Abstract
The lateral septum (LS) is a basal forebrain GABAergic region that is implicated in social novelty. However, the neural circuits and cell signaling pathways that converge on the LS to mediate social behaviors aren’t well understood. Multiple lines of evidence suggest that signaling of brain-derived neurotrophic factor (BDNF) through its receptor TrkB plays important roles in social behavior. BDNF is not locally produced in LS, but we demonstrate that nearly all LS GABAergic neurons express TrkB. Local TrkB knock-down in LS neurons decreased social novelty recognition and reduced recruitment of neural activity in LS neurons in response to social novelty. Since BDNF is not synthesized in LS, we investigated which inputs to LS could serve as potential BDNF sources for controlling social novelty recognition. We demonstrate that selectively ablating inputs to LS from the basolateral amygdala (BLA), but not from ventral CA1 (vCA1), impairs social novelty recognition. Moreover, depleting BDNF selectively in BLA-LS projection neurons phenocopied the decrease in social novelty recognition caused by either local LS TrkB knockdown or ablation of BLA-LS inputs. These data support the hypothesis that BLA-LS projection neurons serve as a critical source of BDNF for activating TrkB signaling in LS neurons to control social novelty recognition.
Subject terms: Neurotrophic factors, Social neuroscience
Introduction
Social deficits are prevalent in many psychiatric and neurodevelopmental disorders, including autism [1, 2] and schizophrenia [3]. While changes in social behavior manifest differently across these disorders, deficits in attention to socially salient stimuli and social recognition are well documented [3–5]. Mice are a highly social species that display robust responses to novel conspecifics, such as attention to, and investigation of, novel individuals [6, 7]. The three-chamber social approach task, which measures the amount of time a mouse spends in close proximity with another mouse, was developed and validated to assess social novelty, social recognition, and social preference [8]. Although rodent models cannot fully recapitulate the complexity of human social behavior, they are important for studying neural circuits that are linked to social behaviors observed in human disorders.
The lateral septum (LS) is a spatially complex, predominantly GABAergic region that extends across a significant portion of the rostral-caudal axis of the basal forebrain, and is subdivided into dorsal, intermediate and ventral subregions [9]. The LS is a potent modulator of social behaviors in humans [10–13] and rodents [9, 14] with documented roles in social saliency [15, 16], social recognition [17–20], and social aggression [21, 22]. The LS is innervated by many brain regions with established roles in social behaviors, including the basolateral amygdala (BLA) [20, 23], ventral hippocampus CA1 (vCA1) [24], ventral tegmental area [9, 25], as well as the infralimbic and prelimbic cortices [9, 26, 27]. However, the inputs to the LS that mediate social recognition are not well understood, and the cell-signaling mechanisms in the LS that transmit this social information remain unclear.
Multiple lines of evidence suggest that brain-derived neurotrophic factor (BDNF) is required for a variety of social behaviors [28–31]. Signaling through its cognate receptor tropomyosin kinase B (TrkB), BDNF regulates dendritic morphology, synapse formation and synaptic plasticity [32–34]. Although GABAergic neurons rarely synthesize BDNF, they robustly express TrkB [35, 36], and BDNF plays a critical role in supporting their function [37–41]. For example, GABAergic neurons rely on BDNF released from other cell types to activate TrkB signaling, which is critical for their maturation and physiological function [40, 42–44]. In line with its GABAergic composition, the LS is largely devoid of Bdnf expression, but projection neurons innervating the LS densely express BDNF [45, 46], suggesting they serve as a BDNF source for the region. Previous research showed that BDNF-expressing catecholaminergic projections to the LS influence the morphology and gene expression of a subpopulation of calbindin-expressing LS neurons [47]. However, whether BDNF-TrkB signaling in the LS is important for the social behaviors mediated by the LS has not been investigated.
We hypothesized that (1) TrkB signaling in LS GABAergic neurons is important for regulating social behavior, and that (2) neuronal projections from BDNF-rich limbic regions serve as a source of BDNF to activate TrkB signaling in LS to control social behavior. Key candidate regions included the BLA and vCA1 because both regions highly express BDNF [45], send projections to the LS [21, 23, 27], and have established roles in regulating both social novelty [9, 16, 20, 48] and recognition [24, 49, 50]. Our data demonstrate that local TrkB knockdown in the LS is critical for social novelty recognition behavior in mice. Moreover, we identify BLA-LS projections as critical for these behaviors, and show that this behavior depends on BDNF expression within these projection neurons. In summary, the data demonstrate a role for LS TrkB signaling in social novelty recognition, and support the possibility that BLA-LS neurons serve as a source of BDNF for activating LS TrkB receptors to control this behavior.
Materials and methods
Animals
Wild-type mice (C57BL/6 J; stock # 000664) and mice carrying a loxP-flanked Bdnf allele (Bdnftm3Jae; stock # 004339, referenced in text as BDNFfl/fl) were purchased from The Jackson Laboratory. Mice carrying a loxP-flanked TrkB allele (strain fB/fB, referenced in text as TrkBfl/fl [51, 52]) were used for some experiments. TrkBfl/fl mice were maintained on a C57BL/6 J background. Adult male mice were housed in a temperature-controlled environment with a 12:12 light/dark cycle and ad libitum access to food and water. All experimental animal procedures were approved by the Sobran Biosciences Institutional Animal Care and Use Committee.
Behavior
The three-chamber social interaction test arena consisted of three adjacent chambers separated by two clear plastic dividers, and connected by two open doorways. Mice were habituated for 3 consecutive days (10 min per day) prior to testing days. Mice were transferred to the testing room 1 h prior to testing. The test consisted of 5 min of habituation and two 10 min sessions (trial 1 and 2) with a 5 min inter-trial-interval. The subject mouse was kept in the center chamber during habituation. In the first 10 min session (trial 1), the subject mouse was allowed to freely investigate the three chambers. A novel mouse (stranger 1) was placed under an inverted metal cup in one side chamber, an identical empty cup was placed in the other side chamber. After the trial 1 session, the doorways were closed and the subject mouse was kept in the center chamber for 5 min. In the second session (trial 2), another novel object mouse (stranger 2) was put in the empty cup and the subject mouse freely investigated the three chambers. Age-matched adult C57BL/6 J male mice were used as object mice in this study, and housed under the same conditions as the subject mice. Each object novel/stranger mouse was used once a day in the three-chamber social interaction test. Time spent sniffing each cup was manually scored as interaction time in a blind manner using the Stopwatch+ program developed by the Center for Behavioral Neuroscience (cbn-atl.org) at Emory University [53, 54].
For full description of methods, see Supplementary information.
