Abstract
Background
Pancreatic cancer is the third leading cause of cancer death in the United States, which is attributed to limited treatment options. Complementary and alternative medicine (CAM) therapies have been proposed to provide benefits in treating pancreatic cancer. Despite its importance in treatment, clinicians are not generally well equipped to counsel their patients about CAM therapies. This review identified the quantity and assessed the quality of clinical practice guidelines (CPGs) providing CAM recommendations for the treatment and/or management of pancreatic cancer.
Methods
A systematic review was conducted to identify pancreatic cancer CPGs. MEDLINE, EMBASE and CINAHL were searched from 2011 to 2022. The Guidelines International Network (GIN) and the National Center for Complementary and Integrative Health (NCCIH) websites were also searched. Eligible CPGs published by non-profit agencies on treatment and/or management of pancreatic cancer for adults were assessed using the Appraisal of Guidelines, Research and Evaluation II (AGREE II) instrument.
Results
From 31 eligible search results, 7 CPGs mentioned CAM and 3 CPGs made CAM recommendations. The mean scaled domain percentages of the CPGs in this study (overall, CAM-specific) were as follows: scope and purpose (81.3%, 77.8%), stakeholder involvement (63.9%, 42.6%), rigor-of-development (51.0%, 40.3%), clarity-of-presentation (83.3%, 54.6%), applicability (42.3%, 30.5%), and editorial independence (58.3%, 58.3%).
Conclusions
Evaluation of the CPGs demonstrated that quality varied both within and between CPGs. CPGs that scored well could be used by patients and clinicians as the basis for discussion for the use of CAM therapies. Future research should identify other appropriate CAM therapies for further development of CPGs for pancreatic cancer.
Registration
The protocol was registered on PROSPERO (registration number: CRD42022334025).
Keywords: Pancreatic cancer, Complementary and alternative medicine, Systematic review, AGREE II, Clinical practice guideline
1. Introduction
The pancreas is a vital organ that secretes essential digestive enzymes and regulates blood sugar levels. Pancreatic cancer is the third leading cause of cancer death as of 2022 and is expected to become the second by the year 2030.1,2 The high mortality rate is due to a lack of specific screening tests, early metastases, aggressive local invasion, and resistance to chemotherapy and radiation therapy.3 Most patients with pancreatic cancer present with non-specific symptoms and are at an advanced stage when diagnosed, which leads to a poor prognosis.3,4 Due to most patients presenting at an advanced stage, conventional therapies such as surgery, chemotherapy, and radiation therapy have limited success.3 As a result, patients with cancer often resort to complementary and alternative medicine (CAM) to relieve their physical and emotional symptoms, in hopes that such therapies may supplement their fight against cancer.5 The use of CAM approaches for adjuvant cancer therapy and pain management is prevalent among patients with various types of cancers. Up to 30–40% of surveyed cancer patients in general and over 56% of patients with pancreatic cancer self-reported the use of at least one CAM therapy.6,7 Complementary medicine is defined as a non-mainstream therapeutic approach that is used together with conventional medicine, whereas alternative medicine is defined as a non-mainstream therapeutic approach used in replacement of conventional medicine.8,9
Among the available CAM therapies, Chinese herbal medicines have displayed promising therapeutic benefits for patients with pancreatic cancer. The noted advantages of Chinese herbal medicines in patients with cancer include the enhancement of the immune system, limitation of tumor progression, and suppression of the side effects from chemotherapy and radiation therapy.10 Preclinical studies involving Qingyihuaji (QYHJ), a seven-herb Chinese medicine formula, have demonstrated these benefits in pancreatic cancer mice models, which has subsequently led to its wider use in clinical practice.11 A systematic review of 86 experimental studies looking at 74 different herbal derivatives, published in 2022, suggested that all of them exhibited therapeutic potential for pancreatic cancer.12 Other commonly used CAM therapies include acupuncture and electroacupuncture, which are known to be beneficial in cancer pain management. A randomized controlled trial examining the efficacy of electroacupuncture treatment for pancreatic cancer pain displayed significant results in analgesic effects compared to placebo.13 Despite CAM being a significant component of cancer treatment, up to 40–50% of patients with cancer do not disclose CAM use to their healthcare providers.14 The miscommunication between healthcare providers and patients may lead to foregone benefits and avoidable risks.15
There is widespread acceptance and use of CAM in most medical specialties including oncology.16 Though some conventional healthcare providers may be aware of the CAM therapies available, many are not familiar with the evidence-based CAM resources that can be used to provide clinical guidance.17 Therefore, to ensure patient safety and the best standard of care, it is imperative for healthcare providers to be supplied with information regarding the credibility of CAM therapies. With the advent of evidence-based medicine, healthcare providers heavily rely on CPGs to evaluate the appropriateness of the treatments their patients receive. CPGs assist healthcare providers in weighing the risks and benefits of therapies, which ultimately leads to better patient outcomes.18 Therefore, assessing the quality of CPGs is important in helping healthcare providers understand their reliability. There are a few reviews that have assessed the quality of pancreatic cancer CPGs.19,20 However, none of these CPGs’ quality assessments has specifically focused on CAM therapies for pancreatic cancer. In this systematic review, we determined the mention of CAM in the CPGs for pancreatic cancer and assessed the quality of CAM recommendations.
2. Methods
2.1. Approach
We conducted a systematic review using methods described in the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria to identify CPGs for the treatment of pancreatic cancer.21,22 Our protocol was registered on PROSPERO (registration number: CRD42022334025). The quality assessment of eligible CPGs containing CAM recommendations was carried out with the internationally accepted and validated guideline appraisal tool, the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.23 Moreover, the AGREE II assessment of CPGs was repeated exclusively for CAM-specific sections. The AGREE II instrument is comprised of 23 grading criteria organized within six domains including the following: scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability, and editorial independence.
