Summary
Here, we provide a step-by-step protocol for the collection and intracerebroventricular infusion of cerebrospinal fluid (CSF) in mice. We describe steps to withdraw CSF quickly and abundantly while avoiding blood contamination. Using the Lynch coil technique, we gain functional insights into the collected CSF by slowly infusing minimal amounts of CSF directly to the lateral ventricles of aged mice. This protocol is versatile and can be used to infuse drugs, antibodies, or scarce biological compounds.
For complete details on the use and execution of this protocol, please refer to Iram et al. (2022).1
Subject areas: Model Organisms, Neuroscience
Graphical abstract

Highlights
-
•
Withdrawal of mouse cerebrospinal fluid (CSF) from the cisterna magna
-
•
Quality control of CSF to detect blood contamination
-
•
Intracerebroventricular infusion of CSF using a micro-osmotic pump
-
•
Detailed guide for the Lynch coil technique for delivery of scarce biological compounds
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we provide a step-by-step protocol for the collection and intracerebroventricular infusion of cerebrospinal fluid (CSF) in mice. We describe steps to withdraw CSF quickly and abundantly while avoiding blood contamination. Using the Lynch coil technique, we gain functional insights into the collected CSF by slowly infusing minimal amounts of CSF directly to the lateral ventricles of aged mice. This protocol is versatile and can be used to infuse drugs, antibodies, or scarce biological compounds.
Before you begin
CSF provides the brain with various nourishing compounds that were shown to regulate numerous aspects of brain development and function.2,3 Moreover, physiological and pathological processes in the brain are directly reflected in CSF composition. Therefore, CSF is routinely used in the clinic for diagnosis and assessment of disease progression and efficacy of therapeutic interventions.4 However, CSF withdrawal and functional examination in rodents is challenging because of the low total CSF volumes and the difficulty of administering it directly into the brain ventricles.
We have recently performed a proteomic analysis of young and aged mouse CSF5 and CSF transfers from young to aged mice to functionally assess the effects of young CSF proteins on the aged brain.1 The protocol below describes the specific steps for systematically withdrawing CSF with minimal blood contamination and infusing it intracerebroventricularly using a micro-osmotic pump. Notably, the micro-osmotic pump can be filled with other compounds such as antibodies or drugs.
Institutional permissions
All animal care and procedures complied with the Animal Welfare Act and were in accordance with institutional guidelines and approved by the VA Palo Alto Committee on Animal Research and the institutional administrative panel of laboratory animal care at Stanford University. Aged C57BL/6 mice (18–22 months old) were obtained from the National Institute on Aging rodent colony. Young male C57BL/6 mice (2 months old) were obtained from Charles River Laboratories or Jackson Laboratories. All experiments used male mice. All mice were housed at the Palo Alto VA animal facility under a 12-h light/12-h dark cycle with dark hours from 18:30–06:30 and housed at 68–73 °F under 40–60% humidity.
Pull capillaries
Timing: 30 min
-
1.
In a 15 cm petri dish attach two rows of double-sided tape to store pulled capillaries.
-
2.
Load a borosilicate glass tube onto a P-97 Flaming Micropipette Puller. Use the following capillary pulling settings: P = 500, Heat = 575, Pull = 49, Vel. = 39, Time = 250.
Note: optimal settings drift over time, especially Heat. Refer to manual for parameter optimization, e.g., the ramp test.
-
3.
Each glass tube will be pulled into two tapered capillaries. Store capillaries in the 15 cm petri dish prepared in step 1.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| 2-month-old C57BL/6J mice (male) | The Jackson Laboratory | Strain #:000664 |
| 2-month-old C57BL/6 mice (male) | Charles River Laboratory | Strain: #027 |
| 18-month-old C57BL/6J mice (male) | The Jackson Laboratory | Strain #:000664 |
| Chemicals, peptides, and recombinant proteins | ||
| Artificial CSF (aCSF) | Torcis Bioscience | Cat: 3525/25ML |
| Hydrogen peroxide solution (H2O2) | Sigma-Aldrich | Cat: 1009 |
| Baytril | ElancoDVM | Cat: 140-913 |
| Buprenorphine slow release | ZooPharm LLC | N/A |
| Isoflurane | Dechra | ANADA #200-129 |
| PBS | Thermo Fisher | 10010049 |
| Other | ||
| Plastic Petri dish, 150 × 15 mm | Boekel Scientific | Cat: 120032 |
| Borosilicate glass tube without filament, inner diameter 1.30 mm, outer diameter 1.70 mm, length 10.15 cm | King Precision Glass | Cat: 8250 |
| Flaming/Brown pipette puller | World Precision Instruments | Cat: SU- P97 |
