Abstract
Background & Aims
Tenofovir is recommended as part of the first-line antiretroviral therapy (ART) to treat people living with HIV (PLWH) with HBV coinfection. However, the effects of tenofovir-containing ART on hepatocellular carcinoma (HCC) risk among PLWH with/without chronic hepatitis virus infections remain unclear.
Methods
This study included 23,838 PLWH. All of them were males aged ≥20 years and followed prospectively during 2000–2017. Four major nationwide registries – the Human Immunodeficiency Virus surveillance database, Taiwan Cancer Registry, Death Certification System, and National Health Insurance Database – were applied to define ART and comorbidities and ascertain newly diagnosed HCC. Tenofovir-containing ART was identified through prescription records. Cox proportional hazards models were used to determine the association of tenofovir use with HCC incidence.
Results
HCC incidence was lower among ever users of tenofovir than among never users (24.2 and 85.7 per 100,000 person-years, respectively). Ever users had significantly reduced HCC risk (adjusted hazard ratio 0.20, 95% CI 0.13–0.31). The effect of tenofovir use on reduced risk for HCC consistently favored never users across many prespecified subgroups, including HBV or HCV coinfection (p <0.05). The findings were consistent in subgroups of PLWH diagnosed with HIV before tenofovir’s approval and in those born before the nationwide roll-out of neonatal HBV vaccination.
Conclusions
Our findings underscore the need for randomized controlled trials of tenofovir in combination with long-acting injectable ART regimens to assess its safety and efficacy in PLWH, particularly in those with HBV or HCV coinfection.
Impact and implications
Tenofovir’s effect on the risk of hepatocellular carcinoma (HCC) among people living with HIV with hepatitis B or C coinfection remains under investigated. This nationwide prospective cohort study, comprising 23,838 men living with HIV, showed that tenofovir-containing antiretroviral therapy was associated with reduced risk of HCC (adjusted relative risk: 0.20, 95% CI 0.13–0.31), which was consistently observed across many prespecified subgroups. The effect of tenofovir use on HCC risk should be further investigated in PLWH, particularly following the development of long-acting injectable ART regimens.
Keywords: AIDS, chronic hepatitis, risk assessment, nationwide registry, prospective study
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HCC, hepatocellular carcinoma; HRs, hazard ratios; NHI, National Health Insurance; PLWH, people living with HIV
Graphical abstract
Highlights
-
•
Impact of tenofovir-containing ART on HCC risk among PLWH with HBV/HCV coinfection has rarely been investigated.
-
•
This nationwide cohort study found that tenofovir-containing ART reduced the risk of HCC (adjusted HR 0.20, 95% CI 0.13–0.31).
-
•
Compared with never use, tenofovir use was associated with reduced HCC risk across many subgroups, including those with HBV/HCV.
-
•
Our study implied the need for RCTs to assess the effects of tenofovir in combination with long-acting ART on HCC risk.
Introduction
Globally, approximately 38 million people are infected with HIV. People living with HIV (PLWH) are at an increased likelihood of HBV or HCV infection,1,2 and this coinfection may exacerbate morbidity and mortality beyond that caused by either infection alone.3 Among PLWH, 40% of liver-related causes of death are hepatocellular carcinoma (HCC).4 Compared with the general population, PLWH have a lifelong increased risk of HCC.5
Tenofovir is a nucleotide analogue reverse transcriptase inhibitor that is used in antiretroviral therapy (ART) for HIV treatment.6 On the basis of its established efficacy and tolerability against HIV and HBV,7,8 the World Health Organization recommends a tenofovir-containing regimen as the first-line ART, particularly for PLWH with HBV coinfection.9 However, the TREAT Asia HIV Observational Database revealed that only half of PLWH with HBV coinfection received tenofovir-containing ART, even in high-income countries.10
Only a few studies have investigated HCC incidence or mortality changes after tenofovir became available. A prospective study that followed up PLWH with HBV coinfection reported that all-cause mortality had markedly decreased over time, coinciding with the introduction of tenofovir.11 In addition, the difference in overall mortality in HIV/HBV coinfection vs. HIV monoinfection has significantly diminished since tenofovir’s approval.12 Limited studies have compared PLWH with or without tenofovir-containing ART to determine whether tenofovir affects HCC risk.
In this study, we used nationwide registries to evaluate whether tenofovir affects HCC incidence in PLWH with or without HBV/HCV coinfection.
