCC compared to controls. miR-9-5p was also found upregulated in both OSCC and OSMF. Further, miR-133a-3p expression was negatively correlated with age, smoking, drinking status, and AJCC staging, whereas miR-9-5p expression was only positively associated with tobacco/ areca nut chewing. The ROC plots, logistic regression model generated, and the correlation between the expression of miR-9-5p and miR-133a-3p in blood and tissue suggests that these could be used as risk stratification biomarkers.
Keywords: Oral submucous fibrosis, Oral squamous cell carcinoma, microRNAs, Interactomics, Risk stratification biomarkers
Introduction
Oral squamous cell carcinoma (OSCC) is the most of up to 15% and differs with ethnicity, region, habits, head and neck cancer (HNSCC). OSCC has a low 5-year survival rate because of late diagnosis and frequent recurrence [1]. Its progression is mainly seen from a group of precursor lesions called oral potentially malignant disorders (OPMDs) [2, 3]. Many retrospectives and epidemiological studies on OSCC suggest that OPMDs such as OSMF, Oral lichen planus, oral leukoplakia, and human papillomavirus significantly contribute to OSCC development [4, 5]. OSMF has a high malignant transformation rate of around 8%, which differs with ethnicity, region, habits, and culture [6, 7]. Chronic inflammation of oral mucosa, excessive collagen deposition in the connective tissue, local inflammation of lamina propia are the major pathological characteristics of OSMF [8]. OSMF, a probable malignant lesion, comprises various epithelial alterations that vary from atrophy with hypoplasia to hyperplasia and dysplasia [9]. The mechanisms participating in multistep carcinogenesis and progression are regulated genetically and epigenetically. Protein coding genes and their contribution to carcinogenesis are constantly being explored. Still, noncoding genomes, especially miRNAs, and their involvement in the initiation, progression, metastasis, chemoresistance, and recurrence remain relatively unexplored.
In the past decade, studies have established miRNAs as critical regulators of the oncogenic potential of cells, and also they are predicted to regulate the expression of at least 60% of human genes [10, 11]; therefore, the aberrant expression of miRNAs in OSMF can’t be ignored in malignant transformation of OSCC. Many studies are done to explore the role of miRNAs in OSCC tumors, especially in the invasion, migration angiogenesis, and their related pathways [12–16] but, here are few studies with analyses of change in miRNAs expression associated with OSMF pathogenesis [17–19]. A recent systematic review by El-Sakka et al. has extensively explored miRNA studies and their correlation with OPMDs. In most included studies, their findings have no specific relation between differentially expressed miRNAs in OSCC with OPMDs [2]. There are a limited number of reports which talk about the similarities or distinguishing characteristics of OSMF that may lead to OSCC. Therefore, identification of early-stage molecular signatures that predicts tumorigenesis is the need of the hour. We have performed a three-phase database screening to obtain commonly deregulated miRNAs in OSMF and OSCC (publication under journal consideration). We have selected three miRNAs from the literature search and our screening results in the present study, i.e., miR-221-3p, miR-133a-3p, and miR-9-5p. Studies show downregulation of miRNA-133a [20–22] and miRNA-133b [20] but no significant expression profiling with OPMDs. Studies have shown that overexpression of miRNA-221-3p/222-3p increases growth and tumorigenesis in OSCC [23], but their significance to OPMDs is not mentioned to the best of our knowledge. miRNA-9 was selected in this study due to its controversial expression profile in many cancer types such as down-regulated in Breast cancer, Renal cell carcinoma, gastric cell carcinoma, and HNSCC) on the other hand is found to be up-regulated in hepatocellular, gliomas, and colorectal cancers [24]. In a study by Kim et al., compression induced miRNA-9 downregulation and reversal were seen in breast cancer tissues [25]; this suggests that mechano-transduction is followed in miRNA-9, which may be associated with OSMF also. Therefore we decided to evaluate a miRNA-9 expression in OSCC and OSMF tissues. Also, there is a recent study that found miRNA-9 enriched in HPV infected HNSCC patients and downregulates PPARD to induce macrophage polarization [26]. Considering the earlier studies, miR-9-5p, miR-221-3p, and miR-133a-3p might have a role in OSMF and OSCC pathogenesis, therefore in the present study, the expression of these miRNAs and their correlation with the clinicopathological parameters were studied in OSMF and OSCC patients.
Materials and Methods
Clinical Samples
A total of 93 participants were recruited in the study, out of which 34 were histopathologically confirmed OSCC patients, 30 were OSMF, and 29 were healthy individuals treated as controls. Informed written consent was obtained from all the participants. Demographic characteristics of the participants such as age, sex, exposure to risk factors (smoking, drinking, tobacco chewing), duration of exposure, mouth opening (OSMF patients) were recorded. Tissue and blood samples from OSCC and OSMF patients have been collected post operation from the Departments of Onco-surgery and Dentistry, All India institute of medical sciences (Jodhpur, India) from August 2018 to February 2020. Control blood samples were collected from healthy volunteers (patients, relatives, and healthcare workers) who consented to participate in the study. Control tissue samples were collected from patients undergoing dental procedures or orofacial surgery in the Department of Dentistry with informed consent. Histopathological examinations were performed to obtain tumor differentiation, OSCC stage, node involvement, metastasis, OSMF grade, and lesion characteristics.
