Abstract
There are a plethora of antibiotic resistance cases and humans are marching towards another big survival test of evolution along with drastic climate change and infectious diseases. Ever since the first antibiotic [penicillin], and the myriad of vaccines, we were privileged to escape many infectious disease threats. The survival technique of pathogens seems rapidly changing and sometimes mimicking our own systems in such a perfect manner that we are left unarmed against them. Apart from searching for natural alternatives, repurposing existing drugs more effectively is becoming a familiar approach to new therapeutic opportunities. The ingenious use of revolutionary artificial intelligence-enabled drug discovery techniques is coping with the speed of such alterations. D-Mannose is a great hope as a nutraceutical in drug discovery, against CDG, diabetes, obesity, lung disease, and autoimmune diseases and recent findings of anti-tumor activity make it interesting along with its role in drug delivery enhancing techniques. A very unique work done in the present investigation is the collection of data from the ChEMBL database and presenting the targetable proteins on pathogens as well as on humans. It shows Mannose has 50 targets and the majority of them are on human beings. The structure and conformation of certain monosaccharides have a decisive role in receptor pathogen interactions and here we attempt to review the multifaceted roles of Mannose sugar, its targets associated with different diseases, as a natural molecule having many success stories as a drug and future hope for disease management.
Graphical abstract

Keywords: Mannose, Drug delivery, Immune regulation, Antibiotic, Urinary tract infections, Congenital disorder of glycosylation (CDG)
Introduction
Carbohydrates are the most common hydrates of carbon widely used in biological systems, to modify structures of other biomolecules for specific functions, to mediate metabolic activities, as biosynthetic intermediates, and most importantly in energy generation and storage. The carbohydrate linkage to protein in glycoproteins and nucleic acids for linear scaffolding is a prerequisite for the functioning of the proteins. The critical functions thus include cell-cell communication and adhesion, protein structure conformations, membrane structure, and cellular signaling [1, 2].
Functional sugars notably D-Mannose, show broad applications in pharmaceutical and food industries, also as alternatives to sucrose in terms of calories and sweetness [3]. D-Mannose is a hexose sugar and it is mainly seen in the plant cell wall as a component of mannan [4]. D-Mannose is involved in the synthesis of glycoproteins necessary to regulate the body’s immune system and thus is an important dietary supplement [5]. D-Mannose also plays a major physiological role including wound healing, antitumor, and antibacterial properties [6]. Further, D-Mannose is extensively used as a precursor for vitamins and antitumor drug synthesis [7, 8].
Mannose is essential in human metabolism as it has the key function to glycosylate certain proteins [9]. Apart from this, Mannose has been reported to relieve intestinal cystic pain [10] and to treat urinary tract infections caused by bacteria [11, 12]. Mannose is found effective in the treatment of lipopolysaccharide-induced acute lung injury in rats [13]. Recently, studies revealed that Mannose is an effective suppressor of autoimmune and inflammatory diseases and effectively suppresses a number of diseases which include Type I diabetes, asthma, colitis, obesity, osteoarthritis, chronic graft-versus-host disease, and lupus [14–18]. Immune regulatory functions of Mannose have also been revealed [19]. Mannose-containing polymeric delivery systems for targeted specific delivery of antileishmanial drugs are reported by Narayanaswamy et al. [10]. The immunotherapy as well as the radiotherapy of triple-negative breast cancer, TNBC is found to be enhanced by oral administration of D-Mannose by promoting programmed death-ligand-1 (PD-L1) degradation [20]. Zhang et al. have reported recently that inflammation can be suppressed by a novel strategy using Mannose which is safe and promising to treat inflammatory and allergic diseases [21]. Nan et al. also have studied that the molecule Mannose can play a fruitful role in the treatment of a wide variety of diseases [22]. Wei et al. in a comprehensive review of pharmacotherapy reports include areas to be explored more but studies going on in the fields of treatment of pancreatic fistula, improving the ability of MRI for acute pancreatitis, and preventing obesity which is caused by a high-fat diet [23]. Elena Dalle Vedove discusses the perspective of Mannose and Mannose-6-Phosphate receptor in targeted drug delivery, particularly in cancer Therapy [24].
Even though there are few detailed reviews [21–24] they have a specific focus on a few areas in which Mannose is found to be effective, and here we try to incorporate most of the important findings accompanying this molecule as a drug that will be worth reporting as this research is finding momentum recently. The importance of the Mannose targets and connected diseases are discussed which are having a significant role in pathogenesis and immune regulation and should be carefully studied for polypharmacology/promiscuity concerns. In the present work, a collection of data from the ChEMBL database listing out the targetable proteins on pathogens as well as on humans are investigated for its role in diseases. Among the 50 targets of Mannose, the majority of them are on human beings and should be taken very good care of while discussing a new drug’s role.
The breakthrough findings are shown in the timeline Fig. 1. Due to the excellent properties of D-Mannose and its functioning for health, the sugar gained great attention from researchers in recent years.
Fig. 1.

Mannose in research as medicine for various diseases
In nature, Mannose occurs mainly as a component of storage and structural carbohydrate polymers and of glycolipids and glycoproteins [25, 26]. More often, Mannose occurs in homo or heteropolymers like yeast mannans or in galactomannans [27, 28]. Mannose is a universal secondary cell wall component and is the main component of the neutral sugar fraction of the matrix polysaccharides in gymnospermous woods. Mannose contributes a significant percentage of the sugar residues of glycoproteins and glycolipids. Mannose in these glycoproteins and glycolipids is α-linked and which is in contrast to its β-linkages in polysaccharides [29]. A small amount of Mannose has been found in free form in fruits like apples, oranges, and peaches and in animals notably in mammalian plasma at 50–100 µM [9, 30]. Ivory nuts, guar gums, fenugreek, and coffee beans are rich sources of Mannose. Ivory nuts mainly contain β- mannans whereas the other three mainly have galactomannans [31].
The two ring-structured forms of the sugar are typically the six-membered pyranose form and the five-membered furanose form. At the anomeric location, each ring closure can take the form of an alpha or beta configuration. The C-2-epimer of glucose called D-Mannose is mostly found in the sweet-tasting [67%] and bitter-tasting [33%] anomeric forms of the pyranose [32, 33], whereas the furanose form is present in only 2% of cases. Although L-Mannose is typically not used in biological systems, some plant enzymes can utilize it as an artificial substrate in-vitro because of its structural resemblance to natural L-rhamnose [33]. Figure 2 depicts the Mannose pyranose and furanose variants.
Fig. 2.
Pyranose and furanose forms of Mannose
In the biological system, monosaccharides with a number of stereogenic centers exist in cyclic structures and its attacking OH group will figure out intramolecular cyclization and the ring size preferably as pentoses and hexoses [34]. The conformational stability of Mannose derivatives to stay in the open chain or cyclic form is found to be influenced by the factors such as the ability to form maximum intramolecular hydrogen bonding, keeping electrostatic potential and total energy in the minimal range [35].
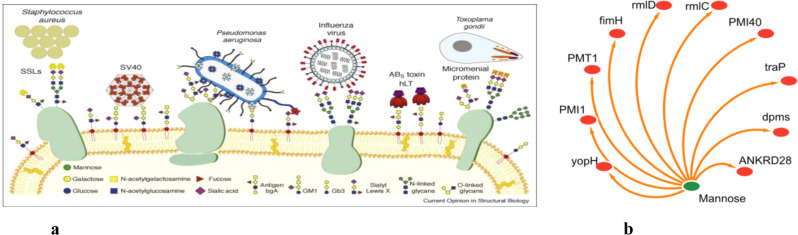
Mannose is a major sugar for microbial recognition in humans and Fig. 3a shows human cell surface glycoconjugates [36] and also found to have targetable proteins on pathogens [Fig. 3b].
Fig. 3.
a Microbial recognition involving Mannose of human cell surface glycoconjugates, adopted with permission from 10.1016/j.sbi.2008.08.001. b Target proteins of Mannose on pathogens. Data were taken from ChEMBL repository https://www.ebi.ac.uk/chembl/g/#search_results/targets/query=Mannose%20targets
Biosynthesis of Mannose
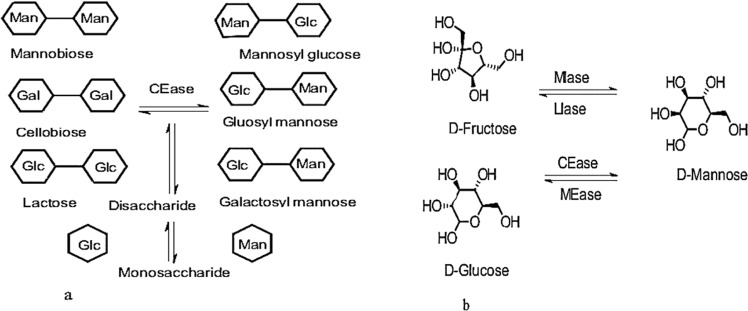
Guanosine diphosphate Mannose [GDP Mannose], an active Mannose donor molecule is the usual donor of nucleotide sugar for the biosynthesis of Mannose polymers and this can be isolated from numerous plants [25]. GDP-Mannose is further, the source of mannosyl units of glycoproteins, glycolipids, and dolichol phosphoMannose. It also functions as an essential biochemical feedstock for deoxy sugars synthesis (e.g., GDP-fucose). GDP-Mannose is a precursor for the biosynthesis of L-ascorbic acid in plants [37]. GDP-Mannose is synthesized from fructose-6-phosphate by three enzymes. The first enzyme phosphoMannose isomerase converts fructose-6-phosphate to Mannose-6-phosphate which is further converted to Mannose-1-phosphate by the catalytic activity of the second enzyme phosphomannomutase. Mannose-1- phosphate is finally converted to GDP-Mannose by the third enzyme Mannose-1-phosphate guanylyltransferase [38]. The biosynthesis of GDP Mannose is diagrammatically represented in Fig. 4.
Fig. 4.

Biosynthesis of GDP-Mannose
Production
Currently, different methods are used to produce D-Mannose which include extraction from plants, chemical synthesis, and biological production using microbial enzymes [3]. The direct extraction of Mannose from plants is quite difficult and is normally complex and involves multiple hydrolytic reactions like thermal hydrolysis, acid hydrolysis, and enzymatic hydrolysis [39, 40]. In the laboratory, Mannose can be produced by mannitol oxidation or by base-catalyzed glucose epimerization through fructose [40]. Currently, a popular method of Mannose production is the chemical conversion of glucose which is normally performed under high temperatures in presence of excess acid and molybdate [41]. However, this method uses complex downstream purification leading to by-product formation that causes environmental pollution, and high energy consumption. Hence alternative strategies are being experimented. Among these, enzymatic methods have received much attention as they enable the production of Mannose in mild conditions with high efficiency and eco-friendliness [42]. D-Mannose isomerase (MIase, EC 5.3.1.7), D-lyxose isomerase (LIase, EC 5.3.1.15), D-Mannose 2-epimerase (MEase, EC 5.1.3), and cellobiose 2-epimerase (CEase, EC 5.1.3.11) are the four major enzymes that produce Mannose [3]. The first two enzymes catalyze the isomerization between Mannose and fructose, whereas the latter two involve the C-2 epimerization between Mannose and glucose (Fig. 5a, b). Among these enzymes, lyxose isomerase and cellobiose 2- epimerase are not a choice for Mannose production because of their low substrate specificity and metal ion dependence [43, 44]. However, Mannose isomerase can specifically isomerize fructose to Mannose without metal ion dependence [45]. The exploration of new microbial enzymes is still an emerging area for the production of functional sugar, and D-Mannose is a case in point [3, 29], reported PsMIaseA- a novel Mannose isomerase from P. syringae as a good enzyme for Mannose production. Wu et al. also reported a promising D-lyxose isomerase (D-LIase) from C. polysaccharolyticus for the direct production of D-Mannose from D-fructose [46].
Fig. 5.
a Enzymatic methods: Epimerization at the C-2 position of β-1,4-linked disaccharide and disaccharides break into individual monosaccharides. b The production of mannose from different isomers
Mannose in genetic diseases
In our blood, around 50–100 μM Mannose is present and 3–5-fold is gained from food [47]. Mannose enters the cell via direct absorption even though the amount is lesser than that obtained from glucose interconversion through hexokinase transporters. It then immediately changes to Mannose-6-phosphate for fixing the stereocenter of the anomeric carbon by utilizing ATP. Glycogen also forms glucose-6-phosphate [or fructose-6-phosphate] and using isomerase enzymes converts to Mannose-6-phosphate. This is the primary reaction of the Mannose metabolic pathway and it can be influenced by two parameters, one is the source of Mannose and the other is the availability of isomerase enzymes.