Patients and methods
Study design
This nationwide cohort study was conducted to determine HCC incidence among PLWH who received ART with or without tenofovir in 2000–2017. We integrated four national health registries provided by the Taiwanese Ministry of Health and Welfare. The administrative and claims data stored in these registries are complete, accurate, and up-to-date.13 The HIV surveillance database was used to identify people who received a diagnosis of HIV between 2000–2016. We obtained their treatment information through computerized data linkage with the National Health Insurance (NHI) Database. In addition, the Taiwan Cancer Registry was used to identify PLWH with newly diagnosed HCC and their diagnosis dates. The death certification system was used to determine if any PLWH in our cohort died between 2000 and December 31, 2017. The patient data were linked using citizenship identification numbers and birthdates. The study protocol (YM109165E) was approved by the Institutional Review Board of National Yang Ming Chiao Tung University, Taipei, Taiwan.
Study cohort
According to Taiwanese law, all cases of newly diagnosed HIV or acquired immunodeficiency syndrome (AIDS) must be reported to the Taiwan Centers for Disease Control within 24 h. The HIV surveillance database includes all reported HIV cases with seropositive antibodies against HIV or detectable HIV RNA on a PCR test. The route of transmission is also recorded. For the period 2000–2016, a total of 30,328 PLWH aged ≥20 years were identified in the surveillance database. Of them, 28,531 (93.5%) were men. Patients with any cancer diagnosis before HIV diagnosis were excluded. Eventually, 23,838 male PLWH were included in the analysis.
Definitions of tenofovir use and comorbidities
In Taiwan, NHI is compulsory and covers >99% of all residents.14 The NHI database contains detailed claims information on drug prescriptions, medical procedures, outpatient visits, inpatient hospitalizations, and dates. ART prescriptions were determined using Anatomical Therapeutic Chemical codes in the patients’ post-HIV-diagnosis prescription records (Table S1). The comorbidities of chronic HBV or HCV infection, cirrhosis, and diabetes were identified using ICD-9 or ICD-10 codes (Table S2) provided the following criterion was met: at least one hospital admission code with the diagnosis or with ≥3 outpatient visits; this criterion has good validity.15
Follow-up to ascertain newly diagnosed HCC
The patients were followed up from the date of HIV diagnosis. After computerized data linkage with the National Cancer Registry, HCC incidence and dates of diagnosis were determined using ICD-9 code 155 and ICD-10 code C22. In all cases, the diagnosis of HCC was established based on pathological or radiographic criteria.
Statistical analysis
The baseline characteristics of users and non-users of tenofovir-containing ART regimens were compared using chi-square tests. The duration of follow-up was calculated for each patient as the time from the HIV diagnosis date to the date of HCC diagnosis, death, or end of the study period (i.e. December 31, 2017), whichever came first. HCC incidence was derived by dividing the number of cases by the person-years of follow-up. The cumulative risk of HCC in terms of tenofovir use was estimated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards models were used to obtain the crude and adjusted hazard ratios (HRs) with 95% CIs for tenofovir use on HCC risk. The proportionality assumptions (non-changing HRs over time) of the Cox models were examined, and the assumptions were found to not be violated. The potential confounders associated with HCC included age, AIDS, HBV/HCV, diabetes, cirrhosis, and HIV diagnostic year. We initially evaluated these covariates in the univariate analyses separately. The covariates significant in the univariate models were further included in the multiple Cox’s regression models to evaluate the impacts of tenofovir-containing regimens on HCC incidence. Tenofovir was approved for HIV treatment and reimbursement of its cost began in Taiwan in 2011; thus, the year of HIV diagnosis (before or after 2011) was treated as a covariate in the multiple regression models. In the subgroup of patients receiving an HIV diagnosis before 2011 (n = 14,407, 60.4%), we evaluated the effect of tenofovir on HCC risk. We repeated the subgroup analyses after stratifying by baseline characteristics and chronic hepatitis virus infection status (HBV, HCV, or both). Taiwan launched a nationwide neonatal hepatitis B immunization program in 1986; thus, we performed sensitivity analyses by repeating all analyses in the patients born before 1986 (i.e. without HBV vaccination at birth; n = 18,020; 75.6%). There were three and zero cases of HCC among the men receiving a HIV diagnosis after 2011 and HBV vacinees born after 1986, respectively. We did not perform additional analyses by restricting these groups. Statistical significance was defined as a two-sided p value of <0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
The patients’ baseline characteristics are presented in Table 1. The mean age was 32.2 years, and 81.1% of the patients were 20–39 years old. Among the study population, 48.1% received a diagnosis of AIDS, and 29.3% received a diagnosis of HBV or HCV infection. Compared with non-users, users of tenofovir were older (p <0.001); had a lower prevalence of AIDS (p <0.001), cirrhosis (p = 0.03), and diabetes (p = 0.003); and had a higher prevalence of HBV (p <0.001).
Table 1.