2.2. Eligibility criteria
The CPG eligibility criteria were informed by the Population, Intervention, Comparison, and Outcomes (PICO) framework. The eligible population was adults (>19 years) diagnosed with pancreatic cancer. The intervention included recommendations for the treatment and/or management of pancreatic cancer. With respect to comparisons, we first identified CPGs that provided CAM therapy recommendations using the comprehensive reference list of CAM therapies available on the Cochrane Complementary Medicine website.24 Next, we quality assessed the CAM-specific sections of the CPGs. The outcomes were AGREE II scores reflecting the credibility of CPGs’ content and format. Based on the applicability criteria of the AGREE II instrument, CPGs included for quality assessment had to include evidence-based recommendations. In the case of CPGs containing both evidence-based and consensus-based recommendations, they were still included, however, CPGs containing solely consensus-based recommendations were excluded.25 Additionally, eligible CPGs had to have provided recommendations for the treatment and/or management of pancreatic cancer. CPGs that mentioned CAM therapies without providing a recommendation were ineligible to be assessed. Moreover, eligible CPGs were published between 2011 and May 19, 2022, and their full version (e.g., not abridged) had to have been published in English. We made all attempts to gain access to the full text of CPGs that were not publicly available via institutional library access and/or with the further assistance of an academic librarian. Other types of items such as, primary studies, systematic reviews, conference/abstract proceedings, protocols, letters, or editorials were excluded.
2.3. Searching and screening
MEDLINE, EMBASE, and CINAHL databases were searched on May 20, 2022 to obtain all CPGs published between 2011 and May 19, 2022. Our search strategy included key terms (Supplement 1) synonymously used in the literature to refer to pancreatic cancer, including “pancrea* cancer” and “pancreatic neoplasms”. Additionally, the Guidelines International Network (GIN) and the National Center for Complementary and Integrative Health (NCCIH) websites were searched using the key terms described previously. GIN is a repository of CPGs and the NCCIH is the United States’ government agency that leads scientific research on CAM. HAB and MR screened relevant titles, abstracts, and full-text items to confirm eligibility. Furthermore, JYN reviewed the screened titles, abstracts, and full-text items to resolve selection differences between the two screeners and to standardize the screening process.
2.4. Data extraction and analysis
The data extraction process involved the retrieval of information from eligible CPGs including date of publication, country of the first author, type of guideline developer (academic institutions, government agencies, disease-specific foundations, or professional associations or societies), and the presence of CAM recommendations. For CPGs that included CAM recommendations, further information was extracted including the types of CAM mentioned, CAM recommendations, CAM funding sources, and the names of CAM providers that were part of the CPG panel. Furthermore, the websites of CPG publishers were reviewed to extract any associated knowledge-based resources provided in supplementation of the CPGs.
2.5. Guideline quality assessment
Methods described in the AGREE II instrument's user manual were followed for the evaluation of eligible CPGs.20 Initially, a pilot test was conducted with the AGREE II instrument where 3 separate CPGs were evaluated independently by two reviewers. The discrepancies between individual evaluations were discussed and resolved to standardize the CPG appraisal process using the AGREE II instrument. Next, HAB and MR individually assessed all eligible CPGs (CPGs with CAM recommendations were assessed twice, once for the overall CPG and once for only the CAM-specific sections) on 23 grading items across a total of 6 domains. The assessment of each item was based on a seven-point Likert scale from strongly disagree “1″ to strongly agree “7″, and the scores were judged overall to recommend for or against each CPG. The adapted AGREE II questions were used in evaluating CAM sections (Supplementary File 2). JYN facilitated the resolution of scoring differences between reviewers. Average appraisal scores were obtained by calculating the average scores across 23 items scored by each reviewer, and further averaging between the independent averages. Average overall assessment scores were obtained by averaging the “overall guideline assessment” of each reviewer. For inter-domain comparison, scaled domain percentages were calculated by summing both reviewers’ ratings of items within each domain, and then scaling the total as a percentage of maximum score possible for each domain. All scoring measurements including average appraisal scores, average overall assessment scores, and scaled domain percentages for each CPGs were organized in a tabular format for comparison.
3. Results
3.1. Search results
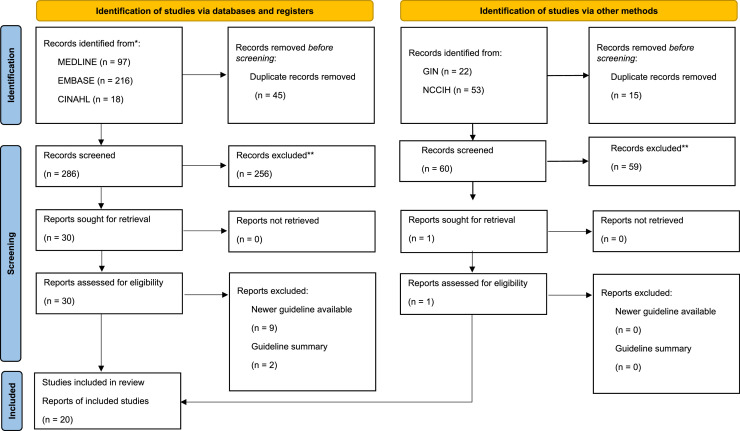
From the 407 search items that were retrieved, 346 were unique, and 315 titles and abstracts were excluded, resulting in 31 full-text items that were further considered (Fig. 1). Of those, 11 were deemed ineligible for evaluation, due to the availability of newer CPG versions (n = 9), or because they were CPG summaries (n = 2). This left 20 eligible CPGs.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Of these CPGs, 7 mentioned CAM therapies and 3 included CAM therapy recommendations.
Fig. 1.
PRISMA Diagram.
