Abstract
The invasive raccoon (Procyon lotor) is an abundant carnivore and considered as an important potential vector of infectious diseases and parasites in Europe. Raccoons show a broad, opportunistic, omnivorous food spectrum. Food supply and habitat quality in urban areas are very attractive for the generalist raccoon. This inevitably leads to increased interaction with humans, domestic animals and livestock, making the raccoon a potentially suitable zoonosis vector. In its autochthonous range, especially in the Eastern and Midwestern United States, the raccoon has been studied very intensively since the beginning of the 20th century. Whereas, basic field biology and parasitology studies in Germany and Europe are lacking and have only been conducted sporadically, regionally and on small sample sizes. In the presented study 234 raccoons from central Germany were comprehensively examined for their metazoan parasite fauna. The present study shows for the first time an extremely diverse parasite fauna in raccoons outside their native range and proves their essential role as intermediate hosts and hosts for ecto- and endoparasites. A total of 23 different parasite species were identified, five of which are human pathogens, 14 of which are new for the parasite fauna of raccoons in Europe. The human pathogenic raccoon roundworm Baylisascaris procyonis is the most common parasite species in this study, with a prevalence of up to 95%. The digenetic trematode Plagiorchis muris, another human pathogenic parasite species, was detected for the first time in raccoons. The ongoing spread of invasive carnivores and the associated spread and transmission of their parasites and other pathogens increases the potential health risk of wild and farmed animals as well as humans. An increase in parasitic diseases in humans (e.g. raccoon roundworm) is to be expected, especially in urban areas, where raccoons are becoming more and more abundant.
Keywords: Raccoon (Procyon lotor), Invasive species, Metazoan parasite fauna, Baylisascaris procyonis, Plagiorchis muris, Zoonotic diseases
Graphical abstract
Highlights
-
•
234 raccoons were dissected, 23 parasite species could be detected.
-
•
14 new parasite species have been identified for raccoons in Europe.
-
•
Compared to literature, a very high prevalence (95%) of B. procyonis was detected.
-
•
The human pathogenic trematode P. muris was detected in raccoons for the first time.
1. Introduction
Invasive alien species (IAS) cause significant changes to species communities and ecosystems and are considered one of the most important threats to biodiversity worldwide (Clavero et al., 2009; Dueñas et al., 2018, 2021; Falaschi et al., 2020; Haubrock et al., 2021). In addition, they can cause considerable economic damage and endanger both human and animal health. The European Commission estimates the economic and health damage caused by IAS in Europe at 9.6–12.7 billion euros annually. In the course of globalization and a steadily increasing population and settlement density, invasive species are becoming increasingly important in cities. In Europe, the raccoon (Procyon lotor) is considered invasive and is included in the Union list of invasive species (Regulation (EU) No. 1143/2014). Originally from North America, the omnivorous raccoon was introduced to Europe for its fur. Its popularity in 20th century fashion, resulted in an increase of raccoon fur-farming. They were purposefully released into the wild in 1934 at Lake Edersee, northern Hesse, Germany (Leicht, 2009). From there, as well as through escaped animals from fur farms in eastern Germany, the raccoon spread in the following decades. The species is widely distributed and established in Germany and neighboring European countries and will probably continue to spread in the future (Kochmann et al., 2021).
The raccoon's high dispersal ability and feeding ecology means that it can colonize almost all natural habitats (Hunter and Barrett, 2012).Therefore, it is suspected to be responsible for the regional decline of numerous native species. The raccoon shows a broad, opportunistic, omnivorous food spectrum that includes plants, insects, small mammals, eggs, young birds, reptiles, amphibians, as well as carrion (Stubbe and Krapp, 1993). Thus, it poses a potential threat to native biodiversity and leads to significant feeding damage in agriculture (Beasley, 2008; Demeny et al., 2019). It also invades urban areas where it uses anthropogenic resources and can reach very high population densities. The raccoon is known to carry a large number of parasites and pathogens, which can be transmitted to wildlife, livestock, domestic animals, and humans (Beltrán-Beck et al., 2012; Karamon et al., 2014). Due to its proximity to humans, there is an increased risk of transmission of zoonotic pathogens (including the raccoon roundworm - Baylisascaris procyonis, Plagiorchis muris and more), human pathogenic viruses (including West Nile virus, SARS-CoV-2 and other corona viruses), and microorganisms (including multi-drug resistant bacteria (e.g. MRSA)) (Strausbaugh et al., 2004; Beltrán-Beck et al., 2012; Maas et al., 2022). Recent studies on raccoon populations from areas with high infection rates of the zoonotic pathogen B. procyonis, demonstrate a sharp increase in human infections. According to Zeveloff (2002), parasites and pathogens are the main mortality factors of raccoons. Population regulating events are mainly caused by distemper viruses (Kilham et al., 1956; Giacinti et al., 2021) and rabies (Rosatte et al., 1997).
In its autochthonous range, the raccoon has been studied very intensively since the early 20th century, especially in the Eastern and Midwestern United States (Lotze and Anderson, 1979; Hall, 1981; Schaffer et al., 1981; Baskin, 1998; Larivière, 2004; Karamon et al., 2014), whereas basic field biology studies in Europe are lacking and have been conducted only regionally and sporadically (Lutz, 1981; Hohmann, 1998; Gehrt, 2003; Michler, 2003; Helbig, 2011; Beltrán-Beck et al., 2012; Heddergott et al., 2020). Known are only a handful of studies in Germany (Priemer and Lux, 1994; Lux and Priemer, 1995; Gey, 1998; Rentería-Solís, 2015) where, similar to studies from other European countries, for example non-invasive fecal examinations only show punctual observations of the current prevalence of individual parasite infections (Popiołek et al., 2009; Maas et al., 2022). Methodologically comparable studies to the current work, albeit with a smaller study scope, provide evidence for the expanding metazoan endoparasite fauna of raccoons (Karamon et al., 2014; Cybulska et al., 2018; Piróg et al., 2018; Romeo et al., 2021). This shows that to this date the metazoan parasite fauna of raccoons in Europe has only been marginally studied.
The present study aims to fill this gap and provides a first detailed overview of the parasite fauna occurring in raccoons in Germany.
2. Material and methods
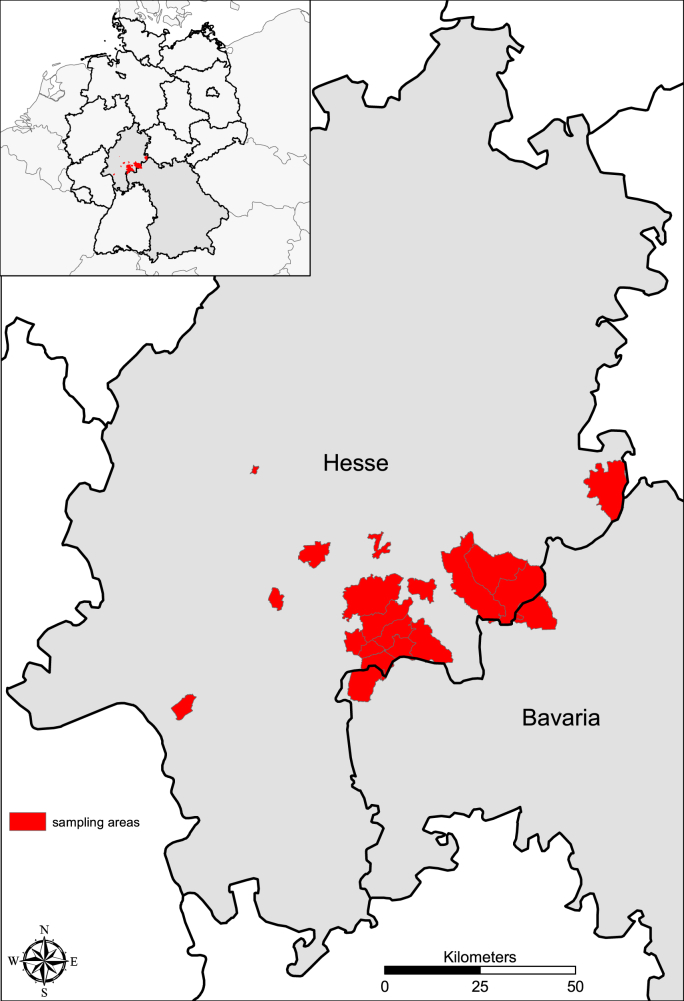
Between September 2017 and November 2021 234 free-ranging raccoons (Procyon lotor) were captured in an area of about 1100 km2 in Central Germany (federal states of Hesse and Bavaria). The location where the animals were captured was provided by the hunters at postcode level. A total number of 20 postcode areas were covered (Fig. 1). The raccoons were hunted or trapped. The selection of the trapping sites was based on reports of raccoon sightings from foresters, private hunters, and house owners. Sampling was carried out in accordance with the applicable legal regulations. Throughout the study area no special permits (other than a general hunting license) were required to legally hunt raccoons. None of the authors was involved in hunting and no animal was killed with the aim of providing samples for this study. Samples were obtained directly from licensed hunters. All captured animals were given a defined sample identification number. Metadata including date of capture, site, etc. were recorded on an accompanying document. Specimens were deep-frozen in stable PE bags including the accompanying sheet at minus 20° until they were examined.
Fig. 1.
Geographic origin of examined Procyon lotor (N = 234).
2.1. Recording of morphometric data and parasitological examinations
During the examination, laboratory coats, nitrile gloves, mouth and eye protection were worn as personal protective equipment to avoid contamination. Section and preparation utensils as well as work surfaces were disinfected with 1.5% incidin solution between work steps and after each laboratory day.