Defects may occur while assembling, transporting, or even during the processing itself of N-linked oligosaccharide glycosylation on proteins and causes serious genetic disorders which are collectively known as congenital disorder of glycosylation (CDG1 or Jaeken syndrome) [48–51]. Jaeken et al. in a letter to the editors of the American journal of human genetics have discussed these defects and their presentations on patients [52]. According to their recent review, CDG is “still hot in 2020” and another similar review clearly states that CDG has only three possible ways to treat and oral Mannose comes in the first place. They have placed CDG in a poorly treatable family of disorders even in 2022 [53, 54]. The phosphoMannose isomerase which catalyzes the process of interconversion of fructose-6-phosphate into Mannose-6-phosphate has a crucial role in glycosylation reactions as it maintains the number of Mannose derivatives by providing as per the demand.
In 1998, Niehues et al. [55] studied phosphoMannose isomerase deficiency and Mannose therapy in CDG-lb (congenital disorder of glycosylation-1b) patients. Another manifestation of carbohydrate-deficient glycoprotein syndrome, hyperinsulinemic hypoglycemia is reported by De Lonlay et al. [56] and Babovic-Vuksanovic [57]. In all these cases, oral Mannose was used for treatment. As per their reports, it was revealed that protein-losing enteropathy, hypoglycemia, and coagulopathy are being reversed by Mannose supplements. Additionally, it also reported improvement of the transferrin IEF pattern in three children with CDG-Ib. Davis et al. [58] studied long-term Mannose supplementation in mouse models and found no ill effects, but Sharma et al. warned in 2014 [30, 59], that Mannose supplements may induce embryonic lethality and blindness during pregnancy again in mouse models, but not yet confirmed in humans, but cautioned not be given during pregnancy.
Dr. Hudson Freeze, Director of Stanford Children’s Health Research Centre has been working on the “identification and understanding of human glycosylation disorders” for the past 20 years and their group discovered the first patient with an inherited deficiency in phosphoMannose isomerase (CDG-Ib) and successfully treated him with oral Mannose supplements. The most relevant studies start from the finding that the specific transporter through which Mannose enters the cell is insensitive to glucose [60], oral intake of Mannose can increase blood Mannose level [61], Mannose in fibroblasts having CDG syndrome can correct altered N-glycosylation [62], the N-glycosylation of human fibroblasts prefers transported Mannose than that is derived from glucose as a source of Mannose [63], the metabolism of Mannose in families of CDG-1 is found to be abnormal [64], failures in Mannose therapy reported are due to short-term Mannose supplementation [65], dyserythropoiesis can result from the deficiency of alpha-mannosidase-II, genetic deficiency of αM-II should abolish complex N-glycan production as reportedly does inhibition of αM-II by swainsonine [66], evidence supporting Mannose is directly utilized in glycoprotein biosynthesis in mammals [9], and finally, the pros and cons of Mannose metabolism studies were a great help in Mannose supplementation by which they could change many miserable lives of CDG patients [67–75].
Mannose in chemotherapy
The increase in glucose levels along with the decrease in cell growth in tumor cells during the Mannose treatment was reported by Gonzalez et al. [6]. The accumulated Mannose-6-phosphate causes an inhibition in the enzyme’s hexokinase and phosphoglucose isomerase involved in the first and second steps of glycolysis in tumor cells due to Mannose intake. Mannose could perform better than fructose, fucose, galactose, and glucose and also showed improvement in combination with doxorubicin or cisplatin while treating mice with tumors than they were used alone, and hence the efficacy of conventional chemotherapy is enhanced by Mannose. Major Mannose-modified formulations used in chemotherapy are tabulated in Table 1. The acute leukemia drug methotrexate (MTX) in combination with nanoparticles was found to be effective in the treatment of rheumatoid arthritis and different types of cancers. MTX-Man NPs, which consist of MTX and MTX-Mannose NPs may be a more effective option for treating tumors [23]. Along with many forms of carbohydrates, Mannose is being targeted for antigen receptors on APCs for effective vaccine delivery [76]. A number of reports are available on the regulatory effects of Mannose on lung cancer, breast cancer, osteosarcoma, etc., while according to a recent report Mannose treatment via diet was found to suppress mitochondrial metabolism in leukemia cells, suggesting a potential therapeutic agent [77].
Table 1.
Mannose-modified formulations used in chemotherapy
| Mannose-modified formulations | Application in chemotherapy |
|---|---|
| MTX-Man NPs | Mannose is linked to Methotrexate (MTX) by ester bonds, Biosafe, Dual-self-recognizing, Stimulus-responsive, Carrier-free MTX–MAN NPs Used in Chemotherapy [128]. |
| BTM-NMs | Targeted photodynamic treatment using a 3-arm distyryl BODIPY derivative coupled with Mannose units (BTM) and Tween 80 nanomicelles (BTM-NMs) (PDT). Specifically, Mannose-receptor-mediated endocytosis internalized BTM-NMs with a preference for lysosomal accumulation. When exposed to light, these NMs may disintegrate in cell lysosomes and then induce very effective singlet oxygen production. Through PDT, singlet oxygen effectively and selectively killed cancer cells by rupturing the lysosomal membrane and promoting BTM’s escape from the lysosome into the cytoplasm [129]. |
| MSN-M6C-Man | There is increased endocytosis and enhanced efficacy by grafting Dimannoside-carboxylate (M6C-Man) on the surface of mesoporous silica nanoparticles (MSNs) for photodynamic therapy, particularly to target the overexpressed cation-independent Mannose-6-phosphate receptor in prostate cancer [130]. |
| NP-R@M-M | Nanoparticles made of poly-d,l-lactide, and glycolic acid that contain the toll-like receptor 7 agonist imiquimod (R837). Then, cancer cell membranes (NP-R@M), whose surface proteins may serve as tumor-specific antigens, are coated onto adjuvant nanoparticles (NP-R). Following Mannose moiety alteration to get a nanovaccine, NP-R@M-M has improved absorption by antigen-presenting cells, such as dendritic cells, to stimulate the maturation state and elicit anticancer immune responses [131]. |
| PLA-b-(PTA-g-Mannose) | A regulated ring-opening polymerization of O-carboxy anhydride (OCA) and extremely effective “Click” chemistry are used to create PLA-b-(PTA-g-Mannose). The Mannose moiety enables for active targeting of the micelles to cancer cells that specifically express Mannose receptors, which in turn increases the anticancer efficacy of the medicine. Doxorubicin (DOX), a representative lipophilic anticancer agent, can be successfully encapsulated into the micelles. This micellar system, which is entirely made of biodegradable and biocompatible polyesters, has intriguing potential for targeted drug administration and cancer therapy [113]. |
| Ulvan on GO–CH–Ma | A novel D-Mannose-mediated targeted drug delivery system (GO–CH–Ma) for targeting glioblastoma cancer was developed by loading Ulvan lactua as the anticancer model drug onto functionalized graphene oxide. The entrapment of ulvan on GO–CH–Ma has been observed to be 88% and demonstrated a promising targeted drug delivery system to treat in vitro glioblastoma [118]. |
D-Mannose as antibiotics
D-Mannose has been utilized for the treatment of urinary tract infections (UTIs) tracing all the way back to the 1970s [78, 79]. All around the world, D-Mannose is promoted and consumed as a dietary enhancement and is predominantly focused on supporting the health of the urinary tract. It is utilized either as an independent item or joined with cranberry concentrate or probiotics. Intense and recurrent UTIs (rUTIs) are generally treated with antibiotics, the emergence of antibiotic-resistant strains limited their application [80]. D-Mannose, a natural sugar, has the ability to prevent bacterial binding to urothelial cells by masking the adhesion of fimH (a protein on the tip of Escherichia coli) and is considered an alternative to antibiotics used for UTIs [81]. The predominance of bacterial etiology winds up in a huge utilization of an expansive range of anti-infection agents, which thus prompts expanded paces of safe uropathogens. Likewise, the non-antibiotic strategies for prevention and cure prevail.
Wagenlehner [82] performed a non-interventional prospective study among female patients with acute cystitis and reported that treatment with D-Mannose monotherapy was better than that of antibiotics. They showed that patients using D-Mannose in monotherapy mode in acute uncomplicated cystitis (AUC) scored significantly higher cure rates compared to that of patients who were administered antibiotics. It clearly indicated that the effectiveness of D-Mannose is closely comparable to that of antibiotics in the prevention and treatment of UTIs.
In a study, Kranjcec et al. divided three groups of UTI-affected female patients at random. Patients in the first group (n = 103) received 2 g of powdered D-Mannose daily; those in the second group (n = 103) received 50 mg of nitrofurantoin daily, and those in the third group (n = 102) received no preventative treatment for 6 months. The study’s findings showed that administering D-Mannose with nitrofurantoin decreased the risk of UTI. The D-Mannose group had fewer adverse effects. After conducting more thorough research, the study came to the conclusion that D-Mannose is helpful for UTI prevention [12].
Domenici et al. led a pilot clinical study among women aged 18–65 years with severe cystitis history of rUTI’s (n = 43) [83]. For such severe cases, 1.5 g of D-Mannose was supplied twice a day for 3 days, and once per day for 10 days. And for the long term, once a day for a week for every alternate month for 6 months. It was found that the D-Mannose has potential as an effective agent for both acute UTI and as a prophylactic for rUTI in a specific population. Phe V et al. conducted a clinical trial to find the effect of D-Mannose in patients with multiple sclerosis and rUTIs [84]. The participants were administered D-Mannose powder 1.5 g twice daily for 16 weeks. The results of using D-Mannose in patients with MS experiencing rUTIs were safe and feasible. The author suggests further studies to establish the efficacy of the same.
D.Porru et al. led a pilot investigation of the adequacy of D-Mannose in repetitive urinary plot contaminations (r UTIs) on which the customary prophylactic antitoxin systems do not change the drawn-out hazard of a repeating infection [85]. In his randomized get-over clinical preliminary, 60 female patients between 22 and 54 years of age with intense suggestive UTI and at least three rUTIs during the former year were selected. Patients were arbitrarily allocated to antimicrobial treatment with trimethoprim/sulfamethoxazole or to a routine of oral D-Mannose 1 g three times each day at a time span hour for quite a long time, and followed by 1 g two times every day for a very long time. The admission of D-Mannose was displayed as powerful and protected in forestalling rUTIs in ladies. The number of disease-free ladies was more noteworthy in the D-Mannose bunch in examination with the anti-microbial gathering. The mean time to UTI recurrence was noted to be 4 times than compared to the antibiotic treatment and oral Mannose treatment. However, the utilization of D-Mannose as a component in the antimicrobial movement was not explored. It could be acting by forestalling bacterial development and digestion or by choosing fimH variations. Scribano et al. utilized the vital elements of bacteria of the model UPEC (Uropathogenic Escherichia coli) strain CFT073 treated with D-Mannose and examined by standard microbiological techniques [81]. FimH usefulness was inspected by human bladder cell attachment examinations and yeast agglutination. In all cases, the outcomes showed that high D-Mannose fixations have no impact on bacterial development and do not obstruct various anti-infection agent exercises. D-Mannose was positioned as the most unfavoured carbon source that could uphold bacterial digestion and development in comparison to D-glucose, D-fructose, and L-arabinose. As modest quantities of glucose are physiologically recognizable in urine, it tends to be inferred that the presence of D-Mannose is unessential for bacterial digestion. Further, evacuation of D-Mannose after long haul openness did not change FimH’s ability to tie to mannosylated proteins. In general, the outcomes demonstrated that D-Mannose is a decent option in the avoidance and treatment of UPEC-related UTIs.
Parazzini et al. took seven studies, in which D Mannose was taken to clinical studies. Among them, two were of D-Mannose alone, and others were Cranberry extract, Morinda citrifolia fruit extract, Pomegranate extract, Fructooligosaccharides, Lactobacilli, and N-acetylcysteine. Then they reviewed the effect of D‑Mannose with or without other drugs in the treatment of symptoms of urinary tract infections and reported that symptoms decreased after treatment with D-Mannose [86]. D-Mannose also holds many patents (Table 2) including D-Mannose contraceptives (US-2014256656-A1) which is invented by Dale L. Benedict and Martha Benedict and Bio Tech Pharmacal Inc. hold the property rights to the patent.
Table 2.
