Summary
A mouse model of hindlimb ischemia is an important tool for studying diverse therapeutic approaches for vascularization with high surgical success and low mortality rates. Here, we present a protocol for the induction of hindlimb ischemia in mice, including the surgery procedure and steps to analyze blood perfusion in the ischemic area using a laser speckle contrast analyzer. We also detail the isolation of endothelial cells from thigh muscles using flow cytometry after ischemic surgery.
For complete details on the use and execution of this protocol, please refer to Park et al. (2016).1
Subject areas: Cell Biology, Cell Isolation, Flow Cytometry/Mass Cytometry, Health Sciences, Model Organisms
Graphical abstract
Highlights
-
•
Specific instructions for the hindlimb ischemic surgery in mice
-
•
Determination of blood perfusion recovery with LASCA
-
•
An optimized approach to dissociate hindlimb tissues for cell sorting
-
•
Flow-cytometry-based sorting of the mouse endothelial cells
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
A mouse model of hindlimb ischemia is an important tool for studying diverse therapeutic approaches for vascularization with high surgical success and low mortality rates. Here, we present a protocol for the induction of hindlimb ischemia in mice, including the surgery procedure and steps to analyze blood perfusion in the ischemic area using a laser speckle contrast analyzer. We also detail the isolation of endothelial cells from thigh muscles using flow cytometry after ischemic surgery.
Before you begin
Ligation of the femoral artery in rodents is an important model used to study vessel formation, yielding data that provide novel therapeutic options for peripheral vascular diseases such as atherosclerosis, acute arterial occlusion and diabetic ulcers.1,2,3,4,5,6,7 The following protocols describe the steps used in the mouse model of hindlimb ischemia and its subsequent analysis. The protocols focus on induction of hindlimb ischemia, blood perfusion analysis and endothelial cell sorting from the ischemic region of the hindlimbs. The protocols are established using 8 to 12-week-old C57BL/6J mice.
Institutional permissions
All animal husbandry, generation and handling were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Louisiana State University Health, Shreveport.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE anti-mouse CD31 (Clone #. MEC13.3) (1:300 dilution) | BioLegend | Cat# 102508 |
| FITC anti-mouse CD45 (Clone #. QA17A26) (1:300 dilution) | BioLegend | Cat# 157608 |
| Chemicals, peptides, and recombinant proteins | ||
| Collagenase IV | Worthington | Cat# LS004188 |
| Ethanol 200 proof | Decon | Cat# DSP-MD.43 |
| Fetal bovine serum (FBS)-premium, heat inactivated | Biotechne | Cat# S11150H |
| Hair remover cream | Nair® | |
| Isoflurane, USP | Piramal Critical Care | NDC 66794-013-25 |
| Iodine prep pads | Dynarex | |
| Lidocaine and prilocaine cream, 2.5%/2.5% | Fougera | NDC 0168-0357-30 |
| Meloxicam, 5 mg/mL | Norbrook | NDC 55529-040-10 |
| PBS pH 7.4 (10×) | Gibco | Cat# 70011-044 |
| Red blood cell (RBC) lysing buffer | Sigma-Aldrich | Cat# R7757 |
| Water, cell culture grade | VWR | Cat# VWRL0200-0500 |
| Deposited data | ||
| Blood perfusion analysis, cell sorting and analyzed data | (Park et al.1) | https://www.ahajournals.org/doi/10.1161/ATVBAHA.115.306430 |
| Experimental models: Organisms/strains | ||
| 8 to 12-week-old C57BL/6J mice (male, female) | Jackson Laboratory | Strain code: 000664 |
