Abstract
The ability of genetic vaccination to protect against a lethal challenge of anthrax toxin was evaluated. BALB/c mice were immunized via gene gun inoculation with eucaryotic expression vector plasmids encoding either a fragment of the protective antigen (PA) or a fragment of lethal factor (LF). Plasmid pCLF4 contains the N-terminal region (amino acids [aa] 10 to 254) of Bacillus anthracis LF cloned into the pCI expression plasmid. Plasmid pCPA contains a biologically active portion (aa 175 to 764) of B. anthracis PA cloned into the pCI expression vector. One-micrometer-diameter gold particles were coated with plasmid pCLF4 or pCPA or a 1:1 mixture of both and injected into mice via gene gun (1 μg of plasmid DNA/injection) three times at 2-week intervals. Sera were collected and analyzed for antibody titer as well as antibody isotype. Significantly, titers of antibody to both PA and LF from mice immunized with the combination of pCPA and pCLF4 were four to five times greater than titers from mice immunized with either gene alone. Two weeks following the third and final plasmid DNA boost, all mice were challenged with 5 50% lethal doses of lethal toxin (PA plus LF) injected intravenously into the tail vein. All mice immunized with pCLF4, pCPA, or the combination of both survived the challenge, whereas all unimmunized mice did not survive. These results demonstrate that DNA-based immunization alone can provide protection against a lethal toxin challenge and that DNA immunization against the LF antigen alone provides complete protection.
Anthrax is a well-known disease and was one of the first to be described in association with its causative organism, Bacillus anthracis (18). Although the disease is well characterized, it is only in recent years that we have begun to understand the molecular basis of anthrax. The principal virulence factor of B. anthracis is a multicomponent toxin secreted by the organism that consists of three separate gene products designated protective antigen (PA), lethal factor (LF), and edema factor (EF). The genes encoding these toxin components (pag, lef, and cya, respectively) are located on a 184-kb plasmid designated pXO1, carried by all strains of B. anthracis (26). PA (735 amino acids [aa]; Mr, 82,684) is a single-chain protein which binds to an as yet unidentified receptor on the cell surface and subsequently undergoes furin-mediated cleavage to yield a 63-kDa receptor-bound product (8, 16, 21, 31). The 63-kDa PA fragment forms a heptameric complex on the cell surface which is capable of interacting with either the 90-kDa LF protein or the 89-kDa EF protein, which is subsequently internalized (27, 31). LF (776 aa; Mr, 90,237) is a zinc metalloprotease that cleaves several isoforms of mitogen-activated protein kinase kinase (Mek1, Mek2, and MKK3), thereby disrupting signal transduction events within the cell and eventually leading to cell death (6, 30). The EF protein (767 aa; Mr, 88,808) is a calmodulin-dependent adenylate cyclase that causes deregulation of cellular physiology, leading to clinical manifestations that include edema (19). The LF protein combines with PA to form what is referred to as lethal toxin (Letx), which is considered to be the primary factor responsible for the lethal outcome of anthrax infection.
One of the earliest successful vaccines was an attenuated strain of B. anthracis used by Louis Pasteur to vaccinate sheep against anthrax (29). The current Food and Drug Administration-approved anthrax vaccine in the United States is produced from the culture supernatant fraction of the V770-NP1-R strain of B. anthracis and consists principally of PA adsorbed onto aluminum hydroxide. Protection against anthrax infection is associated with a humoral immune response directed against PA (14, 15). Some evidence suggests that EF and LF may also contribute to specific immunity (15, 24, 32), although these components have not been formulated into a subunit vaccine. At this time, there is significant interest in the development of a more highly defined anthrax vaccine and numerous efforts directed toward that goal are in progress. In this regard, in recent years there has been substantial interest in the development of DNA-based vaccines for genetic immunization due to the potential advantages associated with this approach (5, 25). With respect to DNA-based immunization against anthrax, it was demonstrated that one can obtain a protective response to an Letx challenge by immunization with a plasmid encoding the 63-kDa protease-cleaved fragment (PA63) of PA (9). In the present study, our goals were to extend those observations and to explore whether DNA-based immunization against the LF gene product would contribute to or provide protection against an Letx challenge. In addition, we sought to explore whether combined immunization with genes encoding PA and LF would provide additional protection against the effects of Letx. In order to establish a baseline response for future epitope mapping considerations, we chose to utilize the minimum PA and LF structures which could form a functional binding complex while eliminating the metalloprotease function of LF. Therefore, these experiments were carried out using the gene fragment encoding PA63, which is capable of binding to the PA receptor and to LF, and the gene fragment encoding LF4 (aa 1 to 254), which contains the N-terminal one-third of the LF antigen but lacks the domain associated with the LF metalloprotease function yet retains the ability to bind to PA63 (2,12).
