Abstract
Background:
Microplastics (MPs) are small fragments from any type of plastic formed from various sources, including plastic waste and microfibers from clothing. MPs degrades slowly, resulting in a high probability of human inhalation, ingestion and accumulation in bodies and tissues. As its impact on humans is a prolonged event, the evaluation of its toxicity and influence on human health are critical. In particular, MPs can enter the human digestive system through food and beverage consumption, and its effect on the human colon needs to be carefully examined.
Methods:
We monitored the influence of small MPs (50 and 100 nm) on human colon cells, human colon organoids and also examined their toxicity and changes in gene expression in vivo in a mouse model.
Results:
The data suggested that 5 mg/mL concentrations of 50 and 100 nm MPs induced a > 20% decrease in colon organoid viability and an increase in the expression of inflammatory-, apoptosis- and immunity-related genes. In addition, in vivo data suggested that 50 nm MPs accumulate in various mouse organs, including the colon, liver, pancreas and testicles after 7 d of exposure.
Conclusion:
Taken together, our data suggest that smaller MPs can induce more toxic effects in the human colon and that human colon organoids have the potential to be used as a predictive tool for colon toxicity.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-022-00496-8.
Keywords: Microplastics, Colon organoids, Differentiation, Normal colon cells, Toxicity
Introduction
The impact of microplastics (MPs) on human health has become a great concern as their abundance in the environment has increased dramatically [1, 2]. MPs can be defined as any type of plastic less than 5 mm in length. [3, 4]. MPs cause serious pollution in natural ecosystems and are derived from a variety of sources, including clothing, cosmetics, industrial processes and food packaging. As most MPs decompose very slowly in undisrupted natural environments, their impact on the environment, ecosystem and human health needs to be evaluated carefully given their long-term existence in nature [5, 6].
Humans can be exposed to MPs in various ways, including through the skin, digestive system and airways[7–9]. In particular, small MPs from food packaging and other plastic products, including straws, can enter the human body through the digestive system, which can influence human organs, including the human colon[10–12]. Therefore, systematic evaluation of the effect of small MPs on human colon cells and human colon organoids is important to provide valuable information about the effects of MPs on human health.
Human organoids are simplified and miniaturized three-dimensional assemblies of cells that show realistic microanatomy of actual organs[13, 14]. Organoids can be derived from either stem cells or cells from human/animal tissues and can be maintained and differentiated for several days up to several years[15]. Toxicity studies with animals have become difficult due to ethical issues, and because organoids closely mimic actual human organ structures, they can serve as a valuable research tool for studying complex human organs, including the influences of various substances on human organs[16, 17].
Here, we evaluated the effect of MPs on human colon fibroblasts, human colon organoids and a mouse model. By comparing the size-dependent impact of MPs on fibroblasts, organoids and within an in vivo system, information on how accurately human fibroblasts and organoids can predict in vivo toxicity was deduced. In addition, the potential of using human organoids as a predictive toxicity system is assessed.
Materials and methods
MPs preparation and characterization
Fifty and 100 nm microplastic beads conjugated with fluorescent dyes (Fluoresbrite® dyed particles, MP) were purchased from Polysciences (Warrington, PA, USA). MPs were sterilized by autoclaving and stored at 4 °C before the experiment. Field emission scanning electron microscopy (FE-SEM) images of MPs were obtained with a Sigma HD camera (Carl Zeiss AG, Jena, Germany). The particle size and zeta potential of MPs were obtained with a Litesizer 500 particle size analyzer (Anton Paar, Graz, Austria).
MPs cytotoxicity assay using CCD18-co cells
Normal human colon fibroblast CCD18-Co cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained with Eagle’s minimum essential medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin–streptomycin (Thermo Fisher Scientific) in a 37 °C, 5% CO2 incubator (Thermo Fisher Scientific). For the MPs-induced cytotoxicity test, CCD-18Co cells were seeded in 96-well cell culture plates at a density of 5000 cells/well (Corning, Corning, NY, USA). After 24 h of seeding, the cells were treated with the designated concentrations of 50 and 100 nm MPs (0.008, 0.04, 0.2, 1, 5 and 10 mg/mL). After 48 h of incubation, cell viability was monitored with a cell counting kit (CCK-8, Dojindo, Kummamoto, Japan). For the cell proliferation inhibition assay, 5,000 CCD-18Co cells were seeded on 96-well plates, and 5 mg/mL 50 and 100 nm MPs were added. After 12, 24, 72, 120 and 168 h, cell viability was monitored with CCK-8. For dead cell staining, 50 and 100 nm MPs-treated CCD-18Co cells were incubated for 48 h, and 4 µM ethidium homodimer-1 (EthD-1, Thermo Fisher Scientific) was added to the cells. Fluorescence images of cells were obtained with a Lionheart FX automated microscope (BioTek, Winooski, VT, USA).