For the examination, frozen raccoons were thawed in shallow pans for at least 18 h at room temperature. Four areas were defined on the animal body for the systematic recording of ectoparasites: Head area with ears, nose and muzzle, forechest, trunk dorsal (dorsal area with cauda) and trunk ventral (abdomen with extremities). Sampling of ectoparasites was performed by systematic parting of the hair coat. Similarly, the flat bags used to store the animals were examined for fallen parasites such as fleas and ticks. Combing out with conventional flea combs was discarded for lack of functionality, due to the specific coat structure with guard hairs and woolly hairs (Schmidt, 1980) and frequent matting and contamination of the woolly hairs. Found individuals, separated by species groups and area, were first transferred into block dishes containing 0.9% sodium chloride solution. At the same time, a visual check was made with regard to the general condition of the animal, possible injuries, discharge from body orifices, as well as checking the sex determination based on the external sex organs. Age determination was carried out according to Stuewer (1943) as well as on the basis of tooth development and specimens were divided into two age classes, "adult" and "juvenile". Subsequently, the following biometric data were determined for each raccoon specimen based on Goldman and Jackson (1950) and Stubbe and Krapp (1993): total weight, carcass weight, total length, standard length, tail length, hind foot length, ear length, tooth development and any abnormalities.
Endoparasites were isolated by necropsy only. To remove the organs, the raccoon was opened medially from the tip of the mandible, with the tongue detached and the trachea and esophagus exposed down to the pelvic bone (Storch and Welsch, 2014). The anus was dissected free, symphysis pubica and os hyoideum were severed, and the complete organ composite was removed after cutting the diaphragm and transferred to a laboratory dish. The organs were separated and the following morphometric data were collected: liver weight, stomach weight full and empty. All organs were weighed to the nearest 0,01 g. The empty body cavity was examined for cysts and encapsulated parasites and subsequently the carcass weight was determined. Samples for Trichinella sp. examination (from foreleg + diaphragm pillar), os baculum, tongue, fecal sample ans tissue sample from hind leg were collected and transferred to the prepared sample containers for further studies. Blood vessels in liver, spleen, kidneys, lungs, heart as well as the organs themselves were opened and examined with a binocular for abnormalities. In suspected cases, squeeze preparations were made by squeezing conspicuous organ areas between two petri dishes to the extent that any abnormalities could be checked under the binocular. The trachea, esophagus, and stomach were dissected out individually using bandage scissors with a rounded tip and opened with a medial longitudinal incision. The stomach contents were transferred to a Petri dish. The weights of the stomach and stomach contents were determined. The inner surfaces of the stomach as well as the trachea were examined for parasites under binocular vision. Intestinal loops were disentangled and arranged on a cutting board. Small and large intestines were cut lengthwise using bandage scissors with a rounded tip to avoid destroying any parasites that might be present and transferred section by section (10 cm length each) to large petri dishes, rinsed with 0.9% sodium chloride solution and the inner intestinal wall and rinsed solution were examined under a binocular. Intestinal contents were transferred in portions to petri dishes and examined for parasites under a light microscope. Collected parasites were separated according to organ affiliation and stored in block dishes containing 0.9% NaCl solution until end of dissection and afterwards were preserved in 70% ethanol and for further genetic study transferred to 100% ethanol. Examined carcasses and organs were disposed of through the rendering plant.
For identification purposes, nematodes were dehydrated in a gradated ethanol series and transferred to 100% glycerine (Klimpel et al., 2019). Ectoparasites were cleared in 10% KOH, dehydrated and mounted in Canada balsam. Fleas were left in potassium hydroxide (10%) for 2 h at 95 °C or 24 h at 35 °C. Afterwards they were rinsed in xylene and stored in 70% ethanol. The parasitological terms prevalence, mean intensity, intensity and abundance follow the recommendations of Bush et al. (1997) and Klimpel et al. (2019).
2.2. Species identification
Most species were determined based on morphological characteristics only. Parasite identification literature included original descriptions, as well as identification keys (Neumann and Mayer, 1914; Peus, 1938; Sprent, 1968; Priemer and Lux, 1994; Hong et al., 1996; Anderson, 2000; Brinck-Lindroth and Smit, 2007; Anderson et al., 2009). If no species level could be determined, genetic species identification was performed. DNA of 11 ecto- and endoparasite species (12 individuals from 12 different raccoons) could be extracted according to the protocol of the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). PCR was performed at a volume of 25 μl (12.5 μl Taq PCR Master Mix (Qiagen, Hilden, Germany), 7 μl H2O distilled, 2.5 μl DNA extract, 1 μl MgCl2 25 MM (VWR, Darmstadt, Germany), 1 μl each forward and reverse primer). The primers as well as the thermocycler settings for the PCR reactions were systematically selected according to the parasite groups and performed following already established protocols (Folmer et al., 1994; Powers et al., 1997; Littlewood and Olson, 2001; Whiting, 2002; Holterman et al., 2006; Laurimaa et al., 2016; Nugaraitė et al., 2017; Hornok et al., 2018; Gérard et al., 2020) (see Table 1). Agarose gel electrophoresis followed to check the quality of the PCR products. Samples with clearly recognizable DNA fragments of the correct size were purified using NucleoSpin Gel and PCR Clean-up (Macherey-Nagel, Düren, Germany). Primers used for the PCR reaction were also used for sequencing of the samples at Microsynth Seqlab GmbH (Göttingen, Germany) using Sanger sequencing. For species identification, the sequenced samples were compared with existing data using the BLAST algorithm of the GENBANK NCBI sequence database and results were compared according to Query length (length of the submitted sequence in bases), the Query Coverage (percentage of submitted sequence that could be compared with sequence from database) and Percent Identity (how similar in percent was transmitted sequence with the present sequence in database).
Table 1.
Overview of used primers, methods and results of genetically species identification including literature sources. Species marked with * were additionally confirmed with morphological methods.
| Order | Accession number | Species | Primer | Thermocycling | Query Length | Query Cover | Per. Ident. | Sources |
|---|---|---|---|---|---|---|---|---|
| Nematoda | SAMN32641565 | Porrocaecum ensicaudatum* | forward primer rDNA2 (5′-TTGATTACGTCCCTGCCCTTT-3′) reverse primer rDNA1. 5.8s (5′-ACGAGCCCGAGTGATCCACCG-3′) | 120s at 94 °C, 40 x (60s at 94 °C, 60s at 57 °C, 120s at 72 °C) | 603 | 77% | 98.51 |
Powers et al. (1997) Morphology: Anderson et al. (2009), Osche (1959) |
| SAMN32641562 | Baylisascaris procyonis | forward primer 988F (5′-CTCAAAGATTAAGCCATGC-3′) reverse primer 1912R (5′-TTTACGGTCAGAACTAGGG-3′) | 94 °C for 5 min; 5 x (94 °C, 30 s; 45 °C, 30 s; 72 °C, 70 s) 35 x (94 °C, 30 s; 54 °C, 30 s; 72 °C, 70 s) 300s at 72 °C | 934 | 99 | 100 | Holterman et al. (2006) | |
| SAMN32641568 | Baylisascaris procyonis | forward primer 28S-rD1.2a (5′- CCCSSGTAATTTAAGCATATTA-3′) reverse primer 28SB (5′-TCGGAAGGAACCAGCTACTA-3′) | 180s at 95 °C, 40 x (30s at 95 °C, 30s at 54 °C, 60s at 72 °C) 600s at 72 °C | 1067 | 99 | 99.81 | Whiting (2002) | |
| Digenea | SAMN32641558 | Brachylaima mesostoma* | forward primer dig12 (5′- AAGCATATCACTAAGCGG-3′) reverse primer 1500R (5′- GCTATCCTGAGGGAAACTTCG-3′) | 98 °C for 10 s, 40x (50 °C for 20 s, 68 °C for 90 s, 60s at 72 °C) | 1096 | 99 | 99 |
Gérard et al. (2020) Morphology: Heneberg et al. (2016) |
| SAMN32641566 | Euryhelmis squamula* | forward primer WormA (5′-GCGAATGGCTCATTAAATCAG-3′) reverse primer WormB (5′-CTTGTTACGACTTTTACTTCC-3′) | 180s at 94 °C; 40x (30s at 94 °C, 30s at 56 °C, 120s at 72 °C); 420s at 72 °C | 1145 | 100 | 99.56 |
Nugaraitė et al. (2017), Littlewood and Olson (2001) Morphology: Anderson and Pratt (1965) |
|
| SAMN32641560 | Alaria alata | 1239 | 99 | 97.68 | ||||
| Acanthocephala | SAMN32641567 | Polymorphus minutus | forward primer LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) reverse primer HCO 2198 (5′-ACTAAAAAACCAGTGGGACTTCAAAT-3′) | 120s at 95 °C; 35x (60s at 95 °C, 60s at 40 °C, 90s at 72 °C); 420s at 72 °C | 608 | 100 | 93.09 | Folmer et al. (1994) |
| Arachnida | SAMN32641559 | Ixodes hexagonus | 641 | 99 | 99.58 | |||
| SAMN32641569 | Ixodes ricinus | 649 | 100 | 99.08 | ||||
| Insecta | SAMN32641561 | Chaetopsylla globiceps | forward primer F-Leu (5′-TCT AAT ATG GCA GAT TAG TGC-3′) reverse primer R-Lys (5′- GAG ACC AGT ACT TGC TTT CAG TCA TC- 3′) | 300s at 95 °C; 40x (40s at 94 °C, 60s at 53 °C, 60s at 72 °C); 420s at 72 °C | 726 | 95 | 99.42 |
Hornok et al. (2018) Morphology: Brinck-Lindroth and Smit (2007) |
| SAMN32641564 | Ctenocephalides felis | 711 | 99 | 99.58 | ||||
| SAMN32641563 | Paraceras melis* | forward primer 18Sa1.0 (5′-GGT GAA ATT CTT GGA YCG TC-3′) reverse primer 18S9R (5′-GAT CCT TCC GCA GGT TCA CCT AC-3′) | 300s at 95 °C; 40x (40s at 94 °C, 60s at 55 °C, 60s at 72 °C); 420s at 72 °C | 839 | 100 | 98.21 |
2.3. Screening for Trichinella spp
All raccoons were analyzed for Trichinella spp. muscle larvae using pepsin digestion. At least 50 g of tissue derived from skeletal muscles (tongue, masseter, diaphragm, and forelimb muscles; approx. 10 g each) were digested in 250 ml of a solution containing 1:100 diluted HCl (37% HCl; Carl Roth GmbH, Germany) and 1% pepsin (Merck, Darmstadt, Germany) while stirring at 35–38 °C until meat particles dissolved (approximately 45 min). Afterwards, the solution was centrifuged at 1500g for 5 min. The supernatant was discarded and the pellet resuspended in physiological saline solution. The resuspension was microscopically examined for the presence of Trichinella spp. larvae at 40–100 times magnification. For additional testing of Trichinella spp., we used the PrioCHECK™ Trichinella Alternative Artificial Digestion (AAD) Kit (Thermo Fisher Scientific, Langenselbold, Germany) according to the manufacturer's protocol.