| Software and algorithms | ||
| FlowJo | FlowJo | https://www.flowjo.com/ |
| Pimsoft | Perimed | https://www.perimed-instruments.com/ |
| SQ software | Thermo Fisher Scientific | https://www.thermofisher.com/us/en/home/life-science/cell-analysis/flow-cytometry/flow-cytometers/bigfoot-spectral-cell-sorter/features.html |
| Other | ||
| Cell strainer (40 μm) | Fisher Scientific | Cat# 22-363-547 |
| Cell strainer (70 μm) | Fisher Scientific | Cat# 22-363-548 |
| Cell strainer (100 μm) | Fisher Scientific | Cat# 22-363-549 |
| Centrifuge | Thermo Fisher Scientific | Model: Sorvall Legend X1R |
| Cell sorter | Thermo Fisher Scientific | Model: Bigfoot |
| Cotton tip applicators (sterile) | VWR | Cat# 76407-736 |
| Forceps (Dumont, #5SF) | Fine Science Tools | Cat# 11252-00 |
| Forceps (standard) | Fine Science Tools | Cat# 11000-12 |
| Forceps (angled 10°) | Fine Science Tools | Cat# 00125-11 |
| Forceps (angled 45°) | Fine Science Tools | Cat# 00276-13 |
| Isoflurane anesthesia vaporizer | HME | Model, Vapor 19.1 |
| Laser speckle contrast analyzer | Perimed | Model, PeriCam PSI HR |
| Microscope | Nikon | Model: SMZ 745T |
| Microscope slides | Fisher Scientific | Cat# 12-550-15 |
| Needle 16G × 1′ | BD | Cat# 305197 |
| Needle 18G × 1 1/2′ | BD | Cat# 305196 |
| Petri dish, 100 × 15 mm | Genesee | Cat# 32-107G |
| Razor blades, single edged | VWR | Cat# 76457-428 |
| Retractor tip Blunt - 5 mm | Carfil Quality | Cat# ACD-012 |
| Retractor tip Blunt – 7.5 mm | Carfil Quality | Cat# ACD-013 |
| Shaking incubator | Thermo Fisher | Model: MaxQ 4000 |
| 15 mL conical centrifuge tubes, polypropylene | Genesee | Cat# 28-103 |
| 1.7 mL microtubes, clear | Genesee | Cat# 24-282 |
| 3 mL syringe, luer-lok tip | BD | Cat # 309657 |
| 37°C incubator | Quincy Lab | Model: 12E incubator |
| 50 mL conical centrifuge tubes, polypropylene | Genesee | Cat# 28-108 |
| 5 mL culture tubes, 12 × 75 mm | Genesee | Cat# 21-128 |
| Suture 6-0, silk | Sofsilk | Cat# S-1172 |
| Suture 7-0, silk | Covidien | Cat# S1733K |
Materials and equipment
Analgesic solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Meloxicam (5 mg/mL) | 500 μg/mL | 100 μL |
| PBS | 1× | 900 μL |
| Total | 1 mL |
Note: Use meloxicam at a dose of 5 mg/kg body weight for analgesic injections given to mice. Analgesic solution should be prepared immediately before use.
CRITICAL: Meloxicam is classified as a non-steroidal anti-inflammatory drug (NSAID); potential side effects include heart attacks, intestinal ulceration, and strokes.
Tissue dissociation buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Collagenase IV | 2 mg/mL | 10 mg |
| FBS | 20% | 1 mL |
| PBS (10×) | 1× | 0.5 mL |
| Water, cell culture grade | 3.5 mL | |
| Total | 5 mL/mouse |
Note: Incubate the buffer at 37°C and 100 rpm in a shaking incubator (Thermo Fisher; Model, MaxQ 4000) until the collagenase IV has dissolved completely. If needed, the buffer can be kept at 4°C for up to 1 day. The buffer must be incubated at 37°C for 30 min before use.
20% FBS/1× PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 20% | 20 mL |
| PBS (10×) | 1× | 10 mL |
| Water, cell culture grade | – | 70 mL |
| Total | 100 mL |
Note: Prepare in a sterile manner and store at 4°C for up to 1 month.
4% FBS/1× PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 4% | 4 mL |
| PBS (10×) | 1× | 10 mL |
| Water, cell culture grade | – | 86 mL |
| Total | 100 mL |
Note: Prepare in a sterile manner and store at 4°C for up to 1 month.
