Abstract
This report explores the participation of some afferent mechanisms in the immune response induced by the Cuban anti-meningococcal vaccine VA-MENGOC-BC. The induction of delayed-type hypersensitivity in nursing babies and lymphocyte proliferation after immunization is demonstrated. The presence of gamma interferon IFN-γ and interleukin-2 (IL-2) mRNAs but absence of IL-4, IL-5, and IL-10 mRNAs were observed in peripheral blood mononuclear cells from immunized subjects after in vitro challenge with outer membrane vesicles. In addition, some effector functions were also explored. The presence of opsonic activity was demonstrated in sera from vaccinees. The role of neutrophils as essential effector cells was shown. In conclusion, we have shown that, at least in the Cuban adult population, VA-MENGOC-BC induces mechanisms with a T-helper 1 pattern in the afferent and effector branches of the immune response.
Neisseria meningitidis is a human pathogen and one of the major causes of bacterial meningitis (29). Infection may result in the development of septicemia and/or meningitis, with severe clinical symptoms. Natural immunity in humans is acquired by meningococcal colonization of the upper respiratory tract and increases with age (13). In 1969, Goldschneider et al. described an age-related inverse relationship of the incidence of meningococcal disease and the presence of bactericidal antibodies (14).
Polysaccharide-based vaccines against some serogroups are available, but these antigens cannot be used to protect against serogroup B due to the low immunogenicity of the B polysaccharide in humans (49); therefore, protein-based vaccines have been developed. VA-MENGOC-BC is the registered trademark of the Cuban vaccine against serogroup B and C N. meningitidis (2, 44). One of the most important findings of the Cuban vaccine trial was the demonstration, for the first time, that antibodies induced to noncapsular surface antigens can protect against meningococcal disease (10). Another important observation was that VA-MENGOC-BC is innocuous and safe. The vaccine efficacy surpassed 80% in a double-blind placebo-controlled vaccine trial conducted in junior high school students (11 to 15 years old) (24, 44). Yet another finding was the reduction in the morbidity and mortality rates caused by group B N. meningitidis after its application in all Cuban provinces since 1988 (48). Last but not least was the decreased incidence in children less than 5 years old from 67 to 120 in 1983 to 0.05 to 0.09 per 105 inhabitants in 1997 (24).
The presence of bactericidal antibodies has been shown to correlate with natural protection against the disease (14). Such antibodies are observed after infections by serogroup A, C, Y, and W-135 N. meningitidis and correlate with the protection induced by their polysaccharide-based vaccines (9, 31, 50). Nevertheless, the presence of bactericidal activity after immunization with outer membrane vesicle (OMV)-based vaccines such as VA-MENGOC-BC is controversial (5, 30, 44, 47), but the induction of such antibodies by noncapsular antigens (9, 10, 38, 39) remains the goal.
The complement-fixing antibodies may have other effector functions and come from a cellular pattern of immune response. Based on cytokine production, CD4+ T lymphocytes have been classified as T-helper 1 (Th1) cells, which produce and favor gamma interferon (IFN-γ) and interleukin-2 (IL-2)-mediated cellular immune responses, and Th2 cells, which produce and favor IL-4-, IL-5-, and IL-10-mediated humoral responses (25, 35). The cytokine production associated with T-cell proliferation has also become an important way to evaluate immune responses. Therefore, the cellular responses induced by VA-MENGOC-BC, including in vivo and in vitro responses were evaluated. Delayed-type hypersensitivity (DTH) and lymphocyte proliferation (LP) have been widely accepted as measures of T-cell activity. The antibodies that fix complement also have opsonic activity (36), and the specific immune response is amplified by the T-helper cascade, which includes intercellular and cellular responses known as a nonspecific amplification. This means that the participation of macrophages and neutrophils (polymorphonuclear leukocytes [PMN]) could be very important in regulation of the immune response as well as part of the effector mechanisms against N. meningitidis B infection.
Our main goal here has been to further our understanding of the triggering by this vaccine of the afferent and effector branches of the immune response, considering that serum bactericidal activity is only one of the multiple mechanisms involved in protection against N. meningitidis B. Particular attention was given to the mechanism related to Th1 cellular responses. In the afferent branch, DTH, LP, and production of cytokines at the mRNA level were explored. In the effector branch, the presence of opsonizing antibodies was demonstrated, and the role of PMN as effectors was also evaluated.
MATERIALS AND METHODS
Vaccine and immunization.
N. meningitidis strain B:4:P1,19,15 (Cuban vaccine strain) was grown until early stationary phase, and OMVs were extracted with 0.1 M Tris-HCl [pH 8.6]–10 mM EDTA–0.5% (wt/vol) deoxycholate. This preparation was purified by sequential centrifugation steps at 20,000 × g for 30 min. Following ultracentrifugation at 125,000 × g for 2 h, the pelleted OMVs were homogenized in phosphate-buffered saline (PBS; pH 7.2) with 3% (wt/vol) sucrose and further purified by column chromatography. In addition, the vaccine contains purified capsular polysaccharide of serogroup C meningococcus, both adsorbed on Al(OH)3 gel (16). The immunization schedule for humans comprises two doses applied with a 6- to 8-week interval, whereas for rats there was a 5-week interval. One dose of vaccine (0.5 ml) contained 50 μg of proteins, 50 μg of polysaccharide, and 2 mg of Al(OH)3 and was administered intramuscularly, deep in the deltoid muscle in humans and in the posterior extremity of rats.