| Model 940 Small Animal Stereotaxic Instrument with Digital Display Readout Console includes: Stereotaxic “U” Frame Assembly Model 960 Electrode Manipulator with A.P. Slide (mounted on animals left side) With Model 940-B Linear Scale Assembly with Digital Display Readout Console (10-micron resolution) WITHOUT Model 1770 Electrode Holder with Corner Clamp WITHOUT Model 920 Rat Adaptor includes Nose/Tooth Bar WITHOUT Model 920 Rat Ear Bars 18° Tip Model 900C Base Plate Assembly (base dimensions 17″ × 10″) |
David Kopf Instruments | Cat: Model 940 |
| Mouse gas anesthesia head holder | David Kopf Instruments | Cat: Model 923B |
| Non-rupture ear bars 60° tip | David Kopf Instruments | Cat: Model 922 |
| Upper bracket clamp | David Kopf Instruments | Cat: Model 1770-C |
| Adjustable stage platform | David Kopf Instruments | Cat: Model 901 |
| Straight clamp with pad | David Kopf Instruments | Cat: Model 1271-C-Mod |
| 90° electrode holder | David Kopf Instruments | Cat: Model 1769 |
| High speed stereotaxic drill | David Kopf Instruments | Cat: Model 1474 |
| High speed rotary hand piece | Foredom | Cat: MH170 |
| 2 LV temperature controller | David Kopf Instruments | Cat: Model PH TCAT |
| Heating plate mouse | David Kopf Instruments | Cat: Model PH HP4M |
| Mouse rectal probe | David Kopf Instruments | Cat: Model PH RET-3-ISO |
| Cannula holder (Alzet & Plastic Holder) | David Kopf Instruments | Cat: Model 1766-AP |
| Alcohol swabstick | Medline | Cat: mds093810 |
| Povidone Iodine swabstick | Medline | Cat: mds093902 |
| Cotton tipped applicators | Uline | Cat: S-21102-S1 |
| Magnetic fixator retraction system - blunt retractors | Fine Science Tools | Cat: 18200-10 |
| Magnetic fixator retraction system – elastomer | Fine Science Tools | Cat: 18200-07 |
| 2× Iris forceps, 10 cm, curved, serrated, German | World Precision Instruments | Cat: 15915-G |
| Hardened fine scissors | Fine Science Tools | Cat: 14090-09 |
| 1.5 mL attached screw cap microcentrifuge tube, with O-ring, polypropylene, sterile | Corning | Cat: 430909 |
| Disposable cotton-plugged borosilicate-glass Pasteur pipets | Fisher Scientific | Cat: 13-678-8B |
| Scotch Heavy duty shipping packaging tape | Office Depot | Cat:363792 |
| Micro-osmotic pump Model 1007D | Alzet | Cat: 0000290 |
| Brain Infusion Kit 3 | Alzet | Cat: 0008851 |
| Loctite 454 for cannula attachment | Alzet | Cat: 0008670 |
| 1469SB surgery tissue adhesive 3 ML bottle | 3M | Cat: 70200742529 |
| Polyethylene tubing PE-60 (up to 15 cm) | Alzet | Cat: 0007750 |
| PE60 - Polyethylene .030″ × .048" (Per Ft.) | Braintree Scientific | Cat: PE60 36 FT |
| 1 mL Norm-Ject Disposable Syringe | Henke Sass Wolf | Cat: NJ-9166017-02-MEA |
| Puralube vet ointment | MWI Veterinary | Cat: 027505 |
| Magnifying lamp | Amazon | Cat: B088BK22KJ |
Materials and equipment
Mice
In our studies we used aged C57BL/6 mice (18–22 months old) received from the National Institute on Aging. These mice are available only through NIA/ NIH grants. As an alternative, aged mice can be bred and aged in-house or are available to purchase through Jackson Laboratories (see key resources table for details). Of note, in CSF transfer studies we recommend matching the strain of young and aged mice to avoid immune reactions.
Step-by-step method details
Set up procedure room for CSF collection
Timing: 45 min
This section describes the preparation of the stereotactic instrument and glass capillaries for CSF collection.
-
1.
Disinfect procedure area with 70% ethanol.
-
2.
Set down area mats.
-
3.Fix attachments to stereotactic instrument:
-
a.Attach anesthesia tubes to the nose cone.
-
b.Attach blunt retractors to the stereotactic instrument with Elastomer as shown in Methods video S1.
-
c.Attach arm with the capillary holder at a 90-degree angle to the nosecone; adjust arm using the Allen wrench if necessary (Figure 1A).
-
a.
-
4.
Look through a magnifying lamp and use scissors to trim the tip of the pulled glass capillary to achieve an inner diameter of 0.25 mm as shown in Figures 1B and 1C.
CRITICAL: Make sure that the cut capillary end is flat. If there are uneven sharp ends, tap it gently against the flat surface of the scissors to flatten it.
-
5.
Fix a trimmed capillary to the bottom of the instrument’s arm so that it is horizontal. Attach the aspirator tube to the untampered back end of the pulled glass capillary.
-
6.
Set aside a 20 μL micropipette set to 20 μL and a 200 μL micropipette set to 200 μL both with the corresponding tip attached.
Figure 1.
Surgery set up
(A) Stereotactic set up for CSF collection to step 3 “set up procedure room for CSF collection”. A- Anesthesia tubes attached to the nose cone. B- Blunt retractors. C- Arm with a capillary holder. D- Ear bars.
(B and C) Capillary Before (B) and after (C) trimming with scissors in reference to step 4 “set up procedure room for CSF collection”. Scale bar represents 0.5 cm.
Anesthesia for CSF collection
Timing: 3 min
In this section, we will anesthetize the mouse by exposing it to isoflurane.
-
7.
Turn on isoflurane for the induction chamber at 3% isoflurane at 0.5 L/min.
-
8.
Transfer mouse to the isoflurane chamber.
-
9.
Wait for ∼2 min until the mouse’s breath is slow, at least 1 s per breath.
