Abstract
To develop a Pseudomonas aeruginosa vaccine that allows the host immune system to select the antigens, we hypothesized that dendritic cells (DC) pulsed with P. aeruginosa would induce protective immunity against pulmonary infections with P. aeruginosa. Incubation of murine bone marrow-derived DC with P. aeruginosa in vitro led to uptake of P. aeruginosa and activation of the DC. Spleen-derived CD4+ cells from mice immunized with P. aeruginosa-pulsed DC showed increased proliferation, demonstrating that DC pulsed with P. aeruginosa were capable of eliciting a P. aeruginosa-specific immune response. To evaluate if P. aeruginosa-pulsed DC can induce protective immunity against P. aeruginosa pulmonary infection, DC incubated with P. aeruginosa in vitro were administered systemically to syngeneic mice, and the mice were then challenged by intrapulmonary infection with P. aeruginosa (5 × 104 CFU/mouse) 13 days later. Unimmunized control mice and mice who had previously received naive DC or DC stimulated with lipopolysaccharide or Escherichia coli died within 72 h. In contrast, 45% of mice receiving P. aeruginosa-pulsed DC demonstrated prolonged survival (>14 days). Finally, DC-pulsed with heat-inactivated P. aeruginosa protected CD8−/− but not CD4−/− mice, demonstrating that CD4+ T cells were required for the DC pulsed with P. aeruginosa to induce protective immunity.
Pseudomonas aeruginosa, an environmentally ubiquitous, gram-negative, opportunistic pathogen, is commonly associated with progressive, chronic respiratory infection in patients with cystic fibrosis (CF) and other causes of airway derangement (6). Once colonization of the airways is established, P. aeruginosa is rarely eliminated, despite an exuberant host inflammatory response (10). The treatment of P. aeruginosa infection by antibiotic therapy is limited due to a high incidence of drug resistance and the inability to completely eradicate infection in CF patients (1, 6). Bacterial virulence factors (12), as well as CF-specific host factors, may play a role in the persistence of this organism (29, 35). Despite considerable effort, vaccines against P. aeruginosa infection involving conventional immunization strategies have not been efficacious (18, 21, 33), although recent novel approaches show some promise (14, 34, 36, 40). The lack of progress toward the development of a vaccine against P. aeruginosa infection has been due, in part, to an incomplete understanding of the optimal P. aeruginosa antigens for the vaccine, as well as of the host immune mechanisms that mediate protective immunity against this pathogen (5, 6, 10).
The focus of this study is to assess a new paradigm in the development of a vaccine to protect against Pseudomonas infection, using Pseudomonas-pulsed dendritic cells (DC) as the immunizing biologic agent. DC are potent antigen-presenting cells that play a central role in the induction of T-cell immunity in vivo (2) and in lung host defense (9, 27). Large numbers of DC with powerful in vivo antigen-presenting properties can be propagated in vitro using recombinant cytokines (16). Ex vivo antigen-pulsed DC are effective inducers of tumor-specific (25) or antiviral (22) protective immunity, and a variety of bacterial pathogens have been reported to be taken up and processed for effective antigen presentation by DC (15, 24, 26, 39, 41).
The present study analyzes the interaction of P. aeruginosa with DC and evaluates the use of DC pulsed with Pseudomonas to induce protection against fatal pulmonary infections with P. aeruginosa. The data presented here demonstrate that murine bone marrow-derived DC interact with and are activated by P. aeruginosa in vitro and that DC pulsed with P. aeruginosa administered to syngeneic mice lead to induction of a CD4+ T cell proliferative response and prolonged survival following a lethal intrapulmonary challenge with P. aeruginosa in a process that is dependent on the presence of CD4+ T cells.
MATERIALS AND METHODS
Experimental animals.
Female C57BL/6 (He-2b) and CD4 and CD8 knockout mice (both on the C57BL/6 background), 4 to 6 weeks old, were purchased from Jackson Laboratories (Bar Harbor, Maine) and housed under pathogen-free conditions.
DC.
DC were generated from mouse bone marrow precursors harvested from C57BL/6 mice in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, and 100 μg of streptomycin, with recombinant murine granulocyte-macrophage colony-stimulating factor (100 U/liter; Sigma, St. Louis, Mo.) and recombinant murine interleukin-4 (IL-4) (2 ng/ml; R&D Systems, Minneapolis, Minn.), for 8 days as previously described (37).