3.2. Guideline characteristics
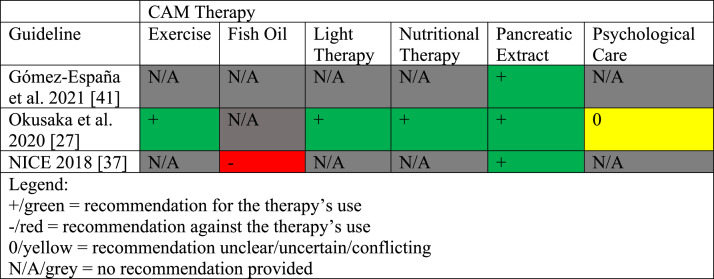
The guideline characteristics are shown in Table 1. The eligible CPGs were published between 2011 and 2021 and originated from the following countries: the United States (n = 5), China (n = 3), Switzerland (n = 3), England (n = 2), Canada (n = 1), France (n = 1), Germany (n = 1), Japan (n = 1), Saudi Arabia (n = 1), Singapore (n = 1), and Spain (n = 1) .27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Of these CPGs, many were funded and/or developed by professional associations or societies (n = 19), or by an international agency (n = 1). CAM was mentioned in 7 of the 20 CPGs examined and included: pancreatic enzyme replacement therapy (n = 4), nutritional interventions (n = 2), psychological care (n = 2), diet therapy (n = 1), spiritual counselling (n = 1), and exercise (n = 1). From these 7 guidelines, only 3 CPGs made CAM recommendations relating to: pancreatic extract (n = 3), exercise therapy (n = 1), fish oil (n = 1), light therapy (n = 1), nutritional therapy (n = 1), and psychological care (n = 1). The AGREE II instrument was used to assess only these 3 CPGs. Additionally, no CAM funding sources, nor the inclusion of CAM providers as part of the CPG panel were specified in any CPGs. We provide a summary of CAM recommendations made across pancreatic cancer CPGs for the benefit of healthcare providers and researchers in Fig. 2.
Table 1.
Characteristics of Eligible Guidelines.
| Guideline | Country (First Author) | Developer | CAM Category | Guideline Topic |
|---|---|---|---|---|
| Gómez-España et al. 202141 | Spain | Spanish Society of Medical Oncology | Pancreatic extract | Pancreatic cancer and biliary tract cancer |
| Tempero et al. 202135 | United Kingdom | National Comprehensive Cancer Network | N/A | Pancreatic adenocarcinoma |
| Li 202128 | China | China Alliance of Cellular and Interventional Therapy Techniques for Diabetic Foot | N/A | Advanced pancreatic cancer treatment |
| Yang et al. 202130 | China | Chinese Pancreatic Surgery Association | N/A | Pancreatic cancer diagnosis and treatment |
| Sohal et al. 202033 | United States | American Society of Clinical Oncology | Pancreatic extract | Metastatic pancreatic cancer |
| Delpero et al. 202045 | France | French National Institute of Cancer | N/A | Pancreatic cancer treatment |
| Okusaka et al. 202027 | Japan | Japan Pancreas Society | Pancreatic extract, Nutritional therapy, Light therapy, Exercise, Psychological care, Fish oil | Pancreatic cancer |
| Zhang et al. 202044 | China | Chinese Pancreatic Surgery Association | Psychological care | Pancreatic cancer treatment |
| Khorana et al. 201938 | United States | American Society of Clinical Oncology | N/A | Potentially curable adenocarcinoma |
| Palta et al. 201939 | United States | American Society of Radiation Oncology | Light therapy | Radiation therapy for pancreatic cancer |
| NICE 201837 | England | National Institute for Health and Care Excellence | Pancreatic extract, Fish oil | Pancreatic cancer diagnosis and managment |
| Singh et al. 201743 | Canada | Cancer Care Ontario | N/A | Gastroenteropancreatic neuroendocrine tumours (NETs) treatment |
| Balaban et al. 201632 | United States | American Society of Clinical Oncology | Pancreatic extract | Advanced pancreatic cancer |
| Ducreux et al. 201526 | France | European Society of Medical Oncology | Pancreatic extract | Pancreatic cancer diagnosis, treatment, follow-up |
| SCAN 201542 | Singapore | Singapore Cancer Network | N/A | Pancreatic adenocarcinoma treatment |
| Seufferlein et al. 201429 | Germany | Association of Scientific Medical Societies | N/A | Ductal Pancreatic Adenocarcinoma |
| Rahal et al. 201440 | Saudi Arabia | Saudi Oncology Society | N/A | Pancreatic cancer management |
| Seufferlein et al. 201236 | Germany | European Society of Medical Oncology | N/A | Pancreatic adenocarcinoma diagnosis, treatment, follow-up |
| Öberg et al. 201234 | Sweden | European Society of Medical Oncology | N/A | Gastroenteropancreatic neuroendocrine tumours (NETs) diagnosis, treatment, follow-up |
| Ramage et al. 201231 | England | UK and Ireland Neuroendocrine Tumour Society | N/A | Gastroenteropancreatic neuroendocrine tumours (NETs) management |
Fig. 2.
Summary of CAM Recommendations in Clinical Practice Guidelines for Pancreatic Cancer.
3.3. Guidelines mentioning CAM without recommendations
Four CPGs mentioned CAM interventions, without recommending them. These CPGs did not state any advantages or disadvantages of CAM intervention usage to the target audience; therefore, they were not assessed with the AGREE II instrument. These CPGs mentioned pancreatic enzyme replacement therapy to manage symptoms such as anorexia/weight loss,32, 33 increasing fruit and folate intake to minimize the risk of developing pancreatic cancer,26 managing pain through oral supplementation of pancreatic enzyme,26 and psychological and spiritual counselling to improve patients’ quality of life.30
3.4. Average appraisal scores, average overall assessments and recommendations regarding use of guidelines: overall guideline
Table 2 indicates the average appraisal scores, average overall assessments, and recommendations regarding use for each of the 3 pancreatic cancer CPGs assessed. The average appraisal scores for the CPGs ranged from 3.7 to 5.7 on the seven-point Likert scale (where 7 means strongly agree that the item is met); only 1 CPG achieved or exceeded an average appraisal score of 5.0. Of the remaining CPGs, only 1 exceeded or achieved an average score of 4.0. The average overall assessments for the CPGs ranged between 4.0 and 6.5; only 1 CPG achieved or exceeded an average score of 5.0.
Table 2.
