Highlights
-
•
No significant white matter changes following 8-week iTBS over bilateral pSTS in ASD.
-
•
Symptom changes do not correlate with white matter metrics following 4-week iTBS.
-
•
It is imperative to have more studies on brain markers linked to responses to rTMS.
Keywords: Autism, Theta burst stimulation, Transcranial magnetic stimulation, Posterior superior temporal sulcus, Randomized controlled trial, Diffusion MRI
Abstract
Following the published behavioral and cognitive results of this single-blind parallel sham-controlled randomized clinical trial, the current study aimed to explore the impact of intermittent theta burst stimulation (iTBS), a variant of excitatory transcranial magnetic stimulation, over the bilateral posterior superior temporal sulci (pSTS) on white matter macro/microstructure in intellectually able children and adolescents with autism. Participants were randomized and blindly received active or sham iTBS for 4 weeks (the single-blind sham-controlled phase). Then, all participants continued to receive active iTBS for another 4 weeks (the open-label phase). The clinical results were published elsewhere. Here, we present diffusion magnetic resonance imaging data on potential changes in white matter measures after iTBS. Twenty-two participants in Active-Active group and 27 participants in Sham-Active group underwent multi-shell high angular resolution diffusion imaging (64-direction for b = 2000 & 1000 s/mm2, respectively) at baseline, week 4, and week 8. With longitudinal fixel-based analysis, we found no white matter changes following iTBS from baseline to week 4 (a null treatment by time interaction and a null within-group paired comparison in the Active-Active group), nor from baseline to week 8 (null within-group paired comparisons in both Active-Active and Sham-Active groups). As for the brain-symptoms relationship, we did not find baseline white matter metrics associated with symptom changes at week 4 in either group. Our results raise the question of what the minimal cumulative stimulation dose required to induce the white matter plasticity is.
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social communication deficits and repetitive/restricted behaviors and affects 1–2 % of children worldwide (Lord et al. 2018). Although most people with ASD suffer from long-term enduring distress and poor general outcomes (Lai et al. 2014), there has been no effective biological intervention for the core autistic symptoms (Lord et al. 2022).
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique that stimulates the cortex via relatively focused electromagnetic pulses at a typical frequency (Klomjai et al. 2015). For the past three decades, rTMS has been applied to several psychiatric disorders and has demonstrated therapeutic efficacy in refractory major depressive disorder (Blumberger et al. 2018) and obsessive–compulsive disorder (Carmi et al. 2019). Theta burst stimulation (TBS) is a modified rTMS protocol where pulses are applied and delivered in a burst-firing pattern (3 pulses at 50 Hz) with a 200 ms inter-burst interval (Huang et al. 2005). TBS has a comparable therapeutic effect to conventional rTMS in treating depression (Blumberger et al., 2022, Blumberger et al., 2018) but with the advantages of a shorter stimulation duration and intensity (Huang et al., 2005, Schwippel et al., 2019). Moreover, Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT) demonstrated that the accelerated and high-dose intermittent TBS (iTBS) protocol was well tolerated, safe and effective in people with treatment-resistant depression (Cole et al. 2020).
The application of rTMS/TBS in ASD has been investigated over the past 15 years. Although an earlier meta-analysis has shown a positive therapeutic effect of dorsolateral prefrontal rTMS/TBS on repetitive behaviors in ASD, its impacts on social communication deficits remain inconsistent (Barahona-Correa et al. 2018). The posterior superior temporal sulcus (pSTS) is a brain region important in computing the dynamic aspects of the theory of mind and social perception (Pitcher and Ungerleider, 2021, Yang et al., 2015). The pSTS is also considered one of the key nodes within the default-mode network (Power et al. 2011), which essentially corresponds to the social brain network that is altered in ASD (Padmanabhan et al. 2017). The morphology of the caudal branches of the pSTS is associated with social cognitive function in ASD (Hotier et al. 2017). Therefore, the expert consensus suggests that the pSTS could be a potential target of rTMS in ASD (Cole et al. 2019). However, whether unilateral or bilateral pSTS should be the stimulation target remains inconclusive. Earlier studies demonstrated altered neural correlates of right pSTS in ASD as compared to typically developing controls, including cortical thickness (Shih et al., 2011) and hypoactivation in functional MRI during biological perception (against the scrambled motion control condition) (Freitag et al., 2008, Kaiser et al., 2010). A large-scale meta-analysis review confirmed the integral role of right pSTS in three social information processing networks, including social perception, action observation, and theory of mind (Yang et al. 2015). However, several studies also showed the essential role of left pSTS in ASD. For example, the left pSTS activation could predict the changes in emotion recognition in adults with ASD receiving virtual reality social cognition training (Yang et al. 2017). Another study on neurotypical populations found that children tend to recruit bilateral pSTS, but adults would show right-lateralized pSTS activation during the facial processing task (Hildesheim et al. 2020). Further complicating the issue, Sliwinska and Pitcher (2018) found that the level of pSTS activation and the extent of TMS effects varied across individuals. Some participants showed greater activation in the right pSTS, but others in the left pSTS. Sliwinska et al. (2020) further demonstrated causal functional connectivity between the left and the right pSTS during facial expression recognition following the dual-site TMS manipulation. Taken together, to maximize the impact of iTBS, we decided to target the bilateral pSTS herein.
Regarding the direction of stimulation, an earlier study found that applying inhibitory TMS over the right pSTS could lead to transient disruption in the behaviors of orienting toward the eyes (Saitovitch et al. 2016). Moreover, an inhibitory TMS protocol (i.e., continuous TBS) delivered to the right pSTS would interfere with functional connectivity between the amygdala and right pSTS, disrupting facial processing function (Pitcher et al. 2017). On the contrary, based on the hypothesis that the excitatory protocol (i.e., iTBS) over the bilateral pSTS might enhance their function, resulting in decreasing social communication deficits in youth and adults with ASD (Afzali et al., 2021, Ni et al., 2021a, Ni et al., 2017, Ni et al., 2021b), our pilot study found that compulsive behaviors significantly decreased and social awareness increased marginally after one session of iTBS over the pSTS in adults with ASD (Ni et al. 2017). Although our 4-week randomized blind controlled trial did not support the therapeutic impacts of iTBS over the pSTS on clinical symptoms and social cognitive function, the exploratory analyses of the data from a 4-week open-label intervention following the first part of the 4-week blind trial found that there may exist sizeable inter-individual variability in therapeutic efficacy, which might be moderated by more cumulative iTBS sessions (8 weeks > 4 weeks), baseline full-scale intelligence quotient, baseline autistic severity and concurrent use of psychotropic medications (Ni et al., 2021a, Ni et al., 2021b). Moreover, individuals with higher full-scale intelligence quotient, better social cognitive performance, and less comorbid with attention-deficit hyperactivity disorder were prone to be therapeutic responders of iTBS over the pSTS (Ni et al., 2021a). Neurophysiologically, baseline MRI metrics may link to the therapeutic effect of rTMS/TBS in clinical populations such as depression and posttraumatic stress disorder (Barredo et al., 2019, Ge et al., 2020, Philip et al., 2019). Nonetheless, whether brain metrics could be linked to individual responses in autistic populations has not been tested.