Step-by-step method details
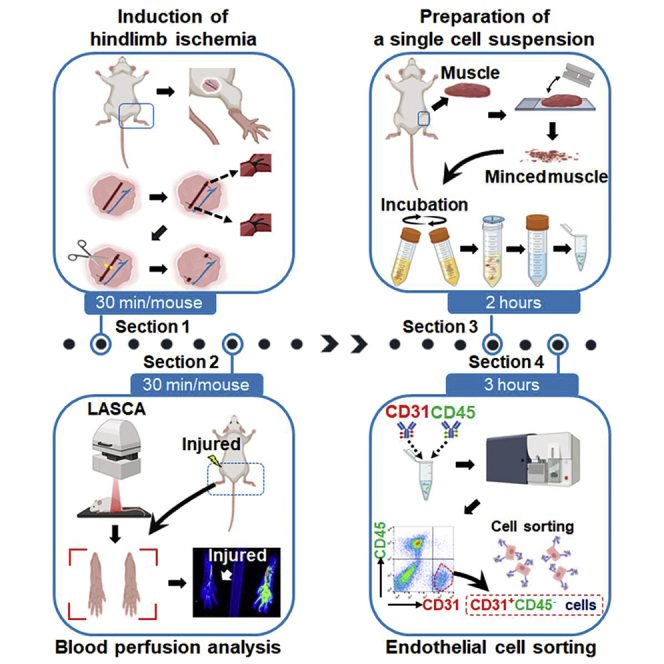
Induction of hindlimb ischemia
Timing: up to 30 min per mouse
This section describes the detailed steps used to induce ischemic injury in the mouse hindlimb by amputating the femoral artery. Two- to three-month-old-mice are used for this procedure.
-
1.
Anesthetize the mouse with 3% isoflurane in 100% oxygen in the induction chamber to ensure the mouse is well anesthetized in the induction chamber prior to being positioned on the board.
-
2.
Observe the mouse to see whether there is any sign of movement. Confirm the depth of anesthesia by pinching the mouse’s paw.
-
3.
If the mouse does not respond, remove the mouse from the chamber and place it in the prone position on a 37°C temperature heating pad.
-
4.
Insert the mouse’s muzzle into an anesthesia mask for anesthesia maintenance with 1.5% isoflurane and 100% oxygen.
-
5.
Apply hair removal cream to remove fur from the hindlimb and treat the skin with iodine and 70% ethanol.
Note: We have not found any potential influence of meloxicam on the vascular endothelial cell viability under hypoxic condition.
-
6.
Make a small incision (1 cm) in the thigh from which the fur has been removed.
-
7.
Use a retractor to pull back the wound. For the next steps (8–12), use a dissection microscope with more than 10× magnification to allow an enlarged view of the surgery region (Figure 1).
-
8.
Expose the neurovascular bundle by carefully moving the peritoneum proximally with forceps and cotton-tipped applicators.
-
9.Dissect the femoral artery and vein using forceps and cotton-tipped applicators.
-
a.Separate them from femoral nerve to avoid nerve damage during femoral artery ligation.
-
b.Continue to apply sterile 1× PBS to the area to prevent it from drying out.
-
a.
-
10.
Separate the femoral artery from the femoral vein using angled forceps so as not to damage the femoral vein during femoral artery ligation. Continue to apply sterile 1× PBS to the area to prevent it from drying out.
-
11.
Thread a strand of 7-0 suture under the proximal and distal femoral artery and ligate using both strands of the suture with 2 knots in each. Cut off the excess suture.
-
12.
Transect the femoral artery in the section located between the distal and proximal knots using spring scissors.
Note: Take care not to cause any damage to the femoral vein or bleeding from the severed vessel.
-
13.
Remove the retractor and close the incision size 6-0 silk sutures.
-
14.
Inject the mouse via intraperitoneal (IP) injection with Meloxicam (5 mg/kg body weight).
Alternatives: Carprofen or Ketoprofen at a dose of 5 mg/kg body weight can be used through subcutaneous (SC) injection as analgesics instead of meloxicam.
CRITICAL: Treat the mouse with Meloxicam (5 mg/kg body weight) every 24 h for 4 days from the day of surgery.