MATERIALS AND METHODS
Construction of PA and LF expression plasmids.
The eucaryotic expression plasmid pCI (Promega, Inc., Madison, Wis.) was used in this study for the expression of truncated versions of the PA and LF proteins. The gene fragment encoding aa 175 to 764 of the PA protein was PCR amplified using the plus-strand primer 5′-ACA AGT CTC GAG ACC ATG GTT CCA GAC CGT GAC-3′ and the minus-strand primer 3′-CTC TAT CCT ATT CCA TTA AGA TCT ACT AAA-5′, with pYS2 as a template (33, 35). Included in the primer sequences are XhoI and XbaI restriction sites (underlined), respectively. The PA gene fragment expressed in this study corresponds to the biologically active, protease-cleaved PA63 fragment of the full-length 83-kDa protein (8). The PCR product was digested with XhoI and XbaI and ligated into the pCI vector, which had been cut with the same two restriction enzymes. The plasmid construct pCLF4 encodes aa 10 to 254 of LF, which constitutes the PA binding domain. This plasmid was constructed from a PCR-amplified fragment using the primers 5′-GT CAT GGT CTA GAA ACC ATG CAC GTA AAA GAG-3′ and 3′-TTG CTT GTT CTT TAT ATT TAG ATA TCA GAT CTG CAT-5′, which incorporate XbaI cleavage sites (underlined). The XbaI-digested PCR and pCI plasmid fragments were ligated to form the pCLF4 plasmid used in this study. Neither of the resulting plasmid constructs, pCPA and pCLF4, contains a signal sequence for secretion of the expressed gene product. All plasmids were purified from Escherichia coli DH5α using Endo-free plasmid preparation kits (Qiagen) and were dissolved before use in phosphate-buffered saline (0.15 M NaCl, 0.01 M Na phosphate, pH 7.3).
Protein preparations.
PA, LF, and LFE687C (LF7) used in this study were expressed and purified as previously described (20, 28). LFE687C is the full-length, enzymatically inactive LF protein containing the indicated amino acid substitution within the zinc-binding active site (17).
DNA vaccination.
One-micrometer-diameter gold particles were coated with purified plasmid DNA according to the instructions of the manufacturer (Bio-Rad Laboratories, Richmond, Calif.). Separate groups of female BALB/c mice at 4 to 5 weeks of age (Jackson Laboratories, Bar Harbor, Maine) were immunized intradermally in the abdomen via biolistic particle injection (Helios gene gun; Bio-Rad Laboratories) on days 0, 14, and 28 with approximately 1 μg of plasmid DNA-coated gold particles for each injection. Immunization groups included mice injected with the same microparticles coated with pCPA, pCLF4, a 1:1 mixture of the pCPA and pCLF4 plasmids, or, as a vector control, the pCI plasmid. For the prime-boost immunization experiments, groups of BALB/c mice were first immunized twice with plasmid DNA as described above and then with a third and final boost of purified antigen (12.5 μg) (PA and/or LF7) emulsified in Freund's incomplete adjuvant (1:1 ratio of adjuvant to protein, vol/vol). The protein immunizations were administered intramuscularly. Blood samples were obtained 2 weeks following each vaccination, and the sera were pooled and stored at −20°C until analyzed.
Mouse macrophage protection assay.