Baseline characteristics of the study population, stratified by the use of tenofovir-containing antiretroviral therapy.
| Baseline characteristic | Total (N = 23,838) No. (%) |
Tenofovir users (n = 14,865) No. (%) |
Tenofovir non-users (n = 8,973) No. (%) |
|---|---|---|---|
| Age (years), mean ± SD | 32.2 ± 9.3 | 32.9 ± 10.2 | 31.7 ± 8.7 |
| 20–29 | 11,306 (47.4) | 7,214 (48.5) | 4,092 (45.6) |
| 30–39 | 8,034 (33.7) | 5,091 (34.3) | 2,943 (32.8) |
| 40–49 | 3,229 (13.6) | 1,944 (13.1) | 1,285 (14.3) |
| 50–59 | 942 (4.0) | 493 (3.3) | 449 (5.0) |
| ≥60 | 327 (1.4) | 123 (0.8) | 204 (2.3) |
| Diagnosis of AIDS | |||
| No | 12,373 (51.9) | 8,061 (54.2) | 4,312 (48.1) |
| Yes | 11,465 (48.1) | 6,804 (45.8) | 4,661 (51.9) |
| Transmission route | |||
| MSM | 16,566 (69.5) | 10,547 (71.0) | 6,019 (67.1) |
| People who inject drugs | 4,081 (17.1) | 2,576 (17.3) | 1,505 (16.8) |
| Heterosexual | 3,120 (13.1) | 1,703 (11.5) | 1,417 (15.8) |
| Other | 71 (0.3) | 39 (0.3) | 32 (0.4) |
| Chronic hepatitis virus infection | |||
| HBV only | 2,099 (8.8) | 1,733 (11.7) | 366 (4.1) |
| HCV only | 3,724 (15.6) | 2,279 (15.3) | 1,445 (16.1) |
| HBV and HCV | 1,155 (4.9) | 878 (5.9) | 277 (3.1) |
| None | 16,860 (70.7) | 9,975 (67.1) | 6,885 (76.7) |
| Cirrhosis | |||
| No | 23,701 (99.4) | 14,792 (99.5) | 8,909 (99.3) |
| Yes | 137 (0.6) | 73 (0.5) | 64 (0.7) |
| Diabetes | |||
| No | 23,231 (97.5) | 14,529 (97.7) | 8,702 (97.0) |
| Yes | 607 (2.6) | 336 (2.3) | 271 (3.0) |
AIDS, acquired immunodeficiency syndrome; MSM, men who have sex with men.
In our cohort, 14,865 (62.4%) had ever received a tenofovir-containing regimen; of them, 14,193 (95.5%) received it after 2011. Table S3 presents the number of tenofovir users by HIV diagnostic year and various chronic hepatitis virus infection statuses. After 2011, higher proportions of PLWH were tenofovir users. However, the proportion of tenofovir use was still substantial (54.2%) among male PLWH diagnosed before 2011 (n = 8,065), indicating a switch in ART regimens after 2011. Moreover, 82.6% of male PLWH with HBV coinfection were prescribed a tenofovir-containing regimen (p <0.001).
Incidence of HCC
The mean follow-up duration was 8.5 years. After 201,936 person-years of follow-up, 97 cases of HCC were identified, yielding an incidence rate of 48 per 100,000 person-years. The HCC incidence was lower among users of tenofovir than among non-users (24.2 and 85.7 per 100,000 person-years, respectively). HCC incidence was higher among patients with advanced age, AIDS, cirrhosis or diabetes, and HIV diagnostic year before 2011 (p <0.05; Table 2). The incidence rate was higher for patients who acquired HBV or HCV coinfection than those without coinfection (p <0.001). At the end of follow-up, approximately 0.62% of users had received an HCC diagnosis compared with 1.67% of non-users (p <0.0001; Fig. 1A). Among the male PLWH before 2011, tenofovir use was still associated with significantly lower cumulative risk of HCC (0.61% vs. 1.76%, p <0.0001; Fig. 1B) throughout follow-up.
Table 2.
Number and incidence of hepatocellular carcinoma cases by baseline characteristics.