Interaction of DC with P. aeruginosa in vitro
To analyze the interaction of DC with P. aeruginosa in vitro, bone marrow-derived DC (2 × 105 cells) were incubated with 20 CFU per cell of PAO1-GFP, a nonmucoid laboratory P. aeruginosa strain that expresses green fluorescent protein (gift from Alice Prince, Columbia University, New York, N.Y.). The bacteria were grown at 37°C in tryptic soy broth (TSB) (Difco Laboratories, Detroit, Mich.) to the mid-log phase and washed four times in phosphate-buffered saline (PBS [pH 7.4]). Following incubation in RPMI 1640 for 3 h at 37°C, the DC were washed three times in PBS and fixed with 4% paraformaldehyde in PBS (23°C, 15 min) on Cytospin preparations. Nuclei were counterstained with the DNA dye 4′,6′-diamino-2-phenylindole (DAPI) (1 μg/ml; Molecular Probes, Eugene, Oreg.) in PBS with 0.1% Triton X-100 for 5 min to visualize and quantify DC-associated bacteria. The DC were then evaluated by fluorescence and differential interference microscopy using a Nikon Microphot SA microscope and a 60× N.A. 1.4 objective.
Activation of DC incubated with P. aeruginosa in vitro.
To assess whether coincubation of P. aeruginosa with DC leads to activation of the DC, DC were incubated for 3 h with 10 CFU of PAO1 per cell in RPMI 1640–10% FBS. The DC were then washed and incubated for 3 h with 200 mg of gentamicin (Sigma) per ml to kill live bacteria, and the cultures were contained for an additional 48 h in RPMI 1640–10% FBS. The DC were then washed three times with PBS, and 4 × 105 cells were stained (30 min, 4°C) with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MAb) to the costimulatory molecule CD80 (B7.1, 16–10A1) or CD86 (B7.2, GL1). An isotype-matched FITC-labeled MAb was used as a control (all antibodies from Pharmingen, San Diego, Calif.). Stained DC were analyzed by flow cytometry (EPICS XL apparatus; Coulter Corp., Miami, Fla.).
To analyze the secretion of IL-12 following coincubation of DC with PAO1, DC were incubated with either live or heat-inactivated (1 h, 56°C) PAO1 (10 CFU/cell) for 3 h. The DC were then washed with PBS, incubated with gentamicin for 60 min as described above, and cultured for 48 h in 48-well dishes with RPMI 1640–10% FBS. The supernatants of the cultures were then harvested and centrifuged to remove debris, and the amount of IL-12 remaining was assessed by enzyme-linked immunosorbent assay (ELISA) for mouse IL-12 p40 (R&D Systems).
CD4+ T-cell proliferation following transfer of Pseudomonas-pulsed DC.
To analyze whether the transfer of DC pulsed with P. aeruginosa would lead to Pseudomonas-specific proliferation of CD4+ T cells in vivo, the proliferative responses of CD4+ cells derived from spleens of mice that were primed with antigen (mice injected with DC pulsed with heat-inactivated or live PAO1) or not primed with antigen (unstimulated mice or mice injected with naive DC) were compared to those of PAO1-pulsed, irradiated DC. DC were incubated with live or heat-inactivated PAO1 (10 CFU/cell) for 3 h, washed three times with PBS, and incubated with gentamicin (200 mg/ml) for 1 h to kill remaining live bacteria. The DC were then washed and resuspended in PBS, and 2 × 105 cells were injected intravenously (via the internal jugular vein) into syngeneic 4-to-6-week-old C57BL/6 mice. Naive animals and animals injected with naive DC served as controls. To control for any remaining live bacteria, an aliquot of the DC preparation was plated on MacConkey agar plates, with and without lysis of the cells with 0.5% Triton-X (Sigma). No colonies were detected after 24 h of incubation. After 14 days, the spleens were harvested and CD4+ cells were isolated using magnetic beads (Miltenyi, Auburn, Calif.). The purity of the isolated CD4+ cells was assessed by flow cytometry using FITC-labeled MAb to murine CD4. The CD4+ cells (2 × 105 cells) were cocultured in 96-well plates with 2 × 104 irradiated DC (3,000 rad) that had been generated from naive syngeneic mice and pulsed with heat-inactivated PAO1 (10 CFU/cell) for 60 min prior to the culture with the CD4+ cells. After 48 h, [3H]thymidine (90 Ci/mmol, 1 μCi/well; DuPont-NEN, Boston, Mass.) was added, and incubation was continued for 14 h at 37°C, after which the cells were harvested, and the [3H]thymidine incorporation was measured using a β-counter. Cell proliferation was expressed as a stimulation index (disintegrations per minute [dpm] for CD4+ cells cultured with Pseudomonas-pulsed DC/dpm for CD4+ cells cultured with unpulsed DC). Control experiments were carried out to determine the proliferative responses of CD4+ cells derived from mice immunized with P. aeruginosa-pulsed DC to irradiated DC pulsed with E. coli and of CD4+ cells derived from mice immunized with E. coli-pulsed DC to irradiated DC pulsed with P. aeruginosa.
Serum antibody response following transfer of Pseudomonas-pulsed DC.
