Abstract
Alzheimer disease (AD) is currently the leading cause of cognitive decline and dementia worldwide. Recently, studies have suggested that other neurodegenerative comorbidities such as limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), Lewy body disease (LBD), and cerebrovascular disease frequently co-occur with Alzheimer disease neuropathologic change (ADNC) and may have significant cognitive effects both in isolation and synergistically with ADNC. Herein, we study the relative clinical impact of these multiple neurodegenerative pathologies in 704 subjects. Each of these pathologies is relatively common in the cognitively impaired population, while cerebrovascular pathology and ADNC are the most common in cognitively normal individuals. Moreover, while the number of concurrent neuropathologic entities rises with age and has a progressively deleterious effect on cognition, 44.3% of cognitively intact individuals are resistant to having any neurodegenerative proteinopathy (compared to 15.2% of cognitively impaired individuals) and 83.5% are resistant to having multiple concurrent proteinopathies (compared to 64.6% of cognitively impaired individuals). The presence of at least 1 APOE ε4 allele was associated with impaired cognition and the presence of multiple proteinopathies, while APOE ε2 was protective against cumulative proteinopathies. These results indicate that maintenance of normal cognition may depend on resistance to the development of multiple concurrent proteinopathies.
Keywords: Alzheimer disease, Cognitive reserve, Lewy body dementia, Limbic-predominant age-related TDP-43 encephalopathy, Resilience, Vascular dementia, National Alzheimer’s Coordinating Center (NACC)
INTRODUCTION
Originally described in the early 20th century (1), Alzheimer disease (AD) remains the most common neurodegenerative disease and underlying cause of dementia worldwide. The strongest associated risk factor for AD is aging, although a number of other genetic and environmental risk factors have been identified (2, 3). Alzheimer disease neuropathologic change (ADNC) is characterized by β-amyloid plaques (Aβ) and neurofibrillary tangles composed of phosphorylated tau (p-tau), which is thought to progress in stereotypical patterns (4–6) and correlate with clinical symptoms (7). More recent work has shown that other relatively common neurodegenerative diseases, including Lewy body disease (LBD), limbic-predominant age-related TDP-43 encephalopathy (LATE), and cerebrovascular disease frequently co-occur with ADNC and contribute to cognitive decline (8–26). A recent study found that the presence of “quadruple misfolded proteins” (i.e. p-tau, Aβ, TDP-43, and p-α-synuclein) was a relatively common finding in a community-based cohort and individuals with all 4 of these proteins (representing concurrent degrees of ADNC, LATE, and LBD), performed worse on cognitive measures than individuals with fewer comorbid neuropathologies (27).
Current clinical concepts of cognitive impairment and cognitive reserve, as well as neuropathologic concepts of resilience and resistance have been widely used across different studies and disciplines with significant variation in the application and definition of terms (28). It can be agreed that in the context of AD pathology, “resistance” may refer to subjects with intact cognition who had significantly less ADNC than their age-matched cognitively intact counterparts, while “resilience” may to refer to subjects with intact cognition with significantly more ADNC than their age-matched cognitively intact counterparts (28–32). Given the frequency of comorbid neuropathologic findings that may contribute to cognitive decline; however, these concepts become more complex as cognitive function may be more closely related to unassessed diseases. For example, a cognitively intact individual who has significant ADNC might be considered “resilient” against the ADNC but may have a lower degree of LATE-NC or less of a cerebrovascular disease burden than a cognitively impaired individual with similar levels of ADNC, and so would better be described as “apparent resilience” (31). Similarly, an individual with “resistance” to the development of ADNC might simultaneously be considered “resilient” against the presence of LATE-NC, LBD, or cerebrovascular disease.
In this report, we utilized the National Alzheimer’s Coordinating Center (NACC) dataset to study 704 total cases (including 79 cognitively normal individuals) with available Clinical Dementia Rating (CDR) scores and sufficient neuropathologic data to determine levels of ADNC, LATE-NC, LBD, and cerebrovascular disease. We investigated the impact of these 4 common neurodegenerative findings on cognition in terms of CDR, CDR sum of boxes, and Mini-Mental State Examination (MMSE) with a focus on cases that are resistant to and resilient against multiple neurodegenerative pathologies. We also establish approximate levels of ADNC and multiple concurrent pathologies that could be expected in cognitively normal individuals by age.
MATERIALS AND METHODS
Case selection, neuropathologic variable definition, and exclusion criteria
Using the Uniform data set (UDS) and neuropathology (NP) data set from the NACC, established with funding from the National Institute on Aging (U01 AG016976), we identified a total of 7709 cases with unique NACC identification numbers that had patient evaluation within the final 24 months of life. UDS and NP data were downloaded from NACC (https://naccdata.org/). Standardized UDS variable definitions (https://naccdata.org/data-collection/forms-documentation/uds-3) and NP variable definitions (https://naccdata.org/data-collection/forms-documentation/np-11) from NACC were used, as described previously (33, 34). All participants provided written informed consent at each Alzheimer’s Disease Research Center that contributed tissue and clinical data to NACC. We initially excluded 361 cases diagnosed with FTLD-tau (NPFTDTAU), 67 cases with Pick disease (NACCPICK), 4 cases MAPT mutation (NPFTDT2), 93 cases with corticobasal degeneration (NACCCBD), 177 with progressive supranuclear palsy (NACCPROG), 40 with amyotrophic lateral sclerosis/motor neuron disease (NPALSMND), 136 with frontotemporal lobar degeneration (FTLD)-tau (NPFTDTAU), 87 with prion disease (NACCPRIO), 5 with multiple system atrophy (NPPDXB), 2 with trinucleotide repeat diseases (NPPDXD), and 6 with Down syndrome (NACCDOWN) (Fig. 1).