Human colon organoid culture
Human colon organoids were suspended in an ice-cold mixture of 40% human colon organoid growth media (Stemcell Technologies, Vancouver, Canada) and 60% Matrigel (Corning). Colon organoid crypts were plated in a 96-well plate and incubated at 37 °C for 10 min. Next, 100 µL human colon organoid growth media was added and cultured at 37 °C; the medium was changed every other day. Organoids were passaged every 7–10 d with gentle cell dissociation reagents (Stemcell Technologies). Human colon organoids on 96-well cell culture plates were treated with the designated concentrations of MPs.
Human colon organoid staining and viability assay
For live/dead staining, colon organoids were incubated with 20 mM calcein-AM (green, Thermo Fisher Scientific) and 10 mg/ml EthD-1 (red, Thermo Fisher Scientific) for 60 min at 37 °C and then imaged by microscopy (Eclipse Ti2, Nikon, Tokyo, Japan). Additionally, 50 nm and 100 nm MPs were GFR-tagged colon organoids, and CCD-18Co cells only confirmed dead cells by EthD-1. For the viability assay, the colon organoids and CCD-18Co cells were quantitatively analyzed using the CellTiter-Glo® 3D cell viability assay kit (Promega, Madison, WI, USA). At the indicated concentration points after MPs treatment, the luminescence generated by the reaction was measured using a microplate reader (SpectraMax iD5, Molecular Devices, San Jose, CA, USA) and presented as a dose–response curve. The IC50 values of the MPs were determined from concentration-dependent inhibition curves using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA).
Immunofluorescence (IF) staining
For IF staining, organoids were fixed in 4% paraformaldehyde for 30 min at room temperature, permeabilized, blocked with blocking solution (1% serum + 0.1% Triton X-100 in Dulbecco's phosphate buffered saline), and then stained with the primary mucin-2 antibody (Thermo Fisher Scientific) at 4 °C for 16 h and then the RFP-conjugated secondary antibody (Thermo Fisher Scientific) at room temperature for 2 h. Nuclei were counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (Invitrogen, Waltham, MA, USA), and images were acquired on a microscope.
Total RNA isolation and quantitative PCR
Total RNA isolation of colon organoids was performed using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocols. RNA quality and quantity were assessed using a NanoDrop 2000 instrument (Thermo Fisher Scientific). RNA (2 μg) was reverse transcribed to cDNA using the RT2 First Strand Kit (Qiagen) according to the manufacturer's protocols. The expression of 84 key colon inflammation-related genes was evaluated using Human CD RT2 Profiler PCR Arrays (#PAHS-169Z; Qiagen, Table S1) and SYBR Green chemistry (RT2 SYBR Green ROX qPCR Mastermix; Qiagen) according to the manufacturer's protocols using the Roter gene Q instrument (Qiagen). The expression level of each gene was normalized to the geometric mean of five housekeeping reference genes (ACTB, B2 M, GAPDH, HPRT1 and RPLP0) based on RefFinder algorithm (GeneGlobe, Qiagen) analysis.
Animal care and experimentation
All animal experiments were carried out using C57BL/6 mice according to the established guidelines of the Institutional Animal Care and Use committee of the Korea Research Institute of Chemical Technology. Seven-week-old C57BL/6 male mice were purchased from Orient Bio. (Seongnam, Korea). The mice were placed in a specific pathogen-free animal room under controlled conditions (temperature, 23 ± 2 °C and humidity, 55–60%) and a 12/12 h light/dark cycle. All animals were allowed to acclimate for at least 1 week before use. Standard laboratory animal feed was purchased from Biopia (Gunpo, Korea), and animal feed and water were freely provided. The 50 nm MPs dose was determined based on the concentration at which the highest toxicity was found in colon organoids. Mice were orally administered once on Day 0. The mice were exposed to MPs for a total of 7 d and were weighed before the intervention and on the final day of exposure. At the end of the exposure on the final day, the mice were euthanized, and the blood, colon, kidney and testes of the mice were collected. The plasma concentrations of glucose,, aspartate transaminase (AST), alanine transferase (ALT), cholesterol (CHO), HLD-cholesterol, and LDL-cholesterol were measured by colorimetric assays using an automatic biochemical analyzer (Selectra 2, Vital Scientific N.V., The Netherlands). The plasma concentrations of interleukin 8 (IL-8) were measured by a colorimetric assay using an IL-8 ELISA kit (R&D Systems, Minneapolis, MN, USA) with concentrations expressed in units of ng/mL. All in vivo experimental procedures were approved by the Animal Research Committee of the Korea Research Institute of Chemical Technology (approval number: 2022-7A-09–01).