The OSCC and OSMF patients with HPV infection OSMF patients with mouth opening greater than 25 mm were excluded. Blood samples of all the participants were withdrawn and collected in EDTA vacutainers, and RNA was isolated from fresh blood and tissue samples.
RNA Isolation
Total RNA was isolated from all OSCC, OSMF, and Control tissue and blood samples using TRIzol reagent (Himedia) according to the manufacturer’s instructions. Quality and quantity of total RNA were analyzed spectrometry, and samples with a 260/280 ratio of 1.9 ± 0.5 were taken for further analysis.
Quantitative Real-Time PCR (qPCR)
SYBR-based qPCR was used to quantify mature miRNA expression using the miScript PCR system by QIAGEN. This three-component system includes a reverse transcription kit with an optimized blend of poly (A) polymerase and reverse transcriptase for cDNA synthesis and an SYBR-Green PCR kit. miRNAs specific primers were commercially obtained from Qiagen (miscript primer assay). The qPCR reaction mix was made according to the manufacturer’s instructions. The conditions followed were: Initial activation for 15 min at 95 °C followed by 40 cycles of 94 °C for 15 s, 55 °C for 30 section 70 °C for 30 s using a Bio-Rad CFX96DX Real-Time quantitative PCR machine. The expression of miRNAs was normalized using RNU6 The relative miRNA expression that is fold change (FC) was calculated using the 2−ΔΔCt cycle threshold method [27]. Log2FC values were plotted in the graph reporting upregulation or downregulation of each miRNA.
Statistical Analyses
Descriptive statistics were used to compare the case’s and controls’ baseline parameters and demographic data. Normality tests (Kolmogorov–Smirnov and Shapiro–Wilk) were performed for analyzing data distribution. Due to the non-normal distribution of data, nonparametric tests were performed for log2 FC for miRNA expression (1. Kruskal–Wallis test-within the three groups, i.e., OSCC, OSMF, controls, and 2. Mann–Whitney between two groups, i.e., OSCC-controls, OSCC-OSMF, and OSMF-Controls). Two-tailed Spearman’s Rho was performed to correlate clinicopathological parameters and habitual attributes with the miRNA expressions. Also, the spearman rho’s correlation test was used to correlate ∆∆Ct in tissue with the blood miRNA expression. ROC was plotted for calculating the AUC for distinguishing between the groups. The multinomial logistic regression was performed using the dependent variable as a group of the patients (OSCC, OSMF, and controls), miRNA expressions as covariates, and risk factors (tobacco/pan masala chewing, smoking, alcohol abuse, etc. and duration of exposure) as factors. Further, the main effects and interactions between the covariates and factors were also analyzed to see the impact on the generated models. The developed model was evaluated by model fitting information, the goodness of fit, and pseudo-R square values. The software SPSS 25.0 was used for statistical analysis. Python and Excel were used to generate graphs and pie charts.
The institutional Ethics committee approved the present study. The institutional ethical committee number is AIIMS/IEC/2018/618, and the approval date is 23-08-2018.
Results
Patients and Demographic Data
Out of 93 individuals, 34 OSCC patients, 12 were females, and 22 were males. The mean age of the OSCC patients was 47 ± 9.7 years. The disease was moderately differentiated in 32 (88%) and well-differentiated in 2 (5%) at presentation. 30 OSMF and 29 control samples had mean ages 36 ± 13.6 and 33 ± 5.1, respectively. OSCC patients were classified according to AJCC staging. In the OSCC group, there were no stage I patients in our participants, and stage II, III, and IV were 17, 60, and 23%, respectively. The OSMF patients were graded according to Khanna JN and Andrade NN classification [28]. Only 7 (23%) OSMF patients belonged to grade III, and 23 (76%) of OSMF patients in our study had mouth opening less than 15 mm; they belong to class IVa and IVb. All the OSCC patients and OSMF patients were tobacco chewers. The OSMF and OSCC study participants belonged to age up to 75 years, but our controls mainly were the hospital staff and patient relatives, they were of younger ages (up to 45 years) than OSCC and OSMF patients. Therefore, if any correlation of the age with expression or any other parameter appears in the statistical analysis, that would not be considered clinically relevant. Chewing habits were present in all the OSMF and OSCC groups with more than 5 years of frequent habit, but only 14% of controls had chewing habits and less than 5 years at a lower frequency. The frequencies of other demographic parameters and risk factors are shown in Table 1.
Table 1.