Patents history of Mannose and their derivatives
| Patent ID | Title | Grant date | Reference Link |
|---|---|---|---|
| CA-2894536-C | Mannose derivatives for treating bacterial infections | 2020-07-28 | https://patents.google.com/patent/CA2894536C/en |
| US-10669298-B2 | Mannose derivatives for treating bacterial infections | 2020-06-02 | https://patents.google.com/patent/US10669298B2/en |
| KR-102075885-B1 | Mannose derivatives for treating bacterial infections | 2020-02-11 | https://patents.google.com/patent/KR102075885B1/en |
| ES-2732311-T3 | Mannoside compounds and procedures for their use | 2019-11-21 | https://patents.google.com/patent/ES2732311T3/en |
| EP-2672820-B1 | Mannoside compounds and methods of use thereof | 2019-04-17 | https://patents.google.com/patent/EP2672820B1/en |
| JP-6412506-B2 | Mannose derivatives for treating bacterial infections | 2018-10-24 | https://patents.google.com/patent/JP6412506B2/en |
| ES-2685975-T3 | Mannose derivatives to treat bacterial infections | 2018-10-15 | https://patents.google.com/patent/ES2685975T3/en |
| CN-104936969-B | Mannose derivative for treating bacterium infection | 2018-08-17 | https://patents.google.com/patent/CN104936969B/en |
| DK-2935302-T3 | Mannose derivatives for treating bacterial infections | 2018-08-13 | https://patents.google.com/patent/DK2935302T3/en |
| TW-I626247-B | Mannose derivatives for treating bacterial infections | 2018-06-11 | https://patents.google.com/patent/TWI626247B/en |
| EP-2935302-B1 | Mannose derivatives for treating bacterial infections | 2018-06-06 | https://patents.google.com/patent/EP2935302B1/en |
| US-9963478-B2 | Mannose derivatives for treating bacterial infections | 2018-05-08 | https://patents.google.com/patent/US9963478B2/en |
| AU-2013361602-B2 | Mannose derivatives for treating bacterial infections | 2018-02-01 | https://patents.google.com/patent/AU2013361602B2/en |
| US-9616084-B2 | Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids | 2017-04-11 | https://patents.google.com/patent/US9616084B2/en |
| US-9598454-B2 | Mannose derivatives for treating bacterial infections | 2017-03-21 | https://patents.google.com/patent/US9598454B2/en |
| AU-2010333017-B2 | Mannose derivatives as antagonists of bacterial adhesion | 2016-06-16 | https://patents.google.com/patent/AU2010333017B2/en |
| JP-5799022-B2 | Mannose derivatives as antagonists of bacterial adhesion | 2015-10-21 | https://patents.google.com/patent/JP5799022B2/en |
| EP-1809328-B1 | Mannooligosaccharide composition for body fat reduction | 2013-03-13 | https://patents.google.com/patent/EP1809328B1/en |
| EP-0804599-B2 | Mannose or xylose based positive selection | 2012-10-10 | https://patents.google.com/patent/EP0804599B2/en |
| CA-2157470-C | Mannose or xylose based positive selection | 2012-04-17 | https://patents.google.com/patent/CA2157470C/en |
| DE-60319354-T2 | Mannose-6-phosphate receptor mediated gene transfer to muscle cells | 2009-03-26 | https://patents.google.com/patent/DE60319354T2/en |
| EP-1495769-B1 | Mannose-6-phosphate receptor mediated gene transfer into muscle cells | 2008-02-27 | https://patents.google.com/patent/EP1495769B1/en |
| DE-60307701-T2 | Mannose binding protein-containing pharmaceutical compositions | 2007-10-11 | https://patents.google.com/patent/DE60307701T2/en |
| EP-0804599-B1 | Mannose or xylose based positive selection | 2006-05-24 | https://patents.google.com/patent/EP0804599B1/en |
| US-7018824-B2 | Mannosyl transfer with regeneration of GDP-Mannose | 2006-03-28 | https://patents.google.com/patent/US7018824B2/en |
| US-5767378-A | Mannose or xylose based positive selection | 1998-06-16 | https://patents.google.com/patent/US5767378A/en |
| AU-682495-B2 | Mannose or xylose based positive selection | 1997-10-09 | https://patents.google.com/patent/AU682495B2/en |
Mannose in drug delivery
The efficiency of a drug is also determined by its ADMET (Absorption, distribution, metabolism, excretion, and toxicity) profiles along with bioavailability and side effects. The trends like combinational therapy and targeted delivery etc. have also made the researchers adopt better drug delivery methods to increase bioavailability and reduce side effects significantly reducing the time of intake and course of medication. Latif et al. studied the role of sugars on the surface of liposomes in immuno-potentiation and the mechanism was explained as rapid and specific recognition of macrophages by receptor-mediated endocytosis that among the two sugars they have studied α-Mannose and β galactose, the former was rapidly taken up [87]. The glycosylation ligands like Mannose, galactose, and even glucose have the advantages of non-toxicity, and no immunogenicity as well as good biocompatibility and biodegradation which can bind to drug delivery systems enabling the process of targeted delivery more effectively. Chen, et al in their recent review discussed in detail the mechanisms of targeting, methods adopted for synthesis, and profound characteristics in the glycosylation-modified drug-delivery targeting systems [88]. Pei et al. worked with Mannose-functionalized antigen nanoparticles that triggered MHC-1 presentation in dendritic cells in the cancer cell microenvironment [89]. In the ChEMBL database, [https://www.ebi.ac.uk/chembl/g/#search_results/targets/query=Mannose%20targets], Mannose has 50 targets and the majority of them are on human beings experimenting with the targeted delivery on a few of them yielded excellent results (Fig. 6). Important targets and their connections to diseases are tabulated in Table 3.
Fig. 6.

Important Mannose targets on human
Table 3.
Mannose targets and related diseases
| Mannose Target | Associated in disease |
|---|---|
| Mannose-6-phosphate isomerase (MPI) | Tumor cell apoptosis [132, 133] |
| Hepatic fibrosis [134]. | |
| Macrophage Mannose receptor 1 (MRC1/CD206) | Allergen-induced lung inflammation [135]. |
| Regulator of (Meta)Inflammation [136]. | |
| Predicts Prognosis in Community-acquired Pneumonia [137]. | |
| Targeting protein for Xklp2 (TPX2) | Tumor angiogenesis in pancreatic cancer [138]. |
| Prognostic marker and therapeutic target for gastric cancer [139]. | |
| Mannose-binding protein C (MBL2) | Biomarker for Many Human Diseases [140, 141]. |
| Role in the susceptibility to infections [142]. | |
| CD81 antigen (CD81) | A tumor target [143–145]. |
| Mannan-binding lectin serine protease 2 (MASP2) | MASP-2 deficiency and tuberculosis [146]. |
| Post-ischemic brain injury [147] | |
| Roles in the development of lupus-like glomerulonephritis [148]. | |
| N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase, (NAGPA) | Human stuttering mutations [149, 150]. |
| Regulatory-associated protein of mTOR, (RPTOR) | Peripheral blood and breast cancer [151]. |
| Rheumatoid Arthritis [152]. | |
| Autophagy and Apoptosis in Renal Carcinoma [153]. | |
| Squamous cell carcinoma [154]. | |
| Hsp90 co-chaperone Cdc37 (CDC37) | Neurodegenerative Diseases [155]. |
| Agent in longevity [156]. | |
| Aurora kinase A (AURKA) | Hepatocellular Carcinoma [157]. |
| The modulator of Lung Fibrosis [158]. | |
| The target for GC treatment [159]. | |
| Lung adenocarcinoma [160]. | |
| Cation-dependent Mannose-6-phosphate receptor (M6PR) | Regulator of T cell immunity [161]. |
| Controlling tumor-infiltrating innate immune cells [162]. | |
| Involvement in sperm cell-HIV-1 interaction [163]. | |
| Mannan-binding lectin serine protease 1Mannan-binding lectin serine protease 1 (MASP1) | Support the hemostatic system [164]. |
| Functions as enzymes and as pattern recognition molecules [165]. | |
| Promote the fibrinolytic activity destabilizes the clot [166]. | |
| Importin subunit beta-1 (KPNB1) | Target for Glioblastoma [167]. |
| Prognostic biomarker in Colorectal cancer patients [168]. | |
| The therapeutic target for Prostate cancer [169]. | |
| Histone-lysine N-methyl transferase EZH2, (EZH2) | Increase chemosensitivity by inhibiting cell viability in lung cancer cells [170]. |
| Therapeutic Target for Kidney Diseases [171]. | |
| Cancer epigenetics [172]. | |
| Epithelioid sarcoma and follicular lymphoma [173]. | |
| Polycomb protein EED, (EED) | Malformation of the dentate gyrus and intellectual disability [174]. |
| Regulation of epithelial-mesenchymal transition of cancer cells [175]. | |
| Histone-lysine N-methyltransferase EZH1 (EZH1) | Genetic markers for Thyroid tumors with EZH1 mutations [176]. |
| Effective biomarker for Triple-negative breast cancer (TNBC) [177]. | |
| GDP-Mannose 4,6 dehydratase (GMDS) | Biomarker for lung adenocarcinoma [178]. |
| Colorectal cancer and GMDS mutation [179]. | |
| Perilipin-3 (PLIN3) | Response to Radiation Therapy of Prostate Cancer [180]. |
| Biomarker for Type2 Diabetes Mellites [181]. | |
| The therapeutic target for Non-alcoholic fatty liver disease [182]. | |
| Diagnostic and prognostic biomarkers in Renal cancer [183]. | |
| Heat shock protein HSP 90-alpha (HSP90AA1) | Therapeutic target for peri-implantitis. [184]. |
| Repairing DNA damage and chemoresistance of ovarian cancer cells [185]. | |
| Biomarker for Oral Squamous Cell Carcinoma [186]. | |
| As regulatory axis in osteosarcoma [187]. |
In the book chapter “Mannose receptor and targeting strategies”, the authors have comprehensively discussed examples of strategies adopted in drug delivery mechanisms for the treatment of cancer and infectious diseases as the MR receptors which is by far the most studied member of the Mannose family receptors belonging to the C-type lectin family which is having unique multi-domain, multifunctional endocytic receptors [90].
Banerjee et al. could find that the hamycin used in the experimental Leishmaniasis in hamster models can increase its therapeutic efficacy by making it a Mannose-coated liposomal hamycin than just a liposomal modification [91]. Chaubay et al. suggested that Rifampicin (RIF)-loaded Mannose-conjugated chitosan nanoparticles (mCNPs) is showing better accumulation in macrophage-rich organs than the free drug and can be a promising method to treat visceral Leishmaniasis [92]. Shahnaz et al. designed a Mannose-anchored thiolated chitosan Amphotericin B nanocarrier which showed improved drug bio-distribution and half-life again in the treatment of visceral Leishmaniasis [93]. Francis et al. suggested Amphotericin B-poly Mannose conjugates could inhibit C. Albicans, C. Parapsilosis, and C. Neoformans, and have a possible therapeutic potential for the treatment of diseases like leishmaniasis [94]. Sohrabi and Lipoldova revealed the misery of the Leishmania major Seidman (LmSd) parasite and the role of the Mannose receptor, that ability of non-healing LmSd to infect and survive in host cells may evolve a change in cellsurface oligosaccharide structures on MRC1 (Mannose Receptor C-Type-1) and P4 dermal macrophages [95]. Martínez-López et al. discussed the need to understand the interactions between the Leishmania parasite and the host myeloid cells where the Mannose receptor is overexpressed such as macrophages, neutrophils, and dendritic cells, as leishmaniases continue to be a major neglected infectious disease worldwide and that we need new therapeutic approaches and better vaccination in the light of increased microbial resistance and limited treatment measures [96].
Oral therapy of Mannosylated thiolated chitosan-coated Paromomycin [PM]-loaded Poly[lactide-co-glycolide] PLGA nanoparticles are a promising strategy for the treatment of visceral leishmaniasis [97]. It was also understood that mannosylated Pegylated [pegylated polyethylenimine] dendritic polyglycerol-based conjugates can selectively target Leishmania-infected macrophages [98]. Sony et al. prepared and characterized mannosylated liposomes of Amp B, and the Vesicle size, zeta potential, and the % drug release from Mannose-coupled liposomes were established to be 1.62 ± 0.08 μm, 15.7 ± 0.8 mV and 41.9%, respectively after 24 h [99].
The “Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis” by Prasanna et al. clearly mentioned that among the three sugars (fucose, galactose, and Mannose) used to design liposomal systems Mannose-coated liposomal systems were found to have reduced toxicity more effective in the in-vivo studies compared to conventional liposomes and the free drugs [100]. Among the very many surface labeling ligands used on the nanoparticles to decorate in order to attract their corresponding receptors to target amastigote on the macrophage including D-Mannose, and p-aminophenyl-α-D-Mannopyranoside along with other molecules and occupy the front row [101]. Tiwari et al. and Mareti et al. studied the Mannose functionalized carriers for Mycobacterium tuberculosis (Mtb) [102, 103]. Compared to Rifampicin-loaded unmodified nanostructured lipid carriers (REP-NLCs) those modified with cationic mannosylated (RFP-Man-NLCs) were showing a significant increase in uptake in alveolar macrophages resulting in no inflammatory responses, safer formulation strategies and reduced toxicity [104].