The cytotoxicity of the purified Letx was established using a previously described macrophage cytotoxicity assay (28, 34). For the protection assay, J774A.1 mouse macrophage cells were placed in flat-bottom 96-well microtiter plates at a concentration of 6 × 104 cells/well in Dulbecco's modified Eagle's medium (Sigma, St. Louis, Mo.) with 7% fetal bovine serum, 4.5 g of glucose per liter, and 2 mM l-glutamine and incubated for 24 h at 37°C. Serum from a pCLF4-immunized New Zealand White rabbit was serially diluted and incubated with LF protein for 1 h to allow neutralization to occur. Following this incubation, the LF–anti-LF mixture was added to PA protein to achieve a final concentration of 3 μg of Letx per ml. This preparation was incubated at room temperature for 1 h prior to being added to the cells, which were then incubated for an additional 7 h at 37°C. At the end of the incubation, 100 μl of 0.5-mg/ml MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) per well was added, followed by a 1-h incubation. Cells that survive exposure to Letx are able to oxidize MTT to an insoluble purple pigment, thus providing a proportional measure of the viability of the cells. At the end of the incubation period, the culture supernatant was aspirated, 50 μl of a solution containing 0.5% (wt/vol) sodium dodecyl sulfate and 25 mM HCl in 90% (vol/vol) 2-propanol was added, and the suspension was vortexed. The A450 was determined using a microplate reader (Bio-Tek Instruments, Inc.).
In vivo protection experiment.
Plasmid-immunized BALB/c mice that had received a total of three injections were challenged with purified Letx 2 weeks following the third and final injection. The challenge was conducted by tail vein injection of a previously mixed combination of purified PA and LF proteins (60 μg of PA and 25 to 30 μg of LF per mouse), the equivalent of approximately 5 50% lethal doses (LD50) of Letx.
ELISA for anti-PA and anti-LF antibodies.
Titers of antibodies to PA and LF were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, Immulon 4 96-well plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated at 4°C overnight with 100 ng of purified PA or LF7 protein dissolved in 0.1 M carbonate buffer, pH 9.6. Plates were washed with TBS (Tris-buffered saline; 50 mM Tris-HCl, 0.15 M NaCl, pH 7.3) and blocked with 1% (wt/vol) bovine serum albumin in TBS. Serum samples were serially diluted in TBS containing 0.05% Tween 20 and added to the plates. All incubations were carried out at 37°C for 1 h. Anti-mouse immunoglobulin G (IgG) conjugated to horseradish peroxidase (Amersham Life Science, Arlington Heights, Ill.) was added as the secondary antibody. The presence of bound antibody was detected following a 30-min incubation in the presence of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Zymed, South San Francisco, Calif.), and absorbance was read at 405 nm using a Bio-Rad model 550 plate reader. Antibody titers were defined as the highest dilution in serum that resulted in an absorbance value two times greater than that of a serum sample from a nonimmune control, with a minimum value of 0.05. Antibody isotypes were determined in a similar manner, except that anti-mouse IgG1 or anti-mouse IgG2a conjugated to alkaline phosphatase was used as the secondary antibody (Zymed Laboratories). Antibody quantitation was done by ELISA analysis using a standard curve with purified IgG1 and IgG2 antibody reagents.
RESULTS
Immunization with plasmids encoding portions of PA or LF.
This study utilized the pCI mammalian expression vector (Promega), in which the human cytomegalovirus immediate-early enhancer-promoter region drives strong, constitutive expression of the incorporated gene (Fig. 1). In this study we chose to express only partial sequences of the PA and LF genes, as shown in Fig. 1. The pCPA plasmid expresses a truncated version of the PA gene product, aa 175 to 735, which is PA63 lacking the furin cleavage site (aa 164 to 167), yet is fully functional in vivo (8). The pCLF4 plasmid expresses a truncated form of LF, aa 10 to 254, which lacks the catalytic domain of LF yet retains PA63 binding activity and is therefore capable of interacting with the truncated form of PA expressed from pCPA (1).
FIG. 1.
Plasmid vectors used in this study. Plasmid pCI (Promega, Inc.), a eucaryotic expression vector, was used to express aa 10 to 254 of B. anthracis LF protein and aa 175 to 764 of B. anthracis PA protein. CMV, cytomegalovirus; SV40, simian virus 40.