Immune response induction. (i) DTH response.
A group of 50 healthy nursing babies without history of meningococcal disease were included in the study after written consent of the parents. They were immunized twice, at 3.5 months of age and 42 days later. DTH was measured 28 days after the second dose by multipuncture application of nonadsorbed OMVs (14 μg) as antigen. The OMV preparation was diluted (vol/vol) in PBS-glycerol, plus phenol (0.05%); it and the control (without antigen) were applied in the forearm. Induration areas were measured 48 h later, and differences greater than 3 mm between antigen and control were considered positive.
(ii) LP assay.
A group of 20 healthy young adults without history of meningococcal disease and negative for immunoglobulin G (IgG)-specific (anti-OMV) antibodies before immunization participated in this study. They were recruited and included in the study after written consent. Volunteers were immunized intramuscularly with two doses of VA-MENGOC-BC at a 6-week interval. Twenty-one days later fresh peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood by sedimentation on a Ficoll-Hypaque (Pharmacia) gradient. PBMC were cultured in 96-well round-bottom microtiter plates (Costar) at a density of 105 cells per well in 200 μl of RPMI 1640 (Sigma) supplemented with 5% heat-inactivated autologus serum, 10 μg of gentamicin per ml, and 0.3 mg of l-glutamine per ml. Nonadsorbed OMVs from strain B:4:P1.19,15 were added at doses of 0.2, 1, 5, and 10 μg per ml. As a control, PBMC were incubated without the antigen or with phytohemagglutinin (PHA) at a 1% concentration (Gibco). These cells were incubated for 5 days at 37°C and 5% CO2 (ASSAB, Sweden) and pulsed with 1 μCi of [3H]thymidine (Amersham, International Little Chalfont, United Kingdom) over the last 18 h of culture. Cells were harvested, and the incorporated radioactivity was measured in a liquid scintillation counter (Pharmacia, LKB). Results are expressed as the mean counts per minute of triplicate cultures. Volunteers with a stimulation index of ≥2 and ≥1,000 cpm were considered positive. To avoid exclusion of appropriate antigen-presenting cells, unfractionated PBMC were used in the 5-day proliferation assay, which is widely accepted as a measure of T-cell activity.
(iii) Stimulation of cytokine mRNA.
Six adult healthy volunteers without history of meningococcal disease participated in this study. They were recruited and included in the study by regular written consent. They had been immunized with two doses of VA-MENGOC-BC 3 years before. PBMC of each individual were freshly isolated and cultured individually as described for the LP assay. Two milliliters of cellular suspension (8 × 106 per well) was incubated with 9.6 μg of OMV antigen (1.2 μg/106 cells); PHA (2 μg/106 cells) and medium were used as controls. The cells incubated with antigen were harvested after 8, 10, 12, 14, 16, 18, 24, and 72 h of culture. Cells cultured with the mitogen or medium alone were harvested after 10 h. They were washed three times and lysed for RNA isolation.
Total RNA isolation was performed using the guanidine isothiocyanate and organic solvents method (29). Briefly, PBMC were washed three times with PBS and scraped into 1 ml of solution D (4 M guanidine isothiocyanate [Fluka], 0.25 M sodium citrate [pH 7.0; Sigma], 0.5% Sarkosyl [Sigma], 0.1 M 2-mercaptoethanol [Merck]). After homogenization, 0.1 ml of 2 M sodium acetate (pH 4), 1 ml of water-saturated phenol (Fluka), and 0.2 ml of chloroform-isoamyl alcohol (49:1, vol/vol; Merck) were added. RNA was precipitated from the aqueous phase with an equal volume of isopropanol for 1 h at −20°C. The pellet was resuspended in 400 μl of solution D and precipitated again under the same conditions. Finally, the pellet was resuspended in an adequate volume of diethyl pyrocarbonate-treated H2O (Sigma).
The RNase protection assay (RPA) using PharMingen's RiboQuant Multi-Probe RPA kit was performed according to the manufacturer's instructions. A mixture of plasmids containing cDNA templates of different lengths for human cytokines IL-4, IL-5, IL-10, IL-13, IL-14, IL-15, IL-9, IL-2, and IFN-γ and the L32 and GAPDH (glyceraldehyde phosphate dehydrogenase) housekeeping genes were used (12). Briefly, using the templates, radiolabeled antisense RNA probes were synthesized by in vitro transcription reaction using T7 RNA polymerase under appropriate conditions and an excess [α-32P]UTP (Amersham) (27). The radiolabeled probes were purified by phenol-chloroform extraction and ethanol precipitation after the transcription reaction was stopped by adding 1 μl of RNase-free DNase. Excess purified probes were hybridized to 5 μg of purified total RNA from samples in hybridization buffer overnight at 56°C. The free probe and single-stranded RNA (nonprotected) were digested with RNases A and T1 in adequate buffer at 37°C for 45 min. The reaction was stopped by the addition of proteinase K; the remaining RNase-protected probes were purified with phenol-chloroform and ethanol precipitation, resolved on 5% denaturing polyacrylamide gel, and autoradiographed. All the reagents and enzymes were provided by the PharMingen kit. The films were scanned and analyzed using the Molecular Analyst software (Bio-Rad). Since the undigested radiolabeled RNA probe contains flanking frames of plasmid DNA, it migrates at lower rates than protected fragments due to the elimination of nonprotected flanking frames during RNAse digestion. Because the lengths of probes and protected fragments for each cytokine are known, a standard curve was plotted with undigested radiolabeled probes (migration distance versus log nucleotide lengths) and used to establish the identity of RNase-protected bands in experimental samples. The quantity of each mRNA species expression level was determined by the intensity of appropriately sized protected probes and homogenized using the signal from an L32 probe used as the standard.