Figure 1.

Flow chart demonstrating NACC cases excluded from study. *Note: numerous cases were missing data for multiple ADNC/LATE-NC/LBD/cerebrovascular variables.
Given that our experimental design required data for ADNC, limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), LBD, and cerebrovascular disease, we excluded all cases without some data for each of these variables (Fig. 1). We then collapsed variables for each of these neuropathologic entities into binary variables for data analysis. ADNC levels were identified using NACC variable NPADNC. For cases in which the NPADNC variable was not available, ADNC levels 0–1 (“not” or “low”) or 2–3 (“intermediate” or “high”) were derived from a combination of Braak stage (NACCBRAA), Thal phase (NPTHAL), and CERAD neuritic plaque score (NACCNEUR) (6, 35). For our purposes, ADNC scores of 0–1 were assigned the score 0, and ADNC scores of 2–3 were assigned the score 1. Cases for which ADNC level could not be determined were discarded. For assessing LATE-NC stage, we used NACC variables NPTDPB (TDP-43-immunoreactive inclusions—amygdala), NPTDPC (TDP-43-immunoreactive inclusions—hippocampus), NPTDPD (TDP-43-immunoreactive inclusions—entorhinal/inferior temporal cortex), and NPTDPE (TDP-43-immunoreactive inclusions—neocortex) to assign LATE-NC stage 0 (no TDP-43-positive pathology), stage 1 (amygdala only), stage 2 (+hippocampus), and stage 3 (+middle frontal gyrus) (10). LATE-NC scores of 0–1 were assigned the score 0, and LATE-NC scores of 2–3 were assigned the score 1. Cases for which LATE-NC level could not be determined were discarded. For Lewy body pathology, we used the McKeith staging system (36, 37) and the NACC variable NACCLEWY, where 0 = none, 1 = brainstem predominant, 2 = limbic (transitional), 3 = neocortical (diffuse), 4 = amygdala predominant, and 5 = olfactory bulb. Absence, brainstem predominant, or olfactory bulb Lewy bodies were assigned the score 0, and more advanced LBD was assigned the score 1. Cases for which Lewy body pathology could not be determined were discarded.
Given the variety of cerebrovascular pathologies and variables provided by NACC, we performed multivariate regression analysis to identify which of these were significantly associated with cognitive impairment using all available NACC cases with cerebrovascular variables. The presence of infarcts and lacunes (NACCINF; p = 0.0412), single or multiple old hemorrhages (NPHEMO; p = 0.0410), white matter rarefaction (NPWMR; p = 0.0293), and moderate-severe arteriolosclerosis (NACCARTE; p < 0.0001) were included, similar to previous research (38). Subjects with 1 or more of these findings were assigned vascular pathology score 1, if none were present the subject was assigned the vascular pathology score 0 (39–42). Cases for which vascular pathology could not be determined were discarded. The main NP variables (ADNC, LATE-NC, LBD, and vascular) were dichotomized and used as binary variables to allow for combining variables from different types of pathologies into a single system for comparative analysis between pathologies and in cohorts with multiple comorbid pathologies and to help simplify otherwise more complicated and noisy data.
Finally, we screened all cases for age and availability of global CDR. Cases were excluded if they lacked CDR, had CDR = 0 at their last patient encounter and were less than 50 years old at the time of death, and/or if they had CDR = 0 at their last patient encounter and the last patient encounter occurred >24 months before death since we could not be certain of the cognitive status at the time of death. In total, 704 cases met our experimental inclusion criteria. Demographic information on these cases can be found in Table 1. Of note, however, for linear models of age versus ADNC, LATE-NC, and Lewy body pathology, additional NACC cases were included that may not have complete data on the other neuropathologic variables.
Table 1.
Demographic data in cognitively normal and cognitively impaired individuals
| Overall cohort (n = 704) | Cognitively normal (CDR = 0; n = 79) | Cognitively impaired (CDR ≥ 0.5; n = 625) | p value | |
|---|---|---|---|---|
| Mean age (years) | 74.1 ± 0.3 | 72.1 ± 1.2 | 74.4 ± 0.4 | 0.0563 |
| Sex (M:F) | 393:311 | 48:31 | 345:280 | 0.4004 |
| Race | 0.4074 | |||
| Caucasian | 89.8% | 87.8% | 90.3% | |
| African American/Black | 7.5% | 2.7% | 8.2% | |
| Asian | 2.0% | 6.8% | 1.2% | |
| Hawaiian/Pacific Islander | 0.7% | 2.7% | 0.3% | |
| Education (years) | 15.4 ± 0.1 | 15.8 ± 0.4 | 15.3 ± 0.1 | 0.1129 |
| APOE status | ||||
| APOE ε2 presence | 9.7% | 15.2% | 9.0% | 0.1023 |
| APOE ε4 presence | 40.1% | 24.1% | 42.1% | 0.0022 |
Data analysis
All univariate and multivariate statistical analyses have been completed using GraphPad Prism version 9 (GraphPad Software, Inc., La Jolla, CA) and MedCalc (http://medcalc.org; MedCalc Software Ltd, Ostend, Belgium). All linear modelings (age vs ADNC, age vs LATE-NC, age vs Lewy body pathology) were created using the Pearson correlation coefficient. Analysis of number of concurrent pathologies and clinical variables (number of concurrent pathologies vs age, number of concurrent pathologies vs CDR, number of concurrent pathologies vs CDR sum of boxes, number of concurrent pathologies vs MMSE) was performed using analysis of variance. Analyses of NP and UDS variables between CDR = 0 and CDR ≥ 0.5 were performed with multiple t-tests. All the resulting p values were adjusted for multiple comparisons using Bonferroni correction, and the cutoff was 0.05. These models were adjusted by age at death (NACCDAGE).
