Abstract
Immune-related adverse events following treatment with immune checkpoint inhibitors (ICIs) can occur at any time during therapy, with onset occurring most frequently during the first 3 months of treatment. However, they rarely occur after treatment cessation. An awareness of delayed immune-related events following the termination of immunotherapy is paramount for optimal tumour management. The present study reports a case of a 69-year-old male patient with right lung adenocarcinoma. He suffered from psoriasis for ~40 years and was suspected of developing immune checkpoint inhibitor-related pneumonitis (CIP) 6 months after the cessation of treatment with the anti-programmed cell death-1 receptor antibody sintilimab. The present case study is, to the best of our knowledge, the first case of late-onset CIP after the cessation of sintilimab. Subsequently, the report also reviews previously reported cases of late-onset CIP after the cessation of ICI treatment. The present report highlights the finding that CIP can develop, although rarely reported, months or even years after the termination of immunotherapy. Therefore, CIP should always be considered as one of the possibilities and addressed accordingly once the pulmonary infection is ruled out. Careful monitoring, timely diagnosis and administration of corticosteroids are essential in controlling this condition, particularly for patients with pre-existing autoimmune diseases.
Keywords: sintilimab, immune-related adverse events, late-onset, immune checkpoint inhibitor-related pneumonia, case report
Introduction
Over the last decade, immune checkpoint inhibitors (ICIs) have shown considerable potential due to reports of their impressive efficacy in the field of oncotherapy (1), which has resulted in the development of radical novel strategies for treating various malignancies (2). Instead of killing tumour cells by direct exposure, ICIs function by regulating the behaviour of immune cells, such as T-cells (3). Unlike conventional chemotherapeutics, ICIs are generally well tolerated by patients and give rise to a specific but distinct profile of toxicity, namely immune-related adverse events (irAEs) (4). However, despite the promising outcomes of immunotherapy based on ICIs for the majority of solid tumours, irAEs occur in ~70% of patients, which is not negligible and must be addressed (5). Although a wide array of organs and body tissues have been reported to be involved, the skin, gastrointestinal tract, endocrine glands, lung, liver and joints are generally the most frequently affected (6). Whilst the majority of irAEs develop during ongoing therapy (7), irAEs occurring >90 days after the final administration of ICIs, defined as delayed immune-related events (8), are becoming increasingly common. These types of delayed immune-related events include adrenocorticotropic hormone deficiency (9), hypophysitis (10), type 1 diabetes (11,12), hepatitis (13) and thrombocytopenia (14).
Immune checkpoint inhibitor-related pneumonitis (CIP) has not been frequently observed in previous clinical trials testing ICIs (15), despite it being one of the leading causes of ICI-related mortality (16). CIP appears to occur later than other types of irAEs, with a median time to onset of 2.8 months since the initiation of treatment (range, between 9 days to 19.2 months) (17,18). Specific cases of late-onset CIP >90 days following the cessation of ICI treatment are rare according to the literature, with only seven reported to date (15,19-22). Therefore, it remains to be an under-reported adverse event associated with cancer immunotherapy.
The present report documents a case of a male patient with late-onset CIP occurring 6 months after the discontinuation of sintilimab therapy for lung adenocarcinoma. Other previous reports of late-onset CIP were also reviewed to facilitate late-onset CIP characterisation and raise awareness of this condition.
Case report
A 69-year-old male, with a 40-year history of psoriasis for which he was being treated with acitretin, presented with a cough and expectoration, accompanied by bloody sputum for >1 month. He was diagnosed with adenocarcinoma of the lung at a local hospital (Ningjin County Hospital, Xingtai, China; Fig. 1). To get better medical care, he underwent a positron emission tomography (PET)/CT scan at Hebei General Hospital (Shijiazhuang, China; performed in November 2020). They revealed high uptake by the upper lobe of the right lung, consistent with lung cancer. In addition, high uptake by metabolic lymph nodes in the right hilum and right mediastinum was observed, indicating metastasis. The patient subsequently underwent right upper lobectomy and mediastinal lymph node dissection, aided by thoracoscopy, followed by the diagnosis of stage pT2bN2M0 IIIa infiltrating adenocarcinoma. Postoperative recovery was favourable and no antitumor adjuvant therapy was performed.
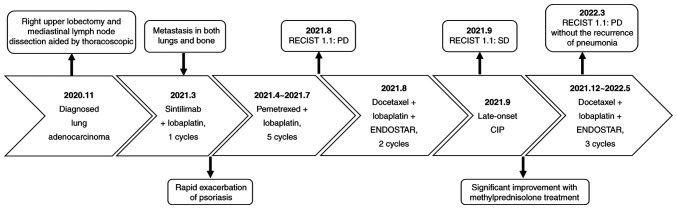
Figure 1.
Timeline of patient diagnosis and treatment of immune checkpoint inhibitor-related pneumonitis. SD, stable disease; PD, progressive disease; ENDOSTAR, recombinant human endostatin; CIP, immune checkpoint inhibitor-related pneumonitis.