Average Appraisal Scores and Average Overall Assessments of Each Guideline.
| Guideline | Metric | Appraiser 1 | Appraiser 2 | Average | Standard Deviation |
|---|---|---|---|---|---|
| Okusaka 202027 (Overall) |
Appraisal Score | 4.4 | 4.4 | 4.4 | 0.0 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 | |
| Okusaka 202027 (CAM Section) |
Appraisal Score | 3.5 | 3.2 | 3.3 | 0.2 |
| Overall Assessment | 3.0 | 3.0 | 3.0 | 0.0 | |
| NICE 201837 (Overall) |
Appraisal Score | 6.1 | 5.3 | 5.7 | 0.6 |
| Overall Assessment | 7 | 6 | 6.5 | 0.7 | |
| NICE 201837 (CAM Section) |
Appraisal Score | 5.3 | 4.3 | 4.8 | 0.7 |
| Overall Assessment | 4.0 | 5.0 | 4.5 | 0.7 | |
| Gómez-España 202141 (Overall) |
Appraisal Score | 3.7 | 3.7 | 3.7 | 0.2 |
| Overall Assessment | 4.0 | 4.0 | 4.0 | 0.0 | |
| Gómez-España 202141 (CAM Section) |
Appraisal Score | 3.1 | 2.4 | 2.8 | 0.5 |
| Overall Assessment | 3.0 | 3.0 | 3.0 | 0.0 |
3.5. Average appraisal scores, average overall assessments and recommendations regarding use of guidelines: CAM sections
Table 2 indicates the average appraisal scores, average overall assessments, and recommendations regarding use for each of the 3 pancreatic cancer CPGs assessed. The average appraisal scores for the CPGs ranged from 2.8 to 4.8 on the seven-point Likert scale (where 7 means strongly agree that the item is met); only one CPG achieved or exceeded an average appraisal score of 4.0. Whereas from the remaining CPGs, only 1 exceeded or achieved an average score of 3.0. The average overall assessments for the CPGs ranged between 3.0 and 4.5; only 1 CPG achieved or exceeded an average score of 4.0.
3.6. Overall recommendations: overall guideline
Of the 3 CPGs assessed, only 1 CPG was recommended and rated as “Yes” by both appraisers.37 The remaining CPGs were both independently rated as “Yes with modifications” and “No” by the two appraisers, respectively27, 41 (Table 3).
Table 3.
Overall Recommendations for Use of Appraised Guidelines.
3.7. Overall recommendations: CAM sections
Of the 3 CPGs assessed, only 1 CPG was recommended by both appraisers and independently rated as “Yes” and “Yes with modifications” .37 The remaining CPGs were both independently rated as “Yes with modifications” and “No” by the two appraisers, respectively27, 41 (Table 3).
3.8. Scaled domain percentage quality assessment
The following list includes scaled domain percentage scores of the three CPGs assessed (overall guideline, CAM section): scope and purpose scores (61.1–94.4%, 69.4–88.9%), stakeholder involvement scores (25.0–94.4%, 11.1–80.6%), rigor-of-development scores (30.2–72.9%, 20.8–66.7%), clarity-of-presentation scores (75.0–86.1%, 33.3–38.9%), applicability scores (27.1–60.4%, 18.8–39.6%), and editorial independence scores (45.8–75.0%, 45.8–75.0%) (Table 4).
Table 4.
Scaled Domain Percentages for Appraisers of Each Guideline.
| Guideline | Domain score (%) |
||||||
|---|---|---|---|---|---|---|---|
| Scope and purpose | stakeholder involvement | rigor of development | Clarity of presentation | Applicability | Editorial Independence | ||
| Gómez-España 202141 | Overall Guideline | 61.1 | 25.0 | 30.2 | 88.9 | 39.6 | 45.8 |
| CAM Section | 69.4 | 11.1 | 20.8 | 88.9 | 33.3 | 45.8 | |
| NICE 201837 | Overall Guideline | 94.4 | 94.4 | 72.9 | 86.1 | 60.4 | 75.0 |
| CAM Section | 88.9 | 80.6 | 66.7 | 38.9 | 39.6 | 75.0 | |
| Okusaka 202027 | Overall Guideline | 88.3 | 72.2 | 50.0 | 75.0 | 27.0 | 54.2 |
| CAM Section | 75.0 | 36.1 | 33.3 | 36.1 | 18.6 | 54.2 | |
3.8.1. Scope and purpose
Across all CPGs, the overall objective was specific and well-defined in both the overall guideline and the CAM-specific sections. Similarly, except for 1 CPG,41 the health questions covered were also specifically and clearly stated for both the overall and CAM-specific sections. The population to whom the CPG was intended to apply for both the overall and CAM-specific sections was specific across all CPGs.
3.8.2. Stakeholder involvement
For all the appraised CPGs, characteristics of members from relevant professional groups were clearly stated in the overall CPGs including members’ institutional affiliation, geographical location, and subject discipline. However, there were no CAM providers specified in any of the CPGs. The views and preferences of the target population for both the overall and CAM-specific section were not detailed or defined in 1 CPG41 but were considered and sought for both sections in another CPG by incorporating lay members.37 One CPG sought the views of the target population in the overall guideline through the incorporation of a patient representative but did not consider additional population preferences for CAM-specific sections.27 Additionally, the target users were clearly defined in only 2 CPGs.27, 37
3.8.3. Rigor of development
Except for 1 CPG,41 systematic methods were used to search for evidence.27, 31 The criterion for selecting the evidence, the strengths and limitations of the evidence, as well as the health benefits, side effects, and risks were specifically described and considered for both the overall and CAM-specific section of only 1 CPG.37 The methods for formulating the recommendations for both CAM-specific and non-CAM specific sections were clearly described, apart from 1 CPG.41 Additionally, an explicit link between the recommendations and the evidence is clearly presented in 2 CPGs for both the overall and CAM-specific sections.37, 41 Only 1 of the CPGs explicitly declared to be externally reviewed by experts prior to its publication.27 All CPGs failed to provide a detailed or thorough procedure on future updates.
3.8.4. Clarity of presentation
All CPGs presented specific and unambiguous recommendations for both CAM-specific and non-specific sections. However, in comparison to CAM-specific sections, the different options for the treatment and/or management of pancreatic cancer for non-CAM specific sections were presented more clearly and with a greater amount of detail. There were also more alternative options presented for non-CAM specific sections in comparison to CAM-specific recommendations. Notably, overall key recommendations were easily identifiable for all CPGs, but CAM-specific recommendations were considerably difficult to locate and identify.
3.8.5. Applicability
Although some CPGs clearly described facilitators and barriers to the implementation of their respective overall recommendations more thoroughly37, 41 than others27; the details of CAM-specific recommendations in all CPGs were substantially less thorough and specific. All CPGs incorporated advice and/or tools to support the practice of the recommendations. The potential resource implications of applying the recommendations were only addressed in one CPG discussing cost-effectiveness and economic barriers.37 All CPGs presented little to no monitoring or auditing criteria for both CAM-specific and non-CAM specific recommendations.