Details of group-wise sample size, demographic data, and clinicopathological parameters
| Parameters | OSCC | OSMF | Control |
|---|---|---|---|
| Blood samples | n = 34 | n = 30 | n = 29 |
| Tissue samples | n = 34 | n = 30 | n = 4 |
| Age | |||
| Mean (range) |
47 ± 9.742 30–75 years |
36 ± 13.686 20–70 years |
33 ± 5.242 25–45 years |
| Gender | |||
|
Female Male |
12 (35.7%) 22 (64.7%) |
11 (36.7%) 19 (63.3%) |
7 (24.1%) 22 (75.9%) |
| Smoking status | |||
|
Yes No |
13 (38.2%) 21 (61.8%) |
3 (10.0%) 27 (90.0%) |
8 (27.6%) 21 (72.4%) |
| Alcohol abuse | |||
|
Yes No |
11 (32.4%) 23 (67.6%) |
5 (16.7%) 25 (83.3%) |
17 (58.6%) 12 (41.4%) |
| Tobacco Consumption | |||
|
Yes No |
34 (100%) 0 (0%) |
30 (100%) 0 (0%) |
8 (27.5%) 21 (72.5%) |
| Frequency of habits | |||
|
Less than 5 years 5–10 years More than 10 years |
2 (5.9%) 14 (41.2%) 18 (52.9%) |
11 (36.7%) 15 (50.0%) 4 (13.3%) |
8 (58.6%) 4 (27.6%) 0 (13.8%) |
| AJCC staging (OSCC) | |||
|
Stage I Stage II Stage III Stage IV |
0 (0%) 6 (17%) 20 (60%) 8 (23%) |
||
| Khanna and Andrade grading (OSMF) | |||
|
Grade III Grade IVa Grade IVb |
8 (26.6%) 17 (53.3%) 6 (20%) |
||
| Histologic grading | |||
|
Well-differentiated Moderately differentiated Poorly differentiated |
2 (5)% 32 (88%) 0% |
Expression Profiles of miRNA and Their Correlation with Clinicopathological Parameters and Risk Factors
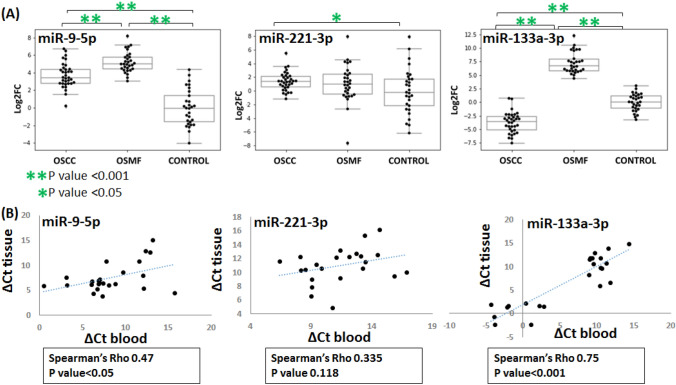
The differential expression of the miRNAs compared to controls in OSMF and OSCC groups is shown in Fig. 1. The log2FC values between the two groups are also mentioned with their significance in Table 2. In the clinical samples, miRNA-9 was up-regulated in both OSMF (log2FC; 5.2) and OSCC (log2FC; 3.7). Compared to OSMF, miR-9-5p was downregulated in OSCC with log2FC of − 1.5. miR-221-3p expression was found to be up-regulated in OSCC compared to controls, but the Ct values were found scattered in all the groups. The statistical test (Mann–Whitney) performed has given a significant p-value (0.042) for miR-221-3p upregulation in OSCC compared to controls with a log2FC of 1.4. miR-133a-3p was found to be significantly downregulated in OSCC, and interestingly, it was up-regulated in OSMF that too with a log2FC value of − 3.7 and 7.2 significantly (p-value < 0.001), respectively. Collectively, significant downregulation of miRNA-133a-3p in oral cancer compared to controls and interesting up-regulation in oral submucous fibrosis compared to oral cancer, miR-9-5p up-regulation in oral submucous fibrosis, as well as Oral cancer compared to controls, was observed. miR-221-3p was observed to be upregulated in oral cancer.
Fig. 1.
(A) Log2Fc plots for OSMF and OSCC expression compared to normal and (B) ∆Ct of tissue plotted against ∆Ct of blood for each miRNA
Table 2.
Relevant expression of selected miRNAs (miR-133a-3p, miR-9-5p, and miR-221-3p)—expression in OSCC concerning OSMF, expression in OSCC with respect to controls and expression in OSMF with respect to controls
| miRNAs | OSCC-OSMF | OSCC-Control | OSMF- Control |
|---|---|---|---|
|
miRNA-133a-3p (Log2FC) |
Down regulation − 10.9 (P < 0.001) |
Down regulation − 3.7 (P < 0.001) |
Up regulation 7.2 (P < 0.001) |
|
miRNA-9-5p (Log2FC) |
Down-regulation − 1.5 (P < 0.001) |
Up regulation 3.7 (P < 0.001) |
Up regulation 5.2 (P < 0.001) |
|
miRNA-221-3p (Log2FC) |
Up regulation 0.4 (P = 0.192) |
Up regulation 1.4 (0.042) |
Up regulation 1.0 (P = 0.536) |
We have further performed spearman’s correlation analysis to obtain the association of this miRNA expression with patients’ risk factors, stage (OSCC), and grade (OSMF). miR-133a-3p expression was found negatively associated with tobacco consumption, alcohol abuse, and duration of these habits. AJCC staging of OSCC patients was also correlated with the downregulation of miR-133a-3p. miR-9-5p upregulation is associated with tobacco consumption and the time of its intake. Although the expression of miR-221 was not significantly different between the groups, its expression was found to be substantially associated with alcohol abuse. The correlation of risk factors OSCC staging and OSMF grading with miRNA expressions are shown in Table 3.