| Baseline characteristic | Number of patients (n = 23,838) | Number of HCC cases (n = 97) | Person-years of follow-up | Incidence rate† | p value |
|---|---|---|---|---|---|
| Tenofovir-based regimen | |||||
| Non-users | 8,973 | 67 | 78,174 | 85.7 | |
| Users | 14,865 | 30 | 123,762 | 24.2 | <0.0001 |
| Age (years) | |||||
| 20–29 | 11,306 | 9 | 92,936 | 9.7 | |
| 30–39 | 8,034 | 25 | 71,508 | 35.0 | |
| 40–49 | 3,229 | 41 | 27,644 | 148.3 | |
| 50–59 | 942 | 11 | 7,557 | 145.6 | |
| ≥60 | 327 | 11 | 2,290 | 480.3 | <0.0001 |
| Diagnosis of AIDS | |||||
| No | 12,373 | 32 | 101,528 | 31.5 | |
| Yes | 11,465 | 65 | 100,407 | 64.7 | 0.0002 |
| Chronic hepatitis virus infection | |||||
| HBV only | 2,099 | 26 | 18,862 | 137.8 | |
| HCV only | 3,724 | 27 | 40,183 | 67.2 | |
| HBV + HCV | 1,155 | 31 | 12,619 | 245.7 | |
| None | 16,860 | 13 | 130,273 | 10.0 | <0.0001 |
| Cirrhosis | |||||
| No | 23,701 | 90 | 201,041 | 44.8 | |
| Yes | 137 | 7 | 895 | 782.4 | <0.0001 |
| Diabetes | |||||
| No | 23,231 | 90 | 197,511 | 45.6 | |
| Yes | 607 | 7 | 4,425 | 158.2 | 0.0165 |
| HIV diagnosis year | |||||
| Before 2011 | 14,407 | 93 | 160,494 | 58.0 | |
| After 2011 | 9,431 | 4 | 41,442 | 9.7 | <0.0001 |
AIDS, acquired immunodeficiency syndrome; HCC, hepatocellular carcinoma.
per 100,000 person-years.
Fig. 1.
Cumulative risk of HCC in people living with HIV who have ever or never used tenofovir-containing ART.
(A) Total population; (B) people receiving an HIV diagnosis before 2011. Log-rank tests used to examine the differences in cumulative risks, which demonstrated significance (p <0.0001). ART, antiretroviral therapy; HCC, hepatocellular carcinoma.
Relative risk of HCC given tenofovir use
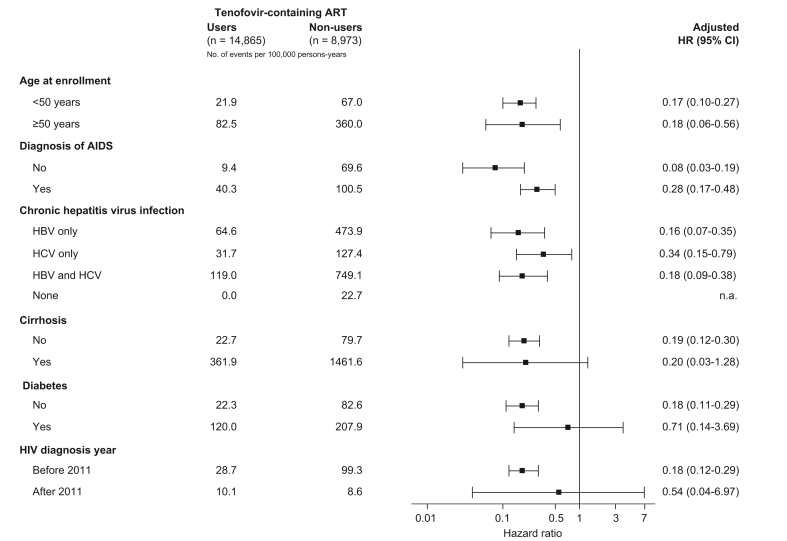
Users of tenofovir-containing ART had decreased risk of HCC compared to non-users, with a crude HR of 0.29 (0.19-0.45). Age, AIDS, chronic hepatitis virus infection, cirrhosis, diabetes and HIV diagnostic year showed significant associations with HCC and were included in the multivariate models. Ever users of tenofovir exhibited a significantly reduced risk of HCC (adjusted HR 0.20, 95% CI 0.13–0.31) after adjustment for age, AIDS, chronic hepatitis virus infection, cirrhosis, diabetes, and HIV diagnostic year (Table 3). In the multiple models, advanced age, HBV/HCV coinfection, and cirrhosis were associated with significant risk of HCC (p <0.05). Among male PLWH before 2011, tenofovir use was consistently associated with decreased HCC risk (adjusted HR 0.18, 95% CI 0.12–0.29) when non-users were taken as the reference group. Among PLWH who used tenofovir-containing regimens, taking PLWH who had used tenofovir-containing ART for <1 year as a reference group, those who used tenofovir-containing ART for longer durations had a decreased risk of HCC, with a corresponding adjusted HR of 0.23 (0.09-0.62) for 1 year to <2 years and 0.05 (0.02-0.13) for ≥2 years. In the subgroup analysis (Fig. 2), tenofovir use was shown to be beneficial with respect to HCC, and the HCC incidence was generally lower among tenofovir users than non-users. The effect of tenofovir use was associated with a consistent reduced HCC risk across many prespecified subgroups. Among PLWH with hepatitis B infection only, tenofovir-containing regimens were associated with a decreased risk of HCC, with an adjusted HR of 0.16 (95% CI 0.07–0.35).