3. Results
3.1. Ecto- and endoparasite fauna
Of the 234 examined raccoons, 22 individuals (9.4%) were juveniles (13 males, 9 females), 212 (90.6%) were adults (145 males, 67 females). The average total weight for adult males was 5.61 kg, for juvenile males 3.21 kg, for adult females 4.84 kg and 2.82 kg for juvenile females. The average standard length for adult males was 51,47 cm, for juvenile males 43,65 cm, for adult females 48,73 cm and 42,89 cm for juvenile females. Detailed morphometric data was recorded during dissection but since there was no significant connection with parasite infestation they will not be discussed further. The parasite fauna of the investigated raccoons consisted of 23 different ecto- and endoparasite species belonging to six taxonomic groups (Table 2). The most frequent ectoparasites were the louse Trichodectes octomaculatus with 69.2% prevalence (with a mean Intensity of mI = 66.4) as well as the tick Ixodes ricinus with 45.3% prevalence (mI = 8.5). Furthermore, the tick species Ixodes hexagonus, the mite species Sarcoptes scabiei and Neotrombicula autumnalis and the flea species Chaetopsylla globiceps, Ctenocephalides felis, Nosopsyllus fasciatus and Paraceras melis could be identified.
Table 2.
Parasitological data of Procyon lotor (N = 234), N = number of infected host animals, P = prevalence of infection, I = intensity of infection, mI = mean intensity of infection, A - abundance over all examined Procyon lotor.
| N | P | I | mI | A | |||
|---|---|---|---|---|---|---|---|
| Ectoparasites | Insecta | Chaetopsylla globicepsa | 6 | 2.6% | 1–31 | 6 | 0.156 |
| Ctenocephalides felis | 40 | 17.1% | 1–94 | 14 | 2.394 | ||
| Nosopsyllus fasciatusa | 13 | 5.6% | 1–4 | 1.5 | 0.084 | ||
| Paraceras melisa | 63 | 26.9% | 1–27 | 2.5 | 0.673 | ||
| Trichodectes octomaculatus | 162 | 69.2% | 1–1500 | 66.4 | 45.949 | ||
| Arachnida | Ixodes ricinus | 106 | 45.3% | 1–91 | 8.5 | 3.851 | |
| Ixodes hexagonusa | 44 | 18.8% | 1–46 | 5.8 | 1.09 | ||
| Sarcoptes scabiei | 2 | 0.9% | 1–1000 | 500.5 | 4.505 | ||
| Neotrombicula autumnalisa | 5 | 2.1% | 1–188 | 44 | 0.924 | ||
| Endoparasites | Digenea | Alaria alata | 1 | 0.4% | 1 | 1 | 0.004 |
| Brachylaima mesostomaa | 7 | 3.0% | 1–26 | 8 | 0.24 | ||
| Brachylaima erinaceia | 2 | 0.9% | 4–7 | 5.5 | 0.05 | ||
| Euryhelmis squamulaa | 4 | 1.7% | 1–78 | 32.5 | 0.553 | ||
| Plagiorchis murisa | 2 | 0.9% | 1–5 | 3 | 0.027 | ||
| Cestoda | Mesocestoides sp. | 6 | 2.6% | 1–292 | 88 | 2.288 | |
| Atriotaenia incisa | 46 | 19.7% | 1–763 | 50.4 | 9.929 | ||
| Nematoda | Baylisascaris procyonis | 222 | 94.9% | 1–285 | 28.6 | 27.141 | |
| Porrocaecum ensicaudatuma | 90 | 38.5% | 1–114 | 8.9 | 3.427 | ||
| Molineus patensa | 1 | 0.4% | 2 | 2 | 0.008 | ||
| Synhimantus laticepsa | 1 | 0.4% | 47 | 47 | 0.188 | ||
| Trichuris murisa | 1 | 0.4% | 2 | 2 | 0.008 | ||
| Acanthocephala | Echinorhynchus truttaea | 2 | 0.9% | 1 | 1 | 0.009 | |
| Polymorphus minutus | 4 | 1.7% | 17–246 | 99 | 1.683 | ||
New for Raccons in Europe.
The most frequent endoparasites were the nematodes Baylisascaris procyonis with 94.9% prevalence (mI = 28.6) followed by Porrocaecum ensicaudatum with a prevalence of 38,5% (mI = 8.9). Other endoparasitic species that could be determined in this study were the digeneans Alaria alata, Brachylaima erinacei, Brachylaima mesostoma, Euryhelmis squamula and Plagiorchis muris, as well as the cestodes Mesocestiodes sp. and Atriotaenia incisa, the nematodes Molineus patens, Synhimantus laticeps and Trichuris muris and the acanthocephalans Echinorhynchus truttae and Polymorphus minutus. The sequenced data of the genetically examined samples was uploaded to the NCBI Sequence Read Archive (SRA) and are available under the accession numbers given in Table 1.
No Trichinella larvae were identified in any of the 234 analyzed raccoon muscle samples.
4. Discussion
The objective of this study was to identify the parasite fauna of raccoons, to provide a comprehensive overview of parasite diversity, and to highlight the occurrence of parasite species with zoonotic potential. The parasite fauna of raccoons observed in the present study was significantly more diverse than in previous studies from other regions in Central Europe and Germany (Lux and Priemer, 1995; Helbig, 2011; Karamon et al., 2014; Michler, 2017; Stope, 2019; Duscher et al., 2020; Romeo et al., 2021).
In the present study totally 23 different parasite species were determined. With tick species I. hexagonus, mite species N. autumnalis, flea species C. globiceps, N. fasciatus, and P. melis, digenea B. mesostoma, B. erinacei, E. squamula as well as P. muris, nematodes P. ensicaudatum, M. patens, S. laticeps and T. muris and acanthocephalan species E. truttae first findings for European raccoons could be described. Potential human pathogenic parasites, such as S. scabiei, N. autumnalis, A. alata, P. muris and B. procyonis have also been detected. The zoonotic raccoon roundworm B. procyonis was the most abundant parasite species with exceedingly high infestation rates.
Currently, there are only a few studies available on the metazoan parasite fauna of raccoons from Europe, that reported a much less diverse parasite fauna (Priemer and Lux, 1994; Lux and Priemer, 1995; Gey, 1998; Beltrán-Beck et al., 2012; Rentería-Solís, 2015; Michler, 2017). This shows that the metazoan parasite fauna of raccoons in Europe has only been marginally studied. The low parasite diversity in European raccoons known to date is not particularly surprising, as raccoons have only been present in Europe since the 1930s and thus co-evolution of host-specific parasites has not yet taken place in Europe (Torchin et al., 2003). In addition, existing studies so far have been very locally restricted. The results and effort of this study differ significantly in terms of parasite species detected and sample size (n = 234 individuals) compared to previous studies.
4.1. Ectoparasites
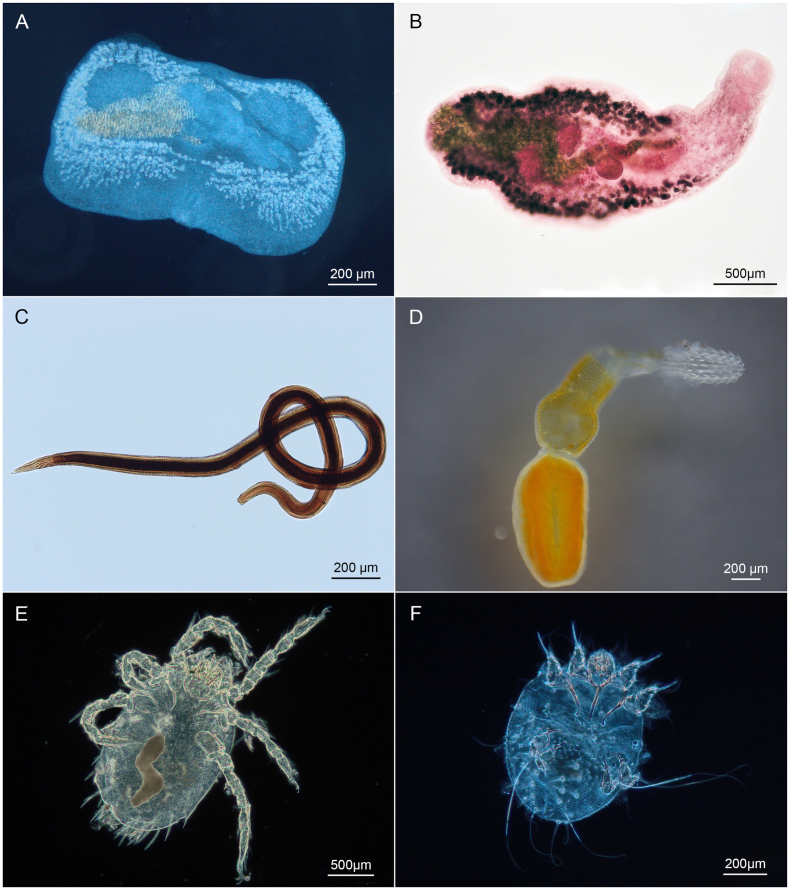
The ectoparasitic species identified in this study were I. ricinus, I. hexagonus, S. scabiei, N. autumnalis, C. globiceps, C. felis, N. fasciatus, P. melis and T. octomaculatus. The most common species were I. ricinus with a prevalence of 45.3% as well as T. octomaculatus with a prevalence of 69.2%. The species I. ricinus and I. hexagonus, belonging to the class Arachnida, are among the most common and widespread tick species in Europe and are known to act as vectors of Tick-borne encephalitis (TBE), Lyme disease, Ehrlichiosis, Anaplasmosis, tick-borne babesiosis and tick-borne rickettsiosis and other various pathogens (Camacho et al., 2003; Nijhof et al., 2007; Bitam and Raoult, 2009; Randolph, 2009; Medlock et al., 2013; Walter et al., 2020). Both species parasitize mainly mammals such as hedgehogs, foxes or dogs (Claerebout et al., 2013; Dziemian et al., 2014; Rizzoli et al., 2014) and infest the host for the blood meal, which is required during their developmental cycle. The two mite species S. scabiei and N. autumnalis (Fig. 2) are distributed worldwide and infest various mammals and use them as feeding hosts (Mehlhorn and Mehlhorn, 2020).