RESULTS
Cognitively normal and cognitively impaired cohorts
Using global CDR score, we first grouped cases by cognitive status, where CDR = 0 was “cognitively normal” and CDR ≥ 0.5 was “cognitively impaired”. Seventy-nine cases had a status of CDR = 0 within 24 months of death and were classified as cognitively normal, while 625 had mild-severe cognitive impairment (CDR = 0.5–3) (43). While there was no significant difference in the age, gender, race, or education level between these 2 groups (Table 1), patients with CDR = 0 had significantly less frequent intermediate-high level ADNC compared to the CDR ≥ 0.5 cohort (34.2% vs 69.6%; p < 0.0001) and less frequent stages 2–3 LATE-NC (13.9% vs 32.6%; p = 0.0004). There were also less frequent instances of at least 1 neuropathologic proteinopathy (55.7% vs 84.8%; p < 0.0001) and significantly less frequent instances of multiple coexisting proteinopathies (16.5% vs 35.4%; p = 0.0006) in the cognitively intact group. Some degree of cerebrovascular pathology was relatively common in both groups (Table 2). After correcting for multiple tests, the CDR = 0 cohort had significantly lower overall ADNC, Braak stage, Thal phase, CERAD NP score, LATE-NC stage, arteriolosclerosis, cerebral cortex atrophy severity, and hippocampal atrophy severity. Lewy body pathology was not significantly different between the 2 groups, although there was a trend toward a higher average stage in the cognitively impaired subjects (Table 3).
Table 2.
Comparison of pathologies in cognitively normal individuals and cognitively impaired individuals
| Cognitively normal (CDR = 0; n = 79) | Cognitively impaired (CDR ≥ 0.5; n = 625) | p value | |
|---|---|---|---|
| ADNC, Int-High | 27 (34.2%) | 435 (69.6%) | <0.0001 |
| LATE-NC, stages 2–3 | 11 (13.9%) | 204 (32.6%) | 0.0004 |
| LBD, stages 2–3 | 17 (21.5%) | 146 (23.4%) | 0.7785 |
| Cerebrovascular disease | 39 (43.8%) | 289 (46.2%) | 0.6331 |
| At least 1 proteinopathy | 44 (55.7%) | 530 (84.8%) | <0.0001 |
| Multiple proteinopathies | 13 (16.5%) | 221 (35.4%) | 0.0006 |
| At least 1 pathology | 46 (58.2%) | 541 (86.6%) | 0.0208 |
| Multiple pathologies | 15 (19.0%) | 260 (41.6%) | <0.0001 |
Table 3.
Significant pathologic variables in CDR = 0 and CDR ≥ 0.5 cohorts
| Pathologic (NP) variable | NACC designation | CDR = 0 mean | CDR ≥ 0.5 mean | Adj. OR (95% CI) | p value | Adj. p value |
|---|---|---|---|---|---|---|
| ADNC level (ABC score) | NPADNC | 1.14 | 2.05 | 4.41 (2.69–7.23) | <0.0001 | <0.0001 |
| Braak stage | NACCBRAA | 2.39 | 4.09 | – | <0.0001 | <0.0001 |
| Thal phase | NPTHAL | 2.24 | 3.36 | – | <0.0001 | <0.0001 |
| CERAD NP score | NACCNEUR | 0.92 | 1.87 | – | <0.0001 | <0.0001 |
| LATE-NC stage | NPTDPB-NPTDPE | 0.34 | 0.77 | 2.99 (1.55–5.79) | 0.0012 | 0.0002 |
| Lewy body pathology | NACCLEWY | 0.17 | 0.82 | 1.11 (0.63–1.96) | 0.6071 | 0.1023 |
| Arteriolosclerosis | NACCARTE | 1.12 | 1.65 | 1.92 (1.23–2.98) | 0.0093 | 0.0031 |
CDR, Clinical Dementia Rating; Adj. OR, adjusted odds ratio; CI, confidence interval; bold, significant to a level of p < 0.05.