Effector immune mechanisms. (i) Opsonophagocytic activity.
Nine of the volunteers used in the LP assay who had no serum anti-OMV IgG class antibodies before immunization were included in this study. Sera were collected before vaccination and 4 weeks after the second dose. During the second extraction heparinized blood was also obtained for the purification of phagocytic cells (PMN and macrophages). The erythrocytes were eliminated with lysing solution, and the leukocytes resuspended at 1.25 × 107 phagocytic cells per ml in RPMI 1640 containing 0.5% bovine serum albumin (Sigma). The serogroup B meningococci (N. meningitidis strain B:4:P1.19,15) were grown overnight on Mueller-Hinton agar in 5% CO2 atmosphere at 37°C, inoculated in Frantz-modified medium to an optical density of 620 nm, using a 10-mm light, and grown to logarithmic phase in an orbital shaker at 37°C. Bacteria were washed three times in 0.9% NaCl (2,500 × g for 10 min at 4°C), resuspended in RPMI 1640 (pH 7.2), and adjusted to an optical density of 1, and CFU per milliliter was determined. Bacteria were labeled with fluorescein isothiocyanate (FITC; 0.25 mg/ml) by stirring the bacteria for 30 min at 37°C in PBS, killed by ethanol (0.1% for 1 h), and filtered through 0.22-μm-pore-size filters (Sartorious). They were extensively washed in PBS at 2,500 × g for 10 min at 4°C, resuspended in the same medium, counted by flow cytometry, and adjusted to 5 × 108 cells per ml, and aliquots were stored at −70°C. Bacterial suspensions (5 × 107) were opsonized with 5% autologous sera with or without active complement (inactivation was performed by treatment for 30 min at 56°C) during 15 min, and the cells were added (1:20, cell/bacteria) and incubated for 30 min. Phagocytosis was stopped by adding 1 ml of ice-cold PBS supplemented with 0.02% of EDTA. The suspensions were analyzed by a Cytofluorograph Ortho 50 H interfaced to a model 2150 computer (Ortho Diagnostic Instrument, Westwood, Mass.) with a 488-nm wavelength. FITC fluorescence was measured at 515 to 575 nm, and forward angle light scatter was measured at 488 nm.
(ii) PMN as effector cells.
Two assays were performed. In the first, the in vitro effector activity of PMN against N. meningitidis B (103 bacteria per well) was tested with human PMN (105 cells per well). The blood was obtained as described above (100:1, cell/bacteria). The sedimented erythrocytes were eliminated with lysing solution, and the PMN were resuspended in RPMI 1640 supplemented with 10% fetal calf serum (Gibco). The cells were activated with IFN-γ (10 U/ml) and lipopolysaccharide (LPS; 1 μg/ml) 30 min before addition of the bacteria, and CFU were measured 24 h later. In the second, a rat neutropenic model was used to determine the role of PMN in vivo. Wistar rats (CENPALAB; Havana, Cuba) weighing 80 ± 20 g were randomized in three groups of 10 animals each. The first group was treated before challenge with an unrelated IgM antibody (control), and the second was immunized with 2 doses of VA-MENGOC-BC (25 μg of protein, intramuscularly) 5 weeks apart; the third group was immunized as was group 2 and 2 h before each dose made neutropenic by intraperitoneal inoculation of 2 ml of ascitic fluid of an anti-PMN monoclonal antibody (MAb) (RP3, IgM class; kindly donated by F. Sendo, Yamagata University, Yamagata, Japan) (20). MAb efficiency was measured every day in the peripheral blood by differential counts. All groups were challenged intraperitoneally with 100 50% lethal doses of N. meningitidis B (strain B:4:P1.19,15). Rat survival was recorded over 3 days.
Ethical considerations.
Since children were included in this work, authorization from the National Group of Paediatrics, health authorities, and the Institutional Ethical Committee were necessary, with prior demonstration of safety and innocuousness. Furthermore, the written consent of each parent or guardian was included.
Statistical analysis.
Differences between groups were tested for significance by a paired two-tailed Student's t test. Survival significance was calculated by the exact Fisher probability. The result of RPA analysis was processed with Molecular Analyst software. The X-ray films were digitized using a Hewlett-Packard scanner, and the lengths were determined by comparing the mobility of the bands with that of the undigested probes. The density/area ratios were calculated, and the relative mRNA levels were determined in relation to the density/area ratio of the housekeeping gene L32. The means and standard deviations for the six volunteers were calculated and plotted for each time point measured.