-
10.
Ensure the animal is adequately anesthetized by testing the hindlimb pedal withdrawal reflex. If the foot pad pinch causes a response, keep the mouse in the chamber for an additional 30 s and re-test reflex again. Quickly follow the steps below to avoid the mouse gaining consciousness.
Fixation to stereotactic instrument for CSF collection
Timing: 2–3 min
In this section, we will fix the mouse to the stereotactic instrument; isoflurane will be delivered through the nosecone to maintain anesthesia and the ear bars will hold the head in place for the surgery.
-
11.
Concurrently, turn off isoflurane to induction chamber and turn on isoflurane to the stereotactic instrument at 2–3% isoflurane at 0.5 L/min.
-
12.
Quickly remove mouse from the induction chamber.
-
13.
Grip the mouse by the scruff with your non dominant hand. Using your dominant hand, open the animal’s mouth with blunt forceps.
-
14.
Gently slide the animal’s mouth onto the mouthpiece.
-
15.
Slide and secure the nosecone onto the nose, making sure the nosecone fits snugly on the nose (pushing mouse’s whiskers back).
Note: Steps 12 through 15 are shown in Methods video S2.
-
16.
Use the lever behind the nosecone to move mouse’s head up, stop when the ear bars reach the mouse’s temples and secure the head with ear bars as shown in Methods video S3.
-
17.
Making sure the nosecone is loose to avoid breaking the nose, move the nosecone downward until the nose is facing slightly down ∼30 degrees relative to horizontal so that cisterna magna membrane is more easily accessible. Slide the nosecone snugly back onto the nose as shown in Methods video S4 and Figure 2.
Figure 2.
Final position of the mouse before CSF collection, in reference to step 17 “Fixation to Stereotactic Instrument for CSF collection”
Exposure of Cisterna Magna
Timing: 2–3 min
In this section, we will make a sagittal incision of the skin inferior to the skull and carefully separate the subcutaneous tissue and neck muscles through the midline to expose the cisterna magna.
-
18.
Use an alcohol and iodide swab to disinfect the back of the skull, neck, and upper back and to keep hair matted down. Repeat two more times.
Note: Hair could be shaved before the procedure.
-
19.
Tug skin on the back of the neck with blunt forceps (Figure 3A); cut with scissors to expose a ∼1–1.5-cm-length area under the skin (Figure 3B).
Note: To avoid bleeding/rupturing blood vessels make sure to thoroughly tug the skin before making the first cut.
-
20.
Holding the scissors horizontal, cut through the superficial connective tissue (white stripe) (Figures 3C–3E).
-
21.
Place the blunt retractors, attached to the stereotactic instrument to pull the muscles apart. After this, a thin layer of muscle above the cisterna magna will be exposed (Figure 3F).
-
22.
Separate the muscles at the midline by positioning closed blunt forceps in the midline and pulling them sideways (in a motion like opening a curtain from the midline) until the cisterna magna is exposed (Figures 3G–3I).
Note: The cisterna magna membrane is very durable. Take care not to cut it with scissors, but blunt forceps can be used forcefully on the overlying tissue to expose the membrane without worry of breaking the membrane itself.
-
23.
Clearing the muscles should ideally occur without any bleeding. If there is bleeding, use cotton swabs to clear blood and tissue from the cisterna magna membrane. Do not proceed until the bleeding resides and the blood is completely cleared off the membrane.
Note: To see whether the membrane is clean, press a cotton applicator directly onto the membrane, twist it, retract it, and examine the center of the tip for pink color. If there is pink, the membrane is not clean.
Figure 3.
Exposure of the Cisterna Magna
(A) Tugging skin on the back of the neck with blunt forceps and positing scissors to make the first cut, in reference to step 19 “exposure of Cisterna Magna”.
(B) Exposing tissue under the skin after first cut, you should see a white stripe in the middle in reference to step 19 “exposure of Cisterna Magna”.
(C–E) Cutting through the first layer of muscle in reference to step 20 “exposure of Cisterna Magna”.
(F) Using blunt retractors to pull away muscle from the skull, exposing a thin layer of muscle above cisterna magna, in reference to step 21 “exposure of Cisterna Magna”.
(G–I) Using the “digging” motion with the blunt forceps to expose the cisterna magna. In panel I, the inverted triangle represents the area where the cisterna magna should be punctured for CSF collection. Scale bar represents 100 μm. Avoid all blood vessels to decrease the risk of contaminating CSF with blood, in reference to step 22 “exposure of Cisterna Magna”.
Extraction of cerebrospinal fluid (CSF)
Timing: 2–10 min
In this section, we will be using a glass capillary to puncture the cisterna magna and withdraw CSF.
-
24.
Revolve the capillary arm toward the mouse so that the capillary is pointed directly at the cisterna magna (Figures 4A and 4B).
-
25.
Look through the magnifying lamp and adjust the position of the capillary so that it is close to the cisterna magna.
CRITICAL: look through the scope / magnifying glass to position the capillary tip as far as possible from blood vessels. The pattern of blood vessels will differ between mice.
-
26.
Cisterna magna membrane puncture: (Methods video S5).
-
27.
Gradually move the capillary forward until it touches the membrane.
-
28.
The membrane is expected to stretch to an extent. Continue moving the capillary forward gently until the membrane is punctured.