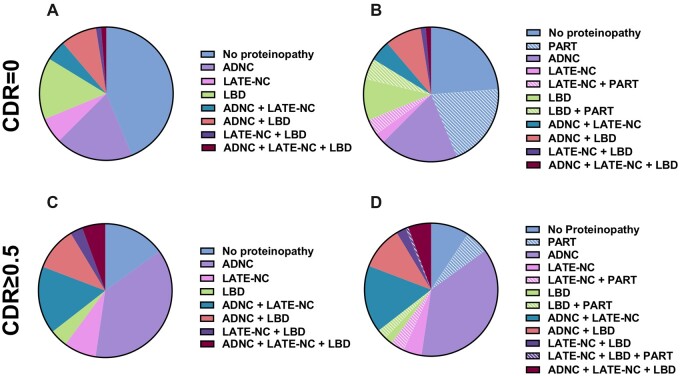
These results suggest that 34.2% of cognitively normal individuals are “resilient” in the face of intermediate/high-level ADNC (they have significant AD pathology, but no cognitive deficit) (Table 2). It is notable that many of the individuals without intermediate/high-level ADNC display lower degrees of neurofibrillary degeneration and diffuse plaque deposition, and 20% of these subjects would qualify as “definite” primary age-related tauopathy (PART), compared to 5.8% of cognitively impaired individuals (Fig. 2) (44–46). Most importantly, however, 44.3% of cognitively normal individuals had no evidence of any of the assessed neurodegenerative proteinopathies (intermediate to high ADNC, LATE-NC, or LBD), and 83.5% did not develop more than 1 neurodegenerative proteinopathy. As expected, there were also a number of clinical variables that differed between the cognitively normal and cognitively impaired cohorts, including Hachinski ischemic score (47), difficulty with language, bills, hobbies, independence, judgement and problem solving, attention, remembering dates, traveling, doing taxes, personal care, preparing food, as well as apathy and agitation, focal neurologic signs/symptoms, and personality/behavioral issues, among others (Supplementary Data Table S1).
Figure 2.
Proportion of CDR = 0 subjects with each proteinopathy (A) excluding and (B) including primary age-related tauopathy (PART) and CDR ≥ 0.5 subjects with each proteinopathy (C) excluding and (D) including PART.
Effect of increasing number of concurrent neuropathologic findings
We next examined the effect of single and multiple concurrent neuropathologic findings on cognition in terms of global CDR, CDR sum of boxes (48), and MMSE. Individuals without any of the assessed pathologies were significantly younger than individuals with at least 1 of ADNC, LATE-NC, LBD, and/or cerebrovascular pathology (66.9 ± 3.1 vs 72.7 ± 0.2 years; p = 0.0046), and subject age becomes progressively older with increasing numbers of concurrent pathologies (p = 0.0156) (Fig. 3A), as previously described (26, 49, 50). Global CDR and CDR sum of boxes are significantly lower and MMSE is significantly higher in individuals without any of the assessed pathologies compared to those with at least 1 of ADNC, LATE-NC, LBD, and/or cerebrovascular pathology (Table 4), and the differences in these variables become more pronounced with increasing numbers of neuropathologic findings (Fig. 3B–D). Despite the frequency of cerebrovascular disease noted in both the cognitively impaired and cognitively normal individuals (Table 2), this pathology does not appear to be without consequence. Global CDR is significantly higher (1.8 ± 0.2 vs 0.9 ± 0.3; p = 0.0141), CDR sum of boxes is higher (10.0 ± 0.9 vs 4.8 ± 1.4; p = 0.0023), and MMSE is significantly lower (20.1 ± 1.1 vs 24.6 ± 1.6; p = 0.0224) in cases with isolated cerebrovascular pathology compared to those without. The effects of additional comorbid pathologies also tend to contribute to cognitive decline even in cases with severe pathology in 1 category (i.e. patients with high ADNC/Braak stage VI had further cognitive impairment in the presence of stages 2–3 LATE-NC). This analysis demonstrates that while each of the assessed proteinopathies is capable of causing cognitive impairment individually, the presence of additional concurrent proteinopathies results in progressively worse cognitive outcomes.
Figure 3.
Correlation of number of concurrent neuropathologic entities with (A) age, (B) global CDR, (C) CDR sum of boxes, and (D) MMSE.
Table 4.