-
15.
Apply topical lidocaine ointment to the sutured area.
-
16.
Once the surgery has been completed, place the mouse on a draped heated pad in a new cage (clean, with fresh bedding) to prevent post-surgical complications.
-
17.
Monitor the mouse continuously until it is awake, and then place the mouse into a new cage until the end of the experiment.
CRITICAL: Perform post-procedural care daily (at least 5 times/week) to monitor for signs of complications by checking the appearance, behavior, physical condition, and body weight of the mouse. Postoperative observation will include eating, drinking, and movement. Euthanize mice developing any of the following signs: weight loss greater than 20%, wound infection, respiratory distress, or intractable dehydration. Based on pain criteria set by the laboratory, you may use another dose of meloxicam. If there are indications of dehydration, you may administer 1 mL sterile 0.9% sodium chloride subcutaneously.
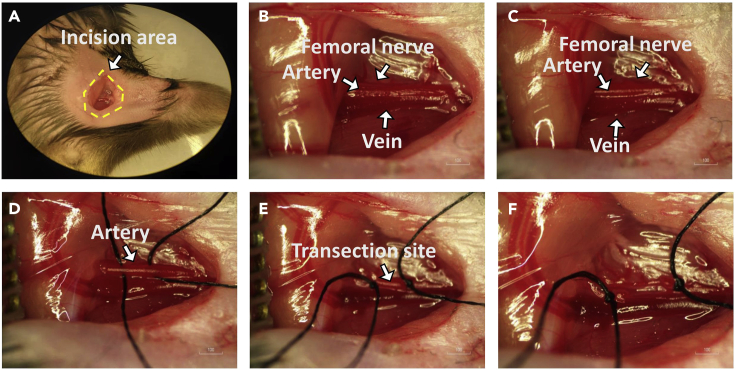
Figure 1.
Anatomy of the mouse hindlimb showing induction of ischemia
Each representative image shows a process from steps 6 to 12 of the ischemic surgery.
(A) The dotted box indicates the incision area in the hindlimb (steps 6 and 7).
(B) Exposure of the neurovascular bundle (step 8).
(C) Separation of the femoral artery from the femoral vein and nerve (steps 9 and 10).
(D) Passage of sutures under the proximal and distal femoral artery (step 11).
(E) Ligation (step 11).
(F) Transection of the artery (step 12).
Blood perfusion analysis
Timing: up to 30 min per mouse
To determine perfusion recovery, measure surface blood perfusion in the hindlimbs using a Laser Speckle Contrast Analyzer (LASCA) on the day of surgery (Figure 2A).
-
18.
Turn on the LASCA and open its software (PIMSoft; Perimed Inc., Las Vegas, NV).
-
19.Set up the LASCA software in a section of the project editor using the following parameters:
-
a.Working distance: 10.4 cm.
-
b.Measurement area: 2.9 cm (width), 2.2 cm (height).
-
c.Point density: high.
-
d.Resolution: 0.02 mm.
-
e.Image capture frame rate: 6 images/s for 1 min.
-
a.
-
20.
Anesthetize the mouse with 3% isoflurane in 100% oxygen in the induction chamber to ensure the mouse is well anesthetized in the induction chamber prior to being positioned on the board.
-
21.
Observe the mouse to see whether there is any sign of movement. Confirm the depth of anesthesia by pinching the mouse’s paw.
-
22.
If the mouse does not respond, remove the mouse from the chamber and place it in the prone position on the LASCA plate with a 37°C temperature heating pad.
-
23.
Administer a continuous flow of isoflurane to the mouse by connecting the mouse’s muzzle to an anesthesia mask with 1.5% isoflurane and 100% oxygen (Figure 2B).
CRITICAL: The mouse must be placed on a 37°C heated surface at least 5 min before the blood perfusion analysis. Keep the room temperature between 20°C and 25°C. Temperature changes will affect the results of the blood perfusion image analysis (see limitations).
-
24.