3.8.6. Editorial independence
All CPGs declared funding sources but failed to state whether the interests of the funding body influenced the content. Similarly, except for 1,37 the CPGs declaring competing interests failed to explicitly state how they were sought or how the competing interests might have influenced the formulation of CPG recommendations.27, 41
4. Discussion
The purpose of this study was to identify the quantity and assess the quality of CAM recommendations in CPGs providing recommendations for the treatment and/or management of pancreatic cancer. This study identified 20 CPGs that were published between 2011 and 2021, of which 7 CPGs mentioned CAM, of which 3 CPGs recommended CAM therapy. The 23-item AGREE II instrument found considerable domain- and overall-wide variation in quality both within and between the CPGs. Within each CPG, there were substantially different scores achieved throughout the 23 items of the AGREE II instruments. The average appraisal scores obtained were found to be 4.4, 5.7, and 3.7 for the overall CPGs but only 3.3, 4.8, and 2.8 for the CAM-specific sections of all three CPGs respectively. 27, 37, 41Additionally, the average overall assessment scores were found to be 4.5, 6.5, and 4.0 for the overall CPGs but only 3.0, 4.5, and 3.0 for the CAM-specific section of all three CPGs respectively.27, 37, 41 Similarly, the mean scaled domain percentages of the CPGs in this study were higher for overall CPG assessment in comparison to the CAM-specific sections. The general trend of these results in all CPGs show that the quality of CAM-specific sections is noticeably poorer, highlighting the lack of emphasis on CAM therapy recommendations.
4.1. Comparative literature
Our findings are comparable and consistent with other research studies that have appraised CPGs relating to pancreatic cancer. Two studies that were both published in 201546, 47 shared similar AGREE II mean domain ranks to our results, ranking ‘scope and purpose’ and ‘clarity of presentations’ as the highest. This suggests that most pancreatic cancer CPGs were successful in specifically describing their clinical questions, stating their intended audience, and clearly presenting recommendations in an unambiguous and easily identifiable manner. However, similar to our findings, many studies also had low ‘rigor of development’ scores47, 48 and the lowest ‘applicability’ scores.47 This is problematic because it questions the validity of pancreatic cancer CPGs and decreases rates of recommendation implementation, respectively. One study48 reasoned their low ‘rigor of development’ scores for the CPGs they assessed, to be due to insufficient detail provided in methods used by those CPGs, to develop and formulate their recommendations. This was also a concern present in one of the CPGs we assessed.41 The low applicability scores shared by all assessed CPGs are due to the lack of consideration towards facilitators or barriers that might impede implementation of the recommendations27, 41, 47 and the exclusion of monitoring and/or auditing criteria in all CPGs. Low ‘editorial independence’ rates are also commonly observed among pancreatic cancer CPGs. For example, a study that evaluated the quality of pancreatic cancer CPGs using the RIGHT checklist found low reporting rates of funding sources, statements of declaration and management of interests.49 Similarly, a study appraising CPGs on the diagnosis of pancreatic cancer found that only 2 of the 9 CPGs assessed explicitly declared that the interests or views of the funding body did not impact the development of the CPG.48 These results are concerning and align with the findings of our study because all the CPGs appraised in this review failed to state whether the interests of the funding body influenced the content of their CPGs. The CPG development committees should take full consideration of conflicts of interest in the development process to promote editorial independence and prevent unnecessary bias. Past research studies have reported that there has been little to no improvement in the quality of pancreatic cancer CPGs over the years.49 This is consistent with our results because the CPGs we assessed were published in the years 2018, 2020, and 2021 with varying scores throughout the years, rather than improvement.
While to our knowledge, there have been no prior studies that have determined the quantity and assessed quality of CAM therapy recommendations in pancreatic cancer CPGs, our findings are concordant with other studies assessing CAM therapy recommendations in CPGs for other types of cancer. One such study examined 34 breast cancer CPGs and found that 5 CPGs mentioned CAM and 4 CPGs provided CAM therapy recommendations which were assessed using the AGREE II instrument.50 This study also had similar scaled domain percentage rankings as our findings for the CAM-specific section, with ‘scope and purpose’ and ‘applicability’ ranked the highest and lowest, respectively.50 Interestingly, another study assessed CAM therapy recommendations in head and neck cancer CPGs and reported similar results to our study, with ‘scope and purpose’ and ‘clarity of presentation’ domains being ranked the highest for both overall and CAM-specific sections.51 Moreover, other studies focused on examining CAM recommendations in CPGs for the treatment and/or management of cancer-related pain52 and lung cancer53 also ranked ‘applicability’ as the lowest scoring domain. Thus, the inconsistent and subpar quality of cancer-related CPGs is a common factor. Although our assessment of only 3 CPGs is represents a small proportion of pancreatic cancer CPGs, studies conducted in the past on colon54 and ovarian cancer55 have found no eligible CPGs that made mention of CAM or provided CAM therapies at all. This emphasizes the lack of evidence-based CAM resources for clinicians to utilize due to the lack of CPGs focused on CAM therapy recommendations across different types of cancer.
By describing the quantity and assessing the quality of pancreatic cancer CPGs containing CAM recommendations, this study has revealed that a low number of CPGs with CAM recommendations are available to support patients and healthcare professionals in making well-informed decisions about this category of therapies. Potential factors that might have contributed to the results of this study include the comparative lack of research on CAM therapies (e.g., versus pharmaceutical, surgical or radiological therapies), and biases against the use of CAM therapies and the publication of CAM research.56 Additionally, substantially limited funding57 and/or resources available to facilitate CAM research56 are among other factors that challenge CAM research and acceptance among healthcare providers. Recent research has demonstrated that many healthcare professionals have limited CAM knowledge, although their interest and positive attitudes towards CAM has been growing.58 Based on the results of a study collecting medical students’ attitudes towards CAM in their education and training, modifying healthcare professional schools’ curriculum to increase CAM exposure may increase their confidence and knowledge in providing counselling and referrals to their patients about this category of therapies.59
Moreover, the results of our study suggest that pancreatic cancer CPG developers must focus on improving the quality of CAM therapy recommendations. In addition to improving the methodological quality, which constitutes the steps involved in developing the CPGs, future CPG developers must also focus on improving the reporting quality, which will ensure effective communication of the recommendations. Apart from the AGREE II instrument, a plethora of resources including the SIGN 50 guideline developer's handbook,60 NICE guideline manual,61 and GRADE handbook62 may be used by CPG developers.