Comparison of cognition in cohorts with various combinations of neuropathologic findings
| n | Global CDR | CDR sum of boxes | MMSE | |
|---|---|---|---|---|
| No pathology identified | 65 | 0.9 ± 0.3 | 4.8 ± 1.4 | 24.6 ± 1.6 |
| ≥1 pathologies | 639 | 2.1 ± 0.06 | 11.9 ± 0.2 | 17.4 ± 0.2 |
| p value | – | 0.0484 | 0.0005 | 0.0004 |
| 1 pathology | 254 | 1.6 ± 0.1 | 9.2 ± 0.4 | 21.0 ± 0.4 |
| p value | – | 0.0223 | 0.0270 | 0.0791 |
| 2 pathologies | 274 | 2.0 ± 0.04 | 11.6 ± 0.3 | 18.2 ± 0.4 |
| p value | – | <0.0001 | 0.0006 | 0.0168 |
| 3 pathologies | 99 | 2.3 ± 0.1 | 13.3 ± 0.3 | 14.6 ± 0.4 |
| p value | – | <0.0001 | <0.0001 | <0.0001 |
| 4 pathologies | 12 | 2.6 ± 0.1 | 15.4 ± 0.4 | 10.2 ± 0.8 |
| p value | – | <0.0001 | <0.0001 | <0.0001 |
| ADNC, Int-high | 134 | 2.0 ± 0.1 | 11.8 ± 0.6 | 19.8 ± 1.3 |
| p value | – | <0.0001 | <0.0001 | 0.0284 |
| LATE-NC, stages 2–3 | 31 | 2.1 ± 0.2 | 12.3 ± 1.2 | 22.1 ± 1.4 |
| p value | – | <0.0001 | 0.0009 | 0.3226 |
| LBD, stages 2–3 | 25 | 1.2 ± 0.2 | 7.0 ± 1.5 | 24.5 ± 1.4 |
| p value | – | 0.5506 | 0.3708 | 0.9708 |
| Vascular | 64 | 1.8 ± 0.2 | 10.0 ± 0.9 | 20.1 ± 1.1 |
| p value | – | 0.0141 | 0.0023 | 0.0224 |
| ADNC + LATE-NC | 76 | 2.3 ± 0.1 | 13.3 ± 0.6 | 15.0 ± 0.9 |
| p value | – | <0.0001 | <0.0001 | <0.0001 |
| ADNC + LBD | 41 | 2.2 ± 0.1 | 12.7 ± 0.8 | 15.3 ± 1.8 |
| p value | – | <0.0001 | <0.0001 | 0.0003 |
| ADNC + Vascular | 115 | 1.9 ± 0.1 | 11.0 ± 0.7 | 20.1 ± 1.2 |
| p value | – | 0.0002 | <0.0001 | 0.0256 |
| LATE-NC + LBD | 6 | 2.2 ± 0.4 | 12.4 ± 2.1 | – |
| p value | – | 0.1980 | 0.1086 | – |
| LATE-NC + Vascular | 23 | 2.0 ± 0.2 | 11.8 ± 1.3 | 19.5 ± 0.7 |
| p value | – | 0.0372 | 0.0060 | 0.0653 |
| LBD + Vascular | 13 | 1.3 ± 0.2 | 7.0 ± 1.4 | 21.1 ± 3.1 |
| p value | – | 0.5581 | 0.4946 | 0.3651 |
| ADNC + LATE-NC + LBD | 34 | 2.6 ± 0.1 | 14.9 ± 0.6 | 10.0 ± 1.2 |
| p value | – | <0.0001 | <0.0001 | <0.0001 |
| ADNC + LATE-NC + Vascular | 29 | 2.5 ± 0.1 | 14.7 ± 0.8 | 13.1 ± 1.6 |
| p value | – | <0.0001 | <0.0001 | <0.0001 |
| ADNC + LBD + Vascular | 22 | 1.6 ± 0.2 | 9.4 ± 1.0 | 19.0 ± 1.4 |
| p value | – | 0.1904 | 0.0674 | 0.0548 |
| LATE-NC + LBD + Vascular | 14 | 2.4 ± 0.2 | 13.8 ± 1.1 | 17.4 ± 0.8 |
| p value | – | 0.0250 | 0.0045 | 0.0421 |
Effect of patient age on neuropathologic findings
The level of ADNC is significantly correlated to age at death in both cognitively impaired (p < 0.0001) and cognitively normal individuals (p = 0.0344) (Fig. 4A, B). Using these linear models, the approximate level of ADNC for each age can be determined, such that a 75-year-old with cognitive impairment would be expected to have an intermediate level of ADNC (∼2), which is considered an adequate explanation for his or her cognitive impairment in isolation (6), while a 75-year-old cognitively intact subject would be expected to have a low level of ADNC (∼1), and those individuals with less than this expected level for each age (many of which have PART) could be considered “resistant” to ADNC (32). Interestingly, we found a significant correlation between age and LATE-NC in the cognitively impaired cohort only (Fig. 4C, D), while LBD was not significantly correlated to age in either group (Fig. 4E, F). The number of neuropathologic diagnoses is significantly correlated with age in linear models of cognitively impaired and cognitively intact individuals both when including (Fig. 4G, H) and excluding (Fig. 4I, J) cerebrovascular disease. With this model, a 75-year-old individual with cognitive impairment would be expected to have 2–3 neuropathologic diagnoses, while a 75-year-old cognitively intact patient would only be expected to have 1 of the neuropathologic diagnoses assessed here. Cognitively intact individuals with fewer neuropathologic diagnoses than would be expected for their given age (Fig. 4H, J) could thus be considered “resistant” to the development of 1 or more proteinopathies or overall neuropathologic entities (including cerebrovascular disease).
Figure 4.

Correlation of age and (A, B) Alzheimer disease neuropathologic change (ADNC), (C, D) Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), (E, F) Lewy body pathology, (G, H) number of concurrent neuropathologic diagnoses, and (I, J) number of concurrent neuropathologic diagnoses (excluding cerebrovascular disease) in cognitively impaired (CDR ≥ 0.5) and cognitively normal (CDR = 0) individuals.