Open a new file and position the visible red lasers (650 nm) at a region encompassing the hind foot to the toe of the mouse (Figure 2B).
-
25.
Press the record button to start acquiring the blood perfusion image.
-
26.
The complete acquisition data images will show a range of colors (blue-green-yellow-red), which indicates the average value of the blood perfusion intensity: closer to the minimum (blue) or maximum (red) (Figure 2C).
-
27.
To analyze the acquired data, select the region of interest (ROI) in each ischemic region and uninjured region (internal control) to calculate a mean value of perfusion (Figure 2D).
-
28.
Repeat these measurements every week or as needed until the experiment ends.
Note: To check whether the femoral artery ligation is successfully performed, analysis with the LASCA must be performed on the same day of the surgery.
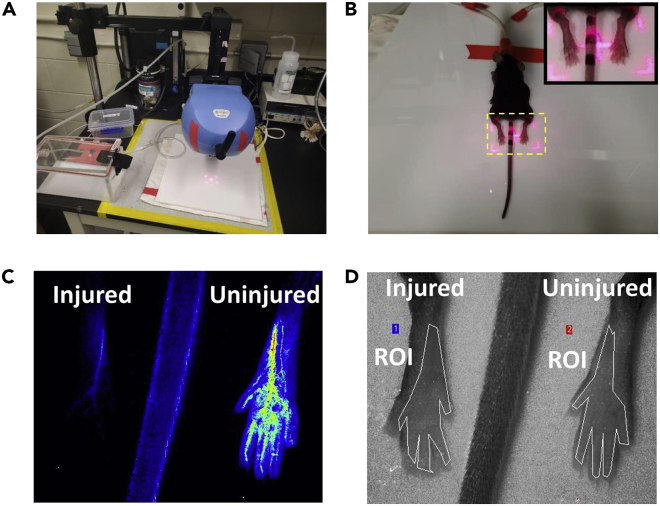
Figure 2.
Laser speckle contrast imaging (LASCA)
(A) Set up LASCA.
(B) Mouse position on the operating table. A rectangular laser (pink) indicates the scanning area for blood perfusion imaging. The inset (dotted box) shows a magnified image.
(C) Blood perfusion imaging on the day of ischemic surgery. The left and right hindlimbs show blood flow in injured and uninjured areas, respectively. The uninjured leg served as an internal control.
(D) Blood perfusion imaging analysis in the region of interest (ROI). Polygonal gates indicate ROI.
Preparation of a single cell suspension from the hindlimb tissue
Timing: up to 2 h
This section describes the detailed steps used to dissociate hindlimb tissues needed to prepare single cell suspensions for subsequent experiments such as cell sorting, gene expression and next generation sequencing (Figure 3).
-
29.
Euthanize the mouse using CO2 for 5 min.
-
30.
Spray the mouse with 70% ethanol to wet down the fur and treat the area around hindlimb aseptically.
-
31.
Use scissors and forceps to remove the skin from the whole femur (Figure 3A).
-
32.
Separate the medial thigh muscles from the femur bones where the distal and proximal suture knots exist and place the isolated muscle in cold 1× PBS (Figure 3B).
-
33.
Transfer the muscle to a microscope slide in a petri dish on ice and mince it with a razor blade for 2–3 min until a fine paste results (Figures 3C and 3D).
-
34.
Transfer the minced muscle to a 50 mL tube containing 5 mL (per mouse) tissue dissociation buffer (2 mg/mL collagenase IV in 20% FBS/1× PBS).
-
35.
Vortex the tube for 3–5 s at the highest speed.
-
36.
Incubate the tube in a shaking incubator at 37°C and 100 rpm for 45 min.
Optional: During incubation, vortex the tube for 3–5 s every 15 min.
CRITICAL: Do not incubate the samples longer than 1 h in the dissociation buffer. Incubation beyond 1 h can cause increased cell death.
-
37.
Pass the single cell suspension solution through a 3 mL syringe attached to a 16 gauge needle 7–8 times and then through an 18 gauge needle attached to the same sized syringe until large particles are no longer observed visually.
