Abstract
This study investigated the effects of phytase and monocalcium phosphate supplementation on the dephosphorylation of phytic acid [myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6] in cecectomized laying hens using total excreta collection. Four corn-soybean meal-rapeseed meal-based diets were mixed with or without 6 g of monocalcium phosphate/kg, with or without supplementation of 1,500 FTU phytase/kg, and had the same calcium concentration at 39 g/kg of feed. Each diet was tested in 5 replicates using a row-column design with 10 cecectomized laying hens in 2 periods. The hens received 120 g/d of feed while being housed individually in metabolism units, and total excreta were collected for a period of 4 d. The monocalcium phosphate × phytase interaction was not significant for InsP6 degradation (P = 0.054). Phytase increased InsP6 disappearance from 13% to 83% (P < 0.001), whereas monocalcium phosphate had no effect. Concentrations of most of the lower inositol phosphate isomers in excreta were higher when monocalcium phosphate was added to the diets. The concentration of Ins(1,2,5,6)P4 in excreta was the highest among the studied partially dephosphorylated inositol phosphates with phytase supplementation and was higher than in diets without phytase supplementation (P < 0.001). Supplementation with phytase increased myo-inositol concentration in excreta (P = 0.002), whereas monocalcium phosphate had no effect. Phosphorus utilization ranged from 4% to 18% and was not significantly affected by the treatments. These results suggest that phytase supplementation markedly increased InsP6 degradation in laying hens. The cecectomized laying hen assay may be suitable for studying the effects of phytase supplementation on phytate dephosphorylation under dietary conditions when performance and phosphorus excretion are unlikely to be affected.
Key words: cecectomized laying hens, dephosphorylation, phytate, myo-inositol
Introduction
In plant seeds, phosphorus (P) is mainly present as phytic acid [myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6] and phytate. Phytase supplementation in feed can increase the release of P from InsP6 and, hence, P digestibility, in nonruminant animals such as poultry beyond the endogenous potential of the digestive system (Rodehutscord et al., 2023). As a consequence, supplementing broiler chickens with phytase often increased growth performance and P utilization, especially when the diets contained no mineral P (Sommerfeld et al., 2018; Siegert et al., 2019).
In laying hens, previous studies have reported that phytase supplementation often had little or no effect on laying performance and P utilization, probably because the P supply in such studies was higher than the P requirement of the hens (Rodehutscord et al., 2023). This suggests that the effects of the enzyme on its target substrate are beneficial for inclusion in phytase efficiency studies. However, studies on InsP6 degradation in laying hens are scarce. A possible reason for this scarcity may be the fact that InsP6 degradation measurements are elaborate. The effects of post-ileal microbial activity should be excluded because post-ileal P absorption is negligible and microbes in the hindgut dephosphorylate inositol phosphates (Rodehutscord et al., 2022). The cecectomized laying hen is an established model in amino acid digestibility research because it minimizes effects of post-ileal fermentation, which largely occurs in the ceca. Hence, cecectomized birds represent an interesting potential model for studying InsP6 degradation in laying hens.
To our knowledge, there is only one published study on InsP6 degradation using cecectomized laying hens. Agbede et al. (2009) investigated the effects of supplementing 500 phytase units (FTU)/kg of feed, and mineral P was varied using monocalcium phosphate (MCP), which also changed the calcium (Ca) concentration of the feed. These authors determined an increase in InsP6 disappearance (meaning a minimum of one phosphate group released from InsP6) by 28 percentage points upon phytase supplementation, with no influence of MCP. However, commercial phytase products have changed since then and higher phytase supplementation levels have been considered by the industry. In addition, InsP6 disappearance and dephosphorylation of lower inositol phosphate isomers were found to be reduced by dietary Ca and mineral P supplements in broiler chickens (Sommerfeld et al., 2018; Krieg et al., 2021), suggesting interactions between phytase and mineral P supplements might exist in laying hens.
Therefore, the objective of this study was to investigate whether 1,500 FTU phytase/kg and the addition of MCP at unchanged Ca concentrations in the feed influence InsP6 degradation and concentrations of lower inositol phosphate isomers and myo-inositol in the excreta of cecectomized laying hens.
MATERIALS AND METHODS
Experimental Setup
Four dietary treatments were investigated in a 2 × 2-factorial arrangement with or without MCP and with or without phytase supplementation. Each diet was tested in 5 replicates in a row-column design with 10 laying hens in 2 periods. The design was optimized using the OPTEX procedure using the SAS software package (version 9.4, SAS Institute, Cary).