Effect of APOE status on cognition and number of concurrent neuropathologic findings
The presence of at least 1 APOE ε4 allele was significantly associated with cognitive impairment (p = 0.0022), and there was a nonsignificant trend toward more frequent instances of at least 1 APOE ε2 allele in cognitively normal individuals (p = 0.1023) (Table 1). Fifty-eight patients in the cognitively impaired group had APOE ε4/ε4 status, while no cognitively normal patients had this allele combination (p = 0.0016). There were 1 cognitively intact and 3 cognitively impaired patients with APOE ε2/ε2 status (p = 0.3795). The presence of at least 1 APOE ε4 allele was significantly associated with the presence of at least 1 proteinopathy (ADNC, LATE-NC, or LBD) (p < 0.0001), as well as the presence of multiple concurrent proteinopathies (p = 0.0143). Conversely, the presence of at least 1 APOE ε2 allele was significantly associated with the absence of any proteinopathy (p = 0.0014) and the absence of multiple concurrent proteinopathies (p = 0.0097). Similar significance patterns were present when analyzing the cohort of cognitively impaired subjects separately but not in the cognitively intact individuals (Table 5).
Table 5.
Impact of APOE allele status on concurrent neuropathologic findings
| APOE ε2 presence | APOE ε4 presence | |
|---|---|---|
| Entire cohort (n = 704) | ||
| Absence of proteinopathy | 17.8% | 10.7% |
| ≥1 proteinopathy | 7.8% | 46.3% |
| p value | 0.0014 | <0.0001 |
| Absence/single proteinopathy | 11.7% | 36.8% |
| Multiple proteinopathies | 5.6% | 46.6% |
| p value | 0.0097 | 0.0143 |
| Cognitively intact cohort (n = 79) | ||
| Absence of proteinopathy | 18.6% | 19.4% |
| ≥1 proteinopathy | 11.1% | 27.9% |
| p value | 0.5309 | 0.4367 |
| Absence/single proteinopathy | 16.7% | 21.2% |
| Multiple proteinopathies | 7.7% | 38.5% |
| p value | 0.6786 | 0.2841 |
| Cognitively impaired cohort (n = 625) | ||
| Absence of proteinopathy | 20.0% | 9.5% |
| ≥1 proteinopathy | 7.0% | 47.9% |
| p value | 0.0003 | <0.0001 |
| Absence/single proteinopathy | 10.9% | 39.6% |
| Multiple proteinopathies | 5.4% | 47.1% |
| p value | 0.0272 | 0.0753 |
DISCUSSION
Although AD remains the most common cause of dementia worldwide, it has become clear that only a minority of these cases represent “pure ADNC.” Most cases have additional pathologies evident at brain autopsy, which may play an additive or synergistic role in cognitive decline (8, 9, 11–14, 16, 18, 20–24, 27, 39, 49, 51, 52). While it is difficult to determine the exact contribution of each disease process to cognitive impairment, in general, the accumulation of additional proteinopathies and cerebrovascular changes results in further cognitive decline on a population level (9, 12, 15, 27, 39, 53). It is similarly important to understand the biology of patients who are cognitively intact in the face of ADNC and other concurrent pathologies (“resilient” individuals) and those who are able to avoid the underlying neuropathologic changes entirely (“resistant” individuals).
The cohort analyzed in this study reveals a number of interesting insights into the interplay between these 4 common neuropathologic findings and APOE status. There is a significant correlation between the number of neurodegenerative pathologies and declining cognition, between APOE status and the number of neurodegenerative pathologies, and between the APOE status and cognitive status (Tables 1, 4, and 5). This suggests that in this cohort the presence of an APOE ε4 allele increases the risk of developing an initial proteinopathy as well as the risk of developing subsequent proteinopathies (14), while the presence of an APOE ε2 allele appears to be protective against both. In addition, the presence of an APOE ε4 allele is significantly more common in the cognitively impaired group. Interestingly, APOE status was not significantly different between cases with and without pathology within the cognitively normal cohort, although this could have been due to the relatively low case numbers in this comparison, or may suggest that other protective factors may play a role in their resistance or resilience (Table 5).
While the frequency of stages 2–3 LATE-NC in cognitively impaired subjects is comparable to what was observed in previous studies (10, 25, 27, 54, 55), the frequency of LATE-NC in cognitively intact individuals is significantly lower (Table 2), and LATE-NC appears to have a significantly deleterious effect on cognition in individuals with concurrent ADNC and LBD, suggesting that LATE-NC may be a major factor in cognitive status (22, 56, 57). There are also notable differences in the specific combinations of pathologies (Table 4). For example, patients with both ADNC and LATE-NC have significantly worse cognitive outcomes that patients with ADNC and cerebrovascular pathology (p = 0.0073) and cases with LBD and LATE-NC have significantly worse cognitive outcomes than patients with LBD and cerebrovascular pathology (p = 0.0370), while there is no significant cognitive difference in patients with LATE-NC and either LBD or cerebrovascular disease (p = 0.6541). This suggests that while all of these pathologies contribute to the overall cognitive state of the patient, they do not all contribute equally and there may be an additional nuance in the interactions between these diseases, including synergistic effects.
Perhaps more importantly, these cognitively normal individuals are significantly more likely to be free of multiple concurrent neurodegenerative proteinopathies and significantly more likely to be free of any identifiable neurodegenerative proteinopathy than cognitively impaired individuals, and these groups of individuals could therefore be considered resistant to the development of multiple proteinopathies and any proteinopathy, respectively (Table 2). Also notable are the more rare “resilient” individuals. We identified 13 cognitively normal individuals who had multiple neurodegenerative proteinopathies, including 7 with ADNC and LBD, 4 with ADNC and LATE-NC, 1 with LATE-NC and LBD, and 1 with all 3 proteinopathies. 24.1% of cognitively normal individuals have a single APOE ε4 allele (Table 1) and so could be considered “resilient” against this genetic risk factor as well. Conversely, many of the cognitively impaired individuals had multiple proteinopathies, and it was rare (15.2%) for these patients to not have at least one of the assessed proteinopathies (in addition to frequent cerebrovascular findings).