CRITICAL: Do not use needles smaller than 18 gauge or syringes larger than 3 mL; either or both can cause increased cell death.
-
38.
Add an equal volume of ice-cold 20% FBS/1× PBS without collagenase IV to the tube.
-
39.
Centrifuge at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
40.
Resuspend the pellet with 10 mL ice-cold 20% FBS/1× PBS without collagenase IV.
-
41.
Transfer the solution sequentially into 100, 70, and 40 μm cell strainers and then centrifuge the filtered solution at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
42.
Resuspend the pellet with 10 mL ice-cold 1× PBS, centrifuge at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
43.
Resuspend the pellets with 2 mL RBC lysis buffer and incubate at room temperature between 20°C and 25°C for 1 min.
-
44.
Add 18 mL ice-cold 20% FBS/1× PBS to the tube.
-
45.
Centrifuge at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
46.
Resuspend the pellet with 10 mL ice-cold 4% FBS/1× PBS, centrifuge at 4°C, 400 × g for 5 min, and aspirate the supernatant.
-
47.
Resuspend the pellet with 1 mL ice-cold 4% FBS/1× PBS.
-
48.
Transfer the cell suspension into a 1.5 mL microcentrifuge tube or tubes appropriate for the subsequent experiments such as endothelial cell sorting.
Figure 3.
Tissue harvesting from thigh muscles for single cell dissociation
(A) Skin removal from the mouse leg.
(B) Dissection of the medial thigh muscles.
(C) Transfer of muscle tissue to a microscope slide in a petri dish on ice.
(D) Chopping the muscle tissue using a razor blade.
Endothelial cell sorting
Timing: up to 3 h
-
49.Staining of cell surface markers.
-
a.Prepare compensation groups as follows:
-
i.Unstained group.
-
ii.Phycoerythrin (PE)-conjugated anti-mouse CD31 (1:300 dilution in 4% FBS/1× PBS).
-
iii.Fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45 (1:300 dilution in 4% FBS/1× PBS).
-
iv.Prepare each group as follows: add 1 × 106 cells in 250 μL 4% FBS/1× PBS to a 1.5 mL microcentrifuge tube and then add the aforementioned antibody into the tubes (PE-CD31 and FITC-CD45), respectively.
-
v.Incubate the tubes at 4°C for 20 min in the dark.
-
vi.Add 1 mL ice-cold 4% FBS/1× PBS to each tube, centrifuge at 4°C, 400 × g for 5 min using a microcentrifuge (model: Legend Micro 21R) and aspirate the supernatant.
-
vii.Resuspend the pellet with 1 mL ice-cold 4% FBS/1× PBS and repeat step 49a vi twice.
-
viii.Add 300 μL 4% FBS/1× PBS and transfer to a 5 mL cell culture tube for each group (unstained, PE-CD31 and FITC-CD45).
-
ix.Keep the tubes on ice in the dark until use. Begin cell sorting within 1 h.
-
i.
-
b.Prepare the sample for endothelial cell sorting as follows:
-
i.Add the rest of the cells to an appropriate tube(s). For better results, prepare several 1.5 mL microcentrifuge tubes containing 1 × 106 cells in 250 μL 4% FBS/1× PBS buffer per tube.
-
ii.Add both PE-conjugated anti-mouse CD31 (1:300) and FITC-conjugated anti-mouse CD45 (1:300) antibodies into each tube.
-
iii.Incubate the tubes at 4°C for 20 min in the dark.
-
iv.Add 1 mL ice-cold 4% FBS/1× PBS to each tube and combine all the tubes into a large appropriate tube(s) such as a 15 mL conical tube.
-
v.Centrifuge the tube(s) at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
vi.Resuspend the pellet with 4 mL ice-cold 4% FBS/1× PBS per tube and repeat step 49 b v twice.
-
vii.Resuspend the pellet with ice-cold 4% FBS/1× PBS (250 μL/1 × 106 cells) and transfer it to a 5 mL cell culture tube.
-
viii.Keep the tube on ice in the dark until use. Begin cell sorting within 1 h.
-
i.