Results
Local knockdown of LS TrkB expression
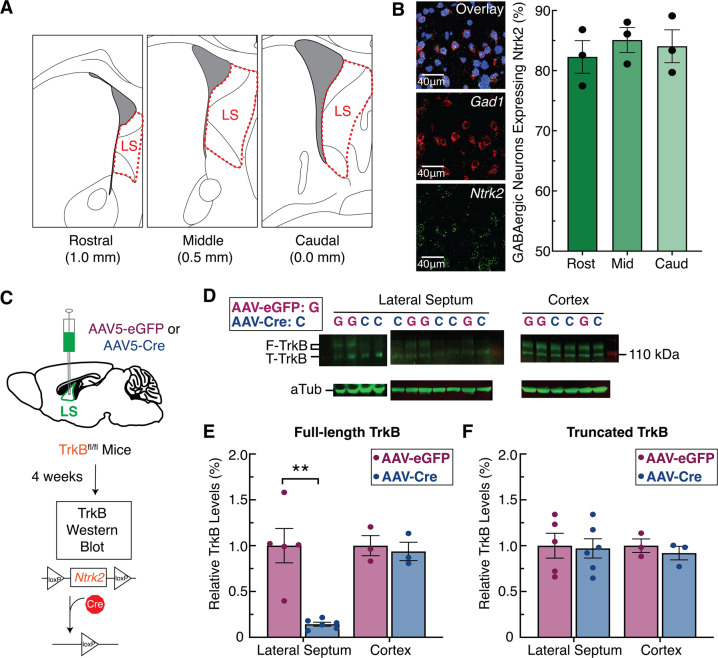
We used RNAscope single-molecule fluorescent in-situ hybridization to quantify expression of the gene encoding TrkB (Ntrk2 probe) in GABAergic neurons (Gad1 probe) across the rostral-caudal axis of the LS (Fig. 1A, B [55]). These data revealed that in wild-type mice, nearly all LS GABAergic neurons (85–90%) expressed Ntrk2, a pattern that was consistent across the rostral-caudal axis (Fig. 1B). Additionally, we confirmed that LS cells do not express Bdnf (Fig. S1A–B). Next, we designed a genetic manipulation strategy to knockdown TrkB expression specifically in the LS. Specifically, for experimental animals we bilaterally injected a cre recombinase-expressing virus (AAV5-EF1a:cre) into the LS of mice carrying a floxed TrkB allele (TrkBfl/fl mice), and for controls that retained intact TrkB expression we injected a non-cre expressing virus (AAV5-EF1a:EYFP) into LS of TrkBfl/fl mice (Fig. 1C). Four weeks following virus injections, we micro-dissected the LS and frontal cortex from each mouse, and quantified protein expression of full-length and truncated TrkB (Fig. 1D–F). In TrkBfl/fl mice expressing cre recombinase in the LS there was no change in TrkB expression in frontal cortex, but full-length TrkB expression was decreased 86% in the LS (Fig. 1D, E). Original characterization of this TrkB floxed allele (TrkBfl/fl) when crossed with a Dlx5/6 cre driver line caused significant decrease in full-length TrkB expression, but also partial loss of truncated expression [52]. In contrast, we observed no significant change in truncated TrkB expression across experimental groups (Fig. 1D, F). This difference may be accounted for by differences in cell composition in the brain regions studied (striatum versus LS), and differences in method of cre delivery (Dlx5/6 cre-driver mouse versus local delivery of AAV) that affected knock-down efficiency of the truncated TrkB receptor, which is highly enriched in astrocyte versus neuronal populations [56]. In summary, GABAergic neurons in the LS highly express TrkB, and the viral methods employed are capable of robust and region-specific knockdown of full-length TrkB.
Fig. 1. Tropomyosin receptor kinase B (TrkB) is highly expressed in the lateral septum (LS) and can be effectively depleted with viral manipulations.
A Illustration depicting the anatomical boundaries across the rostral-caudal axis of the LS. B Fluorescent in-situ hybridization in sections of mouse LS (n = 4) demonstrates abundant Ntrk2 expression in Gad1-positive cells throughout the rostral-caudal divisions. C Schematic of the viral strategy using cre-induced TrkB knockdown in the LS of TrkBfl/fl mice. D Western blot quantification of full-length TrkB (F-TrkB), truncated TrkB (T-TrkB), and α-Tubulin (aTub). E Relative expression levels of F-TrkB protein were significantly lower in the LS of the experimental group (AAV-cre in LS of TrkBfl/fl mice, n = 5) compared to controls (AAV-eGFP in LS of TrkBfl/fl mice, n = 6)(t9 = 5.015, p = 0.0014). F Relative expression levels for T-TrkB protein were similar between experimental and control mice.
Knockdown of LS TrkB abolishes social novelty discrimination
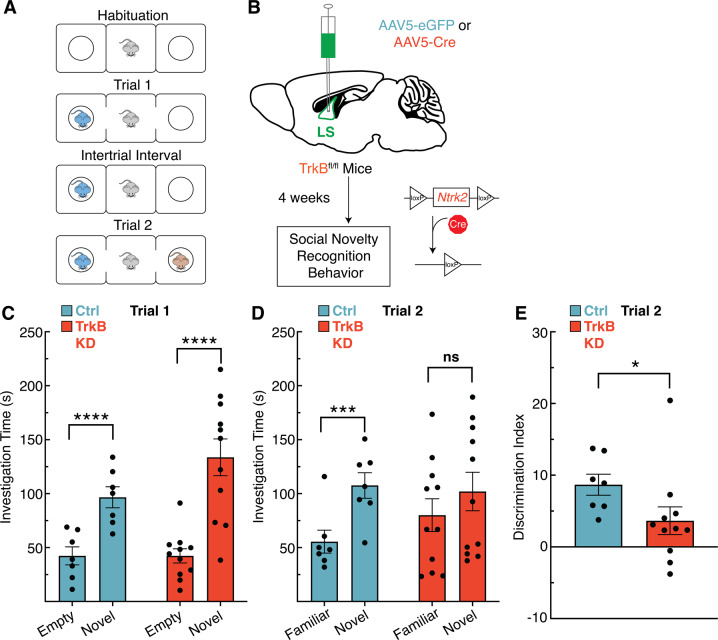
We next used this local knockdown strategy to investigate the necessity of TrkB signaling in the LS in social novelty using the three chamber social interaction task (Fig. 2A, B). This task uses a three-chambered box to quantify the time a mouse spends examining socially novel individuals. In trial 1, the mouse is exposed to a novel mouse (stranger mouse 1) in one of the outer chambers while the other outer chamber remains empty. In trial 2, stranger mouse 1 remains in the same chamber (now the familiar mouse) while a new mouse (stranger mouse 2) is placed in the other chamber. In trial 1 both the TrkB intact (Ctrl) and TrkB knockdown (KD) mice spend significantly more time in the chamber with the novel mouse compared to the empty chamber (Fig. 2C). In trial 2, TrkB intact mice spend significantly more time with the novel mouse compared to the familiar mouse, but TrkB knockdown mice show no difference (Fig. 2D). To evaluate differences in the ability of TrkB intact and TrkB knockdown mice to discriminate between individuals we generated a discrimination index for trial 2, which is calculated as the percentage of time spent with the novel individual minus the time spent with the familiar individual over the total trial time. LS TrkB knockdown mice have a significantly lower discrimination index than TrkB intact mice, suggesting that loss of full-length TrkB may impact the ability to discriminate between individual mice, or increase sociability (Fig. 2E).