Experimental Diets
Diets mainly consisted of corn, soybean meal, rapeseed meal, and grass meal, and were prepared with and without mineral P by adding MCP (Table 1). Limestone was reduced in the MCP-containing diets to maintain the same Ca concentration. Diamol was used to compensate for mass differences. Diets were either supplemented with 1,500 FTU phytase/kg of an E. coli-derived 6-phytase (Quantum Blue™, AB Vista, Marlborough, UK) or left unsupplemented. The complete diets were pelleted through a 3-mm die without using steam. Diets were calculated to meet or exceed the supply recommendations of the Gesellschaft für Ernährungsphysiologie (1999) for laying hens weighing 1,800 g and a daily egg production of 60 g (except for P). The calculated mineral concentrations and phytase activity were confirmed by analysis (Table 1).
Table 1.
Composition and analysis of the experimental diets.
| Monocalcium phosphate | Without |
With |
||
|---|---|---|---|---|
| Phytase | − | + | − | + |
| Diet composition (g/kg, unless otherwise stated) | ||||
| Corn | 593.2 | |||
| Soybean meal | 110 | |||
| Rapeseed meal | 110 | |||
| Grass meal | 50 | |||
| Soybean oil | 30 | |||
| Premix without mineral phosphorus1 | 20 | |||
| NaCl | 1 | |||
| dl-Methionine | 0.5 | |||
| Monocalcium phosphate | - | 6 | ||
| Limestone (coarse) | 41 | 39.65 | ||
| Limestone (fine) | 41 | 39.65 | ||
| Diamol | 3.3 | - | ||
| Phytase2 (FTU/kg) | - | 1,500 | - | 1,500 |
| Diet analysis (g/kg DM, unless otherwise stated) | ||||
| DM (g/kg) | 913 | 914 | 914 | 914 |
| CP | 164 | 162 | 164 | 164 |
| Crude ash | 144 | 139 | 142 | 139 |
| Crude fat | 57 | -3 | - | - |
| Crude fiber | 43 | - | - | - |
| Gross energy (MJ/kg DM) | 17.4 | 17.4 | 17.4 | 17.5 |
| Calcium | 43 | 43 | 43 | 42 |
| Phosphorus (total) | 4.3 | 4.2 | 5.7 | 5.8 |
| InsP6-P | 2.6 | 2.8 | 2.8 | 2.8 |
| Detected inositol phosphate isomers (µmol/g DM) | ||||
| InsP6 | 14.1 | 15.1 | 14.8 | 15.0 |
| Ins(1,2,3,4,5)P5 | 0.6 | 0.5 | 0.6 | 0.5 |
| Ins(1,2,4,5,6)P5 | 1.0 | 1.1 | 1.0 | 1.1 |
| Myo-inositol | 2.2 | 2.2 | 2.2 | 2.2 |
| Phytase activity (FTU/kg) | <50 | 1,620 | <50 | 1,500 |
Provided per kg of diet: 13.2 g calcium carbonate, 1.4 g sodium chloride, 0.6 g sodium carbonate, 10,000 IE vitamin A (retinylacetate), 2,000 IE vitamin D3 (cholecalciferol), 20 mg vitamin E (all-rac-α-tocopheryl acetate), 3 mg vitamin K3 (menadione sodium bisulphite), 1.6 mg vitamin B1 (thiamine mononitrate), 4 mg vitamin B2 (riboflavin), 2.4 mg vitamin B6 (pyridoxine hydrochloride), 16 µg vitamin B12 (cyanocobalamin), 20 mg niacinamide, 6 mg calcium-d-pantothenate, 0.6 mg folic acid, 0.2 mg biotin, 250 mg choline chloride, 60 mg iron from iron-(II)-sulphate monohydrate, 60 mg manganese from manganese-(II)-sulphate monohydrate, 32 mg zinc from zinc-oxide, 16 mg zinc from zinc-sulphate monohydrate, 4 mg copper from cupric-(II)-sulphate pentahydrate, 0.6 mg iodine from calcium iodate anhydrous, and 0.2 mg selenium from sodium selenite.
Added on top of the diets.
Not determined.