Dysregulated activity-dependent synaptic plasticity is believed to be essential pathophysiology of ASD (Bourgeron 2015). Notably, the therapeutic effect of rTMS/TBS may be contributed by long-term potentiation in neurons (Heynen and Bear, 2001, Hoogendam et al., 2010) and synaptic plasticity (Yang and Calakos 2013). The outcomes of these mechanisms may become 'visible,' as shown in brain magnetic resonance imaging (MRI) studies (Allendorfer et al., 2012, Godfrey et al., 2022, Ueda et al., 2021, Zheng et al., 2020). However, the impacts of these types of brain stimulation on brain structures and function in ASD remain unclear. Only one recent study based on magnetic resonance spectroscopy (MRS) found local glutamate changes in the active versus sham rTMS on the left dorsolateral prefrontal cortex (DLPFC) in emerging adults with ASD (Moxon-Emre et al. 2021).
The current study was an exploratory investigation based on the diffusion magnetic resonance imaging (dMRI) data collected in our earlier clinical trial (Ni et al., 2021a) and aimed to investigate the impacts of iTBS of bilateral pSTS on white matter macro/microstructure in intellectually able children and adolescents with ASD. We tested the hypothesis that local white matter tracts connecting the pSTS to other brain regions are affected following 4-week iTBS compared to sham intervention (the blind phase). White matter fiber macro/microstructure was quantified using a novel fixel-based analysis (FBA) framework (Raffelt et al., 2017, Tournier et al., 2019) on high-quality dMRI data. In FBA, quantitative metrics are given to each fiber population within an MRI voxel, the so-called 'fixel.' The fiber-specific measures of FBA can quantify the differences or alterations in local microstructural fiber density and macroscopic fiber-bundle cross-section on a fixel-wise basis. This characteristic allows the FBA to analyze individual fiber-specific properties even in voxels containing multiple fiber populations (i.e., crossing fibers), thereby providing better sensitivity and interpretability than conventional voxel-based analysis (Mito et al., 2018, Raffelt et al., 2017). The study objectives were: (i) To test for a putative white matter change between active and sham iTBS in ASD over a 4-week blinded iTBS course; (ii) to investigate whether longer iTBS interventions (8 weeks vs 4 weeks) would affect levels of white matter changes (if there were any); (iii) to explore differences in baseline white matter macro/microstructure between responders and non-responders; and (iv) to assess if MRI features could link to the changes of social communication deficits after iTBS from baseline to week 4 (the blind phase) for active and sham groups, respectively.
2. Methods
2.1. Study design
The dMRI data were collected from a randomized, parallel, single-blind, and sham-controlled trial investigating the impacts of iTBS over the bilateral pSTS on symptoms and brain in children and adolescents with ASD at a tertiary medical center/university hospital in Taiwan (Ni et al., 2021a). Participants were enrolled between August 2016 and July 2019. Seventy-eight participants completing the baseline assessments were randomized to Active-Active (n = 40) or Sham-Active groups (n = 38). In phase 1 (baseline to week 4), participants received active or sham iTBS over the pSTS twice per week for 4 continuous weeks. In phase 2 (week 5 to week 8), all participants received active iTBS over the pSTS twice per week for 4 successive weeks (Fig. 1). Participants and their caregivers were blind to the allocation of the active and sham conditions in Phase 1. The stimulation frequency of the present study design was decided based on the recruitment feasibility, considering Taiwan's overwhelming education system and tight exam schedules (Chen et al. 2015). Details and results of the clinical trials were reported elsewhere (Ni et al., 2021a).
Fig. 1.
Flow diagram.
The current study was approved by the Research Ethics Committee at this university hospital (104-9413A) and registered with ClinicalTrials.gov (NCT03621189). The purposes and procedures of the study were explained face-to-face to the participants and their parents. Informed consent was obtained from all individual participants and their parents in the study. All studies procedures adhere to the institutional research committee's tenets and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2. Participants
Participants aged 8–17 years with ASD were recruited from this university hospital's child and adolescent psychiatry outpatient clinic. The diagnosis of ASD was corroborated using the Autism Diagnosis Objective Schedule (Lord et al. 2000). We excluded participants with a full-scale intelligence quotient < 70 based on the Wechsler Intelligence Scale for Children-3rd (Wechsler 1991) or Wechsler Adult Intelligence Scale-3rd (Wechsler 1997) (a cutoff at 16 years), any prior history of major neurological (especially epilepsy) or medical illness, or major psychiatric disorder such as mood and anxiety disorders, schizophrenia and substance misuse. Participants with co-occurring attention-deficit hyperactivity disorder were included and assessed by two board-certified child psychiatrists (H.-C.N., H.-Y.L.). All psychotropic medications were continued without change during the trial. All participants had been naïve to any non-invasive brain stimulation treatment.
The autistic symptoms were estimated by the parent-rated social responsiveness scale (SRS) (Constantino JN, 2005) and the repetitive behavior scale-revised (Bodfish and Lewis, 1999).
2.3. Intervention
The biphasic pulses for TBS were generated with the Magstim Super Rapid2 system (Magstim Company, Oxford, UK) and applied with the 70-mm figure-of-eight coil. The coil was placed on the individual's pSTS, which was transformed from MNI coordinates (±50, −55, 10; as defined by a meta-analysis on the role of pSTS during social cognition (Van Overwalle and Baetens 2009)) to the individual's baseline T1 weighted structural imaging using the Navigated Brain Stimulation system (Nexstim®, Helsinki, Finland). Specifically, the individual's T1-weighted structural image was first spatially normalized with SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) to get the transformation matrix. Then we used an inverse transformation matrix, which could accept the normalized coordinates at the input and output the corresponding coordinates in the individual's native structure image. We marked the output coordinates on the native structural image in the Navigated Brain Stimulation system for further stimulation. The coil was oriented approximately 45° to the transverse plane of the head, with the coil handle pointing posteriorly and upwardly. This degree was decided to prevent the stimulation from being blocked by the ears.
The iTBS protocol adopted in the present study is as follows (Huang et al. 2005). For each iTBS session, we first delivered two iTBS courses over the left pSTS with a 3-minute break. Five minutes later, we repeated two additional iTBS courses over the right pSTS. For each iTBS course, the TBS train was delivered every 10 seconds 20 times to have 600 total pulses. That is, we delivered 1200 pulses over each hemisphere per session.