-
29.
After the membrane is punctured, quickly retract the capillary slightly to avoid damage to the brain tissue. Clear CSF should start flowing into the capillary through capillary action.
Optional: For active CSF withdrawal, press the 20 μL micropipette plunger all the way down to the second stop and attach the pipette tip to the end of the aspirator tube connected to the capillary (Methods video S6). Immediately after the plunger release, retract the capillary with one quick motion out of the mouse and twist off the 20 μL micropipette tip from the tube attached to the capillary. Proceed to step 31.
Note: Make sure to form a seal with your fingers between the pipette tip and the aspirator tube and slowly release the P20 plunger, CSF will quickly flow faster.
-
30.
Wait around 10 min until desired volume of CSF is acquired ∼ 8–15 μL and smoothly retract the capillary with one quick motion out of the mouse.
-
31.
Inspect the capillary under the magnifying glass. Look for any traces of blood contamination (Figures 4C and 4D).
-
32.
Disconnect mouse and perform a cervical dislocation. This is a terminal procedure and mouse should be taken down per approved animal protocol procedures.
Note: while this is a terminal procedure, mouse should be breathing under anesthesia during CSF collection. CSF withdrawal will not work in a dead mouse.
Figure 4.
Extraction and collection of CSF
(A and B) Positioning the capillary parallel to the mouse’s nose, in reference to step 24 “extraction of cerebrospinal fluid (CSF)”.
(C and D) CSF collected in the glass capillary, checking under magnifying glass for blood contamination in reference to step 31 “extraction of cerebrospinal fluid (CSF)”.
(E) Dispensing CSF into a sample tube in reference to step 35 “CSF storage, centrifugation and quality control”.
CSF storage, centrifugation, and quality control
Timing: 30 min
In this section, we describe a method to measure blood contamination in the CSF sample.
-
33.
Gently remove the tube attached to the capillary.
-
34.
Detach the capillary from the stereotactic instrument.
-
35.
Plunge air out of a 200 μL micropipette with tip attached onto the circular side of the capillary and collect the dispensed CSF into the sample tube (Figure 4E).
-
36.
Store CSF tube on ice.
-
37.
Centrifuge at 4°C for 15 min at 2,000 g.
-
38.Blood contamination measurement and storage:
-
a.After centrifugation, look for a pellet—some samples that appear clear may have a visible red “dot” of red blood cells that pelleted during the centrifugation. These samples should be discarded.
-
b.Aspirate supernatant very carefully and transfer the centrifuged CSF into a sample tube; seal tightly; store dry ice.
-
c.Add 6 μL of ultra-pure water to the remaining pellet and resuspend—samples are stable on ice.
-
d.Use a Nanodrop instrument and open the UV-vis tab.
-
e.Add the following wavelengths: 415, 455, 540 nm. These wavelengths detect oxyhemoglobin (oxyHb) and bilirubin.
-
f.415 nm for oxyHb is usually the most sensitive, with a clinical cutoff of 0.023 AU for intracranial hemorrhage.
-
g.Blank with 2 μL of the ultra-pure pure water—ensure no air bubbles.
-
h.Load 2 μL of each resuspended pellet.
-
i.Any pellet lysates absorbing past a stringent absorbance threshold of 0.02 AU should be discarded.
-
j.Store the samples at −80°C.
-
a.
Note: For our studies we used CSF within 12 months of storage at -80 avoiding freezing and thawing cycles. We advise to aliquot before freezing if only part of the volume is needed for downstream readouts.
Prepare pumps
Timing: 75 min
In this section, we describe in detail the materials and methods required to prepare micro-osmotic pumps with the polythene catheter tubing coil. Further, we showcase the technique to fill the pumps with artificial CSF and the tubing with young CSF.
Note: We used the Lynch coil method recommended by ALZET (https://www.alzet.com/wp-content/uploads/2019/06/Pulsatile-Delivery.pdf) with some modifications as described below. Using this method, we could load only the minimum amount of CSF necessary for the infusion. If the reagent being infused is not precious, it would be advised to use the regular pump filling method using a 1.5 cm catheter to connect the pump to the cannula.
-
39.
Disinfect lab bench with 70% ethanol.
-
40.
Set down area mats.
-
41.Sterilize and bring the following items to the lab bench:
-
a.Two 2 L glass beakers, one with 1.5 L boiling and the other with 1.5 L ice-cold water.
-
b.Timer, set to 1 min.
-
c.Ruler.
-
d.Marker.
-
e.Pasteur pipette.
-
f.Packing tape.
-
g.Polythene (PE) catheter tubing.
-
h.Micro-osmotic pump.
-
i.Brain infusion cannula (from Brain infusion kit 3).
-
j.Flow moderators.
-
k.1 mL NORM-JECT syringe.
-
l.Blunt needle.
-
a.
-
42.
Determine catheter length: Use the following length-volume conversions and the pump infusion rate to determine the appropriate length of catheter tubing. Each cm of the PE tube holds 4.566 μL.
Note: For example, ALZET model 1007D releases 0.5 μL/h∗ for 7 days. This totals to 84 μL of aCSF or young CSF in a catheter 18.39 cm long. We add to that extra tubing so 91.32 μL in 20 cm.
Note: Check pump rate for each lot of pumps supplied and adjust the calculations appropriately.