Improved internalization of macrophages and biocompatibility were verified in the Mannosylated solid lipid nanoparticles (M-SLNs) due to mannosylation and the results showed promising as well as safe for Tuberculosis therapy [105]. Rifampicin-loaded mannosylated and Pegylated graphene oxide (Rif@GO-Peg-MAN) showed significantly enhanced killing of intracellular macrophages infected by BCG (Bacille Calmette-Guerin) and Mtb bacilli and pharmacokinetics thereby increasing the efficacy of the drug [106]. As per the study conducted by Shrivastava et al. [107] in Balb/C mice was maximum in the case of mannosylated liposomes for the combination of anti-tuberculosis drugs, Isoniazid [INH] and Rifampicin [RIF].
The large dose and adverse effect which was accompanied by the repurposed drug for Mycobacterium tuberculosis Linezolid was solved by loading the drug with mannosylated gelatin nanoparticles and directly targeting alveolar macrophages [108]. Mannose receptors are promising targets for anticancer drug development as they have a low mutation tendency and their endocytic efficiency is high. It is discussed in detail how Mannose and Mannose-6-phosphate-targeted delivery systems in cancer therapy have and their broad applications in direct treatments or by gene therapy and also in immunotherapy and diagnostics purposes [109].
Hong Liang et al. study say for the delivery of anti-atherosclerotic agents will be more effective by Mannose functionalized dendrimeric nanoparticles to target the plaque-associated macrophages directly [110]. Ke et al. suggested carbamate–Mannose modified nano complexes may provide a promising approach for eliminating cancer stem cells to prevent cancer relapses and metathesis [111]. To study mannosylation strategies and the potential of Mannose-targeted drug/antigen delivery systems for vaccination and treatment of diseases localized in macrophages and other antigen-presenting cells, Irache et al. have compiled reports stating that a deeper understanding of the structure–activity relationship is necessary [112]. Yin et al. have used biodegradable and biocompatible polyesters to develop a micellar system which/that can effectively encapsulate the anticancer drug Doxorubicin and the Mannose moiety facilitates the active targeting of the cancer cells that are expressed on the Mannose receptors [113]. Mannose and Mannose-6-phosphate receptors have a potential role in theragnostic purposes like immune-based and gene therapies as high endocytic efficiency, and a low tendency to mutate are two imperative qualities to select potential candidates for targeted delivery systems in cancer therapy and they are used in direct treatments with conventional chemotherapeutics or via gene therapy against cancer cells [24].
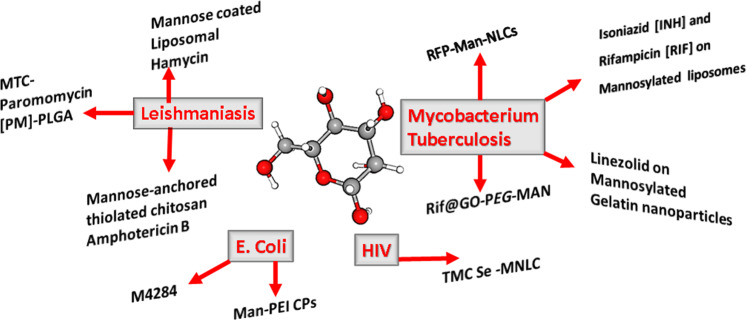
Hu et al. have reviewed the recent advances in biomedical applications to induce cancer cell death how Mannose-based nanomaterials have been used to modulate the tumor microenvironment, stimulate an immune response, and block bacterial adhesion designed for the treatment and prevention of cancer, and several infectious diseases [114]. Mannosylation makes a promising carrier for Etravirine (TMC) together with Selenium nanoparticles, to target the HIV reservoirs [115] and Mannose-modification found to be effective in Fim H antagonist against Escherichia coli as well [116, 117]. The important Mannose decorated drug delivery systems in different infectious diseases are depicted in Fig. 7.
Fig. 7.
Mannose decorated drug delivery systems in different infectious diseases
The major findings in 2021 are a Mannose-decorated chitosan-functionalized graphene oxide carrier loaded with ulvan demonstrated a promising targeted drug delivery system to treat in vitro glioblastoma by Kesavan et al. [118] and Yu et al. [119] claim they could prepare a Mannose-modified liposome drug delivery system that can overcome the poor in vivo stability and low uptake by Dendric Cells (DCs), especially for cancer immunotherapy. The important ones are depicted in Fig. 6.
Mannose in immune regulation
The most crucial stage of the innate immune system’s identification and eradication of foreign invaders is the inflammatory response. The coordinated action of numerous components, including neutrophils and macrophages of the innate immune system, T lymphocytes of the adaptive immune system, vascular endothelium, and smooth muscle cells, is required to complete the inflammatory response from the point of commencement through resolution. Local immune cells identify the harmful substance for the first time during the beginning phase. Endothelial cell activation, clonal growth, proliferation, and the attraction of more immune cells to the injury site follow. The elimination of immune cells that have been activated, the build-up of immune cells with memory, and the restoration of homeostasis all take place during the resolution phase. However, immune-cell hyperactivation’s life-threatening cytokine storm wreaks havoc. However, the life-threatening cytokine storm associated with immune-cell hyperactivation creates havoc very frequently. The role of Mannose-glycan during all these consequences could be considered. In atopic dermatitis, D-Mannose was observed regulating the inflammation in keratinocytes in mice models [120].
During the initiation phase, inherent glycan-binding proteins (GBPs) are typically involved in cell–cell interactions and the recognition of extracellular molecules, apart from the recognition of glycans on the same cell. GBPs recognize and bind specific sequences of glycans to facilitate various cellular processes based on the glycomic profile. The Mannose-binding lectin (MBL) is a GBP, plays a central role in human innate immunity, Dommett et al. [121] targets the terminal Mannose glycans of microorganisms, triggering the opsonophagocytosis and other activities such as activation of the complement pathway and inflammation. Extrinsic GBPs consist of the majority of pathogenic microbial toxins, adhesins, or agglutinins and a few mediate symbiotic relationships also. Furthermore, the complex situation arises from the fact that some microbial pathogens are engaged with “molecular mimicry,” sidestepping immune reactions by covering themselves with glycans typical of their hosts. The entry and exit of Mannose to the cell and its involvement in glycan synthesis have been explained in a number of reports in detail. The level of inflammation depends on the metabolic activities of the immune cells. During the glycolytic switch, glycolysis supplies metabolic intermediates for other biosynthetic pathways necessary for cellular growth and differentiation. In addition to glycolysis, the pentose phosphate pathway (PPP), the hexosamine pathway, and the glutaminolysis are increased upon activation. The cellular levels of metabolites resulting from this metabolic state determine the activation/repression of signaling pathways, the epigenetic and post-transcriptional regulation of inflammatory genes, and the post-translational modification of proteins. According to Gonzalez et al. [6], increased level of Mannose in the form of Mannose-6-phosphate is responsible for the suppression of enzymes of glucose metabolism like glucose phosphate isomerase (GPI), impairing all the glycolytic pathways such as tricarboxylic acid cycle, pentose phosphate pathway, glycan synthesis, etc.
Glucose not only fuels the pentose phosphate pathway, necessary for the maintenance of redox homeostasis during the inflammatory response by promoting the proliferation of proinflammatory macrophages, it also maintains an abundant level of the nonessential amino acid serine that supports LPS-induced IL-1β production during its continuous synthesis. Regular glycolysis process keeps the succinate level at its optimum which also promotes IL-1β production.
In 2017, Zhang et al. discovered the immune-regulatory effect of D-Mannose on T cells in both preventive and therapeutic models of type I diabetes and lung airway inflammation [19]. It was shown that the stimulatory effect of D-Mannose on Treg cell differentiation works in both human and mouse cells. TGF-β activation has been suggested to be the main cause for such stimulation associated with the upregulation of integrin αvβ8 and reactive oxygen species [19]. The D-Mannose-induced proliferation of regulatory T cells and gut microbiota-dependent anti-inflammatory effects have been considered to be the main cause of attenuated bone loss in senile mice as reported by Liu et al. [122]. Tregs induction by D-Mannose has been found to suppress liver damage through reduced inflammatory response in mice [123]. It has been reported that glycolysis inhibition can provide protection to a variety of cells and mouse models from immunopathological conditions.
It is assumed that as the concentration of Mannose in human blood is one-hundredth of that of glucose, it might not have any role in cell biogenesis. However, cancer cells with low MPI levels are supposed to have impaired glucose metabolism due to accumulated levels of Mannose-6-phosphate. Thus, inhibition of glucose metabolism associated with low succinate results in impaired HIF-1α activation. Extensive work accomplished by Torretta et al. demonstrated that LPS-induced Il1b expression was reduced as Mannose is suggested to decrease glucose catabolism in macrophages and impairs succinate-mediated HIF-1α activation [16].
It was suggested that oxidative burst-mediated bactericidal activity could be reduced in presence of excess levels of D-Mannose. Oxygen consumption is increased in the stimulated human neutrophils (PMNs) to enhance the secretion of reactive oxygen species causing inflammation. PMN metabolism was inhibited by D-Mannose in a study involving lectin-mediated bacterial adherence. It was also observed that 100 mM Mannose inhibited oxygen consumption by 82%, and superoxide generation by 84% of these cells after extensive experiments [124].
Conclusion
Mannose is essential in human metabolism, suppresses autoimmune and inflammatory diseases to mention like Type I diabetes, asthma, colitis, osteoarthritis, chronic graft-versus-host disease, lupus, and relieve intestinal cystic pain, used to treat urinary tract infections in the fields of treatment of pancreatic fistula, improving the ability of MRI for acute pancreatitis and to prevent obesity, in the treatment of acute lung injury in rats. It has an important role in the targeted delivery of antileishmanial, antituberculosis antibacterial, antiviral, and also antitumor drugs. Due to the development of system pharmacology the traditional drug discovery approach based on the “one drug, one target, one disease” concept has become a myth. New insight into drug discovery and development based on drug target interaction could not only pave the way for natural products to be considered as drugs but also partially eliminated the threat of off-target toxicity or unintended beneficial effects of a newly predicted drug. Drug–target interactions (DTIs) data of D-Mannose collected from databases may not be quantitative at present.
In the light of increased drug resistance cases and immunodeficiencies, we need to be equipped with alternatives like nutraceuticals and maintain health rather than treating for frequent disease states. Intestinal flora is very important in health and antibiotics have been revealed to cause unfavorable alterations and saccharide probiotics may help to fight this problem. Diseases like Tuberculosis and Leishmaniasis which need a time period for cure can be efficiently managed by nutraceutical combinations along with existing drugs and the intelligent engineering techniques that nanotechnology provides rather than putting them under neglected tropical diseases. E. coli is a threat for antimicrobial resistance (AMR) causing recurrent UTI, especially in women along with Mycobacterium tuberculosis and D-Mannose comes as a hope for the management of these diseases. As per the World Health Organisation, drug-resistant diseases are already responsible for seven lakh deaths worldwide each year, including 230,000 people who die from multidrug-resistant tuberculosis. It’s high time we need to change these statistics by alternative natural molecules like Mannose and develop smart and strategic treatment modalities like that we have seen during the SARS-COV-2 pandemic and in this era of artificial intelligence and machine learning along with network pharmacology [125–127]. The Mannose targets and connected diseases are having a significant role in pathogenesis and immune regulation and should be carefully studied for polypharmacology/promiscuity concerns before we figure out the new role. We look forward to many more successful roles of D-Mannose which is recently gaining momentum in cancerous tumor treatment research and thereby taking the central stage in medicinal research.
Acknowledgements
D. Sruthi acknowledges the Department of Health Research (DHR), Government of India, New Delhi, for her award of Young scientist-HRD Scheme (YSS/2019/000035/PRCYSS). Rest all authors acknowledge the opportunity given by the respective institute to complete this review.