Fig. 2. Tropomyosin receptor kinase B (TrkB) expression in the lateral septum (LS) is required for social novelty recognition behavior in mice.
A Description of three chamber social interaction experiment. B The viral strategy using cre-induced knockdown of TrkB expression in the LS of TrkBfl/fl mice prior to social novelty recognition. C In trial 1, both TrkB intact (Ctrl) and TrkB knockdown (KD) mice spend significantly more time with the novel mouse (2-way RM ANOVA, ns p = 0.0959 interaction of novelty x experimental manipulation, p < 0.0001 main effect of novelty, ns p = 0.2179 main effect of experimental manipulation; Bonferroni post hoc p = 0.0089 for TrkB intact mice, p < 0.0001 for TrkB knockdown mice). D In trial 2, TrkB KD mice do not show a difference between investigation time of the familiar and novel mice (2-way RM ANOVA, ns p = 0.0813 interaction of novelty x experimental manipulation, main effect of novelty p < 0.0003, ns p = 0.6639 main effect of experimental manipulation; Bonferroni post hoc p = 0.0016 for TrkB intact mice, ns p = 0.093 for TrkB KD mice). E Mice with intact LS TrkB (Ctrl) show better social discrimination between the socially novel and familiar mice in Trial 2 when compared toTrkB KD mice (U11,7 = 12, p = 0.0154).
To rule out that the impairment in attention to social novelty discrimination was due to olfactory impairment, both control and LS TrkB knockdown mice were subjected to a four trial odor discrimination task (Fig. S2A). Knockdown of TrkB in LS did not impair odor discrimination (Fig. S2B). We also assessed the necessity of TrkB signaling in LS for a number of fear- and anxiety-related behaviors that engage LS circuitry. LS TrkB knockdown did not significantly decrease anxiety-like behavior in the elevated plus maze (Fig. S2C), nor did it impact the ability to acquire, retrieve or extinguish fear memories (Fig. S2D). These data support the hypothesis that the deficits observed following LS TrkB knockdown are specific to social behavior. Together these data support the hypothesis that LS TrkB expression is important for social novelty recognition behavior.
LS TrkB knockdown abolishes socially induced c-Fos expression
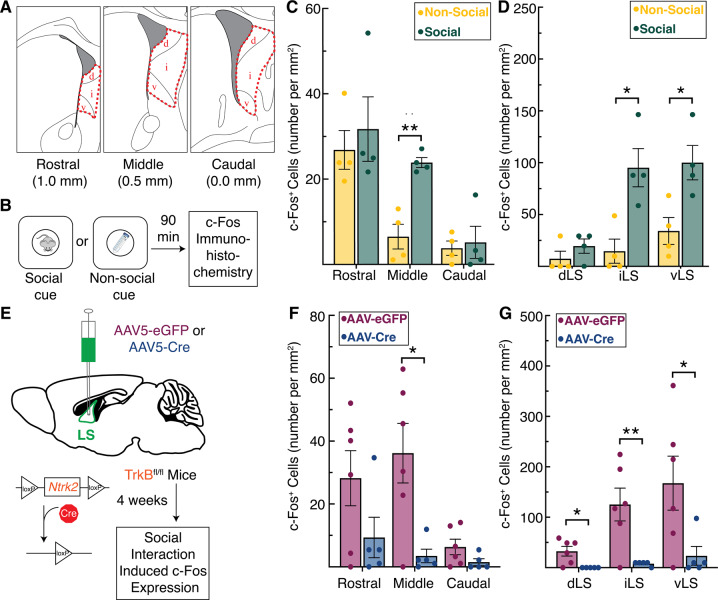
To map neural activity patterns in LS cells following exposure to novel social stimuli, we performed fluorescent immunohistochemistry for c-Fos across the rostral-caudal divide of the LS (Fig. 3A) in wild-type mice exposed to a social stimulus (a novel male mouse) compared to a non-social stimulus (a novel object)(Fig. 3B). c-Fos quantification was performed using the open-source biological imaging software Fiji (for detailed methods, see Supplementary information and Fig. S3A [57]). In the middle portion of the LS, significantly more cells expressed c-Fos in response to social stimuli compared to the non-social stimulus (Fig. 3C). This effect mapped most strongly to the intermediate and ventral subregions of the LS (Fig. 3D). The number of c-Fos expressing cells in all other subregions within the rostral (Fig. S3B) and caudal (Fig. S3C) portions of the LS remained relatively consistent across the social and non-social conditions suggesting that these effects on cell recruitment in the LS are restricted to the middle portion of the LS.
Fig. 3. Tropomyosin receptor kinaseB (TrkB) expression in the lateral septum (LS) is required for social novelty induced c-Fos expression.
A Illustration depicting the anatomical boundaries across the rostral-caudal axis of the LS, denoting the dorsal (d), intermediate (i), and ventral (v) subregions of the LS. B Description of social or non-social cues used to induce c-Fos expression. C Induction of c-Fos is significantly increased by social cues in the middle portion of the LS (t6 = 5.543, p = 0.004), (D) an effect seen specifically in the intermediate (t6 = 3.697, p = 0.030) and ventral subdivisions (t6 = 3.131, p = 0.040) of the middle LS. E The viral strategy using cre-induced TrkB knockdown in the LS of TrkBfl/fl mice prior to examining social interaction induced c-Fos expression. F Knockdown of TrkB in the LS significantly decreases cell recruitment in the middle portion of the LS following social stimuli (t9 = 3.069, p = 0.0396), (G) an effect seen across the dorsal (t9 = 3.049, p = 0.029), intermediate (t9 = 3.254, p = 0.029), and ventral (t9 = 2.342, p = 0.044) subdivisions of the middle LS.
To investigate whether TrkB knockdown in LS would abolish LS-responsivity to socially novel stimuli, we generated an additional cohort of LS TrkB knockdown and control mice using the same viral strategy described above (Fig. 3E). Compared to controls, we detected significantly fewer cells expressing c-Fos in response to novel social stimuli in the middle portion of the LS in LS TrkB knockdown mice (Fig. 3F, representative images in Fig. S4A). All subregions (dorsal, intermediate, and ventral) within the middle portion of the LS displayed blunted c-Fos expression in response to a socially novel individual (Fig. 3G). In contrast, LS TrkB knockdown had no significant effect on induction of c-Fos in response to socially novel individuals within the rostral (although we do note non-significant trend in the rostral iLS data, Fig. S4B) and caudal (Fig. S4C) portions of the LS compared to controls. These data suggest that TrkB expression in LS neurons is critical for their recruitment in response to social novelty.