Animals and Housing
The animal trial was approved by Regierungspräsidium Stuttgart under protocol no. V352/18TE and conducted in accordance with German animal welfare regulations (Anonymous, 2006). Laying hens of the strain LSL-Classic were cecectomized at 23 wk of age. The same animals were used in 2 preceding experiments, and the age of the birds during the present trial was 96 to 99 wk. During the trial periods, hens were kept individually in metabolism units measuring 89 cm × 89 cm × 89 cm, which were equipped with perches, nest boxes, feeding troughs, water cups, and a wire mesh floor. Stainless steel trays were installed under the mesh for excreta collection. When not housed in metabolism units, the hens were housed together with accompanying hens in a floor pen on litter. The lighting was switched on from 7:00 h to 21:00 h, and the temperature was set to 20°C.
Experimental Procedures
Each trial period lasted for 8 d, with 4 d of adaptation and 4 d of excreta collection. The hens spent 2 d in a group between the periods. The daily feed allowance during the periods was 120 g, offered in equal meals at 7:00 h and 15:00 h. Feed residues were collected from the feed troughs. Feed spilled into the water cup also was collected, frozen, and later dried at 105°C to correct feed intake on a DM basis. Egg weights were recorded daily. Excreta were completely collected beginning at 7:00 and 15:00 h each day after spilled pellets, feathers, and shed skin flakes were removed from the trays. The excreta were immediately frozen at –20°C after each collection.
Sample Preparation and Chemical Analyses
A centrifugal mill (ZM 200, Retsch GmbH, Haan, Germany) equipped with a 1.0-mm sieve was used to grind the diets for analyses of crude fiber. Other proximate nutrients were analyzed after grinding through a 0.5-mm sieve using the same mill. All other diet analyses were conducted after grinding to a fine powder using a ball mill (MM40, Retsch GmbH, Haan, Germany). Excreta samples were thawed at 4°C, weighed, homogenized, and DM content was analyzed in triplicate. For further analyses, excreta were freeze-dried and pulverized using a vibrating cup mill (Pulverisette 2, Fritsch GmbH, Idar-Oberstein, Germany).
Analysis of DM and proximate nutrients was performed following methods no. 3.1, 4.1.1, 5.1.1b, 8.1, and 6.1.1 of the official methods for nutrient analyses in Germany (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten, 2007). Bomb calorimetry was used to determine gross energy. Inositol phosphate isomers, myo-inositol, calcium, and phosphorus were analyzed as previously described (Sommerfeld et al., 2018). Co-elutions impaired clear discrimination between the isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3. Because of these unknown proportions, the term InsP3x was used for this isomer. Phytase activity in the diets was analyzed by AB Vista Lab Service (Hengoed, UK) using a validated product-specific ELISA method. The results were calculated from a calibration curve with known activity, as determined by the Quantum Blue product analysis, and the activity was expressed as FTU/kg of feed.
Calculations and Statistical Analysis
Data from 1 hen in 1 period represented 1 observation and was treated as the statistical unit. The InsP6 disappearance and nutrient retention for each observation were calculated as follows:
| (1) |
where intake and excretion were calculated as the nutrient concentrations in feed and excreta (g/kg DM) multiplied by the DM intake (g/d) and DM excretion (g/d), respectively. Data were statistically analyzed with the following model using the MIXED procedure in SAS:
| (2) |
where yijkl is the dependent trait, MCPi is the fixed MCP effect (with or without), Phyj is the fixed phytase supplementation effect (0 or 1,500 FTU/kg), henk is the random hen effect (1–10), periodl is the random period effect (1 or 2), and eijkl is the residual error. The normal distribution and homogeneity of variance were verified graphically. The effects were considered significant at P < 0.050.
RESULTS AND DISCUSSION
Two observations were excluded from the evaluation because feed intake during the collection period was low (<90% of the offered amount of 1 hen in period 1 and another hen in period 2). The feed intake of all other hens was almost complete (≥99% of the offered amount) and did not differ among dietary treatments (P = 0.305). The hens laid eggs on at least 7 out of the 8 d of each period in most observations, resulting in a laying rate of 88% or higher. Hen weight and egg weight averaged 1,714 g and 66.1 g, respectively.