Fig. 2.
Light micrographs of different endo- and ectoparasite species showing the general morphology of the identified parasites in the investigated raccoons; A: Euryhelmis squamula, B: Plagiorchis muris, C: Porrocaecum ensicaudatum, D: Polymorphus minutus, E: Neotrombicula autumnalis, F: Sarcoptes scabiei.
Furthermore, four flea species (C. globiceps, C. felis, N. fasciatus, P. melis) were identified, of which the badger flea P. melis was the most abundant. The host range varies greatly among the different flea species (Brinck-Lindroth and Smit, 2007). Only C. felis has been reported as ectoparasite of raccoons before, although it is mainly found on domestic cats and dogs, but can infest other mammals as well as humans (Brinck-Lindroth and Smit, 2007). The raccoon's infections with the cat flea can easily be explained, as the raccoon becomes more and more urbanized, and its proximity to humans and their pets is decreasing accordingly. The main reasons are that invasive carnivores use anthropogenic food resources (Prange et al., 2004) such as litter or even cat food and that they inhabit urban buildings, including inhabited houses (Michler, 2004). Thus, transmission of the cat flea can occur quickly. Infestation with the other three flea species can be explained in a similar way. Their main hosts are foxes, badgers and rats, and the raccoon interacts with these in its new range. Firstly, by feeding, as the omnivorous raccoon feeds on small mammals, especially rodents (Bartoszewicz et al., 2008) and secondly, by using similar prey species and habitat niches as the fox and badger in the wild.
The louse T. octomaculatus is a highly host-specific ectoparasite species typical for raccoons andcompletes its life cycle on one and the same host (Emerson and Price, 1985). Trichodectes octomaculatus is known to be a common parasite for the raccoon in both its native and invasive of distribution ranges (Richardson et al., 1994; Haitlinger and Łupicki, 2009), which is also reflected in the high infestation rate found in this study.
Most ectoparasites infest hosts for a blood meal, which is essential for their development. As a result, they can cause symptoms such as itching, lesions of the skin or hair loss, which can affect and weaken the host animal. Ectoparasites can also transmit pathogens to other animals, for example, ticks can be responsible for the transmission of Borrelia or Rickettsia (Süss et al., 2004). To date, the ectoparasitic fauna of raccoons in Germany and Europe was not well described and only a few parasite species were known. In the current study we were able to describe five out of the nine ectoparasites as new species for the raccoon in Europe. With the continuous spread of the raccoon, future studies need to pay attention to external parasites, since these species do not only use the raccoon as vector but can transmit various pathogens, thus posing an additional threat to human and animal health. For example, I. ricinus, I. hexagonus, S. scabiei, N. autumnalis, C. globiceps, N. fasciatus and C. felis are all able to parasitize humans and may be of epidemiological importance as vectors for various human pathogenic viruses and microorganisms (TBE, Lyme disease, rickettsia, spirochetes and other bacteria, Mehlhorn, 2012).
4.2. Endoparasites
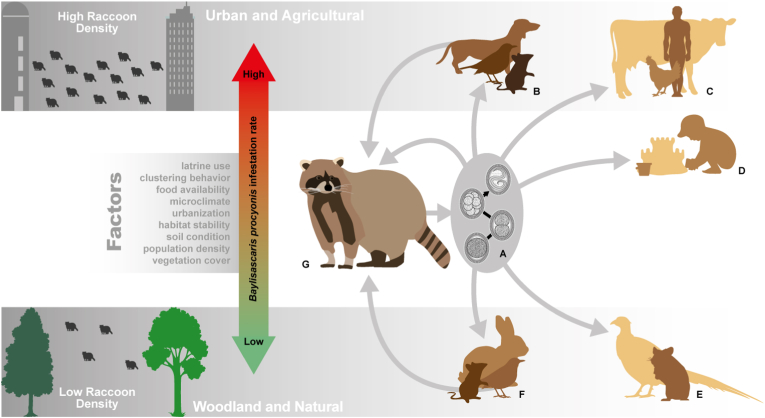
In total, five trematodes, two cestodes, five nematodes, and two acanthocephalans were identified as endoparasites of raccoons in the study area. Predominantly, these were found in the small intestine, but also in the large intestine and stomach. The most common endoparasite was the raccoon roundworm B. procyonis, which occurred with a prevalence of almost 95%, a national and European high in comparison to other studies. For example, no infestations of raccoon roundworm have been previously detected in eastern German raccoon populations (Lux and Priemer, 1995; Michler, 2017), while other studies from central Europe describe infestation frequencies between 39% and 75% (Gey, 1998; Winter, 2004; Anheyer-Behmenburg, 2013; Rentería-Solís et al., 2018). Baylisascaris procyonis is highly host-specific and was introduced with the raccoons from their original range in North America (Bauer, 2011). The females of adult worms are larger than the males, their eggs are excreted in the raccoon's feces and can survive in the environment for several years. The intermediate hosts of this parasite can be birds and smaller mammals, which in turn are preyed upon by the raccoon. Due to the presence of, for example, concentrated anthropogenic food resources in urban and agricultural areas, the adaptable raccoons reach ever higher population densities in human proximity (Prange et al., 2004; French et al., 2019). Since common latrines are usually used, which in turn can be visited by potential intermediate hosts, this increases the likelihood of raccoon roundworm spread in these areas as well as the risk of infection for humans and animals (e.g. Roussere et al., 2003; Kazacos 2001) (Fig. 3). The final host shows almost no signs of disease when infected with this roundworm, while any intermediate or accidental hosts may suffer organ damage (Blizzard et al., 2010). The raccoon roundworm B. procyonis is currently considered a serious zoonotic agent in Germany (Bauer, 2013; French et al., 2019) and a causative agent of human baylisascariosis. Roussere et al. (2003), Graeff-Teixeira et al. (2016) and Weinstein et al. (2017) highlighted the potential risk of infection to children from oral ingestion of eggs in raccoon feces, placing a particular focus on urban and suburban areas for parasitological and serological investigations. For North America, studies in areas with known high infection rates of the zoonotic pathogen B. procyonis in raccoon populations showed an increase in human baylisascariosis (Haider et al., 2012; Hung et al., 2012; Kelly et al., 2012; Peters et al., 2012). Infection can cause neural, visceral, and ocular larva migrans (Kazacos, 2001; Graeff-Teixeira et al., 2016) resulting in severe damage to organic tissues and even death (Gavin et al., 2005). Kazacos (2016) lists 25 cases of clinical neural larva migrans described in North America. Only a few cases have been reported for Germany, but the very high prevalences and intensities of B. procyonis infestation in the study area, together with the ever-increasing spread of raccoons in cities and settlements, suggests an increasing risk of infection and thus a higher threat to human and animal health in certain areas than previously thought.
Fig. 3.
Several factors influence the spread of Baylisascaris procyonis, infestation rates are higher in urban and agricultural environments than in semi-natural sites. Thus, a denser population of raccoons in urban areas has a beneficial effect on infestation numbers. However, other factors such as vegetation density as well as available food resources, microclimate, soil characteristics, and land use also influence the rate of B. procyonis infestation and zoonotic potential. An Unembryonated eggs are shed by the raccoon. Eggs undergo 2–4 weeks of development outside the body until the embryonated and infective stage is reached. Embryonated eggs can remain in the environment for several months and remain infectious. B In urban and agricultural areas, new intermediate hosts are frequently infected. These are either eaten by the raccoon or can become infected like the raccoon (e.g., dogs) and then also excrete eggs. C In urban and agricultural areas, there are usually other off-target hosts than in semi-natural areas. Here, for example, cattle or chickens have been confirmed as false hosts. In these hosts, visceral or ocular larva migrans is induced without the larvae being ingested by the raccoon. D Children can become infected with infectious stages and contract Larva migrans through constructed latrines as well as simple defecation in or near play facilities (e.g., sandboxes, climbing houses). E Due to the steady spread of raccoons, new false hosts are increasingly infected with eggs of B. procyonis, such as chicken birds (pheasants), even in semi-natural areas. F The normal life cycle of B. procyonis includes the prey of P. lotor such as various small mammals or birds. In Europe, these species also play the main role in semi-natural habitats. G Raccoons, as the primary host, become infected with B. procyonis by direct ingestion of embryonated eggs through contact with latrines and via excreted feces of infected raccoons. Numerous small mammals or birds serve as paratenic or intermediate hosts, passing larvae directly to the raccoon. The worms grow in the raccoon and can reach high densities in the raccoon's intestine.