In March 2021, a PET/CT (Discovery Elite; GE Healthcare) scan indicated metastasis in both lungs and bone. Next-generation sequencing was therefore performed to detect the statuses of oncogenes, namely EGFR, anaplastic lymphoma kinase, BRAF V600E, c-ros proto-oncogene 1, neurotrophic tyrosine receptor kinase, human epidermal growth factor receptor 2 and tyrosine-protein kinase Met, which revealed no driver mutations. Detection of the tumour mutational burden and programmed death-ligand 1 (PD-L1) expression were not conducted due to financial reasons. Antinuclear antibodies were negative. Administration of sintilimab [200 mg; intravenous drip (IVD)] combined with lobaplatin (50 mg; IVD) and pemetrexed (800 mg; IVD) was recommended as the first-line chemotherapy every 3 weeks for six cycles. However, the patient strongly refused pemetrexed infusion in the first cycle due to severe nausea and vomiting within 5 days after lobaplatin infusion. In the second cycle, sintilimab was subsequently discontinued due to the rapid exacerbation of psoriasis after the first infusion. Psoriasis gradually improved with methylprednisolone treatment (20 mg per day; IVD; 3 days) and remained stable. Pemetrexed was then reintroduced into the chemotherapy regimen from the second cycle as the patient's physical condition improved. A treatment regimen consisting of pemetrexed (800 mg; IVD, every 3 weeks) and lobaplatin (50 mg; IVD, every 3 weeks) was subsequently administered over five cycles from April 2021 to July 2021, before the patient was admitted to Hebei General Hospital (Shijiazhuang, China) for further tests in August (Fig. 1). Unfortunately, results from the CT scan indicated disease progression according to solid tumor response evaluation criteria version 1.1 (RECIST1.1) (23). After first-line therapy, the patient still had a good performance status and was eligible for further platinum combination chemotherapy (24). To minimise adverse effects, the dosage of lobaplatin was lowered. Accordingly, the patient was treated using an individualised scheme of chemotherapy (docetaxel, 100 mg, day 1, IVD; lobaplatin, 40 mg, day 1, IVD) combined with targeted therapy (recombinant human endostatin, 30 mg, day 1-7, IVD) (Fig. 1). The final treatment was scheduled on 28 August 2021, which was well tolerated.
In September 2021, the patient was readmitted to the hospital after complaining of intermittent cough, yellow phlegm and fever. A CT scan (Fig. 2A-a and A-b) showed interstitial changes in both lungs. The tumor condition was assessed as stable disease (RECIST1.1). Routine blood examinations (Sysmex XN-3000 Automated Hematology Analyzer; Sysmex Corporation) revealed white blood cell (WBC) counts to be 10.62x109/l (normal value, 3.5-9.5x109/l) and neutrophil numbers (NEUT#) of 8.72x109/l (normal value, 1.8-6.3x109/l). Considering the presence of pulmonary infection, the patient was administrated with ceftriaxone (2 g, once a day) for 4 days before being subsequently switched to piperacillin-tazobactam (4.5 g, every 8 h) for 6 days when pseudomonas aeruginosa was detected in the sputum samples. Meanwhile, the patient was treated with inhaled budesonide suspension and acetylcysteine solution aerosol to relieve the symptoms.
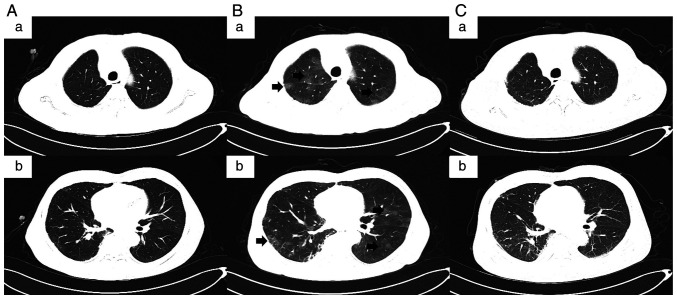
Figure 2.
CT images of the patient. (A-a and A-b) CT images showing interstitial changes in both lungs at admission following 6 months of cessation of immunotherapy (September 2021). (B-a and B-b) CT revealed interstitial changes and multiple ground-glass opacities (indicated by arrows in the images) in both lungs 10 days after anti-infection treatment (September 2021). (C-a and C-b) CT showing progressive improvement of immune checkpoint inhibitor-related pneumonitis after corticosteroid administration without recurrence (December 2021). A-a and A-b, B-a and B-b, and C-a and C-b are two representative examples shown for each case.
After 10 days, routine blood re-examination revealed the following: i) WBC was 4.42x109/l (normal value, 3.5-9.5x109/l); ii) NEUT# was 3.04x109/l (normal value, 1.8-6.3x109/l); iii) C-reactive protein was 58.69 mg/l (normal value, 0-6 mg/l); and iv) normal levels of procalcitonin. Cultures of the patient's blood and sputum were both negative for bacteria. However, the patient remained to be afflicted with an intermittent fever (≤38˚C), cough with shortness of breath and occasionally yellow phlegm. Considering the history of ICI treatment, the development of CIP was not excluded. Therefore, methylprednisolone sodium succinate (80 mg per day) was injected before chest CT was performed on that day.