Abbreviation
- MTX-Man NPs
Methotrexate loaded into nanoparticles modified with Mannose
- BTM-NMs
3-arm distyryl BODIPY derivative coupled with Mannose units and Tween 80 nanomicelles
- PDT
photodynamic treatment
- MSN-M6C-Man
dimannoside-carboxylate on mesoporous silica nanoparticles
- MHC - I
major histocompatibility complex class I
- APC
antigen-presenting cell
- (NP-R@M),
nanoparticles made of poly (d,l-lactide and glycolic acid) that contain imiquimod (R837)
- PLA-b-(PTA-g-Mannose)
poly (Lac- O-carboxyanhydride)-b-(poly(Tyr(alkynyl)-OCA)-g-Mannose)
- GO–CH–Ma
D-Mannose-mediated the chitosan-functionalized graphene oxide
- rUTIs
recurrent urinary tract infections
- AUC
acute uncomplicated cystitis
- UPEC
uropathogenic Escherichia coli
- MS
multiple sclerosis
- ADMET
absorption, distribution, metabolism, excretion, and toxicity
- CDG-lb
congenital disorder of glycosylation-1b
- IEF
isoelectric focusing
- RIF
rifampicin
- INH
isoniazid
- mCNPs
mannose-conjugated chitosan nanoparticles
- AmpB
amphotericin B
- MRC1
mannose receptor C-type-1
- LmSd
Leishmania major Seidman
- (MTC) - [PM]- PLGA
mannosylated thiolated chitosan coated Paromomycin-loaded Poly[lactide-co-glycolide]
- REP-NLCs
rifampicin-loaded unmodified nanostructured lipid carriers
- RFP-Man-NLCs
rifampicin-loaded modified with cationic mannosylated nanostructured lipid carriers
- Mtb
mycobacterium tuberculosis
- M-SLNs
mannosylated solid lipid nanoparticles
- Rif@GO-Peg-MAN
rifampicin-loaded mannosylated and Pegylated graphene oxide
- BCG
Bacille Calmette-Guerin
- TMC
tetramethyl chitosan
- SeNPs
selenium nanoparticles
- DCs
dendric cells
- GBPs
glycan binding proteins
- MBL
mannose-binding lectin
- PPP
pentose phosphate pathway
- GPI
glucose phosphate isomerase
- LPS
lipopolysaccharide
- IL-1β
interleukin-1β
- TGF-β
transforming growth factor beta
- MPI
mannose-6-phosphate isomerase
- HIF-1α
hypoxia-inducible factor 1-alpha
- PMNs
polymorphonuclear neutrophils
- AMR
anti-microbial resistance
- DTI
drug-target interactions
Author contributions
Conception and design by MD. All authors have contributed equally, read, and approved the final manuscript.
Data availability
Publicly available.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper.
Consent to publish
All authors hereby agree to give their consent for publication.
Ethical approval
This manuscript does not contain any studies with human or animal participants performed by any of the authors.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sushma Dave, Email: drsushmadave@gmail.com.
Jayashankar Das, Email: dasjayashankar@gmail.com.
References
- 1.Takahashi M, Kuroki Y, Ohtsubo K, Taniguchi N. Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: their functions and target proteins. Carbohydr Res. 2009;344:1387–90. doi: 10.1016/j.carres.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Ofek I, Kahane I, Sharon N. Toward anti-adhesion therapy for microbial diseases. Trends Microbiol. 1996;4:297–9. doi: 10.1016/0966-842X(96)30023-1. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Zhang W, Mu W. Recent studies on the biological production of D-mannose. Appl Microbiol Biotechnol. 2019;103:8753–61.. doi: 10.1007/s00253-019-10151-3. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Chen Z, Zhang W, Zhang T, Mu W. D-lyxose isomerase and its application for functional sugar production. Appl Microbiol Biotechnol. 2018;102:2051–62.. doi: 10.1007/s00253-018-8746-6. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B. D‐Mannose: properties, production, and applications: an overview. Compr Rev Food Sci Food Saf. 2016;15:773–85. doi: 10.1111/1541-4337.12211. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez PS, O’Prey J, Cardaci S, Barthet VJ, Sakamaki JI, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018;563:719–23.. doi: 10.1038/s41586-018-0729-3. [DOI] [PubMed] [Google Scholar]
- 7.Chen FE, Zhao JF, Xiong FJ, Xie B, Zhang P. An improved synthesis of a key intermediate for (+)-biotin from d-mannose. Carbohydr Res. 2007;342:2461–4. doi: 10.1016/j.carres.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Kamel MM, Ali HI, Anwar MM, Mohamed NA, Soliman AM. Synthesis, antitumor activity and molecular docking study of novel Sulfonamide-Schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur J Med Chem. 2010;45:572–80. doi: 10.1016/j.ejmech.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Alton G, Hasilik M, Niehues R, Panneerselvam K, Etchison JR, Fana F, et al. Direct utilization of mannose for mammalian glycoprotein biosynthesis. Glycobiology. 1998;8:285–95. doi: 10.1093/glycob/8.3.285. [DOI] [PubMed] [Google Scholar]
- 10.Narayanaswamy R, Kanagesan S, Pandurangan A, Padmanabhan P. Basics to different imaging techniques, different nanobiomaterials for image enhancement. In: Nanobiomaterials in medical imaging. William Andrew Publishing. 2016. p. 101–129.
- 11.Michaels EK, Chmiel JS, Plotkin BJ, Schaeffer AJ. Effect of D-mannose and D-glucose on Escherichia coli bacteriuria in rats. Urol Res. 1983;11:97–102. doi: 10.1007/BF00256954. [DOI] [PubMed] [Google Scholar]
- 12.Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32:79–84. doi: 10.1007/s00345-013-1091-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu XL, Xie QM, Shen YH, Jiang JJ, Chen YY, Yao HY, et al. Mannose prevents lipopolysaccharide-induced acute lung injury in rats. Inflamm Res. 2008;57:104–10. doi: 10.1007/s00011-007-7037-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. 2017;23:1036–45.. doi: 10.1038/nm.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma V, Smolin J, Nayak J, Ayala JE, Scott DA, Peterson SN, et al. Mannose alters gut microbiome, prevents diet-induced obesity, and improves host metabolism. Cell Rep. 2018;24:3087–98.. doi: 10.1016/j.celrep.2018.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-Mannose suppresses macrophage IL-1β production. Nat Commun. 2020;11:1–2. doi: 10.1038/s41467-020-20164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z, Miao J, Zhang T, He M, Zhou X, Zhang H, et al. d-Mannose suppresses osteoarthritis development in vivo and delays IL-1β-induced degeneration in vitro by enhancing autophagy activated via the AMPK pathway. Biomedicine Pharmacother. 2021;135:111199. doi: 10.1016/j.biopha.2020.111199. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Teng X, Abboud G, Li W, Ye S, Morel L. D-mannose ameliorates autoimmune phenotypes in mouse models of lupus. BMC Immunol. 2021;22:1–2. doi: 10.1186/s12865-020-00392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. 2017;23:1036–45.. doi: 10.1038/nm.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Yang Y, Dong W, Lin M, He J, Zhang X, et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci. 2022;119:e2114851119. doi: 10.1073/pnas.2114851119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W, Tong A. Mannose treatment: a promising novel strategy to suppress inflammation. Front Immunol. 2021:3954. [DOI] [PMC free article] [PubMed]
- 22.Nan F, Sun Y, Liang H, Zhou J, Ma X, Zhang D. Mannose: a sweet option in the treatment of cancer and inflammation. Front Pharmacol. 2022:1825. [DOI] [PMC free article] [PubMed]
- 23.Wei Z, Huang L, Cui L, Zhu X. Mannose: Good player and assister in pharmacotherapy. Biomed Pharmacother. 2020;129:110420. doi: 10.1016/j.biopha.2020.110420. [DOI] [PubMed] [Google Scholar]
- 24.Dalle Vedove E, Costabile G, Merkel OM. Mannose and Mannose‐6‐phosphate receptor–targeted drug delivery systems and their application in cancer therapy. Adv Healthc Mater. 2018;7:1701398. doi: 10.1002/adhm.201701398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold A, Lewis DH. Mannose and green plants: occurrence, physiology and metabolism, and use as a tool to study the role of orthophosphate. N Phytol. 1977;79:1–40. doi: 10.1111/j.1469-8137.1977.tb02178.x. [DOI] [Google Scholar]
- 26.Shintani T. Food industrial production of monosaccharides using microbial, enzymatic, and chemical methods. Fermentation. 2019;5:47. doi: 10.3390/fermentation5020047. [DOI] [Google Scholar]
- 27.Falcone G, Nickerson WJ. Cell-wall mannan-protein of baker’s yeast. Science. 1956;124:272–3. doi: 10.1126/science.124.3215.272.b. [DOI] [PubMed] [Google Scholar]
- 28.Bardalaye PC, Nordin JH. Chemical structure of the galactomannan from the cell wall of Aspergillus niger. J Biol Chem. 1977;252:2584–91.. doi: 10.1016/S0021-9258(17)40498-4. [DOI] [PubMed] [Google Scholar]
- 29.Hua X, Li Y, Jiang Z, Ma J, Liu H, Yan Q. Biochemical properties of a novel D-Mannose isomerase from Pseudomonas syringae for D-Mannose production. Appl Biochem Biotechnol. 2021;193:1482–95.. doi: 10.1007/s12010-021-03487-y. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Ichikawa M, Freeze HH. Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun. 2014;453:220–8. doi: 10.1016/j.bbrc.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart RA, Carrico CK, Webster RL, Steinhardt RG. Physicochemical stereospecificity in taste perception of α-d-mannose and β-d-mannose. Nature. 1971;234:220. doi: 10.1038/234220a0. [DOI] [PubMed] [Google Scholar]
- 32.Steinhardt RG, Jr, Calvin AD, Dodd EA. Taste-structure correlation with α-D-mannose and β-D-mannose. Science. 1962;135:367–8. doi: 10.1126/science.135.3501.367. [DOI] [PubMed] [Google Scholar]
- 33.Bhuiyan SH, Itami Y, Izumori K. Immobilization of L-rhamnose isomerase and its application in L-mannose production from L-fructose. J Ferment Bioeng. 1997;84:558–62.. doi: 10.1016/S0922-338X(97)81912-5. [DOI] [Google Scholar]
- 34.Izydorczyk M. Understanding the chemistry of food carbohydrates. CRC Press: Boca Raton, FL, USA; 2005.
- 35.Rakhila M, Jinuraj KR, Dhanalakshmi M, Reshmi D, Manuel AT, Jaleel U. A decision-making component in cyclisation of mannose derivatives—a computational approach. IJRPC. 2018;8:217–31. [Google Scholar]
- 36.Imberty A, Varrot A. Microbial recognition of human cell surface glycoconjugates. Curr Opin Struct Biol. 2008;18:567–76. doi: 10.1016/j.sbi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Williams SJ, Stick RV. Carbohydrates: the essential molecules of life. Ed. 2. Elsevier Science; 2009.
- 38.Zhang T, Pan Z, Qian C, Chen X. Isolation and purification of D-mannose from palm kernel. Carbohydr Res. 2009;344:1687–9. doi: 10.1016/j.carres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Monteiro AF, Miguez IS, Silva JP, Silva AS. High concentration and yield production of mannose from açaí (Euterpe oleracea Mart.) seeds via mannanase-catalyzed hydrolysis. Sci Rep. 2019;9:1–2. doi: 10.1038/s41598-019-47401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shallenberger RS, Birch GG. Sugar chemistry. AVI Publishing Co., Inc.; 1975.
- 41.Hu H, Liu S, Zhang W, An J, Xia H. Efficient epimerization of glucose to mannose over molybdenum‐based catalyst in aqueous media. ChemistrySelect. 2020;5:1728–33.. doi: 10.1002/slct.201903417. [DOI] [Google Scholar]
- 42.Zhang W, Zhang C, Liang G. Study on improving the yield of D-mannose by differential isomerization of glucose (in Chinese) Technol Dev Chem Ind. 2017;46:18–21. [Google Scholar]
- 43.Park CS, Kim JE, Choi JG, Oh DK. Characterization of a recombinant cellobiose 2-epimerase from Caldicellulosiruptor saccharolyticus and its application in the production of mannose from glucose. Appl Microbiol Biotechnol. 2011;92:1187–96. doi: 10.1007/s00253-011-3403-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Huang J, Jia M, Guang C, Zhang T, Mu W. Characterization of a novel D-lyxose isomerase from Thermoflavimicrobium dichotomicum and its application for D-mannose production. Process Biochem. 2019;83:131–6. doi: 10.1016/j.procbio.2019.05.007. [DOI] [Google Scholar]
- 45.Hirose J, Kinoshita Y, Fukuyama S, Hayashi S, Yokoi H, Takasaki Y. Continuous isomerization of D-fructose to D-mannose by immobilized Agrobacterium radiobacter cells. Biotechnol Lett. 2003;25:349–52. doi: 10.1023/A:1022301725817. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Chen M, Guang C, Zhang W, Mu W. Characterization of a recombinant D-mannose-producing D-lyxose isomerase from Caldanaerobius polysaccharolyticus. Enzym Microb Technol. 2020;138:109553. doi: 10.1016/j.enzmictec.2020.109553. [DOI] [PubMed] [Google Scholar]
- 47.Sharma V, Freeze HH. Mannose efflux from the cells: a potential source of mannose in blood. J Biol Chem. 2011;286:10193–200. doi: 10.1074/jbc.M110.194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaeken J, Carchon H. The carbohydrate-deficient glycoprotein syndromes: an overview. J Inherit Metab Dis. 1993;16:813–20. doi: 10.1007/BF00714272. [DOI] [PubMed] [Google Scholar]
- 49.Stibler H, Jaeken J, Kristiansson B. Biochemical characteristics and diagnosis of the carbohydrate‐deficient glycoprotein syndrome. Acta Pædiatr. 1991;80:21–31. doi: 10.1111/j.1651-2227.1991.tb12025.x. [DOI] [Google Scholar]
- 50.Jaeken J, Hagberg B, Strømme P. Clinical presentation and natural course of the carbohydrate‐deficient glycoprotein syndrome. Acta Pædiatr. 1991;80:6–13. doi: 10.1111/j.1651-2227.1991.tb12023.x. [DOI] [Google Scholar]
- 51.Matthijs G, Schollen E, Pardon E, Veiga‐Da‐Cunha M, Jaeken J, Cassiman JJ, et al. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13, in carbohydrate‐deficient glycoprotein type I syndrome (Jaeken syndrome) Nat Genet. 1997;16:88–92. doi: 10.1038/ng0597-88. [DOI] [PubMed] [Google Scholar]
- 52.Jaeken J, Matthijs G, Saudubray JM, Dionisi-Vici C, Bertini E, de Lonlay P, et al. Phosphomannose isomerase deficiency: a carbohydrate-deficient glycoprotein syndrome with hepatic-intestinal presentation. Am J Hum Genet. 1998;62:1535. doi: 10.1086/301873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ondruskova N, Cechova A, Hansikova H, Honzik T, Jaeken J. Congenital disorders of glycosylation: Still “hot” in 2020. Biochim Biophys Acta-Gen Subj. 2021;1865:129751. doi: 10.1016/j.bbagen.2020.129751. [DOI] [PubMed] [Google Scholar]
- 54.Blau N, Duran M, Gibson KM, Dionisi-Vici C. editors. Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Berlin/Heidelberg: Springer; 2014.