The second most abundant endoparasite (P = 38.5%) of the raccoon in the present study was the nematode P. ensicaudatum (Fig. 2). This species parasitizes mainly the small intestine of birds (Borgsteede et al., 2003). The genus Porrocaecum represents the only genus of autochthonous avian parasites within the Ascaridoidea that can be transmitted to mammals (Osche, 1959; Baruš et al., 1978; Anderson, 2000; Borgsteede et al., 2003). According to Levin (1961) and Anderson (2000) earthworms or other avian species of the genera Sturnus and Turdus may serve as intermediate hosts in the life cycle of P. ensicaudatum. A probable infection with P. ensicaudatum by intermediate hosts from the family Lumbricidae can also be inferred from the studies of Wharton (1979), according to which gravid female P. ensicaudatum were found in the small intestine of Corvus frugilegus. However, in mammals such as raccoons, this parasite does not undergo further development, but remains free in the intestinal lumen (Osche, 1959). This is also evident in this study, as this parasite was detected freely in infested animals in the small and large intestines, as well as partially in the stomach. It can be concluded that the raccoon does feed on the intermediate host of P. ensicaudatum, like species of the family of Lumbricidae, as well as on birds of the genus Sturnus and Turdus. The nematode M. patens parasitizes mainly martens (Kołodziej-Sobocińska et al., 2021), but occurs also in other mammals (Popiołek et al., 2009). Species of the genus Molineus undergo a simple life cycle, with the next host ingesting the eggs from the environment and thus becoming infected orally or by invasion through the skin (Gupta, 1961, 1963). The very low infestation rate in this study suggests a more incidental infection of the raccoon. Synhimantus laticeps is a nematode species that parasitizes in the stomach of owls and raptors (Santoro et al., 2010) The development and infection routes of S. laticeps have not yet been identified. It is suspected that raptor prey serves as intermediate hosts. Infection of the animals examined in this study can only be explained by the fact that infested bird species were preyed upon by the raccoon. Likewise, infection with T. muris provides information about the feeding habits of the raccoon, since this nematode is found primarily in mice and rats (Fahmy, 1954). However, due to the low infestation numbers of the latter species, only limited conclusions can be made about the possible infection risks of raccoons, because it is not certain whether these findings represent actual infections or just intestinal passage of orally ingested parasites. In terms of feeding ecology, these findings provide a clear indication of the raccoon's broad dietary spectrum.
The cestode species A. incisa was found in 46 of the examined animals, which corresponds to a prevalence of 19.7%. It is known as a small intestine parasite in badgers as well as raccoons and has already been described in raccoons in Germany (Priemer and Lux, 1994). Not much is known about the development, possible intermediate hosts or triggered diseases and symptoms (Torres et al., 2001). However, the occasionally high infestation numbers (max. intensity 763) confirm the importance of raccoons as hosts and vectors of this parasite. The second species of Cestoda, Mesocestoides sp., could only be determined to genus level in this study. Species of this genus are distributed worldwide and use a wide range of hosts, as many species of mammals and birds can be infested. Infection of raccoons with cestodes of this genus have previously been described in Europe (Karamon et al., 2014; Schwarz et al., 2015). The extent to which the raccoon is important as a vector of this parasite cannot be determined due to relatively low infestation numbers and the fact that the exact species could not be identified. However, this particular parasite again points to the broad food spectrum of the raccoon.
The digenean species A. alata, B. mesostoma, B. erinacei, E. squamula and P. muris (Fig. 2) were recorded in raccoons in the study area. Fluke species often use two intermediate hosts in their life cycle. Freshwater or terrestrial snails are often used as the first intermediate host, and second intermediate hosts can be amphibians, birds, mammals and reptiles (Zeller, 1867; Anderson and Pratt, 1965; Heneberg et al., 2016; Gérard et al., 2020). All five digenean species occurred in low infestation numbers for potentially several reasons. On the one hand, the raccoon could have been infected only as an accidental host or the parasites could have been ingested with food and thus it is not a direct infection of the raccoon. On the other hand, the majority of the examined raccoons were hunted or trapped in the winter months. as described above, digeneans use snails and amphibians as intermediate hosts (Anderson and Pratt, 1965; Heneberg et al., 2016; Lucius et al., 2018) and restricted seasonal access to these food sources (Glandt, 2016) could be another reason for the low infestation numbers. Comparative studies in the spring and summer months could provide more information on this inference. With the detection of the digenetic trematode P. muris, the record of another human pathogenic parasite species was established (Lee et al., 2004). So far, only the genus was reported for the raccoon (Lux and Priemer, 1995). Hong et al. (1996) described a case of infection with this parasite in South Korea that may be due to consumption of freshwater fish. Likewise, human infections with the digenea A. alata may occur (Jakšić et al., 2002). The fact that the raccoon can act as a host for these parasites increases the risk of human infection, as the raccoon is increasingly found in cities and the proximity to humans continues to decrease. Thus, transmissions can occur more easily. In the present study, E. squamula was recorded for the first time in a raccoon from Europe. The complex life-cycle requires at least three hosts for the complete development. The first intermediate hosts of E. squamula are snails of the family of Hydrobiidae (Anderson and Pratt, 1965).The second intermediate hosts are frogs and toads, and Metacercariae of this species have already been detected in Europe in Rana temporaria, Rana esculenta, Triturus cristatus and various toad species (Zeller, 1867; Baer, 1931; Anderson and Pratt, 1965). Raccoons belong to the final hosts of E. squamula (Parker, 1950; Babero and Shepperson, 1958; Harkema and Miller, 1964; Bafundo et al., 1980; Cole and Shoop, 1987). The detection of E. squamula is of great nutritional and ecological interest. Anderson and Pratt (1965) indicate the obligate presence of amphibians in the life cycle of E. squamula (Fig. 2), thus, the findings of this parasite provide evidence of the use of amphibian food sources and therefore uncover possible conflict and negative impact on already threatened amphibian populations.
The last two recorded species E. truttae and P. minutus (Fig. 2) belong to the Acanthocephala. Adult P. minutus live in the intestines of various water birds, while E. truttae occurs in the intestines of various freshwater fish. Both species use amphipods and fish as intermediate hosts (Romanovski, 1964; Awachie, 1966).This suggests that the ingestion and infection of the raccoon with these parasites occurred through its diet, thus providing further insight into the wide food spectrum that the omnivorous raccoon utilizes.
4.3. Conclusions
The results of this study indicate that raccoons are now an integral part of the studied ecosystem. According to previous studies, raccoons in Germany had a limited range of metazoan parasite species (Priemer and Lux, 1994; Lux and Priemer, 1995; Gey, 1998; Beltrán-Beck et al., 2012; Rentería-Solís, 2015; Michler, 2017) while 99 metazoan parasite genera are described for their area of origin, the North American region (Gey, 1998). This study shows that the raccoon is a host for more than 20 parasitic metazoan species in the study area. One reason for the different results compared to previous studies could be that the raccoon has only been present in Europe since the 1930s and thus no co-evolution of host-specific parasites has yet take place in Europe (Torchin et al., 2003). The full potential of raccoons as vectors of parasitic pathogens cannot be assessed in such a short time and only random sampling in Germany and Europe. The results of this study differ significantly from those of previous studies in terms of metazoan species numbers, suggesting that the occurrence of parasitic organisms in different raccoon populations might be variable, with potentially significant regional differences. The fact that 14 new parasite species were described for the raccoons in Germany/Europe in the present study underpins that there are not enough comprehensive studies available. It is highly likely that the raccoon will integrate and adapt more and more in the new area of distribution, which will also expand in the future. Furthermore, raccoons will probably play an important role as an additional vector of native parasites and other pathogens in the years to come.
Funding
The present study was financially supported by the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt - DBU 35524/01–43) and the Uniscientia Foundation (project number P 180–2021).
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We thank all cooperation members of the ZOWIAC Project for the acquisition of samples as well as support for planning the sampling strategies. On behalf of all participating hunters, we would like to express our special thanks to Rudolf Ziegler, Karl-Adam Vey, Timo Spaniol, Mathias Braun and Fabian Best for sampling.