As reported in previous studies (9, 11–13, 15–19, 21, 23, 24, 27, 38, 39, 49, 53, 54), we determined that the number of NP entities present increases with age and has a mounting detrimental effect on cognition (Figs. 3 and 4G, I and Table 4), but we also found a progressive increase in the number of neuropathologic entities present in cognitively normal individuals with increasing age (Fig. 4H, J). This suggests that while some individuals with retained cognition may still accumulate multiple concurrent neuropathologic diagnoses with age, the majority of individuals destined to maintain good cognition might be resistant not only to developing an initial neurodegenerative entity but might harbor some resistance to developing additional pathologies. Importantly, the age of cognitively intact individuals was not significantly different than the age of cognitively impaired individuals (Table 1), reiterating the idea that these differences in pathologic findings cannot be explained by age alone and it should not be assumed that cognitively impaired individuals will necessarily develop cognitive deficits with age.
The primary limitation of this study is that the NACC dataset is not entirely representative of the general population or other community-based cohorts. This cohort is highly enriched for Caucasian patients, patients with dementia, relatively high levels of education, and rare disease states (Table 1), a common issue with clinical and multi-institutional cohorts (58). It contains a higher number of dementia cases and likely contains more significant and more severe neuropathologic findings than would be expected in the general population, although this would likely have less effect on findings derived solely from the CDR = 0 population. In addition, the NACC dataset and NP/UDS variables have undergone many rounds of revision over the years as new variables were added and both the clinical and neuropathologic criteria were refined. As a result, there are many cases that did not have sufficient neuropathologic data to be included in this study (i.e. missing data on TDP-43 distribution or specific vascular variables), leading to the exclusion of many cases, as detailed in the Materials and Methods section. There is also quantifiable pathology that is not fully captured with these neuropathologic variables. For example, while the Braak stage details the location of neurofibrillary degeneration, it does not take into account the density of tau deposition in each assessed region, which has been shown to vary significantly within Braak stages and have a significant independent effect on cognition in tauopathies (59). Other variables associated with ADNC, LATE-NC, LBD, and cerebrovascular disease also primarily address the type and distribution of pathologies instead of the regional severity. In addition, these neuropathologic variables were each dichotomized, which allows for simplification in understanding the interactions between disease processes, however results in loss of some more nuanced information and statistical power (60).
In the context of previous literature, our findings add to the conclusion that the presence of multiple concurrent neuropathologic entities is a major, if not the main, contributor to the decline in cognitive status. These results indicate that while being resistant and resilient against multiple entities is relatively rare for patients in the NACC dataset, these patients do exist and may become more common with more complete neuropathologic analyses in people with normal cognition. The cause for the maintenance of cognitive status in these individuals is unclear, and future studies should focus on repeating these analyses in community-based cohorts and identifying potential environmental factors or additional genetic variants which may, in part, provide protection against the development of these neuropathologic entities and the ability to maintain cognition once this pathology begins to develop. Given the prevalence of multiple concurrent neuropathologic contributors to cognitive status, determining the combination and effects of each will be crucial in the future evaluation of dementia patients and for designing more effective treatment options.
Supplementary Material
Contributor Information
Jamie M Walker, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Timothy E Richardson, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
FUNDING
J.M.W. and T.E.R are supported in part by National Institute on Aging (NIA) R21 AG078505. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the article. The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), and P30 AG072959 (PI James Leverenz, MD).
CONFLICT OF INTEREST
The authors have no duality or conflicts of interest to declare.