-
43.Prepare polythene catheter tubing coil.6
-
a.Mark tube at the 1.5 cm point using a ruler and maker, this is the part that will connect the pump to the cannula (Figure 5A). This length is fixed regardless of the length of the coil. The rest of the tube (determined by the calculation in step 42) will be coiled.
-
b.Tightly coil tubing over a Pasteur pipette (Figure 5B).
-
c.Hold the coiled tubing over the Pasteur pipette in one hand and seal the tubing with packing tape using the other hand (Figure 5C).
-
d.Submerge the Pasteur pipette into the boiling water for 1 min.
-
e.Move Pasteur pipette from boiling water to ice-cold water for 2 min (Figure 5D).Note: Make sure the coil is fully submerged in water during steps d and e.
-
f.Remove Pasteur pipette from ice-cold water and using scissors gently cut off the packing tape around the tubing coil.
-
g.Slide the coil tubing off the Pasteur pipette, if the coil holds its shape (Figure 5E) this step successful, and one can move on to the next step.Note: If the coil does not hold its shape, repeat step 43 before moving on.
-
a.
-
44.Fill micro-osmotic pumps with artificial CSF (aCSF):
-
a.Attach a blunt needle (supplied with the pumps) to a 1 mL NORM-JECT syringe. use only blunt needles to prevent puncturing the pump.
-
b.Fill up syringe with aCSF. Roughly 100 μL of aCSF per pump. Transfer aCSF from the syringe into the micro-osmotic pump by pressing it to the far end of the pump and pushing until aCSF spills over.Note: Make sure to slowly retract needle from the pump while filling it to prevent air bubbles to get trapped in the pump (Methods video S7).
-
c.Place the filled pumps in a clean weigh dish.
-
a.
-
45.Fill coil with CSF:
-
a.Thaw CSF on ice.
-
b.Prepare the flow modulators by cutting off the white plastic caps. We only need the metal part of the flow modulators (Figure 6A).
-
c.Cut a new small piece ∼1 cm of polythene catheter tubing and attach it to one end of the flow modulator.
-
d.Attach the other open end of the flow modulator to the coiled end of tubing coil and loop it through the inside of the coil (Figure 6B).
-
e.Fix the brain infusion cannula on the straight end of coil (Figure 6C).
-
f.On the flow modulator side, attach the NORM-JECT syringe with a blunt needle and fill CSF through the coil (Methods video S8).Note: No air bubbles should be formed inside the coil while filling it with CSF, or else air bubbles instead of the CSF will be infused into the mouse. Re-fill the coil if bubbles form.
-
g.Use two forceps to remove the catheter tubing attached to one end of the flow modulator in step 45c (Methods video S9).
-
a.
-
46.
Insert open end of the flow modulator into the pump filled in step 45.
Note: a small air bubble should be formed separating the aCSF in the pump and fluid in the catheter.
-
47.
Lastly, prime the prepared pumps in ∼5 mL of sterile PBS in a 50 mL canonical tubes at 37°C.
Note: The brain infusion cannula should remain dry, only the pump and the coil should be submerged in the PBS (as shown in Figure 6D).
Note: Remove the pumps from 37°C, only minutes before surgery because the fluid in the pump retracts in lower temperatures.
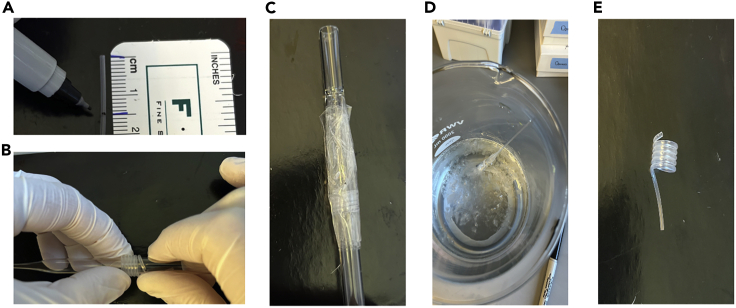
Figure 5.
Preparing pumps
(A) Measuring 1.5 cm of Polythene catheter tubing, in reference to step 43a “prepare pumps”.
(B) Coiling Polythene catheter tubing around a Pasteur pipette, in reference to step 43b “prepare pumps”.
(C) Securing the coil using packaging tape, in reference to step 43c “prepare pumps”.
(D) Coil in ice cold water, in reference to step 43e “prepare pumps”.
(E) Final product, in reference to step 43g “prepare pumps”.
Figure 6.
Preparing coil to fill with CSF
(A) Removing white plastic caps off the flow modulators, in reference to step 45b “prepare pumps”.
(B) Looping flow modulator through the coiled tubing, in reference to step 45d “prepare pumps”.
(C) Cannula attached to the straight end of the coil, in reference to step 45e “prepare pumps”.
(D) Priming the pump and coil in PBS keeping the cannula dry, in reference to step 47 “prepare pumps”.
Set up procedure room for pump implantation
Timing: 45 min
This section describes the preparation of the procedure room for pump implantation surgeries.
-
48.
Disinfect area.
-
49.
Set down area mats.
-
50.Fix attachments to stereotactic instrument (Figure 7).
-
a.Attach anesthesia tubes to the nose cone.
-
b.Connect temperature control system through 3 cords: I) power cord, II) rectal probe blue cord, III) black flat cord that connects to heating plate that is mounted on the elevated platform.