4.2. Strengths and limitations
This review's notable strengths included a systematic search of multiple bibliographic databases and websites (along with the searching of reference lists of relevant reviews) to identify CPGs that provided CAM recommendations for the treatment and/or management of pancreatic cancer. The quality of eligible CPGs was assessed using the AGREE II instrument, which has been found to be both reliable and valid for this purpose.23 After appraising eligible CPGs for this study, all authors met to discuss any concerns and/or ambiguities without unduly modifying legitimate discrepancies between assessors.
With respect to limitations, we acknowledge that every search strategy and choice of sources to search can still fail to capture every existing eligible record. Healthcare providers and patients may refer to other web-based resources such as Physician Data Query (PDQ), a comprehensive database by the National Cancer Institute (NCI) providing peer-reviewed summaries on cancer treatments.63 It must be acknowledged that the use of such resources may influence clinical decision-making in practice, however, for the purpose of this review, we deemed that the searching for and assessing of such resources was beyond the scope of this study. Additionally, it should be noted that based on our eligibility criteria, we excluded CPGs written in languages other than English. However, given that many traditional medical systems originate from world regions where English is not widely spoken (e.g., traditional Chinese medicine from China, Kampo medicine from Japan, among others) and their CPGs are often published in non-English languages. The CPGs were independently evaluated by two appraisers as opposed to four, as suggested by the AGREE II instrument to maximize reliability, which may limit interpretations of these results. To mitigate this concern and standardize scoring, a preliminary pilot test was carried out by JYN, HAB, and MR during which they independently appraised three CPGs, discussed their respective scores and attained consensus on how to apply the AGREE II instrument. Lastly, and while not necessarily a limitation, given that the AGREE II instrument has not been designed to assess the quality of consensus-based CPGs, their assessment may be warranted as a future direction.
5. Conclusions
This study identified 3 CPGs that included CAM recommendations for the treatment and/or management of pancreatic cancer. These CAM therapies included: pancreatic extract, nutritional therapy, light therapy, exercise, and fish oil. All eligible CPGs were assessed twice with the AGREE II instrument: once for the overall CPG (non-CAM specific sections) and once for the CAM-specific sections. An evaluation of these CPGs demonstrated that the quality of all CPGs varied distinctively. Specifically, the applicability domain scored the lowest for both the overall CPG and CAM-specific sections. The scaled domain percentages were notably lower in all domains for the CAM-specific assessment, as compared to the overall CPG assessment, except for editorial independence, for which both assessments had equal percentage scores. CPGs with lower scaled domain percentages and recommendations could be improved in future updates in accordance with the criteria in the AGREE II instrument and with knowledge from the guideline development resources available to support the development and application of CPGs. Healthcare providers may utilize the CPGs that received higher AGREE II ratings and favourable overall recommendations to engage in discussions and explore the usage of CAM. However, the substantial lack of CAM therapy recommendations in pancreatic cancer CPGs limit the facilitation and availability to support patients' and healthcare professionals' informed and collaborative decision-making. The inclusion of additional evidence-based CAM therapy recommendations by future pancreatic cancer CPG developers should be encouraged to facilitate clinician knowledge and support patients’ treatment and/or management.
Conflict of interests
The authors declare that they have no competing interests.
Funding
JYN was awarded a Research Scholarship and an Entrance Scholarship from the Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences at McMaster University.
Ethical statement
This study involved a systematic review of peer-reviewed literature only; it did not require ethics approval or consent to participate.
CRediT authorship contribution statement
Jeremy Y Ng: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Hardil Anup Bhatt: Formal analysis, Investigation, Writing – review & editing. Maheen Raja: Formal analysis, Investigation, Writing – review & editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2023.100921.
Supplement 1. MEDLINE search strategy for pancreatic cancer clinical practice guidelines.
Appendix. Supplementary materials
PRISMA Diagram
Supplement 2: Modified AGREE II Questions Used to Guide Scoring of CAM Sections of Each Guideline
Data availability
All relevant data are included in this manuscript.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Hussain S.P. Pancreatic Cancer: Current Progress and Future Challenges. Int J Biol Sci. 2016;12(3):270–272. doi: 10.7150/ijbs.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park W., Chawla A., O’Reilly E.M. Pancreatic Cancer: A Review. JAMA. 2021;326(9):851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthur K., Belliard J.C., Hardin S.B., Knecht K., Chen C.S., Montgomery S. Reasons to Use and Disclose Use of Complementary Medicine Use – An Insight from Cancer Patients. Cancer Clin Oncol. 2013;2(2):81–92. doi: 10.5539/cco.v2n2p81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molassiotis A., Fernández-Ortega P., Pud D., Ozden G., Scott J.A., Panteli V., et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 7.Buckner C.A., Lafrenie R.M., Dénommée J.A., Caswell J.M., Want D.A. Complementary and Alternative Medicine Use in Patients Before and After a Cancer Diagnosis. Curr Oncol. 2018;25(4):275–281. doi: 10.3747/co.25.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Complementary, Alternative, or Integrative Health: What's In a Name? [Internet]. NCCIH. Accessed on June 19, 2022. Available from: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- 9.Ng J.Y., Boon H.S., Thompson A.K., Whitehead C.R. Making sense of “alternative”, “complementary”, “unconventional” and “integrative” medicine: exploring the terms and meanings through a textual analysis. BMC Complement Altern Med. 2016;16(1):134. doi: 10.1186/s12906-016-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee TCS of COPCE Chinese consensus on pancreatic cancer diagnosis and treatment (2014 version) lcgdbzz. 2014;30(10):970–980. doi: 10.3969/j.issn.1001-5256.2014.10.002. [DOI] [Google Scholar]