RESULTS
Immune response induction. (i) VA-MENGOC-BC induces a DTH response.
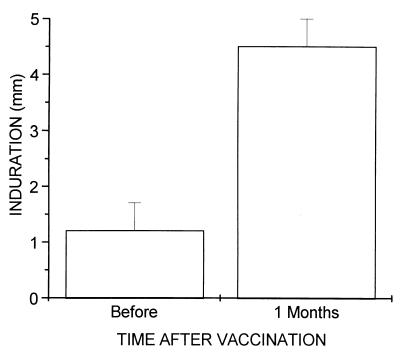
The induction of DTH by VA-MENGOC-BC was evaluated by measuring DTH before immunization of 3.5-month-old nursing babies and 30 days after the second dose. Figure 1 shows the effect of this vaccine on the DTH response. It was negative before immunization, and a remarkable increase was observed after the second dose in all subjects. Six weeks after the first dose, DTH was also positive but much less than after the complete immunization schedule (data not shown).
FIG. 1.
T-cell responses of 50 immunized nursing babies in a DTH assay. The antigens used for stimulation were OMVs from strain 385/83 at 14 μg per test. The data shown represent the mean and standard error of the difference between antigen and control indurations. A response was considered positive if the difference from the control was >3 mm. All babies were DTH positive postvaccination.
(ii) VA-MENGOC-BC induces LP.
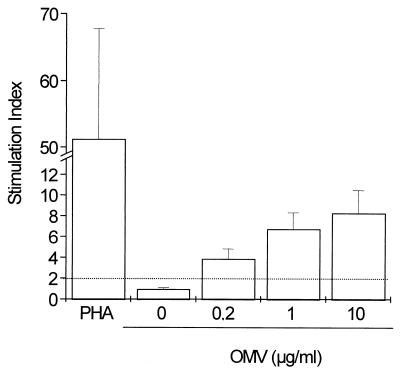
LP assays were carried out to study the T-cell response in vaccinated subjects who were negative for specific IgG before vaccination started. OMVs from the Cuban strain B:4:P1.19,15 were used to study the antigen-specific stimulation of human PBMC 21 days after the second vaccine dose. As shown in Fig. 2, OMVs induced stimulation of PBMC from young adult humans immunized with VA-MENGOC-BC in a dose-response fashion.
FIG. 2.
T-cell responses of 20 immunized adult volunteers in an LP assay. The antigens used for stimulation were OMVs from strain 385/83 at a concentration of 0 to 10 μg per ml. Results are expressed as mean and standard errors of the stimulation index of all cases in triplicate cultures. Dashed lines represent cutoff values.
(iii) The OMVs of VA-MENGOC-BC induce cytokine mRNA.
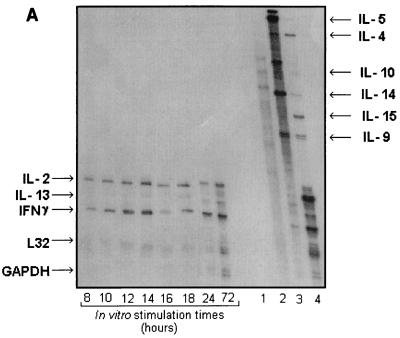
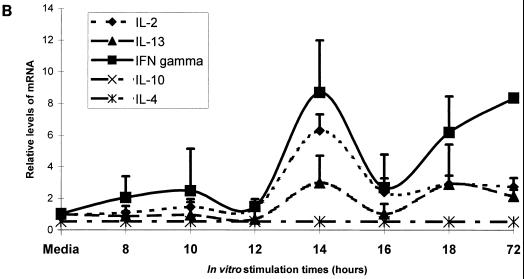
The influence on the cytokine pattern after in vitro OMV challenge of PBMC from VA-MENGOC-BC immunized adult human subjects was evaluated. Figure 3A shows the effects of antigenic stimulation on IFN-γ, IL-2, and IL-13 mRNAs, which appear to follow different kinetics. A graphic representation is shown in Fig. 3B. IFN-γ and IL-2 relative expression levels began to increase at 8 h, reaching a peak at 14 h of stimulation; a decrease was observed at 16 h. IFN-γ transcriptional induction increased again up to at least 72 h, when it reached a level similar to that attained at 14 h. IL-2 mRNA induction remained at similar levels after 16 h. On the other hand, the relative expression of IL-13 showed kinetics similar to those for IFN-γ, but at a much lower level. The induction of IL-4, IL-5, IL-10, IL-14, IL-15, and IL-9 mRNAs were detected in the PHA controls but not in the samples.
FIG. 3.