References
- Anderson G.A., Pratt I. Cercaria and first intermediate host of Euryhelmis squamula. J. Parasitol. 1965;51:13. [PubMed] [Google Scholar]
- Anderson R.C., editor. Nematode Parasites of Vertebrates: Their Development and Transmission. second ed. CABI Pub; Wallingford, Oxon, UK, New York, NY: 2000. p. 650. [Google Scholar]
- Anderson R.C., Chabaud A.G., Willmott S. CAB International; Wallingford (UK): 2009. Keys to the Nematode Parasites of Vertebrates: Archival Volume; p. 463. [Google Scholar]
- Anheyer-Behmenburg H.E. first ed. Tierärztl. Hochsch.; Hannover: 2013. Untersuchungen zum Vorkommen von Zoonoseerregern und dem kaninen Staupevirus in der Waschbärpopulation Niedersachsens, 2011-2013. Dissertation; p. 140. [Google Scholar]
- Awachie J.B. The development and life history of Echinorhynchus truttae Schrank, 1788 (Acanthocephala) J. Helminthol. 1966;40:11–32. doi: 10.1017/s0022149x00034040. [DOI] [PubMed] [Google Scholar]
- Babero B.B., Shepperson J.R. Some helminths of raccoons in Georgia. J. Parasitol. 1958;44 [Google Scholar]
- Baer J.G. Quelques helminthes rares ou peu connus du putois. Rev. Suisse Zool. 1931;38:313–334. [Google Scholar]
- Bafundo K.W., Wilhelm W.E., Kennedy M.L. Geographic variation in helminth parasites from the digestive tract of Tennessee raccoons, Procyon lotor. J. Parasitol. 1980;66:134. [PubMed] [Google Scholar]
- Bartoszewicz M., Okarma H., Zalewski A., Szczęsna J. Ecology of the raccoon (Procyon lotor) from western Poland. Ann. Zool. Fenn. 2008;45:291–298. [Google Scholar]
- Baruš V., Sergeeva T.P., Sonin M.D., Ryzhikov K.M., Ryšavý B. 1978. Helminths of Fish-Eating Birds of the Palaearctic Region. [DOI] [Google Scholar]
- Baskin J.A. In: Evolution of Tertiary Mammals of North America. Janis C.M., Scott K.M., Jacobs L.L., editors. vol. 1. Cambridge University Press; Cambridge: 1998. Procyonidae; pp. 144–151. [Google Scholar]
- Bauer C. Baylisascariosis (Baylisascaris procyonis) - a rare parasitic zoonosis in Europe. Berl. Münchener Tierärztliche Wochenschr. 2011;124:465–472. [PubMed] [Google Scholar]
- Bauer C. Baylisascariosis-infections of animals and humans with 'unusual' roundworms. Vet. Parasitol. 2013;193:404–412. doi: 10.1016/j.vetpar.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Beasley J. Relationship between raccoon abundance and crop damage. Human‐Wildlife Conflicts. 2008;2:248. [Google Scholar]
- Beltrán-Beck B., García F.J., Gortázar C. Raccoons in Europe: disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012;58:5–15. [Google Scholar]
- Bitam I., Raoult D. Other tick-borne diseases in Europe. Curr. Probl. Dermatol. 2009;37:130–154. doi: 10.1159/000213072. [DOI] [PubMed] [Google Scholar]
- Blizzard E.L., Yabsley M.J., Nims T., Garrison L.E. Intestinal roundworm (Baylisascaris procyonis) of raccoons (Procyon lotor): information for public health and wildlife professionals. J. Wildl. Manage. WMS. 2010;10 –11:1–7. [Google Scholar]
- Borgsteede F., Okulewicz A., Zoun P., Okulewicz J. The helminth fauna of birds of prey (Accipitriformes, Falconiformes and Strigiformes) in The Netherlands. Acta Parasitol. 2003;48:200–207. [Google Scholar]
- Brinck-Lindroth G., Smit F.G.A.M. Brill; Leiden: 2007. The Fleas (Siphonaptera) of Fennoscandia and Denmark; p. 185. [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Camacho A.T., Pallas E., Gestal J.J., Guitián F.J., Olmeda A.S., Telford S.R., Spielman A. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet. Parasitol. 2003;112:157–163. doi: 10.1016/s0304-4017(02)00417-x. [DOI] [PubMed] [Google Scholar]
- Claerebout E., Losson B., Cochez C., Casaert S., Dalemans A.-C., Cat A. de, Madder M., Saegerman C., Heyman P., Lempereur L. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasites Vectors. 2013;6:183. doi: 10.1186/1756-3305-6-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavero M., Brotons L., Pons P., Sol D. Prominent role of invasive species in avian biodiversity loss. Biol. Conserv. 2009;142:2043–2049. [Google Scholar]
- Cole R.A., Shoop W.L. Helminths of the raccoon (Procyon lotor) in western Kentucky. J. Parasitol. 1987;73:762–768. [PubMed] [Google Scholar]
- Cybulska A., Skopek R., Kornacka A., Popiołek M., Piróg A., Laskowski Z., Moskwa B. First detection of Trichinella pseudospiralis infection in raccoon (Procyon lotor) in Central Europe. Vet. Parasitol. 2018;254:114–119. doi: 10.1016/j.vetpar.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Demeny K., McLoon M., Winesett B., Fastner J., Hammerer E., Pauli J.N. Food subsidies of raccoons (Procyon lotor) in anthropogenic landscapes. Can. J. Zool. 2019;97:654–657. [Google Scholar]
- Dueñas M.-A., Hemming D.J., Roberts A., Diaz-Soltero H. The threat of invasive species to IUCN-listed critically endangered species: a systematic review. Glob. Ecol. Conserv. 2021;26 [Google Scholar]
- Dueñas M.-A., Ruffhead H.J., Wakefield N.H., Roberts P.D., Hemming D.J., Diaz-Soltero H. The role played by invasive species in interactions with endangered and threatened species in the United States: a systematic review. Biodivers. Conserv. 2018;27:3171–3183. [Google Scholar]
- Duscher G.G., Frantz A.C., Kuebber-Heiss A., Fuehrer H.-P., Heddergott M. A potential zoonotic threat: first detection of Baylisascaris procyonis in a wild raccoon from Austria. Transbound Emerg Dis. 2020;68:3034–3037. doi: 10.1111/tbed.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziemian S., Michalik J., Pi Łacińska B., Bialik S., Sikora B., Zwolak R. Infestation of urban populations of the Northern white-breasted hedgehog, Erinaceus roumanicus, by Ixodes spp. ticks in Poland. Med. Vet. Entomol. 2014;28:465–469. doi: 10.1111/mve.12065. [DOI] [PubMed] [Google Scholar]
- Emerson K.C., Price R.D. In: Coevolution of Parasitic Arthropods and Mammals. Kim K.C., editor. Wiley; New York: 1985. Evolution of mallophaga on mammals; pp. 233–255. [Google Scholar]
- Fahmy M.A. An investigation on the life cycle of Trichuris muris. Parasitology. 1954;44:50–57. doi: 10.1017/s003118200001876x. [DOI] [PubMed] [Google Scholar]
- Falaschi M., Melotto A., Manenti R., Ficetola G.F. Invasive species and Amphibian conservation. Herpetologica. 2020;76:216. [Google Scholar]
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- French S.K., Pearl D.L., Peregrine A.S., Jardine C.M. Baylisascaris procyonis infection in raccoons: a review of demographic and environmental factors influencing parasite carriage. Vet. Parasitol.: Reg. Stud. Rep. 2019;16 doi: 10.1016/j.vprsr.2019.100275. [DOI] [PubMed] [Google Scholar]
- Gavin P.J., Kazacos K.R., Shulman S.T. Baylisascariasis. Clin. Microbiol. Rev. 2005;18:703–718. doi: 10.1128/CMR.18.4.703-718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrt S.D. In: Wild Mammals of North America. Biology, Management, and Conservation. second ed. Feldhamer G.A., editor. Johns Hopkins University Press; Baltimore, Md: 2003. Raccoon (Procyon lotor) and allies; pp. 611–634. [Google Scholar]
- Gérard C., Ansart A., Decanter N., Martin M.-C., Dahirel M. Brachylaima spp. (Trematoda) parasitant Cornu aspersum (Gastropoda) en France et risque potentiel pour la consommation humaine. Parasite. 2020;27:15. doi: 10.1051/parasite/2020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gey A. 1998. Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen: Inaugural-Dissertation zur Erlangung des Doktorgrades beim Fachbereich Veterinärmedizin der Justus-Liebig-Universität Gießen. (Gießen) [Google Scholar]
- Giacinti J.A., Pearl D.L., Ojkic D., Jardine C.M. Comparison of two surveillance components for investigating the epidemiology of canine distemper virus in raccoons (Procyon lotor) J. Wildl. Dis. 2021;57:104–115. doi: 10.7589/JWD-D-19-00001. [DOI] [PubMed] [Google Scholar]
- Glandt D. Springer-Verlag; Berlin, Heidelberg: 2016. Fortpflanzung und Entwicklung der Amphibien. [Google Scholar]
- Goldman E.A., Jackson H.H.T. Raccoons of North and Middle America. North am. fauna. 1950;60:1–153. [Google Scholar]
- Graeff-Teixeira C., Morassutti A.L., Kazacos K.R. Update on baylisascariasis, a highly pathogenic zoonotic infection. Clin. Microbiol. Rev. 2016;29:375–399. doi: 10.1128/CMR.00044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.P. The life history of Molineus barbatus Chandler, 1942. Can. J. Zool. 1961;39:579–587. [Google Scholar]
- Gupta S.P. Mode of infection and biology of infective larvae of Molineus barbatus chandler. Exp. Parasitol. 1963;13:252–255. doi: 10.1016/0014-4894(63)90077-8. 1942. [DOI] [PubMed] [Google Scholar]