- 11.Yin J.H., Shi W.D., Zhu X.Y., Chen Z., Liu L.M. Qingyihuaji formula inhibits progress of liver metastases from advanced pancreatic cancer xenograft by targeting to decrease expression of Cyr61 and VEGF. Integr Cancer Ther. 2012;11(1):37–47. doi: 10.1177/1534735411400315. [DOI] [PubMed] [Google Scholar]
- 12.Triantafillidis J.K., Triantafyllidi E., Sideris M., Pittaras T., Papalois A.E. Herbals and Plants in the Treatment of Pancreatic Cancer: A Systematic Review of Experimental and Clinical Studies. Nutrients. 2022;14(3):619. doi: 10.3390/nu14030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Liu T.Y., Kuai L., Zhu J., Wu C.J., Liu L.M. Electroacupuncture treatment for pancreatic cancer pain: A randomized controlled trial. Pancreatology. 2013;13(6):594–597. doi: 10.1016/j.pan.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Davis E.L., Oh B., Butow P.N., Mullan B.A., Clarke S. Cancer patient disclosure and patient-doctor communication of complementary and alternative medicine use: a systematic review. Oncologist. 2012;17(11):1475–1481. doi: 10.1634/theoncologist.2012-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson A., McGrail M.R. Disclosure of CAM use to medical practitioners: a review of qualitative and quantitative studies. Complement Ther Med. 2004;12(2):90–98. doi: 10.1016/j.ctim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Phutrakool P., Pongpirul K. Acceptance and use of complementary and alternative medicine among medical specialists: a 15-year systematic review and data synthesis. Syst Rev. 2022;11(1):10. doi: 10.1186/s13643-021-01882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S.J., Kemper K.J., Kitzmiller J.P. Physician perspectives on education, training, and implementation of complementary and alternative medicine. AMEP. 2017 Jul 25;8:499–503. doi: 10.2147/AMEP.S138572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djulbegovic B., Guyatt G.H. Progress in evidence-based medicine: a quarter century on. Lancet North Am Ed. 2017;390(10092):415–423. doi: 10.1016/S0140-6736(16)31592-6. [DOI] [PubMed] [Google Scholar]
- 19.Li C.C., Wang Y.Q., Li Y.P., Li X.L. Critical appraisal of clinical practice guidelines for treating pancreatic cancer based on the global disease burden. J Evid-Based Med. 2015;8(1):11–21. doi: 10.1111/jebm.12140. [DOI] [PubMed] [Google Scholar]
- 20.He Z., Tian H., Song A., Jin L., Zhou X., Liu X., et al. Quality Appraisal of Clinical Practice Guidelines on Pancreatic Cancer: A PRISMA-Compliant Article. Medicine (Baltimore) 2015;94(12):e635. doi: 10.1097/MD.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Accessed on June 19, 2022. Available from: https://training.cochrane.org/handbook.
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G., et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broad Operational Definition of Complementary, Alternative, and Integrative Medicine: List of Therapies | Cochrane Complementary Medicine [Internet]. Accessed on October 12, 2022. Available from: https://cam.cochrane.org/broad-operational-definition-complementary-alternative-and-integrative-medicine-list-therapies.
- 25.Djulbegovic B., Guyatt G. Evidence vs Consensus in Clinical Practice Guidelines. JAMA. 2019;322(8):725–726. doi: 10.1001/jama.2019.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducreux M., Cuhna A.S., Caramella C., Hollebecque A., Burtin P., Goéré D., et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 27.Okusaka T., Nakamura M., Yoshida M., Kitano M., Uesaka K., Ito Y., et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas. 2020;49(3):326. doi: 10.1097/MPA.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M. Clinical practice guidelines for the interventional treatment of advanced pancreatic cancer. J Intervent Med. 2021;V4:159–171. doi: 10.1016/j.jimed.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seufferlein T., Porzner M., Heinemann V., Tannapfel A., Stuschke M., Uhl W. Ductal pancreatic adenocarcinoma: Surgery, pathology work-up, and neoadjuvant, adjuvant, and palliative treatments. Deutsches Ärzteblatt Int. 2014;111(22):396. doi: 10.3238/arztebl.2014.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Bai X., Bian D., Cai S., Chen R., Cao F., et al. Guidelines for the diagnosis and treatment of pancreatic cancer in China (2021) J Pancreatol. 2021;4(02):49–66. doi: 10.1097/JP9.0000000000000072. [DOI] [Google Scholar]
- 31.Ramage J.K., Ahmed A., Ardill J., Bax N., Breen D.J., Caplin M.E., et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) BMJ. 2012;61(1):6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balaban E.P., Mangu P.B., Khorana A.A., Shah M.A., Mukherjee S., Crane C.H., et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(22):2654–2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 33.Sohal D.P., Kennedy E.B., Cinar P., Conroy T., Copur M.S., Crane C.H., et al. Metastatic pancreatic cancer: ASCO guideline update. J Clin Oncol. 2020;38(27):3217–3230. doi: 10.1200/JCO.20.01364. [DOI] [PubMed] [Google Scholar]
- 34.Öberg K., Knigge U., Kwekkeboom D., Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 doi: 10.1093/annonc/mds295. vii124-30. [DOI] [PubMed] [Google Scholar]
- 35.Tempero M.A., Malafa M.P., Al-Hawary M., Behrman S.W., Benson A.B., Cardin D.B., et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Netw. 2021;19(4):439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 36.Seufferlein T., Bachet J.B., Van Cutsem E.F., Rougier P. Pancreatic adenocarcinoma: ESMO–ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 doi: 10.1093/annonc/mds224. vii33-40. [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Clinical Excellence. Pancreatic Cancer in adults; Diagnosis and Management [NG85]. https://www.nice.org.uk/guidance/ng85 [PubMed]
- 38.Khorana A.A., McKernin S.E., Berlin J., Hong T.S., Maitra A., Moravek C., et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(23):2082–2088. doi: 10.1200/JCO.19.00946. [DOI] [PubMed] [Google Scholar]