CT scans (Fig. 2B-a and B-b) revealed interstitial changes in both lungs in addition to multiple ground-glass opacities, mainly involving the periphery of both lungs. However, they did not support bacterial infection, and tumour progression could also be ruled out. Further analysis of the bronchoalveolar lavage fluid (BALF) revealed lymphocytosis in 30% of lymphocytes, with no evidence of infection according to the microbiological culture and PCR testing. Infectious pneumonia induced by tuberculosis bacteria, fungi and viruses were therefore eliminated. In addition, 3 days of methylprednisolone treatment resulted in a rapid improvement of clinical symptoms without fever or shortness of breath whilst reducing the frequency of coughing. The combination of clinical manifestations, CT imaging and microbiological assays strongly indicated that the patient's medical condition was consistent with sintilimab-induced CIP, defined as grade two according to American Society of Clinical Oncology Clinical Practice Guideline for management of irAEs in patients treated with ICIs (25). Antibiotics were discontinued and the patient was treated continuously with methylprednisolone (40 mg per day) for a further 3 days, resulting in significant improvement on CT scans. Before discharge, the patient was switched to oral prednisolone (30 mg per day), which was gradually lowered to 2.5 mg per day over 6 weeks without the recurrence of CIP. After the remission of pneumonia, the patient had also been treated with piperacillin sodium and tazobactam sodium for addressing urinary tract infections at Ningjin County Hospital (Xingtai, China) in November 2021, which did not exacerbate the interstitial changes in the lungs. In December 2021, the patient was readmitted to Hebei General Hospital (Shijiazhuang, China). CT scans (Fig. 2C-a and C-b) showed progressive improvement of CIP after corticosteroid administration without recurrence. In the follow-up treatment (between December 2021 and May 2022), the patient continued to receive docetaxel and lobaplatin combined with recombinant human endostatin without the recurrence of pneumonia. The tumor condition was assessed as progressive disease (RECIST1.1) in March 2022. The Naranjo's Probability Scale for Adverse Drug Reactions was used to evaluate the patient's condition (Table I) (26). A score of seven was obtained, classifying sintilimab as the probable cause of the patient's late-onset CIP.
Table I.
Naranjo score of the probability that the late-onset immune checkpoint inhibitor-related pneumonitis was the result of the cessation of treatment with sintilimab.
| Naranjo scoring system for adverse reaction | |
|---|---|
| Question | Answer (points) |
| 1. Are there previous conclusive reports on this reaction? | Yes (1) |
| 2. Did the adverse events appear after the suspected drug was given? | Yes (2) |
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? | Yes (1) |
| 4. Did the adverse reaction appear when the drug was readministered? | Not performed (0) |
| 5. Are there alternative causes that could have caused the reaction? | No (2) |
| 6. Did the reaction reappear when a placebo was given? | Not performed (0) |
| 7. Was the drug detected in any body fluid in toxic concentrations? | Not performed (0) |
| 8. Was the reaction more severe when the dose was increased or less severe when the dose was decreased? | Not performed (0) |
| 9. Did the patient have a similar reaction to the same or similar drugs in a previous exposure? | Not performed (0) |
| 10. Was the adverse event confirmed by any objective evidence? | Yes (1) |
| Score=7 (Probable) | |
Literature review
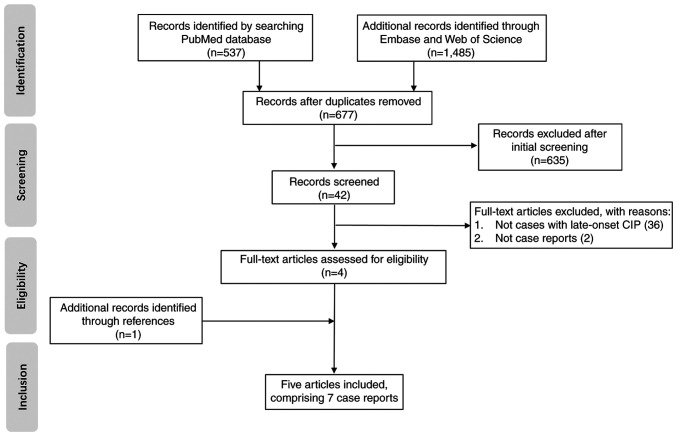
Published studies were identified by searching PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com) and Web of Science (https://www.webofscience.com) until September 2022 for the following combination of terms: (‘immunotherapy’ OR ‘checkpoint inhibitors’ OR ‘checkpoint blockade’ OR ‘anti-CTLA 4’ OR ‘anti-PD-1’ OR ‘anti-PD-L1’ OR ‘ipilimumab’ OR ‘nivolumab’ OR ‘pembrolizumab’ OR ‘sintilimab’ OR ‘camrelizumab’ OR ‘toripalimab’ OR ‘atezolizumab’ OR ‘durvalumab’ OR ‘avelumab’ OR ‘cemiplimab’) AND ‘after’ AND (‘cessation’ OR ‘discontinuation’) AND (‘immune-related adverse event’ OR ‘immune checkpoint inhibitor-related pneumonitis’ OR ‘pneumonitis’). In addition, the reference lists of the retrieved articles were also searched. All studies identified by this procedure were reviewed independently by two reviewers (YPW and YSY) and any disagreements were resolved through discussion. Case reports and case series published in journals or those presented at conferences were included. Included studies reported patients who developed CIP, which manifested ≥90 days after the discontinuation of immunotherapy. Pharmacokinetic/pharmacodynamic studies and randomized controlled trials were excluded due to lacking detailed data of each patient. Furthermore, case series that lacked detailed patient data (≥3 terms of patient's disease, age, sex, type and cycles of ICIs, off-treatment interval treatment, intervention and outcome were missing) were also excluded. A total of five studies (15,19-22) were identified in the review. A total of 3 cases were described in one study (20), bringing the total number of case reports of late-onset CIP to 7. Fig. 3 shows a flow diagram of the screening and selection process.