- Haider S., Khairnar K., Martin D.S., Yang J., Ralevski F., Kazacos K.R., Pillai D.R. Possible pet-associated baylisascariasis in child, Canada. Emerg. Infect. Dis. 2012;18:347–349. doi: 10.3201/eid1802.110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitlinger R., Łupicki D. Arthropods (Acari, mallophaga, siphonaptera) collected from Procyon lotor (linnaeus, 1758) (mammalia, carnivora, Procyonidae) in Poland. Wiad. Parazytol. 2009:59–60. [PubMed] [Google Scholar]
- Hall E.R., editor. The Mammals of North America. second ed. Wiley; New York: 1981. [Google Scholar]
- Harkema R., Miller G.C. Helminth parasites of the raccoon, Procyon lotor in the southeastern United States. J. Parasitol. 1964;50:60. [PubMed] [Google Scholar]
- Haubrock P.J., Turbelin A.J., Cuthbert R.N., Novoa A., Taylor N.G., Angulo E., Ballesteros-Mejia L., Bodey T.W., Capinha C., Diagne C., Essl F., Golivets M., Kirichenko N., Kourantidou M., Leroy B., Renault D., Verbrugge L., Courchamp F. Economic costs of invasive alien species across Europe. NeoBiota. 2021;67:153–190. [Google Scholar]
- Heddergott M., Steinbach P., Schwarz S., Anheyer-Behmenburg H.E., Sutor A., Schliephake A., Jeschke D., Striese M., Müller F., Meyer-Kayser E., Stubbe M., Osten-Sacken N., Krüger S., Gaede W., Runge M., Hoffmann L., Ansorge H., Conraths F.J., Frantz A.C. Geographic distribution of raccoon roundworm, Baylisascaris procyonis, Germany and Luxembourg. Emerg. Infect. Dis. 2020;26:821–823. doi: 10.3201/eid2604.191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig D. Untersuchungen zum Waschbären (Procyon lotor Linné, 1758) im Raum Bernburg. Naturschutz im Land Sachsen-Anhalt. 2011;48:3–19. [Google Scholar]
- Heneberg P., Sitko J., Bizos J. Molecular and comparative morphological analysis of central European parasitic flatworms of the superfamily Brachylaimoidea Allison, 1943 (Trematoda: Plagiorchiida) Parasitology. 2016;143:455–474. doi: 10.1017/S003118201500181X. [DOI] [PubMed] [Google Scholar]
- Hohmann U. Dissertation. Hainholz-Verl.; Göttingen: 1998. Untersuchungen zur Raumnutzung des Waschbären (Procyon lotor L. 1758) im Solling, Südniedersachsen, unter besonderer Berücksichtigung des Sozialverhaltens; p. 153. [Google Scholar]
- Holterman M., van der Wurff A., van den Elsen S., van Megen H., Bongers T., Holovachov O., Bakker J., Helder J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown Clades. Mol. Biol. Evol. 2006;23:1792–1800. doi: 10.1093/molbev/msl044. [DOI] [PubMed] [Google Scholar]
- Hong S.-J., Woo H.-C., Chai J.-Y. A human case of Plagiorchis muris (Tanabe, 1922: digenea) infection in the Republic of Korea: freshwater fish as a possible source of infection. J. Parasitol. 1996;82:647. [PubMed] [Google Scholar]
- Hornok S., Beck R., Farkas R., Grima A., Otranto D., Kontschán J., Takács N., Horváth G., Szőke K., Szekeres S., Majoros G., Juhász A., Salant H., Hofmann-Lehmann R., Stanko M., Baneth G. High mitochondrial sequence divergence in synanthropic flea species (Insecta: siphonaptera) from Europe and the Mediterranean. Parasites Vectors. 2018;11:221. doi: 10.1186/s13071-018-2798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T., Neafie R.C., Mackenzie I.R.A. Baylisascaris procyonis infection in elderly person, British Columbia, Canada. Emerg. Infect. Dis. 2012;18:341–342. doi: 10.3201/eid1802.111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter L., Barrett P. first ed. Haupt Verlag; Bern: 2012. Raubtiere der Welt: Ein Feldführer. [Google Scholar]
- Jakšić S., Uhitil S., Vučemilo M. Nachweis von Mesozerkarien des Saugwurms Alaria alata im Wildschweinefleisch. Z. Jagdwiss. 2002;48:203–207. [Google Scholar]
- Karamon J., Kochanowski M., Cencek T., Bartoszewicz M., Kusyk P. Gastrointestinal helminths of raccoons (Procyon lotor) in western Poland (Lubuskie province) - with particular regard to Baylisascaris procyonis. Bull. Vet. Inst. Pulawy. 2014;58:547–552. [Google Scholar]
- Kazacos K.R. Baylisascaris procyonis and related species. Parasitic. Dis. Wild Mammal. 2001;2:301–341. [Google Scholar]
- Kazacos K.R. Baylisascaris larva migrans. US Geol. Surv. Circular. 2016;1412 [Google Scholar]
- Kelly T.G., Madhavan V.L., Peters J.M., Kazacos K.R., Silvera V.M. Spinal cord involvement in a child with raccoon roundworm (Baylisascaris procyonis) meningoencephalitis. Pediatr. Radiol. 2012;42:369–373. doi: 10.1007/s00247-011-2151-y. [DOI] [PubMed] [Google Scholar]
- Kilham L., Habermann R.T., Herman C.M. Jaundice and bilirubinemia as manifestations of canine distemper in raccoons and ferrets. Am. J. Vet. Res. 1956;17:144–148. [PubMed] [Google Scholar]
- Klimpel S., Kuhn T., Münster J., Dörge D.D., Klapper R., Kochmann J. Springer International Publishing; Cham: 2019. Parasites of Marine Fish and Cephalopods. [Google Scholar]
- Kochmann J., Cunze S., Klimpel S. Climatic niche comparison of raccoons Procyon lotor and raccoon dogs Nyctereutes procyonoides in their native and non‐native ranges. Mamm Rev. 2021;51:585–595. [Google Scholar]
- Kołodziej-Sobocińska M., Tokarska M., Zalewska H., Popiołek M., Zalewski A. Digestive tract nematode infections in non-native invasive American mink with the first molecular identification of Molineus patens. Int. J. Parasitol. Parasites Wildl. 2021;14:48–52. doi: 10.1016/j.ijppaw.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larivière S. Grzimek's Animal Life Encyclopedia; 2004. pp. 309–317. (Raccoons and Relatives (Procyonidae)). [Google Scholar]
- Laurimaa L., Süld K., Davison J., Moks E., Valdmann H., Saarma U. Alien species and their zoonotic parasites in native and introduced ranges: the raccoon dog example. Vet. Parasitol. 2016;219:24–33. doi: 10.1016/j.vetpar.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Lee S.-U., Huh S., Sohn W.-M. Molecular phylogenic location of the Plagiorchis muris (Digenea, Plagiorchiidae) based on sequences of partial 28S D1 rDNA and mitochondrial cytochrome C oxidase subunit I. Kor. J. Parasitol. 2004;42:71–75. doi: 10.3347/kjp.2004.42.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht E. AFZ-Der Wald; 2009. Waschbär - kleiner Feldversuch mit großer Wirkung; pp. 570–573. [Google Scholar]
- Levin N.L. Life history studies on Porrocaecum ensicaudatum (Nematoda), an avian nematode. I. Experimental observations in the chicken. J. Parasitol. 1961;47:38. [PubMed] [Google Scholar]
- Littlewood D.T., Olson P.D. Interrelationships of the Platyhelminthes; 2001. Small Subunit rDNA and the Platyhelminthes: Signal, Noise, Conflict and Compromise; pp. 262–278. [Google Scholar]
- Lotze J.-H., Anderson S. Procyon lotor. Mamm. Species. 1979;1–8 [Google Scholar]
- Lucius R., Loos-Frank B., Lane R.P. Springer Berlin Heidelberg; Berlin, Heidelberg: 2018. Biologie von Parasiten. [Google Scholar]
- Lutz W. Die Verbreitung des Waschbären Procyon lotor (Linné, 1758) im mitteleuropäischen Raum. Z. Jagdwiss. 1981;30:218–228. [Google Scholar]
- Lux E., Priemer J. Zur Parasitierung wildlebender Waschbären unter dem Aspekt ihrer nordamerikanischen Herkunft. Verhandlungsbericht des Internationalen Symposiums über die Erkrankungen der Zootiere. 1995;37:429–434. [Google Scholar]
- Maas M., Tatem-Dokter R., Rijks J.M., Dam-Deisz C., Franssen F., van Bolhuis H., Heddergott M., Schleimer A., Schockert V., Lambinet C., Hubert P., Redelijk T., Janssen R., Cruz A.P.L., Martinez I.C., Caron Y., Linden A., Lesenfants C., Paternostre J., van der Giessen J., Frantz A.C. Population genetics, invasion pathways and public health risks of the raccoon and its roundworm Baylisascaris procyonis in northwestern Europe. Transbound Emerg Dis. 2022;69:2191–2200. doi: 10.1111/tbed.14218. [DOI] [PubMed] [Google Scholar]
- Medlock J.M., Hansford K.M., Bormane A., Derdakova M., Estrada-Peña A., George J.-C., Golovljova I., Jaenson T.G.T., Jensen J.-K., Jensen P.M., Kazimirova M., Oteo J.A., Papa A., Pfister K., Plantard O., Randolph S.E., Rizzoli A., Santos-Silva M.M., Sprong H., Vial L., Hendrickx G., Zeller H., van Bortel W. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors. 2013;6(1):1–11. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. Spektrum Akademischer Verlag; Heidelberg: 2012. Die Parasiten des Menschen. [Google Scholar]
- Mehlhorn H., Mehlhorn B. fourth ed. Springer; Berlin, Heidelberg: 2020. Zecken, Milben, Fliegen, Schaben: Schach Dem Ungeziefer. [Google Scholar]
- Michler B.A. Dissertation; Dresden: 2017. Koproskopische Untersuchungen zum Nahrungsspektrum des Waschbären Procyon lotor (Linné, 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern) unter spezieller Berücksichtigung des Artenschutzes und des Endoparasitenbefalls. [Google Scholar]