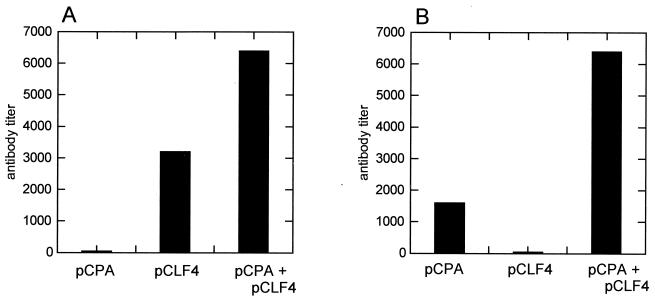
Groups of female BALB/c mice were administered 1-μm-diameter gold beads coated with plasmid DNA (pCPA, pCLF4, or pCI) according to the instructions of the manufacturer (Bio-Rad Laboratories) and introduced via biolistic particle injection (gene gun). Each injection introduced approximately 1 μg of plasmid DNA. Injections were given at 2-week intervals for a total of three injections. Separate groups of mice received plasmid injections of pCPA, pCLF4, a 1:1 mixture of these two plasmids, or a vector control consisting of the pCI plasmid. Two weeks following the third and final injection, pooled antisera were evaluated for their antibody responses to PA and/or LF. Figure 2 demonstrates that each immunized group produced significant titers of antibody to the antigen against which it had been immunized. Significantly, at day 42 titers of antibody to the LF antigen, following DNA immunization, appeared to be about twice the titers of antibody to the PA antigen observed following pCPA immunization. This result suggests that the LF antigen may induce a greater antibody response due to the increased immunogenicity of the LF protein. It is also to be noted that coimmunization with the pCPA and pCLF4 plasmids resulted in a significantly greater overall antibody response to either PA or LF compared to antibody responses following separate immunizations with either gene alone. This result suggests the possibility of some form of synergistic effect when these two genes are coadministered. This observation is also supported by the results of a second series of pCPA and pCLF4 immunizations of a separate group of BALB/c mice (Fig. 3). These results demonstrate that significantly higher endpoint titers of antibody to both PA and LF are obtained when mice are coimmunized with the PA and LF genes.
FIG. 2.
Titers of antibodies to purified LF protein (A) or PA (B) in the sera of BALB/c mice immunized with pCPA, pCLF4, or a combination of pCPA and pCLF4.
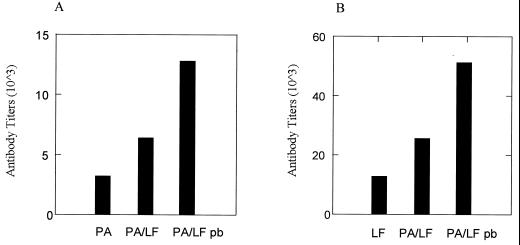
FIG. 3.
Serum antibody titers in BALB/c mice immunized against PA with pCPA (PA), pCPA and pCLF4 (PA/LF), and pCPA and pCLF4 and boosted with PA and mutant LF proteins on day 28 (PA/LF pb) (A) or against LF with pCLF4 (LF), pCLF4 and pCPA (PA/LF), and pCPA and pCLF4 and boosted with PA and mutant LF protein on day 28 (PA/LF pb) (B).
Plasmid immunization results in a protective response.
Small groups of BALB/c mice were immunized three times with pCPA, pCLF4, a 1:1 combination of pCPA and pCLF4, or the plasmid vector (pCI). In an effort to determine whether DNA-based immunization alone can provide protection against exposure to Letx, these mice were challenged with 5 LD50 of Letx administered intravenously. The results of this challenge study are presented in Table 1, where it can be seen that all animals immunized with a plasmid containing the gene for either PA or LF survived. Control mice succumbed to the Letx challenge within hours. These results demonstrate that DNA-based immunization alone can provide a protective response to exposure to the lethal anthrax toxin.
TABLE 1.