To assess if transfer of PAO1-pulsed DC would lead to a specific anti-Pseudomonas antibody response, the sera of C57BL/6 mice which had received 3 × 105 DC pulsed with heat-inactivated or live PAO1 were analyzed 14 days after immunization by ELISA. Sera obtained from naive control mice or from mice that had received naive DC served as the controls. Flat-bottomed 96-well plates (Bio-Rad Laboratories, Hercules, Calif.) were coated at 4°C with 107 CFU of heat-killed PAO1 in 0.05 M carbonate buffer (pH 9.6; Sigma) with 0.2% sodium azide. Serial dilutions of the serum samples were incubated at 23°C for 1 h following the addition of 1% bovine serum albumin. The plates were washed with washing buffer (0.05% Tween 20 in PBS), and rabbit anti-mouse subtype-specific immunoglobulin (immunoglobulin M [IgM], IgG1, IgG2a, IgG2b, IgG3, or IgA; Bio-Rad) was added. After incubation for 1 h and rinsing with washing buffer, the plates were incubated with diluted goat anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Bio-Rad) at 23°C for 1 h. After washing out unreacted conjugated antibodies, the plates were developed with a peroxidase substrate solution (Bio-Rad) for 30 min at 23°C and then evaluated in an ELISA reader at 425 nm.
Immunization with Pseudomonas-pulsed DC.
To evaluate whether P. aeruginosa-pulsed DC would lead to protective responses against intrapulmonary infection with P. aeruginosa, DC generated as described above were incubated with live or heat-inactivated PAO1 for 3 h, washed, and then administered intravenously to syngeneic mice (2 × 105 cells/mouse). Naive mice, mice receiving naive DC, or mice receiving DC that had been incubated with either lipopolysaccharide (LPS) (200 ng/ml; Sigma) or heat-inactivated E. coli strain ATCC 25922 (50 CFU/cell) for 3 h intravenously were used as controls. Additional control experiments were done with intravenous injection of heat-inactivated PAO1 (2 × 106 CFU). After 14 days, the animals were challenged by an intratracheal injection of 5 × 104 CFU of agarose-encapsulated PAO1, as described by Stevenson et al. (38). For encapsulation in agarose, PAO1 cells were grown in TSB (Difco Laboratories) to mid-log phase, washed in PBS, and mixed (dilution ratio, 1:9) with 2% TSB agar in PBS at 52°C. This mixture (10 ml) was added to 100 ml of preheated (52°C) heavy mineral oil under constant stirring for 5 min, followed by rapid cooling to 4°C. After being washed three times in PBS, the beads were evaluated for size using a hemocytometer; only preparations with beads having diameters that were <100 μm were used. The number of bacteria encapsulated in the agarose was evaluated by 18-h cultures of serial dilutions at 37°C on 1.5% MacConkey agarose plates (Difco Laboratories). Following intratracheal administration of the Pseudomonas beads, the survival of the animals was assessed over time.
Role of CD4+ T cells.
To evaluate the role of CD4+ and CD8+ T cells in establishing immunity by P. aeruginosa-pulsed DC, CD4−/−, CD8−/−, and wild-type C57BL/6 mice were immunized with 105 DC which had been pulsed with heat-killed P. aeruginosa (10 bacteria/cell) for 3 h. Wild-type mice without immunization were included as controls. Mice were challenged intratracheally with 105 CFU of PAO1 enmeshed in agar beads 3 weeks after the immunization, as described above. Survival was evaluated over time, and the results were averaged.
Statistical analysis.
All data reported here are means ± standard errors of the means. Statistical comparisons were made using the unpaired Student's t test. Survival evaluation was carried out using Kaplan-Meier analysis (Statview; SAS Institute, Cary, N.C.).
RESULTS
Interaction of P. aeruginosa with DC.
Following incubation of DC with PAO1 at 20 CFU/cell, GFP-expressing PAO1 could be seen associated with or in the DC, but no fluorescence was visible in naive cells (data not shown). Although the determination of the localization of the bacteria within or at the cell surface was not possible using this technology, the data demonstrate that the PAO1 was associated with the DC.
Activation of DC following incubation with P. aeruginosa
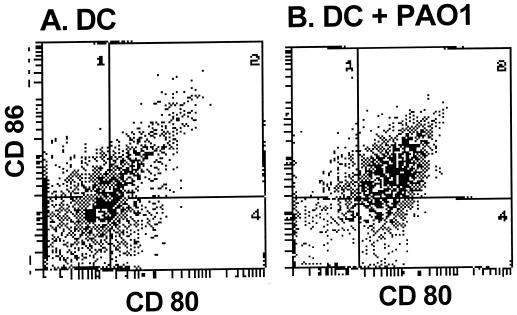
To analyze whether coincubation of P. aeruginosa with DC leads to activation of the DC, the surface expression of the costimulatory molecules relevant to the interaction of DC with T cells (CD80 and CD86), as well as the secretion of the cytokine IL-2, was evaluated. DC incubated with PAO1 demonstrated an increase in the expression of the costimulatory molecules CD86 (74% increase) and CD80 (67% increase) that was even greater than that observed with naive DC (31% increase for CD86 and 40% increase for CD80 [Fig. 1]). Analysis of the culture supernatants showed increased secretion of IL-12 in DC coincubated with PAO1 (152 ± 18 ng/ml) or heat-inactivated PAO1 (253 ± 42 ng/ml) compared to controls (71 ± 13 ng/ml) (for both comparisons, the P value was <0.001). The IL-12 levels in the supernatants of the DC incubated with heat-inactivated PAO1, which were increased compared to those in the supernatants of the DC incubated with viable PAO1, may be a reflection of some toxic effects of the high dose of live bacteria on these cells. Analysis of the number of nonviable DC using trypan blue exclusion showed 9% ± 4% dead cells for the control DC versus 13% ± 2% dead cells for DC incubated with heat-inactivated PAO1 and 24% ± 4% dead cells for DC incubated with live PAO1 (for both comparisons, the P value was >0.05). In our system, IL-12 served as a marker for the activation of the DC. Overall, these data demonstrate that coincubation of DC with P. aeruginosa leads to activation of DC, presumably priming them for interaction with CD4+ T cells.