Tissue histology
Paraffin Sects. (8 µm) of mouse colons were attached to poly-L-lysine-coated glass slides. After incubation at 60 °C for 30 min, the slides were dewaxed and hydrated stepwise using xylene followed by several solutions of distilled water containing decreasing amounts of ethanol (100–60%, vol/vol). The sections were stained with hematoxylin and eosin (H&E, Abcam, Cambridge, UK) and an Alcian blue periodic acid-Schiff (PAS) staining kit (Abcam) according to the manufacturer's protocol. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was conducted according to the manufacturer’s protocol (Abcam).
Statistical analysis
All data are expressed as the mean ± standard error, and the results were generated from three independent experiments (n = 4 ~ 8/group) with a minimum of three technical replicates unless otherwise stated. The statistical analysis included a one-way analysis of variance and t tests, which were performed using the GraphPad Prism program, Qiagen GeneGlobe and Excel. Values below p < 0.05 are considered statistically significant (i.e., *p < 0.05, **p < 0.01 and ***p < 0.001).
Results
MPs Characterization after autoclaving
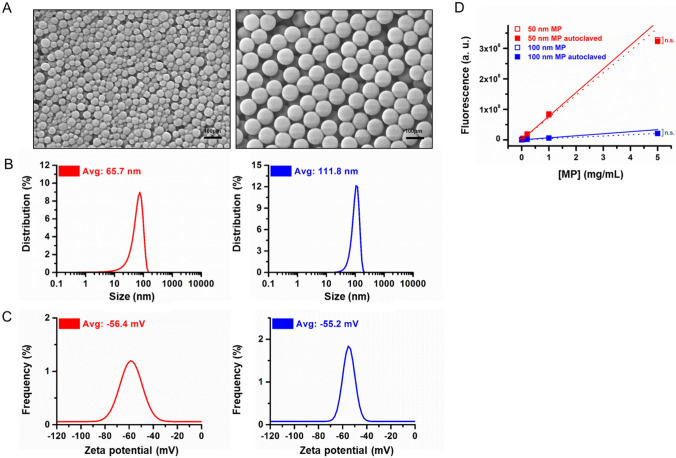
MPs (50 nm and 100 nm) were autoclaved to remove potential contaminating factors before cell and organoid treatment. The size and surface charge of the MPs were monitored before and after autoclaving. The data suggest that autoclaving does not induce significant changes in MPs size (66.1 vs. 65.5 nm for 50 nm MPs and 113.7 vs. 111.8 nm for 100 nm MPs before and after autoclaving, respectively) or surface charge (− 54.5 vs. − 56.4 mV for 50 nm MPs and− 55.6 vs. − 56.2 mV for 100 nm MPs before and after autoclaving, respectively, Fig. 1A–C and Figure S1). The fluorescence intensity from fluorophores conjugated to MPs also showed no significant changes before and after autoclaving (Fig. 1D). Overall, these data indicate that autoclaving does not induce significant changes in MPs properties.
Fig. 1.
Characterization of 50 and 100 nm MPs after autoclaving. A FE-SEM images of 50 (left) and 100 (right) nm MPs. B Size distribution of 50 and 100 nm MPs. C Zeta potential distribution of 50 and 100 nm MPs. D Fluorescence intensities from 50 and 100 nm MPs with increasing concentration. The results are presented as the mean ± S.D. (n = 6)
Effect of MPs on cytotoxicity in human normal colon cells
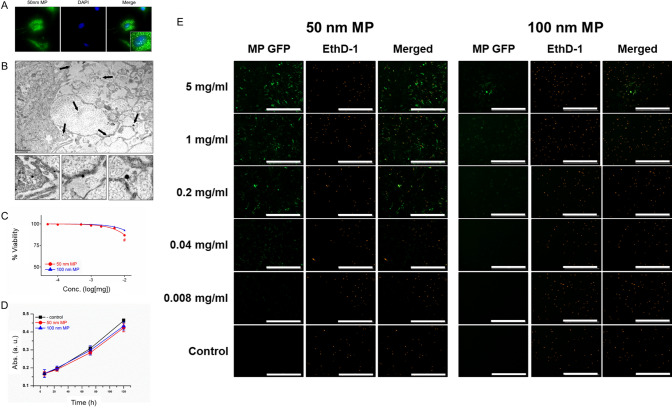
After MPs characterization, we monitored MPs-induced toxicity in normal colon CCD-18Co cells. Fluorescence from the MPs-conjugated fluorophore was detected in the cytosol and nucleus of CCD-18Co cells, suggesting that MPs can successfully penetrate/endocytosis into cells (Fig. 2A). TEM images of CCD-18Co cells incubated with 50 nm MPs also suggested MPs infiltration inside the cells (Fig. 2B). Forty-eight hours of exposure to 10 mg/mL 50 nm MPs induced a 13.2% decrease in CCD-18Co cell viability, whereas the same concentration of 100 nm MPs induced a 7.2% decrease in colon cell viability (Fig. 2C). Figure 2D shows that 168 h of exposure to 10 mg/mL MPs decreased CCD-18Co cell proliferation by 16.5 and 13.8%, respectively. Dead cell staining with EthD-1 also suggested only a small increase in the percentage of dead cells in the presence of 5 mg/mL 50 and 100 nm MPs compared with that of the control (Fig. 2E). Thus, our data indicate that although both MPs can successfully infiltrate cells, CCD-18Co cell viability and proliferation do not change significantly after short (12 h) or long (168 h) exposure to MPs.