Table 3.
Correlation of miRNAs expression with clinicopathological parameters and habitual attributes
| clinicopathological parameters and risk factors | miRNA-133a-3p | miRNA-9-5p | miRNA-221-3p |
|---|---|---|---|
| Smoking status | − 0.24 (P = 0.03) | − 0.141 (0.213) | − 0.204 (P = 0.07) |
| Alcohol abuse | − 0.252 (P = 0.024) | − 0.132 (P = 0.244) | − 0.260 (P = 0.017) |
| Tobacco/areca nut chewing | − 0.02 (P = 0.847) | 0.486 (P < 0.001) | 0.059 (P = 0.575) |
| Duration of habits | − 0.306 (P = 0.006) | 0.497 (P < 0.001) | 0.138 (P = 0.223) |
| AJCC staging (OSCC) | − 0.508 (P = 0.003) | 0.083 (P = 0.64) | − 0.112 (P = 0.528) |
| Khanna and Andrade grading (OSMF) | − 0.205 (P = 0.278) | − 0.04 (P = 0.803) | 0.198 (P = 0.295) |
(−) and (+) values show a negative and positive correlation, respectively
Correlation between ∆Ct values obtained for tissues and blood was performed to understand if these miRNAs have any similarities in the expression profile in blood and tissue. Spearman’s correlation test was performed for a total of 24 tissue and blood samples (10 OSCC, 10 OSMF, and 4 controls) reported in Fig. 1B. The correlation was found to be significant for miR-133a-3p (p < 0.001) and miR-9-5p (p < 0.05).
Determining Diagnostic Caliber of miRNA Expression by Plotting Receiver Operating Curve (ROC) and Generating Logistic Regression Model
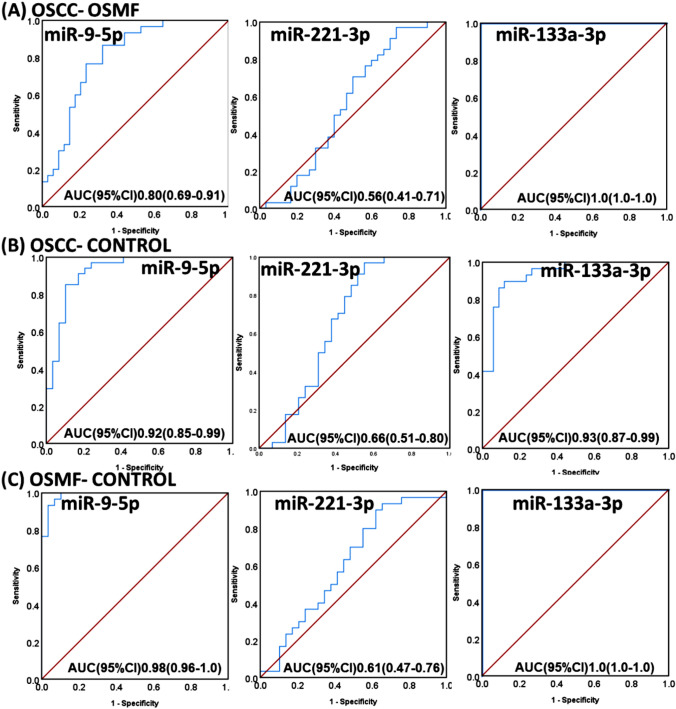
ROC was plotted for each miRNA, and associated AUC calculations were done to obtain specificity and sensitivity miRNA expression to differentiate OSCC from OSMF, OSMF from Controls, and OSCC from controls. We got a comparable specificity and sensitivity for each miR-9-5p and miR-133a-3p using ROC curves (Fig. 2) with AUC greater than 0.80 in each case. Further, we attempted to build a multinomial logistic regression model using patient groups as dependent variables and miRNA expressions (continuous values) as a covariate and other risk factors (categorical data) as factors. The step summary results have shown the interaction of Log2FCmiR-9*alcohol has the chi-square (χ2) 192, p < 0.05, indicating that this interaction has a significant effect on the group of patients. The model fitness was accessed using χ2 stastic. χ2, degree of freedom and significance of the generated model were 106, 12, and < 0.001, respectively. This proves the relationship between the dependent and independent variables. The person (148.641) and deviance (136.640) in the goodness of fit statistic test table were not significant, showing the model is fit. Pseudo R2 measures are cox and snell (0.35), Nagelkerke’s (0.22), and McFadden’s (0.17). Therefore the model accounts for 22 and 17% of the variance and represents relatively decent-sized effects. The likely hood ratio test proves that independent or predictor variables like Log2FCmiR-9*alcohol, tobacco/areca nut chewing, and Log2FC miR-133a-3p respondents were significant, proving that the predictors contribute significantly to the final final decision model.