- Michler F.U. Diplomarbeit; Halle-Wittenberg: 2003. „Untersuchungen zur Raumnutzung des Waschbären (Procyon lotor Linné, 1758) im urbanen Lebensraum am Beispiel der Stadt Kassel (Nordhessen)“. [Google Scholar]
- Michler F.U. vols. 1–15. Infodienst Wildbiologie & Ökologie; Zürich: 2004. (Waschbären im Stadtgebiet. Wildbiologie. Wildbiologie International 5/12). [Google Scholar]
- Neumann R.O., Mayer M. fourth ed. J.F. Lehmann's Verlag; München: 1914. Atlas und Lehrbuch wichtiger tierischer Parasiten und ihrer Überträger mit besonderer Berücksichtigung de Tropenpathologie. [Google Scholar]
- Nijhof A.M., Bodaan C., Postigo M., Nieuwenhuijs H., Opsteegh M., Franssen L., Jebbink F., Jongejan F. Ticks and associated pathogens collected from domestic animals in The Netherlands. Vector Borne Zoonotic Dis. 2007;7:585–595. doi: 10.1089/vbz.2007.0130. [DOI] [PubMed] [Google Scholar]
- Nugaraitė D., Mažeika V., Paulauskas A. Molecular and morphological characterization of isthmiophora melis (schrank, 1788) luhe, 1909 (digenea: Echinostomatidae) from American mink (Neovison vison) and European polecat (Mustela putorius) in Lithuania. Helminthologia. 2017;54:97–104. [Google Scholar]
- Osche G. Über Zwischenwirte, Fehlwirte und die Morphogenese der Lippenregion bei Porrocaecum- und Contracaecum-Arten (Ascaridoidea, Nematoda) Z. Parasitenkd. 1959;19:458–484. [Google Scholar]
- Parker M.V. Euryhelmis squamula (Rudolphi) 1819, reported from a racoon. J. Parasitol. 1950;36:89. [PubMed] [Google Scholar]
- Peters J.M., Madhavan V.L., Kazacos K.R., Husson R.N., Dangoudoubiyam S., Soul J.S. Good outcome with early empiric treatment of neural larva migrans due to Baylisascaris procyonis. Pediatrics. 2012;129:e806–e811. doi: 10.1542/peds.2011-2078. [DOI] [PubMed] [Google Scholar]
- Peus F. Schöps; Leipzig: 1938. Die Flöhe: Bau, Kennzeichen und Lebensweise, hygienische Bedeutung und Bekämpfung der für den Menschen wichtigen Floh-Arten. [Google Scholar]
- Piróg A., Kuśmierek N., Popiołek M. The occurrence of avian acanthocephalan Polymorphus minutus (Goeze, 1782) in raccoons (Procyon lotor L.) introduced to Europe. Ann. Parasitol. 2018;64:249–252. doi: 10.17420/ap6403.160. [DOI] [PubMed] [Google Scholar]
- Popiołek M., Jarnecki H., Łuczyński T. The first record of Molineus patens (Dujardin, 1845) (Nematoda, Molineidae) in the ermine (Mustela erminea L.) in Poland. Wiad. Parazytol. 2009;55 4:433–435. [PubMed] [Google Scholar]
- Powers T.O., Todd T.C., Burnell am, Murray P.C., Fleming C.C., Szalanski A.L., Adams B.A., Harris T. The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. J. Nematol. 1997;29:441. [PMC free article] [PubMed] [Google Scholar]
- Prange S., Gehrt S.D., Wiggers E.P. Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J. Mammal. 2004;85:483–490. [Google Scholar]
- Priemer J., Lux E. Atriotaenia incisa (Cestoda), a parasite of the badger, Meles meles, and the raccoon, Procyon lotor, in Brandenburg, Germany. Can. J. Zool. 1994;72:1848–1853. [Google Scholar]
- Randolph S.E. Tick-borne disease systems emerge from the shadows: the beauty lies in molecular detail, the message in epidemiology. Parasitology. 2009;136:1403–1413. doi: 10.1017/S0031182009005782. [DOI] [PubMed] [Google Scholar]
- Rentería-Solís Z. Dissertation. mbv Mensch-und-Buch-Verl.; Berlin: 2015. Disease Occurrence in Free-Ranging Raccoons (Procyon lotor) from Rural and Urban Populations in North-eastern Germany; p. 94. [Google Scholar]
- Rentería-Solís Z., Birka S., Schmäschke R., Król N., Obiegala A. First detection of Baylisascaris procyonis in wild raccoons (Procyon lotor) from leipzig, saxony, eastern Germany. Parasitol. Res. 2018;117:3289–3292. doi: 10.1007/s00436-018-5988-2. [DOI] [PubMed] [Google Scholar]
- Richardson D.J., Durden L.A., Snyder D.E. Ectoparasites of the raccoon (Procyon lotor) from North-central Arkansas. J. Kans. Entomol. Soc. 1994:208–212. [Google Scholar]
- Rizzoli A., Silaghi C., Obiegala A., Rudolf I., Hubálek Z., Földvári G., Plantard O., Vayssier-Taussat M., Bonnet S., Spitalská E., Kazimírová M. Ixodes ricinus and its transmitted pathogens in urban and Peri-urban areas in Europe: new hazards and relevance for public health. Publ. Health Forum. 2014;2:251. doi: 10.3389/fpubh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovski A. Life-cycle of Polymorphus minutus. Veterinariya. 1964;41:40–41. [Google Scholar]
- Romeo C., Cafiso A., Fesce E., Martínez-Rondán F.J., Panzeri M., Martinoli A., Cappai N., Defilippis G., Ferrari N. Lost and found: helminths infecting invasive raccoons introduced to Italy. Parasitol. Int. 2021;83 doi: 10.1016/j.parint.2021.102354. [DOI] [PubMed] [Google Scholar]
- Rosatte R.C., MacInnes C.D., Williams O., Williams R.T. A Proactive Prevention strategy for raccoon rabies in Ontario, Canada. Wildl. Soc. Bull. 1997;25:110–116. [Google Scholar]
- Roussere G.P., Murray W.J., Raudenbush C.B., Kutilek M.J., Levee D.J., Kazacos K.R. Raccoon roundworm eggs near homes and risk for larva migrans disease, California communities. Emerg. Infect. Dis. 2003;9:1516–1522. doi: 10.3201/eid0912.030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M., Tripepi M., Kinsella J.M., Panebianco A., Mattiucci S. Helminth infestation in birds of prey (accipitriformes and falconiformes) in southern Italy. Vet. J. 2010;186:119–122. doi: 10.1016/j.tvjl.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Schaffer G.D., Davidson W.R., Nettles V.F., Rollor E.A. Helminth parasites of translocated raccoons (Procyon lotor) in the southeastern United States. J. Wildl. Dis. 1981;17:217–227. doi: 10.7589/0090-3558-17.2.217. [DOI] [PubMed] [Google Scholar]
- Schmidt F. second ed. Boldt; Berlin: 1980. Das Buch von Pelztieren und Pelzen: Eine Pelztier- und Rauchwarenkundekunde; p. 416. [Google Scholar]
- Schwarz S., Sutor A., Mattis R., Conraths F.J. Der Waschbärspulwurm (Baylisascaris procyonis)--kein Zoonoserisiko für Brandenburg? Berl. Munch. Tierarztl. Wochenschr. 2015;128:34–38. [PubMed] [Google Scholar]
- Sprent J.F. Notes on Ascaris and Toxascaris, with a definition of Baylisascaris gen.nov. Parasitology. 1968;58:185–198. doi: 10.1017/s0031182000073534. [DOI] [PubMed] [Google Scholar]
- Stope M. Wild raccoons in Germany as a reservoir for zoonotic agents. Eur. J. Wildl. Res. 2019;65 [Google Scholar]
- Storch V., Welsch U. 27th ed. Springer Spektrum; Berlin, Heidelberg: 2014. Kükenthal Zoologisches Praktikum; p. 552. [Google Scholar]
- Strausbaugh L.J., Murray W.J., Kazacos K.R. Raccoon roundworm encephalitis. Clin. Infect. Dis. 2004;39:1484–1492. doi: 10.1086/425364. [DOI] [PubMed] [Google Scholar]
- Stubbe M., Krapp F. Aula Verlag Gmbh; Wiesbaden: 1993. Handbuch der Säugetiere Europas: Raubsäuger (Teil I) [Google Scholar]
- Stuewer F.W. Raccoons: their habits and management in Michigan. Ecol. Monogr. 1943;13:203–257. [Google Scholar]
- Süss J., Fingerle V., Hunfeld K.-P., Schrader C., Wilske B. Durch Zecken übertragene humanpathogene und bisher als apathogen geltende Mikroorganismen in Europa. Teil II: Bakterien, Parasiten und Mischinfektionen. Bundesgesundheitsblatt - Gesundheitsforsch. - Gesundheitsschutz. 2004;47:470–486. doi: 10.1007/s00103-004-0837-0. [DOI] [PubMed] [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Torres J., Miquel J., Motjé M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol. Res. 2001;87:259–263. doi: 10.1007/s004360000316. [DOI] [PubMed] [Google Scholar]
- Walter M., Vogelgesang J.R., Rubel F., Brugger K. Tick-borne encephalitis virus and its European distribution in ticks and Endothermic mammals. Microorganisms. 2020;8:1065. doi: 10.3390/microorganisms8071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S.B., Lake C.M., Chastain H.M., Fisk D., Handali S., Kahn P.L., Montgomery S.P., Wilkins P.P., Kuris A.M., Lafferty K.D. Seroprevalence of Baylisascaris procyonis infection among humans, santa Barbara county, California, USA, 2014-2016. Emerg. Infect. Dis. 2017;23:1397–1399. doi: 10.3201/eid2308.170222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton D.A. The structure of the egg-shell of Porrocaecum ensicaudatum (Nematoda: ascaridida) Parasitol. Int. 1979;9:127–131. [Google Scholar]
- Whiting M.F. Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scripta. 2002;31:93–104. [Google Scholar]
- Winter M. Sachsen-Anhalt. Diplomarbeit; Martin-Luther-Universität Halle-Wittenberg: 2004. Zur Ökologie des Waschbären (Procyon lotor L., 1758) [Google Scholar]
- Zeller E. Über das enzystierte Vorkommen von Distomum squamula Rud. in braunen Grasfrosh. Z. Wiss. Zool. 1867;17:215–220. [Google Scholar]
- Zeveloff S.I. Smithsonian Institution Press; Washington: 2002. Raccoons: A Natural History; p. 200. [Google Scholar]