Cytokine mRNA stimulation. Results of the RPA of one representative case. Five micrograms of total RNA isolated from PBMC stimulated in vitro with OMVs from VA-MENGOC-BC was analyzed with PharMingen's RiboQuant Multi-Probe RPA system. Lane 1, background control: lane 2, RNA control; lane 3, radiolabeled undigested probes; lane 4, PHA-positive control. Note that since undigested radiolabeled RNA probe contains flanking frames of plasmid DNA, it migrates at lower rates than protected fragments due to the elimination of nonprotected flanking frames during RNase digestion (A). (B) Quantification of specific cytokine bands upon autoradiography of RPA of total RNA from PBMC in vitro stimulated with the OMVs from VA-MENGOC-BC, using the Molecular Analyst software. The average and standard errors of six independent cases are represented.
Effector immune mechanism. (i) Immunization with VA-MENGOC-BC induces serum opsonic activity.
The influence of antibodies from VA-MENGOC-BC-immunized subjects (before and 21 days after the second vaccine dose) and autologous complement as opsonins were studied. As shown in Fig. 4, opsonic activity increased after vaccination, and a high percentage of phagocytosed bacteria were observed in postimmunization specimens. The addition of autologous complement before or after immunization significantly increased the opsonizing activity.
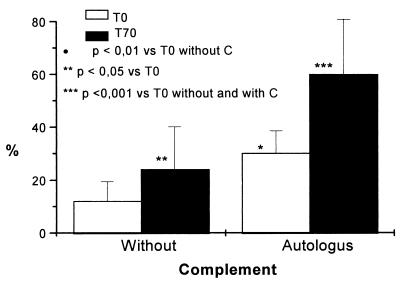
FIG. 4.
Serum opsonic activity of nine immunized adult volunteers as detected by the fluorescence-activated cell sorting assay. Sera were taken before immunization (T0) and 28 days after the second dose (T70). Purified white cells were also obtained the second time. Neisseria meningitidis B was FITC labeled and fixed with methanol. Bacteria were opsonized with 5% heat-inactivated serum in the presence or absence of autologous serum as the complement (C) source, and the nonlymphoid cells were added (1:20, cell/bacteria) for 30 min. The data shown represent the mean and standard errors of all cases in duplicate experiments. Statistical significance is represented.
(ii) Neutrophils appear to be essential cells in the defense against N. meningitidis B.
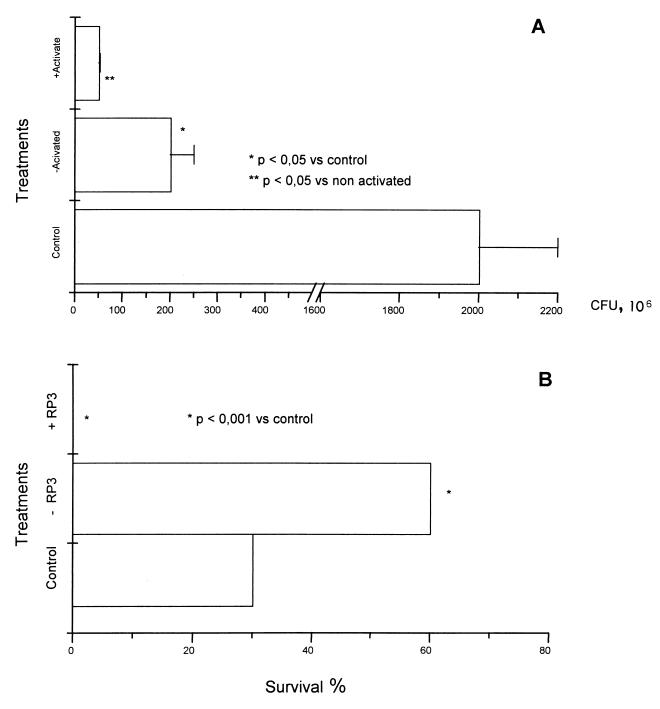
The killer activity of human PMN from immunized volunteers was evaluated in vitro and in vivo. As shown in Fig. 5A, the PMN are efficient in killing N. meningitidis B whether they are activated (IFN-γ plus LPS) or not. In vivo, treatment of rats with MAb RP3 produces peripheral neutropenia (data not shown) as reported (20). Figure 5B shows that vaccination (−RP3 group) significantly increases (P < 0.001) survival in rats; in contrast, 100% mortality was observed in the neutropenic animals after treatment with RP3 at the moment of challenge with N. meningitidis. Survival in animals treated with an irrelevant IgM (control) was 30%.
FIG. 5.
Essential role of neutrophils against Neisseria meningitidis B infection. Activated (LPS plus IFN-γ) or nonactivated PMN are capable of killing N. meningitidis (A). VA-MENGOC-BC (−RP3 group) also protects the rats (P < 0.001), but the elimination of PMN by RP3 treatment before challenge with N. menigitidis B decreased (P < 0.001) their survival.
DISCUSSION
VA-MENGOC-BC is a vaccine that was developed two decades ago; nevertheless, not much is known about its fine mechanisms of action. Indeed, the assumption that this OMV-based vaccine necessarily produces its effect only by development of bactericidal antibodies is premature.