Vaccination with plasmid pCPA or pCLF4 or a combination of these plasmids confers protection against lethal anthrax toxin challenge
| Challenge dose/mouse | LD50 | No. of survival/no. of mice immunized with plasmid DNAa
|
|||
|---|---|---|---|---|---|
| Vector | pPA | pLF4 | pLF4 + pPA | ||
| 60 μg of PA, 25 μg of LF4 | 5 | 0/3 | 3/3 | 3/3 | 4/4 |
A mixture of PA and LF was injected intravenously into multiply immunized or vector-treated BALB/c mice.
Comparison of prime-boost and DNA-only immunizations.
In a separate set of experiments we investigated the ability to enhance titers of antibodies to PA and LF using the prime-boost method. This method consists of priming the immune system with a series of three plasmid-based immunizations followed by a final booster immunization with the protein antigen. In Fig. 3 it can be seen that coadministration of the pCPA and pCLF4 plasmids followed by a final protein booster immunization with the recombinant PA and LF7 antigens produced a substantially higher endpoint titer against either PA or LF at the same time point than the antibody titers resulting from DNA-based immunization alone. It was also observed that a consistently higher antibody titer formed against the LF antigen regardless of the immunization regimen used.
Further analysis of the antisera from plasmid-immunized mice indicated that the predominant antibody type produced as a result of these immunizations is of the IgG1 subclass (Table 2), although significant levels of subclass IgG2 antibodies were also produced. Importantly, protection against anthrax toxin has been associated with the production of subclass IgG1 antibodies or a TH2-type response (23). Thus, while the majority antibody response is characteristic of a TH2-type immune response, it is clear that there is also a significant TH1-type response as well. These results are consistent with those of a previous report by Gu et al. (9).
TABLE 2.
Levels of IgG1 and IgG2a antibodies to purified mutant LF and PA proteins
| Treatment | Anti-PA
|
Anti-LF
|
||
|---|---|---|---|---|
| IgG1 | IgG2a | IgG1 | IgG2a | |
| PAa | 0.6 | 0.5 | ||
| LFb | 38 | 0.2 | ||
| LF and PAc | 0.3 | 0.1 | 69 | 0.1 |
| PA prime boostd | 2 | 0.1 | ||
| LF prime booste | 1,164 | 2.7 | ||
| PA and LF prime boostf | 13 | 4 | 538 | 2.5 |
Sera collected from mice immunized with a DNA vaccine plasmid encoding PA.
Sera collected from mice immunized with a DNA vaccine plasmid encoding LF.
Sera collected from mice immunized with a DNA vaccine plasmid encoding PA and LF.
Sera collected from mice immunized with a DNA vaccine plasmid encoding PA and boosted with 12.5 μg of purified PA protein.
Sera collected from mice immunized with a DNA vaccine plasmid encoding LF and boosted with 12.5 μg of purified LF protein.
Sera collected from mice immunized with a DNA vaccine plasmid encoding PA and LF and boosted with 12.5 μg of purified PA and LF protein.
Neutralization of Letx using anti-LF4 serum.
To determine whether anti-LF antibodies in the sera of pLF4-vaccinated animals could protect against the effects of Letx challenge, an in vitro neutralization assay was performed using murine J774A.1 cells, which are sensitive to Letx. As shown in Fig. 4, a 1:4 dilution of serum from a rabbit immunized two times with pCLF4 and then with a single protein boost with recombinant LF7 conferred 100% protection against the cytotoxic effects of Letx. The results of this assay confirm the macrophage cytotoxicity of the Letx preparations used in this study and demonstrate that the anti-LF antibody inhibits Letx cytotoxicity by blocking the incorporation of LF into the cell.
FIG. 4.
Neutralization of anthrax toxin by rabbit anti-LF4 antibody. Various dilutions of anti-LF4 serum were preincubated with recombinant LF (•) for 1 h. The mixture was added to J774A.1 cells in the presence of Letx for 7 h, and cell viability was measured. ▪, absence of MTT; ▴, negative Letx control.