Figure 3.
Flow diagram of the screening and selection process for the literature review. CIP, immune checkpoint inhibitor-related pneumonitis.
The major clinical features of reported in the seven cases of late-onset CIP that qualified from the present screening process are summarized in Table II (15,19-22). The median patient age (three females and five males) was 63 years (range, 25-69 years). Immunotherapy included nivolumab (n=4) (19-21), pembrolizumab (n=1) (20), atezolizumab (n=1) (15), sintilimab (n=1) and not specified (n=1) (22). The types of cancer treated were stage IV adenocarcinoma of the lung (n=3) (20), stage III melanoma (n=2) (19,22), stage IV squamous cell carcinoma of the lung (n=1) (20), metastatic renal cell carcinoma (n=1) (21) and metastatic para-osteal osteosarcoma (n=1) (15). In particular, the majority of the cases of late-onset CIP occurred during the recurrent or metastatic stages. Notably, the median off-treatment interval for late-onset CIP was 6.5 months (range, 4-28 months) whereas the median cumulative immunotherapy exposure was four doses (range, 1-35 doses). Prior on-treatment irAEs (5/8), including abnormal liver function (n=1), pneumonitis (n=2), colitis (n=1) and exacerbation of psoriasis (n=1), was the most common cause of immunotherapy discontinuation. Clinicians should be mindful of recurrent CIP, since two patients experienced an intermittent recurrence of CIP over a timescale of months or even years following ICI cessation (15,20). In addition, one patient with late-onset CIP concurrently suffered from delayed hepatitis and renal dysfunction (21). CIP (3-4 grade) was present in only two cases (20,22) whereas grades 1 and 2 were observed in the other six. Among the eight patients with late-onset CIP, seven were managed with corticosteroids with the addition of mycophenolate mofetil for one patient. A clear improvement was observed in seven of the eight enrolled patients, but one patient succumbed to CIP despite the administration of high doses of methylprednisolone (20).
Table II.
Clinical data of patients presenting with late-onset CIP following treatment cessation.
| First authors, year | Patient's disease | Age, years | Sex | Type of ICIs | Combinati on medication | Cycles of ICIs | Off-treatment interval | Reasons for discontinuation | CIP grade | Treatments | Outcome | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diamantopoulos et al, 2017 | Melanoma | 62 | F | Nivolumab | Monotherapy | 5 | 6 months | Abnormal liver function | 2 | Moxifloxacin and prednisone | Improved | (19) |
| Kimura et al, 2021 | Adenocarcinoma | 62 | M | Nivolumab | Monotherapy | 4 | 7 months | Financial reasons | 2 | Prednisolone | Improved | (20) |
| Kimura et al, 2021 | Squamous cell carcinoma | 68 | M | Nivolumab | Monotherapy | 2 | 8 months | Pneumonitis | 1 | No treatment | Improved | (20) |
| Kimura et al, 2021 | Adenocarcinoma | 69 | M | Pembrolizumab | Monotherapy | 4 | 4 months | Progression of brain metastases | 4 | Methylprednisolone | Succumbed to pneumonitis | (20) |
| Nakai et al, 2021 | Renal cell carcinoma | 50 | M | Nivolumab | Monotherapy | Not specified | 142 days | Multiple metastases of tumour | 2 | Methylprednisolone and mycophenolate mofetil | Improved | (21) |
| Kucukarda et al, 2022 | Osteosarcoma | 25 | F | Atezolizumab | Monotherapy | 35 | 24 months | Pneumonitis | 2 | Methylprednisolone | Improved | (15) |
| Mandala et al, 2018 | Melanoma | 64 | F | Not specified | Monotherapy | Not specified | 8 months | Colitis | 3 | Wide-spectrum antibiotics and methylprednisolone | Improved | (22) |
| Present study | Adenocarcinoma | 69 | M | Sintilimab | Lobaplatin | 1 | 6 months | Exacerbation of psoriasis | 2 | Methylprednisolone | Improved | (-) |
M, male; F, female; CIP, immune checkpoint inhibitor-related pneumonitis; ICI, immune checkpoint inhibitor.