Fig. 2.
Effect of MPs on human colon fibroblasts. A Fluorescence microscopic images of CCD-18Co cells after incubation with 50 nm MPs. B TEM images. C Viability. D Proliferation and E. Live-dead cell staining results of CCD-18Co cells after incubation with MPs. The scale bar represents 50 µm. The results are presented as the mean ± S.D. (n = 6)
Effect of MPs on cytotoxicity in human colon organoids
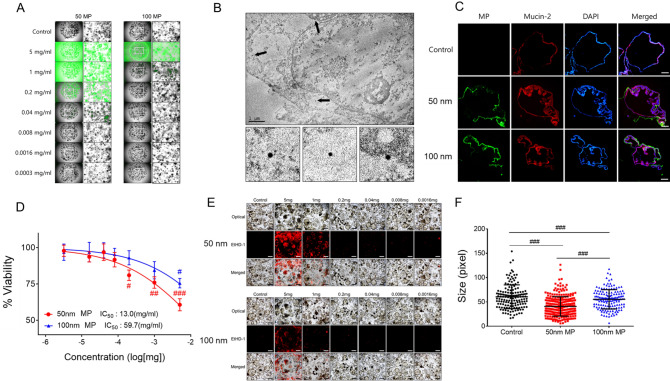
Next, we monitored MPs-induced toxicity in human iPSC-derived colon organoids. Although fluorescence from both 50 and 100 nm MPs-conjugated fluorophores was detected inside colon organoids, more infiltration of 50 nm MPs inside colon organoids was observed compared with that of 100 nm MPs upon exposure to various concentrations (0.0003–5 mg/mL, Fig. 3A). We also confirmed that 50 nm MPs can infiltrate the cytosol of colon organoids, but MPs penetration into the nucleus of colon organoids was not observed with TEM (Fig. 3B). The effect of MPs exposure on gut-specific marker mucin-2 expression in colon organoids was monitored with immunofluorescence staining. Colon organoids treated with 50 and 100 nm MPs showed an increase in mucin-2 expression compared with that of the control (Fig. 3C). The effect of MPs on the viability of colon organoids showed a more prominent decrease in colon organoid viability when treated with 50 nm MPs compared with that of 100 nm MPs (Fig. 3D). The dead cell staining results suggested that 50 nm MPs induced a more prominent increase in the percentage of dead cells in colon organoids than that of 100 nm MPs (Fig. 3E). When comparing the size of colon organoids after exposure to MPs, 50 nm MPs induced a more prominent decrease in colon organoid size compared with that of 100 nm MPs (Fig. 3F). Taken together, 50 nm MPs induced more significant effects on human colon organoid viability, size and gut-specific marker expression than those of 100 nm MPs.
Fig. 3.
Effect of MPs on viability of human colon organoids. A Fluorescence microscopic images. B TEM. C Fluorescence from MPs and mucin-2 in colon organoids. D % viability changes (with IC50 13. 0 mg/ml and 59.7 mg/ml for 50 nm and 100 nm MPs, respectively), E dead cells from EtHD-1 staining and F size changes of colon organoids after incubation with 50 and 100 nm MPs. Fluorescence images were taken under magnification × 100. The results are presented as the mean ± S.D. (n = 6). #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control
Effect of MPs on the expression of apoptosis-, immune response- and inflammation-related genes and cytokines in human colon organoids
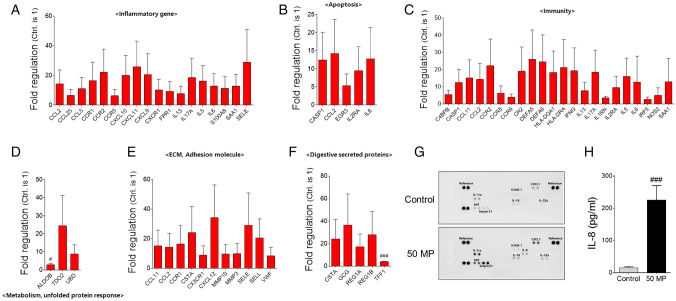
As MPs-induced toxicity in human colon organoids was observed, we evaluated changes in gene and cytokine expression after incubation with MPs. The expression levels of 77 genes associated with inflammation, apoptosis, immunity, metabolism, the extracellular matrix (ECM), adhesion molecules and digestive secreted proteins were evaluated. The data suggested that out of 29 inflammation-related genes, the expression levels of 18 genes were increased more than twofold when incubated with 50 nm MPs (14 genes increased more than twofold with 100 nm MPs) compared with levels of control organoids (Fig. 4A). For apoptosis-related genes, the expression levels of 5 out of 10 genes were increased more than twofold with 50 nm MPs (4 genes showed increased expression with 100 nm MPs) compared with levels in the control (Fig. 4B). The 50 nm MPs-induced increase in 22 immune-related genes (out of a total of 32 genes) was increased more than twofold, whereas 100 nm MPs induced a greater than twofold increase in 17 genes (Fig. 4C). For genes related to metabolism and unfolded protein responses, both 50 and 100 nm MPs induced a greater than twofold increase in 3 genes out of 4 (Fig. 4D). The expression levels of 11 ECM and adhesion molecules