BLA projections, but not vCA1 projections, to the LS are necessary for social novelty discrimination
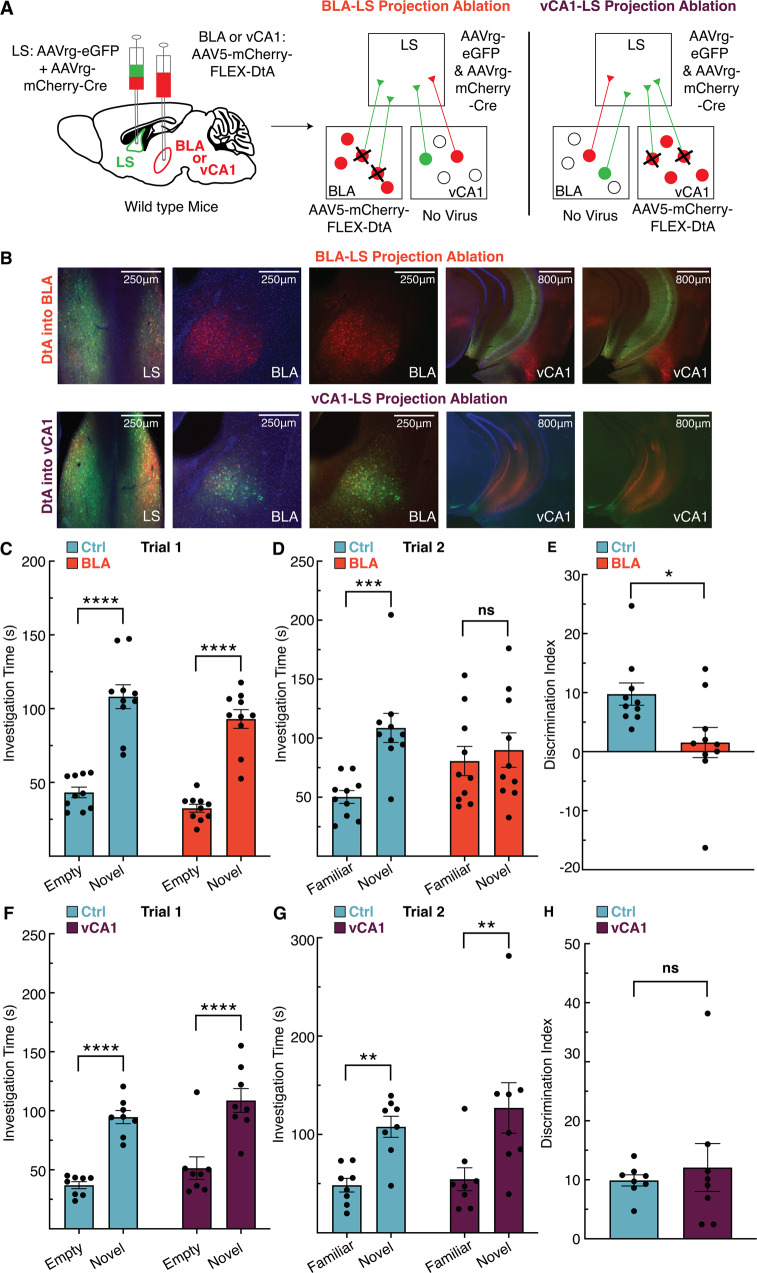
While the LS is critical for controlling responses to social novelty [16–19], the brain regions that transmit information to the LS for proper execution of social novelty discrimination have not been identified. We identified the BLA and vCA1 as candidate regions based on their established roles in regulating social novelty [9, 16, 20, 48] and recognition [24, 49, 50], as well having direct projections to LS [20, 23, 27]. To investigate a role for projections from the BLA or vCA1 to the LS in controlling social novelty behavior, we used a diphtheria toxin A (DtA)-mediated circuit elimination strategy. Specifically, we bilaterally injected two viruses with retrograde tropism (AAVrg-CB7.CI:EGFP and AAVrg-EF1a:mCherry-IRES-cre) into the LS, causing expression of eGFP, mCherry, and cre in cells projecting to LS (Fig. 4A). In the two regions of interest (separate cohorts for BLA and vCA1) we also bilaterally injected a virus (AAV5-EF1a:mCherry-FLEX-dtA) expressing mCherry and cre-dependent DtA (Fig. 4A). In this scenario, cell bodies in the BLA or vCA1 that also send projections to the LS express cre-recombinase, activating cre-mediated expression of DtA in BLA-LS or vCA1-LS neuronal projections. This manipulation caused elimination of the eGFP signal in ablated projections, but retention of the mCherry signal in non-projecting cells (Fig. 4B). In trial 1, both mice with intact BLA-LS projections (Ctrl) and ablated BLA-LS (BLA) projections spent more time with the socially novel mouse compared to the empty chamber (Fig. 4C). In trial 2, however, mice with intact BLA-LS projection neurons spent more time with the novel mouse over the familiar mouse, while mice with ablated BLA-LS projections spent similar time with the familiar and novel mouse (Fig. 4D). In addition, mice with intact BLA-LS projections discriminate better than mice with ablated BLA-LS projection neurons according to their relative percentage of time spent with the novel versus familiar mouse in trial 2 (Fig. 4E). In the vCA1-LS manipulation experiment, mice with intact vCA1-LS projections (Ctrl) and mice with ablated vCA1-LS projections (vCA1) showed expected social novelty behavior in trial 1 (Fig. 4F). Both the vCA1-LS intact and the vCA1-LS ablated groups also show similar increased time with the novel mouse over the familiar mouse in trial 2 (Fig. 4G). Neither group differed in ability to discriminate between the novel and familiar mice in trial 2 (Fig. 4H). These data support a role for BLA-LS neurons, but not vCA1-LS neurons, in regulating social novelty recognition behavior.
Fig. 4. Ablating inputs to the lateral septum (LS) from basolateral amygdala (BLA), but not ventral CA1 (vCA1), abolishes social novelty recognition behavior in mice.
A Viral strategy to selectively eliminate inputs to the LS from either the BLA or vCA1 utilizing retrograde labeling and cre-mediated expression of diphtheria toxin A (DtA) (left). Schematics depict ablation in the cohort where BLA-LS projections are targeted (middle), and ablation in the cohort where vCA1-LS projections are targeted (right). B Representative images show that in the cohort where DtA is expressed in BLA (top row), BLA-LS projections are ablated, while vCA1-LS projections are intact, while in the cohort where DtA is expressed in vCA1 (bottom row), vCA1-LS projections are ablated while BLA-LS projections remain intact. C In trial 1, both mice with BLA-LS projections intact (Ctrl) and BLA-LS projections ablated (BLA) spend significantly more time with the novel mouse (2-way RM ANOVA, ns p = 0.6498 interaction of novelty x experimental manipulation, p < 0.0001 main effect of novelty, ns p = 0.1261 main effect of experimental manipulation; Bonferroni post hoc p < 0.0001 for BLA-LS intact mice, p < 0.0001 for BLA-LS ablated mice). D In trial 2, there is an interaction between the novelty of the mouse and experimental manipulation. BLA-LS intact mice showed significantly more interaction time with the novel mouse compared to the familiar mouse, while BLA-LS ablated mice did not (2-way RM ANOVA, p = 0.0189 interaction of novelty x experimental manipulation, main effect of novelty p = 0.0023, ns p = 0.6712 main effect of experimental manipulation; Bonferroni post hoc p = 0.0008 for BLA-LS intact mice, ns p = 0.9996 for BLA-LS ablated mice). E Mice with intact BLA-LS projections show better social discrimination between the socially novel and familiar mice in Trial 2 than mice with an ablated BLA-LS circuit (U10,10 = 17, p = 0.0115). F In trial 1, both mice with intact vCA1-LS projections (Ctrl) and ablated vCA1-LS projections (vCA1) spend significantly more time with the novel mouse (2-way RM ANOVA, ns p = 0.9803 interaction of novelty x experimental manipulation, p < 0.0001 main effect of novelty, ns p = 0.1212 main effect of experimental manipulation; Bonferroni post hoc p < 0.0001 for vCA1-LS intact mice, p < 0.0001 for vCA1-LS ablated mice). G In trial 2, both mice with intact vCA1-LS projections and ablated vCA1-LS projections spend significantly more time with the novel mouse (2-way RM ANOVA, ns p = 0.6083 interaction of novelty x experimental manipulation, p = 0.001 main effect of novelty, ns p = 0.4976 main effect of experimental manipulation; Bonferroni post hoc p = 0.0093 for vCA1-LS intact mice, p = 0.0022 for vCA1-LS ablated mice). H Mice with intact vCA1-LS projections and mice with ablated vCA1-LS projections show no difference in social discrimination (U9,9 = 30, p = 0.8527).