-
a.
Note: CD45 is a pan-leukocyte marker. CD31 is expressed in endothelial cells and in some hematopoietic cells.
Alternatives: Other combinations of fluorophores including FITC-allophycocianin (APC), FITC-peridinin-chlorophyll-protein (PerCP), PE-APC, Alexa Fluor® 488-Alexa Fluor® 594 or 647 can be used for the cell staining (i.e., CD31 and CD45) instead of the FITC-PE combination.
CRITICAL: Use PBS which does not contain any Ca2+ and Mg2+ to reduce cell aggregation for step 47.
-
50.Endothelial cell sorting using a cell sorter (Figure 4).
-
a.Prepare the compensation settings.
-
i.An unstained group (approximately 10,000 cells) must be recorded first as a negative control for each fluorescent intensity.
-
ii.Run the PE-conjugated anti-mouse CD31 group (approximately 10,000 cells) for the positive PE gate.
-
iii.Run the FITC-conjugated anti-mouse CD45 group (approximately 10,000 cells) for the positive FITC gate.
-
iv.Adjust the compensation settings with the built-in program.
-
i.
-
b.Prepare a 15 mL tube containing 7–8 mL 20% FBS/1× PBS for the endothelial cell collection.
-
c.Set both tubes (sample tube and collection tube) on the cell sorter and run the sample to sort the targeted cell population (CD31+CD45-).
-
i.Cell sorting rate (cell number/s): 1,000–2,000 cells/s.
-
ii.Efficiency: > 80%.
-
iii.Sorter nozzle size: 100 μm.
-
iv.Pressure: 25 psi.
-
i.
-
d.Centrifuge the collection tube at 4°C, 400 × g for 5 min and aspirate the supernatant.
-
e.Store the pellet at −80°C for use in subsequent experiments or resuspend the pellet with the appropriate solutions for any planned experiments.
-
a.
Note: It is difficult to calculate the exact total cell number per thigh muscle before cell sorting due to the presence of the debris. Generally, we obtain a total cell number of 2.0 × 106–3.0 × 106 per mouse (thigh muscle). The cell sorting rate can be increased with decreased efficiency.
CRITICAL: Invert the collection tube containing 7–8 mL 20% FBS/1× PBS several times before the sorting for better cell recovery.
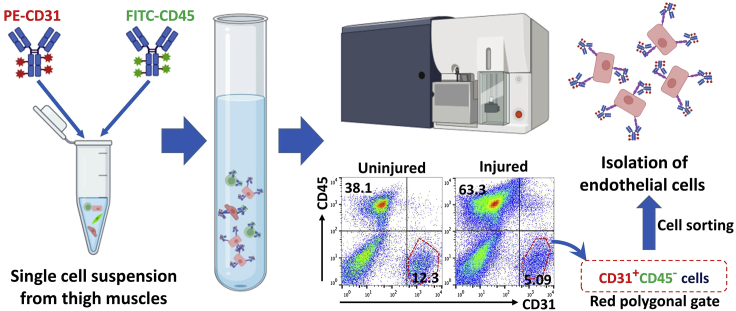
Figure 4.
Endothelial cell sorting process
PE conjugated-anti-mouse CD31 and FITC conjugated-anti-mouse CD45 antibodies were added to a tube containing a single cell suspension followed by incubation at 4°C for 20 min in the dark. The cells were then plotted for CD31 (PE) and CD45 (FITC) against an unstained control. Endothelial cells were sorted from the red polygonal gates on the plotted data. Figure reuse (flow cytometry) from Park et al.1 according to the policy of the American Heart Association. Others created with BioRender.com.
Expected outcomes
Our femoral artery ligation protocol has a mouse survival rate of 100% with greater than 90% success rate. We have not experienced any complications related to the surgery. The injured leg shows 70%–80% blood perfusion recovery day 28 post-surgery compared to the uninjured leg (internal control) in C57BL/6J mice. We routinely obtain 4–8 × 104 endothelial cells (CD31+CD45-) per mouse from cell sorting at day 7 after the injury.