The humoral response induced by VA-MENGOC-BC in humans consists of specific IgG antibody, mainly of IgG subclass (7), with bactericidal activity against some of the most frequent serotype B N. meningitidis pathogens. Nevertheless, the induction of bactericidal antibodies, mainly in babies, remains controversial (2, 30, 47), and no efficacy study has been conducted in this age group. In our opinion, the induction of bactericidal antibodies, the hallmark of the polysaccharide-based vaccines and main goal of the Neisseria vaccinologist (9, 10, 38, 39), does not seem to be totally applicable to OMV-based vaccines, and we feel that this is the principal limitation in the development of such a vaccine. The criteria are based on the necessity of close contact between IgG antibodies (the main antibody class that should be induced by an effective parenteral vaccine) in order to activate complement; the relative dispersion of outer membrane proteins on the bacterial surface; and the relative inaccessibility of these proteins, not only structural but also in the antigenic sense, created by a nonxenogenic capsular B polysaccharide (8, 22, 38). The IgG antibodies with bactericidal activity also have other important biological functions (e.g., as opsonins), and the induction of complement-fixing antibodies is more related to the Th1 pattern of cytokine production (15). This implies that other mechanisms could be present, such as IFN-γ-mediated phagocyte activation (28) in addition to bactericidal antibodies. That is why particular attention was given to the mechanism related to the Th1 cellular response at the afferent and effector branches of the immune response.
We have investigated antigen-specific immune responses after immunization with VA-MENGOC-BC. The cellular responses were measured by in vivo DTH and in vitro proliferation assays against OMVs, the main vaccine component. A strong DTH response was observed against the OMVs in all nursing babies after vaccination. All vaccinees, selected on the basis of low antibody levels against OMVs in order to obtain subjects as naive as possible (26), were positive in the LP assay after vaccination. Although the LP response observed was not very high, the presence of a strong DTH and the in vivo characteristic of this test mean that a functional cell-mediated immune response is induced. T-cell responses were also reported for humans immunized with the Norwegian (26) and Dutch (37) vaccines. The Norwegian vaccine is similar to VA-MENGOC-BC in its fundamental composition (both are OMV-based vaccines), but the Cuban vaccine has a twofold-higher protein content, N. meningitidis purified polysaccharide C is also present, it has a high concentration of A1(HO)3, it does not contain sucrose, and P4 and P5 are less represented (11, 42). The role of these differences and other components at the molecular level of immune induction has not been explored.
The influence of VA-MENGOC-BC immunization on the cytokine pattern of PBMC from immunized subjects was evaluated after in vitro restimulation with OMVs. The increase in IFN-γ and IL-2 mRNAs, but not in IL-4, IL-5, or IL-10 mRNA, strongly suggests that a Th1 pattern of response was stimulated at least in the six adult Cuban volunteers studied. The first peak, observed after 14 h, might be related to the complexity of the OMVs used as antigen for in vitro restimulation. The characteristic wave of mRNA response could be related to the beginning of protein synthesis and secretion or with transcriptional IFN-γ gene regulation. IL-13 is a cytokine related to IL-4 and therefore associated with Th2 responses, but this cytokine has no direct influence on T cells (4, 6, 19, 32, 33).
At the effector level of the immune response, we evaluated the presence of antibodies with opsonic activity and the participation of other cells. An increase in opsonic activity was evident in immunized sera. In addition, the influence of complement on this activity is a well-known phenomenon confirmed in this study. Opsonins against N. meningitidis B have also been reported by others (15, 46). Opsonic activity was also detected in sera from subjects immunized with the Norwegian vaccine (1).
Neutrophils are important effector cells because they are the major leukocytes present during the acute phase of inflammation, represent a high percentage of human white blood cells, and have receptor for Fc immunoglobulins, known to be effective at killing opsonized bacteria. For that reason, the participation of PMN as effector cells was evaluated. The in vitro experiment shows that nonactivated or activated (LPS plus IFN-γ) cells were effective in killing N. meningitidis B, but more important, the elimination of PMN in vivo by treatment with a specific MAb produced 100% mortality in animals challenged with N. menigitidis B. This result suggests that PMN are essential in the defense against this infection, at least in rat models. It is important to note that peripheral blood in the rat consists of only 25% PMN (15); thus, PMN may be even more influential in humans, where the percentage is higher (60 to 70%).
The importance of the Th1 pattern induced by VA-MENGOC-BC seems to be relevant against N. meningitidis B infection, because high levels of IL-10 were associated with fatality in meningococcal disease (21) and the intrathecal production of IL-12 and IFN-γ was observed in patients who had recovered from bacterial meningitis (18).
The relevance of cell-mediated immunity in a Bordetella pertussis animal model has been demonstrated (23, 34). Recently, the importance of the Th1 response has also been reported for patients who have recovered from B. pertussis infection (40). In addition, the response induced by a protective vaccine in humans was also of Th1 pattern (41).
Even though the induction of a Th1-like pattern was demonstrated in this study, the majority of subjects were healthy Cuban adult volunteers; it would be appropriate to carry out a similar study of the primary response against VA-MENGOC-BC in a population of Cuban nursing babies, where the effects of environmental antigens can be decreased, or in other countries, where the circulation of Neisseria and other cross-reactive microorganisms is low, work that is in progress.
ACKNOWLEDGMENTS
This work was supported by Finlay Institute.
We are indebted to E. LeRiverend for English corrections and to D. I. Stoot for criticism, suggestions, and English corrections.