FIG. 1.
Activation of DC following infection with P. aeruginosa. DC were incubated with PAO1 at 10 CFU/cell for 2 h. The DC were then incubated with gentamicin to kill live bacteria. After 48 h, the DC were stained with anti-B7.2–FITC (CD86) and anti-B7.1–phycoerythrin (CD80). Shown are results of flow cytometry of naive DC (A) and PAO1-pulsed DC (B). The gates were set at <1% positive cells for DC stained with an FITC-labeled isotype control IgG.
Pseudomonas-specific CD4+ T-cell proliferation following in vivo immunization with P. aeruginosa-pulsed DC.
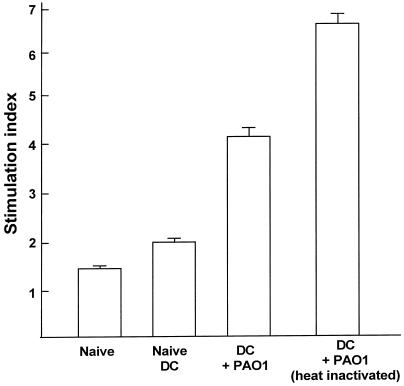
To analyze if DC pulsed with PAO1 were capable of eliciting a P. aeruginosa-specific immune response, CD4+ T cells were harvested from spleens of mice 10 days after immunization with PAO1-pulsed DC. The CD4+ cells were coincubated with sensitized and irradiated syngeneic DC pulsed with PAO1 and analyzed for proliferation of the CD4+ cells. Spleen-derived CD4+ T cells from mice immunized with live PAO1 or heat-inactivated PAO1-pulsed DC showed increased proliferation compared to T cells from naive mice or mice that had received naive DC (in comparison with controls, the P value was <0.002 [Fig. 2]). However, the CD4+ cells of all four groups (i.e., cells derived from naive animals, from animals that had received naive DC, from animals that had received DC pulsed with heat-inactivated PAO1, and from animals that had received DC pulsed with live PAO1) incubated with syngeneic unstimulated DC showed no proliferation (data not shown). The absolute counts of unstimulated CD4+ were low (<500 dpm) and comparable for all groups. These results demonstrate that immunization with P. aeruginosa-pulsed DC leads to induction of a CD4+ T-cell proliferative response against DC presenting a P. aeruginosa antigen(s). However, CD4+ cells derived from mice immunized with DC pulsed with PAO1 previously exposed to irradiated, E. coli-pulsed DC and CD4+ cells derived from mice immunized with DC pulsed with E. coli previously exposed to irradiated, PAO1-pulsed DC showed levels of proliferation (stimulation index values, 3.01 ± 0.2 and 3.2 ± 0.2, respectively) even lower than those shown by CD4+ cells derived from mice immunized with DC pulsed with PAO1 exposed to irradiated, PAO1-pulsed DC (stimulation index, 4.4 ± 0.2, P < 0.001). This suggests that, in part, the proliferative in vitro responses could be due to endotoxin adjuvant effects. As seen with the secretion of IL-12, the heat-inactivated PAO1 leads to a higher proliferation response than does live PAO1, which may be a reflection of some toxicity of the live bacteria on the DC during the 3-h incubation period.
FIG. 2.
Levels of T-cell proliferation in mice immunized with P. aeruginosa-pulsed DC CD4+ cells who also received P. aeruginosa-pulsed DC of spleen-derived CD4+ cells of mice which were primed with antigen (mice injected with DC pulsed with heat-inactivated or live PAO1) were increased compared to those of similarly immunized mice who received DC of spleen-derived CD4+ cells of mice which were not primed with antigen (unstimulated mice or mice injected with naive DC). C57BL/6 mice were injected intravenously with 2 × 105 DC previously pulsed in vitro with live or heat-inactivated PAO1 (10 CFU/cell). Controls included naive mice or mice receiving naive DC. Two weeks following immunization, CD4+ cells were isolated from the spleens and coincubated for 48 h with irradiated DC previously pulsed with PAO1 for 48 h. CD4+-cell proliferation was evaluated by [3H]thymidine incorporation during incubation for 12 h. Proliferation is expressed as a stimulation index calculated in relation to CD4+ cells cultured with unpulsed DC.