Discussion
CIP is one of the major causes of ICI-associated mortality (16). Symptoms of CIP include dry cough, shortness of breath with exertion, reduced oxygen saturation and bilateral ground-glass opacities or patchy nodular infiltrations in the lung on CT imaging (27,28). The incidence of pneumonia secondary to ICIs is <5%, with fatal CIP being reported in 0.2-0.5% patients (16,29). Although relatively infrequent in occurrence, CIP is complex and unpredictable in terms of both clinical and radiological manifestations, which may overlap with those of COVID-19 or other viral infections (30). This therefore provide a challenge for oncologists during the early diagnosis of lung diseases (30). Diagnosis of CIP is considered to be a ‘process of elimination’ in the majority of cases (31). Radiation-induced pneumonitis, all types of infectious pulmonary inflammation and lung cancer progression, should all be considered and excluded (2). If making a differential diagnosis is difficult, CIP can be confirmed by bronchoscopy or lung biopsy (32). Lymphocytosis in BALF samples without evidence of infectious aetiologies can facilitate the confirmation of this diagnosis (32). However, late-onset CIP after ICI cessation is easily misdiagnosed due to the brief patient exposure to ICI and long ICI-free interval, interventions overlapping with the toxicity patterns, reduced medical vigilance after discontinuing treatment and the lengthy process of diagnosis-by-elimination (8).
In the present case report, the patient was admitted due to fever and coughing with sputum, which persisted after anti-infection treatment. However, the patient showed neither signs of tumour progression nor infections by tuberculosis bacteria, fungi or viruses. The patient had no previous history of autoimmune diseases other than psoriasis, whereas antinuclear antibodies tested negative before sintilimab initiation. Psoriasis remained stable when the patient developed pneumonia. To the best of our knowledge, there were no previous reports in the literature associating psoriasis with interstitial pneumonia. It would have been interesting to explore drug-induced interstitial lung disease (DIILD). To date, >400 drugs have been reported to cause DIILD, with anti-cancer drugs, rheumatology drugs, amiodarone and antibiotics being the most common causes (33,34). On referring to the patient's treatment and medication history, docetaxel, recombinant human endostatin, piperacillin sodium and tazobactam sodium have been reported to cause pneumonia or interstitial pneumonia in the medicine specifications or literature (35,36). In fact, after remission for pneumonia, the patient had also been treated with β-lactam drugs for infections of the urinary tract at a local hospital and continued to receive three courses of docetaxel and recombinant human endostatin in follow-up treatment, without the recurrence of pneumonia. Therefore, pneumonia induced by chemotherapy, targeted therapy or antibiotics could be excluded. The patient received one dose of the sintilimab immunotherapeutic 6 months previously (March 2021). Methylprednisolone treatment resulted in a significant improvement of pneumonia in both clinical and radiological manifestations. Therefore, all evidence pointed to late-onset CIP caused by the administration of sintilimab.
Sintilimab, co-developed by Innovent Biologics and Eli Lilly, is a fully humanized IgG4 anti-programmed cell death protein 1 (PD-1) monoclonal antibody (37). Sintilimab binds to PD-1 and restores endogenous antitumour T-cell responses by blocking the interaction of PD-1with its ligands PD-L1 and PD-L2(37). The primary adverse events associated with sintilimab treatment reported in clinical trials are similar to those following nivolumab and pembrolizumab treatment, which include pyrexia, hypothyroidism, hepatitis and CIP (38). The occurrence of irAEs in general may be attributed to the increased activity of immune cells that target antigens common to both tumours and normal tissues (39), which might also serve a role in the development of CIP. Enrichment of CD8+ T or CD4+ T-lymphocytes, along with high expression levels of PD-1, have been detected in the lung or bronchoalveolar lavage samples of patients with CIP in several previous studies (40,41). When the immune homeostasis of the lung is altered, typically characterised by lymphocyte infiltration, an autoimmune reaction may be triggered, with the first key reaction occurring when the lymphocytes are being assembled (42). However, the complexity of the subject is underlined by the fact that radiotherapy, pulmonary infection, cryoablation of lung metastasis or chemotherapy can all alter immune homeostasis and cause hyperactivation of the immune system, leading to CIP (42).
Individuals with pre-existing autoimmune diseases may harbour a genetic susceptibility, leading to a significantly increased risk of irAEs (43,44). Psoriasis is a common immune-mediated skin condition (45). In a retrospective cohort study, Halle et al (46) noted that 57% patients with pre-existing psoriasis experienced flare-ups after receiving ICIs, including cutaneous flare, exacerbation of arthritis and exacerbation of iritis, whereas 59% experienced other irAEs. The patient in the present case report had a 40-year history of psoriasis, which was aggravated by sintilimab immunotherapy. It is considered that pre-existing autoimmune diseases, previous exposure to cytotoxic drugs and pulmonary damage from cancer and inflammation are major risk concerns for the patient in contracting CIP.