Fig. 2.
ROC plots for miR-9-5p, miR-221-3p and miR-133a-3p to obtain diagnostic values within (A) OSCC and OSMF, (B) OSCC and controls and (C) OSMF and Control
So, the overall results look promising to consider miR-133a-3p and miR-9-5p as biomarkers for diagnosing and demarcating OSMF and OSCC patients. Further follow-up study and study in more extensive categorical data may promise their role in OSCC and OSMF demarcation.
Discussion
OSCC is a preventable disease if diagnosed at earlier stages, and OPMD’s transition, including many epithelial alterations, plays a vital role in the tumorigenesis of OSCC. In the present study, an attempt has been made to study the expression of miRNAs in OSMF and OSCC, expecting they can be used as risk stratification biomarkers. Studies are done to explore the role of miRNAs in OSCC tumors, especially in the invasion, migration angiogenesis, and other carcinogenic pathways [12–15]. miRNA profiling is also done in OSCC tissue and blood for investigating its correlation with a clinicopathological profile of tumors and prognosis to investigate if they could serve the purpose of diagnostic biomarkers [2, 14, 15, 29]. Few studies have analyzed miRNA expression changes associated with OSMF pathogenesis [17–19, 30]. Some studies have shown the correlation between the expression of miRNAs with OPMDs, such as a study by Brito et al. done with both formalin-fixed tissue and blood showed significant upregulation of miR-21, miR-181b, and miR-345 in OSCC compared to oral leukoplakia [31]. In a study by Harrandah et al., they found miR-21 up-regulated, miR-494 downregulated, and miR-375 downregulated in OSCC tissue compared to potentially malignant lesions [17]. Heravi et al. showed downregulation of miR-29a (p < 0.05), miR-146a (p < 0.05), miR-223 and up-regulation of miR-27b (p < 0.05) in OLP compared to OSCC [32]. We identified the gap of having rare evidence for miRNA deregulated in OSCC and OSMF; in our earlier database-dependent search of deregulated miRNA between OSCC and OSMF, we screened out five common miRNAs. These are hsa-miR-133a, hsa-miR-133b, hsa-miR-221, hsa-miR-451and hsa-miR-375; which may have a positive correlation to the OSMF to OSCC transition (unpublished data). The targets of these miRNA’s were enriched extensively in the crucial pathways related to the tumorigenesis, such as EMT, cell cycle arrest, cell-cell adhesion, etc. Some earlier reports on these miRNA’s involvement in carcinogenesis, such as miR-375, are already reported as a biomarker for malignant transformation of oral lesions [33–35]. miR-451 is a pro-apoptotic suppressor of angiogenesis that inhibits aggressiveness features in hepatocellular and squamous lung cancer [36, 37]. miR-133b along with miR-1 and miR-206 are found to inhibit the tumor-presenting receptors EGFR and c-MET both in HNSCC [38]. miR-133b is downregulated in many cancers [39, 40]. miR133-b is reported to be downregulated recently in OSCC, and its downregulation is correlated with the poor prognosis [41]. Various studies have shown that overexpression of miR-221-3p/222-3p increases growth and tumorigenesis in OSCC [23], but their significance to OPMDs is not mentioned to the best of our knowledge. Studies show downregulation of miR-133a [21] and miR-133b [20] but no significant expression profiling with OPMDs. miR-133 was significantly downregulated in OSCC, similar to previous studies by Chattopadhyay et al. [21]. They also observed an upregulation in oral leukoplakia, but they could not distinguish between the groups significantly. An essential role of miR-133a-3p in OSMF to OSCC pathogenesis may be present. There are many reports on the expression and function of miR-375 and miR-451 and miR-133b; therefore, we have selected the expression analysis of miRNAs in the patient’s sample miR-133a and miR-221 with miR-9.
miR-9 was selected in this study due to its controversial expression profile in many cancer types such as down-regulated in Breast cancer, Renal cell carcinoma, gastric cell carcinoma, and HNSCC) on the other hand is found to be up-regulated in hepatocellular, gliomas, and colorectal cancers [24]. In a study by Kim et al., compression-induced miR-9 downregulation and reversal were seen in breast cancer tissues [25]; this suggests that mechano-transduction is followed in miR-9 associated with OSMF. Therefore, we decided to explore miR-9 in OSCC and OSMF tissues. Also, a recent study found miR-9 enriched in HPV infected HNSCC patients and downregulates PPARD to induce macrophage polarization [26]. miR-9, which has a dynamic expression profile through different cancer types, was found to be up-regulated in our study compared to controls that may be justified as miR-9 upregulation is involved in angiogenesis metastasis in various cancer types [24]. Also, it was found to be up-regulated in OSMF compared to OSCC, which is an exciting outcome and probably supports the mechano-transduction of miR-9 [25] as miR-9 is said to be down-regulated due to mechanical compression in the tissue and may be OSMF has higher mechanical stress than OSCC due to very high extracellular matrix (ECM) deposition. However, mechanistic studies using cell lines and animal models may help validate the course of pathogenesis.