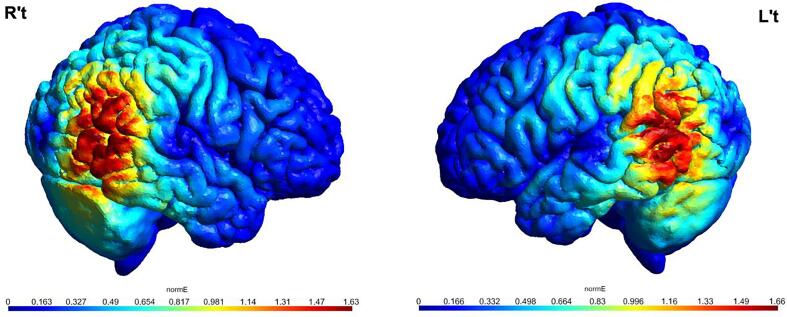
Active motor threshold (AMT) was determined based on standard methods. The active stimulation intervention used an intensity of 80 % AMT (Handwerker et al., 2020, Ni et al., 2021a). Given the unavailability of a bona fide sham coil at the time of this trial, we came up with an alternatives sham condition as used in a similar context (when a sham coil is not available) in previous trials across major psychiatric disorders (Lefaucheur et al., 2020, Ni et al., 2021a). Specifically, to avoid any stimulation effect on participants, the sham intervention was delivered with 60 % AMT with the coil tilted one-wing 90° off the head (Lisanby et al. 2001) because an earlier study demonstrated that a single theta burst with intensity lower than 70 % AMT had no effect on motor evoked potentials (Huang et al. 2009). No participants assigned to the active or sham group in Phase 1 actively disclosed or guessed that he/she/they may receive the active or sham stimulation. Notably, the scalp-to-cortex distance differs between the motor cortex and pSTS. This difference may also vary between individuals, likely resulting in inappropriate and heterogenous dosing of TBS/TMS. Therefore, following the completion of the stimulation intervention, we conducted a post-hoc and exploratory analysis using the SimNIBS toolbox v3.2 (Saturnino et al. 2019), which allowed us to estimate the individual normalized electric fields induced by TBS (with the individual intensity determined by AMT) over the bilateral pSTS via electric field calculations/modeling. The ratio between normalized induced electric fields of pSTS (the average of both hemispheres) and motor cortex (First Dorsal Interosseous) was calculated for each participant. We also computed a metric to illustrate the total individual cumulative dose of TBS that includes the duration (4 vs 8 weeks) and the ratio of electric fields. This summarized metric was used as a covariate for statistical analyses. The exemplary stimulation model is illustrated in Fig. 2. The resultant electric field metrics are provided in Supplementary Table 1; the parameter details of the SimNIBS are described in its footnote.
Fig. 2.
Model of the normalized electric field (normE) induced by iTBS over the bilateral posterior superior temporal sulci in a given participant. The coordinates of bilateral posterior superior temporal sulci were translated from MNI (±50, −55, 10) to the native space of this exemplary participant. Models were computed using SimNIBS 3.2 (Saturnino et al. 2019) based on the study's protocol and default parameters in SimNIBS 3.2.
2.4. MRI scans
Each participant underwent an MRI scan at baseline (t1), week 4 (t2), and week 8 (t3). MRI data were acquired on a 3 Tesla Siemens Tim Trio scanner with a 12-channel head coil. Anatomical T1-weighted images were collected using the three-dimensional MPRAGE sequence, with 160 sagittal slices scanned at repetition time (TR) = 2000 ms, echo time (TE) = 2.63 ms, inversion time = 900 ms, flip angle = 9°, matrix size = 224 × 256, in-plane resolution = 1.0 mm, and slice thickness = 1.0 mm. Diffusion-weighted images were collected using a single-shot spin-echo diffusion-weighted echo-planar imaging sequence with the following parameters: 2.3-mm isotropic voxel, TR/TE = 8300/100 ms, matrix size = 96 × 96, two b-values of 1000 and 2000 s/mm2 and each with 64 diffusion gradient directions. Two b = 0 volumes with opposite phase encoding polarity were acquired to correct image distortion during preprocessing.
2.5. Longitudinal fixel-based analysis (FBA)
The analysis of dMRI data was performed mainly using MRtrix3 (Tournier et al. 2019), with the recommended steps and default parameters (https://www.mrtrix.org/) unless specified otherwise.
A. Preprocessing of dMRI data.
Preprocessing procedures for dMRI data included denoising (Veraart et al. 2016), Gibbs ringing removal (Kellner et al. 2016), corrections for image distortions induced by eddy currents and susceptibility effects, inter-volume motion artifacts (Andersson et al., 2018, Andersson et al., 2017, Andersson and Sotiropoulos, 2016), bias field (Tustison et al. 2010), and upsampling. Output quality was performed with the quality control tool from FSL (Bastiani et al. 2019), including visual inspection. We assessed the percentage of total outliers (indicating image volumes with excessive signal loss) in each participant's dMRI data and excluded those with artifacts or in-scan motion (based on the average root-mean-square displacements between image volumes, relative root-mean-square, >1mm). Twenty-two children in the Active-Active group and 27 in the Sham-Active met the data quality inclusion criteria across three time points. Despite the identical study, the sample sizes of our earlier clinical paper (Active-Active n = 40, Sham-Active n = 35) and the current study (Active-Active n = 22, Sham-Active n = 27) were different because of dMRI data quality. Nonetheless, all demographic data, symptoms, and symptom changes were comparable between the included and excluded participants, regardless of the grouping assignment (Supplementary Table 2). Hence, only their data were employed in the longitudinal FBA below.
B. Longitudinal fixel template & metrics.
Fiber orientation distributions (FODs) were reconstructed based on constrained spherical deconvolution (Tournier et al., 2007, Tournier et al., 2008). For each preprocessed dMRI data at the baseline t1, tissue response functions were first estimated for white matter, gray matter, and cerebrospinal fluid using an unsupervised algorithm (Dhollander et al. 2019). For each data at each time point, the multi-shell multi-tissue constrained spherical deconvolution was then adopted to compute FODs of white matter and tissue compartments of gray matter, and cerebrospinal fluid via per-tissue averaged response functions (Jeurissen et al. 2014), followed by intensity normalization to correct for compartmental inhomogeneities (Dhollander et al., 2021).
To enable fixel-wise analysis across three time points, this study extended the design of longitudinal FBA proposed by Genc et al. (2018) as follows:
-
•
Group longitudinal fixel template – For each participant in either the Active-Active or Sham-Active group, rigid registration was first performed to align the FOD images between t1 and t3, followed by the transformations of the FODs at both time points to the participant's own mid-way space. The FODs at t2 were then registered to the transformed FODs at the mid-way space also via rigid registration. This yielded the FODs of all three time points aligned in an individual's common mid-way space, which were averaged to create intra-participant mean FODs. These per-participant mean FOD images were employed to construct an inter-participant group representative FOD template for the longitudinal data (via MRtrix3′s population_template command) (Raffelt et al. 2017), followed by the segmentation of template FODs (Smith et al. 2013) to produce the group template fixels.
-
•
Longitudinal fixel metrics – First, a FOD-guided registration was conducted between an individual's FODs of each time point and the group template FODs to identify the corresponding fixels between individuals and the template (Raffelt et al. 2011). Next, the standard fixel-wise metrics were computed for each participant at each time point, including fiber density (FD), fiber-bundle cross-section (FC), and combined measurement of FD and FC (FDC). FD measured the intra-axonal volume per fixel; FC measured the macroscopic/volumetric change of an entire local fiber bundle for a fixel; FDC quantified the overall 'connectivity' through microscopic density and macroscopic cross-section of a fixel (see (Raffelt et al. 2017) for more details). All participants' fixel-wise measurements were mapped onto the corresponding template fixels. Finally, two additional metrics were calculated to quantify longitudinal changes from the baseline t1 to a selected time point t (t2 or t3 in this study) as follows:
| (1) |
| (2) |
Eqs. (1), (2) were FD's actual and relative (percentage change) differences, respectively. This exact computation was applied to FC and FDC metrics, only that FC was log-transformed before those longitudinal measures.
-
•
Supplementary analysis – The different cumulative doses in each group may bias the results of comparisons at each time point. Therefore, we implemented additional analyses using the original longitudinal FBA approach (Genc et al. 2018), which created group longitudinal fixel templates based on the intra-participant mean FODs between t1 and t2 and between t1 and t3, respectively. These two templates were separately used to generate the aforementioned longitudinal fixel metrics for the statistical analyses on t1/t2 and t1/t3 comparisons, as explained below.