Late-onset CIP remains an under-recognized and complex diagnostic challenge. The lasting effects of immunotherapy, even after ICI withdrawal, have been verified by several studies (47-50) and may be associated with the underlying mechanism of immunological ‘memory’ (48). If a long-term immunological ‘memory’ with a positive outcome does exist, it may also have implications for late-occurring T cell-mediated toxicities (22,27). Brahmer et al (51) previously reported a serum half-life of 12-20 days for nivolumab after a single dose treatment, with a mean PD-1 receptor occupancy on T-cell plateaus of 72% for ≤57 days. This suggests that the long-lasting pharmacodynamic effects of ICIs generally outlasts their pharmacokinetic half-life (27,51). Furthermore, the receptor occupancy remained at 40% for >8 months after three doses (51). This finding may explain the median off-treatment interval to late-onset CIP of 6.5 months following the cessation of immunotherapy in the present study. Compared with nivolumab, sintilimab has a higher binding affinity with PD-1 molecules (52,53). Wang et al (53) reported a PD-1 receptor occupancy by sintilimab of >95% in patients 4 weeks after a single intravenous infusion. This high binding affinity and sustained receptor occupancy may contribute to persistent T-cell activation following the cessation of therapy even when serum levels of this drug become undetectable.
The present case report specifically highlights the finding that CIP can manifest in a period following the cessation of immunotherapy, even following patient exposure to only one dose. The median off-treatment interval to late-onset CIP was 6.5 months (range, 4-28 months), which is consistent with the finding made by Couey et al (8). Careful monitoring, timely diagnosis and administration of corticosteroids are essential for controlling the condition. Clinical practice guidelines for the management of toxicities from immunotherapy allude to monitoring for delayed immune-related events ≥12 months following the discontinuation of ICI-based treatment (54,55). However, to the best of our knowledge, studies on delayed immune-related events after discontinuation of immunotherapy are scarce due to limited follow-up periods (8). It would have been interesting to explore stringent evidence-based surveillance protocols. Individualized surveillance strategies based on a patient's risk profile for irAEs after treatment cessation are recommended, particularly for those patients with pre-existing autoimmune diseases.
It should be noted that a platinum-based dual-drug regimen, is generally started at 4-6 weeks after surgery and no later than 3 months. Unfortunately, the present patient was not admitted on time due to disruption caused by the COVID-19 pandemic, personal and objective factors. It is considered that the delay is one of the major reasons for the recurrence and metastasis of the tumour only 4 months after surgery. Further accumulation of case data is required to both enhance confidence in diagnostic predictors and to improve the clinical outcomes of late-onset CIP following ICI cessation.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YW and YY analysed and interpreted the data, performed the literature review, and were major contributors in drafting the manuscript. XY and LF collected the clinical data and performed the literature research. JS participated in the data acquisition and interpretation, was involved in drafting the manuscript and critically revised the manuscript. YW and YY confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Hebei General Hospital (Shijiazhuang, China) and informed consent was obtained from the patient.
Patient consent for publication
The patient provided written informed consent regarding the publication of the case details and any associated images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bai J, Li D, Yang P, Xu K, Wang Y, Li Q, Liu J, Du W, Zhang F, Feng R. Camrelizumab-Related myocarditis and myositis with myasthenia gravis: A case report and literature review. Front Oncol. 2022;11(778185) doi: 10.3389/fonc.2021.778185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin B, Xiao J, Li J, Liu X, Wang J. Immune-related organizing pneumonitis in non-small cell lung cancer receiving PD-1 inhibitor treatment: A case report and literature review. J Cancer Res Ther. 2020;16:1555–1559. doi: 10.4103/jcrt.JCRT_971_20. [DOI] [PubMed] [Google Scholar]
- 3.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 2019;58 (Suppl 7):vii59–vii67. doi: 10.1093/rheumatology/kez308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Liang D, Liu J, Zeng J, Zeng Y. The breakthroughs in cancer immune checkpoint based therapy: A review of development in immune checkpoint study and its application. Comb Chem High Throughput Screen. 2017;20:430–439. doi: 10.2174/1386207320666170315121728. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Zhou C, Yuan F, Guo L, Yang L, Shi Y, Zhang J. Case report: Severe immune-related cholestatic hepatitis and subsequent pneumonia after pembrolizumab therapy in a geriatic patient with metastic gastric cancer. Front Med (Lausanne) 2021;8(719236) doi: 10.3389/fmed.2021.719236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safa H, Bhosale P, Weissferdt A, Oliva ICG. Difficulties in differentiating between checkpoint inhibitor pneumonitis and lung metastasis in a patient with melanoma. Immunotherapy. 2020;12:293–298. doi: 10.2217/imt-2019-0122. [DOI] [PubMed] [Google Scholar]