In our study, miR-133 was downregulated as reported by Chattopadhyay et al. in OSCC, similar to earlier reports in many cancers [21, 39–41]. They also observed an upregulation in oral leukoplakia, but they could not distinguish between the groups significantly. Our study group observed a significant upregulation of miR-133-a-3p in OSMF compared to OSCC and Controls with a Log Fc value of 10.9 and 7.2 with p value < 0.001, respectively. An essential role of miR-133a-3p in OSMF to OSCC pathogenesis may be present. This may be because collagen-associated genes COL1A1 COL25A1 are direct targets of miR-133a-3p shown in string PPI networks even if they are not enriched. Previous studies have shown that upregulation of these genes increases collagen production, which is mainly involved in OSMF pathogenesis [42] miR-133a-3p acts as a positive regulator of apoptosis, so its downregulation in OSCC may be justified; hence it can act as a double marker for OSMF to OSCC pathogenesis. A recent report confirms that miR-133 regulates epithelial to mesenchymal transitions [43]. The interesting upregulation of miR-133 in OSMF may also be because miR-133 cluster miRNAs inhibit the tumor-presenting receptors EGFR and c-MET [38], and these markers are downregulated in OSMF and get reactivated in OSCC [44].
The sample Ct data for controls and OSMF were scattered for miR-221. A categorical comparison may be helpful to see the difference in expression compared to age, sex, etc., to obtain a personalized diagnostic test in OSMF. Still, unfortunately, we could not do that because of the small sample size and the differences in mean age between the OSMF and control groups. The targets of mir-221 obtained from our enrichment study were mostly chaperonins family members such as TCP-1, CCT-3, CCT6A are involved in the folding of various proteins like telomere maintenance protein, ubiquitin pathway proteins, actin-tubulin proteins, and alternate transcriptional splice variants [45]. Also, miR-221 is reported to be elevated in many cancers such as hepatocellular, gastric, breast, and lung squamous cell carcinoma with targets such as SOD2 MMP1 genes, which are also predominantly studied in OSCC [46]. The involvement of miR-221 in multiple cancer-associated processes makes it a putative therapeutic target. Therefore, we suggest miR-221-3p needs be explored more with more extensive categorical data in OSCC and OSMF. The current study indicates that transition from OSMF to OSCC may be regulated by complex miR’s interaction with their target genes and precisely the significant expression of miR-133a-3p, miR9-5p makes them useful as biomarkers for early diagnosis of OSCC in OSMF patients, as is also reinforced by the sensitivity and specificity observations of the current study in Fig. 2. These may be explored further using experimental validation for the target genes associated with uncovering the overall miRNA role in the pathogenesis of OSMF to OSCC for early diagnosis and hence better prognosis.
Availability of data and materials
The data are available from the corresponding author on a reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The Institutional Ethics committee, AIIMS, Jodhpur, has approved the research. The institutional ethical committee number is AIIMS/IEC/2018/618, and the approval date is 23-08-2018.
Consent for publication
Not applicable
Informed consent
Informed consent was obtained from all the participants after properly describing the procedure through an information sheet.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.El-Sakka H, Kujan O, Farah CS. Assessing miRNAs profile expression as a risk stratification biomarker in oral potentially malignant disorders: a systematic review. Oral Oncol. 2018;77:57–82. doi: 10.1016/j.oraloncology.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles M, Kerr AR, et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020. [DOI] [PubMed]
- 4.Dionne KR, Warnakulasuriya S, Zain RB, Cheong SC. Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer. 2015;136(3):503–15. doi: 10.1002/ijc.28754. [DOI] [PubMed] [Google Scholar]
- 5.Shih Y-H, Wang T-H, Shieh T-M, Tseng Y-H. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int J Mol Sci [Internet]. 2019 Jun 16 [cited 2020 Jul 22];20(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6627879/. [DOI] [PMC free article] [PubMed]
- 6.Hashibe M. Risk Factors for Cancer of the Mouth: Tobacco, Betel Quid, and Alcohol. In: Warnakulasuriya S, Greenspan JS, editors. Textbook of Oral Cancer: Prevention, Diagnosis and Management [Internet]. Cham: Springer International Publishing; 2020 [cited 2020 Jul 22]. p. 23–30. (Textbooks in Contemporary Dentistry). Available from: 10.1007/978-3-030-32316-5_3.