- 39.Palta M., Godfrey D., Goodman K.A., Hoffe S., Dawson L.A., Dessert D., et al. Radiation therapy for pancreatic cancer: Executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2019;9(5):322–332. doi: 10.1016/j.prro.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Rahal M.M., Bazarbashi S.N., Kandil M.S., Al-Shehri A.S., Alzahrani A.M., Aljubran A.H., et al. Saudi Oncology Society clinical management guideline series: Pancreatic cancer 2014. Saudi Med J. 2014;35(12):1534. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4362172/ [PMC free article] [PubMed] [Google Scholar]
- 41.Gómez-España M., Montes A.F., Garcia-Carbonero R., Mercadé T.M., Maurel J., Martín A.M., et al. SEOM clinical guidelines for pancreatic and biliary tract cancer (2020) Clinic Translat Oncol. 2021;23(5):988–1000. doi: 10.1007/s12094-021-02573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Workgroup S.C. Singapore cancer network (SCAN) guidelines for systemic therapy of pancreatic adenocarcinoma. Ann Acad Med Singapore. 2015;44(10):388–396. https://pubmed.ncbi.nlm.nih.gov/26763056/ [PubMed] [Google Scholar]
- 43.Singh S., Sivajohanathan D., Asmis T., Cho C., Hammad N., Law C., et al. Systemic therapy in incurable gastroenteropancreatic neuroendocrine tumours: a clinical practice guideline. Curr Oncol. 2017;24(4):249–255. doi: 10.3747/co.24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang T., Wu W., Yang Y., Zhao Y. The Chinese guidelines for neoadjuvant therapy of pancreatic cancer (2020) J Pancreatol. 2021;4(04):135–145. doi: 10.1097/JP9.0000000000000077. [DOI] [Google Scholar]
- 45.Delpero J.R., Sauvanet A. Vascular resection for pancreatic cancer: 2019 French recommendations based on a literature review from 2008 to 6-2019. Front Oncol. 2020;10:40. doi: 10.3389/fonc.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C.C., Wang Y.Q., Li Y.P., Li X.L. Critical appraisal of clinical practice guidelines for treating pancreatic cancer based on the global disease burden. J Evid-Base Med. 2015;8(1):11–21. doi: 10.1111/jebm.12140. Available at: [DOI] [PubMed] [Google Scholar]
- 47.He Z., Tian H., Song A., Jin L., Zhou X., Liu X., et al. Quality appraisal of clinical practice guidelines on pancreatic cancer: a PRISMA-compliant article. Medicine (Baltimore) 2015;94(12) doi: 10.1097/MD.0000000000000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X.J., Yang T., Shi X., Xiao B.H., An L.Y., Zheng S.Y., et al. Systematic appraisal of guidelines for the diagnosis of pancreatic cancer. Gland Surg. 2021;10(4):1487. doi: 10.21037/gs-20-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Lu J., Jia M., Ma Y., Sun M., Chen X., et al. Evaluation of the reporting quality of clinical practice guidelines on pancreatic cancer using the RIGHT checklist. Ann Transl Med. 2021;9(13) doi: 10.21037/atm-21-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng J.Y., Sahak H.&., Lau S.K.C. A systematic review and quality assessment of breast cancer clinical practice guidelines providing complementary and alternative medicine recommendations. Curr Oncol Rep. 2021;23(112):1–13. doi: 10.1007/s11912-021-01109-8. [DOI] [PubMed] [Google Scholar]
- 51.Ng J.Y., Dogadova E. The presence of complementary and alternative medicine recommendations in head and neck cancer guidelines: Systematic review and quality assessment. Curr Oncol Rep. 2021;23(32):1–13. doi: 10.1007/s11912-021-01024-y. [DOI] [PubMed] [Google Scholar]
- 52.Ng J.Y .&, Sharma A.E. Guidelines for cancer-related pain: A systematic review of complementary and alternative medicine recommendations. Pain Practice. 2020;21(4):454–467. doi: 10.1111/papr.12964. [DOI] [PubMed] [Google Scholar]
- 53.Ng J.Y., Nault H., Nazir Z. Complementary and integrative medicine mention and recommendations: A systematic review and quality assessment of lung cancer clinical practice guidelines. Integr Med Res. 2020;10(1):1–9. doi: 10.1016/j.imr.2020.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng J.Y., Thakar H. Complementary and alternative medicine mention and recommendations are lacking in colon cancer clinical practice guidelines: A systematic review. Adv Integr Med. 2020;8(1):3–8. doi: 10.1016/j.aimed.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng J.Y .&, Lau S.K.C. Complementary and alternative medicine status in ovarian cancer guidelines: A systematic review. Eur J Integr Med. 2020;40:1–6. doi: 10.1016/j.eujim.2020.101227. [DOI] [Google Scholar]
- 56.Franck L., Chantler C., Dixon M. Should NICE evaluate complementary and alternative medicine? BMJ. 2007;334(7592):506. doi: 10.1136/bmj.39122.512211.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer F.H., Lewith G., Witt C.M., Linde K., von Ammon K., Cardini F., et al. High prevalence but limited evidence in complementary and alternative medicine: guidelines for future research. BMC Complement Altern Med. 2014;14(1):1–9. doi: 10.1186/1472-6882-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jafari A., Zanganeh M., Kazemi Z., Lael-Monfared E., Tehrani H. Iranian healthcare professionals’ knowledge, attitudes, and use of complementary and alternative medicine: a cross sectional study. BMC Complement Med Therap. 2021;21(1):1. doi: 10.1186/s12906-021-03421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joyce P., Wardle J., Zaslawski C. Medical student attitudes towards complementary and alternative medicine (CAM) in medical education: a critical review. J Complement Integr Med. 2016;13(4):333–345. doi: 10.1515/jcim-2014-0053. [DOI] [PubMed] [Google Scholar]
- 60.Scottish Intercollegiate Guidelines Network (SIGN). A Guideline Developer's Handbook. 2019. Available from URL: https://www.sign.ac.uk/what-we-do/methodology/
- 61.National Institute for Clinical Excellence (NICE). Developing NICE guidelines: the Manual. 2022. URL: https://www.nice.org.uk/process/pmg20/chapter/introduction
- 62.Grading of Recommendations, Assessment, Development and Evaluation (GRADE). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. URL: https://gdt.gradepro.org/app/handbook/handbook.html
- 63.PDQ® Health Professional Cancer Information - NCI [Internet]. 2015. Accessed on December 23, 2022. Available from: https://www.cancer.gov/publications/pdq/information-summaries</bib>
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Diagram
Supplement 2: Modified AGREE II Questions Used to Guide Scoring of CAM Sections of Each Guideline
Data Availability Statement
All relevant data are included in this manuscript.