DISCUSSION
It was previously demonstrated that DNA-based expression vectors encoding PA of B. anthracis are immunogenic and can produce a protective response to Letx challenge in a mouse model system (9). In the present study, we have not only repeated these observations concerning PA but also demonstrated that it is possible to produce protective immunity against anthrax Letx by DNA-based immunization using a truncated form of the LF gene which produces an N-terminal fragment of LF lacking metalloproteinase activity. Furthermore, we observed a distinct synergistic effect associated with coimmunization using plasmids encoding fragments of the PA and LF genes, which results in significantly higher antibody responses to both PA and LF. In this study we demonstrated that a significant antibody response is generated using DNA-based immunization alone and that the levels of antibody produced are sufficient to protect animals against a Letx challenge that is five times the LD50.
Of potential significance, it is worth noting that, in the previous DNA immunization study, PA was expressed in a form that may be secreted from the cell but that, in the present study, the expressed PA and LF proteins were not expected to be secreted. In vitro transfection experiments using human UM449 cells demonstrated the expression of these antigens but did not provide evidence of their release from the cell (data not shown). In spite of the apparent lack of secretion of PA and LF, a substantial antibody response was generated. This result is not surprising in view of the study previously reported by Haddad et al. (13). That study demonstrated that, while attachment of a secretion signal sequence resulted in differential intracellular targeting of an encoded antigen, the presence of a signal sequence had no effect on B-cell recognition of the antigen and the subsequent production of an antibody response. Thus, it is apparently not necessary to attach a secretion signal sequence to PA or LF in order to obtain a protective antibody response to Letx. We have recently reported similar findings in a DNA-based immunization study using Pseudomonas aeruginosa exotoxin A (4). At this time it is not clear how the truncated PA and LF proteins may exit the cell for interaction with B cells.
What also remains unclear at this point is how PA63 and the LF4 antigen are processed within the cell in a manner that enables them to induce an enhanced antibody response when administered together. Nonetheless, we consistently observed a significantly greater titer of antibody to either PA or LF whenever the two genes were coadministered (Fig. 2 and 3). Although this observation was not supported in one instance by the data in Table 2 (comparison of PA-specific IgG levels between pCPA- and pCPA- or pCLF4-immunized groups), the data in Table 2 generally support the observation that coimmunization with the pCPA and pCLF4 plasmids produces an enhanced response. It is perhaps worth noting in this regard that the comparison between levels of PA-specific IgG (Table 2) and titers of antiserum to PA (Fig. 2 and 3) may not be directly comparable as determined in these separate assays. Nevertheless, an enhancement of the immune response following coimmunization with PA and LF has recently been reported by others (3). Of possible significance with regard to the enhanced antibody response observed following coimmunization with both the PA and LF4 genes is the reported mitogenic activity associated with LF (3, 10, 11, 32). Brossier et al. reports that the adjuvant effect requires binding activities between LF and PA but does not depend on the subsequent binding of the Letx complex to cells (3). In this regard, the ability of LF4 to bind to PA63 would be consistent with the requirement for a PA-LF complex in the development of a synergistic immune response when these antigens are coadministered. This hypothesis would also predict that use of a mutated form of PA that is unable to bind to the cellular receptor would not diminish the synergistic immune response following coimmunization with the PA and LF antigens. Such experiments are in progress. A significant difference in the results of experiments reported in the present study is that when PA63 and the LF4 antigen are first expressed within the cell, as under the conditions of these experiments, we also observe a synergistic immune response. In addition, the immune response to the LF antigen is generally greater than the response to PA, unlike the response reported by Brossier et al. when they used spore-based immunizations (3). Also, we find that intracellular expression of the LF4 antigen alone is sufficient to produce a significant immune response to the LF antigen without any requirement for previous binding to PA. Clearly, the synergism observed upon coimmunization with these genes does not depend on any metalloprotease activity associated with the LF antigen since a truncated form of the protein is expressed.
As expected, the majority of the immune response appears to be a TH2-type response, consistent with previously reported results of a study using plasmid-based immunization with the intact PA gene (9). This is clearly seen in the data presented in Table 2, where it can be seen that the predominant response is the production of IgG1 antibody in all cases. In the previous study plasmid immunizations were conducted via intramuscular injection, whereas in this study plasmids were delivered by means of a gene gun. This difference in results with various techniques is interesting in view of the fact that it has been reported that intramuscular vaccination with DNA generally produces a predominant TH1 response but that gene gun vaccination produces a TH2 response (7). However, it was noted by Gu et al. that both TH1 and TH2 cytokines were induced following DNA-based immunization with the intact PA gene (9).