-
c.Connect drill through 3 cords: I) power cord, II) foot control (connected to the back), III) hand piece (connected to the front).
-
d.Connect digital axis reader to the stereotactic frame with the red dot facing up.
-
a.
-
51.
Dispense eye lubricant in weigh boat with applicators nearby.
-
52.
Place tubes with 70% EtOH, PBS and 30% H2O2 in a rack.
Figure 7.
Setting up procedure room for pump implantation in reference to step 50 “Set up procedure room for pump implantation”
A- PBS and H2O2. B- Digital axis reader. C- Cannula Holder. D- Platform with heating pad. E- Drill in stereotactic holder. F- Ear bars. G- Controls for drill. H- Temperature control. I- Isoflurane machine. J- Foot pedal for drill.
Anesthesia for pump implantation
Timing: 3 min
In this section, we will anesthetize the mouse by exposing it to isoflurane.
-
53.
Turn on isoflurane for the induction chamber at 3%.
-
54.
Transfer mouse to the isoflurane chamber.
-
55.
Wait for ∼2 min until the mouse breath is slow and shallow, roughly one breath per second.
-
56.
Ensure the animal is adequately anesthetized by testing the hindlimb pedal withdrawal reflex. If the foot pad pinch causes a response, keep the mouse in the chamber for an additional 30 s and re-test reflex again.
Fixation to stereotactic instrument for pump implantation
Timing: 5 min
In this section, we will fix the mouse to the stereotactic instrument; isoflurane will be delivered through the nosecone to maintain anesthesia and the ear bars will hold the head in place for the surgery.
-
57.
Concurrently, turn off isoflurane to induction chamber and turn on isoflurane to the stereotactic instrument at 3% isoflurane.
-
58.
Quickly remove mouse from the induction chamber.
-
59.
Scruff the mouse with your non dominant hand, using your dominant hand open the animal’s mouth with blunt forceps.
-
60.
Gently slide the animal’s mouth onto the mouthpiece.
-
61.
Slide and secure the nosecone onto the nose, making sure the nosecone fits snugly on the nose (pushing mouse’s whiskers back).
-
62.
Secure the head centrally with ear bars. The head is positioned at the midline of the frame with both ear bars are pointing to the same number (Figure 8).
Note: Do not use too much pressure as mouse skull can crack at this position, too much pressure can also interfere with breathing.
-
63.
Adjust nose upwards/downwards so that the head is horizontal. When moving head downward make sure nosecone is loose to avoid breaking the nose.
Note: Make sure the eye ointment is covering the entire eye to prevent the eye from drying during this survival surgery.
-
64.
Inject appropriate dose of Buprenorphine slow release (analgesic, 1 mg/kg) and Baytril (antibiotic, 5 mg/kg) prior to surgery per animal protocol.
Figure 8.
Mouse’s head secured centrally with ear bars, in reference to step 62 “Fixation to stereotactic instrument for pump implantation”
Exposure of the skull
Timing: 2 min
In this section, we will make a sagittal incision to expose the skull.
-
65.
Throughout surgery, use aseptic technique as per protocol requirements and keep the mouse on a warm surface area (37°C).
-
66.
Use an alcohol and iodide swab to disinfect the incision area on the top of the skull and neck. Repeat 2 more times.
Note: Hair may be shaved before this step.
-
67.
Tug skin on between the ears and eyes with blunt forceps; cut with scissors to expose a ∼1–1.5-cm-length area (Figure 9A).
-
68.
Wipe away connective tissue covering the skull with cotton swabs.
-
69.
Gently stroke the exposed skull with a cotton applicator soaked in H2O2 and wipe – this will enhance the connections between the skull plate and will make it easy to find bregma (the area where the sagittal and coronal sutures cross) (Figure 9B).
Figure 9.
Exposure of the skull
(A) Tugging and cutting skin between the ears and eyes, in reference to step 67 “exposure of the skull”.
(B) Bregma and Lambda exposed, in reference to step 69 “exposure of the skull”.
Drilling
Timing: 3 min
In this section, we will position the drill to injection coordinates and drill a hole in the skull.
-
70.
Place drill in stereotaxic holder, position above Bregma, and zero X / Y / Z readings on the axis reader (Figure 10A). Move to lambda and make sure skull is horizontal in the anterior posterior axis and adjust the nose cone height if necessary.
-
71.
Move drill to the injection coordinates: Anterior-Posterior (AP) 0; Medio-Lateral (ML) 1 (Figure 10B).
-
72.
Place foot on the foot controller and press all the way, move Dorsal-Ventral (DV) axis gently in pulses up and down to gently drill hole just deep enough to penetrate the skull without damaging the cortex (Methods video S10 and Figure 10C).
Note: Use a pecking motion—penetrate a bit, then withdraw, penetrate a bit more, then withdraw.
Note: In case of bleeding- use cotton tip applicators to dry up blood or bone material.
-
73.
Disconnect the drill from the stereotactic instrument.
Figure 10.
Drill position and drilling
(A) Drill positioned above the bregma and zeroed at all three coordinates: ML 0; AP 0; DV 0, in reference to step 70 “drilling”.
(B) Drill positioned at injection coordinates: ML 1; AP 0; DV 0, in reference to step 71 “drilling”.