2.6. Statistics
Independent t-test and chi-square test were used to evaluate all the demographic data. The Wilcoxon test was used for root-mean-square displacements and total outliers, and ANOVA was used for intracranial volume.
For the statistical inferences of whole-brain fixel-wise longitudinal metrics, the general linear model incorporating the connectivity-based fixel enhancement approach (Raffelt et al. 2015) was used. To enable connectivity-based fixel enhancement, a whole-brain tractogram was generated on the FOD template, post-processed with spherical-deconvolution informed filtering of tractograms (Smith et al. 2013), and then used to compute fixel-to-fixel connectivity for fixel data smoothing and enhanced statistics (Raffelt et al. 2015). To investigate the effects of iTBS, four main types of analysis below were conducted via modifying the design matrix in a general linear model:
-
•
Between-group comparisons (for the phase 1 blind trial) – We assessed the Treatment × Time effect: whether there were differences in longitudinal fixel measures between treatment types from t1 to t2.
-
•
Within-group pairwise comparisons – We assessed the longitudinal fixel metrics (e.g., ΔFDactual) for each treatment type, from t1 to t2 or from t1 to t3.
-
•
Brain-symptom relationships – The autistic symptom of social communication was indicated based on the Social Responsiveness Scale (SRS) scores:
| (3) |
where ΔSRSrelative denotes the relative alteration of SRS from the baseline t1 to a time point t; SRSt1 and SRSt denote the score at t1 and at a time point t, respectively. We assessed the dependency of symptom changes on the baseline condition of white matter structure by testing whether ΔSRSrelative varied with the standard FBA metrics at t1. We tested the relationship between the baseline white matter metrics and symptom changes from baseline to week 4 in the Active and Sham groups, respectively.
-
•
Responders vs non-responders – Within the Active-Active group and Sham-Active group, we assessed whether responders and non-responders differed in the standard FBA metrics at t1. As per our previous study (Ni et al., 2021a), there were 6 participants in the Active-Active group and 3 participants in the Sham-Active group, defined as the responder based on Reliable Change Index (Jacobson and Truax 1991) > 1.64 calculated using the SRS total scores (Supplementary Tables 3 and 4).
Each general linear model controlled for age, in-scanner head motion (quantified by relative root-mean-square; averaged when two time points were selected), and the cumulative dose metric (considering both the ratio between the normalized induced electric fields of the pSTS and motor cortex and treatment duration; Supplementary Table 1). The log of intracranial volume was included as an additional covariate in the statistical models on FC and FDC (Smith et al. 2019). The statistical significance was defined by a family-wise error-corrected P-value (PFWE) < 0.05 based on non-parametric tests of 20,000 permutations to provide a more precise estimate of the error within each test. We also carried out a Supplementary statistical inference based on PFWE set at 0.01 (considering five lines of analysis) to quench the concern that the current results may be type I errors introduced by multiple tests.
3. Results
3.1. Demographic data
After the quality control of dMRI data and the attrition, there were 22 participants in the Active-Active group and 27 participants in the Sham-Active group. In the final analysis, all participants had three time points of dMRI data with acceptable quality (Fig. 1). The demographic data, including age, sex, intelligence, clinical severity, psychiatric comorbidity, and concurrent medication use, was comparable between Active-Active and Sham-Active groups (Table 1). The simulation of individual normalized induced electric fields of TBS ranged 63–98 % of pSTS relative to the motor cortex (Supplementary Table). There was no significant difference in the pSTS/motor ratio of normalized induced electric fields between two groups (Table 1).
Table 1.
Demographics and baseline characteristics of participants with autism spectrum disorder of the adequate diffusion MRI data quality across baseline, week 4, and week 8.
| Active-Active (n = 22) |
Sham-Active (n = 27) |
P value | |
|---|---|---|---|
| Age (years), mean (S.D.) | 13.0 (3.2) | 12.9 (2.8) | 0.939 |
| Male, n (%) | 18 (82) | 23 (85) | 0.751 |
| RMSt1 (mm), mean (S.D.) | 0.316 (0.163) | 0.331 (0.220) | 0.976 |
| Total outlierst1 (%), mean (S.D.) | 0.98 (0.86) | 1.07 (0.83) | 0.482 |
| RMSt2 (mm), mean (S.D.) | 0.326 (0.261) | 0.226 (0.062) | 0.164 |
| Total outlierst2 (%), mean (S.D.) | 0.92 (0.73) | 0.70 (0.40) | 0.340 |
| RMSt3 (mm), mean (S.D.) | 0.334 (0.212) | 0.238 (0.107) | 0.090 |
| Total outlierst3 (%), mean (S.D.) | 1.01 (0.79) | 0.85 (0.87) | 0.433 |
| ICVt1 (cm3), mean (S.D.) | 1440.1 (144.1) | 1409.4 (106.5) | 0.396 |
| pSTS/motor proportion of NormE (%), mean (S.D.) | 78.6 (7.7) | 76.5 (11.3) | 0.447 |
| Intelligence quotient (IQ), mean (S.D.) | |||
| Full intelligence | 91.1 (18.2) | 88.1 (14.3) | 0.519 |
| Verbal Comprehension index | 90.2 (19.0) | 90.1 (17.2) | 0.976 |
| Perceptual Reasoning Index | 101.9 (22.3) | 90.4 (22.8) | 0.083 |
| Working Memory Index | 93.8 (19.3) | 89.1 (15.8) | 0.360 |
| Processing Speed Index | 82.8 (16.0) | 85.4 (23.8) | 0.659 |
| Social Responsiveness Scale, mean (S.D.) | 105.8 (22.9) | 99.0 (20.5) | 0.278 |
| Repetitive Behavior Scale-Revised, mean (S.D.) | 34.4 (20.0) | 32.7 (20.7) | 0.770 |
| Comorbidity with ADHD, n (%) | 13 (59) | 9 (33) | 0.071 |
| Medication, n (%) | |||
| Methylphenidate | 8 (36) | 5 (19) | 0.159 |
| Atomoxetine | 1 (5) | 1 (4) | 0.882 |
| Antipsychotics | 1 (5) | 3(11) | 0.404 |
Acronyms: S.D. – standard deviation; RMS – relative Root-Mean-Square framewise displacement during diffusion MRI; pSTS – posterior superior temporal sulcus; NormE – normalized induced electric field; ICV – Intra-Cranial Volume.
3.2. White matter macro/microstructural metrics before and after iTBS
3.2.1. Between-group comparisons (for the phase 1 blind trial)
There were no significant baseline white matter differences between the two treatment groups, and we did not find a significant Treatment × Time interaction from baseline to week 4. The supplementary analysis based on the t1/t2 group longitudinal fixel template yielded an identical null result.
3.2.2. Within-group pairwise comparisons
We did not identify any significant white matter changes based on pairwise comparisons within both groups over 4 weeks or 8 weeks. These null findings were replicated in the supplementary analyses based on the t1/t2 and t1/t3 group longitudinal fixel templates, respectively.
3.2.3. Brain-symptom relationships
From baseline to week 4, we did not find any significant association of baseline white matter FBA metrics and symptom changes in either group. Because the trial was not blind from week 5 to week 8 and there was no complete control group de facto during this period, we did not explore either group's brain-symptom relationship from baseline to week 8.
3.2.4. Responders vs non-responders
Responders and non-responders within the Active-Active group and Sham-Active group did not differ in white matter macro/microstructure (PFWE > 0.05), demographic data, or the pSTS/motor ratio of normalized induced electric fields at baseline (Supplementary Tables 3 and 4).