REFERENCES
- 1.Aase A, Bjune G, Hoiby E A, Rosenqvist E, Pedersen A K, Michaelsen T E. Comparison among opsonic activity, antimeningococcal immunoglobulin G response, and serum bactericidal activity against meningococci in sera from vaccinees after immunization with a serogroup B outer membrane vesicle vaccine. Infect Immun. 1995;63:3531–3536. doi: 10.1128/iai.63.9.3531-3536.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campa H C, Sierra V G. VA-MENGOC-BC®. Vacuna contra la enfermedad meningocóccica de los grupos B y C. La Habana, Cuba: Centro Nacional de VAM. Ministerio de Salud Pública; 1989. [Google Scholar]
- 3.Canadian Council on Animal Care. Guide to the care and use of experimental animals. Vol. 1. Ottawa, Ontario, Canada: Canadian Council on Animal Care; 1984. p. 86. [Google Scholar]
- 4.Coffman R L, Lebman D A, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 5.De Moraes J C, Perkins B A, Camargo M C C, Hidalgo N T R, Barbosa H A, Sacchi C T, Landgraf I M, Gattas V L, de G. Vasconcelos H, Plikaytis B D, Wenter J D, Broome C V. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Figdor C G, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, de Vries J E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 7.Feriol X. Graduate thesis. Havana, Cuba: University of Havana; 1992. [Google Scholar]
- 8.Fine J, Bitter-Suerman D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extra-neural tissues. J Immunol. 1987;138:4402–4407. [PubMed] [Google Scholar]
- 9.Frasch C E. Vaccines for prevention of meningococcal disease. Clin Microbiol Rev. 1989;2:S134–S138. doi: 10.1128/cmr.2.suppl.s134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasch C E. Meningococcal vaccine: past, present and future, p 245–283. In: Cartwright K, editor. Meningococcal disease. New York, N.Y: John Wiley & Sons Ltd.; 1995. [Google Scholar]
- 11.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Froholm L O, Lindbak A K, Mogster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-Vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–80. [PubMed] [Google Scholar]
- 12.Gilman M. Ribonuclease protection assay. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons Ltd.; 1988. pp. 4.7.1–4.7.8. [Google Scholar]
- 13.Goldschneider I, Gotschlich E C, Artenstein M A. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschneider I, Gotschlich E C, Artenstein M A. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guttormsen H K, Bjerknes R, Naess A, Lehmann V, Halstensen A, Sornes S, Solberg C O. Cross-reacting serum opsonins in patients with meningococcal disease. Infect Immun. 1992;60:2777–2783. doi: 10.1128/iai.60.7.2777-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huergo, C. C., V. G. Sierra Gonzalez, M. M. Gutierrrez Vazquez, G. Bisset Jorrin, L. G. Garcia Imia, G. de la Caridad Puentes Rizo, M. del Carmen Sampedro Herrera, F. Sotolongo Padron, E. X. Le Riverend Morales, and M. A. Galguera Dominguez. January 1997. U.S. patent 5,597,572.
- 17.Jones D M, Borrow R, Fox A J, Gray S, Cartwright K A, Poolman J T. The lipooligosaccharide as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992;13:219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 18.Kornelisse R F, Hack C E, Savelkoul H F, van der Pouw Kraan T C, Hop W C, van Mierlo G, Suur M H, Neijens H J, de Groot R. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect Immun. 1997;65:877–881. doi: 10.1128/iai.65.3.877-881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse N, Tony H P, Sebald W. Conversion of human interleukin-4 into high affinity antagonist by a single aminoacid replacement. EMBO J. 1991;11:3237–3244. doi: 10.1002/j.1460-2075.1992.tb05401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo C, Yamashita T, Araki A, Tershita M, Watanabe T, Atsumi M, Tamura M, Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP3. Inhibition by RP3 treatment of the priming and effector phases of delayed type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993;150:3728–3738. [PubMed] [Google Scholar]
- 21.Lehmann A K, Halstensen A, Sornes S, Rokke O, Waage A. High levels of interleukin 10 are associated with fatality in meningococcal disease. In: Zollinger W D, Frasch C E, Deal C D, editors. Proceedings of the 10th International Pathogenic Neisseria Conference. Baltimore, Md. 1996. pp. 291–292. [Google Scholar]
- 22.Mandrell R E, Kim J J, John C M, Gibson B W, Sugai J V, Apicella M A, Griffiss J M, Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministerio de Salud. Anuario Estaístico de Salud. Havana, Cuba: Dirección Nacional de Estadística del Ministerio de Salud Pública de Cuba no. 27. Ministerio de Salud Pública; 1998. [Google Scholar]
- 25.Mossman T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cells clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 26.Naess L M, Oftung F, Aase A, Wetzler L M, Sandin R, Michaelsen T E. Human T-cell responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine. Infect Immun. 1998;66:959–965. doi: 10.1128/iai.66.3.959-965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor M S, Relf M, Balkwill F R. Northern analysis, ribonuclease protection, and in situ analysis of cytokine messenger RNA. In: Balkwill F R, editor. Cytokines: a practical approach. Oxford, England: Oxford University Press; 1995. pp. 35–36. [Google Scholar]