Pseudomonas-specific antibody response following immunization with P. aeruginosa-pulsed DC.
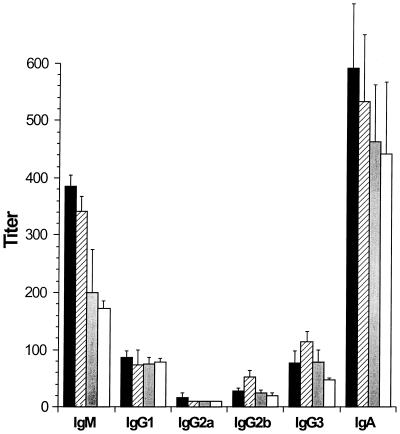
To analyze whether DC pulsed with PAO1 induced an increase in Pseudomonas-specific antibodies in the serum, the sera of the mice were evaluated 14 days following immunization with PAO1-pulsed DC for the presence of Pseudomonas-specific IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA by ELISA. Although increased levels of IgM and IgA were found in the sera of mice immunized with DC pulsed with live or heat-inactivated PAO1, the differences were not statistically significant when data were compared to those of either control group (mice not immunized or immunized with naive DC [Fig. 3]).
FIG. 3.
Pseudomonas-specific antibody response in serum following immunization with P. aeruginosa-pulsed DC. DC were incubated with heat-inactivated (solid bars) or live (hatched bars) PAO1 for 3 h, washed, and then readministered intravenously to syngeneic mice. Naive mice (grey bars) and mice receiving naive DC (open bars) served as controls. Two weeks following immunization, the sera of the mice were collected and analyzed for the presence of Pseudomonas-specific antibodies by ELISA.
Protection against P. aeruginosa following immunization with P. aeruginosa-pulsed DC.
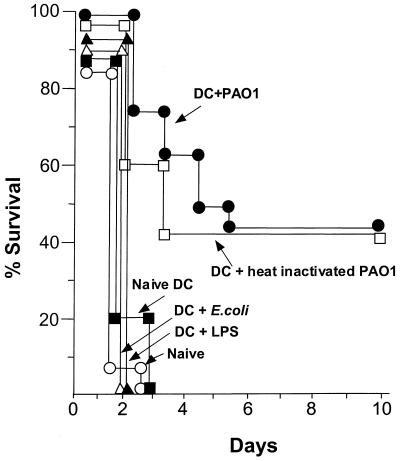
To determine whether mice immunized with P. aeruginosa-pulsed DC were protected against a lethal, intratracheal dose of P. aeruginosa, the survival of mice that had been immunized intravenously with P. aeruginosa-pulsed DC (2 × 105) were analyzed following intrapulmonary infection with a lethal dose of P. aeruginosa encapsulated in agarose beads. Naive mice and mice receiving naive DC, DC pulsed with LPS, or DC pulsed with E. coli all died within 72 h following intrapulmonary challenge with PAO1 (Fig. 4). However, the animals which had previously been immunized with DC pulsed with either live or heat-inactivated PAO1 demonstrated prolonged survival (45% survived for more than 10 days; for both, comparison with the controls resulted in a P value of <0.0001 [Fig. 4]). Animals which had received heat-inactivated PAO1 without DC did not survive longer than 72 h following intrapulmonary challenge with PAO1 (data not shown).
FIG. 4.
Immunization with P. aeruginosa-pulsed DC partially protects against subsequent infection with a lethal dose of P. aeruginosa pulmonary infection. DC were incubated with live (●) or heat-inactivated (□) PAO1 for 3 h, washed, and then readministered intravenously to syngeneic mice. Naive mice (○) and mice receiving naive DC (▪), LPS-stimulated DC (▵), or DC pulsed with E. coli (▴) served as controls. Two weeks later, PAO1 encapsulated in agarose beads (2 × 104 CFU) was administered to the animals intratracheally and survival was evaluated over time (n = 15 animals/group for animals who received PAO1-pulsed or naive DC, n = 5 animals/group for animals who received LPS-stimulated or E. coli-pulsed DC).
Role of CD4+ T cells.