- 8.Couey MA, Bell RB, Patel AA, Romba MC, Crittenden MR, Curti BD, Urba WJ, Leidner RS. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: Diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7(165) doi: 10.1186/s40425-019-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeno A, Yamamoto M, Morita M, Tanaka S, Kanazawa I, Yamauchi M, Kaneko S, Sugimoto T. Late-onset isolated adrenocorticotropic hormone deficiency caused by nivolumab: A case report. BMC Endocr Disord. 2019;19(25) doi: 10.1186/s12902-019-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoniou S, Bazazo G, Rockl L, Papadakis M, Berg C. Late-onset hypophysitis after discontinuation of nivolumab treatment for advanced skin melanoma: A case report. BMC Endocr Disord. 2021;21(191) doi: 10.1186/s12902-021-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mae S, Kuriyama A, Tachibana H. Diabetic ketoacidosis as a delayed immune-related event after discontinuation of nivolumab. J Emerg Med. 2021;60:342–344. doi: 10.1016/j.jemermed.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Yaura K, Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Fulminant type 1 diabetes mellitus developed about half a year after discontinuation of immune checkpoint inhibitor combination therapy with nivolumab and ipilimumab: A case report. Tohoku J Exp Med. 2021;254:253–256. doi: 10.1620/tjem.254.253. [DOI] [PubMed] [Google Scholar]
- 13.Kanaoka K, Moriizumi K, Okada H, Iwahashi K, Tsuji H, Yasuoka H, Minami S. Pembrolizumab-Induced delayed-onset hepatitis. Case Rep Gastroenterol. 2020;14:586–592. doi: 10.1159/000509953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu S, Wang T, Xu F. Delayed immune thrombocytopenia after discontinuation of nivolumab therapy: A case report and literature review. J Oncol Pharm Pract. 2021;27:1548–1552. doi: 10.1177/1078155220981155. [DOI] [PubMed] [Google Scholar]
- 15.Kucukarda A, Gokmen I, Ozcan E, Peker P, Akgul F, Cicin I. Recurrent delayed immune-related pneumonitis after immune-checkpoint inhibitor therapy for advanced osteosarcoma. Immunotherapy. 2022;14:395–399. doi: 10.2217/imt-2021-0275. [DOI] [PubMed] [Google Scholar]
- 16.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 18.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamantopoulos PT, Gaggadi M, Kassi E, Benopoulou O, Anastasopoulou A, Gogas H. Late-onset nivolumab-mediated pneumonitis in a patient with melanoma and multiple immune-related adverse events. Melanoma Res. 2017;27:391–395. doi: 10.1097/CMR.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 20.Kimura H, Sone T, Araya T, Murata A, Yamamura K, Ohkura N, Hara J, Abo M, Kasahara K. Late-onset programmed cell death protein-1 inhibitor-induced pneumonitis after cessation of nivolumab or pembrolizumab in patients with advanced non-small cell lung cancer: A case series. Transl Lung Cancer Res. 2021;10:1576–1581. doi: 10.21037/tlcr-20-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai Y, Otsuka T, Inoue T, Nawa T, Hatano K, Yamamoto Y, Nagahara A, Nakayama M, Kakimoto KI, Nishimura K. Two cases of delayed onset of immune-related adverse events after discontinuation of nivolumab in patients with metastatic renal cell cancer. IJU Case Rep. 2021;4:326–329. doi: 10.1002/iju5.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandala M, Merelli B, Indriolo A, Tondini C. Late-occurring toxicity induced by an immune checkpoint blockade in adjuvant treatment of a stage III melanoma patient. Eur J Cancer. 2018;95:130–132. doi: 10.1016/j.ejca.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Yi Y, Liu Z, Fang L, Li J, Liu W, Wang F, Fu P, Xie C, Liu J, Song B. Comparison between single-agent and combination chemotherapy as second-line treatment for advanced non-small cell lung cancer: A multi-institutional retrospective analysis. Cancer Chemother Pharmacol. 2020;86:65–74. doi: 10.1007/s00280-020-04091-3. [DOI] [PubMed] [Google Scholar]
- 25.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu Y, Wang Z, Feng J, Wang L, Liu H, Liu D, Zhao Y, Yu R, Li W, Sun D, Yu H. Pneumonitis, appendicitis, and biliary obstruction during toripalimab treatment in a patient with extensive-stage small-cell lung cancer: A case report. Ann Palliat Med. 2021;10:9267–9275. doi: 10.21037/apm-21-858. [DOI] [PubMed] [Google Scholar]
- 29.Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, Pennell NA, Velcheti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan RJ, Johnson DB, Rini BI, Neilan TG, Lovly CM, Moslehi JJ, Reynolds KL. COVID-19 and immune checkpoint inhibitors: Initial considerations. J Immunother Cancer. 2020;8(e000933) doi: 10.1136/jitc-2020-000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Lou A, Yu J. Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: A case report and review of literature. Ann Palliat Med. 2021;10:8460–8466. doi: 10.21037/apm-21-23. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Guo X, Zhou J, Li Y, Duan L, Si X, Zhang L, Liu X, Wang M, Shi J, Zhang L. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer. 2020;11:191–197. doi: 10.1111/1759-7714.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skeoch S, Weatherley N, Swift AJ, Oldroyd A, Johns C, Hayton C, Giollo A, Wild JM, Waterton JC, Buch M, et al. Drug-Induced interstitial lung disease: A systematic review. J Clin Med. 2018;7(356) doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spagnolo P, Bonniaud P, Rossi G, Sverzellati N, Cottin V. Drug-induced interstitial lung disease. Eur Respir J. 2022;60(2102776) doi: 10.1183/13993003.02776-2021. [DOI] [PubMed] [Google Scholar]