SUPPLEMENTARY DATA
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Alzheimer A, Stelzmann RA, Schnitzlein HN, et al. An English translation of Alzheimer's 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat 1995;8:429–31 [DOI] [PubMed] [Google Scholar]
- 2. Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019;15:565–581 [DOI] [PubMed] [Google Scholar]
- 3. Loeffler DA. Modifiable, non-modifiable, and clinical factors associated with progression of Alzheimer's disease. J Alzheimers Dis 2021;80:1–27 [DOI] [PubMed] [Google Scholar]
- 4. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 5. Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 6. Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 2012;71:362–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White LR, Edland SD, Hemmy LS, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 2016;86:1000–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabinovici GD, Carrillo MC, Forman M, et al. Multiple comorbid neuropathologies in the setting of Alzheimer's disease neuropathology and implications for drug development. Alzheimers Dement (N Y) 2017;3:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019;142:1503–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAleese KE, Colloby SJ, Thomas AJ, et al. Concomitant neurodegenerative pathologies contribute to the transition from mild cognitive impairment to dementia. Alzheimers Dement 2021;17:1121–33 [DOI] [PubMed] [Google Scholar]
- 12. Boyle PA, Yu L, Wilson RS, et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018;83:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson JL, Richardson H, Xie SX, et al. The development and convergence of co-pathologies in Alzheimer's disease. Brain 2021;144:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018;141:2181–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapasi A, DeCarli C, Schneider JA.. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 2017;134:171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res 2007;1184:284–94 [DOI] [PubMed] [Google Scholar]
- 17. Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–204 [DOI] [PubMed] [Google Scholar]
- 18. Rahimi J, Kovacs GG.. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 2014;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAleese KE, Walker L, Erskine D, et al. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol 2017;27:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spires-Jones TL, Attems J, Thal DR.. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 2017;134:187–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawas CH, Kim RC, Sonnen JA, et al. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ study. Neurology 2015;85:535–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James BD, Wilson RS, Boyle PA, et al. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain 2016;139:2983–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer's contributions to dementia and cognitive resilience in the 90+ study. Acta Neuropathol 2018;136:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Flores R, Wisse LEM, Das SR, et al. Contribution of mixed pathology to medial temporal lobe atrophy in Alzheimer's disease. Alzheimers Dement 2020;16:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas DX, Bajaj S, McRae-McKee K, et al. Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and Lewy bodies with cognitive impairment in individuals with or without Alzheimer's disease neuropathology. Sci Rep 2020;10:14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forrest SL, Kovacs GG.. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci 2022;1–17 [DOI] [PubMed] [Google Scholar]
- 27. Karanth S, Nelson PT, Katsumata Y, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol 2020;77:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arenaza-Urquijo EM, Vemuri P.. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 2018;90:695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aiello Bowles EJ, Crane PK, Walker RL, et al. Cognitive resilience to Alzheimer's disease pathology in the human brain. J Alzheimers Dis 2019;68:1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latimer CS, Burke BT, Liachko NF, et al. Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montine TJ, Cholerton BA, Corrada MM, et al. Concepts for brain aging: Resistance, resilience, reserve, and compensation. Alzheimers Res Ther 2019;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walker JM, Kazempour Dehkordi S, Schaffert J, et al. The spectrum of Alzheimer-type pathology in cognitively normal individuals. J Alzheimer's Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beekly DL, Ramos EM, van Belle G, et al. ; NIA-Alzheimer's Disease Centers. The National Alzheimer's Coordinating Center (NACC) Database: An Alzheimer disease database. Alzheimer Dis Assoc Disord 2004;18:270–7 [PubMed] [Google Scholar]
- 34. Beekly DL, Ramos EM, Lee WW, et al. ; NIA Alzheimer's Disease Centers. The National Alzheimer's Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord 2007;21:249–58 [DOI] [PubMed] [Google Scholar]
- 35. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005;65:1863–72 [DOI] [PubMed] [Google Scholar]
- 37. Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: A multi-centre study. Acta Neuropathol 2021;141:159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arvanitakis Z, Capuano AW, Leurgans SE, et al. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol 2016;15:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer's disease. Acta Neuropathol 2016;131:659–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McAleese KE, Alafuzoff I, Charidimou A, et al. Post-mortem assessment in vascular dementia: Advances and aspirations. BMC Med 2016;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): The contribution of cerebrovascular pathology to cognitive impairment. Brain 2016;139:2957–2969 [DOI] [PubMed] [Google Scholar]
- 42. Skrobot OA, O'Brien J, Black S, et al. ; VICCCS Group. The vascular impairment of cognition classification consensus study. Alzheimers Dement 2017;13:624–633 [DOI] [PubMed] [Google Scholar]
- 43. Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993;43:2412–4 [DOI] [PubMed] [Google Scholar]
- 44. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: The exception that proves the rule? J Neurol Neuromed 2016;1:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Walker JM, Richardson TE, Farrell K, et al. Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol 2021;80:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fischer P, Jellinger K, Gatterer G, et al. Prospective neuropathological validation of Hachinski's Ischaemic Score in dementias. J Neurol Neurosurg Psychiatry 1991;54:580–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: A Texas Alzheimer's Research Consortium Study. Arch Neurol 2008;65:1091–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beach TG, Malek-Ahmadi M.. Alzheimer's disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis 2021;79:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spina S, La Joie R, Petersen C, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer's disease. Brain 2021;144:2186–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol 2017;16:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tome SO, Thal DR.. Co-pathologies in Alzheimer's disease: Just multiple pathologies or partners in crime? Brain 2021;144:706–708 [DOI] [PubMed] [Google Scholar]
- 53. Boyle PA, Yang J, Yu L, et al. Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain 2017;140:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nelson PT, Brayne C, Flanagan ME, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: Combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol 2022;144:27–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gauthreaux KM, Teylan MA, Katsumata Y, et al. Limbic-predominant age-related TDP-43 encephalopathy: Medical and pathologic factors associated with comorbid hippocampal sclerosis. Neurology 2022;98:e1422–e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Katsumata Y, Abner EL, Karanth S, et al. Distinct clinicopathologic clusters of persons with TDP-43 proteinopathy. Acta Neuropathol 2020;140:659–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montine TJ, Corrada MM, Kawas C, et al. Association of cognition and dementia with neuropathologic changes of Alzheimer disease and other conditions in the oldest-old. Neurology 2022;99:e1067–e1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider JA, Aggarwal NT, Barnes L, et al. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009;18:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iida MA, Farrell K, Walker JM, et al. Predictors of cognitive impairment in primary age-related tauopathy: An autopsy study. Acta Neuropathol Commun 2021;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Altman DG, Royston P.. The cost of dichotomising continuous variables. BMJ 2006;332:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.