Table 3.
Tenofovir-containing regimen associated with decreased risk of hepatocellular carcinoma among men with HIV infection.
| Total (N = 23,838) |
HIV diagnosis before 2011 (n = 14,407) |
|||
|---|---|---|---|---|
| Characteristics | Crude HR (95% CI) | Adjusted HR∗ (95% CI) | Crude HR (95% CI) | Adjusted HR∗ (95% CI) |
| Tenofovir-based regimen | ||||
| Non-users | Reference | Reference | Reference | Reference |
| Users | 0.29 (0.19–0.45) | 0.20 (0.13–0.31) | 0.28 (0.18–0.44) | 0.18 (0.12–0.29) |
| Age (years) | 1.10 (1.08–1.11) | 1.08 (1.06–1.10) | 1.09 (1.07–1.10) | 1.09 (1.07–1.10) |
| Diagnosis of AIDS | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 1.99 (1.30–3.03) | 1.41 (0.91–2.18) | 1.86 (1.21–2.87) | 1.39 (0.89–2.17) |
| Chronic hepatitis virus infection | ||||
| None | Reference | Reference | Reference | Reference |
| HBV only | 12.98 (6.67–25.27) | 18.58 (9.45–36.54) | 10.70 (5.42–21.12) | 17.03 (8.54–33.96) |
| HCV only | 5.99 (3.08–11.65) | 6.39 (3.28–12.47) | 4.92 (2.52–9.60) | 5.93 (3.03–11.59) |
| HBV and HCV | 21.80 (11.36–41.82) | 30.95 (15.77–60.74) | 18.38 (9.60–35.21) | 31.02 (15.86–60.67) |
| Cirrhosis | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 19.29 (8.93–41.67) | 2.51 (1.09–5.77) | 13.98 (5.67–34.46) | 1.74 (0.66–4.56) |
| Diabetes | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 3.67 (1.70–7.92) | 0.78 (0.35–1.77) | 3.30 (1.44–7.55) | 0.75 (0.32–1.78) |
| HIV diagnosis year | ||||
| Before 2011 | Reference | Reference | ||
| After 2011 | 0.29 (0.10–0.81) | 0.75 (0.26–2.17) | ||
HR, hazard ratio. Cox's proportional hazards models used in the analyses.
Adjusted for age, diagnosis of AIDS, chronic hepatitis virus infection, cirrhosis, diabetes.
Fig. 2.
Subgroup analysis of tenofovir-containing ART for the reduced risk of hepatocellular carcinoma.
Stratified by one of the following parameters and adjusted for the remaining following parameters: age, AIDS, cirrhosis, diabetes, and HIV diagnosis year; individuals with HBV or HCV diagnosis were additionally adjusted for anti-HBV or anti-HCV treatments. Cox’s proportional hazards models used in the analyses. AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HR, hazard ratio.
Sensitivity analysis
Among male PLWH who did not receive HBV vaccination at birth (born before 1986; n = 18,020; 75.6%), users of tenofovir had a lower risk of HCC than non-users (adjusted HR: 0.20, 95% CI 0.13–0.31; Table S4). Among men with a diagnosis of HIV before 2011 (n = 13,073), users had significantly lower HCC risk than non-users (adjusted HR: 0.18, 95% CI 0.12–0.29). The findings were consistent when stratifying the patients with HBV, HCV, or HBV-HCV coinfection, with adjusted HRs of 0.16 (95% CI 0.07–0.36), 0.34 (95% CI 0.15–0.79), and 0.18 (95% CI 0.08–0.37), respectively.
Discussion
This large-scale nationwide prospective study discovered that PLWH who had ever used tenofovir-containing ART had substantially lower risk of incident HCC than non-users. The apparent benefits of tenofovir were consistent, regardless of age, AIDS diagnosis, and HBV/HCV coinfection, which suggests that the benefits of tenofovir may apply to a broad PLWH population. The findings were consistent when the analyses were restricted to male PLWH before approval of tenofovir-containing ART and to those who did not receive HBV vaccination at birth.