The MCP × phytase interaction was not significant for InsP6 disappearance (P = 0.054) (Table 2). Phytase increased InsP6 disappearance from 13% to 83% (P < 0.001), whereas MCP had no significant effect. Lower concentrations of Ins(1,2,3,4,5)P5 and Ins(1,2,4,5,6)P5 were detected in the excreta when phytase was supplemented to the diets (P < 0.001), and MCP increased the concentration of Ins(1,2,3,4,5)P5 (P = 0.005). There was a significant interaction detected for the Ins(1,2,5,6)P4 concentrations in excreta (P = 0.031). When the diets were not supplemented with phytase, the Ins(1,2,5,6)P4 concentration in the excreta was low and was not influenced by MCP. A higher Ins(1,2,5,6)P4 concentration was found in the excreta upon phytase supplementation (P < 0.001), and the addition of MCP to phytase-supplemented diets further increased the Ins(1,2,5,6)P4 concentration (P < 0.001). Overall, the concentrations of inositol phosphates with a lower degree of phosphorylation than 4 were low and, in many cases, below the limit of detection.
Table 2.
Influence of monocalcium phosphate (MCP) and phytase (Phy) supplementation on InsP6 disappearance, retention of phosphorus and calcium, and concentrations of inositol phosphate isomers, and myo-inositol in excreta of cecectomized laying hens.1
| MCP | Without |
With |
MCP3 |
Phy3 |
ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phy2 | − | + | − | + | SEM | Without | With | SEM | − | + | SEM | MCP | Phy | MCP × Phy |
| InsP6 disappearance (%) | 10 | 83 | 16 | 82 | 1.6 | 46 | 49 | 1.2 | 13B | 83A | 1.2 | 0.100 | <0.001 | 0.054 |
| Phosphorus utilization (%) | 14 | 18 | 11 | 4 | 4.9 | 16 | 7 | 3.5 | 12 | 11 | 3.5 | 0.114 | 0.798 | 0.287 |
| Calcium utilization (%) | 34 | 34 | 36 | 39 | 4.0 | 35 | 37 | 3.2 | 35 | 36 | 3.2 | 0.485 | 0.720 | 0.647 |
| Inositol phosphate isomers in excreta (µmol/g DM) | ||||||||||||||
| InsP6 | 35.0 | 6.9 | 34.2 | 7.5 | 0.79 | 21.0 | 20.9 | 0.57 | 34.6A | 7.2B | 0.57 | 0.898 | <0.001 | 0.361 |
| Ins(1,2,3,4,5)P5 | 1.8 | 0.6 | 2.0 | 1.2 | 0.08 | 1.3B | 1.6A | 0.06 | 1.9A | 1.1B | 0.06 | 0.005 | <0.001 | 0.164 |
| Ins(1,2,4,5,6)P5 | 2.6 | 0.6 | 2.9 | 0.8 | 0.17 | 1.6 | 1.9 | 0.13 | 2.8A | 0.7B | 0.13 | 0.162 | <0.001 | 0.639 |
| Ins(1,3,4,5,6)P5 | ND | 0.4 | <LOQ | 0.2 | 0.19 | 0.4 | 0.2 | 0.19 | . | 0.3 | 0.19 | 0.469 | . | . |
| Ins(1,2,3,4,6)P5 | 0.9 | <LOQ | 1.0 | ND | 0.03 | 0.9 | 1.0 | 0.03 | 1.0 | . | 0.03 | 0.055 | . | . |
| Ins(1,2,5,6)P44 | 0.2c | 3.2b | 0.5c | 4.8a | 0.28 | -5 | - | - | - | 0.005 | <0.001 | 0.031 | ||
| Ins(1,2,3,4)P4 | 0.2b | ND | 0.4a | <LOQ | 0.06 | 0.1B | 0.4A | 0.06 | 0.3 | . | 0.06 | 0.022 | . | . |
| InsP3x6 | ND | 1.1b | ND | 1.8a | 0.10 | 1.1B | 1.8A | 0.10 | . | 1.4 | 0.10 | 0.001 | . | . |
| Ins(1,2)P2 | ND | 0.5b | ND | 1.4a | 0.12 | 0.5 | 1.4 | 0.12 | . | 1.0 | 0.08 | <0.001 | . | . |
| Myo-inositol | 1.1 | 4.0 | 1.2 | 3.1 | 0.60 | 2.5 | 2.2 | 0.43 | 1.2B | 3.5A | 0.43 | 0.541 | 0.002 | 0.434 |
<LOQ = below the limit of quantification in the majority of samples; ND = below the detection limit in the majority of samples. The concentrations of other measured inositol phosphate isomers were below the respective detection limits in the majority of samples in all treatments.
Without (−) or with (+) supplementation of 1,500 FTU phytase/kg.
Presented if the interaction was not significant (P > 0.050).