4. Discussion
This study explored targeted changes of white matter macro/microstructure following 4-week iTBS over the bilateral pSTS in intellectually able children and adolescents with ASD. In line with the clinical results of this trial (Ni et al. 2021a), the current iTBS protocol was insufficient to induce significant changes in white matter macro/microstructure in children and adolescents with ASD. The baseline white matter FBA metrics were not associated with symptom changes at the end of the blind trial (week 4; Phase 1) in both groups.
Against our hypothesis, we did not find significant macro/microstructural changes in white matter after 4 or 8 weeks of iTBS. This result was inconsistent with previous work suggesting that rTMS could induce white matter and structural brain changes. For example, Peng et al. (2012) demonstrated that the left middle frontal gyrus FA increased after 4 weeks of rTMS (20 sessions) over the left DLPFC in people with depression. Tateishi et al. (2019) reported that the right superior frontal gyrus FA increased after 6 weeks of rTMS (30 sessions) over the left DLPFC in people with depression. Voineskos et al. (2021) demonstrated increased gray matter cortical thickness in the right DLPFC but no white matter microstructural changes after 4 weeks of rTMS (20 sessions) over the bilateral DLPFC in people with schizophrenia. The lack of iTBS effect on white matter reported herein may be partly driven by the greater heterogeneity in ASD (Ameis et al., 2020, Ni et al., 2021a, Ni et al., 2021b).
In addition to the preceding explanation framework based on the heterogeneity, the following four postulations may further explain the present null result of iTBS-induced white matter changes. Firstly, in line with what has been found in depressive disorder (Lefaucheur et al. 2020), two iTBS sessions per week (despite involving four standard TBS stimulations) may be inadequate to induce clinically-meaningful white matter changes. This hypothesis is indirectly supported by two rTMS trials in adults with ASD, reporting that five sessions of rTMS per week were sufficient to improve social communication (Enticott et al. 2014) and depression symptoms (Gwynette et al. 2020). A second explanation for the reported null finding may be related to the influence of the spaced TBS (Goldsworthy et al. 2012). Yu et al. (2020) indicated that the interval between each iTBS session might produce different after-effects. Namely, continuous iTBS 1800 (600 × 3) on the primary motor cortex (M1) without intervals surprisingly reduced cortical excitability, while iTBS 1800 (600 × 3) on M1 with a 30-minute interval enhanced more cortical excitability than those with a 10-minute interval. Our study applied iTBS 1200 (600 × 2) on the bilateral pSTS with a 3-minute interval. Although the appropriate interval between two iTBS blocks remains unknown, a short interval adopted in our study might reduce the iTBS-induced excitability and long-term potentiation, leading to fewer synaptic changes and the subsequent null change in white matter macro/microstructure following rTMS. Third, we could not exclude the possibility that the current null findings could be partly attributed to insufficient stimulation dosing. Here, we used the active motor threshold to guide the stimulation intensity over the pSTS. Although this is common practice, it is important to note that the cytoarchitectural properties are unique in the motor cortex from the temporal cortex (Palomero-Gallagher and Zilles 2019). The scalp-to-cortex distance between M1 and the pSTS might differ among participants, likely resulting in the suboptimal and heterogenous dosing of TBS. Notably, the numerical modeling (Saturnino et al. 2019) revealed that the present TBS intensity could only induce 63–98 % of the normalized electric field at the pSTS relative to that was induced in the motor cortex using the same absolute stimulation intensity. This discrepancy between the desired and induced stimulation effects remains elusive. However, when investigating the participants who received the intended dose (pSTS/motor proportion of NormE > 90 %, n = 6) in our study, only one participant responded to iTBS. In addition, there were no significant differences in pSTS/motor proportion of NormE between iTBS responders and non-responders in either Active-Active or Sham-Active group. Whether the conventional AMT approach could consistently induce an effective change in the electric field, which leads to meaningful synaptic plasticity, across different stimulation sites warrants systemic investigations in the future, especially in clinical studies. Lastly, we attempted to target a sulcus (pSTS) and localized the region based on the coordinates defined in a meta-analysis (Van Overwalle and Baetens 2009). Nonetheless, based on the previous electric field modeling reports, TMS likely activates primarily gyral/lip regions in the targeted cortex (Siebner et al. 2022). This neurophysiological peculiarity may further compromise the desired and achieved intensity discrepancy.
In addition, this study has some other limitations and caveats that need to be considered while interpreting the results. First, the sample size in the final analysis is relatively small, which may be underpowered to result in type I/II errors. Nonetheless, the within-person and interventional approach adopted in the current study generally has more favorable statistical power than observation designs in the common brain-wide association study (Marek et al. 2022). Second, our study utilized a study-based FOD template. We acknowledge that using a population-matched FOD template may help reduce the inter-subject variability and thus improve the accuracy of the statistical analysis (Yang et al. 2020). However, the evaluation of this approach, such as harmonizing and addressing the discrepancies associated with the dMRI acquisition schemes, remains an ongoing challenge in the field (Pinto et al. 2020) and is beyond the scope of this study. Third, given the unavailability of a bona fide sham coil, to avoid any possible active effect on participants during the sham condition, we adjusted both the tilt of the coil and the intensity of the stimulation in our sham group. Nonetheless, this approach might alter both the somatosensory and auditory perception since peripheral co-activation might also contribute to TMS effects. Fourth, although participants and parents were blind to the allocation in Phase 1, the SRS might not be sensitive enough to detect subtle changes before and after TBS. More sensitive and objective outcome measurements should be developed and adopted in the future (Cole et al. 2019).
In conclusion, given the small sample size and unique clinical trial design, our finding of null multi-iTBS effect on white matter macro/microstructure in autistic children and adolescents features the issues of the selection of optimal dose (i.e., stimulation intensity) and protocols (e.g., spaced TBS and treatment duration). The development of personalized neuromodulation approaches, as implemented in studies of depression (Cash et al. 2021), should be warranted.
Funding
This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST-105–2628-B-182A-005-MY3 and MOST-108–2628-B-182A-006) and Chang Gung Medical Foundation (BMRP844). Hsiang-Yuan Lin is supported by the Azrieli Adult Neurodevelopmental Centre at Centre for Addiction and Mental Health, and an Academic Scholar Award from the Department of Psychiatry, University of Toronto. The funders have no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Ethics approval
All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee at Chang Gung Memorial Hospital, Linkou, Taiwan (104-9413A), and registered with ClinicalTrials.gov (NCT03621189).
Consent to participate
The purposes and procedures of the study were explained face-to-face to the participants and their parents. Informed consent was obtained from all individual participants and their parents in the study.