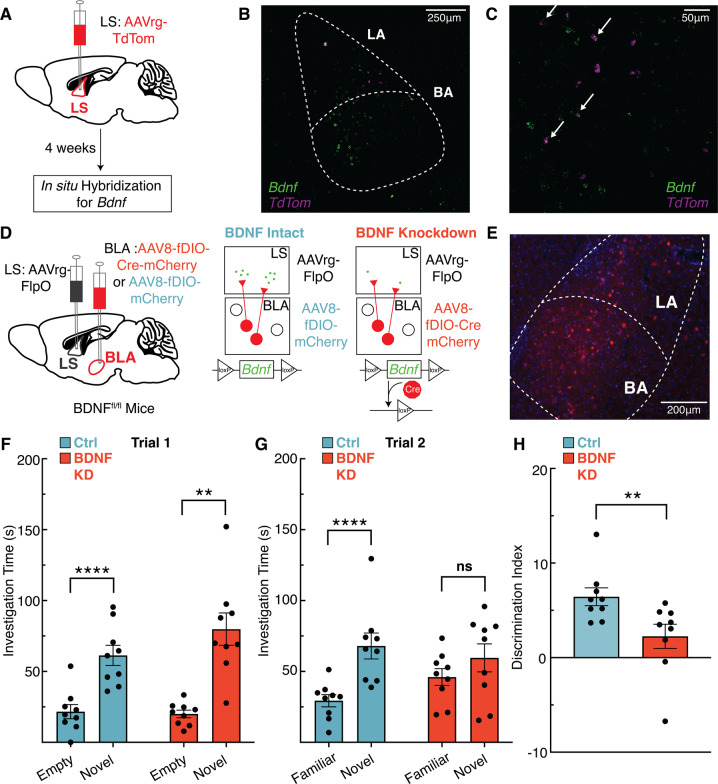
Expression of BDNF within BLA projections to the LS is necessary for social novelty discrimination
BLA-LS projection neurons are a strong candidate source for providing BDNF to LS TrkB-expressing neurons that regulate social novelty because BDNF expression in the BLA is high [45], and the BLA sends projections to the LS [23]. To assess Bdnf expression specifically in BLA-LS neurons, we injected a retrograde virus (AAVrg-CAG-TdTom) in the LS of wild-type mice in order to express TdTomato in all inputs to the LS (Fig. 5A). Following viral expression, we used RNAscope single-molecule fluorescent in-situ hybridization to visualize expression of Bdnf in TdTom-positive neurons in the BLA (Figs. 5B-C, S5B-G). To test whether BDNF expression in BLA-LS projections is necessary to control social novelty behavior, we used a viral strategy combining the Flp recombinase (FlpO) and cre recombinase systems in mice carrying a floxed Bdnf allele (BDNFfl/fl). This strategy allowed us to eliminate BDNF selectively from BLA-LS projections (Fig. 5D). We bilaterally injected a retrograde virus in the LS (AAVrg-EF1a:Flpo) of BDNFfl/fl mice, causing FlpO expression in all neuronal inputs to the LS. We simultaneously injected a virus (AAV8-EF1a:fDIO-mCherry-P2A-cre) expressing Flp-dependent mCherry and cre-recombinase in the BLA. Control mice were injected with a virus (AAV8-EF1a:fDIO-mCherry) into the BLA that expressed Flp-dependent mCherry. In the experimental group, BLA neurons projecting to the LS express cre-recombinase in a FlpO-dependent manner. Expression of cre leads to excision of the floxed BDNF allele and cre-dependent expression of mCherry (Fig. 5E). In trial 1, both mice with intact BDNF in BLA-LS projections (Ctrl) and mice with BDNF knockdown in BLA-LS projections (BDNF KD) spent significantly more time interacting with the novel mouse compared to the empty chamber (Fig. 5F). In trial 2, mice with intact BDNF expression in BLA-LS projections spent more time interacting with the novel mouse compared to the familiar mouse, while mice with BDNF KD in BLA-LS projections spent roughly equal time interacting with both novel and familiar mice (Fig. 5G). Moreover, mice with BDNF KD in BLA-LS projections display poorer discrimination between individuals compared to animals with intact BDNF expression in BLA-LS projections according to their relative percentage of time spent with the novel versus familiar mouse in trial 2 (Fig. 5H). In summary, BDNF expression in BLA-LS neurons is critical for social novelty discrimination.
Fig. 5. Knockdown of brain-derived neurotrophic factor (BDNF) in basolateral amygdala (BLA) inputs to the lateral septum (LS) abolishes social novelty recognition behavior in mice.
A Schematic of strategy to label BLA-LS projections and demonstrate Bdnf expression in BLA-LS neurons. B Representative ×20 image of the topographic location of Bdnf and TdTom transcripts within the BLA. (C) ×40 image of Bdnf + and TdTom + cells, arrows point to co-expressing cells. D Schematic of viral strategy to deplete BDNF expression in BLA-LS projections in BDNFfl/fl mice. E Representative images of viral expression of mCherry within the BLA. F In trial 1, both mice with intact BDNF in BLA-LS projections (Ctrl) and BDNF knockdown in BLA-LS projections (BDNF KD) spend significantly more time interacting with the novel mouse (2-way RM ANOVA, ns p = 0.1397 interaction of novelty x experimental manipulation, p < 0.0001 main effect of novelty, ns p = 0.3072 main effect of experimental manipulation; Bonferroni post hoc p = 0.0010 for BDNF intact mice, p < 0.0001 for BDNF knockdown mice). G In trial 2, there is an interaction between the novelty of the mouse and experimental manipulation. Mice with intact BDNF in BLA-LS projections spent significantly more interacting with the novel mouse compared to the familiar mouse, while mice with BDNF KD in BLA-LS projections mice did not (2-way RM ANOVA, p = 0.0179 interaction of novelty x experimental manipulations, main effect of novelty p < 0.0001, ns p = 0.6761 main effect of experimental manipulation; Bonferroni post hoc p < 0.0001 for mice with intact BDNF in BLA-LS projections, ns p = 0.1237 for mice with BDNF KD in BLA-LS projections). H Mice with intact BDNF expression in BLA-LS projections display better social discrimination between the socially novel and familiar mice in trial 2 than mice with BDNF KD in BLA-LS projections (U9,9 = 9, p = 0.004).