- 55.Niehues R, Hasilik M, Alton G, Körner C, Schiebe-Sukumar M, Koch HG, et al. Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J Clin Investig. 1998;101:1414–20. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lonlay P, Cuer M, Vuillaumier-Barrot S, Beaune G, Castelnau P, Kretz M, et al. Hyperinsulinemic hypoglycemia as a presenting sign in phosphomannose isomerase deficiency: a new manifestation of carbohydrate-deficient glycoprotein syndrome treatable with mannose. J Pediatr. 1999;135:379–83.. doi: 10.1016/S0022-3476(99)70139-3. [DOI] [PubMed] [Google Scholar]
- 57.Babovic-Vuksanovic D, Patterson MC, Schwenk WF, O’Brien JF, Vockley J, Freeze HH, et al. Severe hypoglycemia as a presenting symptom of carbohydrate-deficient glycoprotein syndrome. J Pediatr. 1999;135:775–81. doi: 10.1016/S0022-3476(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 58.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta (BBA)-Gen Subj. 2001;1528:116–26. doi: 10.1016/S0304-4165(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 59.Sharma V, Nayak J, DeRossi C, Charbono A, Ichikawa M, Ng BG, et al. Mannose supplements induce embryonic lethality and blindness in phosphoMannose isomerase hypomorphic mice. FASEB J. 2014;28:1854–69. doi: 10.1096/fj.13-245514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panneerselvam K, Freeze HH. Mannose enters mammalian cells using a specific transporter that is insensitive to glucose (∗) J Biol Chem. 1996;271:9417–21. doi: 10.1074/jbc.271.16.9417. [DOI] [PubMed] [Google Scholar]
- 61.Alton G, Kjaergaard S, Etchison JR, Skovby F, Freeze HH. Oral ingestion of mannose elevates blood mannose levels: a first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem Mol Med. 1997;60:127–33. doi: 10.1006/bmme.1997.2574. [DOI] [PubMed] [Google Scholar]
- 62.Panneerselvam K, Freeze HH. Mannose corrects altered N-glycosylation in carbohydrate-deficient glycoprotein syndrome fibroblasts. J Clin Investig. 1996;97:1478–87. doi: 10.1172/JCI118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panneerselvam K, Etchison JR, Freeze HH. Human fibroblasts prefer mannose over glucose as a source of mannose for N-glycosylation: evidence for the functional importance of transported mannose. J Biol Chem. 1997;272:23123–9. doi: 10.1074/jbc.272.37.23123. [DOI] [PubMed] [Google Scholar]
- 64.Panneerselvam K, Etchison JR, Skovby F, Freeze HH. Abnormal metabolism of mannose in families with carbohydrate-deficient glycoprotein syndrome type 1. Biochem Mol Med. 1997;61:161–7. doi: 10.1006/bmme.1997.2599. [DOI] [PubMed] [Google Scholar]
- 65.Kjaergaard S, Kristiansson B, Stibler H, Freeze HH, Schwartz M, Martinsson T, et al. Failure of short‐term mannose therapy of patients with carbohydrate‐deficient glycoprotein syndrome type 1A. Acta Paediatr. 1998;87:884–8. doi: 10.1111/j.1651-2227.1998.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 66.Chui D, Oh-Eda M, Liao YF, Panneerselvam K, Lal A, Marek KW, et al. Alpha-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–67.. doi: 10.1016/S0092-8674(00)80322-0. [DOI] [PubMed] [Google Scholar]
- 67.Freeze HH. Human glycosylation disorders and sugar supplement therapy. Biochem Biophy Res Commun. 1999;255:189–93. doi: 10.1006/bbrc.1998.9945. [DOI] [PubMed] [Google Scholar]
- 68.Rush JS, Panneerselvam K, Waechter CJ, Freeze HH. Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with Congenital Disorders of Glycosylation (CDG) Glycobiology. 2000;10:829–35. doi: 10.1093/glycob/10.8.829. [DOI] [PubMed] [Google Scholar]
- 69.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta (BBA)-Gen Subj. 2001;1528:116–26. doi: 10.1016/S0304-4165(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 70.Westphal V, Kjaergaard S, Davis JA, Peterson SM, Skovby F, Freeze HH. Genetic and metabolic analysis of the first adult with congenital disorder of glycosylation type Ib: long-term outcome and effects of mannose supplementation. Mol Genet Metab. 2001;73:77–85. doi: 10.1006/mgme.2001.3161. [DOI] [PubMed] [Google Scholar]
- 71.Freeze HH. Sweet solution: sugars to the rescue. J Cell Biol. 2002;158:615. doi: 10.1083/jcb.200207155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujita N, Tamura A, Higashidani A, Tonozuka T, Freeze HH, Nishikawa A. The relative contribution of mannose salvage pathways to glycosylation in PMI‐deficient mouse embryonic fibroblast cells. FEBS J. 2008;275:788–98. doi: 10.1111/j.1742-4658.2008.06246.x. [DOI] [PubMed] [Google Scholar]
- 73.Higashidani A, Bode L, Nishikawa A, Freeze HH. Exogenous mannose does not raise steady-state mannose-6-phosphate pools of normal or N-glycosylation-deficient human fibroblasts. Mol Genet Metab. 2009;96:268–72. doi: 10.1016/j.ymgme.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu J, Mir A, Gao N, Rosa S, Monson C, Sharma V, et al. A zebrafish model of congenital disorders of glycosylation with phosphomannose isomerase deficiency reveals an early opportunity for corrective mannose supplementation. Dis Models Mech. 2013;6:95–105. doi: 10.1242/dmm.010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ichikawa M, Scott DA, Losfeld ME, Freeze HH. The metabolic origins of mannose in glycoproteins. J Biol Chem. 2014;289:6751–61.. doi: 10.1074/jbc.M113.544064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nahar UJ, Toth I, Skwarczynski M. Mannose in vaccine delivery. J Controlled Release. 2022;351:284–300. doi: 10.1016/j.jconrel.2022.09.038. [DOI] [PubMed] [Google Scholar]
- 77.Saito Y, Kinoshita M, Yamada A, Kawano S, Liu HS, Kamimura S, et al. Mannose and phosphomannose isomerase regulate energy metabolism under glucose starvation in leukemia. Cancer Sci. 2021;112:4944. doi: 10.1111/cas.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballou CE, Lipke PN, Raschke WC. Structure and immunochemistry of the cell wall mannans from Saccharomyces chevalieri, Saccharomyces italicus, Saccharomyces diastaticus, and Saccharomyces carlsbergensis. J Bacteriol. 1974;117:461–7. doi: 10.1128/jb.117.2.461-467.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spencer JF, Gorin PA. Mannose‐containing polysaccharides of yeasts. Biotechnol Bioeng. 1973;15:1–2. doi: 10.1002/bit.260150102. [DOI] [PubMed] [Google Scholar]
- 80.Barber AE, Norton JP, Spivak AM, Mulvey MA. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57:719–24. doi: 10.1093/cid/cit284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scribano D, Sarshar M, Prezioso C, Lucarelli M, Angeloni A, Zagaglia C, et al. D-Mannose treatment neither affects uropathogenic Escherichia coli properties nor induces stable fimh modifications. Molecules. 2020;25:316. doi: 10.3390/molecules25020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagenlehner F, Lorenz H, Ewald O, Gerke P. Why d-Mannose may be as efficient as antibiotics in the treatment of acute uncomplicated lower urinary tract infections—preliminary considerations and conclusions from a non-interventional study. Antibiotics. 2022;11:314. doi: 10.3390/antibiotics11030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Domenici L, Monti M, Bracchi C, Giorgini M, Colagiovanni V, Muzii L, et al. D-mannose: a promising support for acute urinary tract infections in women. A pilot study. Eur Rev Med Pharm Sci. 2016;20:2920–5. [PubMed] [Google Scholar]
- 84.Phé V, Pakzad M, Haslam C, Gonzales G, Curtis C, Porter B, et al. Open label feasibility study evaluating D‐mannose combined with home‐based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol Urodyn. 2017;36:1770–5. doi: 10.1002/nau.23173. [DOI] [PubMed] [Google Scholar]
- 85.Porru D, Parmigiani A, Tinelli C, Barletta D, Choussos D, Di Franco C, et al. Oral D-Mannose in recurrent urinary tract infections in women: a pilot study. J Clin Urol. 2014;7:208–13. doi: 10.1177/2051415813518332. [DOI] [Google Scholar]
- 86.Parazzini F, Ricci E, Fedele F, Chiaffarino F, Esposito G, Cipriani S. Systematic review of the effect of D‑mannose with or without other drugs in the treatment of symptoms of urinary tract infections/cystitis. Biomed Rep. 2022;17:1–1. doi: 10.3892/br.2022.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Latif N, Bachhawat BK. The effect of surface sugars on liposomes in immunopotentiation. Immunol Lett. 1984;8:75–8. doi: 10.1016/0165-2478(84)90053-1. [DOI] [PubMed] [Google Scholar]
- 88.Chen F, Huang G, Huang H. Sugar ligand-mediated drug delivery. Fut Med Chem. 2020;12:161–71.. doi: 10.4155/fmc-2019-0114. [DOI] [PubMed] [Google Scholar]
- 89.Pei M, Xu R, Zhang C, Wang X, Li C, Hu Y. Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids Surf B: Biointerfaces. 2021;197:111378. doi: 10.1016/j.colsurfb.2020.111378. [DOI] [PubMed] [Google Scholar]
- 90.Sánchez A, Mejía SP, Orozco J. Recent advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules. 2020;25:3760. doi: 10.3390/molecules25163760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banerjee G, Bhaduri AN, BAsU MK. Mannose-coated liposomal hamycin in the treatment of experimental leishmaniasis in hamsters. Biochem Med Metab Biol. 1994;53:1–7. doi: 10.1006/bmmb.1994.1050. [DOI] [PubMed] [Google Scholar]
- 92.Chaubey P, Mishra B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr Polym. 2014;101:1101–8. doi: 10.1016/j.carbpol.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 93.de Carvalho Oliveira SS, Branquinha MH, E Cruz MD, dos Santos AL, Sangenito LS. Trendings of amphotericin B-loaded nanoparticles as valuable chemotherapeutic approaches against leishmaniasis. Appl Nanobiotechnol Negl Trop Dis. 2021:291–327.