- 35.Min BD, Kang HW, Kim WT, Kim YJ, Yun SJ, Lee SC, Kim WJ. Docetaxel-induced fatal interstitial pneumonitis in a patient with castration-resistant prostate cancer. Korean J Urol. 2012;53:371–374. doi: 10.4111/kju.2012.53.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CJ, Chang HT, Chang CY. Docetaxel-related interstitial pneumonitis. Ther Clin Risk Manag. 2015;11:1813–1816. doi: 10.2147/TCRM.S90488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoy SM. Sintilimab: First global approval. Drugs. 2019;79:341–346. doi: 10.1007/s40265-019-1066-z. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, Zhang W, Gao Y, Jin Z, Zhou J, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6:e12–e19. doi: 10.1016/S2352-3026(18)30192-3. [DOI] [PubMed] [Google Scholar]
- 39.Shibata Y, Murakami S, Kato T. Overview of checkpoint inhibitor pneumonitis: Incidence and associated risk factors. Expert Opin Drug Saf. 2021;20:537–547. doi: 10.1080/14740338.2021.1898584. [DOI] [PubMed] [Google Scholar]
- 40.Leroy V, Templier C, Faivre JB, Scherpereel A, Fournier C, Mortier L, Wemeau-Stervinou L. Pembrolizumab-induced pneumonitis. ERJ Open Res. 2017;3:00081–2016. doi: 10.1183/23120541.00081-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N, Guisier F, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(1700050) doi: 10.1183/13993003.00050-2017. [DOI] [PubMed] [Google Scholar]
- 42.Pozzessere C, Bouchaab H, Jumeau R, Letovanec I, Daccord C, Bourhis J, Prior JO, Peters S, Lazor R, Beigelman-Aubry C. Relationship between pneumonitis induced by immune checkpoint inhibitors and the underlying parenchymal status: A retrospective study. ERJ Open Res. 2020;6:00165–2019. doi: 10.1183/23120541.00165-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21–29. doi: 10.1016/j.ejca.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, Bersanelli M, Michiara M, Grassadonia A, Brocco D, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: A real-world transverse study. Oncologist. 2019;24:e327–e337. doi: 10.1634/theoncologist.2018-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 46.Halle BR, Betof Warner A, Zaman FY, Haydon A, Bhave P, Dewan AK, Ye F, Irlmeier R, Mehta P, Kurtansky NR, et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: Safety and efficacy. J Immunother Cancer. 2021;9(e003066) doi: 10.1136/jitc-2021-003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura H, Sone T, Murata A, Koba H, Tambo Y, Hara J, Abo M, Kasahara K. Long-lasting shrinkage in tumour mass after discontinuation of nivolumab treatment. Lung Cancer. 2017;108:7–8. doi: 10.1016/j.lungcan.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Pesola G, Murianni V, Rebuzzi SE, Banna GL, Cerbone L, Catalano F, Borea R, Gandini A, Cremante M, Puglisi S, et al. Durable response after immunotherapy discontinuation for delayed and severe immune-related adverse events: A case report. Immunotherapy. 2021;13:1379–1386. doi: 10.2217/imt-2021-0085. [DOI] [PubMed] [Google Scholar]
- 49.Umihira S, Koyanagi T, Tamura K, Takahashi Y, Yoshiba T, Takahashi S, Taneichi A, Saga Y, Takei Y, Fujiwara H. Durable response after the discontinuation of pembrolizumab treatment due to an adverse event in a patient with advanced endometrial cancer: A case report. Exp Ther Med. 2022;23(409) doi: 10.3892/etm.2022.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eguchi S, Shinkawa H, Sato Y, Nakai K, Takemura S, Tanaka S, Amano R, Kimura K, Ohira G, Nishio K, et al. Durable response after discontinuation of pembrolizumab therapy for intrahepatic cholangiocarcinoma: A case report. Clin J Gastroenterol. 2021;14:858–865. doi: 10.1007/s12328-021-01396-5. [DOI] [PubMed] [Google Scholar]
- 51.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumours: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Lin W, Tan F, Li N, Xue Q, Gao S, Gao Y, He J. Sintilimab for the treatment of non-small cell lung cancer. Biomark Res. 2022;10(23) doi: 10.1186/s40364-022-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J, Fei K, Jing H, Wu Z, Wu W, Zhou S, Ni H, Chen B, Xiong Y, Liu Y, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11:1443–1451. doi: 10.1080/19420862.2019.1654303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 (suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. ESMO Guidelines Committee. [DOI] [PubMed] [Google Scholar]
- 55.Phan T, Patwala K, Lipton L, Knight V, Aga A, Pianko S. Very delayed acute hepatitis after pembrolizumab therapy for advanced malignancy: How long should we watch? Curr Oncol. 2021;28:898–902. doi: 10.3390/curroncol28010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author on reasonable request.