It is noteworthy that we have been able to produce a protective response by immunizing with a truncated form of the LF antigen, and as far as we know, this is the first time protection following immunization against the LF antigen alone has been reported. Presumably, anti-LF antibodies that result following DNA-based immunization are capable of inhibiting the binding between PA and LF in vivo, thus preventing the formation of the Letx complex. This possibility is supported by the results depicted in Fig. 4, which demonstrate that anti-LF antibodies block the effect of Letx on cultured mouse macrophages. Also of interest is the greater immunogenicity of the LF4 protein. Once again, this is clearly observed in Fig. 2 and 3, as well as in the data presented in Table 2. In related experiments, we have observed that titers of anti-LF antibody remain at high levels for much longer periods of time than do titers of anti-PA antibody (data not shown). Collectively, these results support a possible role for an anti-LF antibody response in protection against anthrax infection. This possibility is a significant result in view of the fact that PA alone has been the primary target for vaccine studies since immunity against PA has been stated to be both necessary and sufficient for protection against anthrax infection (22). One implication of the findings presented in this report is that individuals who have been immunized with the current U.S. anthrax vaccine have undoubtedly produced an antibody response to the LF antigen, which is found in small quantities in the vaccine. The results presented in this paper suggest that the antibody response to LF following the current vaccine immunization series may comprise a significant part of the overall protective response to anthrax infection. This possibility is further supported by the fact that the LF antigen appears to be much more immunogenic and produces an immune response which lasts much longer than the response to the PA antigen.
An additional parameter investigated during this study was the use of a prime-boost strategy to enhance immunization against anthrax. This approach has gained considerable attention since several studies have reported that a combined DNA-protein immunization regimen results in the highest level of immune response overall. In the present study we are able to substantiate these other reports, since we found that the prime-boost approach significantly increases the overall level of antibody response to either PA or the LF antigen. Prime-boost antibody levels are substantially higher than those observed following DNA-only immunizations as observed in both Fig. 3 and Table 2. The results presented in this study indicate that it is feasible to use a DNA-based immunization strategy against anthrax and that any future vaccine against anthrax should consider incorporation of a mutated version of the LF antigen.
REFERENCES
- 1.Arora N, Klimpel K R, Singh Y, Leppla S H. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J Biol Chem. 1992;267:15542–15548. [PubMed] [Google Scholar]
- 2.Arora N, Leppla S H. Residues 1–254 of anthrax toxin lethal factor sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 3.Brossier F, Weber-Levy M, Mock M, Sirard J-C. Role of toxin functional domains in anthrax pathogenesis. Infect Immun. 2000;68:1781–1786. doi: 10.1128/iai.68.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis-Mize K S, Price B M, Baker N R, Galloway D R. Analysis of immunization with DNA encoding Pseudomonas aeruginosa exotoxin A. FEMS Immunol Med Microbiol. 2000;27:147–154. doi: 10.1111/j.1574-695X.2000.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 6.Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel K R, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Woude G F V. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 7.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 8.Gordon V M, Klimpel K R, Arora N, Henderson M A, Leppla S H. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu M-L, Leppla S H, Klinman D M. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine. 1999;17:340–344. doi: 10.1016/s0264-410x(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 10.Guidi-Rontani C, Duflot E, Mock M. Anthrax lethal toxin-induced mitogenic response of human T-cells. FEMS Lett. 1997;157:285–289. doi: 10.1111/j.1574-6968.1997.tb12786.x. [DOI] [PubMed] [Google Scholar]