At least one of the following isomers: Ins(1,2,5,6)P4, Ins(2,3,4,5)P4.
Not presented because the interaction was significant (P < 0.050).
At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
In case of significant interactions (P < 0.050) or in case of treatments below the limit of quantification: different lowercase letters indicate significant differences (P < 0.050) between treatments.
In the case of non-significant interactions (P ≥ 0.050): different capital letters indicate significant differences (P < 0.050) within the main effects MCP or Phy.
The results suggested that MCP did not affect InsP6 disappearance in the excreta but influenced the dephosphorylation of Ins(1,2,5,6)P4 when phytase was supplemented. The higher concentrations of Ins(1,2,5,6)P4 compared to other isomers in phytase-supplemented treatments indicated that hydrolysis of this isomer by phytase and other phosphatases limited complete inositol phosphate dephosphorylation. The hydrolysis of Ins(1,2,5,6)P4 also limited the complete dephosphorylation of InsP6 in broiler chickens (Sommerfeld et al., 2018) when the same phytase product was added to the feed.
Supplementation with phytase increased the myo-inositol concentration in the excreta (P = 0.002), whereas MCP had no significant effect. Higher myo-inositol concentrations suggest that more InsP6 is completely dephosphorylated in phytase-supplemented diets. However, myo-inositol concentrations in excreta may also be determined by myo-inositol absorption (Gonzalez-Uarquin et al., 2020), thus making unambiguous conclusions regarding the amount of completely dephosphorylated InsP6 impossible. Myo-inositol may also be catabolized by microorganisms in the digestive tract (Weber and Fuchs, 2022).
The very low and very high levels of InsP6 disappearance in diets with and without phytase supplementation, respectively, may explain the absence of an effect of MCP on InsP6 disappearance in the present study. It has recently been reviewed that in broiler chickens, endogenous InsP6 disappearance is reduced when mineral P is added to diets that are not supplemented with phytase (Rodehutscord et al., 2022). The InsP6 disappearance in diets without phytase supplementation and mineral P was lower in the present study (10%) than prececal InsP6 disappearance found in many studies on broiler chickens (Rodehutscord et al., 2022). This may be related to the high Ca concentrations in laying hen diets, because Ca can form complexes with InsP6, and increasing dietary Ca has been shown to reduce InsP6 disappearance in broiler chickens (Krieg et al., 2021). The markedly low InsP6 disappearance in the present study in diets without phytase supplementation may have made the negative effect of MCP supplementation unlikely to occur. Sommerfeld et al. (2018) indicated that increasing dietary Ca concentrations from 6 to 10 g/kg DM tended to reduce InsP6 disappearance in broiler chickens fed phytase-supplemented diets. The Ca concentrations in the diets of laying hens investigated in the present study were considerably higher (43 g/kg DM). Nevertheless, InsP6 degradation reached 86%, which is close to the maximum value of prececal InsP6 degradation (87%) found in the study by Sommerfeld et al. (2018). This indicates that high Ca concentration in the feed is not a limiting factor for phytase activity in laying hens.
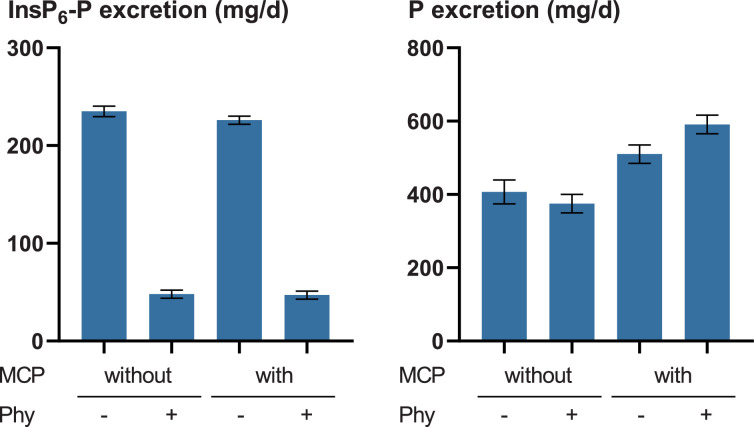
Overall, the values of P utilization were low and were not significantly different (Table 2). While the excretion of InsP6-P was markedly lower when phytase was added to the feed, the total P excretion was not systematically reduced (Figure 1). This indicates that P was excreted by the hens, irrespective of the release of InsP6-P in the digestive tract. Supplemented P from MCP was also excreted to a large extent. The P requirement of the hens was likely not high enough to take advantage of digestible P additionally supplied by MCP or increased InsP6 degradation. This may explain why previous studies found little or no effects of phytase supplementation on laying performance and P utilization in laying hens (Rodehutscord et al., 2023).