Data, Materials, and Code availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Hsing-Chang Ni: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition. Yi-Ping Chao: Methodology, Writing – review & editing. Rung-Yu Tseng: Formal analysis, Writing – review & editing. Chen-Te Wu: Methodology, Writing – review & editing. Luca Cocchi: Writing – review & editing. Tai-Li Chou: Writing – review & editing, Resources. Rou-Shayn Chen: Methodology, Writing – review & editing. Susan Shur-Fen Gau: Writing – review & editing, Resources. Chun-Hung Yeh: Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Hsiang-Yuan Lin: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all of our participants and their family members for partaking in this study and the anonymous reviewers for comments that significantly improved the manuscript. We want to pay tribute to the late Professor Ying-Zu Huang, who guided and inspired this study with wisdom, insight, and tenacity.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103324.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Afzali M., Pieciak T., Newman S., Garyfallidis E., Özarslan E., Cheng H.u., Jones D.K. The sensitivity of diffusion MRI to microstructural properties and experimental factors. J. Neurosci. Methods. 2021;347:108951. doi: 10.1016/j.jneumeth.2020.108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer J.B., Storrs J.M., Szaflarski J.P. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor. Neurol. Neurosci. 2012;30:103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis S.H., Blumberger D.M., Croarkin P.E., Mabbott D.J., Lai M.-C., Desarkar P., Szatmari P., Daskalakis Z.J. Treatment of Executive Function Deficits in autism spectrum disorder with repetitive transcranial magnetic stimulation: A double-blind, sham-controlled, pilot trial. Brain Stimul. 2020;13(3):539–547. doi: 10.1016/j.brs.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Graham M.S., Drobnjak I., Zhang H., Filippini N., Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage. 2017;152:450–466. doi: 10.1016/j.neuroimage.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Graham M.S., Drobnjak I., Zhang H., Campbell J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. Neuroimage. 2018;171:277–295. doi: 10.1016/j.neuroimage.2017.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona-Correa J.B., Velosa A., Chainho A., Lopes R., Oliveira-Maia A.J. Repetitive Transcranial Magnetic Stimulation for Treatment of Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Integr. Neurosci. 2018;12:27. doi: 10.3389/fnint.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J., Bellone J.A., Edwards M., Carpenter L.L., Correia S., Philip N.S. White matter integrity and functional predictors of response to repetitive transcranial magnetic stimulation for posttraumatic stress disorder and major depression. Depress. Anxiety. 2019;36:1047–1057. doi: 10.1002/da.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani M., Cottaar M., Fitzgibbon S.P., Suri S., Alfaro-Almagro F., Sotiropoulos S.N., Jbabdi S., Andersson J.L.R. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184:801–812. doi: 10.1016/j.neuroimage.2018.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberger D.M., Vila-Rodriguez F., Thorpe K.E., Feffer K., Noda Y., Giacobbe P., Knyahnytska Y., Kennedy S.H., Lam R.W., Daskalakis Z.J., Downar J. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683–1692. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- Blumberger D.M., Mulsant B.H., Thorpe K.E., McClintock S.M., Konstantinou G.N., Lee H.H., Nestor S.M., Noda Y., Rajji T.K., Trevizol A.P., Vila-Rodriguez F., Daskalakis Z.J., Downar J. Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs Bilateral Theta Burst Stimulation in Older Adults With Depression: The FOUR-D Randomized Noninferiority Clinical Trial. JAMA Psychiat. 2022;79(11):1065. doi: 10.1001/jamapsychiatry.2022.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, S.F., Lewis M (1999). The Repetitive Behavior Scales: a test manual. North Carolina, Western Carolina Center Research Reports.
- Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- Carmi L., Tendler A., Bystritsky A., Hollander E., Blumberger D.M., Daskalakis J., Ward H., Lapidus K., Goodman W., Casuto L., Feifel D., Barnea-Ygael N., Roth Y., Zangen A., Zohar J. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry. 2019;176(11):931–938. doi: 10.1176/appi.ajp.2019.18101180. [DOI] [PubMed] [Google Scholar]
- Cash R.F.H., Cocchi L., Lv J., Wu Y., Fitzgerald P.B., Zalesky A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Hum. Brain Mapp. 2021;42:4155–4172. doi: 10.1002/hbm.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.Y., et al. (2015). Effects of a selective educational system on fatigue, sleep problems, daytime sleepiness, and depression among senior high school adolescents in Taiwan. Neuropsych Dis Treat. 11, 741-50 doi: 10.2147/ndt.S77179. [DOI] [PMC free article] [PubMed]
- Cole E.J., Enticott P.G., Oberman L.M., Gwynette M.F., Casanova M.F., Jackson S.L.J., Jannati A., McPartland J.C., Naples A.J., Puts N.A.J., Albein-Urios N., Barnett C., Croarkin P., Elliot G., Feinstein C., Francis S., Lai M.-C., Levitt J., McCracken J., Mostofsky S., Pascual-Leone A., Rashtchy S., Rotenberg A., Schultz B., Taylor B., Taylor K.H., Burgt J.V., Veenstra-VanderWeele J., Wu W. The Potential of Repetitive Transcranial Magnetic Stimulation for Autism Spectrum Disorder: A Consensus Statement. Biol. Psychiatry. 2019;85(4):e21–e22. doi: 10.1016/j.biopsych.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole E.J., Stimpson K.H., Bentzley B.S., Gulser M., Cherian K., Tischler C., Nejad R., Pankow H., Choi E., Aaron H., Espil F.M., Pannu J., Xiao X., Duvio D., Solvason H.B., Hawkins J., Guerra A., Jo B., Raj K.S., Phillips A.L., Barmak F., Bishop J.H., Coetzee J.P., DeBattista C., Keller J., Schatzberg A.F., Sudheimer K.D., Williams N.R. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am. J. Psychiatry. 2020;177(8):716–726. doi: 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- Constantino J.N.G.c. Los Angeles; Western Psychological Services: 2005. Social Responsiveness Scale: Manual. [Google Scholar]
- Dhollander T., Tabbara R., Rosnarho-Tornstrand J., Tournier J.-D., Raffelt D., Connelly A. Multi-tissue log-domain intensity and inhomogeneity normalisation for quantitative apparent fibre density. In: Proc. ISMRM, 2472. 2021 [Google Scholar]
- Dhollander T., Mito R., Raffelt D., Connelly A. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. In: Proc. ISMRM, 2019;555 [Google Scholar]
- Enticott P.G., Fitzgibbon B.M., Kennedy H.A., Arnold S.L., Elliot D., Peachey A., Zangen A., Fitzgerald P.B. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul. 2014;7(2):206–211. doi: 10.1016/j.brs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Freitag C.M., Konrad C., Häberlen M., Kleser C., von Gontard A., Reith W., Troje N.F., Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46(5):1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Ge R., Downar J., Blumberger D.M., Daskalakis Z.J., Vila-Rodriguez F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimul. 2020;13:206–214. doi: 10.1016/j.brs.2019.10.012. [DOI] [PubMed] [Google Scholar]
- Genc S., Smith R.E., Malpas C.B., Anderson V., Nicholson J.M., Efron D., Sciberras E., Seal M.L., Silk T.J. Development of white matter fibre density and morphology over childhood: A longitudinal fixel-based analysis. Neuroimage. 2018;183:666–676. doi: 10.1016/j.neuroimage.2018.08.043. [DOI] [PubMed] [Google Scholar]
- Godfrey K.E.M., Muthukumaraswamy S.D., Stinear C.M., Hoeh N. Decreased salience network fMRI functional connectivity following a course of rTMS for treatment-resistant depression. J. Affect. Disord. 2022;300:235–242. doi: 10.1016/j.jad.2021.12.129. [DOI] [PubMed] [Google Scholar]