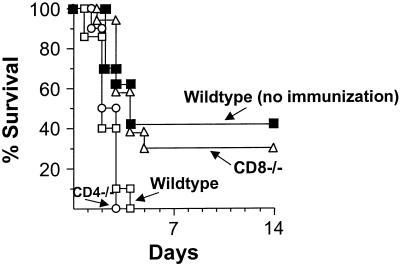
To evaluate the role of CD4+ and CD8+ T cells in the induction of protective immunity following immunization with P. aeruginosa-pulsed DC, immunization with P. aeruginosa-pulsed DC was analyzed in CD4 and CD8 knockout mice. Twenty-one days after immunization, the mice underwent intratracheal infection with agarose-encapsulated PAO1 at a dose of 105 CFU. Immunized wild type mice and CD8 knockout mice showed prolonged survival (percent survival, 40 and 30%, respectively), whereas nonimmunized mice and CD4 knockout mice all died within the first 96 h (P < 0.005 [Fig. 5]). These data demonstrate the necessity of the presence of CD4+ T cells for the protective effect of the immunization of DC pulsed with P. aeruginosa, whereas the presence of CD8+ cells seems to be negligible for the induction of the protective immune response using PAO1-pulsed DC.
FIG. 5.
Role of CD4+ and CD8+ T cells to establish immunity by P. aeruginosa-pulsed DC. CD4−/− (○), CD8−/− (▵), or wild-type (▪) C57BL/6 mice were immunized with 105 DC which had been pulsed with heat-inactivated P. aeruginosa (10 CFU/cell, 3 h). Controls included wild-type mice who were not immunized (□). Three weeks after the immunization, mice were challenged intratracheally with 105 CFU of P. aeruginosa enmeshed in agar beads. Survival was recorded as the percentage of surviving animals (n = 10 animals/group).
DISCUSSION
The present study describes a new strategy to induce immunity against pulmonary infections with P. aeruginosa utilizing the potent antigen-presenting potential of DC. DC activated in vitro with whole P. aeruginosa organisms induced systemic Pseudomonas-specific CD4+ T-cell proliferation in vivo. Importantly, intravenous vaccination of DC pulsed with P. aeruginosa was able to improve survival following a lethal intrapulmonary infection with P. aeruginosa, a process that was dependent on the presence of CD4+ T cells.
Protective immunity against P. aeruginosa
P. aeruginosa is the most common respiratory pathogen found in CF patients and is thought to play a major role in eliciting damage to the pulmonary tract (6, 10). The host response to P. aeruginosa is a complex immunoinflammatory interaction that results in damage to the airways and lung parenchyma. Opsonizing antibodies, as well as cell-mediated immunity, seem to be associated with protective immune responses (3, 5, 11, 17, 18, 20, 21, 28, 33), although an effective vaccine for protection against chronic pulmonary colonization has not been developed. Although the present study does not define all of the mechanisms of DC-pulsed Pseudomonas induction of Pseudomonas-specific protective immunity, there is a clear demonstration of some protection of systemic administration of Pseudomonas-pulsed DC against intrapulmonary infection with P. aeruginosa. Immunization with heat-killed PAO1 at a comparable dose did not lead to protection, suggesting that DC were necessary for the observed effect. CD4+ T cells likely play an important role, as no survival advantage was observed in CD4 knockout mice. Consistent with this observation, mice with an intact immune system immunized with Pseudomonas-pulsed DC had primed CD4+ cells which proliferated in response to Pseudomonas antigens presented by DC in vitro, although part of the in vitro proliferative response could be due to an endotoxin adjuvant effect. Serum anti-Pseudomonas IgM and IgA antibody levels were found to be slightly but not statistically significantly elevated, and thus, these antibodies do not seem to play the major role in the observed protective effect, although the presence of mucosal antibodies in the respiratory tract, which was not evaluated in the present study, could theoretically play a role. Nonspecific cross-reactivity to anti-LPS cannot be completely excluded for the ELISA assay used in the present study; however, with the use of the identical assay, no anti-P. aeruginosa antibodies were detected in mouse serum immunized with E. coli (19). The presence of antibodies in the nonimmunized mice is most likely due to background levels of antibody to P. aeruginosa, due to the ubiquitous nature of this organism. We have recently demonstrated that using DC genetically modified to express CD40 ligand and pulsed with heat-inactivated P. aeruginosa induced protective immunity independent of CD4+ T cells (19). In that study, a lower dose of DC (5 × 104 DC/animal) and a subsequent higher dose of P. aeruginosa (2 × 105 CFU/animal) for the intratracheal challenge were used. Interestingly, we observed a protective effect of DC pulsed with P. aeruginosa modified with a control Ad vector (AdNull) for 10% of the immunized animals.
Investigation of the immune mechanisms leading to host resistance to bronchopulmonary infection with P. aeruginosa have focused on humoral immunity and nonspecific inflammatory responses, such as neutrophil and macrophage responses (3, 11, 17, 20, 21, 28, 33). For humans, a vaccine directed against Pseudomonas have failed to be effective, despite good antibody responses to the vaccine (21, 33). Passive immunization with mucoid exopolysaccharide-specific opsonizing antibody protects against chronic P. aeruginosa infection in rats. Association between the presence of opsonizing mucoid exopolysaccharide antibodies and a lack of colonization with P. aeruginosa in elderly, relatively healthy patients suggests that this antibody plays a protective role (30). Recently, various novel approaches to vaccination, including the use of human MAb against LPS, elastase peptidase, or catalase and genetic vaccination against exotoxin A have demonstrated promising results (14, 34, 36, 40).