Figure 1.
Influence of monocalcium phosphate (MCP) and phytase (Phy) supplementation on excretion of phosphorus (P) and InsP6-P by cecectomized laying hens. The interactions were not significant (P ≥ 0.199) while the Phy and MCP effects were significant for InsP6-P and P excretion, respectively (P < 0.001). Note that the ordinates have a different scale.
In conclusion, the low InsP6 disappearance without phytase and the remarkable effect of phytase supplementation on InsP6 disappearance and InsP6 degradation products in the excreta suggest that cecectomized laying hens are well suited for studying phytase effects on phytate dephosphorylation. The lack of interaction with MCP supplementation indicates that phytase effects on InsP6 degradation can be studied independently of the P concentration of the feed. Whether the enzyme is active on its substrate or not can be determined independently of effects on performance and excretion of the birds.
ACKNOWLEDGMENTS
The authors declare no competing interests and acknowledge the support of I. Kühn (AB Vista, Darmstadt, Germany) for providing the phytase product and analysis of phytase activity. The support of M. Allenbach, P. Hofmann, A. Ibrahim, N. Klein, and S. Wolfrum for assistance with sample collection and preparation is acknowledged.
Funding: This project was conducted using the resources of the University of Hohenheim, and did not receive any external funding.
Disclosures
All authors declare that they have no conflict of interest.
Footnotes
Presented in part by W. Siegert, S. Sommerfeld, and M. Rodehutscord. 2022. Influence of monocalcium phosphate and phytase in the diet on phytate degradation in caecectomised laying hens. Proceedings of the Society of Nutrition Physiology 31, 125.
References
- Agbede J.O., Kluth H., Rodehutscord M. Studies on the effects of microbial phytase on amino acid digestibility and energy metabolisability in caecectomised laying hens and the interaction with the dietary phosphorus level. Br. Poult. Sci. 2009;50:583–591. doi: 10.1080/00071660903196900. [DOI] [PubMed] [Google Scholar]
- Anonymous. Tierschutzgesetz. Bundesgesetzblatt. 2006;25:1206–1222. http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl106s1206.pdf [Google Scholar]
- Gesellschaft für Ernährungsphysiologie . DLG-Verlag; Frankfurt am Main, Germany: 1999. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler) [Google Scholar]
- Gonzalez-Uarquin F., Rodehutscord M., Huber K. Myo-inositol: its metabolism and potential implications for poultry nutrition – a review. Poult. Sci. 2020;99:893–905. doi: 10.1016/j.psj.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg J., Borda-Molina D., Siegert W., Sommerfeld V., Chi Y.P., Taheri H.R., Feuerstein D., Camarinha-Silva A., Rodehutscord M. Effects of calcium level and source, formic acid, and phytase on phytate degradation and the microbiota in the digestive tract of broiler chickens. An. Microb. 2021;3:23. doi: 10.1186/s42523-021-00083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodehutscord M., Sommerfeld V., Kühn I., Bedford M.R. In: Enzymes in Farm Animal Nutrition. 3rd ed. Bedford M.R., Partridge G.G., Hubry M., Walk C.L., editors. CAB International; UK: 2022. Phytases: Potential and limits of phytate destruction in the digestive tract of pigs and poultry Pages 124–152. [DOI] [Google Scholar]
- Rodehutscord M., Sommerfeld V., Angel C.R., Korver D.R. Minimum phosphorus requirements for laying hen feed formulations. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert W., Zuber T., Sommerfeld V., Krieg J., Feuerstein D., Kurrle U., Rodehutscord M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 2019;98:5700–5713. doi: 10.3382/ps/pez355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten . VDLUFA-Verlag; Darmstadt, Germany: 2007. Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Vol. III. Die chemische Untersuchung von Futtermitteln. [Google Scholar]
- Weber M., Fuchs T.M. Metabolism in the niche: a large-scale genome-based survey reveals inositol utilization to be widespread among soil, commensal, and pathogenic bacteria. Microbiol. Spectr. 2022;10:e02013–e02022. doi: 10.1128/spectrum.02013-22. [DOI] [PMC free article] [PubMed] [Google Scholar]