- 7.Saravanan K, Kodanda Ram M, Ganesh R. Molecular biology of oral sub mucous fibrosis. J Cancer Res Ther. 2013;9(2):179–80. doi: 10.4103/0973-1482.113340. [DOI] [PubMed] [Google Scholar]
- 8.Garad A, Joshi SB, Naik CS, Ansari A, Mhatre B. ORAL SUB-MUCOUS FIBROSIS. A REVIEW [Internet]. 2018 [cited 2020 Jul 22].
- 9.Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):612–27. doi: 10.1016/j.oooo.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Seven M, Karatas OF, Duz MB, Ozen M. The role of miRNAs in cancer: from pathogenesis to therapeutic implications. Future Oncol Lond Engl. 2014;10(6):1027–48. doi: 10.2217/fon.13.259. [DOI] [PubMed] [Google Scholar]
- 11.Balatti V, Croce CM. MicroRNA dysregulation and multi-targeted therapy for cancer treatment. Adv Biol Regul. 2020;1:100669. doi: 10.1016/j.jbior.2019.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lajer CB, Nielsen FC, Friis-Hansen L, Norrild B, Borup R, Garnæs E, et al. Different miRNA signatures of oral and pharyngeal squamous cell carcinomas: a prospective translational study. Br J Cancer. 2011;104(5):830–40. doi: 10.1038/bjc.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumoto I, Hanazawa T, Kinoshita T, Kikkawa N, Koshizuka K, Goto Y, et al. MicroRNA expression signature of oral squamous cell carcinoma: functional role of microRNA-26a/b in the modulation of novel cancer pathways. Br J Cancer. 2015;112(5):891–900. doi: 10.1038/bjc.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gai C, Camussi F, Broccoletti R, Gambino A, Cabras M, Molinaro L, et al. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer. 2018;18(1):439. doi: 10.1186/s12885-018-4364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Victoria B, Lopez YN, Suchorska W, Barczak W, Sobecka A, et al. Tissue and serum microRNA profile of oral squamous cell carcinoma patients. Sci Rep. 2018;8(1):675. doi: 10.1038/s41598-017-18945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scapoli L, Palmieri A, Muzio LL, Pezzetti F, Rubini C, Girardi A, et al. MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol. 2010;23(4):1229–34. doi: 10.1177/039463201002300427. [DOI] [PubMed] [Google Scholar]
- 17.Harrandah AM, Fitzpatrick SG, Smith MH, Wang D, Cohen DM, Chan EKL. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(6):743–52.e1. doi: 10.1016/j.oooo.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Yang JM, Ahn S-H, Jeong W-J, Chung J-H, Paik JH. Potential oncogenic role and prognostic implication of microRNA-155-5p in oral squamous cell carcinoma. Anticancer Res. 2018;38(9):5193–200. [DOI] [PubMed]
- 19.B L, J C, X J. [Changes of miRNA after oral submucous fibrosis co-cultured with Salvia and low-dose prednisolone]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(5):471–6. [DOI] [PubMed]
- 20.Cervigne NK, Reis PP, Machado J, Sadikovic B, Bradley G, Galloni NN, et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum Mol Genet. 2009;18(24):4818–29. doi: 10.1093/hmg/ddp446. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay E, Singh R, Ray A, Roy R, De Sarkar N, Paul RR, et al. Expression deregulation of mir31 and CXCL12 in two types of oral precancers and cancer: importance in progression of precancer and cancer. Sci Rep. 2016;6(1):32735. doi: 10.1038/srep32735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Sarkar N, Roy R, Mitra JK, Ghose S, Chakraborty A, Paul RR, et al. A quest for miRNA bio-marker: a track back approach from gingivo buccal cancer to two different types of precancers. PLoS ONE. 2014;9(8):e104839. doi: 10.1371/journal.pone.0104839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C-J, Shen WG, Liu C-J, Chen Y-W, Lu H-H, Tsai M-M, et al. miR-221 and miR-222 expression increased the growth and tumorigenesis of oral carcinoma cells: miR-221 and miR-222 in OSCC. J Oral Pathol Med. 2011;40(7):560–6. doi: 10.1111/j.1600-0714.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 24.Khafaei M, Rezaie E, Mohammadi A, Shahnazi Gerdehsang P, Ghavidel S, Kadkhoda S, et al. miR-9: From function to therapeutic potential in cancer. J Cell Physiol. 2019. [DOI] [PubMed]
- 25.Kim BG, Gao M-Q, Kang S, Choi YP, Lee JH, Kim JE, et al. Mechanical compression induces VEGFA overexpression in breast cancer via DNMT3A-dependent miR-9 downregulation. Cell Death Dis. 2017;8(3):e2646–e2646. doi: 10.1038/cddis.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong F, Mao X, Zhang S, Xie H, Yan B, Wang B, et al. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020;28:34–44. doi: 10.1016/j.canlet.2020.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Khanna JN, Andrade NN. Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases. Int J Oral Maxillofac Surg. 1995;24(6):433–9. doi: 10.1016/S0901-5027(05)80473-4. [DOI] [PubMed] [Google Scholar]