were increased more than twofold when incubated with 50 nm MPs (7 molecules for 100 nm MPs) out of a total of 17 genes (Fig. 4E). MPs also induced an increase in digestive secreted proteins (> twofold for 5 genes with 50 nm MPs and 4 genes with 100 nm MPs out of a total of 6 genes, Fig. 4F). We also monitored changes in cytokine expression in colon organoids after incubation with MPs. Upon the addition of 50 nm MPs, the expression levels of interleukin-1 receptor antagonist (IL1-Rα), C-X-C motif chemokine ligand 1 (CXCL1), macrophage migration inhibitory factor (MIF) and plasminogen activator inhibitor-1 (serpin E1) were increased in colon organoids compared with levels in the control (Fig. 4G). Noticeably, 50 nm MPs induced the expression of IL-8, which was not detected in control organoids (Fig. 4G). Exposure to 50 nm MPs also induced an increase in IL-8 in colon organoid culture media compared with that of the control based on an enzyme-linked immunosorbent assay (ELISA, Fig. 4H). However, fewer changes in gene expression related to inflammation, apoptosis, immunity and digestive secreted proteins were observed on colon organoids exposed to 100 nm (Supplementary Fig. S2). Taken together, our data suggest that 50 nm MPs induced increases in various apoptosis-, immune response- and inflammation-related genes in human colon organoids.
Fig. 4.
PCR array results of colon organoids after incubation with 50 nm MPs. Changes in the gene expression of A inflammatory, B apoptosis, C immunity, D metabolism (unfolded protein response), E ECM and adhesion molecules, and F digestive secreted proteins of colon organoids after incubation with 50 nm MPs. G Dot blot results of control and 50 nm MPs-treated organoids of key inflammatory-related genes. H Amount of IL-8 detected in control and 50 nm MPs-treated colon organoids. The results are presented as the mean ± S.D. (n = 6). #p < 0.05, ###p < 0.001 vs. control
Effect of MPs on in vivo toxicity
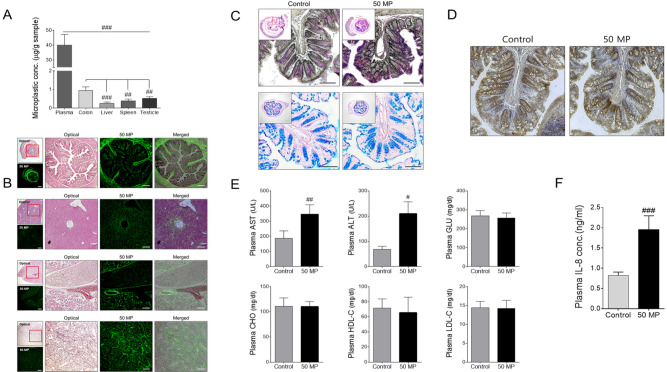
We exposed mice to 50 nm MPs and monitored the accumulation of fluorophore-tagged MPs in organs. After 7 d of 50 nm MPs exposure, the amount of 50 nm MPs detected in mouse plasma increased dramatically compared with that after 2 h of exposure (Fig. 5A) compared to non-treated control (Supplementary Fig. S3). The amount of MPs accumulation was also evaluated after homogenization of each organ. The data also suggested that significant amounts of MPs penetrated into various organs, including the colon, liver, spleen and testes (Fig. 5A). H&E staining of various mouse organs after MPs exposure indicated that intense fluorescence signals from fluorophore-tagged MPs were detected in various organs (Fig. 5B). To evaluate the effect of MPs exposure on colon walls, alcian blue PAS and TUNEL staining were conducted. The degree of PAS staining increased with MPs exposure, whereas no significant changes in alcian blue staining were detected (Fig. 5C). The TUNEL staining results suggested that cells in the colon wall were undergoing apoptosis after MPs exposure (Fig. 5D). Biochemical analysis of mouse blood after 7 d of 50-nm MPs exposure suggested that mice treated with MPs exhibited increased levels of plasma ALT and AST (Fig. 5E), but there were few or no changes in protein levels related to cholesterol and lipogenesis (glucose [GLU], cholesterol [CHO], high-density lipoprotein C [HDL-C] and low-density lipoprotein C [LDL-C]) compared with those of the control. Interestingly, similar to that of colon organoids, the IL-8 level in plasma was increased dramatically in mice after MPs exposure (Fig. 5F). However, no noticeable changes in mouse body weight, colon, thymus, testes or spleen weight were observed in mice treated with 50 nm MPs (Supplementary Fig. S4). Overall, our in vivo MPs toxicity results suggest that mice exposed to MPs show possible toxic effects on various organs.