(C) Hole created by the drill, arrow pointing to the hole, in reference to step 72 “drilling”.
Attaching micro-osmotic pump for I.C.V. infusion
Timing: 5 min
In this section, we will implant the cannula through the hole drilled in the previous section. Further, we will fit the coil and micro-osmotic pump subcutaneously and close the incision.
-
74.
Connect cannula holder to the stereotactic instrument and place it right above the drilled hole in the skull (Figure 11A).
-
75.
Insert big blunt scissors from the incision backwards to make a subcutaneous pocket for the pump (Methods video S11).
-
76.
Fit the pump through this newly exposed area, such that the pump fits snugly under the skin (Figure 11B).
Note: Only the pump and coil fit underneath the skin, the cannula should be sticking out and connected to the cannula holder in the next step.
-
77.
Using forceps prop open the cannula holder with one hand and with the other hand connect cannula to the cannula holder with the needle of the cannula facing downwards (towards the hole in the skull) (Figure 11C).
-
78.
Apply 3 drops of Loctite glue in a triangular pattern on the underside of the cannula (part facing the skull).
-
79.
Gently lower the cannula holder until the cannula is attached to the skull. Secure the cannula in place by applying a thin layer of glue around the circumference of the cannula (Figure 11D).
-
80.
Loosen the cannula holder screw and elevate cannula holder (Figure 11E).
-
81.
Cut out the cannula with a plier while pressing down with the wooden side cotton applicator not to disturb the cannula position (Figure 11F).
-
82.
Align the skin above the catheter and cannula and attach with surgical glue/stitches/ surgical clips as per animal protocol (Figures 11G and 11H).
-
83.
Remove mouse from stereotactic instrument and allow to recover in a paper-lined cage placed half-on and half-off of a heating pad; mouse is monitored every 15 min until they are fully awake (e.g., upright and ambulatory) before returning to their home cage.
-
84.
Monitor the mouse as per post-operative institutional guidelines. Buprenorphine slow-release will be injected 48–72 h post-op if necessary. If the mouse appears lethargic, has a BCS < 2, or if any other abnormal signs are noted, the mouse will be euthanized immediately.
Figure 11.
Attaching micro-osmotic pump for I.C.V. infusion
(A) Cannula holder positioned above the drilled hole, in reference to step 74 “attaching micro-osmotic pump for I.C.V. infusion”.
(B) Micro-osmotic pump is snugly fit under skin such that the cannula is sticking out, in reference to step 76 “attaching micro-osmotic pump for I.C.V. infusion”.
(C) Cannula is attached to the cannula holder, in reference to step 77 “attaching micro-osmotic pump for I.C.V. infusion”.
(D) Securing cannula in place by applying a thin layer of glue around the circumference of the cannula, in reference to step 79 “attaching micro-osmotic pump for I.C.V. infusion”.
(E). Remove the cannula from cannula holder in reference to step 80 “attaching micro-osmotic pump for I.C.V. infusion”.
(F) Using pliers cut the head of the cannula, final product, in reference to step 81 “attaching micro-osmotic pump for I.C.V. infusion”.
(G) Glue the skin above the catheter and cannula, in reference to step 82 “attaching micro-osmotic pump for I.C.V. infusion”.
(H) Mouse at the end of the surgery, after the skin has been glued back together, in reference to step 82 “attaching micro-osmotic pump for I.C.V. infusion”.
Expected outcomes
CSF withdrawal
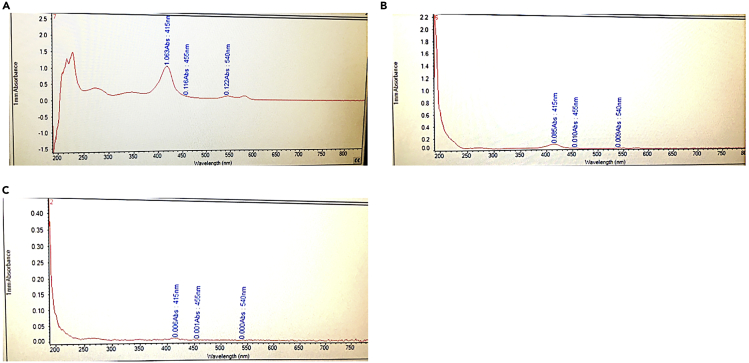
We expect to expose the cisterna magna with no bleeding at all and to collect 8–15 μL of clean CSF (Figure 12). CSF could be used to measure CSF components (proteins, metabolites, lipids, extracellular vesicles, microRNA, etc.) or tested functionally in vitro or in vivo.
Figure 12.
CSF contamination measurement in reference to the “limitations” section
(A) Positive control, CSF contamination is evident by naked eye.
(B) CSF contaminated, but not detected by eye.
(C) Clean CSF.
CSF infusion
We expect to infuse CSF continuously over 7 days directly to the right lateral ventricle of aged mice.
Experimental readouts
At the end of the infusion, mice could be tested in behavioral assays, or taken down for cell isolation, RNA sequencing or histological analysis.
Limitations
Acquiring uncontaminated CSF can be challenging at first and a limitation for those new to rodent surgeries. Thoroughly following our protocol and reading our troubleshooting sections can be of great help. Furthermore, reaching out to us for advice is also welcomed.
Troubleshooting
Problem 1
CSF does not flow out. Related to step 29 in “Extraction of CSF”.