- 29.Chang C-C, Yang Y-J, Li Y-J, Chen S-T, Lin B-R, Wu T-S, et al. MicroRNA-17/20a functions to inhibit cell migration and can be used a prognostic marker in oral squamous cell carcinoma. Oral Oncol. 2013;49(9):923–31. doi: 10.1016/j.oraloncology.2013.03.430. [DOI] [PubMed] [Google Scholar]
- 30.Acharya S, Singh S, Bhatia S. Role of MicroRNA profiling in oral submucous fibrosis pathogenesis and anticarcinogenic action of curcumin in microRNA dysregulation in oral carcinogenesis: a literature update. Indian J Dental Sci. 2019;11:175–9. [Google Scholar]
- 31.Brito JAR, Gomes CC, Guimarães ALS, Campos K, Gomez RS. Relationship between microRNA expression levels and histopathological features of dysplasia in oral leukoplakia. J Oral Pathol Med. 2014;43(3):211–6. doi: 10.1111/jop.12112. [DOI] [PubMed] [Google Scholar]
- 32.Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS. Genomewide study of salivary MicroRNAs for detection of oral cancer. J Dent Res. 2014;93(7_suppl):86S-93S. [DOI] [PMC free article] [PubMed]
- 33.Harrandah AM, Fitzpatrick SG, Smith MH, Wang D, Cohen DM, Chan EKL. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(6):743–52.e1. doi: 10.1016/j.oooo.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Mazumder S, Datta S, Ray JG, Chaudhuri K, Chatterjee R. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019;58:137–45. doi: 10.1016/j.canep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Yoon AJ, Wang S, Shen J, Robine N, Philipone E, Oster MW, et al. Prognostic value of miR-375 and miR-214-3p in early-stage oral squamous cell carcinoma. Am J Transl Res. 2014;11(5):580–92. [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Zhang A, Xiang J, Lv Y, Zhang X. miR-451 acts as a suppressor of angiogenesis in hepatocellular carcinoma by targeting the IL-6R-STAT3 pathway. Oncol Rep. 2016;36(3):1385–92. doi: 10.3892/or.2016.4971. [DOI] [PubMed] [Google Scholar]
- 37.Uchida A, Seki N, Mizuno K, Yamada Y, Misono S, Sanada H, et al. Regulation of KIF2A by antitumor miR-451a inhibits cancer cell aggressiveness features in lung squamous cell carcinoma. Cancers. 2019;11(2):258. doi: 10.3390/cancers11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koshizuka K, Hanazawa T, Fukumoto I, Kikkawa N, Matsushita R, Mataki H, et al. Dual-receptor (EGFR and c-MET) inhibition by tumor-suppressive miR-1 and miR-206 in head and neck squamous cell carcinoma. J Hum Genet. 2017;62(1):113–21. doi: 10.1038/jhg.2016.47. [DOI] [PubMed] [Google Scholar]
- 39.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, et al. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127(12):2804–14. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 40.Nohata N, Hanazawa T, Enokida H, Seki N. microRNA-1/133a and microRNA-206/133b clusters: dysregulation and functional roles in human cancers. Oncotarget. 2012;3(1):9–21. doi: 10.18632/oncotarget.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Wang H, Xu W, Chen Z. Inhibition of miR-133b indicates poor prognosis and promotes progression of OSCC via SOX4.:10. [DOI] [PubMed]
- 42.Arakeri G, Rai KK, Hunasgi S, Merkx M, a. W, Gao S, Brennan PA. Oral submucous fibrosis: An update on current theories of pathogenesis. J Oral Pathol Med. 2017;46(6):406–12. [DOI] [PubMed]
- 43.Jung JE, Lee JY, Park HR, Kang JW, Kim YH, Lee JH. MicroRNA-133 targets phosphodiesterase 1C in drosophila and human oral cancer cells to regulate epithelial-mesenchymal transition. J Cancer. 2021;12(17):5296–309. [DOI] [PMC free article] [PubMed]
- 44.Sharma M, Fonseca FP, Hunter KD, Radhakrishnan R. Loss of oral mucosal stem cell markers in oral submucous fibrosis and their reactivation in malignant transformation. Int J Oral Sci. 2020;12(1):1–10. [DOI] [PMC free article] [PubMed]
- 45.He J, Jing Y, Li W, Qian X, Xu Q, Li F-S, et al. Roles and Mechanism of miR-199a and miR-125b in Tumor Angiogenesis. PLoS ONE. 2013;20(2):e56647. doi: 10.1371/journal.pone.0056647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye H, Wang A, Lee B-S, Yu T, Sheng S, Peng T, et al. Proteomic based identification of manganese superoxide dismutase 2 (SOD2) as a metastasis marker for oral squamous cell carcinoma. Cancer Genomics Proteomics. 2008;5(2):85–93. [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on a reasonable request.