Fig. 5.
Effect of 50 nm MPs on toxicity in C57BL/6 male mice. A Concentration of MPs detected in mouse plasma, colon, liver, spleen and testicles. B Fluorescence of MPs detected in mouse tissue. C PAS and alcian blue staining and D TUNEL staining of 50 nm MPs treated mouse colon tissue. E Plasma AST, ALT, GLU, CHO, HDL-C, and LDL-C concentrations. F Plasma IL-8 concentrations after incubation with 50 nm MPs. The scale bar represents 200 µm. The results are presented as the mean ± S.D. (n = 6). #p < 0.05, ##p < 0.01, ###p < 0.001 vs. control
Discussion
Previous studies have suggest that ingestion of MPs can induce gastrointestinal (GI) disease. Ingested MPs induces change in human gut microbiota as well as induce immunotoxicity [18–20]. MPs accumulation in human GI tract can lead to disruption of GI tract mucus and epithelium [20]. Therefore, effect of MPs consumption on human colon system is need to be carefully evaluated.
By systematically assessing the influence of 50- and 100-nm MPs on 2D cell culture and mouse and colon organoids, our data suggest that MPs size affects its influence. A previous study also suggested that the size of MPs is important in fish toxicity and larger MPs pose relatively little risk [21]; our data confirm that smaller MPs induce more severe toxic effects. Increased level of inflammatory, apoptosis and immunity related-genes in human colon organoids upon addition of MPs suggests that ingestion of MP may potentially induce cytotoxic effects in human GI tract. Ingested MPs leading to an upregulation of immune response genes causing inflammation and toxicity in human GI tract. MPs also induces direct increase of apoptotic related genes leading to a cell death in GI system. Although exact mechanism of MP induced toxicity is still need to be examined, it has been reported that MPs induces inhibition of DNA replication, cell cycle arrest in S phase and G2/ M phase during cell replication inducing cytotoxicity in zebrafish models [22]. High concentration of MPs also induces rupture of lipid bilayer of cell plasma membrane as well as inducing programmed cell death [23]. Exposure to MPs also causes inflammation, oxidative stress and changes in lipid metabolism [24].
Traditionally, 2D human cell culture has been used to evaluate the toxicity of various materials to predict the effect on human health [25]. This type of cell culture has various advantages such as the ease of growing cells and the ability to obtain a reliable result that is relatively less dependent on various experimental conditions. However, translating 2D in vitro results to impacts on humans is a serious obstacle and often leads to false predictions. [26, 27] In vivo experiments are an essential and terminal experimental tool in advance of human clinical trials to evaluate the toxicity of various environmental hazards [28, 29]. Unlike 2D cell culture, in vivo experiments include human-like processes such as blood flow, adsorption, digestion, metabolism and excretion (ADME) mechanisms. This allows a more accurate prediction of the effect of various materials on human health. However, there is a growing concern regarding the use of countless animals in biological experiments [30, 31]. In addition, there is still a biological complexity gap between human and experimental animals; therefore, in vivo experiments also have limitations [32, 33]. Recently, there has been growing interest and ongoing research on using human organoids to evaluate the effects of various materials on human health [34, 35]. Human stem cell-derived organoids can be differentiated into various organs mimicking complex and precise structures. Several recent studies have shown that human stem cell-derived organoids can be used as valuable and accurate tools for evaluating the impact of drugs, toxins and various materials on human health [36, 37]. Recent advances in organoid technology enable high organoid-human correlations and show potential for replacing in vivo experiments. Organoids still have limitations in that although they are derived from human stem cells and closely mimic human organs, they lack the blood flow and ADME of live organisms. [38] However, there is a growing effort to introduce blood vessels and fluid flow into human organoids, and more complex and human-mimicking organoids are expected to be introduced [39].
Our data also suggest a promising future for using organoids as human health impact prediction tools. Compared with the 2D cell culture system, the experimental results of the MPs effect on colon organoids more closely mimicked the in vivo experimental data. Concentration-dependent viability changes in response to MPs were more dramatically observed in human colon organoids than in 2D cultures. In addition, an increase in mucin-2 expression levels in human colon organoids as well as an increase in toxicity upon exposure to MPs were also observed in the in vivo experiment. Although it is too early to conclude that human colon organoids can replace in vivo experiments due to a lack of clinical data on the MPs effect on the human colorectal system, our data suggest that advances in colon organoid culture may help to reduce the use of in vivo experiments for predicting human health impacts. In addition, human-origin colon organoids can overcome in vivo experimental vs. human clinical discrepancies arising from species differences.