- Goldsworthy M.R., Pitcher J.B., Ridding M.C. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur. J. Neurosci. 2012;35:125–134. doi: 10.1111/j.1460-9568.2011.07924.x. [DOI] [PubMed] [Google Scholar]
- Gwynette M.F., Lowe D.W., Henneberry E.A., Sahlem G.L., Wiley M.G., Alsarraf H., Russo S.B., Joseph J.E., Summers P.M., Lohnes L., George M.S. Treatment of Adults with Autism and Major Depressive Disorder Using Transcranial Magnetic Stimulation: An Open Label Pilot Study. Autism Res. : Off. J. Int. Soc. Autism Res. 2020;13(3):346–351. doi: 10.1002/aur.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker D.A., Ianni G., Gutierrez B., Roopchansingh V., Gonzalez-Castillo J., Chen G., Bandettini P.A., Ungerleider L.G., Pitcher D. Theta-burst TMS to the posterior superior temporal sulcus decreases resting-state fMRI connectivity across the face processing network. Netw Neurosci. 2020;4(3):746–760. doi: 10.1162/netn_a_00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen A.J., Bear M.F. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J. Neurosci. 2001;21(24):9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim F.E., Debus I., Kessler R., Thome I., Zimmermann K.M., Steinsträter O., Sommer J., Kamp-Becker I., Stark R., Jansen A. The Trajectory of Hemispheric Lateralization in the Core System of Face Processing: A Cross-Sectional Functional Magnetic Resonance Imaging Pilot Study. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.507199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam J.M., Ramakers G.M., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hotier S., Leroy F., Boisgontier J., Laidi C., Mangin J.-F., Delorme R., Bolognani F., Czech C., Bouquet C., Toledano E., Bouvard M., Petit J., Mishchenko M., d'Albis M.-A., Gras D., Gaman A., Scheid I., Leboyer M., Zalla T., Houenou J. Social cognition in autism is associated with the neurodevelopment of the posterior superior temporal sulcus. Acta Psychiatr. Scand. 2017;136(5):517–525. doi: 10.1111/acps.12814. [DOI] [PubMed] [Google Scholar]
- Huang Y.Z., Edwards M.J., Rounis E., Bhatia K.P., Rothwell J.C. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y.-Z., Rothwell J.C., Lu C.-S., Wang JiunJie, Weng Y.-H., Lai S.-C., Chuang W.-L., Hung J., Chen R.-S. The effect of continuous theta burst stimulation over premotor cortex on circuits in primary motor cortex and spinal cord. Clin. Neurophysiol. 2009;120(4):796–801. doi: 10.1016/j.clinph.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Jacobson N.S., Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Tournier J.D., Dhollander T., Connelly A., Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M., Deen B., Pitskel N.B., Sugrue D.R., Voos A.C., Saulnier C.A., Ventola P., Wolf J.M., Klin A., Vander Wyk B.C., Pelphrey K.A. Neural signatures of autism. PNAS. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner E., Dhital B., Kiselev V.G., Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 2016;76:1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Klomjai W., Katz R., Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS) Ann. Phys. Rehabil. Med. 2015;58:208–213. doi: 10.1016/j.rehab.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383(9920):896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.-P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., Filipović S.R., Grefkes C., Hasan A., Hummel F.C., Jääskeläinen S.K., Langguth B., Leocani L., Londero A., Nardone R., Nguyen J.-P., Nyffeler T., Oliveira-Maia A.J., Oliviero A., Padberg F., Palm U., Paulus W., Poulet E., Quartarone A., Rachid F., Rektorová I., Rossi S., Sahlsten H., Schecklmann M., Szekely D., Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin. Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Lisanby S.H., Gutman D., Luber B., Schroeder C., Sackeim H.A. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol. Psychiatry. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- Lord C., et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C., Elsabbagh M., Baird G., Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–520. doi: 10.1016/S0140-6736(18)31129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Charman T., Havdahl A., Carbone P., Anagnostou E., Boyd B., Carr T., de Vries P.J., Dissanayake C., Divan G., Freitag C.M., Gotelli M.M., Kasari C., Knapp M., Mundy P., Plank A., Scahill L., Servili C., Shattuck P., Simonoff E., Singer A.T., Slonims V., Wang P.P., Ysrraelit M.C., Jellett R., Pickles A., Cusack J., Howlin P., Szatmari P., Holbrook A., Toolan C., McCauley J.B. The Lancet Commission on the future of care and clinical research in autism. Lancet (London, England) 2022;399(10321):271–334. doi: 10.1016/S0140-6736(21)01541-5. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., Donohue M.R., Foran W., Miller R.L., Hendrickson T.J., Malone S.M., Kandala S., Feczko E., Miranda-Dominguez O., Graham A.M., Earl E.A., Perrone A.J., Cordova M., Doyle O., Moore L.A., Conan G.M., Uriarte J., Snider K., Lynch B.J., Wilgenbusch J.C., Pengo T., Tam A., Chen J., Newbold D.J., Zheng A., Seider N.A., Van A.N., Metoki A., Chauvin R.J., Laumann T.O., Greene D.J., Petersen S.E., Garavan H., Thompson W.K., Nichols T.E., Yeo B.T.T., Barch D.M., Luna B., Fair D.A., Dosenbach N.U.F. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito, R., et al. (2018). Fibre-specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain 141, 888-902 doi:10.1093/brain/awx355. [DOI] [PubMed]
- Moxon-Emre I., Daskalakis Z.J., Blumberger D.M., Croarkin P.E., Lyon R.E., Forde N.J., Tani H., Truong P., Lai M.-C., Desarkar P., Sailasuta N., Szatmari P., Ameis S.H. Modulation of Dorsolateral Prefrontal Cortex Glutamate/Glutamine Levels Following Repetitive Transcranial Magnetic Stimulation in Young Adults With Autism. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.711542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H.-C., Hung J., Wu C.-T., Wu Y.-Y., Chang C.-J., Chen R.-S., Huang Y.-Z. The Impact of Single Session Intermittent Theta-Burst Stimulation over the Dorsolateral Prefrontal Cortex and Posterior Superior Temporal Sulcus on Adults with Autism Spectrum Disorder. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H.-C., Chen Y.-L., Chao Y.-P., Wu C.-T., Wu Y.-Y., Liang S.-Y., Chin W.-C., Chou T.-L., Gau S.-F., Huang Y.-Z., Lin H.-Y. Intermittent theta burst stimulation over the posterior superior temporal sulcus for children with autism spectrum disorder: A 4-week randomized blinded controlled trial followed by another 4-week open-label intervention. Autism Int. J. Res. Pract. 2021;25(5):1279–1294. doi: 10.1177/1362361321990534. [DOI] [PubMed] [Google Scholar]
- Ni H.-C., Lin H.-Y., Chen Y.-L., Hung J., Wu C.-T., Wu Y.-Y., Liang H.-Y., Chen R.-S., Gau S.-F., Huang Y.-Z. 5-day multi-session intermittent theta burst stimulation over bilateral posterior superior temporal sulci in adults with autism-a pilot study. Biomed J. 2022;45(4):696–707. doi: 10.1016/j.bj.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A., Lynch C.J., Schaer M., Menon V. The Default Mode Network in Autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:476–486. doi: 10.1016/j.bpsc.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N., Zilles K. Cortical layers: Cyto-, myelo-, receptor- and synaptic architecture in human cortical areas. Neuroimage. 2019;197:716–741. doi: 10.1016/j.neuroimage.2017.08.035. [DOI] [PubMed] [Google Scholar]