In Taiwan, HIV is a notifiable infectious disease and must be reported in electronic registries for management and reimbursement for testing and treatments. The Centers for Disease Control provide PLWH with free-of-charge inpatient and outpatient care, including ART, management of opportunistic illness, and clinical monitoring. Patients with an HIV diagnosis should be periodically monitored for HBV and HCV. Tenofovir disoproxil fumarate and tenofovir alafenamide are two tenofovir prodrugs. The tenofovir-containing regimen defined in our study included both prodrugs. However, the tenofovir alafenamide-containing regimen has only been covered under the NHI since 2017; thus, most of our patients received tenofovir disoproxil fumarate. Tenofovir disoproxil fumarate may cause nephrotoxicity and reduce bone mineral density, whereas a tenofovir alafenamide-containing regimen causes non-inferior viral suppression and leads to improved renal function and bone mineral density.16
Lamivudine has dual antiviral activity against HBV and HIV and may delay liver disease progression.17 Although it has been the most commonly used agent for PLWH with HBV since the mid-90s, approximately 30% of patients developed HBV resistance after 1 year of treatment.18 Tenofovir with a high genetic barrier can achieve sustained HBV suppression even among PLWH with lamivudine-resistant HBV,19 and this HBV suppression can subsequently reduce liver stiffness.20 In the current study, 55% of male PLWH before 2011 may have switched to tenofovir-containing ART after its approval. In addition to HBV suppression, tenofovir-containing ART was associated with a superior HIV virological response and better tolerability.21 Compared with other nucleoside reverse transcriptase inhibitors, tenofovir has less drug substitution rates.22
Besides HBV coinfection, we addressed PLWH with HCV coinfection, which have rarely been investigated. At least 15% of PLWH in our cohort had HCV coinfection. We observed that tenofovir decreased HCC risk among those with HCV coinfection but not HBV coinfection, implying tenofovir may have antitumor activity.23 Tenofovir may induce DNA damage and cell cycle arrest in human cancer cells.24 Comparing the differences of incidence rates on extrahepatic cancers among PLWH received tenofovir-containing regimens or non-users may provide additional evidence to examine the antitumor effects of tenofovir. However, it would likely require a longer follow-up time to obtain sufficient numbers of events, because of the young population (mean age = 32 years old). Tenofovir-containing ART is an effective pre-exposure prophylaxis against HIV25 and HBV.26 A serological follow-up study demonstrated that tenofovir-containing ART had an excellent protective effect against incident HBV,26 which may explain the reduced HCC risk among PLWH without neonatal HBV vaccination. Unfortunately, the claims dataset lacked data on HBV serological markers, making it difficult to evaluate new HBV infections in the study. In the future, monitoring PLWH periodically in clinical settings for the occurrence of either new HBV or HCV infections might be relevant. Moreover, as there were only 30 HCC cases among PLWH who received tenofovir-containing ART, additional subgroups analyses were limited; thus, the impact of time on tenofovir on HCC risk needs to be evaluated in other large cohorts. Taken together, our findings imply that tenofovir-containing ART may have additional benefits even among those without HBV coinfection. Although HCC risk decreased among PLWH who used tenofovir, HCC may still develop, particularly in those with HBV and HCV coinfections, supporting the importance of regular surveillance for HCC.27
Efficacy, durability, safety, additional need for laboratory monitoring, and adherence are key issues in the selection of antivirals for the management of PLWH. Cabotegravir and rilpivirine is a two-drug formulation administered as a monthly intramuscular injection that is approved by the US Food and Drug Administration as a complete regimen for treating HIV. This regimen does not incur a pill burden or stigma relating to HIV treatment.28,29 Randomized trials have indicated that it is non-inferior to standard oral therapy for maintaining HIV suppression28,29 and for HIV prophylaxis.30 Such long-acting injectable regimens may simplify HIV treatment, increase patient satisfaction, and facilitate adherence. These advantages may cause the long-acting injectable regimen to become a priority among the antiretrovirals against HIV. However, trials of long-acting injection regimens have not adequately addressed the issue of HBV or HCV coinfection, but these viruses have similar transmission routes to HIV.[28], [29], [30] Future studies should investigate the role of tenofovir in combination with the long-acting regimen in HIV-HBV/HCV coinfection, particularly in the Asian-Pacific region where chronic HBV is prevalent.31
A strength of our study is the inclusion PLWH from a nationwide surveillance database, with validated and prospectively updated data regarding tenofovir use, comorbidities, and HCC incidence. The complete follow-up of nationwide registries addresses selection bias and minimizes reverse causation. Most PLWH received their HIV diagnosis at a young age;11,12 thus, it may take a long time to compare the effects of various ARTs on HCC incidence. A large population-based study provides an exceptional opportunity to investigate HCC risk, especially in association with tenofovir use and HBV/HCV status. This is the first comprehensive study to evaluate the influence of tenofovir on HCC risk in PLWH with HBV/HCV coinfection.