- 28.Pailbock D M. Macrophage activation by T cells. Curr Opin Immunol. 1992;4:144–349. doi: 10.1016/0952-7915(92)90087-u. [DOI] [PubMed] [Google Scholar]
- 29.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1984;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 30.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Hoiby E A, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa H C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 31.Poolman J T. Polysaccharides and membrane vaccines. In: Robbins J B, editor. Bacterial vaccines. New York, N.Y: Alan R. Liss, Inc.; 1990. pp. 57–86. [PubMed] [Google Scholar]
- 32.Punnonen J, de Vries J E. Characterization of a novel CD2+ human thymic B cell subset. J Immunol. 1993;151:100–110. [PubMed] [Google Scholar]
- 33.Punnonen J, de Vries J E. IL-13 induced proliferation, Ig isotype switching and Ig synthesis by immature fetal B cells. J Immunol. 1994;152:1094–1102. [PubMed] [Google Scholar]
- 34.Redhead K, Watkins J, Barnard A, Mills K H G. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romagnani S. Human Th1 and Th2 subsets: doubt no more. Immunol Today. 1991;12:256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani S. TH1 and TH2 subsets of CD4+ T lymphocytes. Sci Am Sci Med. 1994;May/June:68–77. [Google Scholar]
- 37.Rouppe van-der-Voort E M, van-Dijken H, Kuipers B, van der Biezen J, van der Ley P, Meylis J, Claassen I, Poolman J. Human B- and T-cell responses after immunization with a hexavalent PorA meningococcal outer membrane vesicle vaccine. Infect Immun. 1997;65:5184–5190. doi: 10.1128/iai.65.12.5184-5190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouppe van-der-Voort E M. Meningococcal vaccines. A continuous crusade? Academisch Proefschrift thesis. Bilthoven, The Netherlands: National Institute of Public Health and the Environment; 1998. [Google Scholar]
- 39.Rouppe van-der-Voort E M, van der Ley P, van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J T. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun. 1996;64:2745–2751. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills K H G. Distinct T-cell subtypes induced with a hole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H G. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 42.Sierra V G, Campa H C. Preclinical and clinical studies with the antimeningococcal vaccine BC: VA-MENGOC-BC®. 1990. Rev. Interferón Biotecnol. special volume. [Google Scholar]
- 43.Sierra G V, Campa C, Garcia L, Sotolongo F, Izquierdo L, Valcárcel M, Casanueva V, Baró M, Leguen F, Rodríguez R, Terry H. Proceedings of the 7th International Pathogenic Neisseria Conference. Berlin, Germany: Walter de Gruyter & Co.; 1990. Efficacy Evaluation of the Cuban vaccine VA-MENGOC-BC® against disease caused by serogroup B Neisseria menigitidis; pp. 129–134. [Google Scholar]
- 44.Sierra V G, Campa C, Valcárcel N M, García L, Izquierdo L, Sotolongo F, Casanueva V, Rico C O, Rodríguez C R, Terry H. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–210. [PubMed] [Google Scholar]
- 45.Singh A P, Khuller G K. Induction if immunity against experimental tuberculosis with mycobacterial mannophospho-inositides encapsulated in liposomes containing lipid A. FEEMS Immunol Med Microbiol. 1994;8:119–126. doi: 10.1111/j.1574-695X.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 46.Sjursen H, Bjerknes R, Halstensen A, Naess A, Sernes S, Solberg C O. Flow cytometric assay for the measurement of serum opsonins to Neisseria meningitidis serogroup B, serotype 15. J Immunol Methods. 1989;116:135–243. doi: 10.1016/0022-1759(89)90209-3. [DOI] [PubMed] [Google Scholar]
- 47.Tappero J, Lagos R, Maldonado A, Herrera P, Gheesling L, Williams D, Carlone G, Plikaytis B, Nokleby H, Holst J, Sierra G, Perskins B. Serum bactericidal activity elicited by two outer membrane protein serogroup B meningococcal vaccines among infants, pre-school children, and adults in Santiago, Chile. In: Zollinger W D, Frasch C E, Deal C D, editors. Proceedings of the 10th International Pathogenic Neisseria Conference. Baltimore, Md. 1996. [Google Scholar]
- 48.Valcárcel M, Almeida L, Leguen F, Sotolongo F, Izquierdo L, Campa C, Sierra V G, Galindo A, Rodríguez R, Terry H. Proceedings of the 7th International Pathogenic Neisseria Conference. Berlin, Germany: Walter de Gruyter & Co.; 1990. Epidemiological behavior of meningococcal disease in Cuba; pp. 135–139. [Google Scholar]
- 49.Wyle F E, Artenslein M S, Branck R L, Tramont E C, Kasper D L, Altieri P L, Berman S L, Lowenthal J P. Immunological response in man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;136:514–522. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 50.Zangwill K M, Stout R W, Carlone G M, Pais L, Harekeh H, Mitchell S, Wolfe W H, Blackwood V, Plikaytis B D, Wenger J D. Duration of antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis. 1994;169:847–852. doi: 10.1093/infdis/169.4.847. [DOI] [PubMed] [Google Scholar]