Discussion
BLA projections to the LS control social novelty
The BLA is critical for valence processing in a variety of contexts [58, 59], a function that may extend to processing social stimuli [60]. The BLA is responsive to socially novel conspecifics [16, 48], and controls social discrimination [50], but with which regions the BLA communicates to regulate these behaviors is not well-established. We provide strong evidence that the BLA-LS circuit controls social novelty recognition by demonstrating that this behavior is abolished after ablating neuronal projections from the BLA to the LS. Because BLA neurons can collateralize [61], it is possible that ablating these BLA neurons may partially block transmission of social information to additional regions besides the LS, and this is an important future study. In addition to the BLA, vCA1 has been implicated in controlling responses to social novelty [16] and regulating social memories [24, 49], and LS projections from adjacent subregions of the hippocampus, including dorsal CA2 (dCA2), are critical for controlling social aggression [21]. Surprisingly, ablating projections from vCA1 to the LS did not alter social discrimination behavior. While dCA2 is critical for social recognition [62] through its projections to vCA1 [24], this social information may not be transmitted from ventral hippocampal circuitry to the LS to control social novelty. Further research would be necessary to determine if other portions of the ventral hippocampus, such as dCA2, regulate social novelty and recognition, in addition to aggression. Interestingly, activation versus inhibition of BLA projections to the ventral hippocampus bi-directionally controls time spent socializing [60], which may point to the BLA as a central transmitter of social information to limbic circuitry.
TrkB signaling in the LS critically regulates social novelty
The LS is a central hub for processing social behavior, and controls both social salience [15, 16] and social recognition [17–19], but little is known about the cellular and molecular mechanisms by which social information is relayed to the LS. Knocking down TrkB expression in the LS abolished social novelty recognition in the three-chamber social interaction task, suggesting that TrkB signaling is necessary for discriminating socially novel conspecifics from those that are socially familiar. Importantly, TrkB knockdown did not impair olfactory discrimination, anxiety-like behavior, or fear learning, highlighting the specificity of LS TrkB signaling in controlling the response to social novelty.
The LS is spatially complex, containing dorsal, ventral, and intermediate subregions that span its rostral-caudal axis. Importantly, these subregions have functional distinctions, for example the dorsal and ventral LS control different aspects of social aggression in both males [24] and females [63]. While elevated c-Fos expression in the LS in response to socially novel stimuli is documented [15, 16], how this activity maps to the spatial topography of the LS has not been rigorously explored. Using c-Fos neural activity mapping, we demonstrated that the intermediate and ventral subregions of the middle portion of the LS are the socially responsive loci. Previous results suggested that social novelty-induced activity in the LS was concentrated more rostrally than caudally [15], which is consistent with our results. In addition, we demonstrated that LS TrkB expression is necessary for LS responsiveness to social stimuli. Specifically, LS TrkB knockdown blunts the recruitment of neural activity in the intermediate and ventral subregions of the middle portion of the LS in response to social stimuli.
BDNF expression in BLA-LS neurons is critical for social novelty behavior
The BLA controls a variety of social behaviors in rodents, non-human primates [64] and humans [65–67]. While the cellular and molecular mechanisms that control these behaviors are complex, the prominent role of BDNF-TrkB signaling in many critical components of synaptic function renders it likely that this pathway is involved. Supporting this notion, rare genetic disorders that feature BDNF haploinsufficiency as well as common genetic variation in the human BDNF locus are linked with multiple components of social functioning, including aggression [68–70]. In addition to BDNF, a recent genome-wide association study of anxiety, which included human subjects with social anxiety, identified a single nucleotide polymorphism in NTRK2, the gene encoding the TrkB receptor, as contributing to genetic risk [71].
Our data demonstrate that TrkB expression in the LS is critical for social novelty and recognition behavior (Fig. 1D–G), but because BDNF is not synthesized in the LS (Fig. S1A-B), the source of BDNF for activating LS TrkB receptors is unclear [45]. BDNF is highly expressed in BLA neurons [45], and given our data showing that BLA-LS projection neurons are critical for this behavior, we reasoned that BDNF produced in BLA-LS projection neurons could be a potential source for activating LS TrkB receptors to control this behavior. Indeed, BLA-LS neurons express Bdnf (Figs. 5B-C, S5B-G) and knocking down BDNF selectively in BLA-LS projection neurons phenocopies the behavioral deficits caused by local TrkB knockdown in the LS. While our data support the hypothesis that BDNF release from BLA-LS neurons into the LS engages local TrkB receptors to control social behavior, additional experiments would be needed to causally establish this link. In our experiments, BDNF expression in BLA-LS neurons is chronically depleted, which could lead to longer-term structural and physiological changes to BLA-LS projections due to the loss of neurotrophic support, including from decreased dendritic release of BDNF in the BLA. Such weakening of synaptic connections could contribute to changes in the circuitry that impact social behavior. Moreover, while our data suggests that loss of LS TrkB expression gates the region’s response to social stimuli, further work with acute manipulations of TrkB receptors would be critical to understand the kinetics of how LS BDNF-TrkB signaling controls social behaviors. This is important since the chronic effects of TrkB knockdown in LS in our experiments could impact the physiological function of TrkB-expressing inhibitory neurons and alter the strength of inhibitory synapses. This in turn could impact connectivity within the LS, or inputs to the LS, contributing to the observed impairments in social behaviors.
Ethological considerations on behavior
A limitation of these studies is that they were conducted only in male mice. While male and female mice show substantial overlap across social behaviors, there are documented differences, including establishing social hierarchies [72, 73], neural coding of social behaviors [74], and processing social recognition [75]. Hence, direct comparisons of males and females in social novelty and recognition paradigms poses challenges in behavioral interpretation. Despite these challenges, there are several reasons why future studies should examine a potential role for BDNF-TrkB signaling in BLA-LS circuitry in the context of sex-specific social behaviors. First, prevalence rates [76], as well as genetic risk, for disorders that often feature social deficits are not equal across sex and gender in humans [77–81]. Second, BDNF-TrkB signaling and expression is significantly impacted by sex and sex hormones [82–84]. Conducting these studies, however, will require taking into consideration specific ethological aspects of female social behaviors. For example, female mice establish different social hierarchies depending on the presence of male mice [73]. This manifests in territoriality and aggression in dominant female mice, a behavior normally only seen in postpartum dams in defense of their pups [85]. Conversely, male mice naturally display this territoriality and aggression toward male mice [73, 86], but seldom display these behaviors in male-female interactions [73]. Despite the difficulties in making direct comparisons between male and female mice in studies of social behaviors, well-designed experiments can be implemented to study sex-specific differences in social novelty and recognition. For example, studying female social novelty in postpartum mice or dominant female mice raised in complex social structures will elucidate whether the circuits that enable normal male responses to social novelty are also necessary for controlling female social novelty.