This study has several limitations. First, we lacked information regarding alcohol consumption, immunological recovery, viral load, HCC screening, specific fibrosis stage, and actual adherence. A large cohort study found suppressed HBV DNA decreased HCC risk among PLWH with HBV coinfection,32 suggesting that HBV-related seromarkers were relevant in monitoring PLWH. Unfortunately, the claims dataset lacks personal laboratory information, such as HBV DNA levels. Although we performed analyses according to chronic hepatitis virus infection status, we lacked data on antibodies to hepatitis B core antigen, a critical seromarker that indicates previous HBV infection. Thus, the HCC risk for PLWH without the diagnosis of chronic hepatitis virus infection might be underestimated. However, we performed analyses among HBV non-vaccinees and the results consistently showed reduced HCC risk in tenofovir users compared to non-users. In Taiwan, our government started to reimburse direct-acting antivirals for patients with chronic hepatitis C from 2017. However, strict reimbursement criteria including failure of interferon-based treatment or liver fibrosis ≧F3 was needed. In 2019, all patients with chronic hepatitis C could be reimbursed without criteria for direct-acting antiviral treatment. Thus, most of the PLWH with chronic hepatitis C infection in the study received interferon-based regimen. However, we lacked information on sustained virological response for chronic hepatitis C. As an observational study based on national registries, there were several potential confounders that could not be addressed. Beyond chronic hepatitis virus-related seromarkers, we lacked information on obesity and metabolic dysfunction-associated fatty liver disease. However, we considered the information on diabetes in the multiple regression models as a proxy. Second, women were not included in the study, limiting the generalizability of our results. However, the efficacy of tenofovir should not vary by sex. Finally, most people residing in HBV-endemic areas are exposed to HBV infection through mother-to-infant transmission; thus, the impacts of tenofovir on HCC risk should be further evaluated in other populations.
In conclusion, this large nationwide study indicated that among PLWH, ever use of tenofovir-containing ART was associated with significantly lower risk of incident HCC. Our findings support the need for randomized controlled trials of tenofovir in combination with long-acting ARTs to assess the impact on HCC prevention.
Financial support
This study was supported by the Ministry of Science and Technology, Taipei, Taiwan (109-2926-1010-504 and 109-2628-B-010-010), and the National Health Research Institutes, Taiwan (NHRI-EX111-11117PI). None of the funding organizations played a role in the study design or conduct; data collection, management, analysis, or interpretation; data preparation or review; or manuscript approval.
Authors’ contributions
Study concept and design: Mei-Hsuan Lee; acquisition of data: Mei-Hsuan Lee; analysis and interpretation of data: Mei-Hsuan Lee, Tzu-I Chen; drafting of the manuscript: Mei-Hsuan Lee; critical revision of the manuscript for important intellectual content: Mei-Hsuan Lee, Ping-Feng Wu, Tzu-I Chen, Chi Chan, Hsi-Hsun Lin, Yi-Hsiang Huang, Hsuan-Yu Chen, Yi-Tsung Lin, Chien-Jen Chen; obtained funding and study supervision: Mei-Hsuan Lee.
Data availability statement
The usage of the claims dataset should be approved by the National Health Insurance Database Research committee.
Conflict of interest
The authors disclose no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100634.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Kourtis A.P., Bulterys M., Hu D.J., Jamieson D.J. HIV–HBV coinfection — a global challenge. N Engl J Med. 2012;366:1749–1752. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platt L., Easterbrook P., Gower E., McDonald B., Sabin K., McGowan C., et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 3.Sun J., Althoff K.N., Jing Y., Horberg M.A., Buchacz K., Gill M.J., et al. Trends in hepatocellular carcinoma incidence and risk among persons with HIV in the US and Canada, 1996-2015. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal E., Roussillon C., Salmon-Ceron D., Georget A., Henard S., Huleux T., et al. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalite 2010 survey. HIV Med. 2015;16:230–239. doi: 10.1111/hiv.12204. [DOI] [PubMed] [Google Scholar]
- 5.Shiels M.S., Engels E.A. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6–11. doi: 10.1097/COH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sax P.E., Wohl D., Yin M.T., Post F., DeJesus E., Saag M., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. [DOI] [PubMed] [Google Scholar]
- 7.Benhamou Y., Tubiana R., Thibault V. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N Engl J Med. 2003;348:177–178. doi: 10.1056/NEJM200301093480218. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P., Heathcote E.J., Buti M., Gane E., de Man R.A., Krastev Z., et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 9.WHO . World Health Organization; Geneva: 2021. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. [Google Scholar]