The role of T-cell mediated immunity has received less attention in efforts to develop a vaccine against P. aeruginosa (7). T cells are responsible for many host responses against pulmonary infections, and T cells are necessary to provide help for most antibody responses (8). Normal human T cells proliferate in response to P. aeruginosa (31). Intestinal immunization of rats with killed P. aeruginosa protected the lung against bacterial infection in a T-cell-dependent process (7, 8). The presence of protective T-cell responses in the absence of detectable antibody responses have been described in mice following low systemic doses of live P. aeruginosa (23), and protection against Pseudomonas could be transferred to nonimmune mice by CD8+ and CD4+ T cells (32). Immunized animals demonstrated enhanced macrophage activation and neutrophil recruitment and activation, i.e., Pseudomonas-related immune T cells may enhance elimination of P. aeruginosa from the lung via recruitment and activation of macrophages and neutrophils (32).
DC and immunity.
DC pulsed ex vivo with various protein antigens have the capacity to prime naive T cells in vivo and induce protective immunity against various tumors and microbial infections (2, 22, 24–26, 39, 41). The use of antigen-pulsed DC for the induction of protective immunity against bacteria has been assessed against intracellular pathogens, where a Th1 T-cell-dominant response is thought to be important in controlling the infection (15, 24, 26, 39, 41). Consistent with the observations of the present study, experimental murine eye infection with P. aeruginosa is associated with increased accumulation of DC at the site of infection (13).
Incubation of DC with heat-inactivated PAO1 was as effective as using live bacteria in the induction of a protective effect, suggesting that the processing of the antigens in the DC relevant to induction of anti-Pseudomonas immunity was independent of the viability of the bacteria. Using killed PAO1 in conjunction with DC may be more immunostimulatory, potentially due to lysis or denaturation of immunogens. Studies using DC pulsed with Chlamydia trachomatis to induce protective immunity against genital or pulmonary infection with this organism also used inactivated bacteria (39). As we did not observe any antigen processing and presentation in the PAO1-pulsed DC, the possibility that the observed protective effect might be based on a superantigen effect cannot be ruled out.
The usage of DC pulsed with the intact P. aeruginosa organism as a vaccination strategy has the potential advantage of inducing immunity against multiple antigens simultaneously, as opposed to vaccination strategies using single antigenic components of P. aeruginosa. This is especially relevant in the light of the capacity of P. aeruginosa to commonly mutate in vivo into a mucoid form (10). The appearance of mucoid forms includes overproduction of the polysaccharide alginate, leading to increased bacterial adherence and thus imposing a barrier for phagocytosis, and usually correlates with the formation of a bacterial biofilm containing microcolonies (4). There is genetic diversity among P. aeruginosa strains isolated from patients with CF, with various patterns of colonization, such as colonization with multiple resistant strains, a single strain, or periodically changing dominant strains (10). A vaccination strategy utilizing the whole Pseudomonas organism presented by DC may be able to induce immunity to multiple Pseudomonas antigens before the appearance of a bacterial biofilm. The P. aeruginosa strain used in the present study, PAO1, is a nonmucoid laboratory isolate. Future studies will have to use mucoid strains and various clinical isolates recovered from infected patients with CF at various times during the progression of their disease to further evaluate the efficacy of this strategy.
ACKNOWLEDGMENTS
We thank P. Leopold and R. Ramalingam in our laboratory for helpful discussions and N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by the Will Rogers Memorial Fund, Los Angeles, Calif.; the Cystic Fibrosis Foundation, Bethesda, Md.; and GenVec, Inc., Gaithersburg, Md.