Conclusions
Collectively, the data demonstrate a critical role for TrkB signaling in LS neurons in controlling social novelty recognition, and support the hypothesis that the BLA is a critical source of BDNF for activating TrkB receptors in LS neurons to control this behavior. The data provide novel information about the broader role of amygdala-septal circuits, and establish a foundation for future studies that delineate how molecular and cellular signaling in these circuits impacts social functioning.
Supplementary information
Acknowledgements
We thank members of the Martinowich and Maynard laboratories for helpful comments and suggestions. This work was supported by internal funding from the Lieber Institute for Brain Development, and the National Institute of Mental Health (R01MH105592 to KM). Andrew E. Jaffe is now a full-time employee at Neumora Therapeutics, a for-profit biotechnology company, which is unrelated to the contents of this manuscript. Sun-Hong Kim is now employed by Genentech. Their contributions to the manuscript were made while previously employed by the Lieber Institute for Brain Development.
Author contributions
LAR: Formal analysis, Investigation, Writing - Original Draft, Visualization. SHK: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Review & Editing. SCP: Investigation, Resources. CVN: Investigation. EAP: Investigation. HLH: Investigation. JV: Investigation. KRM: Writing - Review & Editing, Methodology. AEJ: Formal analysis, Resources. KM: Conceptualization, Supervision, Writing - Original Draft, Project administration, Funding acquisition.
Competing interests
KM is the Social Media Editor for Neuropsychopharmacology. No other authors have financial relationships with commercial interests, and the authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lionel A. Rodriguez, Sun-Hong Kim.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01487-y.
References
- 1.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci (Regul Ed) 2012;16:231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Happé F, Ronald A. The “fractionable autism triad”: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 3.Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- 4.Mier D, Kirsch P. Social-cognitive deficits in schizophrenia. Curr Top Behav Neurosci. 2017;30:397–409. doi: 10.1007/7854_2015_427. [DOI] [PubMed] [Google Scholar]
- 5.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev. 2012;36:1060–84. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Pobbe RLH, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 8.Lo S-C, Scearce-Levie K, Sheng M. Characterization of social behaviors in caspase-3 deficient mice. Sci Rep. 2016;6:18335. doi: 10.1038/srep18335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 10.White SF, Brislin S, Sinclair S, Fowler KA, Pope K, Blair RJR. The relationship between large cavum septum pellucidum and antisocial behavior, callous-unemotional traits and psychopathy in adolescents. J Child Psychol Psychiatry. 2013;54:575–81. doi: 10.1111/j.1469-7610.2012.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raine A, Lee L, Yang Y, Colletti P. Neurodevelopmental marker for limbic maldevelopment in antisocial personality disorder and psychopathy. Br J Psychiatry. 2010;197:186–92. doi: 10.1192/bjp.bp.110.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crooks D, Anderson NE, Widdows M, Petseva N, Koenigs M, Pluto C, et al. The relationship between cavum septum pellucidum and psychopathic traits in a large forensic sample. Neuropsychologia. 2018;112:95–104. doi: 10.1016/j.neuropsychologia.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarwar M. The septum pellucidum: normal and abnormal. AJNR Am J Neuroradiol. 1989;10:989–1005. [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan T, Numan M The septal region and social behavior. In: Numan R, editor. The behavioral neuroscience of the septal region, New York, NY: Springer New York; 2000. p. 175–209.
- 15.Shin S, Pribiag H, Lilascharoen V, Knowland D, Wang X-Y, Lim BK. Drd3 signaling in the lateral septum mediates early life stress-induced social dysfunction. Neuron. 2018;97:195–208.e6. doi: 10.1016/j.neuron.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borelli KG, Blanchard DC, Javier LK, Defensor EB, Brandão ML, Blanchard RJ. Neural correlates of scent marking behavior in C57BL/6J mice: detection and recognition of a social stimulus. Neuroscience. 2009;162:914–23. doi: 10.1016/j.neuroscience.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Bielsky IF, Hu S-B, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–53. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99:7–16. doi: 10.1016/S0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara NC, Trask S, Ritger A, Padival M, Rosenkranz JA. Developmental differences in amygdala projection neuron activation associated with isolation-driven changes in social preference. Front Behav Neurosci. 2022;16:956102. doi: 10.3389/fnbeh.2022.956102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, et al. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature. 2018;564:213–8. doi: 10.1038/s41586-018-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, et al. Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr Biol. 2016;26:593–604. doi: 10.1016/j.cub.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hintiryan H, Bowman I, Johnson DL, Korobkova L, Zhu M, Khanjani N, et al. Connectivity characterization of the mouse basolateral amygdalar complex. Nat Commun. 2021;12:2859. doi: 10.1038/s41467-021-22915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun. 2018;9:4163. doi: 10.1038/s41467-018-06501-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, et al. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl Psychiatry. 2016;6:e809. doi: 10.1038/tp.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews GA, Tye KM. Neural mechanisms of social homeostasis. Ann NY Acad Sci. 2019;1457:5–25. doi: 10.1111/nyas.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–95. doi: 10.1016/S0165-0173(97)00009-X. [DOI] [PubMed] [Google Scholar]
- 28.Maynard KR, Hobbs JW, Phan BN, Gupta A, Rajpurohit S, Williams C, et al. BDNF-TrkB signaling in oxytocin neurons contributes to maternal behavior. ELife. 2018;7. [DOI] [PMC free article] [PubMed]
- 29.Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38:522–32. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 31.Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–74. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 34.Song M, Martinowich K, Lee FS. BDNF at the synapse: why location matters. Mol Psychiatry. 2017;22:1370–5. doi: 10.1038/mp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–7. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 36.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–90. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–35. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marty S, Berzaghi MdaP, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/S0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 39.Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- 40.Hill JL, Jimenez DV, Mai Y, Ren M, Hallock HL, Maynard KR, et al. Cortistatin-expressing interneurons require TrkB signaling to suppress neural hyper-excitability. Brain Struct Funct. 2019;224:471–83. doi: 10.1007/s00429-018-1783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maynard KR, Kardian A, Hill JL, Mai Y, Barry B, Hallock HL, et al. TrkB signaling influences gene expression in cortistatin-expressing interneurons. ENeuro. 2020;7. [DOI] [PMC free article] [PubMed]