In summary, we systematically evaluated the effect of MPs on human colon cells, human colon organoids and an in vivo mouse models. Our data suggest that smaller MPs induce more toxic effects and that the results in human colon organoids are more similar to those in the in vivo model than those in human colon cells. Overall, human colon organoids can be used as valuable predictive tools for assess MPs-induced toxicity in humans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors greatly acknowledge financial support from the Technology Innovation Program at the Ministry of Trade, Industry and Energy (20009774) and the Korea Research Institute of Chemical Technology (SI2231-40) of the Republic of Korea.
Declarations
Conflict of interest
The authors have no potential conflicts of interest.
Ethical statements
All animal experiments were carried out using CD-1(ICR) mice according to the established guidelines of the Institutional Animal Care and Use committee of the Korea Research Institute of Chemical Technology. All animals were maintained under a room illuminated daily from 07:00 to 19:00 (12:12 h light/dark cycle), with a temperature of 23 ± 1 °C, a ventilation of 10—12 times per hour, and a humidity of 55 ± 5%. All in vivo experimental procedures were approved by the Animal Research Committee of the Korea Research Institute of Chemical Technology (approval number: 2022-7A-09-01).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Byumseok Koh, Email: bkoh@krict.re.kr.
Ki Young Kim, Email: kykim@krict.re.kr.
References
- 1.Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702:134455. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 2.Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371:672–674. doi: 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- 3.Peñalver R, Arroyo-Manzanares N, López-García I, Hernández-Córdoba M. An overview of microplastics characterization by thermal analysis. Chemosphere. 2020;242:125170. doi: 10.1016/j.chemosphere.2019.125170. [DOI] [PubMed] [Google Scholar]
- 4.Shamskhany A, Li Z, Patel P, Karimpour S. Evidence of microplastic size impact on mobility and transport in the marine environment: a review and synthesis of recent research. Frontiers in Marine Sci. 2021;8:760649. doi: 10.3389/fmars.2021.760649. [DOI] [Google Scholar]
- 5.Corcoran PL. Degradation of microplastics in the environment. Handbook of Microplastics in the Environment; In: Rocha-Santos, T., Costa, M., Mouneyrac, C., editors. Springer International Publishing: Cham, 2020. pp. 1–12.
- 6.Klein S, Dimzon IK, Eubeler J, Knepper TP. Analysis, occurrence, and degradation of microplastics in the aqueous environment. Freshwater microplastics: emerging environmental contaminants? In: Wagner M, Lambert S, editors. The Handbook of Environmental Chemistry; Springer International Publishing: Cham, 2018. pp. 51–67.
- 7.Zhang Q, Xu EG, Li J, Chen Q, Ma L, Zeng EY, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54:3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- 8.Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5:375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamed NNH, Kooi M, Diepens NJ, Koelmans AA. Lifetime accumulation of microplastic in children and adults. Environ Sci Technol. 2021;55:5084–5096. doi: 10.1021/acs.est.0c07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan H, Yue T, Xu Y, Zhao J, Xing B. Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ Sci Technol. 2020;54:12285–12294. doi: 10.1021/acs.est.0c02608. [DOI] [PubMed] [Google Scholar]
- 11.Fournier E, Etienne-Mesmin L, Grootaert C, Jelsbak L, Syberg K, Blanquet-Diot S, et al. Microplastics in the human digestive environment: a focus on the potential and challenges facing in vitro gut model development. J Hazardous Mater. 2021;415:125632. doi: 10.1016/j.jhazmat.2021.125632. [DOI] [PubMed] [Google Scholar]
- 12.Tamargo A, Molinero N, Reinosa JJ, Alcolea-Rodriguez V, Portela R, Bañares MA, Fernández JF, et al. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci Rep. 2022;12:528. doi: 10.1038/s41598-021-04489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Koo BK, Knoblich JA. Human Organoids: Model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d’Aldebert E, Quaranta M, Sébert M, Bonnet D, Kirzin S, Portier G, et al. Characterization of human colon organoids from inflammatory bowel disease patients. Front Cell Dev Biol. 2020;8:363. [DOI] [PMC free article] [PubMed]