- 11.Guidi-Rontani C, Weber-Levy M, Labruyere E, Mock M. Germination of Bacillus anthracis within alveolar macrophages. Mol Microbiol. 1999;31:9–17. doi: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta P, Singh A, Chauhan V, Bhatnagar R. Involvement of residues 147VYYEIGK153 in binding of lethal factor to protective antigen of Bacillus anthracis. Biochem Biophys Res Commun. 2001;280:158–163. doi: 10.1006/bbrc.2000.4099. [DOI] [PubMed] [Google Scholar]
- 13.Haddad D, Liljeqvist S, Stahl S, Andersson I, Perlmann P, Berzins K, Ahlborg N. Comparative study of DNA-based immunization vectors: effect of secretion signals on the antibody responses in mice. FEMS Immunol Med Microbiol. 1997;18:193–202. doi: 10.1111/j.1574-695X.1997.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 14.Ivins B, Fellows P, Pitt L, Estep J, Farchaus J, Friedlander A, Gibbs P. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13:1779–1784. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]
- 15.Ivins B E, Welkos S L. Recent advances in the development of an improved, human anthrax vaccine. Eur J Epidemiol. 1988;4:12–19. doi: 10.1007/BF00152686. [DOI] [PubMed] [Google Scholar]
- 16.Klimpel K R, Molloy S S, Thomas G, Leppla S H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimpel K R, Arora N, Leppla S H. Anthrax toxin lethal factor contains a zinc metalloproteinase consensus sequence which is required for lethal toxin activity. Mol Microbiol. 1994;13:1093–1100. doi: 10.1111/j.1365-2958.1994.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 18.Koch R. Zur Aetiologie des Milzbrandes. Mitt Kaiserl Gesundheits. 1881;1:49–79. [Google Scholar]
- 19.Leppla S H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppla S H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- 21.Leppla S H, Friedlander A M, Cora E M. Proteolytc activation of anthrax toxin bound to cellular receptors. In: Fehrenbach F J, Alouf J E, Falmagne P, Goebel W, Jeljaszewicz J, Jurgens D, Rappuoli R, editors. Bacterial protein toxins. New York, N.Y: Gustav Fischer; 1988. pp. 111–112. [Google Scholar]
- 22.Little S F, Ivins B E. Molecular pathogenesis of Bacillus anthracis infection. Microbes Infect. 1999;2:131–139. doi: 10.1016/s1286-4579(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 23.Little S F, Leppla S H, Cora E. Production and characterization of monoclonal antibodies to the protective antigen of Bacillus anthracis toxin. Infect Immun. 1988;56:1807–1813. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little S F, Knudsen G B. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun. 1986;52:509–512. doi: 10.1128/iai.52.2.509-512.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manickan E, Karem K L, Rouse B T. DNA vaccines—a modern gimmick or a boon to vaccinology? Crit Rev Immunol. 1997;17:139–154. doi: 10.1615/critrevimmunol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 26.Mikesell P, Ivin B E, Ristroph J D, Dreier T M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983;39:371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milne J C, Furlong D, Hanna P C, Wall J S, Collier R J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 28.Park S, Leppla S H. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif. 2000;18:293–302. doi: 10.1006/prep.2000.1208. [DOI] [PubMed] [Google Scholar]
- 29.Pasteur L. De l‘attenuation des virus et de leur retour á la virulence. C R Acad Sci Agric Bulg. 1881;92:429–435. [Google Scholar]
- 30.Pellizari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNγ-induced release of NO and TNFα. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 31.Petosa C, Collier R J, Klimpel K R, Leppla S H, Liddington R C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:8833–8838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 32.Pezard C, Weber M, Sirard J, Berche P, Mock M. Protective immunity induced by Bacillus anthracis toxin-deficicent strains. Infect Immun. 1995;63:1369–1372. doi: 10.1128/iai.63.4.1369-1372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh Y, Klimpel K R, Arora N, Sharma M, Leppla S H. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J Biol Chem. 1994;269:29039–29046. [PubMed] [Google Scholar]
- 34.Varughese M, Chi A, Teixeira A V, Nicholls P J, Keith J M, Leppla S H. Internalization of a Bacillus anthracis protective antigen c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol Med. 1998;4:87–95. [PMC free article] [PubMed] [Google Scholar]
- 35.Welkos S L, Lowe J R, Eden-Mccutshan F, Vodkin M, Leppla S H, Schmidt J J. Sequence and analysis of the DNA encoding protective antigen of Bacillus anthracis. Gene. 1988;69:287–300. doi: 10.1016/0378-1119(88)90439-8. [DOI] [PubMed] [Google Scholar]