Potential solution
There are two possible solutions for this:
-
•
The mouse is dead. Always monitor the mouse’s breath while it is in the induction chamber and when the breathing is ∼1 breath/second, test for hindlimb pedal withdrawal reflex and start the surgery.
-
•
The freshly cut pulled glass capillary in step 4 of “set up procedure room for CSF collection” has a diameter that is too small. Cut another capillary with a wider diameter based on the images in Figure 1.
Problem 2
CSF active withdrawal does not work in aged mice. Related to step 29 Optional in “Extraction of CSF”.
Potential solution
Unfortunately, our active withdrawal method using a 20 μL micropipette and tip only works for young mice age (up to 3 months). You can still use the protocol for withdrawing CSF from an older mouse by capillary action (stop at “Extraction of CSF” step 30 and wait for 10–15 min). This method will yield 8–15 μL of clean CSF.
Problem 3
CSF is contaminated with blood. Related to step 31 in “Extraction of CSF”.
Potential solution
There are several possible solutions for this:
-
•
The freshly cut pulled glass capillary in step 4 of “set up procedure room for CSF collection” has a diameter that is too big. This will lead to puncturing the cisterna magna and blood vessels leading to blood contaminated CSF. Cut another capillary based on the images in Figure 1.
-
•
If the membrane has any blood during the exposure of the cisterna magna blood the CSF will get contaminated. Make sure the membrane is clean, press a cotton applicator directly onto the membrane, twist it, retract it, and examine the center of the tip for pink color. If there is pink, the membrane is not clean. Continue to clean the membrane by using a cotton tip applicator until the center of the tip does not show pink.
-
•
Puncturing a blood vessel during CSF extraction. The cisterna magna is around a highly vascularized area, it is important to view through the magnifying glass and only puncture the part of cisterna magna that does not have any blood vessels.
-
•
Puncturing tissue beyond the cisterna magna. Make sure to retract the capillary after cisterna magna is punctured.
-
•
Please refer to Shuken et al.5 for an alternative method to expose the cisterna magna in case of excessive bleeding from the muscles covering the cisterna magna.
Problem 4
Coil is not holding its shape. Related to step 43g “prepare pumps”.
Potential solution
There are several possible solutions for this:
-
•
Use heavy duty packaging tape to hold the coil in place on the Pasteur pipette. Using lab tape will not work as they do not form a tight seal and hold the coil in place.
-
•
Use boiling hot water. If the water in tepid, the tube will not form the coil shape.
-
•
Make sure PE tubing is used. Coil will not form with vinyl tubing.
Problem 5
Volume of CSF retracting from pump. Related to step 47 “prepare pumps”.
Potential solution
Pumps should not be removed from 37°C until they are needed for the infusion because the fluid in the pump retracts at lower temperatures. We have a water bath in our procedure room for the pumps.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Tal Iram (tal.iram@stanford.edu).
Materials availability
This study did not generate new unique reagents or materials. All the reagents described here are commercially available through the indicated vendors. There are no unique reagents or restrictions to availability of reagents.
Acknowledgments
We thank Prof. Tony Wyss-Coray (T.W.C.) for his support and the Wyss-Coray lab members for discussion and advice. This project was supported by the Department of Veterans Affairs (T.W.C.), the National Institute on Aging (RF1-AG064897-02 to T.W.C.), the Nan Fung Life Sciences Aging Research Fund (T.W.C.), the Big Idea Brain Rejuvenation Project and Interdisciplinary Scholar fellowship from the Wu Tsai Neurosciences Institute (T.W.C. and T.I.), Wu Tsai Neurosciences Interdisciplinary Scholarships, the Zuckerman STEM leadership fellowship, and Tel Aviv University President Award for women postdoctoral scholars (to T.I.). Graphical abstract was created with BioRender.com.
Author contributions
T.I. and S.R.S. established CSF collection protocol with assistance of A.K. T.I. established infusion protocol with assistance of A.K. A.C.Y. established the CSF quality control method. S.R.S. assisted with the development and writing of the CSF collection protocol. A.K. and T.I. wrote the manuscript with feedback from all authors.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.102015.
Data and code availability
This study did not generate new unique code.
References
- 1.Iram T., Kern F., Kaur A., Myneni S., Morningstar A.R., Shin H., Garcia M.A., Yerra L., Palovics R., Yang A.C., et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature. 2022;605:509–515. doi: 10.1038/s41586-022-04722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehtinen M.K., Zappaterra M.W., Chen X., Yang Y.J., Hill A.D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P., et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva-Vargas V., Maldonado-Soto A.R., Mizrak D., Codega P., Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016;19:643–652. doi: 10.1016/j.stem.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M., Hölttä M., Rosén C., Olsson C., Strobel G., et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 5.Shuken S.R., Rutledge J., Iram T., Losada P.M., Wilson E.N., Andreasson K.I., Leib R.D., Wyss-Coray T. Limited proteolysis–mass spectrometry reveals aging-associated changes in cerebrospinal fluid protein abundances and structures. Nat. Aging. 2022;2:379–388. doi: 10.1038/s43587-022-00196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch H.J., Rivest R.W., Wurtman R.J. Artificial induction of melatonin rhythms by programmed microinfusion. Neuroendocrinology. 1980;31:106–111. doi: 10.1159/000123059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate new unique code.