- 42.Itami C, Kimura F, Nakamura S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J Neurosci. 2007;27:2241–52. doi: 10.1523/JNEUROSCI.3345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55. doi: 10.1016/S0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 44.Zheng K, An JJ, Yang F, Xu W, Xu Z-QD, Wu J, et al. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci USA. 2011;108:17201–6. doi: 10.1073/pnas.1114241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo F, Mu Y, Gao C, Xiao Y, Zhou Q, Yang Y, et al. Whole-brain patterns of the presynaptic inputs and axonal projections of BDNF neurons in the paraventricular nucleus. J Genet Genomics. 2019;46:31–40. doi: 10.1016/j.jgg.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Fawcett JP, Alonso-Vanegas MA, Morris SJ, Miller FD, Sadikot AF, Murphy RA. Evidence that brain-derived neurotrophic factor from presynaptic nerve terminals regulates the phenotype of calbindin-containing neurons in the lateral septum. J Neurosci. 2000;20:274–82. doi: 10.1523/JNEUROSCI.20-01-00274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferri SL, Kreibich AS, Torre M, Piccoli CT, Dow H, Pallathra AA, et al. Activation of basolateral amygdala in juvenile C57BL/6J mice during social approach behavior. Neuroscience. 2016;335:184–94. doi: 10.1016/j.neuroscience.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng X, Gu L, Sui N, Guo J, Liang J. Parvalbumin interneuron in the ventral hippocampus functions as a discriminator in social memory. Proc Natl Acad Sci USA. 2019;116:16583–92. doi: 10.1073/pnas.1819133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrido Zinn C, Clairis N, Silva Cavalcante LE, Furini CRG, de Carvalho Myskiw J, Izquierdo I. Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc Natl Acad Sci USA. 2016;113:E4914–9. doi: 10.1073/pnas.1609883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grishanin RN, Yang H, Liu X, Donohue-Rolfe K, Nune GC, Zang K, et al. Retinal TrkB receptors regulate neural development in the inner, but not outer, retina. Mol Cell Neurosci. 2008;38:431–43. doi: 10.1016/j.mcn.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baydyuk M, Russell T, Liao G-Y, Zang K, An JJ, Reichardt LF, et al. TrkB receptor controls striatal formation by regulating the number of newborn striatal neurons. Proc Natl Acad Sci USA. 2011;108:1669–74. doi: 10.1073/pnas.1004744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–8. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manvich DF, Stowe TA, Godfrey JR, Weinshenker D. A Method for Psychosocial Stress-Induced Reinstatement of Cocaine Seeking in Rats. Biol Psychiatry. 2016;79:940–6. doi: 10.1016/j.biopsych.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maynard KR, Tippani M, Takahashi Y, Phan BN, Hyde TM, Jaffe AE, et al. dotdotdot: an automated approach to quantify multiplex single molecule fluorescent in situ hybridization (smFISH) images in complex tissues. Nucleic Acids Res. 2020; 2020. 10.1093/nar/gkaa312. [DOI] [PMC free article] [PubMed]
- 56.Holt LM, Hernandez RD, Pacheco NL, Torres Ceja B, Hossain M, Olsen ML Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. ELife. 2019;8. [DOI] [PMC free article] [PubMed]
- 57.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beyeler A, Chang C-J, Silvestre M, Lévêque C, Namburi P, Wildes CP, et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 2018;22:905–18. doi: 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vrtička P, Sander D, Vuilleumier P. Lateralized interactive social content and valence processing within the human amygdala. Front Hum Neurosci. 2012;6:358. doi: 10.3389/fnhum.2012.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–95. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, et al. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron. 2016;90:348–61. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oliveira VE, de M, Lukas M, Wolf HN, Durante E, Lorenz A, et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat Commun. 2021;12:2900. doi: 10.1038/s41467-021-23064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gothard KM. Multidimensional processing in the amygdala. Nat Rev Neurosci. 2020;21:565–75. doi: 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenberger LA, Eisenegger C, Naef M, Terburg D, Fourie J, Stein DJ, et al. The human basolateral amygdala is indispensable for social experiential learning. Curr Biol. 2019;29:3532–.e3. doi: 10.1016/j.cub.2019.08.078. [DOI] [PubMed] [Google Scholar]
- 66.de Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, van Honk J. The role of human basolateral amygdala in ambiguous social threat perception. Cortex. 2014;52:28–34. doi: 10.1016/j.cortex.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 67.Zheng J, Anderson KL, Leal SL, Shestyuk A, Gulsen G, Mnatsakanyan L, et al. Amygdala-hippocampal dynamics during salient information processing. Nat Commun. 2017;8:14413. doi: 10.1038/ncomms14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spalletta G, Morris DW, Angelucci F, Rubino IA, Spoletini I, Bria P, et al. BDNF Val66Met polymorphism is associated with aggressive behavior in schizophrenia. Eur Psychiatry. 2010;25:311–3. doi: 10.1016/j.eurpsy.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Ernst C, Marshall CR, Shen Y, Metcalfe K, Rosenfeld J, Hodge JC, et al. Highly penetrant alterations of a critical region including BDNF in human psychopathology and obesity. Arch Gen Psychiatry. 2012;69:1238–46. doi: 10.1001/archgenpsychiatry.2012.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han JC, Thurm A, Golden Williams C, Joseph LA, Zein WM, Brooks BP, et al. Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex. 2013;49:2700–10. doi: 10.1016/j.cortex.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purves KL, Coleman JRI, Meier SM, Rayner C, Davis KAS, Cheesman R, et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry. 2020;25:3292–303. doi: 10.1038/s41380-019-0559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Berg WE, Lamballais S, Kushner SA. Sex-specific mechanism of social hierarchy in mice. Neuropsychopharmacology. 2015;40:1364–72. doi: 10.1038/npp.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chovnick A, Yasukawa NJ, Monder H, Christian JJ. Female behavior in populations of mice in the presence and absence of male hierarchy. Aggress Behav. 1987;13:367–75. doi: 10.1002/1098-2337(1987)13:6<367::AID-AB2480130605>3.0.CO;2-X. [DOI] [Google Scholar]
- 74.Li Y, Dulac C. Neural coding of sex-specific social information in the mouse brain. Curr Opin Neurobiol. 2018;53:120–30. doi: 10.1016/j.conb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Karlsson SA, Haziri K, Hansson E, Kettunen P, Westberg L. Effects of sex and gonadectomy on social investigation and social recognition in mice. BMC Neurosci. 2015;16:83. doi: 10.1186/s12868-015-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riecher-Rössler A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017;4:8–9. doi: 10.1016/S2215-0366(16)30348-0. [DOI] [PubMed] [Google Scholar]
- 78.Boyd A, Van de Velde S, Vilagut G, de Graaf R, O’Neill S, Florescu S, et al. Gender differences in mental disorders and suicidality in Europe: results from a large cross-sectional population-based study. J Affect Disord. 2015;173:245–54. doi: 10.1016/j.jad.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785–95. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blokland GAM, Grove J, Chen C-Y, Cotsapas C, Tobet S, Handa R, et al. Sex-dependent shared and nonshared genetic architecture across mood and psychotic disorders. Biol Psychiatry. 2022;91:102–17. doi: 10.1016/j.biopsych.2021.02.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puralewski R, Vasilakis G, Seney ML. Sex-related factors influence expression of mood-related genes in the basolateral amygdala differentially depending on age and stress exposure. Biol Sex Differ. 2016;7:50. doi: 10.1186/s13293-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan CB, Ye K. Sex differences in brain-derived neurotrophic factor signaling and functions. J Neurosci Res. 2017;95:328–35. doi: 10.1002/jnr.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav Neurosci. 2009;123:1169–77. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.