- Peng H., Zheng H., Li L., Liu J., Zhang Y., Shan B., Zhang L.i., Yin Y., Liu J., Li W., Zhou J., Li Z., Yang H., Zhang Z. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J. Affect. Disord. 2012;136(3):249–257. doi: 10.1016/j.jad.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Philip N.S., Barredo J., Aiken E., Larson V., Jones R.N., Shea M.T., Greenberg B.D., van ’t Wout-Frank M. Theta-Burst Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder. Am. J. Psychiatry. 2019;176(11):939–948. doi: 10.1176/appi.ajp.2019.18101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M.S., Paolella R., Billiet T., Van Dyck P., Guns P.-J., Jeurissen B., Ribbens A., den Dekker A.J., Sijbers J. Harmonization of Brain Diffusion MRI: Concepts and Methods. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Japee S., Rauth L., Ungerleider L.G. The Superior Temporal Sulcus Is Causally Connected to the Amygdala: A Combined TBS-fMRI Study. J. Neurosci. 2017;37:1156–1161. doi: 10.1523/JNEUROSCI.0114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Ungerleider L.G. Evidence for a Third Visual Pathway Specialized for Social Perception. Trends Cogn. Sci. 2021;25:100–110. doi: 10.1016/j.tics.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J., Cohen A., Nelson S., Wig G., Barnes K., Church J., Vogel A., Laumann T., Miezin F., Schlaggar B., Petersen S. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt D.A., Smith R.E., Ridgway G.R., Tournier J.-D., Vaughan D.N., Rose S., Henderson R., Connelly A. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40–55. doi: 10.1016/j.neuroimage.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffelt D., Tournier J.D., Fripp J., Crozier S., Connelly A., Salvado O. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage. 2011;56:1171–1180. doi: 10.1016/j.neuroimage.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Raffelt D.A., Tournier J.-D., Smith R.E., Vaughan D.N., Jackson G., Ridgway G.R., Connelly A. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitovitch A., Popa T., Lemaitre H., Rechtman E., Lamy J.-C., Grévent D., Calmon R., Meunier S., Brunelle F., Samson Y., Boddaert N., Zilbovicius M. Tuning Eye-Gaze Perception by Transitory STS Inhibition. Cereb. Cortex. 2016;26(6):2823–2831. doi: 10.1093/cercor/bhw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saturnino, G.B., O. Puonti, J.D. Nielsen, D. Antonenko, K.H. Madsen, A. Thielscher (2019). SimNIBS 2.1: A Comprehensive Pipeline for Individualized Electric Field Modelling for Transcranial Brain Stimulation. In: Makarov, S., M. Horner, G. Noetscher (eds) Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018.(pp3-25). Cham (CH). [PubMed]
- Schwippel T., Schroeder P.A., Fallgatter A.J., Plewnia C. Clinical review: The therapeutic use of theta-burst stimulation in mental disorders and tinnitus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;92:285–300. doi: 10.1016/j.pnpbp.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Shih, P., Keehn, B., Oram, J.K., Leyden, K.M., Keown, C.L., Müller, R.A., 2011. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol Psychiatry 70, 270-7 doi:10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed]
- Siebner H.R., Funke K., Aberra A.S., Antal A., Bestmann S., Chen R., Classen J., Davare M., Di Lazzaro V., Fox P.T., Hallett M., Karabanov A.N., Kesselheim J., Beck M.M., Koch G., Liebetanz D., Meunier S., Miniussi C., Paulus W., Peterchev A.V., Popa T., Ridding M.C., Thielscher A., Ziemann U., Rothwell J.C., Ugawa Y. Transcranial magnetic stimulation of the brain: What is stimulated? - A consensus and critical position paper. Clin. Neurophysiol. 2022;140:59–97. doi: 10.1016/j.clinph.2022.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska M.W., Elson R., Pitcher D. Dual-site TMS demonstrates causal functional connectivity between the left and right posterior temporal sulci during facial expression recognition. Brain Stimul. 2020;13:1008–1013. doi: 10.1016/j.brs.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska M.W., Pitcher D. TMS demonstrates that both right and left superior temporal sulci are important for facial expression recognition. Neuroimage. 2018;183:394–400. doi: 10.1016/j.neuroimage.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Dhollander T., Connelly A. On the regression of intracranial volume in fixel-based analysis. In: Proc. ISMRM, 3385. 2019 [Google Scholar]
- Smith R.E., Tournier J.D., Calamante F., Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298–312. doi: 10.1016/j.neuroimage.2012.11.049. [DOI] [PubMed] [Google Scholar]
- Tateishi H., et al. Improvement Of Frontal Lobe Dysfunction And White Matter Integrity By rTMS In Treatment-Resistant Depression. Neuropsychiatr. Dis. Treat. 2019;15:3079–3087. doi: 10.2147/NDT.S228501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tournier J.-D., Smith R., Raffelt D., Tabbara R., Dhollander T., Pietsch M., Christiaens D., Jeurissen B., Yeh C.-H., Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Yeh C.H., Calamante F., Cho K.H., Connelly A., Lin C.P. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42:617–625. doi: 10.1016/j.neuroimage.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Yuanjie Zheng, Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda R., Yamada N., Abo M., Senoo A. White matter changes follow low-frequency repetitive transcranial magnetic stimulation plus intensive occupational therapy for motor paralysis after stroke: a DTI study using TBSS. Acta Neurol. Belg. 2021;121:387–396. doi: 10.1007/s13760-019-01150-2. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Veraart J., Fieremans E., Novikov D.S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 2016;76:1582–1593. doi: 10.1002/mrm.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos A.N., Blumberger D.M., Schifani C., Hawco C., Dickie E.W., Rajji T.K., Mulsant B.H., Foussias G., Wang W., Daskalakis Z.J. Effects of Repetitive Transcranial Magnetic Stimulation on Working Memory Performance and Brain Structure in People With Schizophrenia Spectrum Disorders: A Double-Blind, Randomized, Sham-Controlled Trial. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6(4):449–458. doi: 10.1016/j.bpsc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- Wechsler D. TX Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III) [Google Scholar]
- Wechsler, D. (1991). Wechsler Intelligence Scale for Children - Third Edition (WISC-III). San Antonio, TX, Psychological Corporation.
- Yang Y.J.D., Allen T., Abdullahi S.M., Pelphrey K.A., Volkmar F.R., Chapman S.B. Brain responses to biological motion predict treatment outcome in young adults with autism receiving Virtual Reality Social Cognition Training: Preliminary findings. Behav. Res. Ther. 2017;93:55–66. doi: 10.1016/j.brat.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Yang Y., Calakos N. Presynaptic long-term plasticity. Front. Synaptic Neurosci. 2013;5:8. doi: 10.3389/fnsyn.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G., et al. (2020). Sample sizes and population differences in brain template construction. Neuroimage 206, 116318 doi:10.1016/j.neuroimage.2019.116318. [DOI] [PMC free article] [PubMed]
- Yang D.Y., Rosenblau G., Keifer C., Pelphrey K.A. An integrative neural model of social perception, action observation, and theory of mind. Neurosci. Biobehav. Rev. 2015;51:263–275. doi: 10.1016/j.neubiorev.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Tang X., Hu R., Liang S., Wang W., Tian S., Wu Y.i., Yuan T.-F., Zhu Y. The After-Effect of Accelerated Intermittent Theta Burst Stimulation at Different Session Intervals. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng A., Yu R., Du W., Liu H., Zhang Z., Xu Z., Xiang Y., Du L. Two-week rTMS-induced neuroimaging changes measured with fMRI in depression. J. Affect. Disord. 2020;270:15–21. doi: 10.1016/j.jad.2020.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.