REFERENCES
- 1.Alonso A, Campanario E, Martinez J L. Emergence of multidrug-resistant mutants is increased under antibiotic selective pressure in Pseudomonas aeruginosa. Microbiology. 1999;145:2857–2862. doi: 10.1099/00221287-145-10-2857. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1α. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Cripps A W, Dunkley M L, Clancy R L, Kyd J. Pulmonary immunity to Pseudomonas aeruginosa. Immunol Cell Biol. 1995;73:418–424. doi: 10.1038/icb.1995.65. [DOI] [PubMed] [Google Scholar]
- 6.Davis P B, Drumm M, Konstan M W. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 7.Dunkley M, Pabst R, Cripps A. An important role for intestinally derived T cells in respiratory defence. Immunol Today. 1995;16:231–236. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- 8.Dunkley M L, Clancy R L, Cripps A W. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83:362–369. [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuis M, McDonald D M. Dendritic-cell regulation of lung immunity. Am J Respir Cell Mol Biol. 1997;17:284–286. doi: 10.1165/ajrcmb.17.3.f136. [DOI] [PubMed] [Google Scholar]
- 10.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin D, DeSanctis J, Boulé M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govan J R, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazlett L D, Moon M M, Dawisha S, Berk R S. Age alters ADPase positive dendritic (Langerhans) cell response to P. aeruginosa ocular challenge. Curr Eye Res. 1986;5:343–355. doi: 10.3109/02713688609025172. [DOI] [PubMed] [Google Scholar]
- 14.Hemachandra S, Kamboj K, Copfer J, Pier G, Green L L, Schreiber J R. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal Pseudomonas sepsis. Infect Immun. 2001;69:2223–2229. doi: 10.1128/IAI.69.4.2223-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain-Vora S, LeVine A M, Chroneos Z, Ross G F, Hull W M, Whitsett J A. Interleukin-4 enhances pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. 1998;66:4229–4236. doi: 10.1128/iai.66.9.4229-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang I J, Kim I S, Park W J, Yoo K S, Yim D S, Kim H K, Shin S G, Chang W H, Lee N G, Jung S B, Ahn D H, Cho Y J, Ahn B Y, Lee Y, Kim Y G, Nam S W, Kim H S. Human immune response to a Pseudomonas aeruginosa outer membrane protein vaccine. Vaccine. 1999;17:158–168. doi: 10.1016/s0264-410x(98)00159-5. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi T, Worgall S, Singh R, Moore M A, Crystal R G. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat Med. 2000;6:1154–1159. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- 20.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun. 1998;66:3164–3169. doi: 10.1128/iai.66.7.3164-3169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langford D T, Hiller J. Prospective, controlled study of a polyvalent pseudomonas vaccine in cystic fibrosis—three year results. Arch Dis Child. 1984;59:1131–1134. doi: 10.1136/adc.59.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markham R B, Powderly W G. Exposure of mice to live Pseudomonas aeruginosa generates protective cell-mediated immunity in the absence of an antibody response. J Immunol. 1988;140:2039–2045. [PubMed] [Google Scholar]
- 24.Marriott I, Hammond T G, Thomas E K, Bost K L. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–1115. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Melief C J, Ildstad S T, Kast W M, Deleo A B. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 26.Mbow M L, Zeidner N, Panella N, Titus R G, Piesman J. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–3390. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McWilliam A S, Nelson D J, Holt P G. The biology of airway dendritic cells. Immunol Cell Biol. 1995;73:405–413. doi: 10.1038/icb.1995.63. [DOI] [PubMed] [Google Scholar]
- 28.Pennington J E, Kuchmy D. Mechanism for pulmonary protection by lipopolysaccharide pseudomonas vaccine. J Infect Dis. 1980;142:191–198. doi: 10.1093/infdis/142.2.191. [DOI] [PubMed] [Google Scholar]
- 29.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pier G B, Saunders J M, Ames P, Edwards M S, Auerbach H, Goldfarb J, Speert D P, Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987;317:793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 31.Porwoll J M, Gebel H M, Rodey G E, Markham R B. In vitro response of human T cells to Pseudomonas aeruginosa. Infect Immun. 1983;40:670–674. doi: 10.1128/iai.40.2.670-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powderly W G, Pier G B, Markham R B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. V. Generation of bactericidal T cells in nonresponder mice. J Immunol. 1987;138:2272–2277. [PubMed] [Google Scholar]
- 33.Schaad U B, Lang A B, Wedgwood J, Ruedeberg A, Que J U, Furer E, Cryz S J J. Safety and immunogenicity of Pseudomonas aeruginosa conjugate A vaccine in cystic fibrosis. Lancet. 1991;338:1236–1237. doi: 10.1016/0140-6736(91)92103-9. [DOI] [PubMed] [Google Scholar]
- 34.Shiau J W, Tang T K, Shih Y L, Tai C, Sung Y Y, Huang J L, Yang H L. Mice immunized with DNA encoding a modified Pseudomonas aeruginosa exotoxin A develop protective immunity against exotoxin intoxication. Vaccine. 2000;19:1106–1112. doi: 10.1016/s0264-410x(00)00335-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 36.Sokol P A, Kooi C, Hodges R S, Cachia P, Woods D E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J Infect Dis. 2000;181:1682–1692. doi: 10.1086/315470. [DOI] [PubMed] [Google Scholar]
- 37.Song W, Kong H L, Carpenter H, Torii H, Granstein R, Rafii S, Moore M A, Crystal R G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson M M, Kondratieva T K, Apt A S, Tam M F, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas L D, Dunkley M L, Moore R, Reynolds S, Bastin D A, Kyd J M, Cripps A W. Catalase immunization from Pseudomonas aeruginosa enhances bacterial clearance in the rat lung. Vaccine. 2000;19:348–357. doi: 10.1016/s0264-410x(00)00146-8. [DOI] [PubMed] [Google Scholar]
- 41.Wick M J, Ljunggren H G. Processing of bacterial antigens for peptide presentation on MHC class I molecules. Immunol Rev. 1999;172:153–162. doi: 10.1111/j.1600-065x.1999.tb01363.x. [DOI] [PubMed] [Google Scholar]