Limitations
As discussed above, the number of sorted endothelial cells is 4–8 × 104 per mouse at day 7 after the injury. Depending on the experiments planned, the number of mice required should be determined. In this protocol, we do not provide the expected number of endothelial cells at different time points after the injury.
Another limitation of this protocol is that the blood perfusion imaging using LASCA is sensitive to both the body temperature of the mice and the temperature of the environment. The mice must be kept warm on heating pads and the room temperature (20°C and 25°C) must be maintained within a narrow range (20°C–25°C). Lower or higher body temperatures will give inaccurate results in blood perfusion (Figure 5).
Figure 5.
Change in blood perfusion in response to mouse body temperature changes
Troubleshooting
Problem 1
(Step 22) Inconsistent blood perfusion results in response to a change in mouse body temperature.
Potential solution
Low mouse body temperature: stop the anesthesia and transfer the mouse to a mouse cage on a 37°C temperature heating pad for 25–30 min.
High mouse body temperature: turn off the 37°C temperature heating pad and wait 5–10 min.
Problem 2
(Step 34) Collagenase IV under- or over-digestion.
Potential solution
Collagenase IV under-digestion: remove the muscle tissue and mince it with a razor blade again. Incubate the sample in a fresh aliquot of tissue dissociation buffer (5 mL/mouse) at 37°C, 100 rpm for 15 min.
Collagenase IV over-digestion: skip step 35. Shear stress can cause severe cell damage when the tissue is over-digested.
Problem 3
(Steps 34–36) Cell aggregation after tissue dissociation.
Potential solution
Add DNase I (final concentration 0.1 mg/mL) into the tissue dissociation buffer.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Changwon Park (changwon.park@lsuhs.edu).
Materials availability
This study did not generate any new unique reagents.
Acknowledgments
This work was partly supported by a post-doctoral fellowship, Center for Cardiovascular Disease and Science at LSU Health Shreveport (to M.K.) and NIH HL119291 (to C.P.). The graphical abstract is created with BioRender.com.
Author contributions
M.K. collected, assembled, and analyzed the data and wrote the manuscript with the help of S.V. C.P. conceptualized and designed this study, analyzed and interpreted the data, wrote the manuscript, and provided financial support.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate/analyze datasets/code.
References
- 1.Park C., Lee T.J., Bhang S.H., Liu F., Nakamura R., Oladipupo S.S., Pitha-Rowe I., Capoccia B., Choi H.S., Kim T.M., et al. Injury-mediated vascular regeneration requires endothelial ER71/ETV2. Arterioscler. Thromb. Vasc. Biol. 2016;36:86–96. doi: 10.1161/ATVBAHA.115.306430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes J.A., Eid M.A., Creager M.A., Goodney P.P. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2020;40:1808–1817. doi: 10.1161/ATVBAHA.120.314595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couffinhal T., Silver M., Zheng L.P., Kearney M., Witzenbichler B., Isner J.M. Mouse model of angiogenesis. Am. J. Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 4.Duff S., Mafilios M.S., Bhounsule P., Hasegawa J.T. The burden of critical limb ischemia: a review of recent literature. Vasc. Health Risk Manag. 2019;15:187–208. doi: 10.2147/VHRM.S209241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantsounga C.S., Lee C., Neverson J., Sharma S., Healy A., Berus J.M., Parry C., Ceneri N.M., López-Giráldez F., Chun H.J., et al. Macrophage IL-1beta promotes arteriogenesis by autocrine STAT3- and NF-kappaB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022;38:110309. doi: 10.1016/j.celrep.2022.110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyake K., Miyagawa S., Harada A., Sawa Y. Engineered clustered myoblast cell injection augments angiogenesis and muscle regeneration in peripheral artery disease. Mol. Ther. 2022;30:1239–1251. doi: 10.1016/j.ymthe.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teraa M., Conte M.S., Moll F.L., Verhaar M.C. Critical limb ischemia: current trends and future directions. J. Am. Heart Assoc. 2016;5:e002938. doi: 10.1161/JAHA.115.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.