- 94.Francis AP, Gurudevan S, Jayakrishnan A. Synthetic polymannose as a drug carrier: synthesis, toxicity and anti-fungal activity of polymannose-amphotericin B conjugates. J Biomater Sci, Polym Ed. 2018;29:1529–48.. doi: 10.1080/09205063.2018.1469186. [DOI] [PubMed] [Google Scholar]
- 95.Sohrabi Y, Lipoldová M. Mannose receptor and the mystery of nonhealing Leishmania major infection. Trends Parasitol. 2018;34:354–6. doi: 10.1016/j.pt.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 96.Martínez-López M, Soto M, Iborra S, Sancho D. Leishmania hijacks myeloid cells for immune escape. Front Microbiol. 2018;9:883. doi: 10.3389/fmicb.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Afzal I, Sarwar HS, Sohail MF, Varikuti S, Jahan S, Akhtar S, et al. Mannosylated thiolated paromomycin-loaded PLGA nanoparticles for the oral therapy of visceral leishmaniasis. Nanomedicine. 2019;14:387–406. doi: 10.2217/nnm-2018-0038. [DOI] [PubMed] [Google Scholar]
- 98.Vossen LI, Domínguez-Asenjo B, Gutiérrez-Corbo C, Pérez-Pertejo MY, Balaña-Fouce R, Reguera RM, et al. Mannose-decorated dendritic polyglycerol nanocarriers drive antiparasitic drugs to leishmania infantum-infected macrophages. Pharmaceutics. 2020;12:915. doi: 10.3390/pharmaceutics12100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soni A, Jain V, Jain SK, Khangar PK. Preparation and characterization of amphotericin B mannosylated liposomes for effective management of visceral leishmaniasis. J Drug Deliv Ther. 2021;11:113–8. doi: 10.22270/jddt.v11i5-S.5114. [DOI] [Google Scholar]
- 100.Prasanna P, Kumar P, Kumar S, Rajana VK, Kant V, Prasad SR, et al. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis—a review. Biomed Pharmacother. 2021;141:111920. doi: 10.1016/j.biopha.2021.111920. [DOI] [PubMed] [Google Scholar]
- 101.Jamshaid H, Khan GM. Nanotechnology based solutions for anti-leishmanial impediments: a detailed insight. J Nanobiotechnol. 2021;19:1–51.. doi: 10.1186/s12951-021-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiwari S, Chaturvedi AP, Tripathi YB, Mishra B. Macrophage-specific targeting of isoniazid through mannosylated gelatin microspheres. Aaps Pharmscitech. 2011;12:900–8. doi: 10.1208/s12249-011-9654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maretti E, Costantino L, Rustichelli C, Leo E, Croce MA, Buttini F, et al. Surface engineering of solid lipid nanoparticle assemblies by methyl α-d-mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int J Pharm. 2017;528:440–51.. doi: 10.1016/j.ijpharm.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 104.Song X, Lin Q, Guo L, Fu Y, Han J, Ke H, et al. Rifampicin loaded mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm Res. 2015;32:1741–51. doi: 10.1007/s11095-014-1572-3. [DOI] [PubMed] [Google Scholar]
- 105.Vieira AC, Chaves LL, Pinheiro M, Lima SA, Ferreira D, Sarmento B, et al. Mannosylated solid lipid nanoparticles for the selective delivery of rifampicin to macrophages. Artif Cells, Nanomed, Biotechnol. 2018;46:653–63. doi: 10.1080/21691401.2018.1434186. [DOI] [PubMed] [Google Scholar]
- 106.Pi J, Shen L, Shen H, Yang E, Wang W, Wang R, et al. Mannosylated graphene oxide as macrophage-targeted delivery system for enhanced intracellular M. tuberculosis killing efficiency. Mater Sci Eng: C. 2019;103:109777. doi: 10.1016/j.msec.2019.109777. [DOI] [PubMed] [Google Scholar]
- 107.Shrivastava P, Gautam L, Sharma R, Dube D, Vyas S, Vyas SP. Dual antitubercular drug loaded liposomes for macrophage targeting: development, characterisation, ex vivo and in vivo assessment. J Microencapsul. 2021;38:108–23.. doi: 10.1080/02652048.2020.1857861. [DOI] [PubMed] [Google Scholar]
- 108.Patil KD, Bagade SB, Bonde SC. Biodistribution, pharmacokinetics and toxicity evaluation of mannosylated gelatin nanoparticles of linezolid for anti-tubercular therapy. Mater Technol. 2022;37:95–103. doi: 10.1080/10667857.2020.1816021. [DOI] [Google Scholar]
- 109.Dalle Vedove E, Costabile G, Merkel OM. Mannose and mannose‐6‐phosphate receptor–targeted drug delivery systems and their application in cancer therapy. Adv Healthc Mater. 2018;7:1701398. doi: 10.1002/adhm.201701398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He H, Yuan Q, Bie J, Wallace RL, Yannie PJ, Wang J, et al. Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis. Transl Res. 2018;193:13–30. doi: 10.1016/j.trsl.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ke X, Yang C, Cheng W, Yang YY. Delivery of NF-κB shRNA using carbamate-mannose modified PEI for eliminating cancer stem cells. Nanomed: Nanotechnol Biol Med. 2018;14:405–14.. doi: 10.1016/j.nano.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 112.Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008;5:703–24. doi: 10.1517/17425247.5.6.703. [DOI] [PubMed] [Google Scholar]
- 113.Yin L, Chen Y, Zhang Z, Yin Q, Zheng N, Cheng J. Biodegradable micelles capable of mannose‐mediated targeted drug delivery to cancer cells. Macromol Rapid Commun. 2015;36:483–9. doi: 10.1002/marc.201400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu J, Wei P, Seeberger PH, Yin J. Mannose‐functionalized nanoscaffolds for targeted delivery in biomedical applications. Chem—Asian J. 2018;13:3448–59.. doi: 10.1002/asia.201801088. [DOI] [PubMed] [Google Scholar]
- 115.Rojekar S, Abadi LF, Pai R, Prajapati MK, Kulkarni S, Vavia PR. Mannose-anchored nano-selenium loaded nanostructured lipid carriers of etravirine for delivery to HIV reservoirs. AAPS PharmSciTech. 2022;23:1–23. doi: 10.1208/s12249-022-02377-8. [DOI] [PubMed] [Google Scholar]
- 116.Spaulding CN, Klein RD, Ruer S, Kau AL, Schreiber HL, Cusumano ZT, et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature. 2017;546:528–32.. doi: 10.1038/nature22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu M, Li J, Li B. Mannose-modificated polyethylenimine: a specific and effective antibacterial agent against Escherichia coli. Langmuir. 2018;34:1574–80.. doi: 10.1021/acs.langmuir.7b03556. [DOI] [PubMed] [Google Scholar]
- 118.Kesavan S, Meena KS, Sharmili SA, Govindarajan M, Alharbi NS, Kadaikunnan S, et al. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J Drug Deliv Sci Technol. 2021;65:102760. doi: 10.1016/j.jddst.2021.102760. [DOI] [Google Scholar]
- 119.Yu J, Wang S, Qi J, Yu Z, Xian Y, Liu W, et al. Mannose-modified liposome designed for epitope peptide drug delivery in cancer immunotherapy. Int Immunopharmacol. 2021;101:108148. doi: 10.1016/j.intimp.2021.108148. [DOI] [PubMed] [Google Scholar]
- 120.Luo J, Li Y, Zhai Y, Liu Y, Zeng J, Wang D, et al. D-Mannose ameliorates DNCB-induced atopic dermatitis in mice and TNF-α-induced inflammation in human keratinocytes via mTOR/NF-κB pathway. Int Immunopharmacol. 2022;113:109378. doi: 10.1016/j.intimp.2022.109378. [DOI] [PubMed] [Google Scholar]
- 121.Dommett RM, Klein N, Turner MW. Mannose‐binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu H, Gu R, Zhu Y, Lian X, Wang S, Liu X, et al. D-mannose attenuates bone loss in mice via Treg cell proliferation and gut microbiota-dependent anti-inflammatory effects. Ther Adv Chronic Dis. 2020;11:2040622320912661. doi: 10.1177/2040622320912661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito D, Ito H, Ideta T, Kanbe A, Shimizu M. d-mannose administration improves autoimmune hepatitis by upregulating regulatory T cells. Cell Immunol. 2022;375:104517. doi: 10.1016/j.cellimm.2022.104517. [DOI] [PubMed] [Google Scholar]
- 124.Rest RF, Farrell CF, Naids FL. Mannose inhibits the human neutrophil oxidative burst. J Leukoc Biol. 1988;43:158–64. doi: 10.1002/jlb.43.2.158. [DOI] [PubMed] [Google Scholar]
- 125.Dhanalakshmi M, Das K, Pandya M, Shah S, Gadnayak A, Dave S, et al. Artificial neural network-based study predicts GS-441524 as a potential inhibitor of SARS-CoV-2 activator protein furin: a polypharmacology approach. Appl Biochem Biotechnol. 2022;194:4511–29. doi: 10.1007/s12010-022-03928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pandya M, Shah S, Dhanalakshmi M, Juneja T, Patel A, Gadnayak A, et al. Unravelling Vitamin B12 as a potential inhibitor against SARS-CoV-2: a computational approach. Inform Med Unlocked. 2022;30:100951. doi: 10.1016/j.imu.2022.100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pinzi L, Tinivella A, Caporuscio F, Rastelli G. Drug repurposing and polypharmacology to fight SARS-CoV-2 through inhibition of the main protease. Front Pharmacol. 2021;12:636989. doi: 10.3389/fphar.2021.636989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fan Z, Wang Y, Xiang S, Zuo W, Huang D, Jiang B, et al. Dual-self-recognizing, stimulus-responsive and carrier-free methotrexate–mannose conjugate nanoparticles with highly synergistic chemotherapeutic effects. J Mater Chem B. 2020;8:1922–34.. doi: 10.1039/D0TB00049C. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Q, Cai Y, Li QY, Hao LN, Ma Z, Wang XJ, et al. Targeted delivery of a mannose‐conjugated BODIPY photosensitizer by nanomicelles for photodynamic breast cancer therapy. Chem–A Eur J. 2017;23:14307–15.. doi: 10.1002/chem.201702935. [DOI] [PubMed] [Google Scholar]
- 130.Bouffard E, Mauriello Jimenez C, El Cheikh K, Maynadier M, Basile I, Raehm L, et al. Efficient photodynamic therapy of prostate cancer cells through an improved targeting of the cation-independent mannose 6-phosphate receptor. Int J Mol Sci. 2019;20:2809. doi: 10.3390/ijms20112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–9. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 132.Yao Y, Liu W, Li J, Zhou M, Qu C, Wang K. MPI-based bioinformatic analysis and co-inhibitory therapy with mannose for oral squamous cell carcinoma. Med Oncol. 2021;38:1–1. doi: 10.1007/s12032-021-01552-4. [DOI] [PubMed] [Google Scholar]
- 133.Shtraizent N, DeRossi C, Nayar S, Sachidanandam R, Katz LS, Prince A, et al. MPI depletion enhances O-GlcNAcylation of p53 and suppresses the Warburg effect. Elife. 2017;6:e22477. doi: 10.7554/eLife.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeRossi C, Bambino K, Morrison J, Sakarin I, Villacorta‐Martin C, Zhang C, et al. Mannose phosphate isomerase and mannose regulate hepatic stellate cell activation and fibrosis in zebrafish and humans. Hepatology. 2019;70:2107–22.. doi: 10.1002/hep.30677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Y, Do DC, Ishmael FT, Squadrito ML, Tang HM, Tang HL, et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511-3p. J Allergy Clin Immunol. 2018;141:350–64.. doi: 10.1016/j.jaci.2017.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Zande HJ, Nitsche D, Schlautmann L, Guigas B, Burgdorf S. The Mannose receptor: from endocytic receptor and biomarker to regulator of (Meta) inflammation. Front Immunol. 2021;12:765034. doi: 10.3389/fimmu.2021.765034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsuchiya K, Suzuki Y, Yoshimura K, Yasui H, Karayama M, Hozumi H, et al. Macrophage mannose receptor CD206 predicts prognosis in community-acquired pneumonia. Sci Rep. 2019;9:1–0. doi: 10.1038/s41598-019-55289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miwa T, Kokuryo T, Yokoyama Y, Yamaguchi J, Nagino M. Therapeutic potential of targeting protein for Xklp2 silencing for pancreatic cancer. Cancer Med. 2015;4:1091–100. doi: 10.1002/cam4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang B, Zheng W, Fang L, Wu L, Zhou F, Yin X, et al. Overexpressed targeting protein for Xklp2 (TPX2) serves as a promising prognostic marker and therapeutic target for gastric cancer. Cancer Biol Ther. 2016;17:824–32. doi: 10.1080/15384047.2016.1195046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sunil Singh S, Chi Fai Cheung R, Ho Wong J, Bun Ng T. Mannose binding lectin: a potential biomarker for many human diseases. Curr Med Chem. 2016;23:3847–60.. doi: 10.2174/0929867323666160817162208. [DOI] [PubMed] [Google Scholar]
- 141.Kalia N, Singh J, Kaur M. The ambiguous role of mannose-binding lectin (MBL) in human immunity. Open Med. 2021;16:299–310. doi: 10.1515/med-2021-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Auriti C, Prencipe G, Moriondo M, Bersani I, Bertaina C, Mondì V et al. Mannose-binding lectin: biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically ill neonates. J Immunol Res. 2017;2017. [DOI] [PMC free article] [PubMed]
- 143.Zhang Y, Qian H, Xu A, Yang G. Increased expression of CD81 is associated with poor prognosis of prostate cancer and increases the progression of prostate cancer cells in vitro. Exp Ther Med. 2020;19:755–61. doi: 10.3892/etm.2019.8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vences-Catalán F, Duault C, Kuo CC, Rajapaksa R, Levy R, Levy S. CD81 as a tumor target. Biochem Soc Trans. 2017;45:531–5. doi: 10.1042/BST20160478. [DOI] [PubMed] [Google Scholar]
- 145.Vences-Catalán F, Kuo CC, Rajapaksa R, Duault C, Andor N, Czerwinski DK, et al. CD81 is a novel immunotherapeutic target for B cell lymphoma. J Exp Med. 2019;216:1497–508.. doi: 10.1084/jem.20190186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sokolowska A, Szala A, St Swierzko A, Kozinska M, Niemiec T, Blachnio M, et al. Mannan-binding lectin-associated serine protease-2 (MASP-2) deficiency in two patients with pulmonary tuberculosis and one healthy control. Cell Mol Immunol. 2015;12:119–21. doi: 10.1038/cmi.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Orsini F, Chrysanthou E, Dudler T, Cummings WJ, Takahashi M, Fujita T, et al. Mannan binding lectin-associated serine protease-2 (MASP-2) critically contributes to post-ischemic brain injury independent of MASP-1. J Neuroinflammation. 2016;13:1–3. doi: 10.1186/s12974-016-0684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Machida T, Sakamoto N, Ishida Y, Takahashi M, Fujita T, Sekine H. Essential roles for mannose-binding lectin-associated serine protease-1/3 in the development of lupus-like glomerulonephritis in MRL/lpr mice. Front Immunol. 2018;9:1191. doi: 10.3389/fimmu.2018.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kang C, Drayna D. A role for inherited metabolic deficits in persistent developmental stuttering. Mol Genet Metab. 2012;107:276–80. doi: 10.1016/j.ymgme.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kornfeld S. N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase (NAGPA). In: Handbook of glycosyltransferases and related genes. Second Edition. Springer: Japan; 2014. p. 1349–1358.