- 15.Giandomenico SL, Sutcliffe M, Lancaster MA. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat Protoc. 2021;16:579–602. doi: 10.1038/s41596-020-00433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsui T, Shinozawa T. Human organoids for predictive toxicology research and drug development. Front Genet. 2021;12:767621. doi: 10.3389/fgene.2021.767621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caipa Garcia AL, Arlt VM, Phillips DH. organoids for toxicology and genetic toxicology: applications with drugs and prospects for environmental carcinogenesis. Mutagenesis. 2022;37:143–154. doi: 10.1093/mutage/geab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamargo A, Molinero N, Reinosa JJ, Alcolea-Rodriguez V, Portela R, Banares MA, et al. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci Rep. 2022;12:528. doi: 10.1038/s41598-021-04489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17:57. doi: 10.1186/s12989-020-00387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier E, Etienne-Mesmin L, Grootaert C, Jelsbak L, Syberg K, Blanquet-Diot S, et al. Microplastics in the human digestive environment: a foucus on the potential and challenges facing in vitro gut model development. J Hazard Mater. 2021;415:125632. doi: 10.1016/j.jhazmat.2021.125632. [DOI] [PubMed] [Google Scholar]
- 21.Asmonaite G, Larsson K, Undeland I, Sturve J, Almroth BC. Size matters: ingestion of relatively large microplastics contaminated with environment pollutants posed little risk for fish health and fillet quality. Environ Sci Technol. 2018;52:14381–14391. doi: 10.1021/acs.est.8b04849. [DOI] [PubMed] [Google Scholar]
- 22.Gao N, Huang Z, Xing J, Zhang S, Hou J. Impact and molecular mechanism of microplastics on zebrafish in the presence and absence of copper nanoparticles. Front Marine Sci. 2021;8:762530. doi: 10.3389/fmars.2021.762530. [DOI] [Google Scholar]
- 23.Yong CQY, Vakuyaveettil S, Tang BL. Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health. 2020;17:1509. doi: 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong J, Choi J. Development of AOP relevant to microplastics based on toxicity mechanisms of chemical additives using ToxCastTM and deep learning models combined approach. Envirion Int. 2020;137:105557. doi: 10.1016/j.envint.2020.105557. [DOI] [PubMed] [Google Scholar]
- 25.Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Zheng YF, Bae SH, Huang Z, Chae SU, Jo SJ, Shim HJ, et al. Lack of correlation between in vitro and in vivo studies on the inhibitory effects of (–)-sophoranone on cyp2c9 is attributable to low oral absorption and extensive plasma protein binding of (–)-sophoranone. Pharmaceutics. 2020;12:328. doi: 10.3390/pharmaceutics12040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas Gómez R, Restrepo Valencia P. In vitro-in vivo pharmacokinetic correlation model for quality assurance of antiretroviral drugs. Colomb Med. 2015;46:109–116. doi: 10.25100/cm.v46i3.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng H, Xia T, George S, Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3:1620–1627. doi: 10.1021/nn9005973. [DOI] [PubMed] [Google Scholar]
- 29.Luker T, Alcaraz L, Chohan KK, Blomberg N, Brown DS, Butlin RJ, et al. Strategies to improve in vivo toxicology outcomes for basic candidate drug molecules. Bioorganic Med Chem Lett. 2011;21:5673–5679. doi: 10.1016/j.bmcl.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 30.Festing S, Wilkinson R. The ethics of animal research: talking point on the use of animals in scientific research. EMBO Rep. 2007;8:526–530. doi: 10.1038/sj.embor.7400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ideland M. Different views on ethics: how animal ethics is situated in a committee culture. J Medical Ethics. 2009;35:258–261. doi: 10.1136/jme.2008.026989. [DOI] [PubMed] [Google Scholar]
- 32.Leenaars CHC, Kouwenaar C, Stafleu FR, Bleich A, Ritskes-Hoitinga M, De Vries RBM, et al. Animal to human translation: a systematic scoping review of reported concordance rates. J Transl Med. 2019;17:223. doi: 10.1186/s12967-019-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eliasof S, Lazarus D, Peters CG, Case RI, Cole RO, Hwang J, et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Nat Acad Sci U S A. 2013;110:15127–15132. doi: 10.1073/pnas.1309566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutson MS, Alexander PG, Allwardt V, Aronoff DM, Bruner-Tran KL, Cliffel DE, et al. Organs-on-chips as bridges for predictive toxicology. Appl Vitro Toxicol. 2016;2:97–102. doi: 10.1089/aivt.2016.0003. [DOI] [Google Scholar]
- 35.Nabi SU, Ali SI, Rather MA, Sheikh WM, Altaf M, Singh H, et al. Organoids: a new approach in toxicity testing of nanotherapeutics. J Appl Toxicol. 2022;42:52–72. doi: 10.1002/jat.4206. [DOI] [PubMed] [Google Scholar]
- 36.Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol Reprod. 2017;96:720–732. doi: 10.1095/biolreprod.116.143446. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Gong J, Gao L, Zou T, Kang J, Xu H. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol Environ Saf. 2022;235:113429. doi: 10.1016/j.ecoenv.2022.113429. [DOI] [PubMed] [Google Scholar]
- 38.Yu J. Vascularized organoids: a more complete model. Int J Stem Cells. 2021;14:127–137. doi: 10.15283/ijsc20143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.