- 10.Boettiger D.C., Kerr S., Ditangco R., Chaiwarith R., Li P.C., Merati T.P., et al. Tenofovir-based antiretroviral therapy in HBV-HIV coinfection: results from the TREAT Asia HIV Observational Database. Antivir Ther. 2016;21:27–35. doi: 10.3851/IMP2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Welzen B.J., Smit C., Boyd A., Lieveld F.I., Mudrikova T., Reiss P., et al. Decreased all-cause and liver-related mortality risk in HIV/hepatitis B virus coinfection coinciding with the introduction of tenofovir-containing combination antiretroviral therapy. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai W.C., Hsu W.T., Liu W.D., Sun H.Y., Chuang Y.C., Huang Y.S., et al. Impact of antiretroviral therapy containing tenofovir disoproxil fumarate on the survival of patients with HBV and HIV coinfection. Liver Int. 2019;39:1408–1417. doi: 10.1111/liv.14059. [DOI] [PubMed] [Google Scholar]
- 13.Chiang C.J., You S.L., Chen C.J., Yang Y.W., Lo W.C., Lai M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45:291–296. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 14.Wen C.P., Tsai S.P., Chung W.S. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008;148:258–267. doi: 10.7326/0003-4819-148-4-200802190-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sheu M.J., Liang F.W., Li S.T., Li C.Y., Lu T.H. Validity of ICD-10-CM codes used to identify patients with chronic hepatitis B and C virus infection in administrative claims data from the Taiwan National Health Insurance outpatient claims dataset. Clin Epidemiol. 2020;12:185–192. doi: 10.2147/CLEP.S236823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills A., Arribas J.R., Andrade-Villanueva J., DiPerri G., Van Lunzen J., Koenig E., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 17.Liaw Y.F., Sung J.J.Y., Chow W.C., Farrell G., Lee C.Z., Yuen H., et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 18.Dienstag J.L., Schiff E.R., Wright T.L., Perrillo R.P., Hann H.-W.L., Goodman Z., et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 19.Benhamou Y., Fleury H., Trimoulet P., Pellegrin I., Urbinelli R., Katlama C., et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 20.Stockdale A.J., Phillips R.O., Beloukas A., Appiah L.T., Chadwick D., Bhagani S., et al. Liver fibrosis by transient elastography and virologic outcomes Aafter introduction of Tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis. 2015;61:883–891. doi: 10.1093/cid/civ421. [DOI] [PubMed] [Google Scholar]
- 21.Sax P.E., Tierney C., Collier A.C., Fischl M.A., Mollan K., Peeples L., et al. Abacavir–lamivudine versus tenofovir–emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velen K., Lewis J.J., Charalambous S., Grant A.D., Churchyard G.J., Hoffmann C.J. Comparison of tenofovir, zidovudine, or stavudine as part of first-line antiretroviral therapy in a resource-limited-setting: a cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi J., Kim H.J., Lee J., Cho S., Ko M.J., Lim Y.S. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruning A., Burger P., Gingelmaier A., Mylonas I. The HIV reverse transcriptase inhibitor tenofovir induces cell cycle arrest in human cancer cells. Invest New Drugs. 2012;30:1389–1395. doi: 10.1007/s10637-011-9704-7. [DOI] [PubMed] [Google Scholar]
- 25.Mayer K.H., Molina J.M., Thompson M.A., Anderson P.L., Mounzer K.C., De Wet J.J., et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396:239–254. doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatanaga H., Hayashida T., Tanuma J., Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013;56:1812–1819. doi: 10.1093/cid/cit145. [DOI] [PubMed] [Google Scholar]
- 27.Wandeler G., Mauron E., Atkinson A., Dufour J.F., Kraus D., Reiss P., et al. Incidence of hepatocellular carcinoma in HIV/HBV-coinfected patients on tenofovir therapy: relevance for screening strategies. J Hepatol. 2019;71:274–280. doi: 10.1016/j.jhep.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Orkin C., Arasteh K., Górgolas Hernández-Mora M., Pokrovsky V., Overton E.T., Girard P.-M., et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 29.Swindells S., Andrade-Villanueva J.-F., Richmond G.J., Rizzardini G., Baumgarten A., Masiá M., et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 30.Landovitz R.J., Donnell D., Clement M.E., Hanscom B., Cottle L., Coelho L., et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385:595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollinger R.C., Thio C.L., Sulkowski M.S., McKenzie-White J., Thomas D.L., Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. The lancet HIV. 2020;7:e443–e448. doi: 10.1016/S2352-3018(19)30342-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H.N., Newcomb C.W., Carbonari D.M., Roy J.A., Torgersen J., Althoff K.N., et al. Risk of HCC with hepatitis B viremia among HIV/HBV-coinfected persons in north America. Hepatology. 2021;74:1190–1202. doi: 10.1002/hep.31839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The usage of the claims dataset should be approved by the National Health Insurance Database Research committee.