- 151.Yin Y, Lei S, Li L, Yang X, Yin Q, Xu T, et al. RPTOR methylation in the peripheral blood and breast cancer in the Chinese population. Genes Genom. 2022;44:435–43.. doi: 10.1007/s13258-021-01182-0. [DOI] [PubMed] [Google Scholar]
- 152.Samimi Z, Izadpanah A, Feizollahi P, Roghani SA, Assar S, Zafari P, et al. The association between the plasma sugar and lipid profile with the gene expression of the regulatory protein of mTOR (Raptor) in patients with rheumatoid arthritis. Immunol Investig. 2021;50:597–608. doi: 10.1080/08820139.2020.1781160. [DOI] [PubMed] [Google Scholar]
- 153.Hou B, Liu S, Li E, Jiang X. Different role of raptor and rictor in regulating rasfonin‐induced autophagy and apoptosis in renal carcinoma cells. Chem Biodivers. 2020;17:e2000743. doi: 10.1002/cbdv.202000743. [DOI] [PubMed] [Google Scholar]
- 154.Kondo S, Hirakawa H, Ikegami T, Uehara T, Agena S, Uezato J, et al. Raptor and rictor expression in patients with human papillomavirus-related oropharyngeal squamous cell carcinoma. BMC Cancer. 2021;21:1–4. doi: 10.1186/s12885-021-07794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gracia L, Lora G, Blair LJ, Jinwal UK. Therapeutic potential of the Hsp90/Cdc37 interaction in neurodegenerative diseases. Front Neurosci. 2019;13:1263. doi: 10.3389/fnins.2019.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Calderwood SK. Cdc37 as a co-chaperone to Hsp90. The Networking of Chaperones by Co-chaperones. 2015:103–12. [DOI] [PMC free article] [PubMed]
- 157.Farid AA, Afify NA, Alsharnoby AA, Abdelsameea E, Bedair HM. Predictive role of AURKA rs 1047972 gene polymorphism and the risk of development of hepatocellular carcinoma. Immunol Investig. 2022;51:1211–21.. doi: 10.1080/08820139.2021.1920609. [DOI] [PubMed] [Google Scholar]
- 158.Yang Y, Santos DM, Pantano L, Knipe R, Abe E, Logue A et al. Screening for inhibitors of YAP nuclear localization identifies aurora kinase a as a modulator of lung fibrosis. Am J Respir Cell Mol Biol. 2022;67. [DOI] [PMC free article] [PubMed]
- 159.Chen R, Zhou S, Fang C, Ye F, Chen J, Jiang P. Targeting AURKA by microRNA-490-3p suppresses gastric cancer cell growth. Histol Histopathol. 2022;37:397–404. doi: 10.14670/HH-18-415. [DOI] [PubMed] [Google Scholar]
- 160.Hong SY, Lu YC, Hsiao SH, Kao YR, Lee MH, Lin YP, et al. Stabilization of AURKA by the E3 ubiquitin ligase CBLC in lung adenocarcinoma. Oncogene. 2022;41:1907–17.. doi: 10.1038/s41388-022-02180-6. [DOI] [PubMed] [Google Scholar]
- 161.Ara A, Ahmed KA, Xiang J. Mannose-6-phosphate receptor: a novel regulator of T cell immunity. Cell Mol Immunol. 2018;15:986–8. doi: 10.1038/s41423-018-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dangaj D, Abbott KL, Mookerjee A, Zhao A, Kirby PS, Sandaltzopoulos R, et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. Plos One. 2011;6:e28386. doi: 10.1371/journal.pone.0028386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cardona-Maya W, Velilla PA, Montoya CJ, Cadavid Á, Rugeles MT. In vitro human immunodeficiency virus and sperm cell interaction mediated by the mannose receptor. J Reprod Immunol. 2011;92:1–7. doi: 10.1016/j.jri.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Golomingi M, Kohler J, Jenny L, Hardy ET, Dobó J, Gál P, et al. Complement lectin pathway components MBL and MASP-1 promote haemostasis upon vessel injury in a microvascular bleeding model. Front Immunol. 2022;13:948190. doi: 10.3389/fimmu.2022.948190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosbjerg A, Würzner R, Garred P, Skjoedt MO. Masp-1 and masp-3 bind directly to aspergillus fumigatus and promote complement activation and phagocytosis. J Innate Immun. 2021;13:211–24.. doi: 10.1159/000514546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choudhary K, Patel PK, Are VN, Makde RD, Hajela K. Mannose-binding lectin-associated serine protease-1 cleaves plasminogen and plasma fibronectin: prefers plasminogen over known fibrinogen substrate. Blood Coagul Fibrinolysis. 2021;32:504–12.. doi: 10.1097/MBC.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 167.Zhu ZC, Liu JW, Yang C, Li MJ, Wu RJ, Xiong ZQ. Targeting KPNB1 overcomes TRAIL resistance by regulating DR5, Mcl-1 and FLIP in glioblastoma cells. Cell Death Dis. 2019;10:1–6. doi: 10.1038/s41419-019-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y, Li KF. Karyopherin β1 deletion suppresses tumor growth and metastasis in colorectal cancer (CRC) by reducing MET expression. Biomed Pharmacother. 2019;120:109127. doi: 10.1016/j.biopha.2019.109127. [DOI] [PubMed] [Google Scholar]
- 169.Yang J, Guo Y, Lu C, Zhang R, Wang Y, Luo L, et al. Inhibition of Karyopherin beta 1 suppresses prostate cancer growth. Oncogene. 2019;38:4700–14.. doi: 10.1038/s41388-019-0745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cao Z, Wu W, Wei H, Zhang W, Huang Y, Dong Z. Downregulation of histone‑lysine N‑methyltransferase EZH2 inhibits cell viability and enhances chemosensitivity in lung cancer cells. Oncol Lett. 2021;21:1–1. doi: 10.3892/ol.2020.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li T, Yu C, Zhuang S. Histone methyltransferase EZH2: a potential therapeutic target for kidney diseases. Front Physiol. 2021;12:640700. doi: 10.3389/fphys.2021.640700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res/Fundamental Mol Mechanisms Mutagenesis. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 173.Groisberg R, Subbiah V. EZH2 inhibition for epithelioid sarcoma and follicular lymphoma. Lancet Oncol. 2020;21:1388–90.. doi: 10.1016/S1470-2045(20)30530-1. [DOI] [PubMed] [Google Scholar]
- 174.Liu PP, Xu YJ, Dai SK, Du HZ, Wang YY, Li XG, et al. Polycomb protein EED regulates neuronal differentiation through targeting SOX11 in hippocampal dentate gyrus. Stem Cell Rep. 2019;13:115–131. doi: 10.1016/j.stemcr.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oktyabri D, Tange S, Terashima M, Ishimura A, Suzuki T. EED regulates epithelial–mesenchymal transition of cancer cells induced by TGF-β. Biochem Biophys Res Commun. 2014;453:124–30. doi: 10.1016/j.bbrc.2014.09.082. [DOI] [PubMed] [Google Scholar]
- 176.Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum Pathol. 2018;81:9–17. doi: 10.1016/j.humpath.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 177.Peng W, Tang W, Li JD, He RQ, Luo JY, Chen ZX, et al. Downregulation of the enhancer of zeste homolog 1 transcriptional factor predicts poor prognosis of triple-negative breast cancer patients. PeerJ. 2022;10:e13708. doi: 10.7717/peerj.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wei X, Zhang K, Qin H, Zhu J, Qin Q, Yu Y, et al. GMDS knockdown impairs cell proliferation and survival in human lung adenocarcinoma. BMC Cancer. 2018;18:1–4. doi: 10.1186/s12885-018-4524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nakayama K, Moriwaki K, Imai T, Shinzaki S, Kamada Y, Murata K, et al. Mutation of GDP-mannose-4, 6-dehydratase in colorectal cancer metastasis. PLoS One. 2013;8:e70298. doi: 10.1371/journal.pone.0070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lamprou I, Kakouratos C, Tsolou A, Pavlidis P, Xanthopoulou ET, Nanos C, et al. Lipophagy-related protein Perilipin-3 and resistance of prostate cancer to radiation therapy. Int J Radiat Oncol* Biol* Phys. 2022;113:401–14.. doi: 10.1016/j.ijrobp.2022.01.033. [DOI] [PubMed] [Google Scholar]
- 181.Alghamdi A, Alhotti DZ, Sabico S, Al-Attas OS, Al-Daghri NM. Associations of perilipin 3 with insulin resistance in arab adults with type 2 diabetes. Dis Markers. 2021;2021. [DOI] [PMC free article] [PubMed]
- 182.Garcia‐Macia M, Santos‐Ledo A, Leslie J, Paish HL, Collins AL, Scott RS, et al. A mammalian target of rapamycin‐perilipin 3 (mTORC1‐Plin3) pathway is essential to activate lipophagy and protects against hepatosteatosis. Hepatology. 2021;74:3441–59.. doi: 10.1002/hep.32048. [DOI] [PubMed] [Google Scholar]
- 183.Wang K, Ruan H, Song Z, Cao Q, Bao L, Liu D et al. PLIN3 is up-regulated and correlates with poor prognosis in clear cell renal cell carcinoma. Urol Oncol: Semin Orig Investig. 2018;6,343-e9. [DOI] [PubMed]
- 184.Zhang H, Huang J, Fan X, et al. HSP90AA1 promotes the inflammation in human gingival fibroblasts induced by Porphyromonas gingivalis lipopolysaccharide via regulating of autophagy. BMC Oral Health. 2022;22:366. doi: 10.1186/s12903-022-02304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang Y, Chen Q, Wu D, Chen Q, Gong G, He L, et al. Lamin-A interacting protein Hsp90 is required for DNA damage repair and chemoresistance of ovarian cancer cells. Cell Death Dis. 2021;12:1–1. doi: 10.1038/s41419-021-04074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Usman M, Ilyas A, Syed B, Hashim Z, Ahmed A, Zarina S. Serum HSP90-alpha and oral squamous cell carcinoma: a prospective biomarker. Protein Pept Lett. 2021;28:1157–63.. doi: 10.2174/0929866528666210616112539. [DOI] [PubMed] [Google Scholar]
- 187.Yao ZP, Zhu H, Shen F, Gong D. Hsp90 regulates the tumorigenic function of tyrosine protein kinase in osteosarcoma. Clin Exp Pharmacol Physiol. 2022;49:380–90.. doi: 10.1111/1440-1681.13613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available.